Given the centrality of GFR in characterizing CKD, much effort has focused on different ways to determine GFR. The gold standard method is a measured GFR (mGFR) with the clearance of an exogenous ideal marker that is biologically inert and exclusively cleared by glomerular filtration without tubular reabsorption or secretion. Inulin clearance has been considered the gold standard, although there may be other exogenous markers with these properties. Iothalamate clearance is much more widespread in its use in the United States and can be considered a gold standard mGFR for the arguments that we will make. The major problem with clinical application of mGFR is that it is invasive, inconvenient, and too expensive for routine use in most settings. In practice, alternative methods to mGFR that are less invasive, more convenient, or less expensive are often used for determining GFR.
With all alternative methods, there is potential bias (i.e., systematic deviation) compared with mGFR. One alternative method with a concern for bias is urinary creatinine clearance (CrCl). Urinary CrCl is believed to overestimate GFR and do so more severely at lower levels of GFR because of increased tubular secretion of creatinine. Another alternative method with a widely held concern for bias is eGFR, which is believed to underestimate GFR at high levels of GFR. Findings from these two examples have been based on analyses where bias was assessed across levels of GFR. However, there are different ways to assess bias across levels of GFR. Should bias be assessed across levels of mGFR or across levels of the alternative method? Is there only one correct approach? We will argue that there is a best approach for comparing clearance-based methods (e.g., comparing CrCl with mGFR) and a different best approach for comparing eGFR with mGFR.
In this issue of the Clinical Journal of the American Society of Nephrology, Zhang et al. (1) use the Chronic Renal Insufficiency Cohort to assess bias between CrCl and mGFR. They challenge the widely held belief that urinary CrCl overestimates GFR more severely at lower levels of GFR (1). This challenge is not on the basis of unique data but rather, a different analytic approach. As with prior studies, they reproduce the finding that has been used to support the claim of increased tubular secretion of creatinine as GFR declines. Specifically, they show that, although the ratio of CrCl to mGFR was, on average, 1.13, this ratio increases as mGFR decreases. They then show that, to some extent, this finding can be explained by a statistical phenomenon known as regression to the mean. Regression to the mean occurs because of measurement error; if you were to obtain mGFR in a group of individuals twice, those with low mGFR will tend to have a higher mGFR on repeat, and those with high mGFR will tend to lower mGFR on repeat. In this analysis, CrCl can be viewed as being similar to repeat mGFR. Because a low mGFR is, in part, caused by measurement error, those with a low mGFR will tend to have a relatively higher CrCl (regression to the mean), and this will increase the ratio of CrCl to mGFR (Table 1).
Table 1.
Assessment of bias between different GFR methods across levels of GFR
| GFR Methods Compared | mGFR Versus CrCl | mGFR Versus eGFR |
| Commonly used x axis | mGFR | mGFR or (mGFR+eGFR)/2 |
| Commonly used y axis | CrCl-to-mGFR ratio | eGFR−mGFR |
| Commonly used assessment of bias | Mean of CrCl-to-mGFR ratio | Median or mean of eGFR−mGFR |
| Methodologic limitations with the common approach | CrCl and mGFR are independent and both affected by measurement error; to some extent, a low mGFR is caused by measure error, and the corresponding CrCl will be higher (regression to the mean), resulting in an increase in the CrCl-to-mGFR ratio | Least squared linear regression is asymmetric, and eGFR was developed to estimate mGFR; thus, eGFR equations were derived to be unbiased across levels of eGFR, not levels of mGFR or (mGFR+eGFR)/2; commonly used eGFR equations were not derived, such that the mean or median of eGFR−mGFR=0; the MDRD Study and CKD-EPI equations were derived, such that the mean of ln eGFR−ln mGFR=0 |
| More correct x axis | (mGFR + CrCl)/2; this is the appropriate setting for application of the Bland–Altman approach; if measurement error with mGFR and CrCl is similar, then (mGFR + CrCl)/2 causes error in the x axis to be equally influenced by mGFR error and CrCl error | eGFR; although eGFR has measurement error from the serum markers, this is already incorporated into how the equation estimates mGFR for the equation population |
| More correct assessment of bias (y axis) | No one correct approach (relative or absolute bias both informative) | eGFR should always be assessed for bias the same way that eGFR was derived to be unbiased; for the MDRD Study and CKD-EPI equations, this would be the mean of ln eGFR−ln mGFR and can be reported as a relative bias (percentage bias) |
| Biologic/clinical relevance | Tubular secretion of creatinine is thought to increase as GFR declines; however, the CrCl-to-mGFR ratio increasing as mGFR declines is caused by regression to the mean to some extent rather than biology | The claim that eGFR underestimates at high levels of GFR is not correct, because eGFR equations were derived to be unbiased at all levels of eGFR for the equation population; rather, eGFR can underestimate GFR in populations that are healthier than the equation population; this is particularly a problem with equations that were developed using all patients with CKD (MDRD Study) or mostly patients with CKD (CKD-EPI) applied to healthy individuals with high-normal marker levels (serum creatinine or cystatin C) |
mGFR, measured GFR; CrCl, creatinine clearance; MDRD, Modification of Diet in Renal Disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Is there a better analytic approach to assess whether bias between CrCl and mGFR changes across levels of GFR? If so, does this better analytic approach clarify whether there is increased tubular secretion of creatinine as GFR declines? The best approach for comparing two independent clearance–based methods may be a Bland–Altman plot (Table 1) (2). A Bland–Altman plot uses the average (mGFR+CrCl)/2 on the x axis, which decreases error by averaging the two GFR methods. More importantly, the average (mGFR+CrCl)/2 causes error on the x axis to be equally influenced by both mGFR error and CrCl error, lessening the effects of regression to the mean. Zhang et al. (1) estimated the measurement error (within–person coefficient of variation) as 10% with mGFR and similarly, 12% with CrCl. Using a Bland–Altman plot, the ratio of CrCl to mGFR did not increase as (mGFR+CrCl)/2 decreased, and this finding argues against increased tubular secretion of creatinine as GFR declines (1). However, another recent study using a Bland–Altman plot did find CrCl increased relative to mGFR as (mGFR+CrCl)/2 declined (3). Although not originally reported, the trend was statistically significant (P<0.01; T. Larson, personal communication) and argues for increased tubular secretion of creatinine as GFR declines. The reason for the discrepancy between these two studies is unclear.
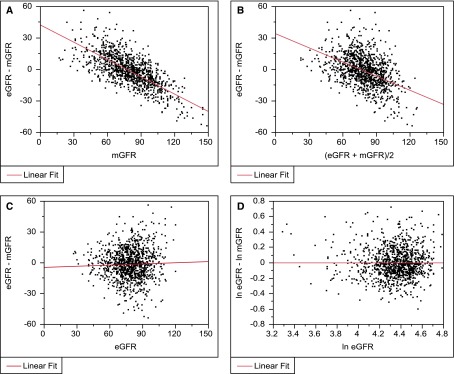
If Bland–Altman plots are the best approach for comparing CrCl with mGFR, are they also the best way to compare eGFR with mGFR? We would argue that the answer is no. Although CrCl and mGFR are independent of each other, mGFR and eGFR have a dependent asymmetric relationship. The eGFR equations commonly used in practice were developed with least squares regression to estimate the average mGFR for a set of predictors (usually some combination of serum creatinine, cystatin C, age, sex, and race) (4,5); the term eGFR can literally be interpreted as the expected value of mGFR. Commonly, the bias between eGFR and mGFR is compared across levels of mGFR (Figure 1, Table 1). Such analysis may explain the commonly held belief that eGFR underestimates at high levels of GFR. However, the correct way to assess whether the eGFR equation is biased is to use the same approach as originally used when deriving the equation. Both the Modification of Diet in Renal Disease (MDRD) Study (4) and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) (5) equations were developed to be unbiased, such that the mean of ln eGFR−ln mGFR is zero across the full range of eGFR for the equation population. For interpretation, this bias can be converted to a percentage using the formula [exp(ln eGFR−ln mGFR)−1]×100%. If the median or mean of eGFR−mGFR is desired to assess bias, then eGFR equations should be derived, such that the median or mean of eGFR−mGFR is zero. This was not done for either the MDRD Study or the CKD-EPI equation. Assessing eGFR bias in a manner consistent with how the eGFR equation was derived to be unbiased allows for a more clear determination of whether eGFR behaves differently in populations that differ from the equation population.
Figure 1.
Differences between eGFR and measured GFR (mGFR) are only unbiased across levels of eGFR on the logarithmic scale. Here, we derive eGFR to estimate mGFR in 1093 subjects from an earlier study (10) using a least squares regression model (ln mGFR regressed on ln serum creatinine, ln serum creatinine squared, age, and sex). We then assess whether bias changes as GFR changes in the same dataset with a linear fit. (A) eGFR−mGFR across levels of mGFR. (B) eGFR−mGFR across levels of (eGFR+mGFR)/2. (C) eGFR−mGFR across levels of eGFR. (D) ln eGFR−ln mGFR across levels of ln eGFR.
An objection to this reasoning may be that eGFR has measurement error, and thus, regression to the mean phenomena should occur when assessing bias across levels of eGFR. Certainly, eGFR has measurement error from the endogenous markers, such as serum creatinine and cystatin C, used in the eGFR equation. However, eGFR measurement error is already incorporated into how eGFR predicts mGFR. For example, a high eGFR level in a patient may be, in part, caused by measurement error from the within–person biologic and assay variation in serum creatinine or cystatin C levels causing a low level. However, the eGFR equation was derived assuming that low serum creatinine or cystatin C levels were, in part, caused by measurement error in the study sample used to derive the equation. Thus, in a similar population to the equation population, measurement error with eGFR will not cause bias.
When eGFR is applied to populations that differ from the equation population, measurement error with eGFR can cause bias. A common example of this is the application of serum creatinine–based equations that were developed using all (MDRD Study equation) or mostly (CKD-EPI equation) patients with CKD to healthy populations. A healthy person with a high normal serum creatinine (about 1.3 mg/dl in men and 1.1 mg/dl in women with a standardized assay) has about a 50% higher mGFR than a patient with CKD with the same high normal serum creatinine level (6). A high normal serum creatinine (or a high normal cystatin C level) in a healthy person is at the tail upper end of the distribution for a healthy population but not for a CKD population, and it is more likely to be high from measurement error in a healthy population than in a CKD population. This contributes to the underestimation of mGFR by about 20% with the CKD-EPI equations in healthy populations with an eGFR of 45–59 ml/min per 1.73 m2 (7). Other than incorrect implicit assumptions about measurement error with eGFR, there are also biologic reasons for eGFR to be biased when applied in populations that differ from the equation population. The primary source of creatinine, muscle mass, is lower in patients with CKD (8), and this also contributes to why creatinine-based equations developed using patients with CKD underestimate GFR in healthy patients (6). Cystatin C–based equations developed in nontransplant populations underestimate GFR in transplant recipients (7), possibly because there are numerous factors that affect the non-GFR determinants of cystatin C levels (9), and the distributions of these factors may differ between transplant and nontransplant populations.
In conclusion, improper assessment of CrCl and eGFR bias can lead to incorrect understanding of renal physiology and the clinical assessment of kidney function. There are two important examples of this occurring. Increased tubular secretion of creatinine at lower levels of GFR may occur, but regression to the mean can explain at least some of the data used to support this claim. The concern that eGFR underestimates at high levels of GFR is unfounded, because equations were developed to be unbiased across all levels of eGFR for the equation population. The problem is that application of eGFR equations developed with mostly patients with CKD to healthy individuals can substantially underestimate GFR.
Disclosures
None.
Acknowledgments
This paper was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grants DK090358 and DK100227.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “Measurement Error as Alternative Explanation for the Observation that CrCl/GFR Ratio is Higher at Lower GFR,” on pages 1574–1581.
References
- 1.Zhang X, McCulloch CE, Lin F, Lin Y-c, Allen IS, Bansal N, Go AS, Hsu C-y: Measurement error as alternative explanation for the observation that CrCl/GFR ratio is higher at lower GFR. Clin J Am Soc Nephrol 11: 1574–1581, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bland JM, Altman DG: Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310, 1986 [PubMed] [Google Scholar]
- 3.Seegmiller JC, Burns BE, Schinstock CA, Lieske JC, Larson TS: Discordance between iothalamate and iohexol urinary clearances. Am J Kidney Dis 67: 49–55, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration: Using standardized serum creatinine values in the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators: Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG: Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease. Ann Intern Med 141: 929–937, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Meeusen JW, Rule AD, Voskoboev N, Baumann NA, Lieske JC: Performance of cystatin C- and creatinine-based estimated glomerular filtration rate equations depends on patient characteristics. Clin Chem 61: 1265–1272, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roshanravan B, Patel KV, Robinson-Cohen C, de Boer IH, O’Hare AM, Ferrucci L, Himmelfarb J, Kestenbaum B: Creatinine clearance, walking speed, and muscle atrophy: A cohort study. Am J Kidney Dis 65: 737–747, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keddis MT, Amer H, Voskoboev N, Kremers WK, Rule AD, Lieske JC: Creatinine-based and cystatin c-based GFR estimating equations and their non-GFR determinants in kidney transplant recipients [published online ahead of print June 23, 2016]. Clin J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rule AD, Bailey KR, Lieske JC, Peyser PA, Turner ST: Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney Int 83: 1169–1176, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]