Abstract
Background and objectives
Assisted peritoneal dialysis is a treatment option for individuals with barriers to self-care who wish to receive home dialysis, but previous research suggests that this treatment modality is associated with a higher rate of hospitalization. The objective of our study was to determine whether assisted peritoneal dialysis has a different rate of hospital days compared to in-center hemodialysis.
Design, setting, participants, & measurements
We conducted a multicenter, retrospective cohort study by linking a quality assurance dataset to administrative health data in Ontario, Canada. Subjects were accrued between January 1, 2004 and July 9, 2013. Individuals were grouped into assisted peritoneal dialysis (family or home care assisted) or in-center hemodialysis on the basis of their first outpatient dialysis modality. Inverse probability of treatment weighting using a propensity score was used to create a sample in which the baseline covariates were well balanced.
Results
The study included 872 patients in the in–center hemodialysis group and 203 patients in the assisted peritoneal dialysis group. Using an intention to treat approach, patients on assisted peritoneal dialysis had a similar hospitalization rate of 11.1 d/yr (95% confidence interval, 9.4 to 13.0) compared with 12.9 d/yr (95% confidence interval, 10.3 to 16.1) in the hemodialysis group (P=0.19). Patients on assisted peritoneal dialysis were more likely to be hospitalized for dialysis-related reasons (admitted for 2.4 d/yr [95% confidence interval, 1.8 to 3.2] compared with 1.6 d/yr [95% confidence interval, 1.1 to 2.3] in the hemodialysis group; P=0.04). This difference was partly explained by more hospital days because of peritonitis. Modality switching was associated with high rates of hospital days per year.
Conclusions
Assisted peritoneal dialysis was associated with similar rates of all-cause hospitalization compared with in-center hemodialysis. Patients on assisted peritoneal dialysis who experienced peritonitis and technique failure had high rates of hospitalization.
Keywords: peritoneal dialysis, hospitalization, social support, family, Cohort Studies, Humans, Outpatients, Peritonitis, Propensity Score, renal dialysis, Self Care
Introduction
Assisted peritoneal dialysis (PD) has emerged as a treatment option for patients who wish to receive home dialysis but have significant barriers to self-care (1–7). Patients on assisted PD are significantly older and have a higher burden of comorbidity compared with patients on self-care PD, and therefore, adverse events may be common, including hospitalization, peritonitis, and death (8,9). However, appropriate risk adjustment was not performed in these studies, and it is unclear whether differences in outcomes were driven by differences in case mix or the treatment modality itself.
The objective of this study was to determine whether patients on chronic dialysis who receive assisted PD as their first outpatient modality have a higher rate of hospital days compared with patients receiving in-center hemodialysis (HD) after controlling for measured baseline differences between the groups. In-center HD was used as the comparator, not self-care PD, because it is the alternative dialysis modality for most of these older patients.
Materials and Methods
Study Design and Data Sources
This multicenter, retrospective cohort was created by linking data from the Dialysis Measurement, Analysis, and Reporting (DMAR) system to provincial health care databases housed at the Institute for Clinical Evaluative Sciences (ICES) in Ontario, Canada (www.ices.on.ca). The DMAR system is a quality assurance system that prospectively measures baseline characteristics and outcomes, including modality changes, hospitalization, transplantation, treatment withdrawal, and death (10–12). Data are entered by trained clinical staff and reviewed every 90 days by the investigators (M.J.O. and R.R.Q.) to ensure data quality. The DMAR dataset was linked to provincial databases, including (1) the Registered Persons Database, which contains demographic, place of residence, and vital status information for the Ontario population; (2) the Ontario Health Insurance Plan Claims database, which contains information regarding inpatient and outpatient physician visits and tests; (3) the Canadian Institute for Health Information (CIHI) Discharge Abstract Database (DAD), which contains diagnostic and procedural information from all acute care hospitalizations; (4) the Home Care Database, which includes information about home care visits; and (5) the Canadian Organ Replacement Register, which is a national registry of patients on dialysis. Linkage used unique encoded identifiers and was analyzed at the ICES. We conducted this study according to published guidelines using a prespecified protocol, which was approved by the Institutional Review Board at Sunnybrook Health Sciences Centre (13).
Patient Population
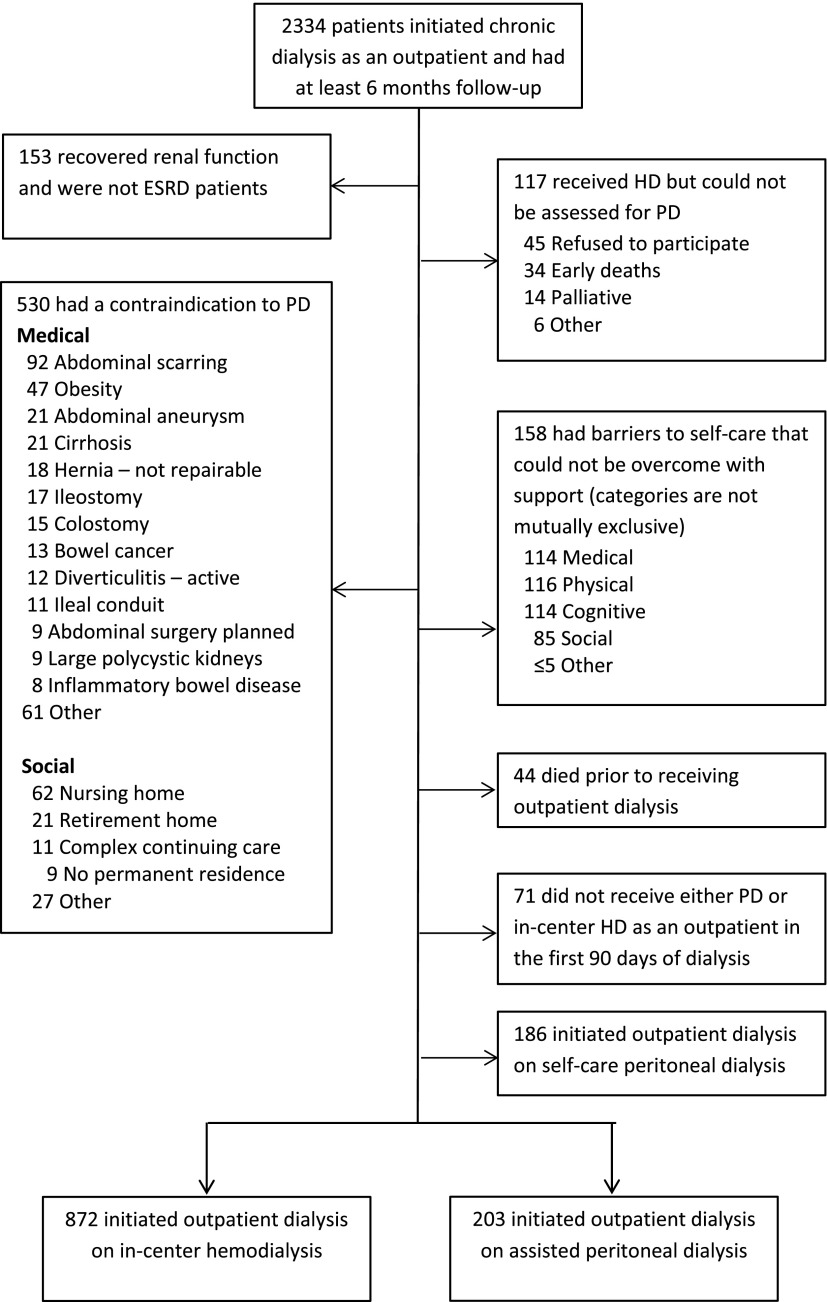
We identified incident patients on chronic dialysis at each study site. The implementation of the DMAR system was staggered, and therefore, timing of accrual varied: Sunnybrook Health Sciences Centre: January 1, 2004; Halton Healthcare: January 1, 2007; London Health Sciences Centre: January 1, 2008; The Ottawa Hospital: October 1, 2009; and St. Michael’s Hospital: September 1, 2011. Enrollment ended on July 9, 2013 at all sites. Patients are registered in the DMAR system if they have a diagnosis of ESRD by a nephrologist, initiated outpatient dialysis, or received at least 30 days of dialysis therapy. Patients who subsequently recovered renal function within the first 6 months were excluded from this study. Minimum follow-up on dialysis was 6 months, but patients who stopped dialysis early for transplant or death were included (Figure 1). Patients were excluded if (1) they could not be assessed for PD, (2) they were not considered eligible for both PD and HD (11), (3) they died prior to receiving outpatient dialysis, (4) they did not receive either outpatient PD or in-center HD within 90 days of the dialysis start, or (5) they initiated outpatient dialysis on self-care PD.
Figure 1.
Reasons for exclusions and process to determine peritoneal dialysis (PD) eligibility. In total, 2334 subjects were initially included in the study cohort if they had a diagnosis of ESRD and initiated dialysis, received at least 30 days of dialysis, or initiated dialysis as an outpatient. These patients were considered appropriate for chronic modality assessment by the sites participating in the Dialysis Measurement, Analysis, and Reporting quality assurance program; 153 (6.6%) recovered renal function, and 117 (5.0%) were not able to be assessed for PD. Of the remaining patients, 530 (26%) had a contraindication to PD, and 158 (7.6%) had barriers to self-care PD that could not be overcome in the judgment of their interdisciplinary dialysis team; 44 patients died before receiving outpatient dialysis, 71 did not received outpatient PD or hemodialysis (HD) within 90 days, and 186 initiated on self-care PD, leaving 872 in the HD control group and 203 in the assisted PD group.
Exposure
Patients who were assessed for PD and eligible were educated about dialysis options and allowed to choose their therapy. Each participating program had established modality education programs, but the study did not standardize the content. Patients who initiated either family-assisted PD or home care assisted PD were assigned to the assisted PD group. In Ontario, home care assistance is funded by the government and provided primarily by registered nurses who are trained by their home care agency. Patients and families were informed of the availability of home care assistance during modality education. but the final determination of need occurred at the end of PD training. Patients who received full care, facility–based HD were assigned to the in–center HD group.
Patient Characteristics
Baseline data included demographics (age, sex, race, and socioeconomic status), comorbidity, receipt of predialysis care, laboratory values, inpatient start, and detailed eligibility assessment for PD. Administrative data included race, etiology of ESRD, John Hopkins Aggregated Diagnosis Groups (http://acg.jhsph.org) (14), neighborhood household income quintile, outpatient visits with nephrologists, family physicians, and hospitalizations in the year before starting dialysis.
Outcomes
The primary outcome was hospital days per person-year of follow-up. Secondary outcomes included hospital admissions per person-year and cause-specific rates of hospitalization, reasons for stopping dialysis (death or transplantation), and rate of home care nursing visits. We described hospitalizations in the subgroups of patients on home care versus family-assisted PD and among those who changed modality.
The cause of hospitalization was classified by M.J.O. and R.R.Q. on the basis of written comments entered by nurses. The cause was first classified as either dialysis related or not. Dialysis-related hospitalizations were then classified as related to peritonitis, central venous catheter bacteremia, other dialysis–related infections, or other noninfectious causes (e.g., dialysis access complications, complications of dialysis therapy [hypotension or arrhythmia], or complications of kidney disease for a patient on dialysis [uremia, volume overload, or electrolyte disturbances]). Central venous catheter bacteremia required evidence of symptoms, a positive blood culture, and no other source of infection. Nondialysis-related hospitalizations were classified as nondialysis-related infection, associated with another major disease category (e.g., cardiovascular, cerebrovascular, peripheral vascular, gastrointestinal/liver, malignancy, or elective surgery), and other. Hospitalizations classified in other categories were crossreferenced against the most responsible diagnosis for the admission as coded in the CIHI DAD to reduce the number of hospitalizations coded in the other category.
Statistical Analyses
Using multivariable logistic regression, we calculated the propensity score as the predicted probability of a patient receiving assisted PD (versus in-center HD) as a function of covariates. Covariates included demographics, predialysis care, comorbidities, predialysis laboratory values, PD program, and year of PD start. Inverse probability of treatment weights using the propensity score was used to standardize baseline covariates between the two groups (15). Baseline differences between the groups were compared using standardized differences (where a value >0.1 was considered clinically meaningful) (16). Our primary analysis used an intention to treat approach on the basis of the first outpatient treatment modality received (assisted PD versus in-center HD). Hospital days and hospital visits per person-year were compared using negative binomial and Poisson regression models, respectively. All model assumptions were tested and satisfied in the final model used. Zero inflated Poisson models were examined, but they did not substantially change the results (17,18). The analysis was not stratified or adjusted for time on dialysis therapy.
To describe hospitalization in patients who switched between treatment modalities, hospital days were reported separately. An as-treated analysis (methods are described in Supplemental Material) was performed using a marginal structural model (19). We conducted all analyses with SAS, version 9.4 (SAS Institute Inc., Cary, NC). We interpreted two–tailed P values <0.05 as statistically significant.
Results
Initial inclusion criteria were met by 2334 patients (Figure 1); 153 (6.6%) recovered renal function, and 117 (5.0%) could not be assessed for PD. Of the remaining patients, 530 (26%) had a contraindication to PD, and 158 (7.6%) had barriers to self-care PD that made them ineligible for PD; 44 patients died before receiving outpatient dialysis, 71 did not receive outpatient PD or in-center HD within 90 days, and 186 initiated on self-care PD, leaving 872 in the HD group and 203 in the assisted PD group. The inclusion rate per site varied from 43% to 67% per site (P=0.001), reflecting, in part, different PD eligibility rates across sites.
Before weighting, patients on assisted PD were older, had higher predialysis hemoglobin and serum albumin, had lower body mass index, had longer predialysis nephrology care, had fewer hospitalizations in the year before dialysis start, and were much more likely to start dialysis as an outpatient (i.e., less acuity) (Table 1). In the assisted PD group, 125 (61%) started with home care assisted PD, and the remainder started with family assistance.
Table 1.
Baseline characteristics before and after weighting in the assisted peritoneal dialysis and hemodialysis groups
| Variable | Before Weighting | After Weighting | ||||
|---|---|---|---|---|---|---|
| Assisted PD, n=203 | HD, n=872 | Standardized Difference | Assisted PD, n=203 | HD,a n=198 | Standardized Difference | |
| Age, yr, mean (SD) | 68.9 (13.2) | 64.2 (15.7) | 0.80 | 68.9 (13.2) | 68.8 (6.6) | 0.02 |
| Men, % | 56 | 62.2 | 0.12 | 56 | 59 | 0.06 |
| Race, white, % | 86 | 90 | 0.12 | 86 | 87 | 0.03 |
| Comorbidity, % | ||||||
| Diabetes | 52 | 52 | 0.01 | 52 | 50 | 0.05 |
| CAD | 34 | 34 | 0 | 34 | 33 | 0.03 |
| CHF | 23 | 27 | 0.10 | 23 | 21 | 0.04 |
| Other cardiac | 24 | 29 | 0.11 | 24 | 23 | 0.02 |
| Cerebrovascular | 17 | 17 | 0.01 | 17 | 18 | 0.01 |
| PVD | 13 | 17 | 0.11 | 13 | 14 | 0.02 |
| Cancer | 14 | 18 | 0.11 | 14 | 15 | 0.03 |
| History of GI bleed | 5.9 | 8.0 | 0.08 | 5.9 | 7.0 | 0.04 |
| Body mass index, mean (SD) | 28.1 (10.2) | 31.4 (30.0) | 0.18 | 28.1 (10.2) | 27.8 (3.86) | 0.04 |
| ADG, mean (SD) | 10.5 (3.76) | 10.9 (3.63) | 0.10 | 10.5 (3.76) | 10.5 (1.67) | 0.01 |
| Hemoglobin before start, g/dl, mean (SD) | 10.7 (1.5) | 9.5 (1.7) | 0.69 | 10.7 (1.5) | 10.6 (0.78) | 0.07 |
| Serum albumin before start, g/dl, mean (SD) | 3.7 (0.5) | 3.4 (1.4) | 0.29 | 3.7 (0.5) | 4.0 (1.6) | 0.15 |
| eGFR, ml/min per 1.73 m2, mean (SD) | 9.0 (4.6) | 8.6 (6.0) | 0.07 | 9.0 (4.6) | 8.8 (2.3) | 0.05 |
| Neighborhood income quintile,b % | ||||||
| 1 | 22 | 23 | 0.19 | 22 | 25 | 0.14 |
| 2 | 23 | 19 | 0.19 | 23 | 23 | 0.14 |
| 3 | 21 | 17 | 0.19 | 21 | 18 | 0.14 |
| 4 | 18 | 20 | 0.19 | 18 | 15 | 0.14 |
| 5 | 16 | 22 | 0.19 | 16 | 18 | 0.14 |
| Rural resident | 17 | 12.7 | 0.09 | 17 | 15 | 0.06 |
| ≥4 mo Nephrology care before start, % | 97 | 76 | 0.48 | 97 | 96 | 0.03 |
| ≥12 mo Nephrology care before start, % | 91 | 65 | 0.52 | 91 | 86 | 0.13 |
| Nephrology visits in prior year, mean (SD) | 13.8 (6.3) | 10.2 (6.7) | 0.59 | 13.8 (6.3) | 14.3 (5.1) | 0.08 |
| Family physician visits in prior year, mean (SD) | 12.6 (14.1) | 14.7 (16.1) | 0.08 | 12.6 (14.1) | 12.6 (5.9) | 0.01 |
| Hospital visits in prior year, mean (SD) | 0.92 (1.38) | 1.47 (1.51) | 0.38 | 0.92 (1.38) | 0.83 (0.7) | 0.08 |
| Hospital days in prior year if admitted, mean (SD) | 13.2 (15.4) | 22.2 (25.1) | 0.37 | 13.2 (15.4) | 15.5 (6.3) | 0.20 |
| Inpatient start, % | 10.8 | 53.6 | 0.91 | 10.8 | 12.6 | 0.06 |
Standardized differences of >0.10 are generally considered meaningful. PD, peritoneal dialysis; HD, hemodialysis; CAD, coronary artery disease; CHF, congestive heart failure; PVD, peripheral vascular disease; GI, gastrointestinal; ADG, John Hopkins Aggregated Diagnosis Group.
The weighting procedure used age, sex, race, comorbidities, ADG, body mass index, predialysis laboratory values, eGFR, income, rural residence, predialysis care, prior visits with nephrologist and family physician, inpatient start, dialysis program, and year of dialysis start; 872 individual patients contributed to the analyses, but after weighting, the sample size was equivalent to 198 patients. However, they were well balanced in terms of baseline covariates.
Neighborhood income quintile is a household size–adjusted measure of household income on the basis of the 2006 Census data. The quintiles were defined within each neighborhood and not across the entire province to minimize the effect of large differences in housing costs and ensure an equal percentage of the population in each income quintile.
Inverse probability of treatment weights using the propensity score balanced baseline covariates but reduced the effective sample size in the HD group to 198 patients. The assisted PD group had a slightly lower albumin (mean =37.7±5.2 g/L assisted PD versus 39.5±16.1 g/L HD), longer predialysis care (91% had 12 months of care for assisted PD versus 86% had 12 months of care for HD), and fewer hospital days in the year before start (13.2±15.4 days assisted PD versus 15.5±6.2 days HD). Patients in the HD group were followed for a mean of 878 compared with 849 days in the assisted PD group, indicating similar survival and transplant rates. The rates of transplant were 0.04 per patient-year (95% confidence interval [95% CI], 0.02 to 0.06) and 0.03 per patient-year (95% CI, 0.01 to 0.06) for assisted PD and HD groups, respectively (P value =0.75). The rates of death were 0.11 per patient-year (95% CI, 0.08 to 0.14) and 0.11 per patient-year (95% CI, 0.07 to 0.16) for assisted PD and HD, respectively (P value =0.94).
Hospitalization
The mean and median number of days in hospital in the assisted PD group were 26.5 and 4.0 days, respectively, compared with 25.1 and 4.0 days for patients on HD (Table 2). Patients on assisted PD had an all-cause rate of 11.1 hospital d/yr (95% CI, 9.4 to 13.0) compared with 12.9 hospital d/yr (95% CI, 10.3 to 16.1) in the HD group (P=0.19). Patients on assisted PD had a rate of 0.80 hospital admissions per year (95% CI, 0.72 to 0.88) compared with 0.71 hospital admissions per year (95% CI, 0.86 to 0.61) in the HD group (P=0.12).
Table 2.
Hospitalization of assisted peritoneal dialysis compared with hemodialysis
| Modality and Outcome | Mean (SD) | Median (IQR) | Rate (95% CI) |
|---|---|---|---|
| Assisted PD, n=203 | |||
| Hospital days | 26.5 (42.3) | 4 (0–37) | 11.1 (9.4 to 13.0)a |
| Hospital visits | 1.9 (1.8) | 1 (0–3) | 0.80 (0.72 to 0.88)b |
| Follow-up, d | 849 (545) | 675 (455–1191) | NA |
| Hemodialysis,c n=198 | |||
| Hospital days | 25.1 (26.6) | 4 (0–26) | 12.9 (10.3 to 16.1) |
| Hospital visits | 1.7 (0.8) | 1 (0–2) | 0.71 (0.61 to 0.86) |
| Total follow-up, d | 878 (278) | 719 (441–1203) | NA |
Total hospital visits in the assisted PD group were 377. Total hospital visits in the hemodialysis group were 1468. IQR, interquartile range; 95% CI, 95% confidence interval; PD, peritoneal dialysis; NA, not applicable.
P value =0.19 compared with hemodialysis.
P value =0.12 compared with hemodialysis.
The weighting procedure used age, sex, race, comorbidities, John Hopkins Aggregated Diagnosis Group, body mass index, predialysis laboratory values, eGFR, income, rural residence, predialysis care, prior visits with nephrologist and family physician, inpatient start, dialysis program, and year of dialysis start; 872 individual patients contributed to the analyses, but after weighting, the sample size was equivalent to 198 patients. However, they were well balanced in terms of baseline covariates.
Adjusting for the remaining baseline differences between the groups in Table 1 did not significantly change the results (HD group compared with PD group; incident rate ratio, 1.06; 95% CI, 0.85 to 1.38). Within the assisted group, patients initiating outpatient dialysis on home care nursing–assisted PD had a rate of 9.2 (95% CI, 5.9 to 14.3) compared 12.1 (95% CI, 8.6 to 17.2) for patients initiating on family-assisted PD (P=0.33).
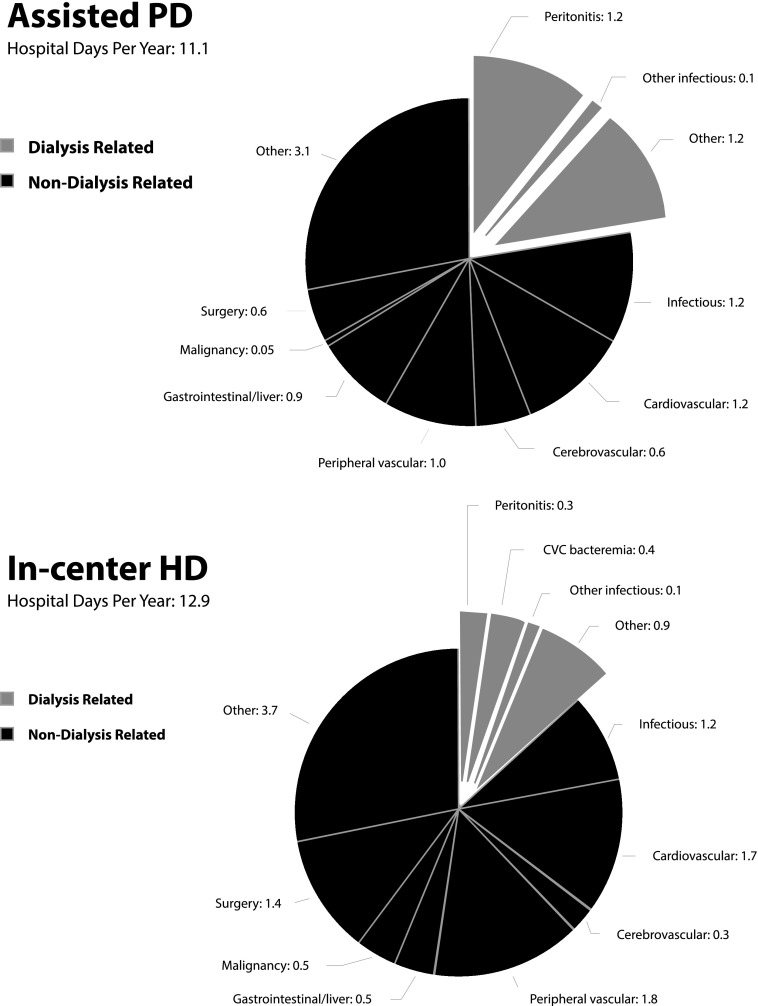
Patients on assisted PD were more likely to be hospitalized for dialysis-related reasons (Figure 2); they were admitted for 2.4 d/yr (95% CI, 1.8 to 3.2) compared with 1.6 d/yr (95% CI, 1.1 to 2.3) in the HD group (P=0.04). The assisted PD group was hospitalized on average for 1.2 d/yr for peritonitis compared with 0.4 d/yr for HD catheter–related bacteremia in the HD group. Within the HD group, patients using an arteriovenous (AV) access (304 [35%] of 872; >98% of AV accesses were fistulas) to start dialysis had an all-cause rate of 12.8 hospital d/yr (95% CI, 8.9 to 18.3) compared with 10.1 hospital d/yr (95% CI, 8.7 to 11.8) for patients using a central venous catheter (P=0.23). Patients using catheters were hospitalized for dialysis-related reasons for 1.72 d/yr (95% CI, 1.4 to 2.2) compared with 0.55 d/yr (95% CI, 0.3 to 1.0; P=0.003) for patients with AV access. The HD group had a greater number of hospital days for nondialysis-related reasons (11.2 versus 8.6 days; P=0.04), specifically elective surgery (1.37 versus 0.57 days; P=0.02) and malignancy (0.54 versus 0.05 days; P=0.02). The assisted PD group had a greater number of hospital days for gastrointestinal/liver disease (0.91 versus 0.49 days; P=0.05).
Figure 2.
Cause–specific hospital days for assisted peritoneal dialysis (PD) compared with in-center hemodialysis (HD). There were no differences between the assisted PD and HD groups in the number of hospital days per year after weighting. Patients on assisted PD were admitted for dialysis-related reasons for 2.4 d/yr (95% confidence interval, 1.8 to 3.2) compared with 1.6 d/yr (95% confidence interval, 1.1 to 2.3) in the HD group (P=0.04). CVC, central venous catheter.
Modality Switches and Hospitalization
In the HD group, 179 of 872 patients (21%) transferred to PD; the median time on HD before the switch was 136 days (Table 3). In the assisted PD group, 51 of 203 (25%) transferred to HD after a median of 350 days, and only 18 (35%) returned to PD. Individuals who transferred from PD to HD spent an average of 17.8 days in the hospital per year (95% CI, 12.5 to 25.5), and individuals that transferred from HD to PD had a similar rate (17.7 days in hospital per year; 95% CI, 11.7 to 26.7). The as-treated analysis showed no difference in hospitalization days between the assisted PD group and the HD group when modality before hospitalization was used to classify the groups (Supplemental Tables 1 and 2).
Table 3.
Hospital days with and without modality switches
| Modality and Outcome | Mean | Median (IQR) | Rate (95% CI) |
|---|---|---|---|
| PD only, n=151 | |||
| Hospital visits | 1.4 | 1 (0–2) | 0.67 (0.59 to 0.77) |
| Hospital days | 20.2 | 2 (0–23) | 8.72 (7.26 to 10.47) |
| PD to HD,a n=52 | |||
| Hospital visits | 3.1 | 2 (1–4) | 1.07 (0.87 to 1.31) |
| Hospital days | 44.9 | 34.5 (6–61) | 17.81 (12.46 to 25.45) |
| HD only, n=693 | |||
| Hospital visits | 1.7 | 1 (0–2) | 0.69 (0.58 to 0.82) |
| Hospital days | 24.9 | 4 (0–24) | 11.77 (9.14 to 15.16) |
| HD to PD,b n=179 | |||
| Hospital visits | 1.8 | 1 (0–2) | 0.81 (0.61 to 1.06) |
| Hospital days | 25.8 | 7 (0–34) | 17.69 (11.73 to 26.7) |
All of the patients in the PD only and PD to HD groups initiated on assisted PD and not self-care PD. Data are not weighted. IQR, interquartile range; 95% CI, 95% confidence interval; PD, peritoneal dialysis; HD, hemodialysis.
In the PD to HD group, the mean time on PD was 592 days and the mean time on HD was 446 days during follow-up.
In the HD to PD group, the mean time on PD was 520 days and the mean time on HD was 259 days during follow-up.
Use of Home Care Assistance
In the assisted group, 125 of 203 patients (61%) initiated outpatient dialysis with home care support. Of the time spent on PD, patients spent an average of 298 days (43%) on home care assisted PD, 240 days (35%) on family-assisted PD, and 156 days (22%) on self-care PD; 48 patients (38%) in the home care assisted group switched to self-care or family-assisted PD, and 22 switched within 30 days of the start of outpatient dialysis. The median time from the switch from home care assisted PD to either self-care PD or family-assisted PD was 31 days.
The rate of home care nursing visits per year of PD (including periods of home care assistance, family assistance, and self-care) in follow-up was 118 (95% CI, 76 to 184) compared with 4.7 (95% CI, 2.9 to 7.5) in the HD group (weighted comparison).
Discussion
This study showed that patients initiating outpatient dialysis on assisted PD have similar rates of hospitalization compared with patients initiating in-center HD after accounting for case mix. The assisted PD group was more likely to be hospitalized for dialysis-related causes, with peritonitis responsible for one half of the hospitalizations. The HD group was admitted for dialysis-related causes at a lower rate, and only one quarter of those admissions were related to HD catheter bacteremia. Modality switching was associated with higher rates of hospitalization. Even after accounting for the high rate of modality switching in the as-treated analysis, the rates in hospitalization days between the two groups remained similar.
The assisted PD group was admitted for 11.1 hospital d/yr, which was similar to the rates in the Canadian studies by Murphy et al. (20) and Lafrance et al. (21) that found that patients on PD were hospitalized for 11.7 and 10.9 d/yr, respectively. This rate is lower than those in prior studies of assisted PD, which range from 18.2 to 52.6 d/yr, although these studies were small (n=22–36) and lacked comparable control groups (1,8,22). Home care assistance in Ontario, Canada is unique, because it is generously funded (two visits per day), is usually provided by registered nurses, and does not have strict eligibility criteria. These features may explain why some patients graduated to self-care PD relatively quickly. To create comparable groups, we restricted the study population to one that was eligible for both modalities and did not include the self–care PD group, because it was younger and healthier. This restriction was required to reduce selection bias but may limit generalizability. The overall eligible rate was similar to those in other large studies, and we felt that excluding patients on HD who were not eligible for PD reduced selection bias (23,24). However, the inclusion rate varied significantly by study site, indicating that some sites were more aggressive at offering PD to patients than others, because the main reason for exclusion from the study was PD noneligibility.
We found that hospital days from peritonitis on assisted PD were three times as common as hospital days from HD catheter–related bacteremia, similar to the finding in a previous study that we conducted (25). In contrast to the work by Lafrance et al. (21), which reported a relative risk of 1.52 (95% CI, 1.34 to 1.74) for infection-related hospitalization, we did not find a higher rate from overall infection in PD. Verger et al. (9) reported a higher risk of peritonitis on assisted PD, but the differences disappeared when PD nurses from the dialysis center regularly visited patients to supervise the home care nurses.
Patients who had a modality switch had higher rates of hospitalization days. PD technique failure increases hospitalization (approximately 9 more d/yr), the cost of PD therapy, and mortality (26–28). Chiu et al. (27) found that technique failure added $7972 ($CAD) in inpatient costs to PD therapy. In this study, technique failure was relatively common, because the assisted PD group was older (29). Interestingly, the subgroup that moved from HD to PD had equally high rates of hospital days per year. All patients in this study were systematically offered PD, which likely increased elective transfers from HD to PD. Receiving HD before PD may be a marker of acuity and comorbidity and is a consistent risk factor for worse outcomes on PD (29–31).
This study was limited, because it was a retrospective, observational study, and enrollment was not balanced among the centers. Larger PD programs and those with more experience with assisted PD may have better results. We also did not have broad measures of all social supports available to patients. Family-assisted PD and home care assisted PD were grouped together to increase the sample size, and we censored follow-up in the as-treated analysis if patients received a modality other than assisted PD or in-center HD. Larger studies could better describe patterns of assistance and relate hospitalizations to a wider variety of modalities, including self-care PD.
Acknowledging these limitations, this study is one of the largest evaluations of assisted PD to date. We were able to describe patterns of family assistance, home care assistance, and self-care PD. The mean rate of home visits was 118 per year, which is less than the maximum offered, because it is supplemented by family and patients can graduate to self-care (2,32). These results should reassure policymakers that assisted PD is a safe and cost-effective strategy to increase home dialysis. Assisted PD is also patient focused, because it expands patient choice among the older dialysis population.
In summary, assisted PD is a widespread therapy that expands home dialysis in the older population with barriers to self-care. It seems to be a safe alternative to in-center HD in terms of hospitalization risk. Peritonitis and technique failure are major drivers of hospitalization and should be monitored closely to maintain equivalent outcomes to in-center HD. Additional research is required to understand how patterns of assistance influence adverse events and how assisted PD compares with other modalities with respect to cost-effectiveness and survival.
Disclosures
M.J.O. and R.R.Q. are coinventors of the Dialysis Measurement, Analysis, and Reporting system. Baxter Healthcare has provided support for the development of a peritoneal dialysis catheter registry for the International Society of Peritoneal Dialysis–North American Chapter. The project is led by M.J.O. and R.R.Q.
Supplementary Material
Acknowledgments
The authors thank the nurses and administrative assistants who entered data into the Dialysis Measurement, Analysis, and Reporting system at the participating study sites. Parts of this material are on the basis of data and information compiled and provided by the Canadian Institute for Health Information (CIHI).
This research was supported by grants from the Change Foundation of Ontario, the Physician Services Incorporated Foundation, and the Canadian Institutes of Health Research. This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC).
The opinions, results, and conclusions reported in this paper are those of the authors and independent from the funding sources. No endorsement by the ICES or the Ontario MOHLTC is intended or should be inferred. Also, the analyses, conclusions, opinions, and statements expressed herein are those of the authors and not necessarily those of the CIHI.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Assisted Peritoneal Dialysis as an Alternative to In-Center Hemodialysis,” on pages 1522–1524.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10130915/-/DCSupplemental.
References
- 1.Oliver MJ, Quinn RR, Richardson EP, Kiss AJ, Lamping DL, Manns BJ: Home care assistance and the utilization of peritoneal dialysis. Kidney Int 71: 673–678, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Dratwa M: Costs of home assistance for peritoneal dialysis: Results of a European survey. Kidney Int Suppl 73: S72–S75, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Hsieh CY, Fang JT, Yang CW, Lai PC, Hu SA, Chen YM, Yu CC, Tian YC, Chien CC, Hung CC: The impact of type of assistance on characteristics of peritonitis in elderly peritoneal dialysis patients. Int Urol Nephrol 42: 1117–1124, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Cheng CH, Shu KH, Chuang YW, Huang ST, Chou MC, Chang HR: Clinical outcome of elderly peritoneal dialysis patients with assisted care in a single medical centre: A 25 year experience. Nephrology (Carlton) 18: 468–473, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Franco MR, Fernandes N, Ribeiro CA, Qureshi AR, Divino-Filho JC, da Glória Lima M: A Brazilian experience in assisted automated peritoneal dialysis: A reliable and effective home care approach. Perit Dial Int 33: 252–258, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Povlsen JV, Ivarsen P: Assisted peritoneal dialysis: Also for the late referred elderly patient. Perit Dial Int 28: 461–467, 2008 [PubMed] [Google Scholar]
- 7.Xu R, Zhuo M, Yang Z, Dong J: Experiences with assisted peritoneal dialysis in China. Perit Dial Int 32: 94–101, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lobbedez T, Moldovan R, Lecame M, Hurault de Ligny B, El Haggan W, Ryckelynck JP: Assisted peritoneal dialysis. Experience in a French renal department. Perit Dial Int 26: 671–676, 2006 [PubMed] [Google Scholar]
- 9.Verger C, Duman M, Durand PY, Veniez G, Fabre E, Ryckelynck JP: Influence of autonomy and type of home assistance on the prevention of peritonitis in assisted automated peritoneal dialysis patients. An analysis of data from the French Language Peritoneal Dialysis Registry. Nephrol Dial Transplant 22: 1218–1223, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Blake PG, Quinn RR, Oliver MJ: Peritoneal dialysis and the process of modality selection. Perit Dial Int 33: 233–241, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver MJ, Garg AX, Blake PG, Johnson JF, Verrelli M, Zacharias JM, Pandeya S, Quinn RR: Impact of contraindications, barriers to self-care and support on incident peritoneal dialysis utilization. Nephrol Dial Transplant 25: 2737–2744, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Oliver MJ, Verrelli M, Zacharias JM, Blake PG, Garg AX, Johnson JF, Pandeya S, Perl J, Kiss AJ, Quinn RR: Choosing peritoneal dialysis reduces the risk of invasive access interventions. Nephrol Dial Transplant 27: 810–816, 2012 [DOI] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med 147: 573–577, 2007 [DOI] [PubMed] [Google Scholar]
- 14.The John Hopkins University Bloomberg School of Public Health : The John Hopkins ACG Case-Mix System Documentation & Application Manual Version 5.0, Baltimore, MD, John Hopkins University, 2001 [Google Scholar]
- 15.Austin PC: An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46: 399–424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PC: Using the standardizing difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 38: 1228–1234, 2009 [Google Scholar]
- 17.Gardner W, Mulvey EP, Shaw EC: Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychol Bull 118: 392–404, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Weaver CG, Ravani P, Oliver MJ, Austin PC, Quinn RR: Analyzing hospitalization data: Potential limitations of Poisson regression. Nephrol Dial Transplant 30: 1244–1249, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Hernán MA, Brumback B, Robins JM: Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 11: 561–570, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Murphy SW, Foley RN, Barrett BJ, Kent GM, Morgan J, Barré P, Campbell P, Fine A, Goldstein MB, Handa SP, Jindal KK, Levin A, Mandin H, Muirhead N, Richardson RM, Parfrey PS: Comparative hospitalization of hemodialysis and peritoneal dialysis patients in Canada. Kidney Int 57: 2557–2563, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Lafrance JP, Rahme E, Iqbal S, Elftouh N, Vallée M, Laurin LP, Ouimet D: Association of dialysis modality with risk for infection-related hospitalization: A propensity score-matched cohort analysis. Clin J Am Soc Nephrol 7: 1598–1605, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smyth A, McCann E, Redahan L, Lambert B, Mellotte GJ, Wall CA: Peritoneal dialysis in an ageing population: A 10-year experience. Int Urol Nephrol 44: 283–293, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Prakash S, Perzynski AT, Austin PC, Wu CF, Lawless ME, Paterson JM, Quinn RR, Sehgal AR, Oliver MJ: Neighborhood socioeconomic status and barriers to peritoneal dialysis: A mixed methods study. Clin J Am Soc Nephrol 8: 1741–1749, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jager KJ, Korevaar JC, Dekker FW, Krediet RT, Boeschoten EW; Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) Study Group : The effect of contraindications and patient preference on dialysis modality selection in ESRD patients in The Netherlands. Am J Kidney Dis 43: 891–899, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Williams VR, Quinn R, Callery S, Kiss A, Oliver MJ: The impact of treatment modality on infection-related hospitalization rates in peritoneal dialysis and hemodialysis patients. Perit Dial Int 31: 440–449, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Boissinot L, Landru I, Cardineau E, Zagdoun E, Ryckelycnk JP, Lobbedez T: Is transition between peritoneal dialysis and hemodialysis really a gradual process? Perit Dial Int 33: 391–397, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chui BK, Manns B, Pannu N, Dong J, Wiebe N, Jindal K, Klarenbach SW: Health care costs of peritoneal dialysis technique failure and dialysis modality switching. Am J Kidney Dis 61: 104–111, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Pajek J, Hutchison AJ, Bhutani S, Brenchley PE, Hurst H, Perme MP, Summers AM, Vardhan A: Outcomes of peritoneal dialysis patients and switching to hemodialysis: A competing risks analysis. Perit Dial Int 34: 289–298, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chidambaram M, Bargman JM, Quinn RR, Austin PC, Hux JE, Laupacis A: Patient and physician predictors of peritoneal dialysis technique failure: A population based, retrospective cohort study. Perit Dial Int 31: 565–573, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Perl J, Pierratos A, Kandasamy G, McCormick BB, Quinn RR, Jain AK, Huang A, Paterson JM, Oliver MJ: Peritoneal dialysis catheter implantation by nephrologists is associated with higher rates of peritoneal dialysis utilization: A population-based study. Nephrol Dial Transplant 30: 301–309, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Portolés J, Del Peso G, Fernández-Reyes MJ, Bajo MA, López-Sánchez P; GCDP : Previous comorbidity and lack of patient free choice of technique predict early mortality in peritoneal dialysis. Perit Dial Int 29: 150–157, 2009 [PubMed] [Google Scholar]
- 32.Olsen J, Bonnevie B, Palmhøj-Nielsen C, Povlsen JV: Economic consequences of an increased number of patients on outgoing dialysis. Scand J Urol Nephrol 44: 452–458, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.