Abstract
Background and objectives
eGFR equations have been evaluated in kidney transplant recipients with variable performance. We assessed the performance of the Modification of Diet in Renal Disease equation and the Chronic Kidney Disease Epidemiology Collaboration equations on the basis of creatinine, cystatin C, and both (eGFR creatinine-cystatin C) compared with measured GFR by iothalamate clearance and evaluated their non-GFR determinants and associations across 15 cardiovascular risk factors.
Design, setting, participants, & measurements
A cross-sectional cohort of 1139 kidney transplant recipients >1 year after transplant was analyzed. eGFR bias, precision, and accuracy (percentage of estimates within 30% of measured GFR) were assessed. Interaction of each cardiovascular risk factor with eGFR relative to measured GFR was determined.
Results
Median measured GFR was 55.0 ml/min per 1.73 m2. eGFR creatinine overestimated measured GFR by 3.1% (percentage of estimates within 30% of measured GFR of 80.4%), and eGFR Modification of Diet in Renal Disease underestimated measured GFR by 2.2% (percentage of estimates within 30% of measured GFR of 80.4%). eGFR cystatin C underestimated measured GFR by −13.7% (percentage of estimates within 30% of measured GFR of 77.1%), and eGFR creatinine-cystatin C underestimated measured GFR by −8.1% (percentage of estimates within 30% of measured GFR of 86.5%). Lower measured GFR associated with older age, women, obesity, longer time after transplant, lower HDL, lower hemoglobin, lower albumin, higher triglycerides, higher proteinuria, and an elevated cardiac troponin T level but did not associate with diabetes, smoking, cardiovascular events, pretransplant dialysis, or hemoglobin A1c. These risk factor associations differed for five risk factors with eGFR creatinine, six risk factors for eGFR Modification of Diet in Renal Disease, ten risk factors for eGFR cystatin C, and four risk factors for eGFR creatinine-cystatin C.
Conclusions
Thus, eGFR creatinine and eGFR creatinine-cystatin C are preferred over eGFR cystatin C in kidney transplant recipients because they are less biased, more accurate, and more consistently reflect the same risk factor associations seen with measured GFR.
Keywords: Cardiovascular Diseases, Creatinine, Cystatin C, diabetes mellitus, glomerular filtration rate, Iothalamic Acid, kidney transplantation, obesity, proteinuria, risk factors, Smoking, Triglycerides
Introduction
The assessment of GFR in kidney transplant recipients is essential for monitoring and management of allograft complications and prognostication of both graft and patient survival. The gold standard measurement of GFR is hampered by the complexity, expense, and invasive nature of the procedure. As such, estimating GFR equations using creatinine (Cr) have become the mainstay for assessment of GFR in transplant recipients. A comparison of Cr–based GFR estimating equations in solid organ transplants showed similar performance between the Modification of Diet in Renal Disease (MDRD) (1) and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Cr equations (2) in two studies (3,4), but superior performance of the MDRD equation in others (5–7). Several generally applicable non–GFR determinants of serum Cr have been recognized, such as muscle mass (8). Moreover, in the native kidney population, Cr-based eGFR has been shown to associate with cardiovascular (CV) risk factors, even after adjusting for measured GFR (mGFR), suggesting that these non-GFR determinants may reflect powerful predictors of adverse outcomes above and beyond kidney function (i.e., GFR) (9,10). Cystatin C (CysC) production and hence, blood levels are less affected by sex and muscle mass (11,12). CysC levels also seem to be associated with CV factors and outcomes (13–17), which are, to some extent, independent of GFR (9,18–23). The association of Cr- and CysC-based eGFR with CV risk factors independent of mGFR in the transplant population has not been well elucidated.
The goals of this study were to (1) assess the performance of CysC–based eGFR equations measured by immunoturbidimetry and calibrated to the standardized CysC reference range compared with Cr–based eGFR equations and mGFR by iothalamate clearance; (2) evaluate the associations between CV risk factors and mGFR; and (3) determine differences in CV risk association between CysC- and Cr-based eGFR compared with mGFR in a stable cohort of kidney transplant recipients >1 year after transplant.
Materials and Methods
Study Population
The Mayo Clinic Institutional Review Board approved this study. Informed consent was waived. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. Outpatient adult recipients of kidney transplants performed at the Mayo Clinic (Rochester, MN) who were at least 1 year post-transplant were included. Consecutive renal transplant recipients who presented to the renal studies unit to undergo GFR measurement by urinary iothalamate clearance were identified between August of 2011 and April of 2013. Chart review was performed to include only patients who had GFR measurement as part of a routine annual evaluation and exclude patients with GFR measurement for clinical concern for acute allograft dysfunction.
Laboratory Assessment
GFR was measured by urinary clearance of nonradiolabeled iothalamate (24) and adjusted for body surface area. Plasma samples for CysC and Cr levels were obtained during the iothalamate clearance study. Cr was measured using a standardized enzymatic assay on a Roche Cobas Chemistry Analyzer (c701 or c501; Roche Diagnostics, Indianapolis, IN), whereas CysC was measured using an immunoturbidimetric assay (Gentian, Moss, Norway) that was traceable to an international reference material. Samples were stored at −80°C and analyzed every 2–4 weeks using the same set of reagents and calibrators over a period of 20 months. GFR was estimated using the Cr–based MDRD equation (eGFR MDRD) (1), the CKD-EPI 2009 equation (eGFR Cr) (2), and the two CysC–based CKD-EPI 2012 GFR estimating equations (eGFR CysC and eGFR Cr-CysC) (13). A subset of these patients with kidney transplants (n=300) was recently reported in a publication comparing eGFR equation performance among different patient groups (kidney donors, patients with CKD, and transplant recipients) (25).
Clinical characteristics were determined from the medical record, including age, sex, race, height, weight, cause of ESRD, time since transplantation, number of previous transplants, diabetes status, hemoglobin A1c (HbA1c), maintenance immunosuppression, and donor characteristics.
CV Risk Factor Assessment
CV risk factors abstracted included diagnoses of diabetes and/or hypertension, CV events (myocardial, cerebral, or peripheral vascular disease), pretransplant dialysis, smoking history, body mass index (BMI), serum lipids (HDL and triglycerides) and serum albumin, Hb, and 24-hour urine protein excretion. Cardiac troponin T (cTnT) was also available as part of each annual transplant visit.
Statistical Analyses
The performance of the estimating equations was assessed by calculating bias, precision, and accuracy compared with mGFR. Bias with eGFR relative to mGFR was assessed as absolute bias (mean difference) and percentage. Precision was defined as the SD of the difference between the estimating equation and the mGFR. Accuracy was defined as the percentage of eGFR that fell within 10% and 30% of the mGFR. Associations of CV risk factors with CysC- and Cr-based equations after adjustment for mGFR were assessed. We began by regressing eGFR or mGFR on each CV risk factor using linear regression. Both eGFR and mGFR were log transformed in the analysis. Thus, regression coefficients were exponentiated to estimate the percentage difference in GFR per risk factor level. Next, we compared these slopes using the generalized estimating equation approach as previously described (19). Interaction between eGFR and each risk factor with mGFR as a reference method was examined to assess whether there was a significantly different association of each CV risk factor with eGFR compared with the same risk factor association with mGFR. All statistical analyses were performed using JMP, version 9 (SAS Institute Inc., Cary, NC).
Results
There were 1139 kidney recipients in the study. This patient cohort was predominately white, living donor recipients, and recipients of first kidney transplants as shown in Table 1. GN was the predominant cause of ESRD. Mean BMI was 29.9±6.9 kg/m2, and 486 (43%) had BMI>30 kg/m2; 96% of patients received prednisone (87% received 5 mg daily). Median time post-transplant was 72 months. Median mGFR was 55.0 ml/min per 1.73 m2; 460 (40%) had mGFR≥60 ml/min per 1.73 m2, 556 (49%) had mGFR between 30 and 59 ml/min per 1.73 m2, and 123 (11%) had mGFR<30 ml/min per 1.73 m2.
Table 1.
Baseline characteristics of kidney transplant recipients and overall kidney function (n=1139)
| Patient characteristics, n=1139 | |
| Recipient age, yr | 55.9±14.3 |
| Men, no. (%) | 640 (56.2) |
| White race, no. (%) | 976 (85.6) |
| Diabetes, no. (%) | 397 (35.0) |
| Cause of ESRD, no. (%) | |
| Diabetes | 217 (19.1) |
| Hypertension | 66 (5.8) |
| PCKD | 171 (15.0) |
| Glomerular disease | 471 (41.4) |
| Other | 214 (18.8) |
| Pretransplant dialysis, no. (%) | 597 (52.5) |
| First transplant, no. (%) | 950 (83.6) |
| Living donor, no. (%) | 862 (75.7) |
| Time post-transplant, mo, median (range) | 72 (11–493) |
| Height, cm | 169.7±10.3 |
| Weight, kg | 86.2±22.0 |
| Body mass index, kg/m2 | 29.9±6.91 |
| Corticosteroid therapy, no. (%) | |
| None | 48 (4.4) |
| 0–5 mg/d | 940 (86.9) |
| >5 mg/d | 94 (8.7) |
| Smoking history, no. (%) | 454 (40.0) |
| Renal function measures, mean±SD; median (min, max) | |
| Creatinine, mg/dl | 1.4±0.5; 1.3 (0.5, 5.1) |
| Cystatin C, mg/L | 1.6±0.6; 1.5 (0.7, 3.9) |
| mGFR, ml/min per 1.73 m2 | 55.6±20.6; 55.0 (8.0, 135) |
| eGFR MDRD | 51.7±19.3; 49.7 (10.3, 171.4) |
| eGFR CKD-EPI creatinine | 54.7±19.6; 52.8 (10.7, 135.9) |
| eGFR CKD-EPI cystatin C | 46.7±18.1; 45.2 (11.4, 117.6) |
| eGFR CKD-EPI creatinine-cystatin C | 49.5±17.9; 48.4 (11.5, 131.3) |
PCKD, polycystic kidney disease; min, max, minimum, maximum; mGFR, measured GFR; MDRD, Modification of Diet in Renal Disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Equation Performance
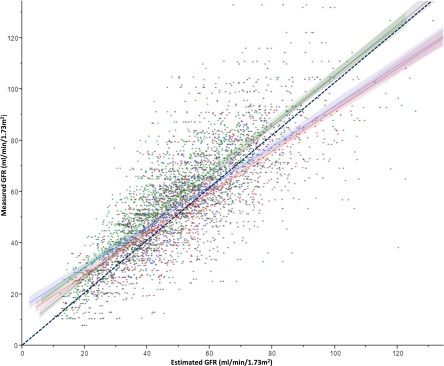
mGFR better correlated with eGFR Cr-CysC (r = 0.86; 95% confidence interval [95% CI], 0.84 to 0.87; P<0.001) and eGFR CysC (r = 0.83; 95% CI, 0.81 to 0.84; P<0.001) than with eGFR Cr (r = 0.77; 95% CI, 0.74 to 0.79; P<0.001) and eGFR MDRD (r = 0.73; 95% CI, 0.71 to 0.76; P<0.001) (Figure 1). eGFR Cr and eGFR MDRD had the lowest bias compared with eGFR CysC and eGFR Cr-CysC. eGFR Cr overestimated mGFR by 3.1%, whereas eGFR MDRD underestimated mGFR by 2.2%, with an accuracy (within 30%) of 80.4% for both equations (Table 2). eGFR CysC had the lowest overall performance. eGFR Cr-CysC performed in between and underestimated GFR by 8.1% with an accuracy (within 30%) of 86.5%. Thus, eGFR Cr-CysC correlated best with mGFR and had the highest accuracy (within 30%), but it had more bias than eGFR Cr and eGFR MDRD (Figure 2).
Figure 1.
Correlation of measured GFR with each of the estimating equations. Measured GFR is plotted on the y axis, and eGFR Modification of Diet in Renal Disease (blue) and Chronic Kidney Disease Epidemiology Collaboration eGFR creatinine (red), cystatin C (green), and creatinine-cystatin C (gray) are plotted on the x axis. The black dashed line represents the line of identity.
Table 2.
Performance of GFR estimating equations
| GFR Equations | Absolute Bias (95% CI), ml/min per 1.73 m2 | Percentage Bias (95% CI) | Accuracy 10%, (N) | Accuracy 30% (N) | Precision, ml/min per 1.73 m2 |
|---|---|---|---|---|---|
| eGFR MDRD | −1.9 (−2.6 to −1.2) | −2.2% (−4.0 to −0.3) | 30.8%a (351) | 80.4% (916) | 14.6 |
| eGFR Cr | 0.8 (0.03 to 1.5) | 3.1% (1.3 to 5.0) | 35.2% (401) | 80.4% (916) | 13.7 |
| eGFR CysC | −8.7 (−9.2 to −8.1) | −13.7% (−15.1 to −12.4) | 27.8%a (317) | 77.1%a (878) | 11.7 |
| eGFR Cr-CysC | −5.0 (−5.6 to −4.5) | −8.1% (−9.4 to −6.8) | 33.2% (378) | 86.5%a (985) | 10.7 |
95% CI, 95% confidence interval; MDRD, Modification of Diet in Renal Disease; Cr, creatinine; CysC, cystatin C; Cr-CysC, creatinine-cystatin C.
P<0.05 compared with eGFR Cr with the McNemar test.
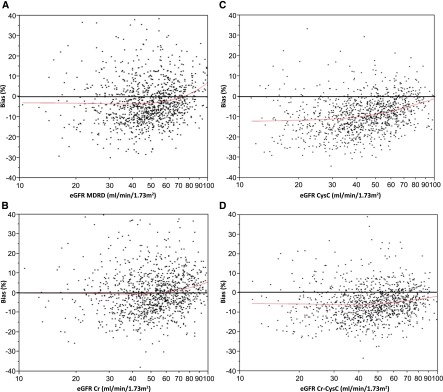
Figure 2.
Equation bias as a function of eGFR. (A) Percentage bias [exp(log eGFR − log measured GFR)−1×100] is on the y axis, and eGFR Modification of Diet in Renal Disease (MDRD) is on the x axis. (B) Percentage bias is on the y axis, and eGFR creatinine (Cr) is on the x axis. (C) Percentage bias is on the y axis, and eGFR cystatin C (CysC) is on the x axis. (D) Percentage bias is on the y axis, and eGFR Cr-CysC is on the x axis.
Next, equation performance was analyzed across CKD stages (26) <15, 15–29, 30–59, and ≥60 ml/min per 1.73 m2 on the basis of eGFR (Table 3). For an eGFR≥60 ml/min per 1.73 m2, both eGFR CysC and eGFR Cr-CysC had higher accuracy (percentage of estimates within 30% of mGFR of 90.9% and 94.0%, respectively) and less bias (−3.7% and −3.5%, respectively) compared with eGFR MDRD (accuracy within 30% of 83.5% and bias of 4.7%) and eGFR Cr (accuracy within 30% of 83.7% and bias of 9.4%). For CKD stage 3 (eGFR=30–59 ml/min per 1.73 m2), eGFR Cr-CysC had superior accuracy within 30% (85.4%) but with significant negative bias (−10.6%) compared with eGFR Cr (accuracy within 30% of 80.4% and bias of −0.8%). For CKD stage 4, eGFR MDRD had the lowest bias (0.4%) but lower accuracy within 30% compared with eGFR Cr and eGFR Cr-CysC. The number of patients with CKD stage 5 was small and ranged from five to 17 patients. Accuracy was similar for eGFR Cr and eGFR Cr-CysC, with persistently less bias for eGFR Cr than for eGFR Cr-CysC. eGFR CysC had the lowest performance for all eGFR stages <60 ml/min per 1.73 m2, with consistently higher negative bias and lower accuracy. In a secondary analysis, when equation performance was stratified on the basis of mGFR, the combined eGFR Cr-CysC equation had higher accuracy than eGFR MDRD and eGFR Cr across all stages of CKD (Supplemental Table 1). The eGFR CysC equation had higher negative bias and lower accuracy for mGFR≥60 ml/min per 1.73 m2 and CKD stage 3 compared with all other estimating equations. For CKD stages 4 and 5, eGFR CysC and eGFR Cr-CysC performed similarly. Both the eGFR MDRD and eGFR Cr had similar accuracy and bias. Therefore, the combined eGFR Cr-CysC equation provided the best estimate of mGFR across all stages of CKD.
Table 3.
Bias, precision, and accuracy of estimating equations stratified for eGFR–based CKD stages (≥60, 30–59, 15–29, and <15 ml/min per 1.73 m2)
| GFR Estimating Equations, ml/min per 1.73 m2 | Percentage Bias (95% CI) | Precision, SD, ml/min per 1.73 m2 | Accuracy 10%, % | Accuracy 30%, % |
|---|---|---|---|---|
| eGFR MDRD | ||||
| ≥60, n=328 | 4.7% (1.0 to 8.3) | 19.5 | 35.0 | 83.5 |
| 30–59, n=697 | −5.7% (−7.9 to −3.6) | 12.1 | 30.1 | 80.5 |
| 15–29, n=109 | 0.4% (−6.8 to 7.6) | 8.1 | 22.9 | 69.7 |
| <15, n=5 | −7.9% (−30.9 to 15.0) | 2.8 | 20.0 | 100 |
| eGFR Cr | ||||
| ≥60, n=404 | 9.4% (6.5 to 12.2) | 16.4 | 39.1 | 83.7 |
| 30–59, n=633 | −0.8% (−3.2 to 1.6) | 11.8 | 33.6 | 80.4 |
| 15–29, n=96 | 3.3% (−4.6 to 11.2) | 8.0 | 28.1 | 66.7 |
| <15, n=6 | −2.4% (−27.5 to 22.8) | 3.3 | 50.0 | 83.3 |
| eGFR CysC | ||||
| ≥60, n=263 | −3.7% (−6.1 to −1.3) | 13.7 | 43.0 | 90.9 |
| 30–59, n=663 | −16.2% (−17.8 to −14.6) | 11.4 | 25.0 | 76.9 |
| 15–29, n=196 | −18.6% (−22.5 to −14.7) | 8.3 | 17.3 | 60.7 |
| <15, n=17 | −17.1% (−31.9 to −2.4) | 6.6 | 23.5 | 58.8 |
| eGFR Cr-CysC | ||||
| ≥60, n=316 | −3.5% (−5.6 to −1.5) | 12.6 | 43.7 | 94.0 |
| 30–59, n=670 | −10.6% (−12.2 to −9.1) | 10.2 | 30.0 | 85.4 |
| 15–29, n=145 | −6.5% (−11.8 to −1.1) | 6.8 | 24.1 | 75.2 |
| <15, n=8 | −5.1% (−19.4 to 9.2) | 2.7 | 50.0 | 87.5 |
95% CI, 95% confidence interval; MDRD, Modification of Diet in Renal Disease; Cr, creatinine; CysC, cystatin C; Cr-CysC, creatinine-cystatin C.
Non–GFR CV Risk Determinants of Cr- and CysC-Based eGFR
The unadjusted association of mGFR with CV risk variables was assessed (Table 4). Lower mGFR is significantly associated with older age, women, obesity, longer time post-transplant, lower HDL cholesterol, lower Hb, lower albumin, higher triglycerides, higher proteinuria, and higher detectable cTnT. Table 4 highlights these relative differences in 15 CV risk factor associations for eGFR compared with mGFR. The association of risk factors with eGFR Cr differed from their association with mGFR for five characteristics (pretransplant dialysis, HDL cholesterol, serum albumin, Hb, and cTnT). The association of risk factors with eGFR MDRD differed from their association with mGFR for six characteristics (age, pretransplant dialysis, time post-transplant, serum albumin, Hb, and cTnT). This was generally similar to the findings with eGFR Cr, with the most notable difference being an attenuated association between lower eGFR MDRD and older age. The association of risk factors with eGFR CysC differed from their association with mGFR for 10 characteristics (age, diabetes, smoking history, CV events, pretransplant dialysis, BMI, time post-transplant, triglycerides, proteinuria, and HbA1c). The association of risk factors with eGFR Cr-CysC differed from their association with mGFR for four characteristics (age, diabetes, triglycerides, and HbA1c).
Table 4.
Associations between cardiovascular risk factor (predictor) and GFR (predicted) described as percentage changes and the test for interaction between the association with eGFR and the association with measured GFR
| Characteristics and GFR Approacha | Difference, % | P Valueb | Test for Interaction (Different Association with eGFR than with mGFR) P Valuec |
|---|---|---|---|
| Age per SD | |||
| mGFR | −4.0 | 0.001 | |
| eGFRCr | −4.9 | <0.001 | 0.24 |
| eGFRCysC | −7.1 | <0.001 | <0.001 |
| eGFRCr-CysC | −5.4 | <0.001 | 0.02 |
| eGFRMDRD | −1.0 | 0.37 | <0.001 |
| Women | |||
| mGFR | −5.1 | 0.04 | |
| eGFRCr | −5.5 | 0.01 | 0.79 |
| eGFRCysC | −7.2 | 0.003 | 0.11 |
| eGFRCr-CysC | −6.8 | 0.003 | 0.16 |
| eGFRMDRD | −7.4 | 0.001 | 0.12 |
| Diabetes | |||
| mGFR | −3.0 | 0.25 | |
| eGFRCr | −3.1 | 0.18 | 0.93 |
| eGFRCysC | −9.2 | <0.001 | <0.001 |
| eGFRCr-CysC | −6.1 | 0.01 | 0.02 |
| eGFRMDRD | −1.5 | 0.53 | 0.36 |
| Smoking history | |||
| mGFR | −3.4 | 0.17 | |
| eGFRCr | −3.2 | 0.16 | 0.91 |
| eGFRCysC | −6.6 | 0.01 | 0.02 |
| eGFRCr-CysC | −4.8 | 0.04 | 0.27 |
| eGFRMDRD | −1.4 | 0.55 | 0.20 |
| CV event | |||
| mGFR | −5.4 | 0.30 | |
| eGFRCr | −6.8 | 0.15 | 0.56 |
| eGFRCysC | −12 | 0.02 | 0.02 |
| eGFRCr-CysC | −9.2 | 0.05 | 0.08 |
| eGFRMDRD | −5.0 | 0.29 | 0.87 |
| Pretransplant dialysis | |||
| mGFR | −2.8 | 0.25 | |
| eGFRCr | 1.3 | 0.56 | 0.01 |
| eGFRCysC | −6.0 | 0.01 | 0.01 |
| eGFRCr-CysC | −2.6 | 0.26 | 0.86 |
| eGFRMDRD | 1.8 | 0.42 | 0.003 |
| Log body mass index per SD | |||
| mGFR | −2.6 | 0.04 | |
| eGFRCr | −2.4 | 0.03 | 0.87 |
| eGFRCysC | −4.8 | <0.001 | 0.001 |
| eGFRCr-CysC | −3.7 | <0.001 | 0.06 |
| eGFRMDRD | −2.3 | 0.04 | 0.72 |
| Log post-transplant months per SD | |||
| mGFR | −3.9 | 0.002 | |
| eGFRCr | −2.6 | 0.02 | 0.06 |
| eGFRCysC | −5.2 | <0.001 | 0.04 |
| eGFRCr-CysC | −3.9 | <0.001 | 0.96 |
| eGFRMDRD | −1.7 | 0.12 | 0.003 |
| Log HDL per SD | |||
| mGFR | 3.2 | 0.01 | |
| eGFRCr | 1.1 | 0.32 | 0.03 |
| eGFRCysC | 4.5 | <0.001 | 0.05 |
| eGFRCr-CysC | 3.0 | 0.01 | 0.84 |
| eGFRMDRD | 1.3 | 0.26 | 0.05 |
| Log triglycerides per SD | |||
| mGFR | −3.3 | 0.01 | |
| eGFRCr | −3.0 | 0.01 | 0.68 |
| eGFRCysC | −5.7 | <0.001 | <0.001 |
| eGFRCr-CysC | −4.5 | <0.001 | 0.03 |
| eGFRMDRD | −2.9 | 0.01 | 0.64 |
| Log hemoglobin per SD | |||
| mGFR | 23.0 | <0.001 | |
| eGFRCr | 18.1 | <0.001 | <0.001 |
| eGFRCysC | 22.7 | <0.001 | 0.72 |
| eGFRCr-CysC | 21.2 | <0.001 | 0.06 |
| eGFRMDRD | 17.9 | <0.001 | <0.001 |
| Log albumin per SD | |||
| mGFR | 13.0 | <0.001 | |
| eGFRCr | 7.4 | <0.001 | <0.001 |
| eGFRCysC | 14.9 | <0.001 | 0.08 |
| eGFRCr-CysC | 11.4 | <0.001 | 0.07 |
| eGFRMDRD | 6.3 | <0.001 | <0.001 |
| Log 24-h urine proteinuria per SD | |||
| mGFR | −12.2 | <0.001 | |
| eGFRCr | −11.0 | <0.001 | 0.13 |
| eGFRCysC | −13.8 | <0.001 | 0.01 |
| eGFRCr-CysC | −12.9 | <0.001 | 0.22 |
| eGFRMDRD | −10.8 | <0.001 | 0.07 |
| Log hemoglobin A1c per SD | |||
| mGFR | −0.6 | 0.65 | |
| eGFRCr | −0.3 | 0.78 | 0.75 |
| eGFRCysC | −4.1 | 0.002 | <0.001 |
| eGFRCr-CysC | −2.1 | 0.09 | 0.03 |
| eGFRMDRD | 0.6 | 0.62 | 0.17 |
| Cardiac troponin T | |||
| mGFR | −37.1 | <0.001 | |
| eGFRCr | −29.8 | <0.001 | 0.01 |
| eGFRCysC | −38.7 | <0.001 | 0.41 |
| eGFRCr-CysC | −35.1 | <0.001 | 0.32 |
| eGFRMDRD | −26.5 | <0.001 | <0.001 |
mGFR, measured GFR; Cr, creatinine; CysC, cystatin C; Cr-CysC, creatinine-cystatin C; MDRD, Modification of Diet in Renal Disease; CV, cardiovascular.
eGFRCr, eGFRCysC, and eGFRCr-CysC are all Chronic Kidney Disease Epidemiology Collaboration–based equations, and eGFRMDRD is the MDRD study equation (1).
P value for testing for association between the CV risk factor and GFR.
P value for testing for interaction between the predictor and the method of deriving GFR.
Discussion
These results show that, in a stable cohort of kidney transplant recipients >1 year after transplantation, the performance of the eGFR Cr-CysC was superior to eGFR MDRD and eGFR Cr in terms of accuracy, but eGFR Cr-CysC showed more bias. The performance of the eGFR CysC equation was inferior to the performances of eGFR MDRD, eGFR Cr, and eGFR Cr-CysC equations in this transplant population, with more bias and less accuracy. Moreover, eGFR CysC was associated with more CV risk factors than all other estimating equations, and it was the least concordant in comparison with the same CV risk associations with mGFR.
Low mGFR was associated with several traditional (age, obesity, and hyperlipidemia) as well as nontraditional (anemia, hypoalbuminemia, proteinuria, and detectable cTnT) risk factors known to correlate with CV disease and mortality. Obese individuals have increased odds of developing CKD, and this relationship is heavily influenced by the presence of other vascular risk factors, such as diabetes, low HDL, and hypertension (27,28). Of importance, the association of low mGFR with higher BMI is likely, in part, because of the indexing of GFR to body surface area (per 1.73 m2) When mGFR is not indexed to body surface area, higher GFR in milliliters per minute associates with higher log BMI (7.2% higher per SD; P<0.001). However, because GFR is usually reported and interpreted in milliliters per minute per 1.73 m2 units, we focused our analysis to reflect how GFR is usually applied in clinical practice.
Low mGFR was also associated with hypoalbuminemia and anemia, both of which are known complications of CKD and have been shown to be significant markers of CV disease and mortality after kidney transplant (29–31). The association of low mGFR with proteinuria has been previously reported in both the native kidney and the kidney transplant populations. A detectable cTnT level significantly associates with CV events and mortality among patients on the waiting list and after kidney transplant (32–34). The relationship between a detectable cTnT and GFR is nonlinear, and the number of kidney transplant recipients with detectable cTnT levels significantly increases at GFR<30 ml/min per 1.73 m2 (32). Therefore, the relationship between low mGFR and a detectable cTnT can be explained by increased myocardial injury secondary to multiple pathophysiologic mechanisms driven by complications of CKD combined with decreased renal clearance of the troponin molecule itself or its metabolites. The lack of association of low mGFR with diabetes and HbA1c may be, in part, because of the fact that diabetes was defined as pretransplant diabetes as well as new-onset diabetes after transplant and therefore, likely included a wide spectrum of diabetic renal manifestations, including hyperfiltration and impaired renal function. Low mGFR was not associated with pretransplant CV events in this study. It should be noted that post–transplant CV events were not reported and presumably, are more likely to correlate with low mGFR (measured post-transplant) than pretransplant events.
As previously found in other populations (19,23), the associations of mGFR with CV risk factors were more similar to those seen with eGFR Cr than with eGFR CysC. Thus, in stable kidney transplant recipients, eGFR Cr is generally better than eGFR on the basis of CysC to understand factors that are dependent on mGFR. Nonetheless, there are still factors that may affect eGFR Cr by influencing the non-GFR determinants of serum Cr, such as protein intake and muscle mass (8,35). In particular, eGFR MDRD and eGFR Cr attenuated the association of lower mGFR with pretransplant dialysis, anemia, hypoalbuminemia, and elevated cTnT, possibly because these CV risk factors may be associated with protein-restricted diet or lower muscle mass in transplant recipients. It is, therefore, not surprising that the association of CysC with Hb, albumin, and cTnT was not different than the association of mGFR with these variables, likely because CysC is not as affected by diet and muscle mass.
It should be noted that the association of eGFR MDRD with age was significantly different than that of mGFR with age. This may be explained by the way that age is modeled in the MDRD equation with log transformation. However, the eGFR Cr equation models age in a linear fashion, which more accurately reflects the association of mGFR with age.
eGFR CysC had a greater number of differing associations with CV risk factors compared with mGFR than eGFR Cr and eGFR MDRD. After mGFR adjustment, CysC has been shown to be significantly associated with hypertension, diabetes, low HDL, high triglyceride level, high uric acid level, albuminuria, and increased BMI independent of mGFR (9,19). In our study, we identified several CV risk factors that were more strongly associated with reduced eGFR CysC than reduced mGFR. In particular, eGFR CysC was more strongly associated with older age, diabetes, smoking history, past CV events, past dialysis, obesity (higher BMI), hypertriglyceridemia, proteinuria, and elevated HbA1c compared with association of these variables with mGFR. This may be explained by the non-GFR biology of CysC and its role in immunomodulation and atherosclerosis (18,20–22).
Studies evaluating the performance of CysC-based equations in kidney transplant recipients have shown variable results, mainly because of prior lack of standardized calibration for the measurement of CysC as well as differing CysC measurement techniques (36). Systematic review of 14 CysC estimating GFR equations in the kidney transplant population showed significant heterogeneity and optimal accuracy of the Le Bricon equation (37). However, the Le Bricon equation was developed in a cohort of only 25 transplant patients, all of whom had a GFR>30 ml/min per 1.73 m2 (38). Moreover, the systematic review only included one study that evaluated the CysC CKD-EPI equations (39). In that study, CysC estimating equations were shown to be more accurate than eGFR Cr equations in kidney transplant recipients (39), but the difference in performance of the eGFR CysC compared with eGFR Cr and eGFR Cr-CysC was not found to significantly affect reclassification of CKD stages (40). The difference in our findings compared with those in the study by Masson et al. (40) may be explained by the gold standard GFR measurement used. Iothalamate clearance was used for GFR measurement in our study versus inulin clearance in the study by Masson et al. (40). It should be noted that the CKD-EPI equations were developed to estimate mGFR by iothalamate clearance and not by inulin clearance. In addition, 42.7% of our patients had BMI>30 kg/m2 compared with 12% in the study by Masson et al. (40). The CysC CKD-EPI equation had improved accuracy in patients with BMI≤30 kg/m2 compared with >30 kg/m2 (accuracy within 30% of 74.9% versus 69.1%) in our cohort. Despite these differences, our findings of the inferior performance of CysC-based eGFR compared with Cr-based eGFR are supported by the significant association of CysC with CV risk factors that do not align with mGFR associations.
There are several strengths of our study. This is the largest single–center study with uniform approach to measurement of GFR and standardized measurements of Cr and CysC in the kidney transplant population. Our patient cohort was 1 year out from transplant with stable allograft function and on stable immunosuppression regimens. The availability of pertinent CV risk factors allowed for analysis of association of mGFR and estimating equations, which had not been previously described in the kidney transplant population. There are also several limitations that warrant discussion. First, the cross–sectional study design prohibited analysis of the temporal relationship between GFR and these risk factors. Second, our patient cohort had several unique characteristics (mostly white, first kidney transplants, and living donor recipients) that may affect the generalizability of the results to other kidney transplant populations. Third, we cannot rule out the possibility that a bias between the immunoturbidimetric and the immunonephelometric assays by which the equations were derived may be present, which would affect the percentage bias of eGFR CysC compared with mGFR. However, the assay used for this study was traceable to the same reference material as was used for the CKD-EPI equations.
The findings of this study confirm that eGFR CysC is an inferior surrogate of mGFR in the kidney transplant population compared with eGFR Cr and eGFR MDRD. Moreover, CysC seems to have several non–GFR determinants that are directly associated with CV risk factors, some of which are similar to those noted in the native kidney population, whereas others are newly reported in this transplant population. Future studies are needed to evaluate CysC in predicting kidney failure, CV outcomes, and mortality in transplant recipients, because it may perform better than GFR as a prognostic marker because of these non-GFR determinants.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Mary Karaus, Cynthia Rendler, and David Dvorak of the Mayo Validation Support Services and Erling Sundrehagen, director of Gentian, for their assistance and support with this study.
This study was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK090358.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11741115/-/DCSupplemental.
References
- 1.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaffi K, Uhlig K, Perrone RD, Ruthazer R, Rule A, Lieske JC, Navis G, Poggio ED, Inker LA, Levey AS: Performance of creatinine-based GFR estimating equations in solid-organ transplant recipients. Am J Kidney Dis 63: 1007–1018, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pöge U, Gerhardt T, Stoffel-Wagner B, Sauerbruch T, Woitas RP: Validation of the CKD-EPI formula in patients after renal transplantation. Nephrol Dial Transplant 26: 4104–4108, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Buron F, Hadj-Aissa A, Dubourg L, Morelon E, Steghens JP, Ducher M, Fauvel JP: Estimating glomerular filtration rate in kidney transplant recipients: Performance over time of four creatinine-based formulas. Transplantation 92: 1005–1011, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Kukla A, El-Shahawi Y, Leister E, Kasiske B, Mauer M, Matas A, Ibrahim HN: GFR-estimating models in kidney transplant recipients on a steroid-free regimen. Nephrol Dial Transplant 25: 1653–1661, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masson I, Flamant M, Maillard N, Rule AD, Vrtovsnik F, Peraldi MN, Thibaudin L, Cavalier E, Vidal-Petiot E, Bonneau C, Moranne O, Alamartine E, Mariat C, Delanaye P: MDRD versus CKD-EPI equation to estimate glomerular filtration rate in kidney transplant recipients. Transplantation 95: 1211–1217, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Rule AD, Bailey KR, Schwartz GL, Khosla S, Lieske JC, Melton LJ 3rd: For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int 75: 1071–1078, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathisen UD, Melsom T, Ingebretsen OC, Jenssen T, Njølstad I, Solbu MD, Toft I, Eriksen BO: Estimated GFR associates with cardiovascular risk factors independently of measured GFR. J Am Soc Nephrol 22: 927–937, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu CY, Propert K, Xie D, Hamm L, He J, Miller E, Ojo A, Shlipak M, Teal V, Townsend R, Weir M, Wilson J, Feldman H; CRIC Investigators : Measured GFR does not outperform estimated GFR in predicting CKD-related complications. J Am Soc Nephrol 22: 1931–1937, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laterza OF, Price CP, Scott MG: Cystatin C: An improved estimator of glomerular filtration rate? Clin Chem 48: 699–707, 2002 [PubMed] [Google Scholar]
- 12.Vinge E, Lindergård B, Nilsson-Ehle P, Grubb A: Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest 59: 587–592, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, Levey AS, Gansevoort RT; CKD Prognosis Consortium : Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 369: 932–943, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menon V, Shlipak MG, Wang X, Coresh J, Greene T, Stevens L, Kusek JW, Beck GJ, Collins AJ, Levey AS, Sarnak MJ: Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med 147: 19–27, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Peralta CA, Katz R, Sarnak MJ, Ix J, Fried LF, De Boer I, Palmas W, Siscovick D, Levey AS, Shlipak MG: Cystatin C identifies chronic kidney disease patients at higher risk for complications. J Am Soc Nephrol 22: 147–155, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu CK, Lin JW, Caffrey JL, Chang MH, Hwang JJ, Lin YS: Cystatin C and long-term mortality among subjects with normal creatinine-based estimated glomerular filtration rates: NHANES III (Third National Health and Nutrition Examination Survey). J Am Coll Cardiol 56: 1930–1936, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Bhavsar NA, Appel LJ, Kusek JW, Contreras G, Bakris G, Coresh J, Astor BC; AASK Study Group : Comparison of measured GFR, serum creatinine, cystatin C, and beta-trace protein to predict ESRD in African Americans with hypertensive CKD. Am J Kidney Dis 58: 886–893, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rule AD, Bailey KR, Lieske JC, Peyser PA, Turner ST: Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney Int 83: 1169–1176, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lafarge JC, Naour N, Clément K, Guerre-Millo M: Cathepsins and cystatin C in atherosclerosis and obesity. Biochimie 92: 1580–1586, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Staun-Ram E, Miller A: Cathepsins (S and B) and their inhibitor Cystatin C in immune cells: Modulation by interferon-β and role played in cell migration. J Neuroimmunol 232: 200–206, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Naour N, Fellahi S, Renucci JF, Poitou C, Rouault C, Basdevant A, Dutour A, Alessi MC, Bastard JP, Clément K, Guerre-Millo M: Potential contribution of adipose tissue to elevated serum cystatin C in human obesity. Obesity (Silver Spring) 17: 2121–2126, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Melsom T, Fuskevåg OM, Mathisen UD, Strand H, Schei J, Jenssen T, Solbu M, Eriksen BO: Estimated GFR is biased by non-traditional cardiovascular risk factors. Am J Nephrol 41: 7–15, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Wilson DM, Bergert JH, Larson TS, Liedtke RR: GFR determined by nonradiolabeled iothalamate using capillary electrophoresis. Am J Kidney Dis 30: 646–652, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Meeusen JW, Rule AD, Voskoboev N, Baumann NA, Lieske JC: Performance of cystatin C- and creatinine-based estimated glomerular filtration rate equations depends on patient characteristics. Clin Chem 61: 1265–1272, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Foster MC, Hwang SJ, Larson MG, Lichtman JH, Parikh NI, Vasan RS, Levy D, Fox CS: Overweight, obesity, and the development of stage 3 CKD: The Framingham Heart Study. Am J Kidney Dis 52: 39–48, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O: Obesity and risk for chronic renal failure. J Am Soc Nephrol 17: 1695–1702, 2006 [DOI] [PubMed] [Google Scholar]
- 29.van Ree RM, Gross S, Zelle DM, van der Heide JJ, Schouten JP, van Son WJ, Gans RO, Bakker SJ: Influence of C-reactive protein and urinary protein excretion on prediction of graft failure and mortality by serum albumin in renal transplant recipients. Transplantation 89: 1247–1254, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Rigatto C, Parfrey P, Foley R, Negrijn C, Tribula C, Jeffery J: Congestive heart failure in renal transplant recipients: Risk factors, outcomes, and relationship with ischemic heart disease. J Am Soc Nephrol 13: 1084–1090, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Rigatto C, Foley R, Jeffery J, Negrijn C, Tribula C, Parfrey P: Electrocardiographic left ventricular hypertrophy in renal transplant recipients: Prognostic value and impact of blood pressure and anemia. J Am Soc Nephrol 14: 462–468, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Keddis MT, El-Zoghby ZM, El Ters M, Rodrigo E, Pellikka PA, Jaffe AS, Cosio FG: Cardiac troponin T before and after kidney transplantation: Determinants and implications for posttransplant survival. Am J Transplant 13: 406–414, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Hickson LJ, Cosio FG, El-Zoghby ZM, Gloor JM, Kremers WK, Stegall MD, Griffin MD, Jaffe AS: Survival of patients on the kidney transplant wait list: Relationship to cardiac troponin T. Am J Transplant 8: 2352–2359, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Apple FS, Murakami MM, Pearce LA, Herzog CA: Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation 106: 2941–2945, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Anastasio P, Cirillo M, Spitali L, Frangiosa A, Pollastro RM, De Santo NG: Level of hydration and renal function in healthy humans. Kidney Int 60: 748–756, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Delanaye P, Pieroni L, Abshoff C, Lutteri L, Chapelle JP, Krzesinski JM, Hainque B, Cavalier E: Analytical study of three cystatin C assays and their impact on cystatin C-based GFR-prediction equations. Clin Chim Acta 398: 118–124, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Harman G, Akbari A, Hiremath S, White CA, Ramsay T, Kokolo MB, Craig J, Knoll GA: Accuracy of cystatin C-based estimates of glomerular filtration rate in kidney transplant recipients: A systematic review. Nephrol Dial Transplant 28: 741–757, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Le Bricon T, Thervet E, Froissart M, Benlakehal M, Bousquet B, Legendre C, Erlich D: Plasma cystatin C is superior to 24-h creatinine clearance and plasma creatinine for estimation of glomerular filtration rate 3 months after kidney transplantation. Clin Chem 46: 1206–1207, 2000 [PubMed] [Google Scholar]
- 39.Masson I, Maillard N, Tack I, Thibaudin L, Dubourg L, Delanaye P, Cavalier E, Bonneau C, Kamar N, Morelon E, Moranne O, Alamartine E, Mariat C: GFR estimation using standardized cystatin C in kidney transplant recipients. Am J Kidney Dis 61: 279–284, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Masson I, Maillard N, Cavalier E, Alamartine E, Mariat C, Delanaye P: KDIGO guidelines and kidney transplantation: Is the cystatin-c based recommendation relevant? Am J Transplant 15: 2211–2214, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.