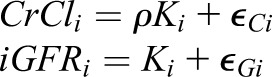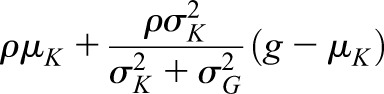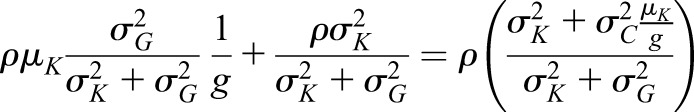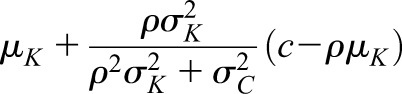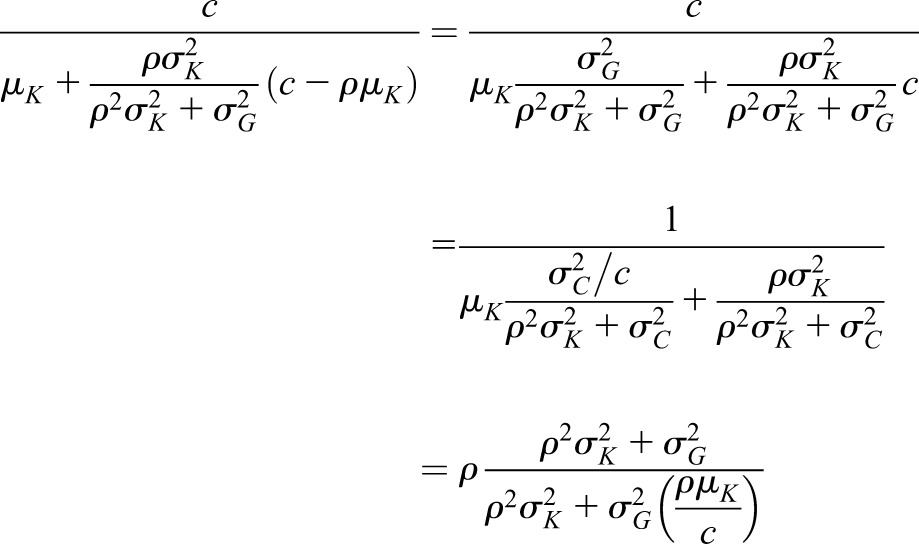Abstract
Background and objectives
Overestimation of GFR by urinary creatinine clearance (CrCl) at lower levels of GFR has long been attributed to enhanced creatinine secretion. However, this does not take into consideration the contribution of errors in measured GFR (and CrCl) due to short-term biologic variability or test imprecision.
Design, setting, participants, & measurements
We analyzed cross-sectional data among 1342 participants from the Chronic Renal Insufficiency Cohort study with baseline measurement of GFR by iothalamate clearance (iGFR) and CrCl by 24-hour urine collection. We examined the CrCl/iGFR ratio classified by categories of iGFR and also by categories of CrCl.
Results
Overall, mean CrCl/iGFR ratio was 1.13. CrCl/iGFR ratio was higher at lower iGFR categories. In contrast, this ratio was lower at lower CrCl levels. We hypothesize these relationships could be due to measurement error, which is bolstered by replicating these trends in a simulation and modeling exercise in which there was no variation in the ratio of CrCl/iGFR with true kidney function but taking into account the effect of measurement error in both CrCl and iGFR (of magnitudes previously described in the literature). In our simulated data, the observed CrCl/iGFR ratio was higher at lower observed iGFR levels when patients were classified by categories of observed iGFR. When the same patients were classified by categories of observed CrCl, the observed CrCl/iGFR ratio was lower at lower observed CrCl levels.
Conclusions
The combined empirical and modeling results suggest that measurement errors (in both CrCl and iGFR) should be considered as an alternative explanation for the longstanding observation that the ratio of CrCl to iGFR gets larger as iGFR decreases.
Numerous textbooks and review articles state that as kidney function declines, the proportion of creatinine cleared by secretion (relative to filtration) increases (Supplemental Table 1) (1–14). This is identified as a major weakness of using creatinine clearance (CrCl) (and serum creatinine) to estimate kidney function (1,3,5,13). For example, the latest edition of Brenner and Rector’s “The Kidney” textbook states: “tubular secretion of creatinine increases with decreased renal function, thus masking a true drop in GFR” (15). Similarly, it is written in UpToDate that: “The increase in creatinine secretion as GFR falls can limit the interpretation of the creatinine clearance” (1).
However, available empirical data supporting this notion primarily consist of studies showing that the CrCl/GFR ratio is higher in patients with lower measured GFR than in patients with higher measured GFR (Supplemental Table 1) (16–20).
A potential alternative explanation for prior observations that the CrCl/GFR ratio increases as measured GFR declines is the contribution of measurement error in both CrCl and GFR. We use the term “measurement error” here to encompass short-term biologic variability as well as imprecisions inherent in the conduct of the test itself, such as under- or over-collection of timed urine specimens or imprecision in the laboratory assays. When there is measurement error, the observed value of any ratio (e.g., CrCl/GFR) will tend to be larger than the true, fixed ratio when the denominator (GFR here) is smaller than its average value; this becomes more extreme as the denominator decreases. For example, when patients are classified into those with measured GFR<30 ml/min per 1.73 m2, that stratum included patients who have truly poor kidney function but also those with low measured GFR due to measurement error.
We examined the ratio of measured CrCl to measured GFR in patients enrolled in the Chronic Renal Insufficiency Cohort (CRIC) study who, at baseline, underwent measurement of GFR by iothalamate clearance (iGFR). In contrast to prior studies, we examined the CrCl/iGFR ratio classified not only by categories of iGFR but also by categories of CrCl. This allowed us to distinguish whether true physiologic changes in creatinine secretion or measurement error is the more likely reason the CrCl/GFR ratio is higher in patients with lower GFR. Specifically, if it were true that tubular secretion rising proportionally with declining levels of kidney function is the only explanation, then the CrCl/iGFR ratio should also be higher among those with lower levels of CrCl (since patients with lower CrCl have worse kidney function). If, however, we had observed that the CrCl/iGFR ratio is lower among those with lower levels of measured CrCl (i.e., the exact opposite trend), such a finding would support measurement error as being an alternative and potentially superior explanation. This is because the presence and propagation of measurement error causes the observed value of any ratio (e.g., CrCl/iGFR) to be lower than the true, fixed ratio when the numerator is smaller than its average value (Table 1), and this becomes more extreme as the numerator decreases.
Table 1.
Theory behind the ratio with fixed values of numerator or denominator
Suppose that we have measurements of CrCl and iGFR for person i that are based on true kidney function (called Ki). iGFR is equal to true kidney function but is measured with error and CrCl is assumed to be equal to ρ times true kidney function (we took ρ=1.13 in the simulation) and is also measured with error:
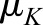 and variance and variance 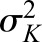 ( (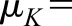 50 and 50 and 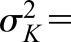 152 are used in the simulation) and that the measurement error terms in (1) are also normally distributed (with variances 152 are used in the simulation) and that the measurement error terms in (1) are also normally distributed (with variances 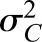 and and 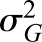 , given by 72 and 52 respectively, in the simulation). We can then calculate the mean of CrCl for given values of iGFR or vice versa using standard properties of a bivariate normal distribution and the equations in (1). , given by 72 and 52 respectively, in the simulation). We can then calculate the mean of CrCl for given values of iGFR or vice versa using standard properties of a bivariate normal distribution and the equations in (1).The mean value of CrCl when iGFR is equal to a specific value, g, is given by
Similarly the mean of iGFR when CrCl is equal to a specific value c is given by
|
Table 1 modified from reference 35.
As a means of confirmation, we conducted a simulation and modeling exercise to assess whether our empirically observed results could be replicated solely by modeling the effects of measurement error in CrCl and measurement error in iGFR (of magnitudes previously described in the literature). The simulation was set such that there was no variation in the ratio of CrCl/iGFR with true kidney function.
Materials and Methods
Study Population
The analysis used baseline data from the CRIC study, a multicenter, observational study of CKD. Anonymized data for this analysis were obtained from the National Institutes of Diabetes and Digestive and Kidney Disease (NIDDK) Data Repository with appropriate institutional review board approval. The design and baseline characteristics of the CRIC study have been previously described (21–23). We studied the subcohort of CRIC study participants who underwent direct measurements of GFR by 125I-iothalamate clearance (n=1423). After excluding participants who were missing CrCl data, our final study population was 1342.
Data Measurement
GFR was measured directly by urinary clearance of 125I-iothalamate (iGFR) (24,25). Median intratest coefficient of variation (CV) for the iGFR was 9.7%, excluding the first period (26).
Serum creatinine measurements were done in the CRIC central laboratory at the University of Pennsylvania on the Hitachi Vitros 950 and calibrated to the IDMS-traceable standardized creatinine (27,28). CrCl was calculated on the basis of urine creatinine concentration×urine volume/serum creatinine concentration from a single 24-hour urine collection and, like iGFR, standardized to body surface area.
Data Analysis
Our main analysis focuses on how CrCl/iGFR ratio varies with categories of CrCl. We compared this with how CrCl/iGFR ratio varies with categories of iGFR.
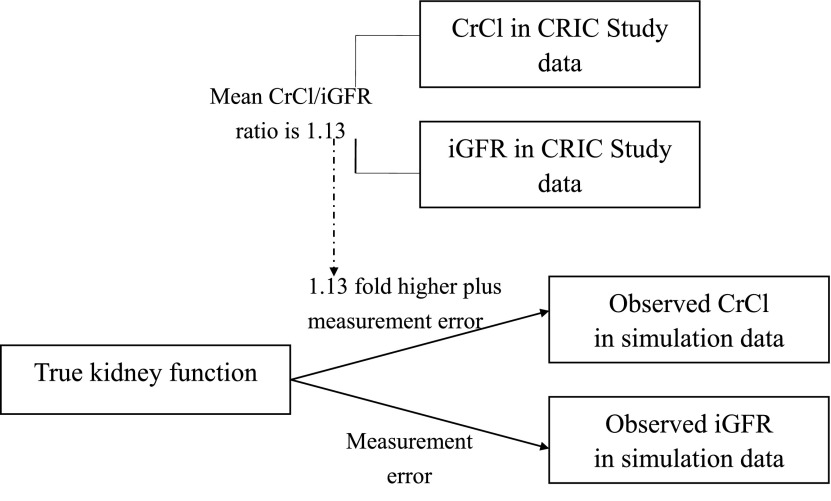
To further assess the relation of CrCl/iGFR ratio with kidney function, we performed a simulation study to generate a random sample of 1400 with “true kidney function” set as 50±15 (mean±SD) ml/min per 1.73 m2. iGFR was set to be equal to “true kidney function” and CrCl set to be 1.13-times “true kidney function.” We simulated the spread of observed CrCl and iGFR readings due to measurement error on the basis of the literature showing that the within-person CV of iGFR was approximately 10% (29–32) and the within-person CV of CrCl was approximately 12% (29,33,34) (Figure 1). Since CV=SD÷mean×100%, SD of iGFR=10%×50=5 ml/min per 1.73 m2 and SD of CrCl=12%×50×1.13=6.78 ≈ 7 ml/min per 1.73 m2. (Please see the Supplemental Appendix for SAS codes.) In the simulation dataset, we also examined how CrCl/iGFR ratio varies with categories of CrCl, and how it varies with categories of iGFR. In addition to the simulation modeling, we used mathematical modeling to calculate the expected ratios for fixed values of the numerator and denominator under measurement error.
Figure 1.
Relationship between true kidney function, observed CrCl, and observed iGFR in simulation data and in CRIC data.
In sensitivity analyses, we repeated the simulation assuming a constant CV for iGFR and CrCl instead of constant SD. We also repeated our simulation model on the basis of a population with a uniform distribution of “true kidney function” from 20 to 80 ml/min per 1.73 m2 (i.e., instead of a bell-shaped distribution, the distribution is rectangle-shaped). (Please see the Supplemental Appendix for SAS codes.)
All analyses were carried out using SAS 9.3 (SAS Institute Inc., Cary, NC) and IBM SPSS Statistics 20.0 (IBM SPSS, Chicago, IL).
Results
Characteristics of the 1342 CRIC participants included in the analysis are shown in Supplemental Table 2. Mean (±SD) serum creatinine was 1.70±0.56 mg/dl and BUN 29±13 mg/dl. The mean CrCl was 52.1±25.8 ml/min per 1.73 m2 and iGFR 48.0±19.9 ml/min per 1.73 m2. The median time lapse between 24-hour urine collection and iGFR measurement was 0 days (interquartile range, 0–11 days).
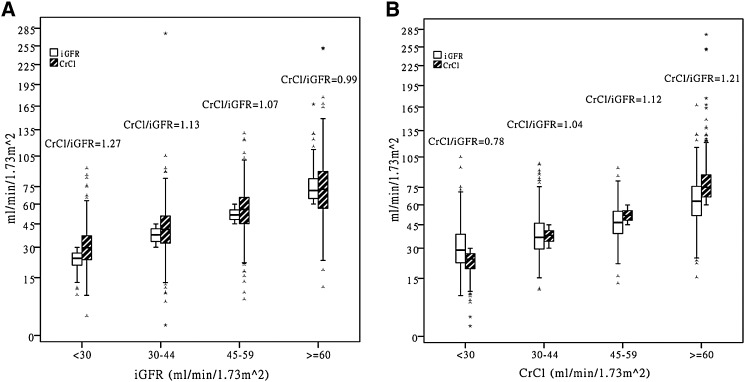
Mean CrCl/iGFR ratio was 1.13±0.46 and median CrCl/iGFR ratio was 1.09 (interquartile range, 0.88–1.32). Similar to prior studies, we found that the CrCl/iGFR ratio progressively increased at lower iGFR level when patients were classified by categories of iGFR (Figure 2A, Table 2). Among CRIC participants with iGFR≥60, 45–59, 30–44, and <30 ml/min per 1.73 m2, the mean CrCl/iGFR ratio was 1.00±0.35, 1.08±0.35, 1.14±0.47, and 1.37±0.60, respectively.
Figure 2.
Distribution of iGFR, CrCl, as well as CrCl/iGFR ratio stratified by categories of iGFR and by categories of CrCl in 1342 CRIC participants (box plots show median, interquartile range, and outliers; whiskers represent the highest and lowest values that are not outliers >1.5 box lengths from one hinge of the box). The much wider spread in the values of the unconstrained kidney function metric versus the constrained values and how this varies by strata of kidney function metric are graphically displayed. For example, in (A), by definition, those in the lowest iGFR category all have iGFR<30 ml/min per 1.73 m2 but the CrCl values among them may be as high as >75 ml/min per 1.73 m2. So the average CrCl/iGFR ratio is >1. By comparison, in (B), those in the lowest CrCl category, by definition, all have CrCl<30 ml/min per 1.73 m2 but the iGFR values among them may be as high as >75 ml/min per 1.73 m2; so, the average CrCl/iGFR ratio is <1. CrCl, urinary creatinine clearance; iGFR, measurement of GFR by iothalamate clearance.
Table 2.
CrCl/iGFR classified by categories based on iGFR and CrCl (CRIC data, n=1342)
| Category | iGFR, ml/min per 1.73 m2, median (5th, 25th, 75th, 95th) | CrCl, ml/min per 1.73 m2, median (5th, 25th, 75th, 95th) | CrCl/iGFR Ratio, median (5th, 25th, 75th, 95th) | CrCl/iGFR, mean±SD |
|---|---|---|---|---|
| iGFR level, ml/min per 1.73 m2 | ||||
| ≥60 (n=330) | 71.5 (60.6, 64.5, 82.2, 101.7) | 72.5 (37.3, 56.5, 89.2, 120.9) | 0.99 (0.45, 0.81, 1.16, 1.50) | 1.00±0.35 |
| 45–59 (n=362) | 51.5 (45.6, 48.1, 55.3, 59.1) | 55.7 (27.3, 45.2, 65.5, 83.3) | 1.07 (0.52, 0.88, 1.26, 1.66) | 1.08±0.35 |
| 30–44 (n=400) | 37.6 (30.8, 33.5, 41.6, 44.2) | 41.2 (19.8, 32.4, 50.6, 67.6) | 1.13 (0.49, 0.89, 1.35, 1.80) | 1.14±0.47 |
| <30 (n=250) | 24.0 (14.6, 20.6, 26.8, 29.4) | 29.8 (13.9, 23.3, 37.0, 54.9) | 1.27 (0.69, 1.01, 1.56, 2.58) | 1.37±0.60 |
| CrCl level, ml/min per 1.73 m2 | ||||
| ≥60 (n=424) | 63.1 (37.7, 51.7, 76.0, 96.1) | 75.2 (61.2, 66.7, 86.8, 120.5) | 1.21 (0.87, 1.05, 1.44, 2.16) | 1.33±0.52 |
| 45–59 (n=333) | 46.6 (27.1, 39.0, 55.0, 67.8) | 51.8 (45.6, 48.2, 55.5, 59.2) | 1.12 (0.75, 0.96, 1.33, 1.90) | 1.18±0.37 |
| 30–44 (n=341) | 36.6 (22.3, 29.6, 46.1, 68.6) | 37.9 (30.7, 34.1, 40.9, 44.3) | 1.04 (0.59, 0.81, 1.26, 1.71) | 1.07±0.38 |
| <30 (n=244) | 28.9 (15.5, 22.2, 38.6, 55.4) | 24.0 (11.4, 19.3, 26.9, 29.6) | 0.78 (0.32, 0.57, 1.02, 1.42) | 0.81±0.35 |
iGFR, measurement of GFR by iothalamate clearance; CrCl, urinary creatinine clearance.
However, when the same patients were classified by categories of CrCl, the ratio of CrCl/iGFR decreased at lower CrCl level (Figure 2B, Table 2). Among CRIC participants with CrCl≥60, 45–59, 30–44, and <30 ml/min per 1.73 m2, the mean CrCl/iGFR ratio was 1.33±0.52, 1.18±0.37, 1.07±0.38, and 0.81±0.35, respectively. Similar results are seen in continuous analysis (Supplemental Figures 1 and 2).
We were able to replicate these features in a simulation exercise on the basis of a hypothetical population of 1400 patients with “true kidney function” of mean 50 ml/min per 1.73 m2 and SD of 15 ml/min per 1.73 m2. As described in Materials and Methods, we simulated the spread of observed CrCl and observed iGFR readings due to measurement error on the basis of the literature (Figure 1). The result of the simulation also showed that the ratio of observed CrCl to observed iGFR was higher at lower observed iGFR level when patients were classified by categories of observed iGFR (Table 3). When the same patients were classified by categories of observed CrCl, the ratio of observed CrCl to observed iGFR was lower at lower CrCl level (Table 3).
Table 3.
CrCl/iGFR classified by categories based on iGFR and CrCl (simulation data, n=1400)
| Category | iGFR, ml/min per 1.73 m2, median (5th, 25th, 75th, 95th) | CrCl, ml/min per 1.73 m2, median (5th, 25th, 75th, 95th) | CrCl/iGFR Ratio, median (5th, 25th, 75th, 95th) | CrCl/iGFR, mean±SD |
|---|---|---|---|---|
| iGFR level, ml/min per 1.73 m2 | ||||
| ≥60 (n=382) | 67.5 (60.6, 63.1, 74.6, 84.9) | 75.0 (59.5, 68.4, 82.6, 97.9) | 1.09 (0.91, 1.01, 1.18, 1.29) | 1.09±0.12 |
| 45–59 (n=508) | 51.8 (45.9, 48.5, 55.7, 58.8) | 59.1 (41.6, 51.6, 66.0, 76.6) | 1.13 (0.85, 1.00, 1.26, 1.44) | 1.13±0.19 |
| 30–44 (n=373) | 38.1 (30.9, 34.4, 41.7, 44.5) | 43.8 (28.2, 36.6, 51.0, 60.2) | 1.15 (0.76, 0.99, 1.32, 1.57) | 1.15±0.23 |
| <30 (n=137) | 23.5 (12.1, 19.4, 27.2, 29.3) | 27.4 (10.6, 21.2, 34.6, 46.1) | 1.23 (0.56, 0.96, 1.56, 2.03) | 1.30±0.75 |
| CrCl level, ml/min per 1.73 m2 | ||||
| ≥60 (n=614) | 62.2 (46.3, 54.5, 70.0, 81.9) | 71.0 (61.0, 65.4, 78.8, 93.1) | 1.17 (0.97, 1.07, 1.27, 1.48) | 1.18±0.16 |
| 45–59 (n=401) | 47.0 (33.5, 41.5, 52.7, 60.6) | 52.8 (45.9, 49.2, 56.5, 59.4) | 1.11 (0.89, 1.00, 1.26, 1.50) | 1.15±0.20 |
| 30–44 (n=273) | 36.8 (22.4, 31.8, 43.2, 49.8) | 38.9 (31.3, 34.9, 42.2, 44.6) | 1.03 (0.79, 0.91, 1.20, 1.59) | 1.09±0.27 |
| <30 (n=112) | 24.3 (11.9, 19.4, 30.2, 40.0) | 24.0 (9.7, 19.7, 27.1, 29.3) | 0.91 (0.47, 0.72, 1.17, 1.74) | 1.03±0.80 |
iGFR, measurement of GFR by iothalamate clearance; CrCl, urinary creatinine clearance.
Similar results were observed when we varied assumptions in the simulation model, such as having a constant CV for iGFR and CrCl instead of a constant SD or making the underlying distribution of true kidney function uniform rather than bell-shaped (data not shown).
Discussion
The key finding of this study is that in a cross-sectional analysis of 1342 CRIC study participants, the ratio of CrCl/iGFR was higher at lower iGFR levels, but this ratio was lower at lower CrCl levels. These results are not easily explained by the conventional conceptual model of enhanced tubular creatinine secretion with lower levels of kidney function. In contrast, this observed pattern can be replicated by a simulation model in which there was no variation in the ratio of CrCl/iGFR with true kidney function but taking into account measurement error in both CrCl and iGFR (of magnitudes previously described in the literature). This is caused by measurement error and is similar to regression to the mean, which has the same underlying cause.
Prior studies neglected the fact that measured GFR (e.g., via iothalamate clearance) is also associated with measurement error, although this has been documented by a number of studies (29–32). For example, one study conducted by Rule et al. showed that the mean between-day CV of iothalamate clearance in the same person is 8.2% (29). Giovannetti et al. observed that the CV of inulin clearance in patients with normal or reduced kidney function is 11.2%–13.9% and the day-to-day variations of CrCl are not greater than those of inulin clearance (33). Direct clearance measurements typically assess filtration rates over only several hours, when in reality GFR is changing throughout the day because of factors such as diurnal variation, posture, and diet (36).
Some prior studies have reported absolute increases in CrCl among patients with worse kidney function. For example, according to Shemesh et al., tubular creatinine secretion was 34±4 versus 21±7 ml/min per 1.73 m2 when inulin clearance was 40–80 versus >80 ml/min per 1.73 m2 (17). We are unaware of experiments showing that as kidney function declines there is augmented expression of the transporters responsible for creatinine secretion which would underlie any compensatory or adaptive physiologic enhancement in creatinine secretion. Creatinine secretion in proximal tubules of the human kidney involves the cation transport pathway comprising the basolaterally expressed organic cation transporter 2 (OCT2) and apically expressed multidrug and toxin extrusion transporter 1 (MATE1) and MATE2-k (37–39). There are also data that the mRNA and protein expression of OCT2 and MATE1 were significantly decreased in rodent CKD models (40,41).
We offer another hypothesis to account for these current and prior observations: there is measurement error in both CrCl and iGFR, although the former is much better appreciated than the latter. Neither CrCl nor iGFR equals “true kidney function”. Therefore, when patients were classified into those with observed iGFR<30 ml/min per 1.73 m2, that stratum included patients who have “true kidney function” <30 ml/min per 1.73 m2 and those who have “true kidney function” >30 ml/min per 1.73 m2 but whose observed iGFR is low due to measurement error. Since the CrCl measurement error may not be in the same direction, the observed CrCl in the latter group will be tend to be >30 ml/min per 1.73 m2. Hence, the average CrCl/iGFR ratio is high among those selected for low observed iGFR. Conversely, patients in the observed CrCl<30 ml/min per 1.73 m2 stratum included those who have “true kidney function” <30 ml/min per 1.73 m2 and those who have “true kidney function” >30 ml/min per 1.73 m2 but whose observed CrCl is low due to measurement error. Since the iGFR measurement error may not be in the same direction, the observed iGFR in the latter group will be tend to be >30 ml/min per 1.73 m2. Hence, the mean CrCl/iGFR ratio is low among those selected for low observed CrCl. The much wider spread in the values of the unconstrained kidney function metric versus the constrained values and how this varies by strata of kidney function metric is illustrated in the Figure 2. (Figure 2A shows the situation when iGFR is the constrained metric and Figure 2B shows the situation when CrCl is the constrained metric.)
We are not disputing that creatinine clearance is achieved via glomerular filtration as well as tubular secretion. We are also not concluding that there can be no enhanced secretion of creatinine with progressive CKD; although, this explanation cannot easily account for the observation that the ratio of CrCl/iGFR is lower among patients with lower CrCl compared with those with higher CrCl. The main motivation of our study is to highlight an alternative interpretation of the literature which has hitherto not been considered.
The purpose of our simulation was not to model data from the CRIC population in the most accurate way possible. Rather, the point of the modeling was to show that even if the true ratio of CrCl/iGFR did not vary by severity of CKD, when there is measurement error, the observed ratio of CrCl/iGFR would increase if patients are classified by progressively lower categories of iGFR. The equation CrCl=1.13×iGFR was taken to be a reasonable starting point to build our simulation model. This simulation model showed that even if the true CrCl is 1.13-times higher than true iGFR throughout the entire range of kidney function, one can still observe—because there are measurement errors in both CrCl and iGFR—that the ratio of CrCl/iGFR is higher among patients with lower observed iGFR than among patients with higher observed iGFR.
Strengths of the analyses include that our results are supported by empirical observation from a large and diverse research study population and theory and simulation exercises under several scenarios. In addition, measurement error rather than true physiologic changes in creatinine secretion is a hypothesis more likely to explain some other observations in the published literature. For example, de Boer et al. analyzed the baseline characteristics of 1441 participants in the Diabetes Control and Complication Trial (42). These patients had relatively preserved kidney function. Yet, in the category of iGFR>130 ml/min per 1.73 m2, the average CrCl/iGFR ratio was <1.0. In the category of iGFR 90–120 ml/min per 1.73 m2, the average CrCl/iGFR ratio was >1.0 and this rose even further above 1.0 when iGFR was <90 ml/min per 1.73 m2. On the basis of the traditional physiologic explanation, one would have to conclude that there is no creatinine secretion—and, in fact, there is creatinine resorption—when kidney function is preserved (or when there is hyperfiltration). And also that “enhanced” creatinine secretion occurs even as GFR falls from 120 to 90 to <90 ml/min per 1.73 m2, a much higher absolute level of kidney function than is generally associated with this concept. In contrast, measurement error is a more plausible alternative explanation for these data from the Diabetes Control and Complication Trial. Because of measurement error, the phenomenon of the CrCl/iGFR ratio increasing across categories defined by progressively lower iGFR levels is expected regardless of the absolute level of kidney function.
We also recognize several limitations. First, iGFR and CrCl were not measured simultaneously using the same blood and urine samples. Second, the study did not include information on the concomitant use of drugs, such as trimethoprim or cimetidine (43,44), which can inhibit the tubular secretion of creatinine, although the use of these medications is likely to be rare at the time period of CRIC enrollment. Our results are consistent with prior studies which have reported that CrCl is approximately 10%–30% higher than simultaneously measured GFR (16–19,45). Third, since there was only one measurement of baseline iGFR and CrCl, we were not able to generate data on within-person CV from CRIC data and had to rely on data from previously published studies. Fourth, there were few patients in the CRIC study data who had very low levels of kidney function (e.g., <15 ml/min per 1.73 m2), so our observations may not generalize to such individuals. Finally, the findings were only assessed in one study and validation in other studies is warranted.
One implication of our analysis is that it provides some reassurance regarding the performance of CrCl (and serum creatinine) as a measure of kidney function. One potential future area of research includes interventional studies to compare measured CrCl values before and after giving a drug like cimetidine to block tubular secretion. However, these studies would not be straightforward to design and interpret. Since cimetidine is cleared by the kidneys, in patients with more advanced CKD less cimetidine is filtered and more cimetidine becomes available in the proximal tubular pericapillary circulation. Thus, more cimetidine enters the proximal tubular cells to compete with creatinine for the brush border (luminal) secretory transporter (46).
We have demonstrated three things. First, using CRIC data, we showed that the CrCl/iGFR ratio was lower at lower CrCl levels (this is novel way of looking at the data). This is not easy to account for on the basis of the accepted explanation that there is enhanced creatinine secretion physiologically as kidney function worsens. It is readily explained, however, by our hypothesis, which highlights the importance of measurement error in both CrCl and iGFR. Second, our simulation model illustrated that even if the true ratio of CrCl/iGFR did not vary by severity of CKD, when there is measurement error the observed ratio of CrCl/iGFR would increase if patients are classified by progressively lower categories of iGFR (i.e., providing an alternative explanation to what has been reported in the literature and also observed with CRIC data). Finally, we provided mathematical formulas (Table 1) showing that, from first principles, we expect that the ratio of CrCl/iGFR will become larger at lower iGFR levels, consistent with the top halves of Tables 2 and 3; and, furthermore, that the ratio of CrCl/iGFR tends to get smaller at lower CrCl levels, as shown in the bottom halves of Tables 2 and 3.
Thus, we believe that measurement error provides an alternative explanation of the existing literature (i.e., the ratio of CrCl/iGFR gets larger at lower iGFR levels) and the novel observation described in this paper (i.e., the ratio of CrCl/iGFR gets smaller at lower CrCl levels).
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to thank Drs. Glenn Chertow and Thomas Hostetter for insightful discussions regarding prior versions of the manuscript.
Supported by grants K23 DK88865 (N.B.) and K24 DK92291 (C.H.) from the National Institutes of Health.
The CRIC study was conducted by the CRIC and supported by the NIDDK. The data from the CRIC reported here were supplied by the NIDDK Central Repositories. This manuscript was not prepared in collaboration with CRIC Steering Committee and does not necessarily reflect the opinions or views of the CRIC study, the NIDDK Central Repositories, or the NIDDK.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “What Is the Correct Approach for Comparing GFR by Different Methods across Levels of GFR?,” on pages 1518–1521.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12821215/-/DCSupplemental.
References
- 1.Inker LA, Perrone RD: Assessment of kidney function, 2014. Available at: http://www.uptodate.com/contents/assessment-of-kidney-function. Accessed February 10, 2016 [Google Scholar]
- 2.Inker LA, Perrone RD: Calculation of the creatinine clearance, 2015. Available at: http://www.uptodate.com/contents/calculation-of-the-creatinine-clearance. Accessed February 10, 2016 [Google Scholar]
- 3.Israni AK, Kasiske BL: Laboratory assessment of kidney disease: glomerular filtration rate, urinalysis, and proteinuria. In: Brenner & Rector’s The kidney, edited by Brenner BM, 9th Ed., Philadelphia, Elsevier Saunders, 2011, pp 872–873 [Google Scholar]
- 4.Lafyette RA, Perrone RD, Levey AS: Laboratory Evaluation of Renal Function. In: Diseases of the Kidney, edited by Schrier RW, Gottschalk CW, 6th Ed., Philadelphia, Lippincott Williams & Wilkins, 1996, pp 307–354 [Google Scholar]
- 5.Levey AS, Perrone RD, Madias NE: Serum creatinine and renal function. Annu Rev Med 39: 465–490, 1988 [DOI] [PubMed] [Google Scholar]
- 6.Levey AS: Measurement of renal function in chronic renal disease. Kidney Int 38: 167–184, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Mandayam S, Mitch WE: Dietary Factors in the treatment of chronic kidney disease. In: Diseases of the Kidney & Urinary Tract, edited by Schrier RW, 8th Ed., Philadelphia, Lippincott Williams & Wilkins, 2007, pp 2672–2696 [Google Scholar]
- 8.Pearlman AM, Gonin JM: Evaluation of Kidney Function: Biochemical and Nuclear Medicine Tests. In: Handbook of Nephrology & Hypertension, edited by Wilcox CS, Tisher CC, 5th Ed., Philadelphia, Lippincott Williams & Wilkins, 2004, pp 20–23 [Google Scholar]
- 9.Perrone RD, Madias NE, Levey AS: Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem 38: 1933–1953, 1992 [PubMed] [Google Scholar]
- 10.Reiser IW, Porush JG: Evaluation of Renal Function. In: Massry & Glassock’s Textbook of Nephrology, edited by Massry SG, Glassock RJ, 3rd Ed., Baltimore, Williams & Wilkins, 1995, pp 1780–1788 [Google Scholar]
- 11.Schwartz GJ, Furth SL: Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol 22: 1839–1848, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Shoskes DA, McMahon AW: Renal Physiology and Pathophysiology. In: Campbell-Walsh Urology, edited by Kavoussi LR, Partin AW, Novick AC, Peters CA, 10th Ed., Philadelphia, Elsevier Saunders, 2012, pp 1025–1046 [Google Scholar]
- 13.Toto RD: Conventional measurement of renal function utilizing serum creatinine, creatinine clearance, inulin and para-aminohippuric acid clearance. Curr Opin Nephrol Hypertens 4: 505–509, discussion 503–504, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Walser M: Assessing renal function from creatinine measurements in adults with chronic renal failure. Am J Kidney Dis 32: 23–31, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Chau K, Hutton H, Levin A: Laboratory assessment of kidney disease: glomerular filtration rate, urinalysis, and proteinuria. In: Brenner & Rector’s The Kidney, edited by Brenner BM, Philadelphia, Elsevier Saunders, 2015, p 783 [Google Scholar]
- 16.Bauer JH, Brooks CS, Burch RN: Clinical appraisal of creatinine clearance as a measurement of glomerular filtration rate. Am J Kidney Dis 2: 337–346, 1982 [DOI] [PubMed] [Google Scholar]
- 17.Shemesh O, Golbetz H, Kriss JP, Myers BD: Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int 28: 830–838, 1985 [DOI] [PubMed] [Google Scholar]
- 18.Kim KE, Onesti G, Ramirez O, Brest AN, Swartz C: Creatinine clearance in renal disease. A reappraisal. BMJ 4: 11–14, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luke DR, Halstenson CE, Opsahl JA, Matzke GR: Validity of creatinine clearance estimates in the assessment of renal function. Clin Pharmacol Ther 48: 503–508, 1990 [DOI] [PubMed] [Google Scholar]
- 20.Coresh J, Toto RD, Kirk KA, Whelton PK, Massry S, Jones C, Agodoa L, Van Lente F: Creatinine clearance as a measure of GFR in screenees for the African-American Study of Kidney Disease and Hypertension pilot study. Am J Kidney Dis 32: 32–42, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer MJ, Go AS, Lora CM, Ackerson L, Cohan J, Kusek JW, Mercado A, Ojo A, Ricardo AC, Rosen LK, Tao K, Xie D, Feldman HI, Lash JP; CRIC and H-CRIC Study Groups : CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis 58: 214–227, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Berg RL, Gassman JJ, Hall PM, Walker WG; Modification of Diet in Renal Disease (MDRD) Study Group : Creatinine filtration, secretion and excretion during progressive renal disease. Kidney Int Suppl 27: S73–S80, 1989 [PubMed] [Google Scholar]
- 25.Hsu CY, Propert K, Xie D, Hamm L, He J, Miller E, Ojo A, Shlipak M, Teal V, Townsend R, Weir M, Wilson J, Feldman H; CRIC Investigators : Measured GFR does not outperform estimated GFR in predicting CKD-related complications. J Am Soc Nephrol 22: 1931–1937, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI; CRIC Study Investigators : Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 60: 250–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joffe M, Hsu CY, Feldman HI, Weir M, Landis JR, Hamm LL; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Variability of creatinine measurements in clinical laboratories: results from the CRIC study. Am J Nephrol 31: 426–434, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration : Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Rule AD, Bailey KR, Lieske JC, Peyser PA, Turner ST: Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney Int 83: 1169–1176, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Greene T, Schluchter MD, Cleary PA, Teschan PE, Lorenz RA, Molitch ME, Mitch WE, Siebert C, Hall PM, Steffes, MW; Modification of Diet in Renal Disease Study Group and the Diabetes Control and Complications Trial Research Group : Glomerular filtration rate measurements in clinical trials. J Am Soc Nephrol 4: 1159–1171, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrone RD, Steinman TI, Beck GJ, Skibinski CI, Royal HD, Lawlor M, Hunsicker LG: Utility of radioisotopic filtration markers in chronic renal insufficiency: simultaneous comparison of 125I-iothalamate, 169Yb-DTPA, 99mTc-DTPA, and inulin. The Modification of Diet in Renal Disease Study. Am J Kidney Dis 16: 224–235, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Kwong YT, Stevens LA, Selvin E, Zhang YL, Greene T, Van Lente F, Levey AS, Coresh J: Imprecision of urinary iothalamate clearance as a gold-standard measure of GFR decreases the diagnostic accuracy of kidney function estimating equations. Am J Kidney Dis 56: 39–49, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giovannetti S, Barsotti G: In defense of creatinine clearance. Nephron 59: 11–14, 1991 [DOI] [PubMed] [Google Scholar]
- 34.Doolan PD, Alpen EL, Theil GB: A clinical appraisal of the plasma concentration and endogenous clearance of creatinine. Am J Med 32: 65–79, 1962 [DOI] [PubMed] [Google Scholar]
- 35.Searle SR, editor: Linear Models, New York, John Wiley & Sons, pp 47, 1971 [Google Scholar]
- 36.Hsu CY, Bansal N: Measured GFR as “gold standard”--all that glitters is not gold? Clin J Am Soc Nephrol 6: 1813–1814, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Urakami Y, Kimura N, Okuda M, Inui K: Creatinine transport by basolateral organic cation transporter hOCT2 in the human kidney. Pharm Res 21: 976–981, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Ciarimboli G, Lancaster CS, Schlatter E, Franke RM, Sprowl JA, Pavenstädt H, Massmann V, Guckel D, Mathijssen RH, Yang W, Pui CH, Relling MV, Herrmann E, Sparreboom A: Proximal tubular secretion of creatinine by organic cation transporter OCT2 in cancer patients. Clin Cancer Res 18: 1101–1108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanihara Y, Masuda S, Sato T, Katsura T, Ogawa O, Inui K: Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol 74: 359–371, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Komazawa H, Yamaguchi H, Hidaka K, Ogura J, Kobayashi M, Iseki K: Renal uptake of substrates for organic anion transporters Oat1 and Oat3 and organic cation transporters Oct1 and Oct2 is altered in rats with adenine-induced chronic renal failure. J Pharm Sci 102: 1086–1094, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM: Renal transporters in drug development. Annu Rev Pharmacol Toxicol 53: 503–529, 2013 [DOI] [PubMed] [Google Scholar]
- 42.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Zinman B, Steffes MW; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group : Longitudinal changes in estimated and measured GFR in type 1 diabetes. J Am Soc Nephrol 25: 810–818, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Acker BA, Koomen GC, Koopman MG, de Waart DR, Arisz L: Creatinine clearance during cimetidine administration for measurement of glomerular filtration rate. Lancet 340: 1326–1329, 1992 [DOI] [PubMed] [Google Scholar]
- 44.Hilbrands LB, Artz MA, Wetzels JF, Koene RA: Cimetidine improves the reliability of creatinine as a marker of glomerular filtration. Kidney Int 40: 1171–1176, 1991 [DOI] [PubMed] [Google Scholar]
- 45.Shannon JA: The Renal Excretion of Creatinine in Man. J Clin Invest 14: 403–410, 1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaltzman JS, Whiteside C, Cattran DC, Lopez FM, Logan AG: Accurate measurement of impaired glomerular filtration using single-dose oral cimetidine. Am J Kidney Dis 27: 504–511, 1996 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.