Abstract
Background and objectives
CKD is a global public health problem with significant mortality and morbidity.
Design, setting, participants, & measurements
We examined the multivariable association of plasma levels of IL-1, IL-1 receptor antagonist, IL-6, TNF-α, TGF-β, high–sensitivity C–reactive protein, fibrinogen, and serum albumin with progression of CKD in 3430 Chronic Renal Insufficiency Cohort study participants.
Results
Over a median follow-up time of 6.3 years, 899 participants reached the composite end point of ≥50% decline in eGFR from baseline or onset of ESRD. Elevated plasma levels of fibrinogen, IL-6, and TNF-α and lower serum albumin were associated with a greater decline in eGFR over time. After adjusting for demographics, BP, laboratory variables, medication use, and baseline eGFR, hazard ratios for the composite outcome were greater for the patients in the highest quartile of fibrinogen (hazard ratio, 2.05; 95% confidence interval, 1.64 to 2.55; P<0.001), IL-6 (hazard ratio, 1.44; 95% confidence interval, 1.17 to 1.77; P<0.01), and TNF-α (hazard ratio, 1.94; 95% confidence interval, 1.52 to 2.47; P<0.001) compared with those in the respective lowest quartiles. The hazard ratio was 3.48 (95% confidence interval, 2.88 to 4.21; P<0.001) for patients in the lowest serum albumin quartile relative to those in the highest quartile. When also adjusted for albuminuria, the associations of fibrinogen (hazard ratio, 1.49; 95% confidence interval, 1.20 to 1.86; P<0.001), serum albumin (hazard ratio, 1.52; 95% confidence interval, 1.24 to 1.87; P<0.001), and TNF-α (hazard ratio, 1.42; 95% confidence interval, 1.11 to 1.81; P<0.001) with outcome were attenuated but remained significant.
Conclusions
Elevated plasma levels of fibrinogen and TNF-α and decreased serum albumin are associated with rapid loss of kidney function in patients with CKD.
Keywords: chronic kidney disease; cytokines; glomerular filtration rate; end-stage renal disease; albuminuria; C-Reactive Protein; Follow-Up Studies; Humans; Inflammation; Interleukin-6; Renal Insufficiency, Chronic; Tumor Necrosis Factor-alpha
Introduction
In the United States, CKD affects >20 million adults; an additional 20 million are at increased risk for developing CKD (1,2). Globally, CKD affects 5%–7% of the population and is more common in developing countries and minority populations (3). Progressive loss of kidney function may be largely caused by secondary factors that are often unrelated to the activity of the initial disease (4,5). Even a modest decrease in kidney function is associated with increased risk for cardiovascular events and death (6). This enormous disease burden and lack of highly effective treatments for CKD create an urgent need to better understand the risk factors and underlying pathophysiology (7).
CKD is characterized by persistent low–grade inflammation (8). Cytokines regulate the inflammatory response and mediate some of their downstream effects through positive acute–phase proteins, such as C-reactive protein (CRP), fibrinogen, and albumin (9,10). In this study, we examined the association between a set of inflammatory biomarkers and progression of CKD in the Chronic Renal Insufficiency Cohort (CRIC) Study, which enrolled a diverse group of adults with a wide range of kidney function. We also report the interaction of these inflammatory biomarkers of race, age, diabetic status, and baseline kidney function with rates of progression of CKD.
Materials and Methods
Study Population
Men and women with eGFR of 20–70 ml/min per 1.73 m2 body surface area were enrolled in the CRIC Study from June of 2003 to August of 2008. The exclusion criteria were cirrhosis, class 3 or 4 heart failure, HIV infection, cancer, active or recent immunosuppression, polycystic kidney disease, and pregnancy. The study protocol was approved by the institutional review board at each participating site. All participants provided written informed consent.
The CRIC Study Data Collection
Demographic characteristics, medical history, anthropometric measures, and medication use were recorded at baseline. Serum creatinine concentrations were measured at baseline and every 6 months thereafter using the Jaffe method on a Beckman Synchron System (Beckman Coulter, Inc., Brea CA). eGFR was computed using the Modification of Diet in Renal Disease estimating equation (11). Albuminuria was estimated as the urine albumin-to-creatinine ratio (UACR). Serum albumin (SAlb) was measured using the Brom Cresol Green–based method. Participants were considered to have diabetes mellitus (DM) if fasting glucose level was ≥126 mg/dl (7.0 mmol/L), if nonfasting glucose level was ≥200 mg/dl (11.1 mmol/L), or if they were treated with insulin or an oral hypoglycemic agent.
Measurement of Biomarkers
Biomarkers were chosen on the basis of their potential role in progression of CKD (8,12,13). High–sensitivity ELISAs (Quantikine HS; R&D Systems, Minneapolis, MN) were used to measure plasma IL-1β, IL-6, and TNF-α levels. Standard ELISAs (Quantikine; R&D Systems) were used to quantify IL-1 receptor antagonist (IL-1RA) and TGF-β levels. The samples were stored at −80°C, and assays were performed at the time of initial thawing. All assays were performed in duplicate, and mean values were used in the analysis. The coefficient of variation was <13% for all cytokines assays except for TNF-α and TGF-β, for which the estimated imprecisions were 15.2% and 21.5%, respectively. High–sensitivity C–reactive protein (hs-CRP) and fibrinogen were quantified in EDTA plasma samples using specific laser–based immunonephelometric methods on the BNII (Siemens Healthcare Diagnostics, Deerfield, IL). The coefficients of variation for hs-CRP and fibrinogen were <5%.
Outcomes
The primary outcomes were (1) a composite outcome defined as occurrence of ≥50% decline in GFR from baseline or onset of ESRD and (2) slope of eGFR over time. Incident ESRD was defined as the new onset of irreversible dependence on RRT (either chronic dialysis or kidney transplantation). ESRD was a secondary outcome.
Statistical Analyses
Continuous variables were checked for skewness and log transformed if non-normal. Biomarkers of inflammation were categorized into quartiles. Cut points for quartile definitions are shown in Supplemental Table 1.
Kaplan–Meier analyses were used to examine the times to event for ESRD and composite outcomes starting at baseline assessment. Statistical significance was determined using the log rank chi–squared test. Participants who did not reach an outcome were censored at last follow-up visit, date of withdrawal, or date of death, whichever came first. For cytokines that were significantly associated with outcomes in univariable analyses (P<0.05), multivariable Cox regression models were used to determine the independent associations of each cytokine, represented as quartiles, and outcomes. Three Cox models were tested. Model 1 is the unadjusted model. In model 2, adjustments were made for age, sex, body mass index (BMI), DM, black race, systolic BP, diastolic BP, alcohol use, smoking, angiotensin–converting enzyme inhibitor/angiotensin receptor blocker (ACEi/ARB) use, total cholesterol, and baseline eGFR. Model 3 included factors in model 2 and also, UACR. We chose model 2 as the primary model, because albuminuria may mediate the association between inflammation and loss of eGFR (8), in which case model 3 only tests the nonmediated pathway. Possible multicollinearity was tested by examining variance inflation using weighted regression (14).
Because death might be a competing risk, we estimated the cumulative incidence function for the composite outcome stratified by biomarker quartile controlling for age, race, sex, DM, baseline eGFR, UACR, hypertension, BMI, alcohol use, smoking, ACEi/ARB use, and total cholesterol (15). This is an estimate of the cumulative probability of reaching the composite outcome as a function of the baseline cytokine level without assuming that death during follow-up is equivalent to censoring without an event.
Interactions between cytokine levels and race (blacks versus whites), age (≤60 versus <60 years old), DM, and baseline eGFR (<30 versus ≥30 ml/min per 1.73 m2) with the composite outcome were examined individually using interaction terms in Cox regression. Here, inflammatory markers were coded as continuous variables.
Each participant’s mean eGFR change per year of follow-up (slope) was calculated by creating an ordinary least squares regression line of eGFR over time using the SAS Autoreg Procedure (SAS Institute Inc., Cary, NC). Extreme outliers were identified by the locations of breaks at the outermost ends of the slope frequency histogram and excluded, because they may represent AKI episodes (Supplemental Figure 1). General linear models were constructed to test the association of cytokine quartiles with GFR slope.
A series of sensitivity analyses was performed. (1) We first examined the association of the biomarkers with ESRD. (2) A second sensitivity analysis examined the additive effect of cytokines using those markers that had significant associations in the earlier analyses. An inflammation score was constructed by adding the baseline quartiles of the relevant markers. For example, a participant with quartiles 2–4 of three relevant markers would receive an inflammation score of 2+3+4=9. The association of this score with the composite outcome was examined using chi-squared tests and Cox regression. (3) The independent associations of significant cytokines were examined by combining them as covariates along with the other patient background variables in a Cox regression model predicting time to the composite outcome. (4) We examined the area under the receiver operating characteristic (ROC) curve using the c statistic from logistic regression to determine whether cytokines improved discrimination for the composite outcome beyond the level achieved by a model including just patient demographics, comorbidities, and baseline eGFR. We also tested whether adding proteinuria further improved discrimination. (5) For cytokines with significant associations in the earlier analyses, in addition to the interactions mentioned above, we examined interactions with sex, BMI, alcohol use, smoking, and hypertension.
SAS, version 9.3 (SAS Institute Inc.) was used for all data analyses, with P<0.05 being considered significant.
Results
Study Population
Of the original 3939 study participants in the CRIC Study dataset, we excluded participants with missing data for follow-up eGFR (n=418), baseline cytokines (n=10), or UACR (n=148), resulting in a sample of n=3430 for additional analysis. Median duration of follow-up was 6.3 years (intraquartile range, 5.1–7.4 years). Baseline study participant characteristics stratified by composite outcome are shown in Table 1.
Table 1.
Characteristics of the patient sample stratified by composite outcome (occurrence of ≥50% decline in GFR from baseline or onset of ESRD)
| Variable | Reached Composite Outcome | P Value | |
|---|---|---|---|
| No, n=2531 | Yes, n=899 | ||
| Age, yr | 59.1±10.5 | 56.2±11.5 | <0.001 |
| Race, no. (%) | <0.001 | ||
| White | 1252 (49.5%) | 243 (27.0%) | |
| Black | 940 (37.1%) | 455 (50.6%) | |
| Other | 339 (13.4%) | 201 (22.4%) | |
| Women, no. (%) | 1181 (46.7%) | 358 (39.8%) | <0.001 |
| BMI, mean±SD, kg/m2 | 32.0±7.5 | 32.4±8.1 | 0.18 |
| Smoker, no. % | 289 (11.4%) | 142 (15.8%) | <0.001 |
| Alcohol use, no. % | 1689 (66.7%) | 502 (55.8%) | <0.001 |
| Diabetes, no. % | 1053 (41.6%) | 585 (65.1%) | <0.001 |
| Hypertension, no. % | 2099 (82.9%) | 839 (93.3%) | <0.001 |
| Mean systolic BP, mmHg (SD) | 124.1 (19.7) | 138.8 (23.0) | <0.001 |
| Mean diastolic BP, mmHg (SD) | 70.4 (12.1) | 74.5 (13.4) | <0.001 |
| Serum creatinine, mean±SD, mg/dl | 1.7±0.5 | 2.3±0.8 | <0.001 |
| Total cholesterol, mean±SD, mg/dl | 181.9±41.8 | 187.9±52.1 | 0.002 |
| eGFR, mean±SD, ml/min per 1.73 m2 | 45.9±12.8 | 35.1±11.7 | <0.001 |
| UACR, mean±SD, natural log | 3.4±1.9 | 6.4±1.8 | <0.001 |
| Biomarkers median (IQR) | |||
| High–sensitivity C–reactive protein, mg/L | 2.53 (1.04–6.05) | 2.50 (1.02–6.43) | 0.42 |
| Fibrinogen, g/L | 3.88 (3.27–4.52) | 4.49 (3.83–5.35) | <0.001 |
| IL-1β, pg/ml | 0.13 (0.06–1.05) | 0.43 (0.06–1.76) | <0.001 |
| IL-1RA, pg/ml | 681.4 (367.7–1483.6) | 825.7 (422.5–1635.4) | <0.001 |
| IL-6, pg/ml | 1.72 (1.04–2.83) | 2.21 (1.41–3.57) | <0.001 |
| TNF-α, pg/ml | 2.00 (1.40–2.90) | 2.80 (2.00–3.90) | <0.001 |
| TGF-β, ng/ml | 10.80 (6.22–17.64) | 10.89 (6.84–18.04) | 0.34 |
| Serum albumin, g/dl | 4.10 (3.80–4.30) | 3.70 (3.40–4.10) | <0.001 |
BMI, body mass index; UACR, urine albumin-to-creatinine ratio; IQR, interquartile range; IL-1RA, IL-1 receptor antagonist.
Univariable and Survival Analyses for Composite End Point
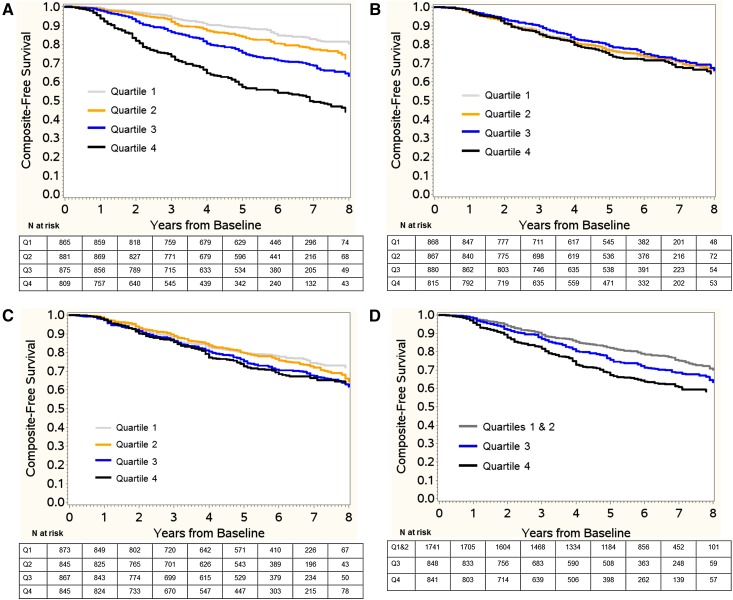
During the study period, 499 study participants (14.6%) died, 707 (20.6%) were diagnosed with ESRD, and 899 (26.2%) reached the composite outcome. In univariable chi–squared analyses, fibrinogen, IL-1β, IL-1RA, IL-6, TNF-α, and SAlb were associated with a composite of ESRD or a reduction of 50% in eGFR from baseline (Table 2). In Kaplan–Meier analyses, higher quartiles of fibrinogen, IL-6, IL-1β, IL-1RA, and TNF-α and lower quartile of SAlb but not hs-CRP or TGF-β were associated with the composite outcome (Figure 1).
Table 2.
Distribution of composite outcome defined as occurrence of ≥50% decline in GFR from baseline or onset of ESRD composite and Cox regression hazard ratios for cytokine quartiles
| Biomarker | N with Composite Outcome (%) | Chi–Squared P Value | Model 1 Hazard Ratio (95% CI) | P Value | Model 2 Hazard Ratio (95% CI) | P Value | Model 3 Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|---|---|---|---|
| Fibrinogen, g/L | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Quartile 1, <3.39 | 126 (14.6) | Reference group | Reference group | Reference group | ||||
| Quartile 2, 3.39 to <4.04 | 171 (19.4) | 1.38 (1.10 to 1.74) | 1.05 (0.84 to 1.33) | 1.00 (0.80 to 1.27) | ||||
| Quartile 3, 4.04 to <4.80 | 247 (28.2) | 2.11 (1.71 to 2.62) | 1.41 (1.13 to 1.76) | 1.18 (0.94 to 1.47) | ||||
| Quartile 4, ≥4.80 | 355 (43.9) | 4.04 (3.30 to 4.95) | 2.05 (1.64 to 2.55) | 1.49 (1.20 to 1.86) | ||||
| IL-1β,a pg/ml | <0.001 | <0.001 | 0.66 | 0.99 | ||||
| Quartiles 1 and 2, <0.21 | 383 (22.0) | Reference group | Reference group | Reference group | ||||
| Quartile 3, 0.21 to <1.29 | 235 (27.7) | 1.33 (1.13 to 1.56) | 1.01 (0.86 to 1.19) | 0.99 (0.84 to 1.17) | ||||
| Quartile 4, ≥1.29 | 281 (33.4) | 1.81 (1.55 to 2.11) | 1.07 (0.92 to 1.26) | 1.00 (0.85 to 1.18) | ||||
| IL-1RA, pg/ml | 0.002 | 0.001 | 0.93 | 0.60 | ||||
| Quartile 1, <390.00 | 196 (22.5) | Reference group | Reference group | Reference group | ||||
| Quartile 2, 390.00 to <715.70 | 207 (24.5) | 1.09 (0.90 to 1.33) | 0.98 (0.81 to 1.20) | 0.99 (0.81 to 1.20) | ||||
| Quartile 3, 715.70 to <1551.00 | 252 (29.1) | 1.32 (1.10 to 1.60) | 0.97 (0.80 to 1.17) | 0.98 (0.81 to 1.18) | ||||
| Quartile 4, ≥1551.00 | 244 (28.9) | 1.39 (1.15 to 1.68) | 0.94 (0.78 to 1.14) | 0.89 (0.73 to 1.08) | ||||
| IL-6, pg/ml | <0.001 | <0.001 | 0.008 | 0.23 | ||||
| Quartile 1, <1.17 | 147 (16.4) | Reference group | Reference group | Reference group | ||||
| Quartile 2, 1.17 to <1.90 | 227 (25.8) | 1.74 (1.41 to 2.14) | 1.26 (1.02 to 1.56) | 1.20 (0.97 to 1.49) | ||||
| Quartile 3, 1.90 to <3.15 | 255 (29.9) | 2.13 (1.74 to 2.61) | 1.32 (1.07 to 1.63) | 1.19 (0.96 to 1.47) | ||||
| Quartile 4, ≥3.15 | 270 (33.8) | 2.57 (2.10 to 3.14) | 1.44 (1.17 to 1.77) | 1.24 (1.00 to 1.53) | ||||
| TNF-α, pg/ml | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Quartile 1, <1.50 | 91 (11.1) | Reference group | Reference group | Reference group | ||||
| Quartile 2, 1.50 to <2.20 | 180 (21.0) | 2.05 (1.59 to 2.64) | 1.22 (0.95 to 1.58) | 0.97 (0.75 to 1.25) | ||||
| Quartile 3, 2.20 to <3.20 | 276 (31.9) | 3.53 (2.79 to 4.48) | 1.57 (1.23 to 2.01) | 1.26 (0.99 to 1.61) | ||||
| Quartile 4, ≥3.20 | 352 (39.7) | 4.99 (3.96 to 6.29) | 1.94 (1.52 to 2.47) | 1.42 (1.11 to 1.81) | ||||
| Serum albumin, g/dl | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Quartile 1, <3.70 | 375 (49.9) | 4.72 (3.93 to 5.67) | 3.48 (2.88 to 4.21) | 1.52 (1.24 to 1.87) | ||||
| Quartile 2, 3.70 to <4.00 | 224 (27.0) | 1.99 (1.63 to 2.44) | 1.81 (1.48 to 2.22) | 1.25 (1.01 to 1.54) | ||||
| Quartile 3, 4.00 to <4.20 | 120 (18.5) | 1.34 (1.06 to 1.69) | 1.35 (1.07 to 1.71) | 1.05 (0.82 to 1.33) | ||||
| Quartile 4, ≥4.20 | 167 (14.5) | Reference group | Reference group | Reference group |
Only biomarkers showing significant association with outcomes in univariate analysis were examined in multivariate analysis. Model 1: unadjusted. Model 2: covariates adjusted for included baseline eGFR, age, sex, race, cholesterol, systolic BP, diabetes, angiotensin–converting enzyme inhibitor/angiotensin receptor blocker, body mass index, alcohol use, and smoking (primary model). Model 3: covariates from model 2 and baseline urine albumin-to-creatinine ratio. Cox regression P values are for each cytokine when coded as a class variable. 95% CI, 95% confidence interval; IL-1RA, IL-1 receptor antagonist.
Because of the skewed distribution of IL-1β, quartiles 1 and 2 are combined for this variable.
Figure 1.
Kaplan–Meier survival estimates for the composite outcome stratified by baseline biomarker quartile (log rank chi–squared P value). (A) High–sensitivity C–reactive protein (CRP) and composite outcome (P=0.42). (B) Fibrinogen and composite outcome (P<0.001). (C) IL-1β and composite outcome (P<0.001). (D) IL-1 receptor antagonist (IL-1RA) and composite outcome (P=0.001). (E) IL-6 and composite outcome (P<0.001). (F) TNF-α and composite outcome (P<0.001). (G) TGF-β and composite outcome (P=0.06). (H) Serum albumin and composite outcome (P<0.001). Q, quartile.
Multivariable Analyses of Composite End Point
After adjusting for baseline eGFR, age, sex, race, cholesterol, systolic BP, DM, ACEi/ARB, BMI, alcohol use, and smoking in Cox regression, a higher hazard for the composite outcome with higher levels of fibrinogen, IL-6, and TNF-α and lower levels of SAlb was observed (model 2 in Table 2). The hazard for the composite outcome was greater for the highest quartiles of fibrinogen (hazard ratio [HR], 2.05; 95% confidence interval [95% CI], 1.64 to 2.55; P<0.001), IL-6 (HR, 1.44; 95% CI, 1.17 to 1.77; P<0.001), and TNF-α (HR, 1.94; 95% CI, 1.52 to 2.47; P<0.001) compared with the respective lowest quartiles. For the lowest SAlb quartile versus the highest quartile, it was 3.48 (95% CI, 2.88 to 4.21; P<0.001). When UACR was added as a covariate in the model (model 3), the risk associations of the highest quartiles of fibrinogen (HR, 1.49; 95% CI, 1.20 to 1.86; P<0.001), IL-6 (HR, 1.24; 95% CI, 1.00 to 1.53; P=0.23), and TNF-α (HR, 1.42; 95% CI, 1.11 to 1.81; P<0.001) with the composite were attenuated along with the association of the lowest SAlb quartile (HR, 1.52; 95% CI, 1.24 to 1.87; P<0.001).
Multivariable Analyses of eGFR Change
The median number of eGFR readings used to compute the slope was six (intraquartile range, 4–7). The mean change in eGFR during follow-up was −1.7 ml/min per 1.73 m2 per year (median =−0.9; interquartile range, −3.1 to +0.6). eGFR declined faster in blacks (median slope =−1.4 versus −0.6), patients with diabetes (median slope =−1.8 versus −0.4), and those with UACR>100 mg/g creatinine (median slope =−3.0 versus −0.1) compared with their counterparts. In the univariable analyses, fibrinogen, IL-1β, IL-1RA, IL-6, and TFN-α were positively associated with rate of eGFR decline, and SAlb was negatively associated (Table 3). In the primary model (model 2), higher quartiles of fibrinogen, IL-6, TNF-α, and SAlb were significantly associated with faster decline in eGFR. The mean adjusted rates of change in eGFR (milliliters per minute per 1.73 m2 per year) in the highest quartile compared with the lowest quartile were −2.59 (95% CI, −2.85 to −2.33) versus −1.06 (95% CI, −1.32 to −0.79) for fibrinogen, −1.94 (95% CI, −2.19 to −1.69) versus −1.35 (95% CI, −1.62 to −1.09) for IL-6, −2.06 (95% CI, −2.31 to −1.81) versus −1.41 (95% CI, −1.67 to −1.14) for TNF-α, and −0.56 (95% CI, −0.92 to −0.21) versus −4.25 (95% CI, −4.63 to −3.86) for SAlb (P<0.001 for all comparisons). When also adjusted for UACR, only fibrinogen and SAlb remained significantly associated with rate of change in eGFR. Mean adjusted eGFR rate of decline in the highest fibrinogen quartile was −2.22 ml/min per 1.73 m2 per year compared with −1.44 ml/min per 1.73 m2 per year in the lowest quartile in model 3 (P<0.001). In the lowest versus highest SAlb quartile, the mean eGFR rate of decline was −3.10 versus −1.00 ml/min per 1.73 m2 per year.
Table 3.
Adjusted eGFR change per year by baseline levels of inflammatory biomarkers
| Biomarker | Model 1 Mean eGFR Change per Year (95% CI) | P Value | Model 2 Adjusted Mean eGFR Change per Year (95% CI) | P Value | Model 3 Adjusted Mean eGFR Change per Year (95% CI) | P Value |
|---|---|---|---|---|---|---|
| hs-CRP, mg/L | 0.71 | 0.73 | 0.42 | |||
| Quartile 1, <1.05 | −1.43 (−1.67 to −1.20) | −1.86 (−2.13 to −1.60) | −1.88 (−2.12 to −1.64) | |||
| Quartile 2, 1.05 to <2.57 | −1.47 (−1.70 to −1.23) | −1.76 (−2.02 to −1.50) | −1.89 (−2.13 to −1.66) | |||
| Quartile 3, 2.57 to <6.51 | −1.47 (−1.70 to −1.24) | −1.69 (−1.94 to −1.44) | −1.69 (−1.90 to −1.46) | |||
| Quartile 4, ≥6.51 | −1.62 (−1.86 to −1.38) | −1.79 (−2.04 to −1.54) | −1.84 (−2.07 to −1.61) | |||
| Fibrinogen, g/L | <0.001 | <0.001 | <0.001 | |||
| Quartile 1, <3.39 | −0.66 (−0.89 to −0.44) | −1.06 (−1.32 to −0.79) | −1.44 (−1.68 to −1.20) | |||
| Quartile 2, 3.39 to <4.04 | −0.96 (−1.18 to −0.73) | −1.26 (−1.51 to −1.01) | −1.54 (−1.77 to −1.31) | |||
| Quartile 3, 4.04 to <4.80 | −1.83 (−2.05 to −1.60) | −1.93 (−2.17 to −1.68) | −1.93 (−2.15 to −1.71) | |||
| Quartile 4, ≥4.80 | −2.73 (−2.98 to −2.49) | −2.59 (−2.85 to −2.33) | −2.22 (−2.46 to −1.98) | |||
| IL-1β,a pg/ml | <0.001 | 0.17 | 0.45 | |||
| Quartiles 1 and 2, <0.21 | −1.22 (−1.38 to −1.06) | −1.69 (−1.89 to −1.49) | −1.76 (−1.95 to −1.58) | |||
| Quartile 3, 0.21 to <1.29 | −1.53 (−1.76 to −1.30) | −1.76 (−2.01 to −1.51) | −2.22 (−2.5 to −1.9) | |||
| Quartile 4, ≥1.29 | −2.05 (−2.29 to −1.81) | −1.94 (−2.20 to −1.69) | −2.26 (−2.6 to −2.0) | |||
| IL-1RA, pg/ml | 0.001 | 0.76 | 0.46 | |||
| Quartile 1, <390.00 | −1.30 (−1.53 to −1.07) | −1.74 (−1.99 to −1.48) | −1.78 (−2.01 to −1.55) | |||
| Quartile 2, 390.00 to <715.70 | −1.31 (−1.55 to −1.08) | −1.70 (−1.95 to −1.45) | −1.77 (−2.00 to −1.54) | |||
| Quartile 3, 715.70 to <1551.00 | −1.62 (−1.85 to −1.39) | −1.79 (−2.04 to −1.54) | −1.84 (−2.06 to −1.61) | |||
| Quartile 4, ≥1551.00 | −1.77 (−2.01 to −1.53) | −1.86 (−2.12 to −1.61) | −1.89 (−2.12 to −1.66) | |||
| IL-6, pg/ml | <0.001 | 0.001 | 0.14 | |||
| Quartile 1, <1.17 | −0.75 (−0.97 to −0.53) | −1.35 (−1.62 to −1.09) | −1.61 (−1.85 to −1.37) | |||
| Quartile 2, 1.17 to <1.90 | −1.58 (−1.80 to −1.35) | −1.83 (−2.08 to −1.58) | −1.91 (−2.13 to −1.68) | |||
| Quartile 3, 1.90 to <3.15 | −1.80 (−2.04 to −1.57) | −1.90 (−2.15 to −1.64) | −1.90 (−2.13 to −1.67) | |||
| Quartile 4, ≥3.15 | −1.96 (−2.20 to −1.71) | −1.94 (−2.19 to −1.69) | −1.83 (−2.06 to −1.60) | |||
| TNF-α, pg/ml | <0.001 | <0.001 | 0.23 | |||
| Quartile 1, <1.50 | −0.71 (−0.94 to −0.48) | −1.41 (−1.67 to −1.14) | −1.74 (−1.98 to −1.49) | |||
| Quartile 2, 1.50 to <2.20 | −1.25 (−1.48 to −1.03) | −1.60 (−1.86 to −1.35) | −1.67 (−1.90 to −1.44) | |||
| Quartile 3, 2.20 to <3.20 | −1.87 (−2.11 to −1.64) | −1.92 (−2.17 to −1.68) | −1.92 (−2.14 to −1.70) | |||
| Quartile 4, ≥3.20 | −2.16 (−2.39 to −1.93) | −2.06 (−2.31 to −1.81) | −1.90 (−2.13 to −1.68) | |||
| TGF-β, pg/ml | 0.17 | 0.99 | 0.82 | |||
| Quartile 1, <6.47 | −1.28 (−1.51 to −1.05) | −1.76 (−2.02 to −1.51) | −1.88 (−2.11 to −1.65) | |||
| Quartile 2, 6.47 to <10.96 | −1.58 (−1.80 to −1.35) | −1.76 (−2.01 to −1.52) | −1.75 (−1.97 to −1.53) | |||
| Quartile 3, 10.96 to <17.86 | −1.52 (−1.76 to −1.29) | −1.78 (−2.04 to −1.53) | −1.84 (−2.07 to −1.62) | |||
| Quartile 4, ≥17.86 | −1.62 (−1.86 to −1.38) | −1.79 (−2.03 to −1.54) | −1.82 (−2.04 to −1.60) | |||
| Serum albumin, g/dl | <0.001 | <0.001 | <0.001 | |||
| Quartile 1, <3.70 | −4.31 (−4.71 to −3.90) | −4.25 (−4.63 to −3.86) | −3.10 (−3.47 to −2.74) | |||
| Quartile 2, 3.70 to <4.00 | −1.52 (−1.78 to −1.26) | −1.72 (−2.09 to −1.35) | −1.56 (−1.90 to −1.22) | |||
| Quartile 3, 4.00 to <4.20 | −1.26 (−1.56 to −0.96) | −1.43 (−1.83 to −1.02) | −1.47 (−1.84 to −1.09) | |||
| Quartile 4, ≥4.20 | −0.40 (−0.60 to −0.20) | −0.56 (−0.92 to −0.21) | −1.00 (−1.33 to −0. 67) |
Adjusted mean eGFR changes (milliliters per minute per 1.73 m2 per year) are shown (with 95% CIs). Model 1: unadjusted. Model 2: covariates adjusted for included baseline eGFR, age, sex, race, cholesterol, hypertension, diabetes, angiotensin–converting enzyme inhibitor/angiotensin receptor blocker, body mass index, alcohol use, and smoking (primary model). Model 3: covariates from model 2 and baseline urine albumin-to-creatinine ratio. 95% CI, 95% confidence interval; hs-CRP, high–sensitivity C–reactive protein; IL-1RA, IL-1 receptor antagonist.
Because of the skewed distribution of IL-1β, quartiles 1 and 2 are combined for this variable.
Competing End Point Analyses
As shown in Supplemental Figure 2, the cumulative incidence of the composite end point remained significant for quartiles of fibrinogen, IL-6, TNF-α, and lower SAlb after accounting for death as a competing event.
Interaction Analyses
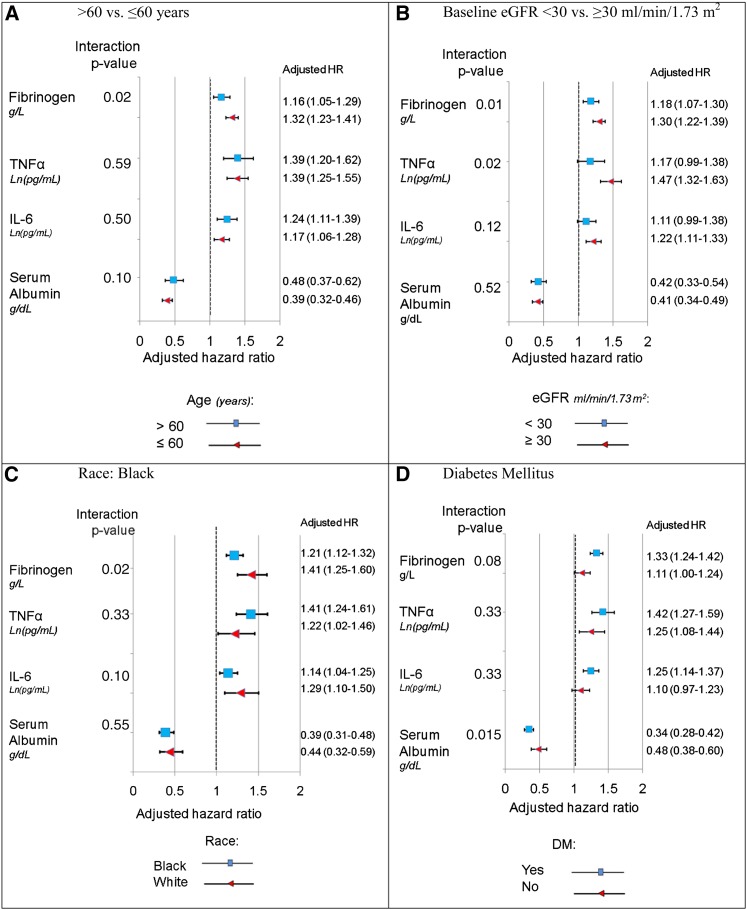
Race, age, DM, and baseline eGFR were tested for interactions in Cox regression models (Figure 2). The associations of fibrinogen and TNF-α with the composite end point were stronger in patients with eGFR≥30 ml/min per 1.73 m2. The association of plasma fibrinogen with the composite outcome was stronger in patients ages ≤60 years old and nonblacks compared with their counterparts. The association of low SAlb with the composite outcome was stronger in patients with DM. Of note, the overall effects of inflammatory markers when coded as continuous variables were very similar to those when coded as quartiles.
Figure 2.
Moderators of Cox hazard ratio (HR) for the composite outcome for baseline cytokine levels. Cytokines are coded as continuous variables. Natural log is used for IL-6 and TNF-α. Covariates include baseline eGFR, age, sex, race, body mass index, urine albumin-to-creatinine ratio, hypertension, diabetes mellitus (DM), alcohol use, smoking, total cholesterol, and angiotensin–converting enzyme inhibitor/angiotensin receptor blocker. (A) Adjusted HR stratified by age ≥60 versus <60 years old. (B) Adjusted HR stratified by baseline eGFR ≥30 versus <30 ml/min per 1.73 m2. (C) Adjusted HR stratified by black race. (D) Adjusted HR stratified by DM.
Sensitivity Analyses
The findings from the sensitivity analysis predicting time to ESRD confirmed a graded increase in risk for ESRD with increasing levels of fibrinogen, IL-6, and TNF-α and decreasing SAlb (model 2 in Supplemental Table 2).
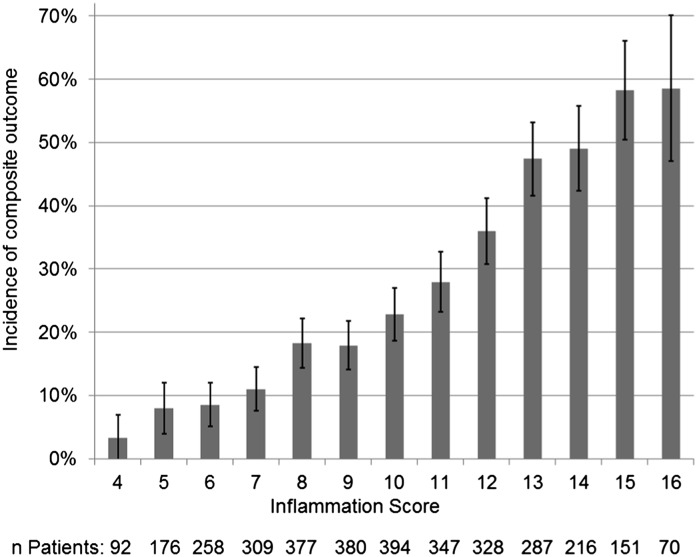
The inflammation score had a strong linear association with incidence of the composite outcome (Figure 3) (Cochran–Armitage trend test P<0.001; Spearman r=0.34; P<0.001). After adjusting for covariates in Cox regression, the inflammation score remained significant (HR, 1.19; 95% CI, 1.16 to 1.22; P<0.001), indicating that each additional point on the inflammation score is associated with a 19% increase in the hazard of reaching the composite outcome (Supplemental Figure 3).
Figure 3.
Unadjusted association between inflammation score and incidence of the composite outcome. Error bars show the 95% confidence intervals. The inflammation score is the sum of baseline quartiles of IL-6, TNF-α, fibrinogen, and serum albumin (reverse scored). The association of these cytokines with the composite outcome is additive (chi square =422.34; P<0.001; Spearman r=0.34), and there is a significant linear trend (Cochran–Armitage trend test P<0.001).
In the Cox regression model using continuous levels of natural log TNF-α, fibrinogen, natural log IL-6, and SAlb together to predict time to the composite outcome, only TNF-α, fibrinogen, and SAlb remained significantly associated with the outcome (Table 4). After adjusting for age, race, sex, hypertension, DM, smoking, alcohol use, ACEi/ARB treatment, total cholesterol, and baseline eGFR, TNF-α, fibrinogen, and SAlb still had independent associations with the composite outcome. When UACR was added as a covariate along with the other covariates, TNF-α and SAlb remained significant. Furthermore, we tested the above models for multicollinearity using weighted regression and found a variance inflation factor ≤1.3, indicating that multicollinearity had little or no effect on the above parameter estimates.
Table 4.
Cox regression hazard ratios for continuous cytokines predicting the composite outcome
| Biomarker | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Fibrinogen, g/L | 1.26 (1.20 to 1.33) | <0.001 | 1.16 (1.10 to 1.23) | <0.001 | 1.03 (0.97 to 1.09) | 0.39 |
| IL-6, natural log pg/ml | 1.03 (0.95 to 1.11) | 0.47 | 0.94 (0.86 to 1.02) | 0.52 | 1.04 (0.96 to 1.13) | 0.36 |
| TNF-α, natural log pg/ml | 1.56 (1.43 to 1.69) | <0.001 | 1.29 (1.17 to 1.42) | <0.001 | 1.18 (1.06 to 1.31) | 0.002 |
| Serum albumin, g/dl | 0.34 (0.30 to 0.39) | <0.001 | 0.36 (0.32 to 0.42) | <0.001 | 0.70 (0.59 to 0.81) | <0.001 |
Model 1: unadjusted model using four cytokines. Model 2: covariates adjusted for included baseline eGFR, age, sex, race, cholesterol, systolic BP, diabetes, angiotensin–converting enzyme inhibitor/angiotensin receptor blocker, body mass index, alcohol use, and smoking (primary model). Model 3: covariates from model 2 and baseline urine albumin-to-creatinine ratio. HR, hazard ratio; 95% CI, 95% confidence interval.
The analysis of area under the ROC curve in prediction models for the composite outcome is presented in detail in Supplemental Material. Adding cytokines to a logistic regression model predicting incidence of the composite outcome significantly improved the area under the ROC curve (from c=0.81 to c=0.84; P=0.01). Adding proteinuria further improved area under the ROC curve (to c=0.88; P<0.001). We found a similar pattern of c statistics using Cox regression for the models with background variables only, cytokines added, and UACR added (c=0.80, c=0.83, and c=0.88, respectively).
Examination of additional interactions found that the association of fibrinogen with the composite outcome was moderated by sex (with men having a stronger effect; HR, 1.33 [95% confidence interval, 1.21 to 1.43] versus 1.18 [95% confidence interval, 1.08 to 1.29] for women; P=0.03 for interaction) (Supplemental Figure 4). Men also had a stronger association of SAlb with outcome than women but only at a trend level of significance (HR, 0.52 [95% confidence interval, 0.40 to 0.69] versus 0.36 [95% confidence interval, 0.30 to 0.43]; P=0.05 for interaction).
Discussion
In this large, well defined cohort of men and women with CKD, including a substantial proportion of blacks and persons with diabetes, elevated circulating levels of fibrinogen, TNF-α, and IL-6 and decreased SAlb were independent predictors of the slope of eGFR decline. After adjusting for traditional risk factors for CKD progression, elevated plasma levels of fibrinogen, IL-6, and TNF-α and decreased SAlb were significantly associated with a composite of ESRD or a reduction of 50% in the eGFR from baseline. Fibrinogen, SAlb, IL-6, and TNF-α remained significantly associated with the composite outcome even after accounting for death as a competing risk. These associations with the composite outcome were stronger at higher levels of kidney function for fibrinogen and TNF-α, thus presenting a unique opportunity for early intervention.
Inflammation is mediated by a group of secreted polypeptides called cytokines. In the kidney, cytokines induce expression of reactive oxygen/nitrogen species (16), bioactive lipids (17), and adhesion molecules (18,19). They also promote aberrant matrix metabolism (20,21), proliferation of resident cells (22), and procoagulant activity of endothelium in the kidney (23). In the Beaver Dam Chronic Kidney Disease Study, a population-based cohort of 4926 predominantly white participants from Wisconsin, circulating levels of IL-6 and TNF-α receptor 2 were associated with incident CKD (24). Tonelli et al. (25) reported that higher CRP and soluble TNF receptor 2 are independently associated with faster rates of kidney function loss in CKD. Recently, two studies confirmed the association of circulating TNF-α receptors with progression of CKD (26,27). The Multi-Ethnic Study of Atherosclerosis Study, consisting of 6814 study participants recruited from six United States communities, noted a small but significant association between the magnitude of change in kidney function and IL-6 levels (28). After adjusting for traditional risk factors for progression of CKD, fibrinogen, SAlb, IL-6, and TNF-α were associated with the composite outcome in our study. However, the standard Cox model, which does not adjust for competing risk of death, can overestimate the absolute risk. After accounting for death as a competing risk event, fibrinogen, SAlb, IL-6, and TNF-α remained significant predictors of the composite outcome.
IL‐6 is a multifunctional cytokine that promotes proliferation of lymphocytes, differentiation of B cells, leukocyte recruitment, and induction of the acute–phase protein synthesis (29). Although generally regarded as a proinflammatory cytokine, IL-6 has many regenerative or anti-inflammatory actions as well (30). TNF-α is produced by macrophages, glomerular mesangial cells, and tubular epithelial cells (31). It induces a respiratory burst in neutrophils with concomitant release of free radicals and thus, promotes renal scaring (32). This is the first study showing significant association between plasma levels of TNF-α and progressive loss of kidney function in a large CKD cohort.
Higher CRP, factor 7, fibrinogen, and white blood cell count were associated with a decline in eGFR in the Cardiovascular Health Study, which included 4620 elderly community–dwelling adults (33). However, only fibrinogen was associated with risk for loss of eGFR in CRIC Study, which only included participants with established CKD. The prognostic importance of elevated fibrinogen levels is independent of and additive to the prognostic influence of CRP that has been reported (34,35). Fibrinogen is a large hexameric plasma glycoprotein, which plays a vital role in a number of pathophysiologic processes, including inflammation, atherogenesis, and thrombogenesis (36). Plasma fibrinogen may influence kidney function through its effect on the coagulation cascade, blood viscosity, and blood flow in the microcirculation (37). Fibrinogen is a receptor-mediated mitogen for renal fibroblasts and a possible facilitator of tubulointerstitial fibrosis (38). Association between low SAlb and progression of CKD has been reported (39). It is speculated that hypoalbuminemia is a surrogate marker for the degree of proteinuria or underlying inflammation, thus explaining the relationship (8).
Sensitivity analysis examining the incidence of the composite outcome across levels of the inflammation score found a linear association that remained significant after adjusting for time at risk and other covariates. However, when these four markers’ effects are examined together, adjusting for UACR, only TNF-α and SAlb had significant independent associations with outcome. Although we found that the inflammatory markers improve discrimination for the composite outcome beyond what is achieved using traditional predictors, more detailed exploration of the causal interplay between cytokines and acute-phase proteins is warranted.
Albuminuria is strongly associated with biomarkers of inflammation and also strongly associated with progression of CKD (8,40). In the Framingham Offspring Cohort, higher TNF-α, IL-6, and TNF receptor 2 levels were associated with UACR (41). Considering that albuminuria could be a causal pathway variable as well as a true confounder, we present models with and without UACR as a covariate. As expected, adjusting for UACR reduced the strength of the associations between both outcomes and the biomarkers that we studied.
Examining interactions of inflammatory biomarkers with other variables revealed that the positive associations between inflammation and progression of CKD were stronger in high baseline eGFR, nonblack race (for fibrinogen), younger age subgroups (for fibrinogen), patients with DM (for SAlb), and men (fibrinogen and SAlb). Intrarenal inflammation may be an important response to initial injury, and loss of kidney function in later phases is possibly mediated through noninflammatory mechanisms (42,43). The effect of inflammation on CKD progression in blacks is possibly obscured by adverse genetic factors and the high prevalence of traditional risk factors seen in this population (44,45). Results from the Cardiovascular Health Study showed that the majority of older people have little or no progression of CKD, explaining, in part, the lack of robust association with inflammation and CKD progression in older individuals (46).
This study is unique in that we show that increased levels of inflammatory biomarkers are associated with faster decline in eGFR and ESRD after adjusting for known risk factors and considering death as a competing risk. This is the first study to show association of circulating levels of TNF-α with progression of CKD. Our study has a number of strengths, including a large cohort of patients with a broad range of kidney function, long–term follow-up, measurement of multiple biomarkers, a large proportion of black participants, and a significant number of outcomes. We acknowledge limitations, including outcome data that were not available in some study participants and the measurement of biomarkers at only one time point. However, findings from at least one other study suggest that a single baseline measure accurately reflects an individual’s inflammatory status over time (47).
To summarize, elevated circulating levels of TNF-α and fibrinogen and decreased SAlb are independent predictors of progression of CKD. These biomolecules could be useful in risk stratification and also, could be potential therapeutic targets. The associations of inflammatory biomarkers and kidney outcomes were stronger at higher levels of eGFR and thus, could possibly represent an opportunity for early intervention.
Disclosures
None.
Supplementary Material
Acknowledgments
D.S.R. is supported by grants R01 DK073665-01A1, 1U01DK099914-01, and 1U01DK099924-01 from the National Institutes of Health (NIH). Funding for the Chronic Renal Insufficiency Cohort (CRIC) Study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported, in part, by Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science NIH/National Center for Advancing Translational Sciences (NCATS) grant UL1TR000003, Johns Hopkins University grant UL1 TR-000424, University of Maryland grant GCRC M01 RR-16500, the Clinical and Translational Science Collaborative of Cleveland, grant UL1TR000439 from the NCATS component of the NIH and the NIH Roadmap for Medical Research, Michigan Institute for Clinical and Health Research grant UL1TR000433, University of Illinois at Chicago Clinical and Translational Science Award grant UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology grant P30GM103337, and Kaiser Permanente NIH/National Center for Research Resources University of California San Francisco-Clinical & Translational Science Institute grant UL1 RR-024131.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13121215/-/DCSupplemental.
References
- 1.United States Renal Data System : Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 2.Gilbertson DT, Liu J, Xue JL, Louis TA, Solid CA, Ebben JP, Collins AJ: Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J Am Soc Nephrol 16: 3736–3741, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Anderson GF, Chu E: Expanding priorities--confronting chronic disease in countries with low income. N Engl J Med 356: 209–211, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Nath KA: Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Yu HT: Progression of chronic renal failure. Arch Intern Med 163: 1417–1429, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Appel LJ, Wright JT Jr., Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X; AASK Collaborative Research Group : Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 363: 918–929, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, Shah VO, Balakrishnan VS, Guzman NJ, Girndt M, Periera BG, Feldman HI, Kusek JW, Joffe MM, Raj DS; CRIC Study Investigators : Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol 7: 1938–1946, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raj DS, Moseley P, Dominic EA, Onime A, Tzamaloukas AH, Boyd A, Shah VO, Glew R, Wolfe R, Ferrando A: Interleukin-6 modulates hepatic and muscle protein synthesis during hemodialysis. Kidney Int 73: 1054–1061, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Kushner I: The phenomenon of the acute phase response. Ann N Y Acad Sci 389: 39–48, 1982 [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Rao M, Wong C, Kanetsky P, Girndt M, Stenvinkel P, Reilly M, Raj DS: Cytokine gene polymorphism and progression of renal and cardiovascular diseases. Kidney Int 72: 549–556, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, Heimbürger O, Cederholm T, Girndt M: IL-10, IL-6, and TNF-alpha: Central factors in the altered cytokine network of uremia--the good, the bad, and the ugly. Kidney Int 67: 1216–1233, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Allison PD: Logistic Regression Using SAS: Theory and Application, Cary, NC, SAS Institute Inc., 1999 [Google Scholar]
- 15.Gray RJ: A class of K-Sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16: 1141–1154, 1988 [Google Scholar]
- 16.Sharma K, Cook A, Smith M, Valancius C, Inscho EW: TGF-beta impairs renal autoregulation via generation of ROS. Am J Physiol Renal Physiol 288: F1069–F1077, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Zager RA, Johnson A: Renal cortical cholesterol accumulation is an integral component of the systemic stress response. Kidney Int 60: 2299–2310, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Park S, Chang YH, Cho YJ, Ahn H, Yang WS, Park JS, Lee JD: Cytokine-regulated expression of vascular cell adhesion molecule-1 in human glomerular endothelial cells. Transplant Proc 30: 2395–2397, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Ramesh G, Reeves WB: TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835–842, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman DL, Ruef C: Interleukin-6: An autocrine regulator of mesangial cell growth. Kidney Int 41: 604–606, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Horii Y, Muraguchi A, Iwano M, Matsuda T, Hirayama T, Yamada H, Fujii Y, Dohi K, Ishikawa H, Ohmoto Y, et al. : Involvement of IL-6 in mesangial proliferative glomerulonephritis. J Immunol 143: 3949–3955, 1989 [PubMed] [Google Scholar]
- 22.Nakamura T, Miller D, Ruoslahti E, Border WA: Production of extracellular matrix by glomerular epithelial cells is regulated by transforming growth factor-beta 1. Kidney Int 41: 1213–1221, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Bevilacqua MP, Pober JS, Majeau GR, Fiers W, Cotran RS, Gimbrone MA Jr.: Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: Characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A 83: 4533–4537, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shankar A, Sun L, Klein BE, Lee KE, Muntner P, Nieto FJ, Tsai MY, Cruickshanks KJ, Schubert CR, Brazy PC, Coresh J, Klein R: Markers of inflammation predict the long-term risk of developing chronic kidney disease: A population-based cohort study. Kidney Int 80: 1231–1238, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G; Cholesterol and Recurrent Events (CARE) Trial Investigators : Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int 68: 237–245, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Carlsson AC, Nordquist L, Larsson TE, Carrero JJ, Larsson A, Lind L, Ärnlöv J: Soluble tumor necrosis factor receptor 1 is associated with glomerular filtration rate progression and incidence of chronic kidney disease in two community-based cohorts of elderly individuals. Cardiorenal Med 5: 278–288, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh YJ, An JN, Kim CT, Yang SH, Lee H, Kim DK, Joo KW, Paik JH, Kang SW, Park JT, Lim CS, Kim YS, Lee JP: Circulating tumor necrosis factor α receptors predict the outcomes of human IgA nephropathy: A prospective cohort study. PLoS One 10: e0132826, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiramoto JS, Katz R, Peralta CA, Ix JH, Fried L, Cushman M, Siscovick D, Palmas W, Sarnak M, Shlipak MG: Inflammation and coagulation markers and kidney function decline: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 60: 225–232, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barton BE: IL-6: Insights into novel biological activities. Clin Immunol Immunopathol 85: 16–20, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S: The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813: 878–888, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Egido J, Gómez-Chiarri M, Ortíz A, Bustos C, Alonso J, Gómez-Guerrero C, Gómez-Garre D, López-Armada MJ, Plaza J, Gonzalez E: Role of tumor necrosis factor-alpha in the pathogenesis of glomerular diseases. Kidney Int Suppl 39: S59–S64, 1993 [PubMed] [Google Scholar]
- 32.Rubin-Kelley VE, Jevnikar AM: Antigen presentation by renal tubular epithelial cells. J Am Soc Nephrol 2: 13–26, 1991 [DOI] [PubMed] [Google Scholar]
- 33.Fried L, Solomon C, Shlipak M, Seliger S, Stehman-Breen C, Bleyer AJ, Chaves P, Furberg C, Kuller L, Newman A: Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J Am Soc Nephrol 15: 3184–3191, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Toss H, Lindahl B, Siegbahn A, Wallentin L: Prognostic influence of increased fibrinogen and C-reactive protein levels in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. Circulation 96: 4204–4210, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Zoccali C, Mallamaci F, Tripepi G, Cutrupi S, Parlongo S, Malatino LS, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Stancanelli B, Nicocia G, Buemi M: Fibrinogen, mortality and incident cardiovascular complications in end-stage renal failure. J Intern Med 254: 132–139, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Kaptoge S, White IR, Thompson SG, Wood AM, Lewington S, Lowe GD, Danesh J; Fibrinogen Studies Collaboration : Associations of plasma fibrinogen levels with established cardiovascular disease risk factors, inflammatory markers, and other characteristics: Individual participant meta-analysis of 154,211 adults in 31 prospective studies: The fibrinogen studies collaboration. Am J Epidemiol 166: 867–879, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Cahill M, Mistry R, Barnett DB: The human platelet fibrinogen receptor: Clinical and therapeutic significance. Br J Clin Pharmacol 33: 3–9, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sörensen I, Susnik N, Inhester T, Degen JL, Melk A, Haller H, Schmitt R: Fibrinogen, acting as a mitogen for tubulointerstitial fibroblasts, promotes renal fibrosis. Kidney Int 80: 1035–1044, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Staples AO, Greenbaum LA, Smith JM, Gipson DS, Filler G, Warady BA, Martz K, Wong CS: Association between clinical risk factors and progression of chronic kidney disease in children. Clin J Am Soc Nephrol 5: 2172–2179, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J; Chronic Kidney Disease Prognosis Consortium : Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Upadhyay A, Larson MG, Guo CY, Vasan RS, Lipinska I, O’Donnell CJ, Kathiresan S, Meigs JB, Keaney JF Jr., Rong J, Benjamin EJ, Fox CS: Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrol Dial Transplant 26: 920–926, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savill J: Regulation of glomerular cell number by apoptosis. Kidney Int 56: 1216–1222, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Cao Q, Harris DC, Wang Y: Macrophages in kidney injury, inflammation, and fibrosis. Physiology (Bethesda) 30: 183–194, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT Jr., Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ; AASK Study Investigators; CRIC Study Investigators : APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarver-Carr ME, Powe NR, Eberhardt MS, LaVeist TA, Kington RS, Coresh J, Brancati FL: Excess risk of chronic kidney disease among African-American versus white subjects in the United States: A population-based study of potential explanatory factors. J Am Soc Nephrol 13: 2363–2370, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Bleyer AJ, Shemanski LR, Burke GL, Hansen KJ, Appel RG: Tobacco, hypertension, and vascular disease: Risk factors for renal functional decline in an older population. Kidney Int 57: 2072–2079, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Snaedal S, Heimbürger O, Qureshi AR, Danielsson A, Wikström B, Fellström B, Fehrman-Ekholm I, Carrero JJ, Alvestrand A, Stenvinkel P, Bárány P: Comorbidity and acute clinical events as determinants of C-reactive protein variation in hemodialysis patients: Implications for patient survival. Am J Kidney Dis 53: 1024–1033, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.