Abstract
Background and objectives
IL-2 receptor antagonist (IL2-RA) is recommended as a first-line agent for induction therapy in renal transplantation. However, this remains controversial in deceased donor renal transplantation (DDRT) maintained on tacrolimus (TAC)/mycophenolic acid (MPA) with or without steroids.
Design, setting, participants, & measurements
We studied the United Network for Organ Sharing Registry for patients receiving DDRT from 2000 to 2012 maintained on TAC/MPA at transplantation hospital discharge (n=74,627) to compare outcomes of IL2-RA and other induction agents. We initially divided the cohort into two groups on the basis of steroid use at the time of discharge: steroid (n=59,010) versus no steroid (n=15,617). Each group was stratified into induction categories: IL2-RA, rabbit antithymocyte globulin (r-ATG), alemtuzumab, and no induction. The main outcomes were incidence of acute rejection within the first year and overall graft failure (defined as graft failure and/or death) post-transplantation. Propensity score (PS), specifically inverse probability of treatment weight, analysis was used to minimize selection bias caused by nonrandom assignment of induction therapies.
Results
Median (25th, 75th percentiles) follow-up times were 3.9 (1.1, 5.9) and 3.2 (1.1, 4.9) years for steroid and no steroid groups, respectively. Acute rejection within the first year and overall graft failure within 5 years of transplantation were more common in the no induction category (13.3%; P<0.001 and 28%; P=0.01, respectively) in the steroid group and the IL2-RA category (11.1%; P=0.16 and 27.4%; P<0.001, respectively) in the no steroid group. Compared with IL2-RA, PS–weighted and covariate–adjusted multivariable logistic and Cox analyses showed that outcomes in the steroid group were similar among induction categories, except that acute rejection was significantly lower with r-ATG (odds ratio [OR], 0.68; 95% confidence interval [95% CI], 0.62 to 0.74). In the no steroid group, compared with IL2-RA, odds of acute rejection with r-ATG (OR, 0.80; 95% CI, 0.60 to 1.00) and alemtuzumab (OR, 0.68; 95% CI, 0.53 to 0.88) were lower, and r-ATG was associated with better graft survival (hazard ratio, 0.86; 95% CI, 0.75 to 0.99).
Conclusions
In DDRT, compared with IL2-RA induction, no induction was associated with similar outcomes when TAC/MPA/steroids were used. r-ATG seems to offer better graft survival over IL2-RA in steroid avoidance protocols.
Keywords: Immunosuppression; kidney transplantation; rejection; Antibodies, Monoclonal, Humanized; Antilymphocyte Serum; Follow-Up Studies; Graft Survival; Humans; Immunosuppressive Agents; Mycophenolic Acid; tacrolimus; Tissue Donors
Introduction
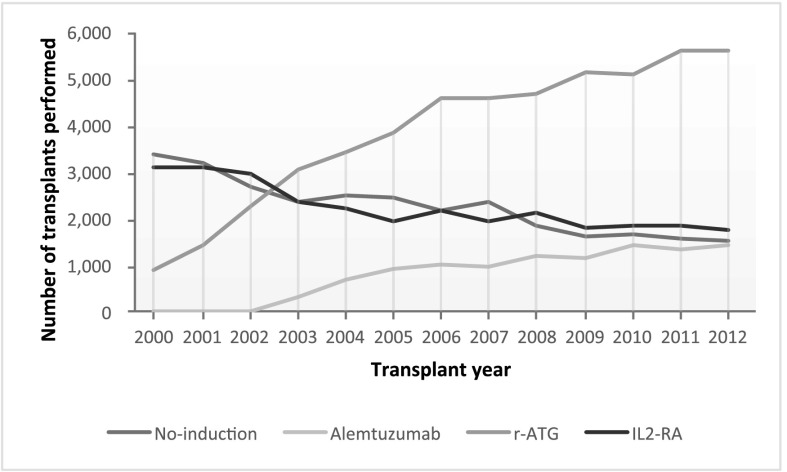
The practice of using combination induction and maintenance immunosuppressive agents to optimize post-transplant outcomes has become increasingly widespread (1,2). Currently available induction immunosuppression agents include lymphocyte-depleting antibodies, such as polyclonal rabbit antithymocyte globulin (r-ATG) and the humanized anti–CD52 mAb (alemtuzumab), and mAbs directed against IL-2 receptor (1,3). Induction regimens are often decided on a patient by patient basis, but in general, lymphocyte-depleting agents are commonly used in high–immunologic risk donor-recipient pairs and steroid-sparing protocols, whereas IL-2 receptor antagonists (IL2-RAs; basiliximab and dacluzimab; the latter was withdrawn from the market in 2009) are commonly used in low–immunologic risk patients (1,4). In the United States, lymphocyte-depleting antibodies seem to be favored over IL2-RA (Figure 1).
Figure 1.
Number of kidney transplants performed in the United States on the basis of induction type and transplant year among deceased donor renal transplantation recipients maintained on tacrolimus/mycophenolic acid at transplantation hospital discharge. IL2-RA, IL-2 receptor antagonist; r-ATG, rabbit antithymocyte globulin.
The current Kidney Disease Improving Global Outcomes (KDIGO) Work Group guidelines recommend IL2-RA as a first–line induction therapy across all types of donor-recipient profiles to reduce acute rejection risk and allograft loss (4,5). However, there has been a lack of randomized, controlled, double–blind clinical trials comparing induction agents when recipients are maintained on tacrolimus (TAC)/mycophenolic acid (MPA) with or without steroids, and the recommendations are mainly made on the basis of meta-analysis data derived from studies using cyclosporin–based maintenance immunosuppression regimens (3,5–7). In the era of TAC/MPA maintenance regimens with or without steroids, controversy exists with respect to the added benefit of IL2-RA induction therapy on outcomes of renal transplantation. In fact, there are recent data that support that no induction therapy could achieve acceptable acute rejection rates (≤20% at 1 year after transplantation), with similar allograft and patient survival compared with IL2-RA induction in the setting of the TAC/MPA maintenance regimen (8–12).
Unfortunately, with the incidence of acute rejection approaching single digits, the sample size required for randomized, controlled, double–blind clinical trials that could discern small differences in observed outcomes between IL2-RA and other induction categories has been estimated to be between 1600 and 7000 (11). Such studies would be too expensive to conduct from the sponsor’s perspective and involve excessive risk because of widespread use of induction agents. Given these obstacles, the best option to compare induction strategies is to use large registry data with careful design (stratification of baseline immunosuppression and advanced statistical adjustments for selection bias).
To address the obvious need for comparison of induction regimens, our group recently reported the analysis of the Organ Procurement and Transplantation Network (OPTN)/United Network for Organ Sharing (UNOS) data regarding the added benefit of induction immunosuppression on outcomes of live donor kidney transplants (LRTs) maintained on a TAC/MPA regimen using propensity score (PS)–weighted analysis (13). In this article, we asked the same question of recipients of deceased donor renal transplantation (DDRT) to find out whether our findings in LRT regarding use of induction immunosuppression apply to DDRT or not.
Materials and Methods
Design and Study Cohort
The research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking. Institutional review board approval was obtained before the study, which was a retrospective cohort analysis of the OPTN/UNOS Registry comprised all adults who received a DDRT between January 1, 2000 and September 30, 2012 (censoring date September 30, 2013) in the United States (n=128,470). Exclusion criteria were as follows: (1) patients ages younger than 18 years old; (2) multiorgan transplantations; (3) two or more previous kidney transplantations; (4) recipients of induction agents other than no induction therapy, alemtuzumab, r-ATG, and IL2-RA; (5) patients with a positive crossmatch (cytotoxic and/or flow cytometry); and (6) recipients of HLA zero mismatch kidneys (representing very low risk for rejection). Data were further limited to the recipients maintained on TAC/MPA immunosuppression at the time of transplantation hospital discharge. In total, 74,627 patients were included in the final analysis. The study population was then divided into two groups on the basis of the use of maintenance steroids at the time of hospital discharge (steroid [n=59,010] versus no steroid [n=15,617] groups). Each group was further divided into subcategories on the basis of induction immunosuppression used: IL2-RA, r-ATG, alemtuzumab, or no induction in the steroid group versus IL2-RA, r-ATG, or alemtuzumab in the no steroid group. The no induction category in the no steroid group was excluded because of small sample size.
Main Outcomes
The primary outcomes were incidence of treated acute rejection within the first year (defined as biopsy proven or clinically indicated) and overall allograft failure (defined as return to dialysis, retransplant, or death with functioning allograft) after transplantation. The acute rejection episodes up to 1 year after transplantation were examined (available for all recipients), whereas overall allograft failures were included through the end of the last follow-up date (September 30, 2013).
Statistical Analyses
Donor and recipient characteristics were described using frequencies or means±SD. Comparison between groups was made using the t test, Kruskal–Wallis test, or chi-squared test. Survival rates were estimated using the Kaplan–Meier product limit method. The log rank test was used for comparison of the unadjusted survival curves. Logistic regression models were used to estimate the odds ratios of acute rejection. Cox regression models were used to estimate the hazard ratios (HRs) associated with overall and death–censored allograft failure risk. P values <0.05 were considered statistically significant. Statistical analyses were performed with SAS software (version 9.3; SAS Institute Inc., Cary, NC) and Stata MP14 software (StataCorp., College Station, TX).
Multivariable logistic and Cox models were adjusted for donor factors (sex and kidney donor profile index [KDPI] [14]), recipient factors (age, sex, race, diabetes status, cardiovascular disease, peak panel reactive antibodies [PRAs], retransplant status, and dialysis exposure), transplant factors (cold ischemia time [CIT], donor-to-recipient weight ratio, HLA mismatch, and transplant year), transplant center (to account for center effect on induction strategy), and the OPTN region (to account for geographic variations). Approximately 40% of peak PRA data were missing across both groups and all categories. Because PRA is a strong predictor of rejection and graft failure, and strongly associated with induction strategy, we included the recipients with missing PRA data as a separate category (in addition to 0%–20%, 20%–80%, and 80%–100% categories) in the multivariable logistic and Cox models. PRA has been reported to the UNOS/OPTN more regularly and accurately since 2007.
PS Analyses.
The PS was derived from multinomial logistic regression using the same covariates as in the adjusted analysis to control for potential selection bias caused by nonrandom assignment of induction treatments. We specifically used the inverse probability of treatment weight, in which the weights were calculated as the inverse of the PS (13,15–18). Details regarding calculation of PS can be found in Supplemental Material and our prior publication.
Subgroup Analyses.
A subgroup analysis was performed for high-risk recipients (including CIT>24 hours, retransplantation, black race, KDPI>85%, and PRA>0%) and low-risk recipients (not having any of above risks factors) regarding primary outcomes in both steroid groups.
Results
Characteristics of the Study Cohort
The changing trend for use of induction therapy in recipients of DDRTs in the United States is illustrated in Figure 1. The use of lymphocyte-depleting antibody (r-ATG and alemtuzumab) has been gradually increased over the past decade. Recipient, donor, and transplant characteristics for both steroid groups and their induction categories are summarized in Tables 1 and 2. Around 15% of the recipients received preemptive transplants across all categories. Before the PS adjustment, most P values were clinically significant. However, after the PS, all P values, with the exceptions of dialysis exposure, CIT, and transplant year in the steroid group and recipient age in the no steroid group, were no longer statistically significant.
Table 1.
Characteristics of donor, recipient, and transplant factors in the steroid group
| Characteristics | Steroid Induction Categories | P Value | ||||
|---|---|---|---|---|---|---|
| IL2-RA | r-ATG | Alemtuzumab | No Induction | Before IPTW | After IPTWa | |
| N=59,010 (%) | 15,549 (26.4) | 27,732 (47) | 2497 (4.2) | 13,232 (22.4) | ||
| Donors | ||||||
| Age, yr | 36.7±16.6 | 36.5±16.6 | 37.5±16.7 | 36.5±16.6 | 0.02 | 0.07 |
| Women, % | 39.2 | 38.5 | 38.5 | 39.3 | 0.34 | |
| Race, % | <0.001 | |||||
| White | 69.4 | 66.9 | 69.7 | 67.4 | ||
| Black | 12.6 | 14.5 | 16.6 | 15.4 | ||
| Hispanic | 14.1 | 14.5 | 11.6 | 14.1 | ||
| Other | 3.9 | 4.1 | 2.1 | 3.1 | ||
| ECD, % | 14.8 | 14.8 | 16.4 | 15 | 0.17 | |
| KDPI, % | 43.2±27.4 | 43.9±27.4 | 46.2±28.1 | 43.6±27.6 | <0.001 | 0.18 |
| Recipients | ||||||
| Age, yr | 51.3±13.4 | 50.1±12.9 | 49.9±12.9 | 49.8±12.9 | <0.001 | 0.89 |
| Women, % | 36.4 | 41.9 | 43.8 | 39.3 | <0.001 | 0.93 |
| Race, % | <0.001 | 0.98 | ||||
| White | 49.8 | 43.2 | 49.1 | 44 | ||
| Black | 27.5 | 35.3 | 37.1 | 36 | ||
| Hispanic | 14 | 14.3 | 9 | 13 | ||
| Other | 8.8 | 7.2 | 4.8 | 7 | ||
| DM (yes), % | 42.1 | 44.1 | 45.8 | 42.8 | <0.001 | 0.66 |
| CVD (yes), % | 6.7 | 7.1 | 5.3 | 5.7 | <0.001 | 0.92 |
| Retransplant, % | 7.2 | 14.1 | 15.4 | 9.3 | <0.001 | 0.12 |
| Dialysis before transplant, % | <0.001 | 0.03 | ||||
| Preemptive | 14.3 | 13.6 | 15.3 | 14.8 | ||
| <1 yr | 6.5 | 4.4 | 7 | 5.7 | ||
| 1–3 yr | 29 | 27.1 | 27.4 | 30 | ||
| >3 yr | 50.3 | 54.9 | 50.3 | 49.5 | ||
| PRA, % | <0.001 | 0.29 | ||||
| 0–20 | 55.8 | 38.2 | 26.3 | 56.5 | ||
| 21–80 | 6.8 | 8.1 | 6 | 8.7 | ||
| 81–100 | 3.4 | 7.3 | 6.8 | 5.6 | ||
| Missing | 34.1 | 46.4 | 60.9 | 29.1 | ||
| Transplants | ||||||
| CIT, h | 16.6±7.9 | 16.5±8 | 20.1±11.8 | 17.2±8.5 | <0.001 | 0.01 |
| Weight ratio D/R | 1.02±0.37 | 1.03±0.37 | 1.03±0.39 | 1.02±0.37 | 0.12 | 0.89 |
| HLA mismatch, % | <0.001 | 0.83 | ||||
| 1 | 2.6 | 2 | 1.7 | 2.8 | ||
| 2 | 5.1 | 4.7 | 6 | 5.1 | ||
| 3 | 14.9 | 14.2 | 16.1 | 14.8 | ||
| 4 | 27.9 | 27.8 | 27 | 27.6 | ||
| 5 | 32.4 | 33.9 | 31.7 | 32.6 | ||
| 6 | 17.1 | 17.4 | 17.4 | 17.2 | ||
| Transplant year, % | <0.001 | <0.001 | ||||
| 2000–2001 | 15.1 | 3.4 | 0 | 16.3 | ||
| 2002–2003 | 15.9 | 9.4 | 1.7 | 16.2 | ||
| 2004–2005 | 12.5 | 13.1 | 12.6 | 17.9 | ||
| 2006–2007 | 14.4 | 17 | 17 | 15.4 | ||
| 2008–2009 | 16.4 | 20.5 | 21.1 | 13.9 | ||
| 2010–2012 | 25.8 | 36.6 | 47.5 | 20.3 | ||
IL2-RA, IL-2 receptor antagonist; r-ATG, rabbit antithymocyte globulin; IPTW, inverse probability of treatment weight; ECD, expanded criteria donor; KDPI, kidney donor profile index; DM, diabetes; CVD, cardiovascular disease; PRA, panel reactive antibody; CIT cold ischemia time; D/R, donor/recipient.
Some of the P values are not reported, because those variables are not included in the propensity score analysis.
Table 2.
Characteristics of donor, recipient, and transplant factors in the no steroid group
| Characteristics | No Steroid Induction Categories | P Value | |||
|---|---|---|---|---|---|
| IL2-RA | r-ATG | Alemtuzumab | Before IPTW | After IPTWa | |
| N=15,617 (%) | 1515 (9.7) | 9378 (60) | 4724 (30.2) | ||
| Donors | |||||
| Age, yr | 38.9±17.1 | 36.6±16.9 | 38.6±16.5 | <0.001 | 0.49 |
| Women, % | 39.0 | 40.2 | 40.2 | 0.66 | |
| Race, % | <0.001 | ||||
| White | 69.6 | 70.6 | 69.1 | ||
| Black | 13.3 | 13.7 | 16.8 | ||
| Hispanic | 13.0 | 12.4 | 11.4 | ||
| Other | 4.1 | 3.3 | 2.7 | ||
| ECD, % | 20 | 15.8 | 18.4 | <0.001 | |
| KDPI, % | 47.4±28.6 | 44.8±27.4 | 47.3±27.6 | <0.001 | 0.91 |
| Recipients | |||||
| Age, yr | 56.3±14.3 | 52±13 | 51.1±12.5 | <0.001 | 0.004 |
| Women, % | 32.9 | 36.6 | 38.5 | <0.001 | 0.62 |
| Race, % | <0.001 | 0.63 | |||
| White | 56.3 | 52.1 | 47.1 | ||
| Black | 21.3 | 25.4 | 34.1 | ||
| Hispanic | 12.2 | 13.5 | 13 | ||
| Other | 10.2 | 9 | 5.8 | ||
| DM (yes), % | 41.1 | 47.3 | 43.9 | <0.001 | 0.93 |
| CVD (yes), % | 8.8 | 9.4 | 7.3 | <0.001 | 0.51 |
| Retransplant, % | 5.1 | 7.4 | 6.4 | 0.001 | 0.60 |
| Dialysis before transplant, % | <0.001 | 0.99 | |||
| Preemptive | 13.9 | 16 | 14.4 | ||
| <1 yr | 5.6 | 4.9 | 3.4 | ||
| 1–3 yr | 29.2 | 28.4 | 21.1 | ||
| >3 yr | 51.3 | 50.7 | 61.2 | ||
| PRA, % | <0.001 | 0.95 | |||
| 0–20 | 50.7 | 43.4 | 33.9 | ||
| 21–80 | 5 | 5.3 | 5 | ||
| 81–100 | 1.9 | 2.9 | 3.9 | ||
| Missing | 42.4 | 48.4 | 57.3 | ||
| Transplants | |||||
| CIT, h | 16.7±8.7 | 17.8±9.6 | 17.6±8.3 | <0.001 | 0.35 |
| Weight ratio D/R | 1.03±0.36 | 1.01±0.36 | 1.00±0.37 | 0.02 | 0.65 |
| HLA mismatch, % | 0.01 | 0.99 | |||
| 1 | 2.4 | 1.7 | 2.5 | ||
| 2 | 4.9 | 4.4 | 5.3 | ||
| 3 | 13.5 | 14 | 14 | ||
| 4 | 27.7 | 29.1 | 29 | ||
| 5 | 34.6 | 34.5 | 32.8 | ||
| 6 | 17 | 16.3 | 16.4 | ||
| Transplant year, % | <0.001 | 0.39 | |||
| 2000–2001 | 3.9 | 0.4 | 0 | ||
| 2002–2003 | 4.6 | 2.8 | 1.1 | ||
| 2004–2005 | 15.6 | 11.5 | 8.1 | ||
| 2006–2007 | 18.8 | 22.6 | 19.5 | ||
| 2008–2009 | 23.6 | 24 | 24.4 | ||
| 2010–2012 | 33.5 | 38.6 | 46.9 | ||
IL2-RA, IL-2 receptor antagonist; r-ATG, rabbit antithymocyte globulin; IPTW, inverse probability of treatment weight; ECD, expanded criteria donor; KDPI, kidney donor profile index; DM, diabetes; CVD, cardiovascular disease; PRA, panel reactive antibody; CIT cold ischemia time; D/R, donor/recipient.
Some of the P values are not reported, because those variables are not included in the propensity score analysis.
Outcomes
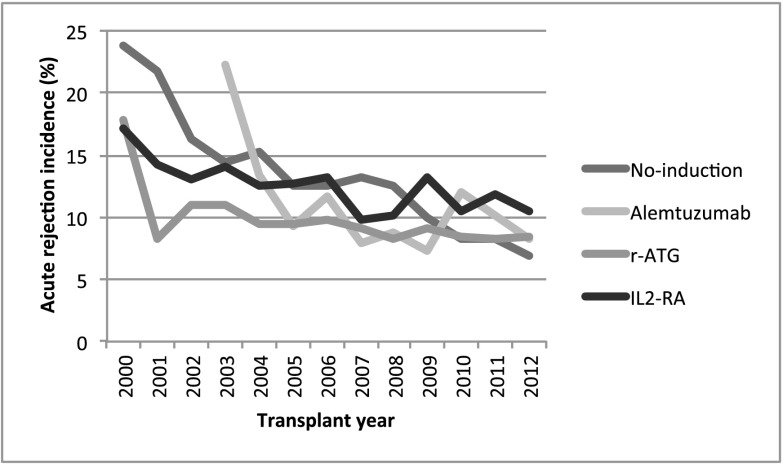
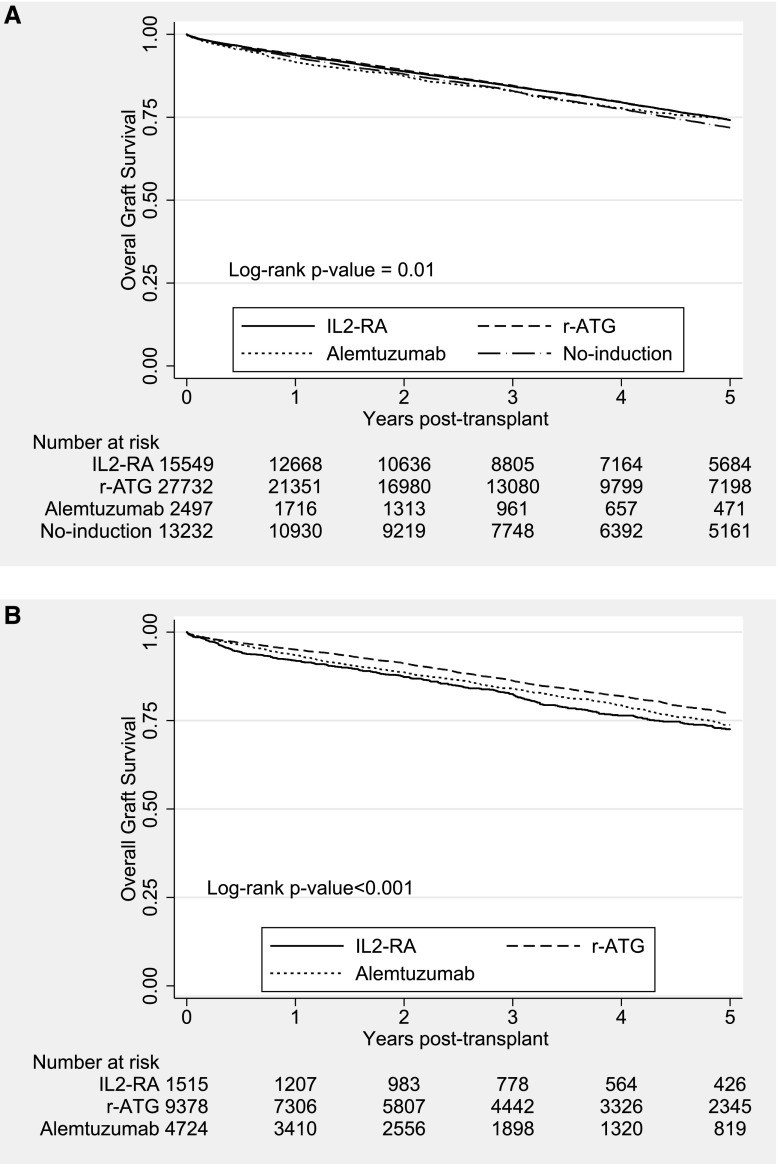
Median (25th, 75th percentiles) follow-up times were 3.9 (1.1, 5.9) and 3.2 (1.1, 4.9) years for the steroid and no steroid groups, respectively. Figure 2 illustrates the trend in incidence of acute rejection within the first year (percentage) among DDRT recipients. There has been a steady decrease in observed rejection rates among all induction categories (≤10% in 2012) over the past decade. However, unadjusted overall allograft survivals at 3 years have stayed stable across all induction categories (approximately 85%) during the study period (Supplemental Figure 1). The primary outcomes were observed more in the no induction category in the steroid group and the IL2-RA category in the no steroid group (Tables 3 and 4). Unweighted Kaplan–Meier curves for overall graft survival are shown in Figure 3. The overall graft survival curves were significantly different in both steroid groups. Regarding secondary outcomes, causes of death and allograft failure are summarized in Supplemental Tables 1 and 2. Incidence of post–transplant lymphoproliferative disorder for each group is shown in Supplemental Table 3.
Figure 2.
Incidence of acute rejection at 1 year (percentage) among deceased donor renal transplantation recipients maintained on tacrolimus/mycophenolic acid at transplantation hospital discharge on the basis of induction type and transplant year in the United States. IL2-RA, IL-2 receptor antagonist; r-ATG, rabbit antithymocyte globulin.
Table 3.
Comparison of the estimated association of induction treatments on acute rejection at 1 year using multivariable logistic regression models
| Induction Type | Acute Rejection Rate within 1 yr, % | ORa | 95% CI | P Value |
|---|---|---|---|---|
| Steroid | ||||
| Logistic regressiona | ||||
| IL2-RA | 12.4 | 1 | ||
| r-ATG | 9.1 | 0.72 | 0.65 to 0.79 | <0.001 |
| Alemtuzumab | 11.6 | 0.93 | 0.76 to 1.12 | 0.44 |
| No induction | 13.3 | 0.99 | 0.89 to 1.11 | 0.91 |
| PS–weighted logistic regressionb | ||||
| IL2-RA | 12.4 | 1 | ||
| r-ATG | 9.1 | 0.68 | 0.62 to 0.74 | <0.001 |
| Alemtuzumab | 11.6 | 0.90 | 0.74 to 1.10 | 0.32 |
| No induction | 13.3 | 0.90 | 0.81 to 1.01 | 0.06 |
| No steroid | ||||
| Logistic regressiona | ||||
| IL2-RA | 11.1 | 1 | ||
| r-ATG | 9.6 | 0.87 | 0.68 to 1.12 | 0.29 |
| Alemtuzumab | 9.1 | 0.71 | 0.54 to 0.94 | 0.02 |
| PS–weighted logistic regressionb | ||||
| IL2-RA | 11.1 | 1 | ||
| r-ATG | 9.6 | 0.80 | 0.60 to 1.00 | 0.05 |
| Alemtuzumab | 9.1 | 0.68 | 0.53 to 0.88 | 0.004 |
OR, odds ratio; 95% CI, 95% confidence interval; IL2-RA, IL-2 receptor antagonist; r-ATG, rabbit antithymocyte globulin; PS, propensity score.
Adjusted for donor factors (sex and kidney donor profile index), recipient factors (age, sex, race, diabetes status, cardiovascular disease, panel reactive antibody, retransplant status, and dialysis exposure), transplant factors (cold ischemia time, donor-to-recipient weight ratio, HLA mismatch, and transplant year), transplant center, and the Organ Procurement and Transplantation Network region.
PS weighted and adjusted for transplant center and the Organ Procurement and Transplantation Network region.
Table 4.
Comparison of the estimated association of induction treatments on overall allograft failure using multivariable Cox regression models
| Induction Type | Overall Graft Failure within 5 yr of Transplant, % | HRa | 95% CI | P Value |
|---|---|---|---|---|
| Steroid | ||||
| Cox regressiona | ||||
| IL2-RA | 25.9 | 1 | ||
| r-ATG | 25.8 | 0.98 | 0.93 to 1.03 | 0.35 |
| Alemtuzumab | 25.7 | 1.06 | 0.94 to 1.19 | 0.33 |
| No induction | 28.1 | 1.01 | 0.95 to 1.07 | 0.84 |
| PS–weighted Cox regressionb | ||||
| IL2-RA | 25.9 | 1 | ||
| r-ATG | 25.8 | 0.97 | 0.93 to 1.02 | 0.29 |
| Alemtuzumab | 25.7 | 0.99 | 0.89 to 1.10 | 0.84 |
| No induction | 28.1 | 1.00 | 0.94 to 1.06 | 0.98 |
| No steroid | ||||
| Cox regressiona | ||||
| IL2-RA | 27.4 | 1 | ||
| r-ATG | 23.1 | 0.84 | 0.73 to 0.96 | <0.01 |
| Alemtuzumab | 26.2 | 0.90 | 0.78 to 1.04 | 0.15 |
| PS–weighted Cox regressionb | ||||
| IL2-RA | 27.4 | 1 | ||
| r-ATG | 23.1 | 0.86 | 0.75 to 0.99 | 0.03 |
| Alemtuzumab | 26.2 | 0.93 | 0.81 to 1.08 | 0.36 |
HR, hazard ratio; 95% CI, 95% confidence interval; IL2-RA, IL-2 receptor antagonist; r-ATG, rabbit antithymocyte globulin; PS, propensity score.
Adjusted for donor factors (sex and kidney donor profile index), recipient factors (age, sex, race, diabetes status, cardiovascular disease, panel reactive antibody, retransplant status, and dialysis exposure), transplant factors (cold ischemia time, donor-to-recipient weight ratio, HLA mismatch, and transplant year), transplant center, and the Organ Procurement and Transplantation Network region.
PS weighted and adjusted for transplant center and the Organ Procurement and Transplantation Network region.
Figure 3.
Unweighted Kaplan–Meier overall graft survival estimates in deceased donor renal transplantation (DDRT) recipients maintained on tacrolimus (TAC)/mycophenolic acid (MPA)/steroids by induction types. (A) Steroid group. (B) No-steroid group. IL2-RA, IL-2 receptor antagonist; r-ATG, rabbit antithymocyte globulin.
Acute Rejection.
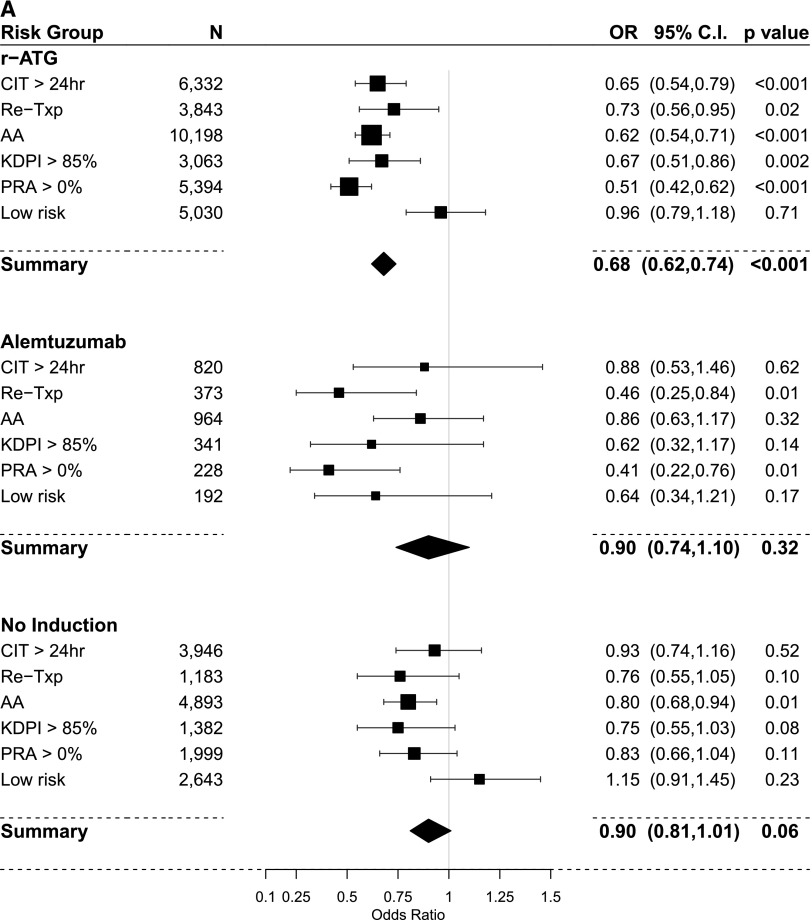
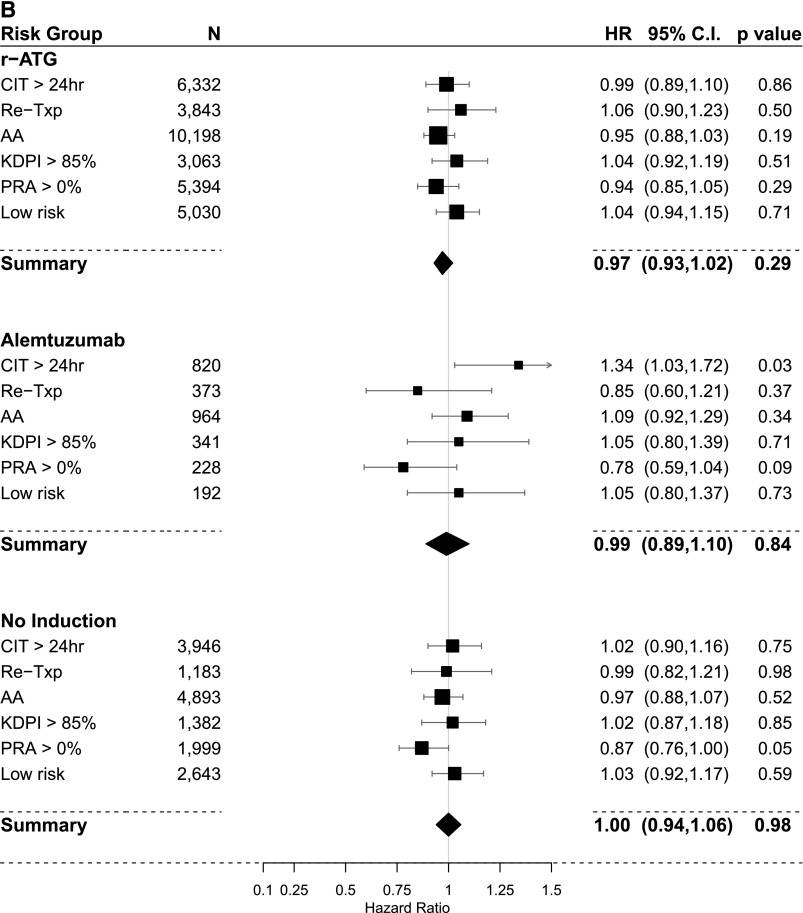
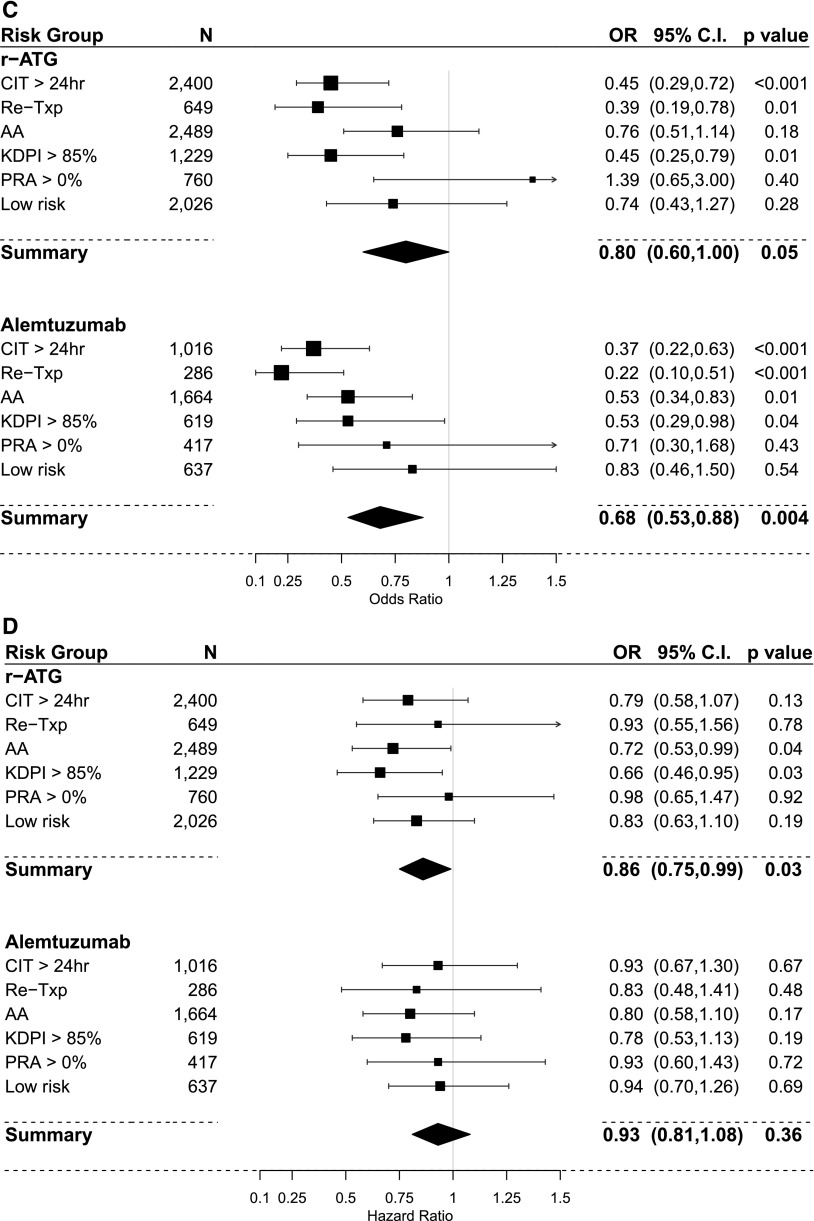
The results of PS–weighted and covariate–adjusted multivariate logistic regression models for acute rejection within 1 year post-transplant compared with IL2-RA as a default reference category are shown in Figure 4, A and C and Table 3. In the steroid group, overall r-ATG was the only induction agent associated with lower rejection rates. In subgroup analysis, r-ATG decreased the rejection rate in all high-risk categories but not in the low-risk category. However, alemtuzumab lowered acute rejection in retransplant and PRA>0% categories. In the no steroid group, overall, both r-ATG and alemtuzumab were significantly associated lower rejection rates. In subgroup analysis, both r-ATG and alemtuzumab had relatively similar effect on successfully decreasing rejection rates in high-risk categories (except the PRA>0% category) but not in the low-risk category.
Figure 4.
Comparison of the estimated association of induction treatments on acute rejection within one-year and overall graft failure (IL-2 receptor antagonist [IL2-RA] is the referent category in all models). (A) Acute rejection within one-year using propensity score (PS) weighted multivariable logistic regression models in the steroid group. (B) Overall graft failure using PS weighted multivariable Cox regression models in the steroid group. (C) Acute rejection within one-year using PS weighted multivariable logistic regression models in the no-steroid group. (D) overall graft failure using PS weighted multivariable Cox regression models in the no-steroid group. 95% CI, 95% confidence interval; AA, black; CIT, cold ischemia time; OR, odds ratio; KDPI, kidney donor profile index; PRA, panel reactive antibody; r-ATG, rabbit antithymocyte globulin; Re-Txp, re-transplant.
Overall Graft Failure.
The results of PS–weighted and covariate–adjusted multivariate Cox models for overall allograft failure compared with IL2-RA as a default reference category are shown in Figure 4, B and D and Table 4. In the steroid group, overall, we did not observe differences in graft failure risk among induction categories. In the subgroup analysis, alemtuzumab was associated with higher risk of graft failure in recipients with CIT>24 hours (HR, 1.34; 95% confidence interval [95% CI], 1.03 to 1.72). In the no steroid group, overall, r-ATG was associated with better graft survival (HR, 0.86; 95% CI, 0.75 to 0.99). Moreover, in the subgroup analysis, r-ATG offered better graft survival in recipients with KDPI>85% (HR, 0.72; 95% CI, 0.53 to 0.99; P=0.04) and recipients of black race (HR, 0.66; 95% CI, 0.46 to 0.95).
Discussion
Our study represents the largest analysis of a national registry of short–term main outcomes in contemporary DDRT recipients maintained on TAC/MPA with or without steroids. The novel use of inverse probability of treatment weight allowed us to compare induction strategies while adjusting for multiple baseline covariates in a way that we feel is superior to previously conducted meta-analyses. Similar to our recent report in LRTs (lack of better outcomes with IL2-RA compared with no induction, r-ATG, and alemtuzumab) (13), it questions current recommendations regarding routine use of IL2-RA in all patients with renal transplants (including deceased and living) suggested by the KDIGO guidelines (5). Below, we discuss currently available meta–analyses and prospective randomized clinical trials.
Steroid Maintenance
Most of the randomized, controlled trials (RCTs) comparing IL2-RA induction with no induction or lymphocyte-depleting agents have been small, low quality, and heterogeneous in terms of donor and recipient characteristics and included cyclosporin–based maintenance regimens. The KDIGO (5) recommends an induction therapy (level 1A recommendation) in all renal transplants recipients, especially in the context of steroid maintenance (preferably IL2-RA as the first-line agent; level 1B recommendation; moderate quality evidence), on the basis of a large meta-analysis (6) (n=4670) that mainly examined the studies using a cyclosporin–based maintenance immunosuppression regimen (6,7). In this meta-analysis, when IL2-RA induction was compared with placebo (no induction), IL2-RA decreased biopsy–proven acute rejection (relative risk [RR], 0.72; 95% CI, 0.64 to 0.81) and overall allograft failure (RR, 0.75; 95% CI, 0.62 to 0.90). Moreover, a subgroup analysis did not show difference in outcomes when cyclosporin and TAC maintenance regimens were compared. However, other prospective studies reported much lower rates of biopsy-proven rejection at 1 year (8%–15%) among kidney transplant recipients receiving IL2-RA induction and TAC/MPA/steroid maintenance (8,19,20). A recently published RCT with a 5-year follow-up (n=227) compared r-ATG with IL2-RA induction in high–risk DDRT recipients (mostly 75%–85% in each arm; maintained on TAC/MPA/steroid) (21). The incidence of rejection at 1 year was low (26% in IL2-RA versus 14% in r-ATG induction; P=0.04). Five-year patient and graft survival and renal function were similar between two groups.
Our findings in this study suggest that IL2-RA was not associated with lower incidence of acute rejection or better overall graft survival compared with no induction therapy in the context of steroid maintenance in DDRT. However, one should interpret this lack of benefit cautiously, because we do not know whether the IL2-RA cohort was treated in the same manner as the no induction cohort in terms of target TAC levels and MPA dosing. Interestingly, the acute rejection incidence did not differ among the recipients receiving IL2-RA and alemtuzumab inductions (one may speculate that late rejections in alemtuzumab induction might have accounted for it). Only r-ATG induction was associated with significantly lower rejection rate at 1 year, although it did not improve overall graft survival.
Steroid Avoidance
Over the last decade, early steroid avoidance has gained interest in the United States, with an aim of minimizing metabolic side effects and negative effects on quality of life. Despite this interest, data looking at minimization of steroids in DDRT are sparse. Early attempts at steroid withdrawal were associated with high rejection rates and early graft failures (22–24). This trend changed with the incorporation of antibody induction into the protocols (25–28). In the context of no steroid protocols, the current KDIGO guidelines recommend using a lymphocyte-depleting agent rather than an IL2-RA for kidney transplant recipients at higher risk for rejection (2B equals a moderate level of evidence).
A randomized, double–blinded study compared acute rejection, graft failure, and death in a cohort of 386 adult renal transplant recipients (DDRT comprising 42% of the cohort) who received antibody induction (IL2-RA versus r-ATG), TAC/MPA maintenance immunosuppression, and steroid cessation within 7 days with those receiving chronic low–dose steroids (29). There was no difference in primary end points (composite of death, graft loss, or moderate/severe acute rejection) at 5 years, but subgroup analysis showed a significantly higher rate of biopsy-proven rejection in recipients induced with IL2-RA (24.4%) compared with r-ATG (14.4%) in the no steroid group. In another steroid withdrawal trial (n=474) using a TAC/MPA maintenance regimen (DDRT comprising 40% of the cohort) (30), alemtuzumab was compared with conventional induction therapy (IL2-RA or r-ATG). The recipients were stratified on the basis of their immunologic risk (low-risk patients [n=335] being randomized to alemtuzumab or IL2-RA and high-risk patients [n=139] assigned to alemtuzumab or r-ATG). In the low-risk cohort, the incidence of rejection at 1 year was lower with alemtuzumab versus IL2-RA (3% versus 20%; P<0.001), but there was no significant difference in the high-risk cohort (10% for alemtuzumab versus 13% for r-ATG; P=0.53). Despite the differences in the low-risk cohort, there was no difference in death–censored graft survival or function at 3 years. A systematic review (four of nine RCTs using TAC/MPA; n=1,282) compared early steroid withdrawal or avoidance with conventional steroid use in renal transplant recipients who received the same antibody induction (mainly IL2-RA or r-ATG) in both arms (31). Acute rejection (risk ratio, 1.06; 95% CI, 0.79 to 1.42) and overall allograft failure risk (risk ratio, 1.29; 95% CI, 0.71 to 2.34) were comparable between both steroid groups. The authors concluded that early steroid avoidance or withdrawal is safe in renal transplant recipients receiving IL2-RA or r-ATG followed with calcineurin inhibitors and MPA maintenance regimen (31).
In our multivariable PS–weighted analysis of DDRT recipients maintained on TAC/MPA without steroids at discharge, induction with r-ATG and alemtuzumab lowered the RR of acute rejection compared with IL2-RA. Only r-ATG was significantly associated with better allograft survival, specifically in recipients receiving marginal kidneys and among those of black race. Our data support the KDIGO recommendation that, in the setting of steroid withdrawal, lymphocyte-depleting agents are more effective at decreasing risk of rejection and that r-ATG seems to be the preferable choice.
Cost and adverse event profile may change preferred induction agent of choice. Overall, IL2-RA is associated with a minimal side effect profile and may offer cost reduction on the basis of shorter hospital stay and lower rate of serious infections (7,32). Similarly, because of cost considerations, many transplant centers are currently using alemtuzumab, which is provided through the Campath Distribution Program free of charge since 2012 (withdrawn from the market and reintroduced as lemtrada for refractory multiple sclerosis) (33). In addition, alemtuzumab causes severe prolonged lymphopenia and is associated with increased late antibody–mediated rejections, opportunistic infections (30,34,35), and higher risk of malignancy (non-Hodgkin lymphoma, colorectal cancer, and thyroid cancer) (36). Adverse events, including leukopenia, thrombocytopenia, cytokine release syndrome, hypersensitivity reactions, serum sickness, and serious infections, are commonly observed with r-ATG use (37–39). A recent transplant and cancer registry reported that r-ATG was associated with melanoma (36). Clinicians need to weight the risk-to-benefit ratio for each recipient before deciding on an induction agent.
Overall, the findings of this study for DDRT recipients and the findings of our prior publication for LRT recipients (13) indicate that, in recipients on TAC/MPA/steroid therapy, incremental benefit of any induction therapy may attain small absolute risk reduction for rejection (approximately 1%–4%) without any significant graft survival benefit (especially true for low–risk DDRT recipients). However, in the setting of steroid avoidance and possibly, in recipients at high risk for rejection, induction therapy, mainly r-ATG, lowers the risk of acute rejection (an RR reduction of 25%–45% compared with IL2-RA induction) and may offer graft survival benefit in some of DDRT recipients, particularly in the steroid avoidance group. Concerns about the safety of r-ATG regarding infection and malignancy have been declining since r-ATG dosing reported in recent studies has been lower (36,40).
Strengths and Limitations of the Study
The main strengths of our study are large sample size, standardization of baseline immunosuppression, and minimization of selection bias using PS weighting. Despite these strengths, it has several limitations: (1) under-reporting of acute rejection episodes by individual transplant centers, (2) lack of data on intensity of maintenance immunosuppression (MPA dosing and TAC trough levels) among induction categories, (3) lack of information on severity of rejection and response to therapy and its effect on overall allograft survival, (4) lack of reliable information on long-term safety of lymphocyte-depleting agents regarding infection and malignancy, (5) lack of information on formation of de novo donor–specific antibody as a marker of immunologic injury resulting in antibody-mediated rejection, and (6) not capturing total exposure to steroids, alemtuzumab, and r-ATG.
We think that the role of IL2-RA in modern renal transplantation has become debatable, especially when TAC/MPA/steroids are used in low-risk DDRT recipients. IL2-RA may be inferior to r-ATG in high–risk rejection situations, such as steroid avoidance protocols, where r-ATG may also offer better graft survival.
Disclosures
B.T. has served for the advisory board meeting and on the Speaker Bureau of Alexion Pharmaceuticals, Inc., New Haven, CT. Other coauthors declare no conflict of interest.
Supplementary Material
Acknowledgments
On the basis of the Organ Procurement and Transplantation Network data as of September 30, 2013, this work was supported, in part, by Health Resources and Services Administration contract 234-2005-370011C. This research is partly supported by University of Texas Southwestern O’Brien Kidney Research Core Center grant P30DK079328.
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, and the mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13171215/-/DCSupplemental.
References
- 1.Hardinger KL, Brennan DC, Klein CL: Selection of induction therapy in kidney transplantation. Transpl Int 26: 662–672, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, Stewart DE, Cherikh WS, Wainright JL, Snyder JJ, Israni AK, Kasiske BL: OPTN/SRTR 2012 Annual Data Report: Kidney. Am J Transplant 14[Suppl 1]: 11–44, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Wiseman AC: Induction therapy in renal transplantation: Why? What agent? What dose? We may never know. Clin J Am Soc Nephrol 10: 923–925, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bia M, Adey DB, Bloom RD, Chan L, Kulkarni S, Tomlanovich S: KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kidney Dis 56: 189–218, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group: KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9[Suppl 3]: S1–S155, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Webster AC, Playford EG, Higgins G, Chapman JR, Craig J: Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst Rev 1: CD003897, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Webster AC, Ruster LP, McGee R, Matheson SL, Higgins GY, Willis NS, Chapman JR, Craig JC: Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst Rev 1: CD003897, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahsan N, Holman MJ, Jarowenko MV, Razzaque MS, Yang HC: Limited dose monoclonal IL-2R antibody induction protocol after primary kidney transplantation. Am J Transplant 2: 568–573, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Woodle ES, Peddi VR, Tomlanovich S, Mulgaonkar S, Kuo PC; TRIMS Study Investigators : A prospective, randomized, multicenter study evaluating early corticosteroid withdrawal with Thymoglobulin in living-donor kidney transplantation. Clin Transplant 24: 73–83, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Gralla J, Wiseman AC: The impact of IL2ra induction therapy in kidney transplantation using tacrolimus- and mycophenolate-based immunosuppression. Transplantation 90: 639–644, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Willoughby LM, Schnitzler MA, Brennan DC, Pinsky BW, Dzebisashvili N, Buchanan PM, Neri L, Rocca-Rey LA, Abbott KC, Lentine KL: Early outcomes of thymoglobulin and basiliximab induction in kidney transplantation: Application of statistical approaches to reduce bias in observational comparisons. Transplantation 87: 1520–1529, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rostaing L, Charpentier B, Glyda M, Rigotti P, Hettich F, Franks B, Houbiers JG, First R, Holman JM: Alefacept combined with tacrolimus, mycophenolate mofetil and steroids in de novo kidney transplantation: A randomized controlled trial. Am J Transplant 13: 1724–1733, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Tanriover B, Zhang S, MacConmara M, Gao A, Sandikci B, Ayvaci MU, Mete M, Tsapepas D, Rajora N, Mohan P, Lakhia R, Lu CY, Vazquez M: Induction therapies in live donor kidney transplantation on tacrolimus and mycophenolate with or without steroid maintenance. Clin J Am Soc Nephrol 10: 1041–1049, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS: A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index. Transplantation 88: 231–236, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, Robins JM: Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol 163: 262–270, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Spreeuwenberg MD, Bartak A, Croon MA, Hagenaars JA, Busschbach JJ, Andrea H, Twisk J, Stijnen T: The multiple propensity score as control for bias in the comparison of more than two treatment arms: An introduction from a case study in mental health. Med Care 48: 166–174, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Rubin DB, Thomas N: Matching using estimated propensity scores: Relating theory to practice. Biometrics 52: 249–264, 1996 [PubMed] [Google Scholar]
- 18.D’Agostino RB, Jr.: Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17: 2265–2281, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyó JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF; ELITE-Symphony Study : Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357: 2562–2575, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Silva HT Jr., Yang HC, Abouljoud M, Kuo PC, Wisemandle K, Bhattacharya P, Dhadda S, Holman J, Fitzsimmons W, First MR: One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients. Am J Transplant 7: 595–608, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Hellemans R, Hazzan M, Durand D, Mourad G, Lang P, Kessler M, Charpentier B, Touchard G, Berthoux F, Merville P, Ouali N, Squifflet JP, Bayle F, Wissing KM, Noël C, Abramowicz D: Daclizumab versus rabbit antithymocyte globulin in high-risk renal transplants: Five-year follow-up of a randomized study. Am J Transplant 15: 1923–1932, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Ahsan N, Hricik D, Matas A, Rose S, Tomlanovich S, Wilkinson A, Ewell M, McIntosh M, Stablein D, Hodge E; Steroid Withdrawal Study Group : Prednisone withdrawal in kidney transplant recipients on cyclosporine and mycophenolate mofetil--a prospective randomized study. Transplantation 68: 1865–1874, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Vanrenterghem Y, Lebranchu Y, Hené R, Oppenheimer F, Ekberg H: Double-blind comparison of two corticosteroid regimens plus mycophenolate mofetil and cyclosporine for prevention of acute renal allograft rejection. Transplantation 70: 1352–1359, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Kasiske BL, Chakkera HA, Louis TA, Ma JZ: A meta-analysis of immunosuppression withdrawal trials in renal transplantation. J Am Soc Nephrol 11: 1910–1917, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, Grinyo J; FREEDOM Study Group : A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplant 8: 307–316, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Woodle ES, Vincenti F, Lorber MI, Gritsch HA, Hricik D, Washburn K, Matas AJ, Gallichio M, Neylan J: A multicenter pilot study of early (4-day) steroid cessation in renal transplant recipients under simulect, tacrolimus and sirolimus. Am J Transplant 5: 157–166, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Heilman RL, Reddy KS, Mazur MJ, Moss AA, Post DJ, Petrides S, Mulligan DC: Acute rejection risk in kidney transplant recipients on steroid-avoidance immunosuppression receiving induction with either antithymocyte globulin or basiliximab. Transplant Proc 38: 1307–1313, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Ciancio G, Gaynor JJ, Guerra G, Sageshima J, Chen L, Mattiazzi A, Roth D, Kupin W, Tueros L, Flores S, Hanson L, Vianna R, Burke GW 3rd: Randomized trial of three induction antibodies in kidney transplantation: Long-term results. Transplantation 97: 1128–1138, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Van Veldhuisen P; Astellas Corticosteroid Withdrawal Study Group : A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg 248: 564–577, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, First MR, Croy R, Holman J; INTAC Study Group : Alemtuzumab induction in renal transplantation. N Engl J Med 364: 1909–1919, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Pascual J, Royuela A, Galeano C, Crespo M, Zamora J: Very early steroid withdrawal or complete avoidance for kidney transplant recipients: A systematic review. Nephrol Dial Transplant 27: 825–832, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Lilliu H, Brun-Strang C, Le Pen C, Büchler M, Al Najjar A, Priol G, Reigneau O, Lebranchu Y: Cost-minimization study comparing Simulect vs. Thymoglobulin in renal transplant induction. Clin Transplant 18: 247–253, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Gundroo A, Zachariah M, Singh N, Sharma R: Alemtuzumab (Campath-1H) experience in kidney transplantation what we have learned; current practices; and scope for the future? Curr Opin Organ Transplant 20: 638–642, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Knechtle SJ, Pirsch JDH, H Fechner J Jr., Becker BN, Friedl A, Colvin RB, Lebeck LK, Chin LT, Becker YT, Odorico JS, D’Alessandro AM, Kalayoglu M, Hamawy MM, Hu H, Bloom DD, Sollinger HW: Campath-1H induction plus rapamycin monotherapy for renal transplantation: Results of a pilot study. Am J Transplant 3: 722–730, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Peleg AY, Husain S, Kwak EJ, Silveira FP, Ndirangu M, Tran J, Shutt KA, Shapiro R, Thai N, Abu-Elmagd K, McCurry KR, Marcos A, Paterson DL: Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clin Infect Dis 44: 204–212, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Hall EC, Engels EA, Pfeiffer RM, Segev DL: Association of antibody induction immunosuppression with cancer after kidney transplantation. Transplantation 99: 1051–1057, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D; Thymoglobulin Induction Study Group : Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med 355: 1967–1977, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Tanriover B, Chuang P, Fishbach B, Helderman JH, Kizilisik T, Nylander W, Shaffer D, Langone AJ: Polyclonal antibody-induced serum sickness in renal transplant recipients: Treatment with therapeutic plasma exchange. Transplantation 80: 279–281, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Mourad G, Garrigue V, Squifflet JP, Besse T, Berthoux F, Alamartine E, Durand D, Rostaing L, Lang P, Baron C, Glotz D, Antoine C, Vialtel P, Romanet T, Lebranchu Y, Al Najjar A, Hiesse C, Potaux L, Merville P, Touraine JL, Lefrancois N, Kessler M, Renoult E, Pouteil-Noble C, Cahen R, Legendre C, Bedrossian J, Le Pogamp P, Rivalan J, Olmer M, Purgus R, Mignon F, Viron B, Charpentier B: Induction versus noninduction in renal transplant recipients with tacrolimus-based immunosuppression. Transplantation 72: 1050–1055, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Hertig A, Zuckermann A: Rabbit antithymocyte globulin induction and risk of post-transplant lymphoproliferative disease in adult and pediatric solid organ transplantation: An update. Transpl Immunol 32: 179–187, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.