Abstract
Background and objectives
Serum creatinine (SCr)–based AKI definitions have important limitations, particularly in very low-birth-weight (VLBW) neonates. Urine biomarkers may improve our ability to detect kidney damage. We assessed the association between 14 different urine biomarkers and AKI in VLBW infants.
Design, setting, participants, & measurements
We performed a prospective cohort study on 113 VLBW infants (weight ≤1200 g or <31 weeks' gestation) admitted to a regional neonatal intensive care unit at the University of Alabama at Birmingham between February 2012 and June 2013. SCr was measured on postnatal days 1, 2, 3, and 4 and was combined with clinically measured SCr to determine AKI according to Kidney Disease Improving Global Outcomes AKI definition (increase in SCr ≥0.3 mg/dl or ≥50% increase from previous lowest value). Urine was collected on the first 4 days (average number of urine collections, 3; range, 1–4). The maximum urine biomarkers and urine biomarker/creatinine levels were calculated for 12 urine biomarkers, and the minimum urine biomarker and biomarker/creatinine levels were assessed for two urine biomarkers. We compared these values between infants with and those without AKI. Ideal cutoffs, area under the receiver-operating characteristic curve , and area under the curve adjusted for gestational age were calculated.
Results
Cumulative incidence of AKI during the first 2 postnatal weeks was 28 of 113 (25%). Infants with AKI had higher maximum levels of urine cystatin C, neutrophil gelatinase-associated lipocalin, osteopontin, clusterin, and α glutathione S-transferase (2.0, 1.8, 1.7, 1.7, and 3.7 times higher, respectively) than infants without AKI. In addition, infants with AKI had lower minimum levels of epithelial growth factor and uromodulin than those without AKI (1.4 and 1.6 times lower, respectively). Most but not all participants had their maximum (or minimum) biomarker values preceding AKI. These associations remained after adjustment for gestational age.
Conclusions
Urine biomarkers measured in the first 4 days of life are associated with AKI during the first postnatal weeks. Further evaluations are necessary to determine whether these biomarkers can predict important clinical outcomes. In addition, intervention studies that use biomarkers to stratify enrollment groups are needed before bedside evaluations can be incorporated into care.
Keywords: NGAL; KIM-1; cystatin c; beta-2 microglobulin; child; acute kidney injury; biomarkers; creatinine; humans; infant, very low birth weight; intensive care units, neonatal; prospective studies
Introduction
AKI in premature infants is common and predicts poor clinical outcomes (1). Current identification of AKI relies on acute elevation of serum creatinine (SCr), but SCr-based definitions are hampered by numerous problems: most important, that SCr is a measure of function, not damage (2). In neonates, SCr-based AKI definitions present additional challenges because SCr levels on postnatal day 1 reflect maternal SCr, which declines over the next week or weeks depending on gestational age (GA) (3). In addition, acute changes in fluid status, which occur during this time period, may have an important effect on SCr values (4,5). Fortunately, urine protein biomarkers show promise of someday being able to help diagnose AKI early in the disease process by noninvasively measuring specific urine proteins (6). Improving the ability to reliably detect AKI would have important implications in the ability to care for critically ill neonates and will also improve the ability to perform clinical research.
In evaluation of urine biomarkers of AKI in neonates, it is critical to adjust for differences in GA. Prior literature has shown that in premature infants without AKI, urine proteins will be highest among those with the lowest GA, probably because of the passive loss of proteins in the context of immature tubular function (6–8). Previously, we (7) and others (8–18) have published data on the ability of urine biomarkers to predict AKI in neonates; however, these studies are limited by the size of the cohort or the use of nested case–control methods, with most of these studies evaluating only one biomarker. In addition, previous studies were subject to risk of misclassification bias, given that many infants had only a few SCr levels measured to determine AKI. This study extends our understanding of urine AKI biomarkers by prospectively collecting SCr data in the entire cohort and increasing the sample size of infants with and without AKI.
To address the limitations of prior literature, as well as to improve the understanding of urine kidney damage biomarkers in premature infants, we performed a prospective cohort analysis on 113 premature infants (birth weight<1200 g and/or GA of ≤31 weeks). We tested the hypothesis that 14 specific candidate urine biomarkers (and urine biomarker/creatinine ratios) are associated with AKI in premature infants after controlling for GA. We tested the maximum value obtained in 12 biomarkers and the minimum value in two biomarkers (uromodulin and epithelial growth factor).
Materials and Methods
Study Population
Preterm infants (birth weight [BW]≤1200 g and/or GA≤31 weeks) admitted to the regional neonatal intensive care unit at the University of Alabama at Birmingham between February 2012 and June 2013 were prospectively followed. Newborns were excluded if they had known congenital kidney disease or if they did not survive beyond the first 48 hours of life. We followed enrolled infants from the time of birth until 36 weeks postmenstrual age (PMA) or hospital discharge, whichever occurred first, as is commonly done to define mortality in premature infants. The study was approved by the University of Alabama at Birmingham Institutional Review Board, and informed parental consent was obtained. The clinical and research activities reported here are also consistent with the Principles of the Declaration of Helsinki.
Definition of Outcomes
To ascertain whether a child developed AKI within the first 2 weeks of life, we measured SCr at postnatal days 1, 2, 3, and 4, in addition to any clinically measured values from the first 2 weeks of life. SCr was measured once in four of 113 (3.5%), twice in six of 113 (5.3%), three times in ten of 113 (8.8%), and at least four times in 93 of 113 (82%) of the cohort during the 2 weeks of life (median, 5 [range, 1–14] days). Neonatal AKI was defined using the Kidney Disease Improving Global Outcomes AKI definition, in which stage 1 was defined as an increase of SCr ≥ 0.3 mg/dl or an increase in SCr by ≥50% within the first 7 days of life, stage 2 was defined as a doubling of SCr, and stage 3 was defined as tripling of SCr (19). We compared each SCr to the lowest previous SCr value to date (this was done because the postnatal day 1 SCr is related to the mother’s SCr value and decreases steadily over the first weeks of life) (3). The four infants who had only one SCr value collected were assumed to not have AKI. We did not include urine output criteria because it is often difficult to measure urine output in babies, and many premature infants with AKI are nonoliguric because of poor tubular function.
Biomarker Analysis
Urine was collected daily during the first 4 days of life using Cuddle Buns (Small Beginnings, Hesperia, CA) diapers placed at the perineum. Samples during the first 4 days of life were collected as follows: all 4 days, 60 of 113 (53%); 3 days, 25 of 113 (32%); 2 days, 13 of 113 (12%); 1 day, five of 113 (4%). The moist part of the diaper was cut out and placed in a syringe. Urine was squeezed into a centrifuge tube using the syringe plunger and centrifuged for 10 minutes. The supernatant was aliquoted and frozen at −70°C until evaluation. Urine biomarker analysis was performed on multiarray plates by electrochemiluminescence using the Sector Image 2400 (Meso Scale Discovery [MSD], Gaithersburg, MD]. Albumin, β-2-microglobulin (B2M), cystatin C, epithelial growth factor (EGF), neutrophil gelatinase-associated lipocalin (NGAL), osteopontin (OPN), and uromodulin (UMOD) were measured in urine using MSD Human Kidney Injury Panel 5 Kit assay; α glutathione S-transferase (α-GST), calbindin, clusterin, kidney injury molecule-1 (KIM-1), osteoactivin, trefoil factor 3, and vascular EGF (VEGF) were measured using MSD Human Kidney Injury Panel 3 Kit Assay. Samples for panel 5 were diluted 500-fold before they were added to the plate. Samples for panel 3 were diluted 10-fold before they were added to the plate. Samples were added to plates and prepared as stated in the manufacturer’s protocol and were analyzed on Sector Imager. All markers detected in the samples were in the range of the standard curve. The inter-run and intrarun percentages of coefficient of variance, as well as the variability of samples and standards (between-runs and in-runs) were below 10% in all cases and below 5% in most sample/standard comparisons.
Statistical Analyses
Infant and maternal demographic characteristics and intervention characteristics were compared between infants with and those without AKI using a chi-squared test (Fisher exact test when expected cell frequencies were <5) for categorical variables; a t test or Mann–Whitney U test was used for normally distributed and non-normally distributed continuous variables, respectively. Univariate statistics were computed for each biomarker of interest, and in a subsequent analysis, a Mann–Whitney U test was used to compare absolute maximum biomarker levels and urine creatinine–corrected biomarker levels (to account for concentration differences) between the AKI and non-AKI groups. Sample data were transformed to their natural logarithm (to account for non-normal distribution). To determine the biomarker threshold level that is best associated with AKI, discriminatory statistics (i.e., area under a receiver-operating characteristic curve [AUC]) were calculated for each biomarker using logistic regression adjusted for GA because of evidence in prior literature of a correlation between biomarker levels and GA (20). To determine the threshold, predicted probabilities were computed for each biomarker, and the biomarker threshold was defined as the threshold level in which the summation of sensitivity and specificity was maximized. P<0.05 was considered to indicate statistically significant differences. SAS software, version 9.3 (SAS Institute Inc., Cary, NC), was used for all statistical analyses.
Results
Bivariate Analysis
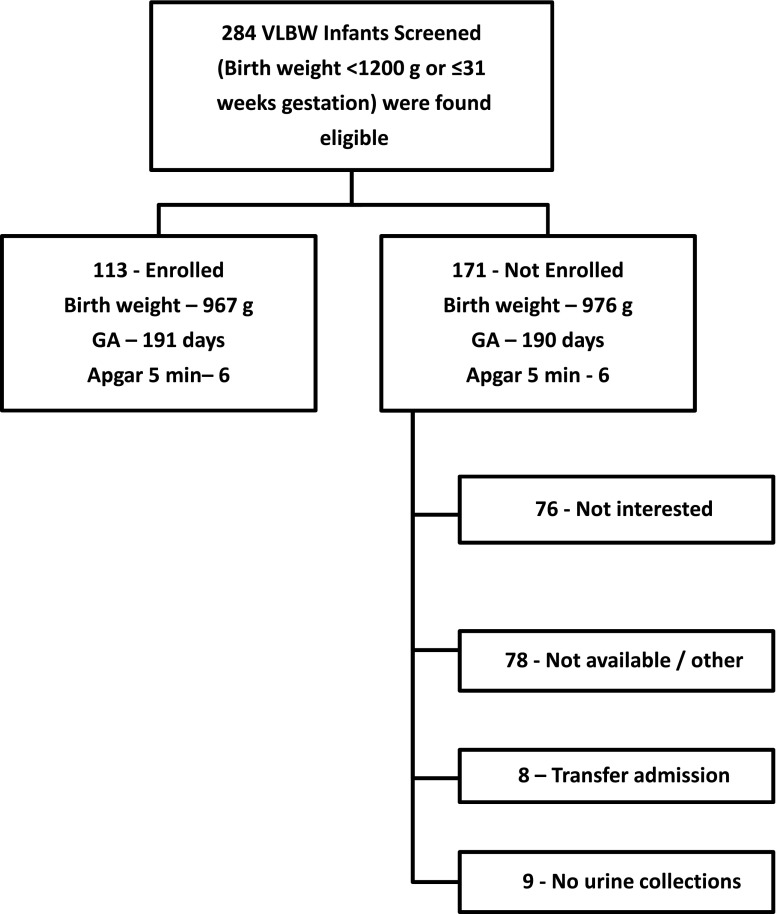
Informed consent was granted in 122 of 284 (43%) very low birth weight (VLBW) infants eligible for the study. The reasons for nonenrollment included lack of interest (n=76), lack of availability (n=78), or transfer to another hospital (n=8). During a 2-week period, nine patients did not have urine collected. We report our findings on the 113 infants whose parents consented for the study and had at least one urine collected for biomarker analysis. Overall, BW, GA, and 5-minute Apgar score did not differ between infants enrolled and those not enrolled (Figure 1).
Figure 1.
Diagram showing enrollment and reasons for non-participation for those who met inclusion and exclusion criteria for the study. GA, gestational age; VLBW, very low birth weight.
Among the 113 infants included in the study, 28 (25%) were diagnosed with AKI (25 with stage 1, one with stage 2, and two with stage 3). Thirteen infants died(11.5%). Babies with AKI had shorter birth length (32.0 cm versus 35.5 cm; P=0.02), were less likely to be born from mothers with pre-eclampsia (one of 28 [4%] versus 32 of 85 [38%]; P=0.001), and were more likely to have an umbilical artery catheter (18 of 28 [64%] versus 29 of 85 [34%]; P=0.02) (Table 1).
Table 1.
Comparison of demographic characteristics between AKI cases and controls
| Characteristic | No AKI (n=85) | AKI (n=28) | P Valuea |
|---|---|---|---|
| Infant | |||
| Birth weight, g | 1000±304 | 894±368 | 0.13 |
| GA, wk | 28.0±2 | 26.4±2 | 0.2 |
| Apgar score at 1 mina | 4 (1, 6) | 4 (2, 6) | 0.8 |
| Apgar score at 5 mina | 7 (6, 8) | 7 (5, 7) | 0.1 |
| Length, cma | 35.5 (31.5, 38) | 32 (29.5, 37) | 0.02 |
| Head circumference, cma | 25 (23, 26.5) | 23.7 (21.5, 25.5) | 0.13 |
| Male, n (%) | 45 (53) | 10 (36) | 0.8 |
| Race, n (%) | 0.36 | ||
| White | 32 (38) | 14 (50) | |
| Black | 46 (54) | 14 (50) | |
| Other | 7 (8) | 0 | |
| Maternal, n (%) | |||
| Prenatal care | 80 (94) | 26 (93) | 0.5 |
| Diabetes | 9 (11) | 3 (11) | 0.6 |
| Hypertension | 24 (28) | 9 (32) | 0.4 |
| Pre-eclampsiab | 32 (38) | 1 (4) | 0.001 |
| Smoking | 10 (12) | 4 (14) | 0.4 |
| Multiparity | 22 (26) | 14 (50) | 0.18 |
| Steroids | 78 (91) | 26 (93) | 0.3 |
| Indomethacin | 5 (7) | 5 (16) | 0.09 |
| Chorioamnionitis | 31 (36) | 15 (54) | 0.28 |
| Interventions, n (%) | |||
| Umbilical artery catheter | 29 (34) | 18(64) | 0.02 |
| Indomethacin | 31(36) | 15 (54) | 0.2 |
| Surfactants | 44 (51) | 19 (68) | 0.4 |
Values expressed with a plus/minus sign are the mean±SD. GA, gestational age.
Non-normally distributed continuous data are expressed as median (25th, 75th percentiles) and were evaluated using Mann–Whitney U test. Otherwise, normally distributed data were evaluated with t test.
Fisher exact test was used when cell number was <5. Otherwise, chi-squared tests for categorical variables were used.
Biomarker Levels Comparison
Table 2 shows the distribution of the levels of the 14 urine biomarkers assessed by percentile groups across all days in all patients. The maximum biomarker levels during the first 4 postnatal days for those with and those without AKI are shown in Table 3. Biomarkers are expressed as pg/ml. Compared with infants without AKI, those with AKI had 2.0 times higher median levels for cystatin C (18.9×105 versus 9.13×105; P=0.01), 1.8 times higher NGAL (6.23×105 versus 3.49×105; P=0.04), 1.7 times higher clusterin (4.25×105 versus 2.45×105; P=0.05), 1.7 times higher OPN (10.4×105 versus 6.14×105; P=0.01), and 3.7 times higher α-GST (9.10×102 versus 2.40×102; P=0.004). On the other hand, compared with infants without AKI, those with AKI had 1.4 times lower median EGF (3.63×103 versus 7.9×103; P=0.05) and 1.6 times lower median UMOD (2.86×105 versus 8.33×105; P=0.001) than those without AKI (Table 3).
Table 2.
Biomarker distribution of all samples among all patients (n=113)
| Biomarker | 2.5th Percentile | 25th Percentile | 50th Percentile | 75th Percentile | 97.5th Percentile |
|---|---|---|---|---|---|
| Albumin | 1.07×107 | 2.20×107 | 3.42×107 | 6.02×107 | 9.33×107 |
| B2M | 2.99×106 | 8.30×106 | 12.3×106 | 16.0×106 | 28.7×106 |
| Cystatin C | 8.60×104 | 37.1×104 | 115×104 | 217×104 | 523×104 |
| EGF | 4.70×102 | 10.1×102 | 14.2×102 | 20.5×102 | 44.6×102 |
| NGAL | 3.23×104 | 21.8×104 | 40.9×104 | 91.6×104 | 578×104 |
| OPN | 1.37×105 | 4.28×105 | 6.36×105 | 13.2×105 | 41.2×105 |
| UMOD | 3.35×105 | 10.4×105 | 16.6×105 | 23.7×105 | 57.2×105 |
| Clusterin | 7.27×104 | 17.1×104 | 27.5×104 | 46.8×104 | 152×104 |
| KIM-1 | 5.13×102 | 20.1×102 | 34.8×102 | 69.0×102 | 217×102 |
| Osteoactivin | 6.72×102 | 14.2×102 | 20.0×102 | 27.5×102 | 82 5×102 |
| TFF3 | 2.08×103 | 7.06×103 | 12.3×103 | 21.3×103 | 66.6×103 |
| VEGF | 5.87×102 | 9.39×102 | 13.9×102 | 22.7×102 | 66.4×102 |
| Calbindin | 3.11×103 | 6.29×103 | 8.72×103 | 16.2×103 | 47.5×103 |
| α-GST | 0.12×102 | 1.17×102 | 3.03×102 | 11.3×102 | 496×102 |
Values are expressed as pg/ml. B2M, β2-microglobulin; EGF, epithelial growth factor; NGAL, neutrophil gelatinase-associated lipocalin; OPN, osteopontin; UMOD, uromodulin; KIM-1, kidney injury molecule 1; TFF3, trefoil factor 3; VEGF, vascular endothelial growth factor; α-GST, α-glutathione-S-transferase.
Table 3.
Comparison of maximum biomarker levels in infants with and without AKI
| Biomarker | Fold Difference for AKI versus No AKI | Median Values (IQR) in AKI Group (n=27) | Median Values (IQR) in Group Without AKI (n=84) | P Value |
|---|---|---|---|---|
| Albumin | 3.84×107 (2.72×107, 8.63×107) | 3.13×107 (2.09×107, 5.41×107) | 0.06 | |
| B2M | 13.4×106 (9.44×106, 19.3×106) | 12.1×106 (8.20×106, 15.6×106) | 0.37 | |
| Cystatin C | 2.0× higher | 18.9×105 (4.38×105, 34.2×105) | 9.13×105 (2.76×105, 18.4×105) | 0.01 |
| EGFa | 4.68×102 (3.63×102, 8.72×102) | 7.9×102 (4.96×102, 12.0×102) | 0.05 | |
| NGAL | 1.8× higher | 6.23×105 (3.43×105, 16.7×105) | 3.49×105 (2.04×105, 8.77×105) | 0.04 |
| OPN | 1.7× higher | 10.4×105 (5.05×105, 20.8×105) | 6.14×105 (4.11×105, 10.0×105) | 0.02 |
| UMODa | 1.6× lower | 5.20×105 (2.86×105, 7.28×105) | 8.33×105 (5.62×105, 11.8×105) | 0.001 |
| Clusterin | 1.7× higher | 4.25×105 (2.15×105, 5.20×105) | 2.45×105 (1.63×105, 3.90×105) | 0.05 |
| KIM-1 | 4.46×103 (2.21×103, 6.93×103) | 3.45×103 (1.97×103, 6.77×103) | 0.75 | |
| Osteoactivin | 1.99×103 (1.19×103, 2.45×103) | 2.09×103 (1.45×103, 2.82×103) | 0.31 | |
| TFF3 | 14.9×103 (6.22×103, 21.3×103) | 12.2×103 (7.10×103, 22.3×103) | 0.83 | |
| VEGF | 14.5×102 (11.1×102, 21.0×102) | 13.5×102 (9.8×102, 24.0×102) | 0.43 | |
| Calbindin | 8.67×103 (5.70×103, 20.1×103) | 8.73×103 (6.30×103, 15.9×103) | 0.79 | |
| α-GST | 3.7× higher | 91.0×101 (19.1×101, 400×101) | 24.0×101 (8.49×101, 95.1×101) | 0.004 |
Unless otherwise noted, values are maximum biomarker levels. Biomarker values are expressed as pg/ml. IQR, interquartile range; B2M, β2-microglobulin; EGF, epithelial growth factor; NGAL, neutrophil gelatinase-associated lipocalin; OPN, osteopontin; UMOD, uromodulin; KIM-1, kidney injury molecule 1; TFF3, trefoil factor 3; VEGF, vascular endothelial growth factor; α-GST, α-glutathione-S-transferase.
Minimum values.
The maximum biomarker/creatinine (Cr) levels during the first 4 postnatal days for infants with and those without AKI are shown in Table 4. These values are expressed as pg/mg creatinine. Compared with those without AKI, those with AKI had 1.7 times higher median albumin/Cr (4.25×108 versus 2.40×108; P=0.01), 1.6 times higher median B2M/Cr (16.0×107 versus 9.93×107; P=0.01), 2.8 times higher median cystatin C/Cr (20.7×106 versus 7.23×106; P=0.002). 1.9 times higher median NGAL/Cr (6.11×106 versus 3.17×106; P=0.003), 1.7 higher median OPN/Cr (7.75×106 versus 4.64×106; P=0.003), 2.0 times higher median clusterin/Cr (3.45×106 versus 1.69×106; P=0.004), 1.6 times higher VEGF/Cr (1.83×104 versus 1.13×104; P=0.03), and 4.6 times higher median α-GST/Cr (10.3×103 versus 2.21×103; P<0.001). In addition, compared with infants without AKI, those with AKI had 1.3 times lower UMOD/creatinine (7.8×106 versus 6.0×106; P=0.02) (Table 4). Controlling for urine creatinine yielded statistically significant differences in three additional biomarkers (albumin, B2M, and VEGF), whereas differences in EGF were no longer statistically significant.
Table 4.
Differences in biomarker/urine creatinine in infants with and without AKI
| Biomarker | Fold Difference for AKI versus No AKI | Median Values (IQR) in AKI Group (n=27) | Median Values (IQR) in Group Without AKI (n=84) | P Value |
|---|---|---|---|---|
| Albumin | 1.7× higher | 4.25×108 (2.28×108, 9.62×108) | 2.40×108 (1.54×108, 4.46×108) | 0.01 |
| B2M | 1.6× higher | 16.0×107 (10.3×107, 23.1×107) | 9.93×107 (6.63×107, 15.4×107) | 0.01 |
| Cystatin C | 2.8× higher | 20.7×106 (4.42×106, 36.3×106) | 7.23×106 (2.43×106, 16.8×106) | 0.002 |
| EGFa | 6.9×102 (4.44×102, 9.1×102) | 7.5×102 (5.4×102, 9.9×102) | 0.2 | |
| NGAL | 1.9× higher | 6.11×106 (3.38×106, 18.9×106) | 3.17×106 (1.59×106, 6.08×106) | 0.003 |
| OPN | 1.7× higher | 7.75×106 (4.91×106, 18.3×106) | 4.64×106 (3.04×106, 6.98×106) | 0.001 |
| UMODa | 1.3× lower | 6.0×106 (4.40×106, 8.7×106) | 7.8×106 (6.1×106, 10.0×106) | 0.02 |
| Clusterin | 2.0× higher | 3.45×106 (2.14×106, 6.04×106) | 1.69×106 (1.14×106, 3.34×106) | 0.004 |
| KIM-1 | 3.59×104 (1.91×104, 7.71×104) | 2.73×104 (1.71×104, 5.09×104) | 0.25 | |
| Osteoactivin | 1.86×104 (1.08×104, 2.67×104) | 1.56×104 (1.20×104, 1.90×104) | 0.43 | |
| TFF3 | 10.2×104 (6.99×104, 24.9×104) | 10.0×104 (5.84×104, 16.2×104) | 0.30 | |
| VEGF | 1.6× higher | 18.3×103 (9.95×103, 21.7×103) | 11.3×103 (6.95×103, 18.2×103) | 0.03 |
| Calbindin | 8.90×104 (5.49×104, 16.1×104) | 6.97×104 (4.94×104, 11.5×104) | 0.21 | |
| α-GST | 4.6× higher | 103×102 (23.6×102, 518×102) | 22.1×102 (7.91×102, 95.4×102) | <0.001 |
Unless otherwise noted, values are maximum biomarker levels. Biomarker values are expressed as pg/mg creatinine. IQR, interquartile range; B2M, β2-microglobulin; EGF, epithelial growth factor; NGAL, neutrophil gelatinase-associated lipocalin; OPN, osteopontin; UMOD, uromodulin; KIM-1, kidney injury molecule 1; TFF3, trefoil factor 3; VEGF, vascular endothelial growth factor; α-GST, α-glutathione-S-transferase.
Minimum values.
Association of Biomarkers and AKI
Of the biomarkers that were significantly different between patients with and without AKI, all had fair discriminative capability (Table 5). In particular, UMOD had the highest AUC (AUC, 0.71), followed by α-GST (AUC, 0.68) and EGF (AUC, 0.68). The biomarkers with the lowest discriminative ability were clusterin (AUC, 0.62) and albumin (AUC, 0.62). Combined with GA in the logistic model, UMOD remained the biomarker with the highest discriminative capability (AUC, 0.73), followed by EGF (AUC, 0.70). The remaining biomarkers had similar AUCs. After correcting for urine creatinine and combined with GA, similar AUCs were observed, although cystatin C had a slightly better discriminative capability (AUC, 0.70) (Table 6).
Table 5.
Performance for biomarker values as potential predictors of AKI
| Biomarker | Ideal Crude Cutoff | Crude AUCa | GA-Adjusted AUCa |
|---|---|---|---|
| Albumin | 3.69×107 | 0.62 | 0.68 |
| Cystatin C | 1.26×106 | 0.65 | 0.68 |
| EGFb | 5.9×102 | 0.68 | 0.70 |
| NGAL | 4.5×105 | 0.63 | 0.67 |
| OPN | 7.12×105 | 0.65 | 0.67 |
| UMODb | 6.7×105 | 0.71 | 0.73 |
| Clusterin | 3.1×105 | 0.62 | 0.67 |
| α-GST | 4.33×102 | 0.68 | 0.68 |
Unless otherwise noted, values are maximum biomarker levels. Biomarker values are expressed as pg/ml. AUC, area under the curve; GA, gestational age; EGF, epithelial growth factor; NGAL, neutrophil gelatinase-associated lipocalin; OPN, osteopontin; UMOD, uromodulin; α-GST, α-glutathione-S-transferase.
Log-transformed before analysis to normalize the distribution.
Minimum values.
Table 6.
Performance for biomarker value/urine creatinine as potential predictors of AKI
| Biomarker | Ideal Crude Cutoff | Crude AUCa | GA-Adjusted AUCa |
|---|---|---|---|
| Albumin | 3.14×108 | 0.67 | 0.67 |
| B2M | 1.2×108 | 0.67 | 0.67 |
| Cystatin C | 1.1×107 | 0.69 | 0.70 |
| NGAL | 4.8×106 | 0.69 | 0.67 |
| OPN | 5.7×106 | 0.71 | 0.69 |
| UMODb | 6.9×106 | 0.64 | 0.68 |
| Clusterin | 2.4×106 | 0.68 | 0.66 |
| VEGF | 1.2×104 | 0.64 | 0.68 |
| α-GST | 4.5×103 | 0.71 | 0.67 |
Unless otherwise noted, values are maximum biomarker levels. Biomarker values are expressed as pg/mg creatinine. AUC, area under the curve; GA, gestational age; β2-microglobulin; NGAL, neutrophil gelatinase-associated lipocalin; OPN, osteopontin; UMOD, uromodulin; VEGF, vascular endothelial growth factor; α-GST, α glutathione-S-transferase.
Log-transformed before analysis to normalize the distribution.
Minimum values.
Timing of Occurrence of Maximum/Minimum Biomarker Levels
AKI diagnosis occurred at a median age of 4 days (range, 2–5; mean, 3.4; SD, 1.1). Maximum biomarker values (or minimum for UMOD and EGF) occurred before the diagnosis of AKI by SCr criteria in 44%–66% of infants for any given biomarker (Table 7). This percentage was even higher (78%–85% for any given biomarker) when the timing of biomarker maximum/minimum on the day of AKI diagnosis or the day before diagnosis was evaluated (Table 7).
Table 7.
Timing of occurrence of maximum biomarker before AKI diagnosis by serum creatinine criteria
| Biomarker | Median Time (Range), d | Mean Time±SD, d | Presented Maximum (or Minimuma) Values Any Time Before AKI Diagnosis, n/n (%) | Presented Maximum (or Minimuma) Values on, or the Day Before, AKI Diagnosis, n/n (%) |
|---|---|---|---|---|
| Albumin | 1 (−4, 2) | 0.8±1.6 | 15/27 (56) | 21/27 (78) |
| B2M | 1 (−4, 2) | 1.4±1.7 | 18/27 (66) | 21/27 (78) |
| Calbindin | 0 (−4, 2) | 0.8±1.6 | 12/27 (44) | 22/27 (81) |
| Clusterin | 0 (−4, 2) | 0.5±1.4 | 15/27 (56) | 22/27 (81) |
| Cystatin C | 1 (−4, 1) | 0.8±1.4 | 13/27 (48) | 22/27 (81) |
| EGFa | 1 (−4, 2) | 1.3±1.7 | 12/27 (44) | 22/27 (81) |
| α-GST | 1 (−4, 1) | 0.9±1.4 | 14/27 (52) | 21/27 (78) |
| KIM-1 | 1 (−4, 2) | 0.9±1.5 | 16/27 (59) | 23/27 (85) |
| NGAL | 0 (−4, 1) | 0.7±1.6 | 12/27 (44) | 21/27 (78) |
| OPN | 1 (−3, 2) | 0.7±1.2 | 15/27 (56) | 23/27 (85) |
| Osteoactivin | 0 (−3, 1) | 0.7±1.5 | 13/27 (38) | 21/27 (78) |
| TFF3 | 0 (−3, 2) | 0.6±1.4 | 12/27 (44) | 21/27 (78) |
| VEGF | 1 (−3, 1) | 0.7±1.5 | 14/27 (52) | 21/27 (78) |
| UMODa | 1 (−4, 2) | 1.3±1.8 | 19/27 (70) | 22/27 (81) |
Day of AKI diagnosis: median, 4; range, 2–5; mean, 3.4; SD, 1.1. Unless otherwise noted, values are maximum biomarker levels. B2M, β2-microglobulin; EGF, epithelial growth factor; α-GST, α-glutathione-S-transferase; KIM-1, kidney injury molecule 1; NGAL, neutrophil gelatinase-associated lipocalin; OPN, osteopontin; TFF3, trefoil factor 3; VEGF, vascular endothelial growth factor; UMOD, uromodulin.
Minimum values.
Discussion
The maximum values of urine biomarkers in the first 4 postnatal days of life were 1.7–3.7 times higher in infants with AKI than those without AKI for cystatin C, NGAL, OPN, clusterin, and α-GST. The minimum EGF and UMOD values were 1.4–1.6 times lower in those with AKI compared with those without AKI. Furthermore, after adjustment for urine creatinine, additional differences were detected for B2M and VEGF, whereas EGF was no longer significantly different. Overall there were moderate performance profiles (with AUC of approximately 0.65–0.7) for most of the biomarkers. These findings enhance our understanding of the potential role of novel urine AKI biomarkers in premature infants.
We have previously reported that urine NGAL, OPN, cystatin C, and B2M levels were higher in those with AKI than those without AKI (7). This study confirms those findings in a larger cohort and, together with our previous findings, provide insights into how AKI affects urine biomarker levels in this population. There were some discrepancies in study design between the present study and our previous published work (7), which may yield differing results. Our previous study had a significantly smaller sample size, with only nine AKI participants in the analysis. In addition, the present study captured more SCr values, minimizing the possibility of misclassification bias. Also, this study evaluated AKI only during the first weeks of life.
Other previous studies that explored the role of AKI biomarkers have suggested that urine biomarkers may be useful candidate biomarkers in premature infants (8–18,21). For example, Sarafidis et al. (10) showed that urine NGAL levels on day 0 of life were higher in those who develop AKI (n=9) by SCr criteira compared with GA-matched controls. Similarly, Tabel et al. (11) showed that urine NGAL levels were independently associated with AKI (n= 6) after adjustment for GA, birthweight, sex, and 1-minute Apgar scores.
Others have also suggested that urine KIM-1 can be an early marker of AKI in premature infants (8–12). For reasons that are not entirely clear, urine KIM-1 was not significantly associated with elevation of SCr. This could be due to differences in tubular development in premature infants, or the reasons for elevation of SCr could have been different in our cohort compared with others.
When exploring the timing of changes in biomarkers in relation to AKI, we found that most infants had maximum/minimum biomarkers values before the diagnosis of AKI. There are several possible explanations of why not all infants had their highest (or lowest) biomarkers before AKI diagnosis. First, it’s possible that the biomarkers continue to rise (or drop) even after a SCr-based diagnosis is made. Second, the limitations of SCr-based AKI definitions (which functioned as our gold standard for AKI in this study) may be causing this effect.
The baseline urine biomarkers of this cohort overall are much higher than the biomarkers in other cohorts. This is likely due to poor tubular function in premature infants. We have previously shown that in infants without AKI, these biomarkers vary by GA. Therefore, this variation needs to be accounted for (as we did in the present study by adjusting for GA in our analysis) in order to make proper inferences.
Interestingly, we found that urine albumin, B2M, and VEGF were not statistically significant when we looked at the biomarkers alone, but they became statistically significantly different when we controlled for urine creatinine. The main reason to control for urine creatinine is to adjust for differences in the dilution of the samples. If two infants had similar amount of biomarker excretion, but one infant produced twice the urine volume of the other, the first infant’s “diluted” urine would make it appear that the amount of biomarker excreted was lower compared with that of the second infant. Controlling for urine concentration allows the investigator to evaluate the biomarker amount more accurately by controlling for dilution of the sample. Alternatively, urine creatinine may differ almost two-fold in premature infants weighting 500 g versus those weighing 1000 g as a direct proportion to muscle mass. Overall, AUC improved slightly for both the crude and adjusted analyses after adjustment for urine creatinine. Additional studies in larger cohorts are needed to better understand these relationships and determine whether correction for dilution status with creatinine (or perhaps other markers) may help optimize these candidate biomarkers. Moreover, it is possible that controlling with other markers (which could help determine developmental stage) could be also useful.
We have previously reported a protective association between AKI and pre-eclampsia in premature infants (22), and here we show again this important association. There are several possible reasons for this association. It is possible that pre-eclampsia could alter the physiology of the premature kidney in such manner that reduces its susceptibility to develop AKI. For example, pre-eclampsia could cause sequential or continuous episodes of ischemia preconditioning, which helps protect against AKI in some human studies (23). Alternatively, the indication for the delivery of a premature infant may differ between those whose mothers had pre-eclampsia and those who are delivered because of other health issues, such as perinatal infection.
The strengths of our study include the relatively large number of infants with AKI (in comparison to other studies of urine biomarkers in premature infants), as well as the fact that each screened infant had ample SCr measurements to determine AKI status. In addition, we have assessed a broad number of candidate urine biomarkers for AKI. Despite these strengths, we also acknowledge several important limitations, which could provide insights as to why the urine biomarker values were not more robust (with AUC consistently around 0.65–0.7). The main potential limitation of this study is that it relies on a rise in SCr to define AKI as a gold standard definition for AKI. We have used the most updated neonatal AKI definition, but it has not been validated against hard clinical endpoints. Thus, it is possible that we have identified as AKI some cases with changes in SCr that may not be due to true renal injury. In addition, it is very possible that the SCr in many infants rises (i.e., functional change) without producing true damage. This is true for any population, and perhaps even truer in this cohort, as the changes in SCr during the first week of life can occur for various reasons besides true kidney injury. Abrupt changes in fluid status (premature infants can lose 15% of their weight in the first week of life) may be causing changes in SCr, without real kidney injury. Finally, we assumed that the four infants with only one SCr measurement did not have AKI.
Future larger cohort studies are needed to solve some of the limitations of our study. Although we adjusted for gestational age, we acknowledge that we were unable to control for all contributing factors that could be associated with both AKI and biomarker values. In addition, the sample size does not allow us to closely evaluate how the trends in the biomarkers may differ in those with true damage versus controls. Although maximum biomarker levels appeared on (or before) the day of AKI diagnosis in our study, future studies are needed to assess the rise/reduction of these biomarkers in the context of confirmed tubular injury.
Future studies with larger cohorts are underway and may close the critical gaps in our understanding of the use of urine biomarkers in premature infants. First, before we can incorporate urine biomarkers into clinical practice, the scientific community needs to show that candidate biomarkers predict significant clinical outcomes, including the development of CKD. Because only 13 infants in our cohort died, we were underpowered to evaluate this association. Second, future studies must also show that interventions guided by urine biomarkers can affect clinical outcomes. Third, studies that include only infants who are at high risk for AKI (as opposed to testing all infants) will likely improve the precision of these tests. Finally, studies to identify the cause of the rise in SCr (i.e., patients who have a transient versus sustained rise) are also needed. Such studies could help to shed light on the challenging task of identifying infants at risk of AKI and will have critical importance in early diagnosis, which can drive clinical change and test interventions in this vulnerable population.
In conclusion, we expanded our understanding of the normative values and changes in biomarker levels between infants with and those without AKI, using the current neonatal AKI definition. Larger well designed studies that can stratify patients at risk, determine the cause of rising SCr, and evaluate clinical outcomes (including CKD) are needed to help validate the use of urine biomarkers in clinical care.
Disclosures
The authors declare no real or perceived conflicts of interest that could affect the study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit for publication. D.J.A. is a speaker for the AKI Foundation, Baxter, and BTG.
Acknowledgments
The authors wish to thank Susan Keeling at University of Alabama at Birmingham Department of Pediatrics for her help with the administrative set-up of the study. We also thank all the neonatal nurses for their assistance in collecting samples. Emma Perez-Costas (Department of Pediatrics, University of Alabama at Birmingham) performed the technical editing and proofreading of this manuscript.
Research reported in this publication was supported by the Norman Siegel Career Development Award from the American Society of Nephrology. D.J.A. also receives funding from the National Institutes of Health (R01 DK13608-01) and the Pediatric and Infant Center for Acute Nephrology, which is sponsored by Children’s of Alabama and the University of Alabama at Birmingham School of Medicine, as well as by the Department of Pediatrics and Center for Clinical and Translational Science under award number UL1TR00165. N.A. receives funding from National Institutes of Health(grant numbers U01 HL122626, R01 HD067126, R01 HD066982, U10 HD34216). R.G. receives funding from University of Alabama at Birmingham Center for Clinical and Translational Science and Pediatric and Infant Center for Acute Nephrology.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, Kent AL: Neonatal acute kidney injury. Pediatrics 136: e463–e473, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Askenazi DJ, Zappittelli M: The role of biomarkers in the management of acute kidney injury. Clin Lab Int 32: 16–18, 2009 [Google Scholar]
- 3.Jetton JG, Askenazi DJ: Update on acute kidney injury in the neonate. Curr Opin Pediatr 24: 191–196, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basu RK, Wong HR, Krawczeski CD, Wheeler DS, Manning PB, Chawla LS, Devarajan P, Goldstein SL: Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol 64: 2753–2762, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu KD, Thompson BT, Ancukiewicz M, Steingrub JS, Douglas IS, Matthay MA, Wright P, Peterson MW, Rock P, Hyzy RC, Anzueto A, Truwit JD; National Institutes of Health National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network : Acute kidney injury in patients with acute lung injury: Impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med 39: 2665–2671, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeidi B, Koralkar R, Griffin RL, Halloran B, Ambalavanan N, Askenazi DJ: Impact of gestational age, sex, and postnatal age on urine biomarkers in premature neonates. Pediatr Nephrol 30: 2037–2044, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Askenazi DJ, Montesanti A, Hunley H, Koralkar R, Pawar P, Shuaib F, Liwo A, Devarajan P, Ambalavanan N: Urine biomarkers predict acute kidney injury and mortality in very low birth weight infants. J Pediatr 159: 907–12.e1, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genc G, Ozkaya O, Avci B, Aygun C, Kucukoduk S: Kidney injury molecule-1 as a promising biomarker for acute kidney injury in premature babies. Am J Perinatol 30: 245–252, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Lavery AP, Meinzen-Derr JK, Anderson E, Ma Q, Bennett MR, Devarajan P, Schibler KR: Urinary NGAL in premature infants. Pediatr Res 64: 423–428, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Sarafidis K, Tsepkentzi E, Diamanti E, Agakidou E, Taparkou A, Soubasi V, Papachristou F, Drossou V: Urine neutrophil gelatinase-associated lipocalin to predict acute kidney injury in preterm neonates. A pilot study. Pediatr Nephrol 29: 305–310, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Tabel Y, Elmas A, Ipek S, Karadag A, Elmas O, Ozyalin F: Urinary neutrophil gelatinase-associated lipocalin as an early biomarker for prediction of acute kidney injury in preterm infants. Am J Perinatol 31: 167–174, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Genc G, Avci B, Aygun C, Ozkaya O, Kucukoduk S: Urinary neutrophil gelatinase-associated lipocalin in septic preterm babies: A preliminary study. Am J Perinatol 30: 655–660, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Gupta C, Massaro AN, Ray PE: A new approach to define acute kidney injury in term newborns with hypoxic ischemic encephalopathy. Pediatr Nephrol 31: 1167–1178, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CN, Chou CH, Jeng SF, Tsai IJ, Chen PC, Chen CY, Chou HC, Tsao PN, Hsieh WS: Urinary neutrophil gelatinase-associated lipocalin levels in neonates. Pediatr Neonatol 57: 207–212, 2016 [DOI] [PubMed]
- 15.Pejović B, Erić-Marinković J, Pejović M, Kotur-Stevuljević J, Peco-Antić A: Detection of acute kidney injury in premature asphyxiated neonates by serum neutrophil gelatinase-associated lipocalin (sNGAL)--sensitivity and specificity of a potential new biomarker. Biochem Med (Zagreb) 25: 450–459, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suchojad A, Tarko A, Smertka M, Majcherczyk M, Brzozowska A, Wroblewska J, Maruniak-Chudek I: Factors limiting usefulness of serum and urinary NGAL as a marker of acute kidney injury in preterm newborns. Ren Fail 37: 439–445, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Smertka M, Wroblewska J, Suchojad A, Majchercyk M, Jadamus-Niebroj D, Owsianka-Podlensny T, Brzozowska A, Maruniak-Chudek I: Serum and urinary NGAL in septic newborns. BioMed Res Int 717318: 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman SB, Massaro AN, Soler-García AA, Perazzo S, Ray PE: A novel urinary biomarker profile to identify acute kidney injury (AKI) in critically ill neonates: A pilot study. Pediatr Nephrol 28: 2179–2188, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group : Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit Care 17: 204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Askenazi DJ, Koralkar R, Levitan EB, Goldstein SL, Devarajan P, Khandrika S, Mehta RL, Ambalavanan N: Baseline values of candidate urine acute kidney injury biomarkers vary by gestational age in premature infants. Pediatr Res 70: 302–306, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McWilliam SJ, Antoine DJ, Sabbisetti V, Turner MA, Farragher T, Bonventre JV, Park BK, Smyth RL, Pirmohamed M: Mechanism-based urinary biomarkers to identify the potential for aminoglycoside-induced nephrotoxicity in premature neonates: A proof-of-concept study. PLoS One 7: e43809, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koralkar R, Ambalavanan N, Levitan EB, McGwin G, Goldstein S, Askenazi D: Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res 69: 354–358, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, Haeberle H, Görlich D, Kellum JA, Meersch M; RenalRIPC Investigators : Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: A randomized clinical trial. JAMA 313: 2133–2141, 2015 [DOI] [PubMed] [Google Scholar]