Abstract
Background and objectives
In the general population, the presence of cerebral microbleeds on T2*–weighted magnetic resonance imaging has been reported to be a predictor of future stroke. Patients with CKD have a high prevalence of microbleeds and are at higher risk of ESRD as well as cardiovascular disease, including stroke. Because endothelial dysfunction is the common pathophysiology among microbleeds, CKD, and cardiovascular disease, we hypothesized that the presence of microbleeds would be an important predictor of composite outcome, including both cardiovascular disease and renal events, in those with CKD.
Design, settings, participants, & measurements
This was a prospective cohort study of 404 patients with CKD who underwent T2*–weighted magnetic resonance imaging for this study between January of 2008 and January of 2011. The primary outcome was composite of cardiovascular and renal outcomes. Cardiovascular outcomes included cardiovascular death, the new onset of myocardial infarction, coronary revascularization, stroke, and amputation/revascularization because of peripheral artery disease. Renal outcomes included doubling of the serum creatinine level and development of ESRD requiring dialysis or transplantation.
Results
At baseline, microbleeds were present in 83 (20.5%) patients. During the follow-up median period of 2.3 years, 124 of the 404 patients experienced the composite outcome. The presence of microbleeds was associated with higher risk for the composite outcome in an unadjusted Cox model, and it remained significant after adjustment for age, sex, diabetes, and systolic BP (hazard ratio [HR], 2.58; 95% confidence interval [95% CI], 1.68 to 3.46 for composite outcome; hazard ratio, 2.41; 95% CI, 1.55 to 3.77 for renal outcome; hazard ratio, 3.46; 95% CI, 1.62 to 7.43 for cardiovascular disease outcome).
Conclusions
In patients with CKD, the presence of microbleeds is a novel and independent predictor of both renal and cardiovascular disease end points.
Keywords: chronic renal disease; cardiovascular disease; microbleeds; magnetic resonance imaging; cerebrovascular disorders; blood pressure; Cohort Studies; Humans; Kidney Failure, Chronic; Peripheral Arterial Disease; Renal Insufficiency, Chronic; Stroke
Introduction
A strong relationship between CKD and cardiovascular disease (CVD) has been reported (1,2). CKD has been shown to be an independent risk factor for CVD, and CKD is associated with adverse outcomes in those with existing CVD (3,4). CKD classification is on the basis of composite outcome, including kidney outcome and CVD risk (1). Recently, several reports have shown that CKD is associated with a high prevalence of stroke, a cerebrovascular disease (5,6).
Cerebral microbleeds (MBs), focal deposits of hemosiderin in the brain, are induced by previous leakage of bleeding from small vessels. The findings suggest that the blood vessels are prone to bleed in an advanced stage of microangiopathy. Indeed, a high prevalence of MBs has been reported in patients with stroke or CVD (7).
Previously, we showed that decreased renal function was a significant factor for MBs in predialysis CKD, independent of the presence of hypertension (8). Interestingly, the prevalence of MBs increased in accordance with the increased severity of CKD stage. Recently, Laible et al. (9) reported that impaired renal function was a factor independently associated with the presence of MBs. The significance of MBs for CKD has not been elucidated. In this study, whether the presence of MBs could be a predictor for composite outcome was investigated in patients with predialysis CKD.
Materials and Methods
Study Population and Protocol
This was a prospective cohort study. All patients in this study were screened from patients with CKD admitted to Osaka City University Hospital or Ohno Memorial Hospital for education, evaluation of complications, and/or treatment for CKD, including elective dialysis initiation, from January of 2008 to January of 2011. CKD was diagnosed by the criteria proposed by the Japanese Society of Nephrology (10) that were on the basis of the definition of CKD by the Kidney Disease Improving Global Outcome (KDIGO) and the eGFR by the equation for Japanese people (11). Patients were not solicited to participate to the study if they were being hospitalized for acute care for unstable conditions, including AKI, febrile diseases, and emergent initiation of dialysis.
This study protocol was developed in accordance with the Declaration of Helsinki and approved by the ethics review committee of Osaka City University Hospital (no. 1415); informed consent was obtained from all patients before their participation in this study. This study was registered with the University Hospital Medical Information Network (UMIN) Clinical Trial Registry as accepted by the International Committee of Medical Journal Editors (UMIN identification no. 000009783).
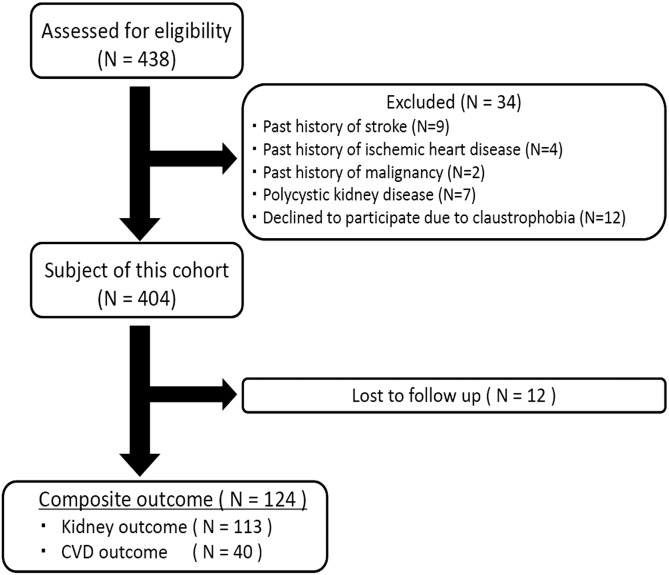
In total, 438 consecutive patients with predialysis CKD were assessed for eligibility. Patients with rapidly progressive GN, polycystic kidney disease, postrenal failure, and malignancy were excluded from this investigation, and 34 patients were excluded because of a past history of stroke (n=9), ischemic heart disease (n=4), malignancy (n=2), or polycystic kidney disease (n=7); 12 declined to participate because of claustrophobia. In total, 404 patients enrolled in this study (Figure 1). These patients were prospectively followed until May of 2012 at intervals of 1–6 months after baseline studies.
Figure 1.
Patient flow diagram. Composite outcome is stroke, cardiovascular event (ischemic heart disease or peripheral arterial disease), doubling of serum creatinine, and development of ESRD. Cardiovascular disease (CVD) outcome is stroke, ischemic heart disease, and peripheral arterial disease. Kidney outcome is doubling of serum creatinine and development of ESRD.
The primary outcome was the composite of CVD outcomes and renal outcomes. The CVD outcomes included cardiovascular death, the new onset of myocardial infarction, coronary revascularization, stroke, and amputation/revascularization because of peripheral arterial event or death. Renal outcomes included doubling of the serum creatinine level and development of ESRD requiring dialysis or transplantation.
We reviewed the medical records for potential events. The cardiovascular events were on the basis of the diagnosis by the attending physicians who did not know the baseline magnetic resonance imaging (MRI) findings. Initiation of dialysis/renal transplantation and decline of eGFR were on the basis of the records of such events and laboratory tests. We did not perform central adjudication of the outcomes for this observational cohort study.
Measurements and Definitions
CKD was defined as the presence of kidney damage as reported by the KDIGO. The baseline characteristics, including a self-administered questionnaire covering medical history and medication completed by the patients, were evaluated when MRI was performed. BP was measured with the patients resting in a supine position for at least 10 minutes on the day of MRI using a standard mercury sphygmomanometer and cuffs appropriate to their arm circumference. In this study, hypertension was defined by (1) administration of antihypertensive agents or a history of hypertension, (2) systolic BP ≥140 mmHg, or (3) diastolic BP ≥90 mmHg. Diabetes mellitus was defined by (1) administration of insulin or oral antidiabetic agents or (2) prior diagnosis according to the Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus of the American Diabetes Association (12). Blood samples were obtained from patients after overnight fasting. Total protein, serum albumin, total cholesterol, triglyceride, LDL cholesterol, HDL cholesterol, BUN, C-reactive protein, and hemoglobin levels were measured by routine laboratory methods.
The eGFR was calculated using the following formula: eGFR (milliliters per minute per 1.73 m2) =194× (serum creatinine)−1.084× (age)−0.287. The value was multiplied by 0.739 for women. This formula represents the eGFR for Japanese people reported by the Japanese Society of Nephrology (11). The eGFR values were stratified into the following ranges according to the KDIGO classification and staging system (13): ≥90 ml/min per 1.73 m2 (stage G1), 60–89 ml/min per 1.73 m2 (stage G2), 30–59 ml/min per 1.73 m2 (stage G3), 15–29 ml/min per 1.73 m2 (stage G4), and <15 ml/min per 1.73 m2 (stage G5).
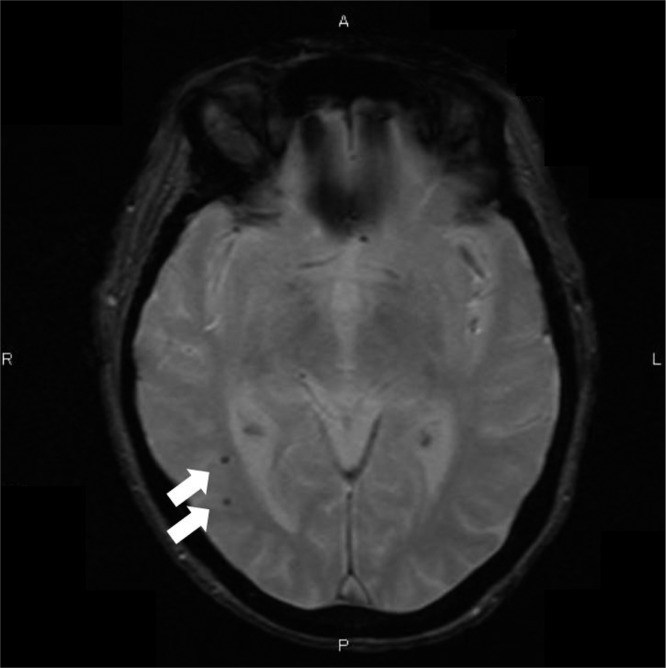
All participating patients underwent a brain MRI that used a superconducting magnet at a field strength of 1.5 T on proton density, T1–weighted fluid-attenuated inversion recovery images, and two–dimensional T2*–weighted MRI in axial planes with 5-mm-thick slices and an interslice gap of 1.5 mm without intravenous administration of paramagnetic substances. MBs (Figure 2) were defined as focal areas of signal loss on T2*-weighted images without a history of stroke or transient ischemic attack. The MRI images were assessed independently by two trained physicians who were not aware of the clinical information. In cases of discordance, although rare, the two raters discussed until they reached consensus.
Figure 2.
Cerebral microbleeds (MBs) examined by T2*–weighted magnetic resonance imaging. T2*–weighted magnetic resonance imaging clearly shows the presence of MBs (allows). MBs, focal deposits of hemosiderin in the brain, are induced by previous leakage of bleeding from small vessels. A, anterior; L, left; P, posterior; R, right.
Statistical Analyses
We did not perform power analyses for the sample size determination by the given event rates in those with and without MBs, the prevalence of MBs, and other factors. Instead, we estimated the needed events for multivariate Cox analysis. We set the events per variable equal to 10. We planned to include approximately 10 variables in the model. Therefore, we needed ≥100 patients experiencing the composite outcomes. We performed this analysis when the number of such patients reached 124.
All data are expressed as means±SD. Differences between groups were examined by the unpaired t test. Categorical variables were compared using the chi-squared test. Log rank analysis was performed to determine whether MBs were associated with a higher end point rate.
Cox regression analysis was performed with the presence of MBs as the key exposure variable and renal, cardiovascular, and composite outcomes as the outcome variables using several models. Renal and CVD outcomes share some risk factors, such as age, sex, diabetes, and hypertension. Dyslipidemia is a risk factor of CVD, whereas it is not an established risk factor of kidney outcomes. Inflammation/protein-energy wasting, renal anemia, and CKD-mineral bone disease (CKD-MBD) are factors affecting CVD and mortality, whereas the contributions of these CKD–related risk factors to kidney outcomes are largely unknown. Therefore, we first used an unadjusted model (model 1), and then, we intended to adjusted the models by the shared risk factors (model 2: the main model adjusted for age, sex, diabetes, and systolic BP) and the CKD-related factors in addition to the shared risk factors (model 3: model 2, eGFR, and urinary protein; model 4: model 2, body mass index, serum albumin, and C-reactive protein; model 5: model 2 and hemoglobin; model 6: model 2, serum calcium, phosphate, and intact parathyroid hormone [PTH]; and model 7: model 2, HDL cholesterol, non-HDL cholesterol, and smoking).
All tests were two tailed, and a probability value of <0.05 was considered significant. These analyses were performed using JMP9 (SAS Institute Inc., Cary, NC).
Results
Baseline Characteristics
Of the 404 patients with CKD, 83 (20.5%) were found to have MBs. The clinical diagnoses of their underlying renal diseases were GN (n=137), diabetic nephropathy (n=121), nephrosclerosis (n=48), and others (n=98). The baseline eGFR ranged between 3.3 and 126.6 ml/min per 1.73 m2. In Table 1, the baseline clinical characteristics according to the absence (without MBs) or presence of MBs (with MBs) are shown. The patients with MBs were significantly older, had higher systolic and diastolic BPs, and had higher pulse pressure than patients without MBs. The higher levels of BUN and serum creatinine and lower eGFR in patients with MBs suggest more decreased renal function than in those without MBs. Furthermore, hemoglobin levels were significantly lower and serum phosphate and intact PTH levels were significantly higher in patients with MBs. Also, the prevalence of MBs was significantly higher in patients with a history of hypertension and those on anticoagulants or antiplatelet agents than in those without a history of hypertension and those not on those agents. There were no significant differences in the other clinical parameters, including the presence of diabetes, the presence of dyslipidemia, and smoking behavior, between those with and without MBs.
Table 1.
Baseline characteristics of patients with and without microbleeds
| Characteristics | Total, n=404 | Without MBs, n=321 | With MBs, n=83 | P Value |
|---|---|---|---|---|
| Age, yr | 60.2±16.4 | 57.9±17.1 | 69.3±8.7 | <0.001 |
| Sex, men | 241 (59.7%) | 180 (56.1%) | 61 (73.5%) | 0.22 |
| BMI, kg/m2 | 23.2±4.3 | 23.3±4.5 | 23.2±3.4 | 0.95 |
| Systolic BP, mmHg | 130.4±22.2 | 125±18 | 152±21 | <0.001 |
| Diastolic BP, mmHg | 70.2±10.8 | 69±11 | 75±10 | <0.001 |
| Pulse pressure, mmHg | 60.2±18.6 | 56±15 | 78±20 | <0.001 |
| Serum creatinine, mg/dl | 2.66±2.48 | 2.30±2.28 | 4.05±2.74 | <0.001 |
| BUN, mg/dl | 34.7±25.7 | 30.4±21.7 | 51.4±32.6 | <0.001 |
| eGFR, ml/min per 1.73 m2 | 38.6±31.0 | 43.1±31.9 | 21.1±18.6 | <0.001 |
| Proteinuria, g/Cre | 2.86±3.42 | 2.78±3.44 | 3.12±3.30 | 0.44 |
| Total protein, g/dl | 6.2±1.0 | 6.2±1.0 | 6.2±0.9 | 0.87 |
| Albumin, g/dl | 3.3±0.7 | 3.3±0.7 | 3.3±0.6 | 0.62 |
| Total cholesterol, mg/dl | 211.2±78.9 | 214.9±81.4 | 196.9±66.9 | 0.06 |
| Triglyceride, mg/dl | 155.5±84.7 | 159.2±85.6 | 141.4±80.0 | 0.09 |
| HDL cholesterol, mg/dl | 50.1±16.9 | 50.6±17.0 | 48.2±16.7 | 0.25 |
| Non-HDL cholesterol, mg/dl | 161.1±73.8 | 164.3±76.6 | 148.7±60.9 | 0.09 |
| CRP, mg/dl | 0.09 (0.03–0.28) | 0.08 (0.03–0.25) | 0.12 (0.06–0.46) | 0.67 |
| Hemoglobin, g/dl | 11.8±2.5 | 12.0±2.5 | 10.9±2.1 | <0.001 |
| Calcium, mg/dl | 9.47±0.63 | 9.50±0.63 | 9.35±0.62 | 0.05 |
| Phosphate, mg/dl | 4.17±1.10 | 4.02±0.92 | 4.72±1.50 | <0.001 |
| iPTH, pg/ml | 133.3±193.2 | 111.3±137.0 | 200.5±298.8 | <0.001 |
| Smoking behavior | 142 (35.1%) | 108 (33.6%) | 34 (41.0%) | 0.25 |
| Presence of diabetes | 166 (41.1%) | 127 (39.6%) | 39 (47.0%) | 0.26 |
| Presence of dyslipidemia | 149 (36.9%) | 114 (35.5%) | 35 (42.2%) | 0.31 |
| Presence of hypertension | 247 (61.1%) | 174 (43.1%) | 73 (88.0%) | <0.001 |
| Anticoagulation and/or antiplatelet therapy | 107 (26.5%) | 77 (19.1%) | 30 (36.1%) | 0.04 |
MB, silent cerebral microbleed; BMI, body mass index; Cre, creatinine; CRP, C-reactive protein; iPTH, intact parathyroid hormone.
Follow-Up and End Points
The median follow-up duration was 2.3 years (25th to 75th percentiles, 1.6–3.4 years). Table 2 shows the outcomes by the presence of MBs in this study. Fifteen patients showed doubling of serum creatinine before being initiated on RRT, and 98 patients developed ESRD. The primary composite outcome was significantly more frequent in patients with MBs than in those without MBs. Patients with MBs had a significantly higher frequency of events of doubling of serum creatinine and ESRD than those without MBs. Cardiovascular events occurred in 44 patients (48 cases: 15 cases were ischemic heart disease, 11 cases underwent amputation [two cases were both ischemic heart disease and amputation], four cases were brain hemorrhage, and 18 cases were brain infarction). Deaths from cardiovascular causes were not different, but rates of ischemic heart disease and amputation or intervention because of arteriosclerosis obliterans were significantly higher in patients with MBs than in those without MBs. In addition, the frequency of stroke events was significantly higher in patients with MBs than in those without MBs.
Table 2.
Outcomes by presence of microbleeds
| Variable | Overall, n=404 | With MBs, n=83 | Without MBs, n=321 | P Valuea |
|---|---|---|---|---|
| Composite outcome | 124 (30.7%) | 56 (67.5%) | 68 (21.1%) | <0.001 |
| Kidney outcome | ||||
| Doubling of S-Cre and/or ESRD | 113 (28.0%) | 51 (61.4%) | 62 (19.3%) | <0.001 |
| CVD outcome | ||||
| (1) Death of CVD | 12 (3.0%) | 4 (4.8%) | 8 (2.5%) | 0.28 |
| (2) Ischemic heart disease and/or PAD | 22 (5.4%) | 11 (13.3%) | 11 (3.4%) | <0.001 |
| (3) Stroke | 22 (5.4%) | 13 (15.7%) | 9 (2.8%) | <0.001 |
| 1 + 2 + 3 | 40 (9.9%) | 22 (26.5%) | 18 (5.6%) | <0.001 |
| Lost to follow-upb | 12 (3.0%) | 2 (2.4%) | 10 (3.1%) | 0.28 |
The CVD outcomes included cardiovascular death, new onset of myocardial infarction, coronary revascularization, stroke, and amputation/revascularization because of PAD. Stroke event is brain infarction or brain hemorrhage. MB, microbleed; S-Cre, serum creatinine; CVD, cerebrovascular and cardiovascular disease; PAD, peripheral arterial disease.
Log rank tests were used to explore the difference in time to each outcome.
Lost patients due to moving or changing hospital.
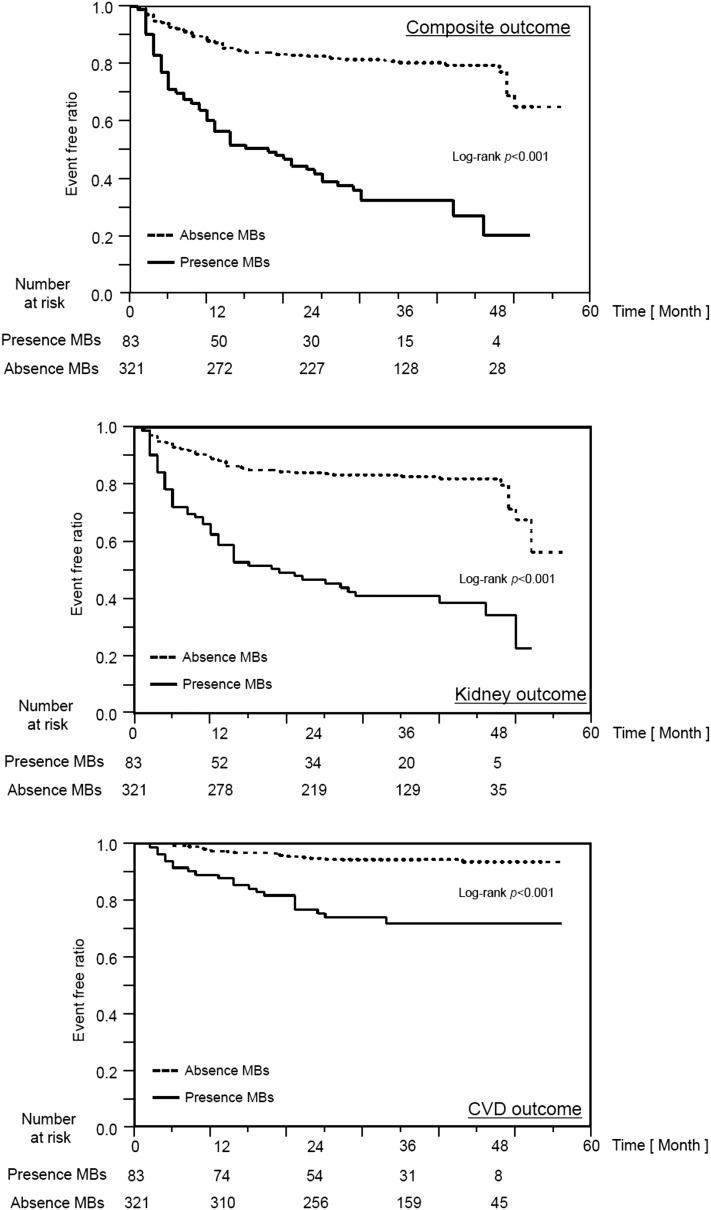
Kaplan–Meier analyses on the basis of the presence of MBs for cardiovascular, kidney, and composite outcomes are shown in Figure 3. The group with MBs had a significantly higher frequency of each outcome than the group without MBs.
Figure 3.
Kaplan–Meier analyses of cumulative rates for each outcome. MB, microbleed.
Predictors of Vascular Morbidity (Kidney and Cardiovascular Outcomes; Model 1)
Table 3 shows the results of univariate Cox proportional hazard analyses of the composite outcome. Among many factors that could predict composite outcome, the presence of MBs showed a very high hazard ratio of 4.62 (95% confidence interval, 3.22 to 6.62).
Table 3.
Univariate regression analyses of composite outcome
| Variable | Unit Change | Univariate | ||
|---|---|---|---|---|
| Hazard Ratio | 95% CI | P Value | ||
| MBs | 4.62 | 3.22 to 6.62 | <0.001 | |
| Age | 1 yr | 1.03 | 1.02 to 1.05 | <0.001 |
| Sex | 0.86 | 0.59 to 1.23 | 0.42 | |
| BMI | 1 kg/m2 | 0.98 | 0.94 to 1.02 | 0.34 |
| Systolic BP | 10 mmHg | 1.31 | 1.23 to 1.40 | <0.001 |
| Diastolic BP | 10 mmHg | 1.17 | 1.00 to 1.36 | 0.05 |
| Pulse Pressure | 10 mmHg | 1.41 | 1.30 to 1.52 | <0.001 |
| eGFR | 1 ml/min per 1.73 m2 | 0.91 | 0.89 to 0.92 | <0.001 |
| Proteinuria | 1 g/Cre | 1.10 | 1.06 to 1.14 | <0.001 |
| Albumin | 1 g/dl | 0.71 | 0.57 to 0.90 | 0.004 |
| Total cholesterol | 1 mg/dl | 0.99 | 0.99 to 1.00 | <0.001 |
| Triglyceride | 1 mg/dl | 1.00 | 0.99 to 1.00 | 0.001 |
| HDL cholesterol | 1 mg/dl | 0.98 | 0.97 to 1.00 | <0.01 |
| Non-HDL cholesterol | 1 mg/dl | 0.80 | 0.70 to 0.90 | <0.001 |
| Hematocrit | 1% | 0.90 | 0.89 to 0.92 | <0.001 |
| Hemoglobin | 1 g/dl | 0.66 | 0.60 to 0.72 | <0.001 |
| Blood glucose | 1 mg/dl | 1.00 | 1.00 to 1.01 | 0.08 |
| HbA1c | 1% | 1.04 | 0.91 to 1.17 | 0.57 |
| Glycoalbumin | 1% | 1.05 | 1.01 to 1.08 | 0.01 |
| C-reactive protein | 1 mg/dl | 1.15 | 1.06 to 1.22 | 0.003 |
| Calcium | 1 mg/dl | 0.39 | 0.32 to 0.49 | <0.001 |
| Phosphate | 1 mg/dl | 2.00 | 1.75 to 2.27 | <0.001 |
| Intact PTH | 1 pg/ml | 1.00 | 1.00 to 1.00 | <0.001 |
| Smoking behavior | 1.14 | 0.79 to 1.65 | 0.45 | |
| Presence of diabetes | 1.77 | 1.24 to 2.54 | 0.002 | |
| Presence of hypertension | 3.31 | 2.14 to 5.33 | <0.001 | |
| Anticoagulation and/or antiplatelet therapy | 1.99 | 1.38 to 2.85 | <0.001 | |
95% CI, 95% confidence interval; MB, silent cerebral microbleed; BMI, body mass index; Cre, creatinine; HbA1c, hemoglobin A1c; PTH, parathyroid hormone.
Multivariate Regression Analyses for Composite Outcome, Kidney Outcome, and CVD Outcome by Presence of MBs
Table 4 shows the results of multivariate Cox proportional hazard analyses for composite, kidney, and CVD outcomes by the presence of MBs. Model 1 is the unadjusted model. Model 2 is adjusted by shared CVD risk factors. Model 3 is adjusted by kidney cause factors in addition to model 2 factors. Model 4 is adjusted by inflammation/protein–energy wasting factors in addition to model 2 factors. Model 5 is adjusted by anemia in addition to model 2 factors. Model 6 is adjusted by CKD-MBD in addition to model 2 factors. Model 7 is adjusted by dyslipidemia factors in addition to model 2 factors. For each outcome except that of model 6, the presence of MBs showed significant differences.
Table 4.
Multivariate regression analyses
| Model | Adjustment | HR | 95% CI | P Value |
|---|---|---|---|---|
| Composite outcome | ||||
| 1 | Unadjusted | 4.62 | 3.22 to 6.62 | <0.001 |
| 2 | Age, sex, DM, and systolic BP | 2.58 | 1.68 to 3.96 | <0.001 |
| 3 | Model 2, eGFR, and UP | 2.61 | 1.69 to 4.04 | <0.001 |
| 4 | Model 2, BMI, Alb, and CRP | 3.18 | 2.04 to 4.97 | <0.001 |
| 5 | Model 2 and Hb | 2.58 | 1.70 to 3.92 | <0.001 |
| 6 | Model 2, Ca, P, and iPTH | 1.67 | 0.99 to 2.80 | 0.06 |
| 7 | Model 2, HDL cholesterol, non-HDL cholesterol, and smoking | 2.51 | 1.64 to 3.87 | <0.001 |
| Kidney outcome | ||||
| 1 | Unadjusted | 4.25 | 2.91 to 6.17 | <0.001 |
| 2 | Age, sex, DM, and systolic BP | 2.41 | 1.55 to 3.77 | <0.001 |
| 3 | Model 2, eGFR, and UP | 2.51 | 1.58 to 4.00 | <0.001 |
| 4 | Model 2, BMI, Alb, and CRP | 3.08 | 1.93 to 4.92 | <0.001 |
| 5 | Model 2 and Hb | 2.51 | 1.62 to 3.89 | <0.001 |
| 6 | Model 2, Ca, P, and iPTH | 1.52 | 0.88 to 2.61 | 0.13 |
| 7 | Model 2, HDL cholesterol, non-HDL cholesterol, and smoking | 2.36 | 1.50 to 3.69 | <0.001 |
| CVD outcome | ||||
| 1 | Unadjusted | 5.34 | 2.87 to 10.09 | <0.001 |
| 2 | Age, sex, DM, and systolic BP | 3.46 | 1.62 to 7.43 | 0.001 |
| 3 | Model 2, eGFR, and UP | 3.37 | 1.57 to 7.30 | 0.002 |
| 4 | Model 2, BMI, Alb, and CRP | 4.41 | 2.01 to 9.79 | <0.001 |
| 5 | Model 2 and Hb | 3.54 | 1.66 to 7.58 | 0.001 |
| 6 | Model 2, Ca, P, and iPTH | 1.96 | 0.74 to 5.25 | 0.18 |
| 7 | Model 2, HDL cholesterol, non-HDL cholesterol, and smoking | 3.33 | 1.53 to 7.25 | 0.003 |
HR, hazard ratio; 95% CI, 95% confidence interval; DM, diabetes mellitus; UP, proteinuria; BMI, body mass index; Alb, albumin; CRP, C-reactive protein; Hb, hemoglobin; Ca, calcium; P, phosphate; iPTH, intact parathyroid hormone; CVD, cardiovascular disease.
Stratified Analyses
Figure 4 shows the association between MBs and the composite outcome stratified by age, sex, diabetes mellitus, systolic BP, proteinuria, and eGFR at baseline using the model adjusted for the four variables as shown in model 2 in Table 2. The higher risk in patients with MBs was almost consistently found, regardless of these factors.
Figure 4.
Forest plots of the composite outcome. Association between baseline cerebral microbleeds (MBs) and composite outcome during follow-up stratified by age, sex, systolic BP, diabetes mellitus, proteinuria, or eGFR. The graphs indicate hazard ratios (95% confidence intervals) adjusted for age, sex, systolic BP, and diabetes mellitus.
Discussion
In this study, the presence of MBs was clearly shown to predict composite outcome, including future cardiovascular events and reduction of renal function, in patients with predialysis CKD.
T2*-weighted MRI for patients with nondialysis CKD showed that 83 patients (20.5%) had MBs. The prevalence of MBs in healthy populations without cerebrovascular disease was reported to be around 3.1%–8.5%; however, the prevalence of MBs was nearly 10-fold greater in cohorts with spontaneous intracerebral hemorrhage compared with in healthy elderly patients (14,15). In patients on hemodialysis, the prevalence was reported to be high at 25% (16). Our previous study showed that 21.6% of patients with predialysis CKD had MBs (8). Indeed, in this study, a similar prevalence of MBs was found in patients with nondialysis CKD, with a higher eGFR than in our previous study.
The presence of MBs has been reported to be a risk for CVDs (8). In patients without CKD, it was reported that a higher rate of CVD events was found in patients with MBs (17,18). Some reports showed that the presence of MBs predicts the progression of systemic atherosclerosis that is manifested in the perforator arteries (19), suggesting higher risk for CVD events in the presence of MBs. Indeed, in this study, the baseline characteristics of patients with MBs included more risk factors for CVDs than in those without MBs. Interestingly, the prevalence of the presence of diabetes, dyslipidemia, and smoking was not different between patients with and without MBs (8); instead, diabetes and dyslipidemia were risk factors for the composite outcome on univariate regression analysis. In general, diabetes, dyslipidemia, inflammation, and anemia are risk factors for CVD. In this study, the multivariate Cox proportional hazard analysis showed that the presence of MBs was a risk factor for CVD events, even adjusted with classic atherosclerotic risk factors. These findings suggest that the presence of MBs is an independent predictor of CVD events. The risk factors for atherosclerosis and microbleeding seem to be somewhat different.
Indeed, the prevalence of hypertension was significantly higher in patients with MBs than in those without MBs. On pathologic examination, MBs represent minor blood leakage from perforating branches of cerebral arteries (19–21). In regard to the effect of high BP on MBs, we had previously reported that age, systolic and diastolic BPs, and pulse pressure were significant independent factors associated with the presence of MBs in patients predialysis (8). High BP has been reported to be a strong risk factor for bleeding from cerebral perforating branches (22).
What is the pathophysiologic mechanism for the observed association of cerebral MBs with both CVD and kidney outcomes? There are small and short vessels called strain vessels (22) exposed to a high pressure to provide a large pressure gradient in a short distance, such as the branches of anterior, middle, and posterior cerebral arteries penetrating into the brain tissues, the central retinal artery in the eye, the coronary arteries in the heart, and juxtamedullary afferent arterioles in the kidneys (22). The sites of hemorrhage or infarction in the brain are frequently the areas of blood supply governed by these perforating arteries (22). There may be a condition that increases stress to and injury of the strain vessels that plays a role in the relationship between the observed relationship of cerebral MBs with CVD and kidney outcomes. Aortic stiffness is one of the candidate factors. Increased aortic stiffness reduces the cushioning function of the aorta, increasing the transmission of energy generated by contraction of the left ventricle to the peripheral organs, possibly resulting in damage of the strain vessels. In support of this notion, aortic stiffness is a known factor associated with cerebral small vessel disease (23) and an independent predictor of both CVD (24) and kidney function decline (25). Thus, the results of this study could be explained by the unique and shared characteristics of the small arteries in the brain, heart, and kidney and the stiffness of the aorta.
Patients with CKD who had MBs had poorer kidney outcomes, including doubling of creatinine level and ESRD, than those without MBs in this study in each CKD stage. Previously, there was no cohort report of MBs that mentioned renal outcomes of patients with predialysis CKD. There were two studies that showed that the presence of silent cerebral infarction was a prognostic factor for reduction of renal function in patients with CKD (26,27). On pathologic examination, MBs represent minor blood leakage from perforating branches of cerebral arteries (19–21). However, silent cerebral infarction represents obstruction of perforating branches of cerebral arteries. On multivariate Cox proportional hazard analysis, the presence of MBs was a risk factor for kidney outcomes adjusted with classic atherosclerotic risk factors. These findings suggest that the presence of MBs is an independent predictor for kidney outcomes.
The presence of MBs was not a predictor for composite and kidney outcomes and CVD events on multivariate regression analysis in model 6 in this study. This model was adjusted with calcium, phosphorus, and intact PTH, factors related to CKD-MBD, in addition to model 2 factors (age, sex, diabetes mellitus, and systolic BP). The phosphorus-to-fibroblast growth factor 23 ratio was reported to be an independent risk factor for CVD (28). However, the relationship between intact PTH/serum calcium and CVD was not clear in patients with predialysis CKD (29). CKD-MBD has been reported to be a strong predictor of future renal events and CVD events (30–33). Although we had no data for fibroblast growth factor 23, MB and CKD-MBD factors seem to share a confounding factor for composite outcome.
This study has several limitations. First, the sample size was relatively small as was the number of CVD events. In this study, there were 22 strokes, 22 cardiovascular events, and 40 CVD events. The small number of CVD events did not permit correction for all potential predictors. The cause of this may be the short observation period. However, there were enough events of kidney function loss during this observation period. Second, there was the possibility of unmeasured or residual confounding. Third, this study was an observational study. These results were not discussed as cause and effect associations.
In conclusion, this is the first report that clearly shows that the presence of MBs is a strong predictor for composite outcome, which included poor kidney outcome and CVD events, in patients with predialysis CKD. This study shows the high prevalence of MBs in patients with predialysis CKD and suggests the brain-kidney association.
Disclosures
None.
Acknowledgments
We thank Dr. Kaeko Kitamura and Dr. Tetsuo Nakayama for their careful readings of brain magnetic resonance imaging.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW; American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention : Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Drey N, Roderick P, Mullee M, Rogerson M: A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis 42: 677–684, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Muntner P, He J, Hamm L, Loria C, Whelton PK: Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 13: 745–753, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Abramson JL, Jurkovitz CT, Vaccarino V, Weintraub WS, McClellan W: Chronic kidney disease, anemia, and incident stroke in a middle-aged, community-based population: The ARIC Study. Kidney Int 64: 610–615, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Muntner P, Judd SE, McClellan W, Meschia JF, Warnock DG, Howard VJ: Incidence of stroke symptoms among adults with chronic kidney disease: Results from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Nephrol Dial Transplant 27: 166–173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidwell CS, Saver JL, Villablanca JP, Duckwiler G, Fredieu A, Gough K, Leary MC, Starkman S, Gobin YP, Jahan R, Vespa P, Liebeskind DS, Alger JR, Vinuela F: Magnetic resonance imaging detection of microbleeds before thrombolysis: An emerging application. Stroke 33: 95–98, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Shima H, Ishimura E, Naganuma T, Yamazaki T, Kobayashi I, Shidara K, Mori K, Takemoto Y, Shoji T, Inaba M, Okamura M, Nakatani T, Nishizawa Y: Cerebral microbleeds in predialysis patients with chronic kidney disease. Nephrol Dial Transplant 25: 1554–1559, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Laible M, Horstmann S, Möhlenbruch M, Wegele C, Rizos T, Schüler S, Zorn M, Veltkamp R: Renal dysfunction is associated with deep cerebral microbleeds but not white matter hyperintensities in patients with acute intracerebral hemorrhage. J Neurol 262: 2312–2322, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Japanese Society of Nephrology : Evidence-based clinical practice guideline for CKD. Clin Exp Nephrol 55: 585–860, 2016 [Google Scholar]
- 11.Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S: Modification of the Modification of Diet in Renal Disease (MDRD) Study equation for Japan. Am J Kidney Dis 50: 927–937, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20: 1183–1197, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Koennecke HC: Cerebral microbleeds on MRI: Prevalence, associations, and potential clinical implications. Neurology 66: 165–171, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Viswanathan A, Chabriat H: Cerebral microhemorrhage. Stroke 37: 550–555, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Naganuma T, Takemoto Y, Yamasaki T, Shima H, Shoji T, Ishimura E, Nishizawa Y, Morino M, Okamura M, Nakatani T: Factors associated with silent cerebral microbleeds in hemodialysis patients. Clin Nephrol 75: 346–355, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Thijs V, Lemmens R, Schoofs C, Görner A, Van Damme P, Schrooten M, Demaerel P: Microbleeds and the risk of recurrent stroke. Stroke 41: 2005–2009, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Naka H, Nomura E, Takahashi T, Wakabayashi S, Mimori Y, Kajikawa H, Kohriyama T, Matsumoto M: Combinations of the presence or absence of cerebral microbleeds and advanced white matter hyperintensity as predictors of subsequent stroke types. AJNR Am J Neuroradiol 27: 830–835, 2006 [PMC free article] [PubMed] [Google Scholar]
- 19.Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, Hartung HP: Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: Evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol 20: 637–642, 1999 [PMC free article] [PubMed] [Google Scholar]
- 20.Kato H, Izumiyama M, Izumiyama K, Takahashi A, Itoyama Y: Silent cerebral microbleeds on T2*-weighted MRI: Correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke 33: 1536–1540, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Kinoshita T, Okudera T, Tamura H, Ogawa T, Hatazawa J: Assessment of lacunar hemorrhage associated with hypertensive stroke by echo-planar gradient-echo T2*-weighted MRI. Stroke 31: 1646–1650, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Ito S, Nagasawa T, Abe M, Mori T: Strain vessel hypothesis: A viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertens Res 32: 115–121, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Van Sloten TT, Mitchell GF, Sigurdsson S, van Buchem MA, Jonsson PV, Garcia ME, Harris TB, Henry RM, Levey AS, Stehouwer CD, Gudnason V, Launer LJ: Association between arterial stiffness, depressive symptoms and cerebral small vessel disease; cross-sectional findings from the AGE-Reykjavik Study. J Psychiatry Neurosci 41: 162–168, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoji T, Emoto M, Shinohara K, Kakiya R, Tsujimoto Y, Kishimoto H, Ishimura E, Tabata T, Nishizawa Y: Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J Am Soc Nephrol 12: 2117–2124, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Sedaghat S, Mattace-Raso FU, Hoorn EJ, Uitterlinden AG, Hofman A, Ikram MA, Franco OH, Dehghan A: Arterial stiffness and decline in kidney function. Clin J Am Soc Nephrol 10: 2190–2197, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uzu T, Kida Y, Shirahashi N, Harada T, Yamauchi A, Nomura M, Isshiki K, Araki S, Sugimoto T, Koya D, Haneda M, Kashiwagi A, Kikkawa R: Cerebral microvascular disease predicts renal failure in type 2 diabetes. J Am Soc Nephrol 21: 520–526, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi M, Hirawa N, Yatsu K, Kobayashi Y, Yamamoto Y, Saka S, Andoh D, Toya Y, Yasuda G, Umemura S: Relationship between silent brain infarction and chronic kidney disease. Nephrol Dial Transplant 24: 201–207, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P; MMKD Study Group : Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF: Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. JAMA 305: 1119–1127, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB Sr., Gaziano JM, Vasan RS: Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879–885, 2007 [DOI] [PubMed] [Google Scholar]
- 31.O’Seaghdha CM, Hwang SJ, Muntner P, Melamed ML, Fox CS: Serum phosphorus predicts incident chronic kidney disease and end-stage renal disease. Nephrol Dial Transplant 26: 2885–2890, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M: Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119: 2545–2552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]