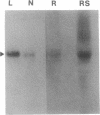
Abstract
Many plants synthesize and accumulate proline in response to osmotic stress. Despite the importance of this pathway, however, the exact metabolic route and enzymes involved in the synthesis of proline in plants have not been unequivocally identified. We report here the isolation of a mothbean (Vigna aconitifolia) cDNA clone encoding a bifunctional enzyme, delta 1-pyrroline-5-carboxylate synthetase (P5CS), with both gamma-glutamyl kinase and glutamic-gamma-semialdehyde dehydrogenase activities that catalyzes the first two steps in proline biosynthesis. The two enzymatic domains of P5CS correspond to the ProB and ProA proteins of Escherichia coli and contain a leucine zipper in each domain, which may facilitate inter- or intramolecular interaction of this protein. The Vigna P5CS enzyme activity is feedback regulated by proline but is less sensitive to end-product inhibition than is the E. coli gamma-glutamyl kinase. The P5CS gene is expressed at high levels in Vigna leaves and is inducible in roots subjected to salt stress, suggesting that P5CS plays a key role in proline biosynthesis, leading to osmoregulation in plants.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel T., Maniatis T. Gene regulation. Action of leucine zippers. Nature. 1989 Sep 7;341(6237):24–25. doi: 10.1038/341024a0. [DOI] [PubMed] [Google Scholar]
- Boggess S. F. Contribution of Arginine to Proline Accumulation in Water-stressed Barley Leaves. Plant Physiol. 1976 Dec;58(6):796–797. doi: 10.1104/pp.58.6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggess S. F., Stewart C. R. Effect of water stress on proline synthesis from radioactive precursors. Plant Physiol. 1976 Sep;58(3):398–401. doi: 10.1104/pp.58.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Plant productivity and environment. Science. 1982 Oct 29;218(4571):443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- Csonka L. N., Gelvin S. B., Goodner B. W., Orser C. S., Siemieniak D., Slightom J. L. Nucleotide sequence of a mutation in the proB gene of Escherichia coli that confers proline overproduction and enhanced tolerance to osmotic stress. Gene. 1988 Apr 29;64(2):199–205. doi: 10.1016/0378-1119(88)90335-6. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol Gen Genet. 1981;182(1):82–86. doi: 10.1007/BF00422771. [DOI] [PubMed] [Google Scholar]
- Dandekar A. M., Uratsu S. L. A single base pair change in proline biosynthesis genes causes osmotic stress tolerance. J Bacteriol. 1988 Dec;170(12):5943–5945. doi: 10.1128/jb.170.12.5943-5945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delauney A. J., Verma D. P. A soybean gene encoding delta 1-pyrroline-5-carboxylate reductase was isolated by functional complementation in Escherichia coli and is found to be osmoregulated. Mol Gen Genet. 1990 May;221(3):299–305. doi: 10.1007/BF00259392. [DOI] [PubMed] [Google Scholar]
- Deutch A. H., Rushlow K. E., Smith C. J. Analysis of the Escherichia coli proBA locus by DNA and protein sequencing. Nucleic Acids Res. 1984 Aug 10;12(15):6337–6355. doi: 10.1093/nar/12.15.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch A. H., Smith C. J., Rushlow K. E., Kretschmer P. J. Escherichia coli delta 1-pyrroline-5-carboxylate reductase: gene sequence, protein overproduction and purification. Nucleic Acids Res. 1982 Dec 11;10(23):7701–7714. doi: 10.1093/nar/10.23.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo R., Nieto C., Fernandez-Tresguerres M. E., Diaz R. Bacterial zipper. Nature. 1989 Dec 21;342(6252):866–866. doi: 10.1038/342866a0. [DOI] [PubMed] [Google Scholar]
- Hayzer D. J., Leisinger T. The gene-enzyme relationships of proline biosynthesis in Escherichia coli. J Gen Microbiol. 1980 Jun;118(2):287–293. doi: 10.1099/00221287-118-2-287. [DOI] [PubMed] [Google Scholar]
- Kohl D. H., Schubert K. R., Carter M. B., Hagedorn C. H., Shearer G. Proline metabolism in N2-fixing root nodules: energy transfer and regulation of purine synthesis. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2036–2040. doi: 10.1073/pnas.85.7.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. J., Henslee J. G., Wakabayashi Y., Jones M. E. Delta 1-pyrroline-5-carboxylate synthase from rat intestinal mucosa. Methods Enzymol. 1985;113:113–120. doi: 10.1016/s0076-6879(85)13025-9. [DOI] [PubMed] [Google Scholar]
- Larosa P. C., Rhodes D., Rhodes J. C., Bressan R. A., Csonka L. N. Elevated Accumulation of Proline in NaCl-Adapted Tobacco Cells Is Not Due to Altered Delta-Pyrroline-5-Carboxylate Reductase. Plant Physiol. 1991 May;96(1):245–250. doi: 10.1104/pp.96.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato R. F., Smith R. J., Valle D. L., Crane K. Mutant cell lines resistant to azetidine-2-carboxylic acid: alterations in the synthesis of proline from glutamic acid. J Cell Physiol. 1984 Apr;119(1):137–143. doi: 10.1002/jcp.1041190122. [DOI] [PubMed] [Google Scholar]
- Mahan M. J., Csonka L. N. Genetic analysis of the proBA genes of Salmonella typhimurium: physical and genetic analyses of the cloned proB+ A+ genes of Escherichia coli and of a mutant allele that confers proline overproduction and enhanced osmotolerance. J Bacteriol. 1983 Dec;156(3):1249–1262. doi: 10.1128/jb.156.3.1249-1262.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayapati P. J., Stewart C. R., Hack E. Pyrroline-5-Carboxylate Reductase Is in Pea (Pisum sativum L.) Leaf Chloroplasts. Plant Physiol. 1989 Oct;91(2):581–586. doi: 10.1104/pp.91.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Handa S., Bressan R. A. Metabolic changes associated with adaptation of plant cells to water stress. Plant Physiol. 1986 Dec;82(4):890–903. doi: 10.1104/pp.82.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Handa S., Bressan R. A. Metabolic changes associated with adaptation of plant cells to water stress. Plant Physiol. 1986 Dec;82(4):890–903. doi: 10.1104/pp.82.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. T. Characterization of a gamma-glutamyl kinase from Escherichia coli that confers proline overproduction and osmotic tolerance. J Bacteriol. 1985 Dec;164(3):1088–1093. doi: 10.1128/jb.164.3.1088-1093.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J., Downing S. J., Phang J. M., Lodato R. F., Aoki T. T. Pyrroline-5-carboxylate synthase activity in mammalian cells. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5221–5225. doi: 10.1073/pnas.77.9.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoke A., Miao G. H., Hong Z., Verma D. P. Subcellular location of delta-pyrroline-5-carboxylate reductase in root/nodule and leaf of soybean. Plant Physiol. 1992 Aug;99(4):1642–1649. doi: 10.1104/pp.99.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Kazazian V., Zogbi V., Bal A. K. Isolation and characterization of the membrane envelope enclosing the bacteroids in soybean root nodules. J Cell Biol. 1978 Sep;78(3):919–936. doi: 10.1083/jcb.78.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd T. C., Dekker B. M., Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989 Mar 25;17(6):2362–2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]