Abstract
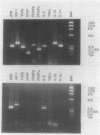
AIM--To analyse patterns of gene expression for peptide regulatory factors in patients with Dupuytren's contracture. METHODS--Tissue samples (palmer fascia) from 12 patients with Dupuytren's contracture and 12 controls were studied using the reverse transcription/polymerase chain reaction (RT/PCR) technique. RESULTS--Tissue from patients with Dupuytren's contracture expressed a higher percentage of peptide regulatory factors than that of controls: interleukin-1 alpha (83% v 16%; p < 0.01); interleukin-1 beta (66% v 8%; p < 0.01); transforming growth factor beta (75% v 25%; p < 0.02); and basic fibroblast growth factor (66% v 25%; p < 0.05). Platelet derived growth factors alpha and beta were also expressed more commonly (66% v 33% and 25% v 16%, respectively), but these differences were not significant. CONCLUSIONS--The increased prevalence of expression for the above mRNAs in Dupuytren's tissue is relevant as interleukin-1, basic fibroblast growth factor, and transforming growth factor beta stimulate the growth of fibroblasts and transforming growth factor beta also enhances production of collagen and other extracellular matrix proteins. Excessive local release of these peptide regulatory factors may have an important role in the pathogenesis of Dupuytren's contracture.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew J. G., Andrew S. M., Ash A., Turner B. An investigation into the role of inflammatory cells in Dupuytren's disease. J Hand Surg Br. 1991 Aug;16(3):267–271. doi: 10.1016/0266-7681(91)90051-o. [DOI] [PubMed] [Google Scholar]
- Azzarone B., Failly-Crepin C., Daya-Grosjean L., Chaponnier C., Gabbiani G. Abnormal behavior of cultured fibroblasts from nodule and nonaffected aponeurosis of Dupuytren's disease. J Cell Physiol. 1983 Dec;117(3):353–361. doi: 10.1002/jcp.1041170310. [DOI] [PubMed] [Google Scholar]
- Barsky S. H., Gopalakrishna R. Isolation of a myofibroblast growth factor from human breast carcinoma cell lines. Biochem Biophys Res Commun. 1987 Dec 31;149(3):1125–1131. doi: 10.1016/0006-291x(87)90525-0. [DOI] [PubMed] [Google Scholar]
- Brickley-Parsons D., Glimcher M. J., Smith R. J., Albin R., Adams J. P. Biochemical changes in the collagen of the palmar fascia in patients with Dupuytren's disease. J Bone Joint Surg Am. 1981 Jun;63(5):787–797. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dallman M. J., Larsen C. P., Morris P. J. Cytokine gene transcription in vascularised organ grafts: analysis using semiquantitative polymerase chain reaction. J Exp Med. 1991 Aug 1;174(2):493–496. doi: 10.1084/jem.174.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Fibroblast growth factor. Chemical structure and biologic function. Clin Orthop Relat Res. 1990 Aug;(257):231–248. [PubMed] [Google Scholar]
- Ignotz R. A., Endo T., Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987 May 15;262(14):6443–6446. [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- LING R. S. THE GENETIC FACTOR IN DUPUYTREN'S DISEASE. J Bone Joint Surg Br. 1963 Nov;45(4):709–718. [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Goustin A. S., Shipley G. D., DiCorleto P. E., Moses H. L. Induction of c-sis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta: a proposed model for indirect mitogenesis involving autocrine activity. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2453–2457. doi: 10.1073/pnas.83.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R. M., Moses H. L. Transforming growth factors and the regulation of cell proliferation. Eur J Biochem. 1990 Feb 14;187(3):467–473. doi: 10.1111/j.1432-1033.1990.tb15327.x. [DOI] [PubMed] [Google Scholar]
- Murrell G. A., Francis M. J., Bromley L. Free radicals and Dupuytren's contracture. Br Med J (Clin Res Ed) 1987 Nov 28;295(6610):1373–1375. doi: 10.1136/bmj.295.6610.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987 Jan 1;165(1):251–256. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Lachman L. B., Kang A. H. Induction of fibroblast proliferation by interleukin-1 derived from human monocytic leukemia cells. Arthritis Rheum. 1984 Sep;27(9):995–1001. doi: 10.1002/art.1780270905. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunn S., Gurr E., Delbrück A., Buhr T., Flory J. The distribution of unsulphated and sulphated glycosaminoglycans in palmar fascia from patients with Dupuytren's disease and healthy subjects. J Clin Chem Clin Biochem. 1988 Jan;26(1):7–14. doi: 10.1515/cclm.1988.26.1.7. [DOI] [PubMed] [Google Scholar]
- di Giovine F. S., Duff G. W. Interleukin 1: the first interleukin. Immunol Today. 1990 Jan;11(1):13–20. doi: 10.1016/0167-5699(90)90005-t. [DOI] [PubMed] [Google Scholar]