Abstract
Background
Dengue is the most common arbovirus infection globally, but its burden is poorly quantified. We estimated dengue mortality, incidence, and burden for the Global Burden of Disease Study 2013.
Methods
We modelled mortality from vital registration, verbal autopsy, and surveillance data using the Cause of Death Ensemble Modelling tool. We modelled incidence from officially reported cases, and adjusted our raw estimates for under-reporting based on published estimates of expansion factors. In total, we had 1780 country-years of mortality data from 130 countries, 1636 country-years of dengue case reports from 76 countries, and expansion factor estimates for 14 countries.
Findings
We estimated an average of 9221 dengue deaths per year between 1990 and 2013, increasing from a low of 8277 (95% uncertainty estimate 5353–10 649) in 1992, to a peak of 11 302 (6790–13 722) in 2010. This yielded a total of 576 900 (330 000–701 200) years of life lost to premature mortality attributable to dengue in 2013. The incidence of dengue increased greatly between 1990 and 2013, with the number of cases more than doubling every decade, from 8∙3 million (3∙3 million–17∙2 million) apparent cases in 1990, to 58∙4 million (23∙6 million–121∙9 million) apparent cases in 2013. When accounting for disability from moderate and severe acute dengue, and post-dengue chronic fatigue, 566 000 (186 000–1 415 000) years lived with disability were attributable to dengue in 2013. Considering fatal and non-fatal outcomes together, dengue was responsible for 1∙14 million (0∙73 million–1∙98 million) disability-adjusted life-years in 2013.
Interpretation
Although lower than other estimates, our results offer more evidence that the true symptomatic incidence of dengue probably falls within the commonly cited range of 50 million to 100 million cases per year. Our mortality estimates are lower than those presented elsewhere and should be considered in light of the totality of evidence suggesting that dengue mortality might, in fact, be substantially higher.
Background
Dengue is the most common arbovirus infection globally, with transmission occurring in at least 128 countries and as many as 4 billion people at risk.1 The number of dengue cases reported to WHO has increased steadily from an average of less than a thousand cases per year globally in the 1950’s to more than 3 million reported cases in 2013.2–5 These reports, however, greatly understate the problem and estimates of the true number of annual apparent infections range from 50 million to 200 million, where apparent infections are defined as all symptomatic infections, including those that are undetected by reporting systems. The most commonly cited range, including by WHO, is 50 million to 100 million apparent cases per year.6,7 Although estimates of dengue deaths are less often reported, the most commonly cited number is around 20 000 deaths per year.8 To our knowledge, these estimates seem largely to be based on expert opinion. The Global Burden of Disease Study 2010 – providing the most recent data-driven estimate of dengue deaths – estimated that more than 14 000 people died from dengue in 2010.9
The disparity between the number of reported cases and estimates of the number of actual cases stems from under-recognition and under-reporting of dengue. Symptomatic dengue infections have a broad range of severity and as many as 70% of patients choose to not seek treatment or treat thmeselves.7 Even for those who are seen by a healthcare professional, the clinical presentation of dengue shares similarities with up to 12 major pathogens making misdiagnosis common, particularly in areas with high incidence of febrile illnesses.10 Population-based cohort studies11,12 have consistently found dengue cases to be greatly under-reported through official passive surveillance and reporting systems. Several studies have attempted to quantify the degree of under-reporting by comparing incidence rates derived from active febrile-illness surveillance with comparable incidence rates derived from official reports. The ratio of these rates is referred to as the expansion factor, and it represents the number by which one would multiply the number of reported cases to derive the number of true apparent dengue infections in a given population. That said, the degree to which dengue is under-reported varies by orders of magnitude across time and space, precluding the use of a simple multiplier. Moreover, many countries where dengue is believed to occur file no official reports, or do so only intermittently, and in these cases there is no number of reported cases to which a multiplier could be applied.
Attempts to estimate the true incidence of symptomatic dengue must, therefore, address these problems. Bhatt and colleagues 7 applied geostatistical methods to the problem: they first developed a global dengue risk map, then geolocated studies of dengue incidence and, finally, modelled the relation between risk and incidence to estimate the incidence for each 5-by-5 km area. Their method yielded an estimate of 96 million (95% credible interval 67 million to 136 million) apparent infections globally. Unfortunately, this method cannot easily be used to estimate changes in dengue incidence over time.
Beyond incidence and mortality, understanding the true burden of dengue demands the estimation metrics that allow for meaningful comparisons with other diseases that have different severity and duration, and that allow for comparisons between fatal and non-fatal outcomes. Among the most common of these burden metrics are years of life lost to premature mortality (YLL), which quantifies health loss due to mortality, giving greater weight to deaths occurring at younger ages; years lived with disability (YLD), which quantifies non-fatal health loss accounting for both the severity and duration of a given condition; and disability-adjusted life years (DALY), which captures the combination of YLLs and YLDs.
We estimated dengue mortality, incidence, and burden by age, sex, and country, as estimated for the Global Burden of Disease Study 2013. The Global Burden of Disease Study 2013 was an effort to comprehensively and systematically estimate death and disability from 306 causes, producing estimates by year, age, sex, and country for 1990–2013. Although summary results have been published previously 13–15, here we present previously unpublished details of our modelling approach and results for dengue and discuss these results in the context of independent attempts to estimate the burden of dengue, with reference to dengue-specific literature.
Methods
Death estimation
The Global Burden of Disease Cause of Death database, contains data for 240 causes of death and was built specifically for the Global Burden of Disease Study from a combination of publicly available and restricted sources, including vital registration, verbal autopsy, and, surveillance data. The raw data were processed to reconcile disparate coding schemes (e.g. International Classification of Diseases 9 and 10), and redistribute garbage codes, among other corrections.15 We modelled dengue mortality using data in the Cause of Death database and the Cause of Death Ensemble Modelling tool, which has been described elsewhere.15,16 Briefly, we selected covariates on the basis of expected associations with dengue mortality and biological plausibility. Among these covariates, we included environmental variables (rainfall, proportion of the population living between latitudes 15° north and 15° south, the proportion of the population living below 100 m elevation, and the proportion of the population living in urban areas), and variables related to each country’s level of development (lag-distributed income per capita, health system access, and mean years of education). Finally we included the population-weighted mean probability of dengue transmission derived from Bhatt and colleagues.7 The appendix (p 3) shows the full list of covariates, and the number of Cause of Death Ensemble Modelling sub-models in which each covariate was used. Within the Global Burden of Disease Study, all-cause mortality was estimated first and then, within each age-sex-country-year group, the sum of all cause-specific death estimates were constrained to equal the number of all-cause deaths through a process called CodCorrect.15
Incidence estimation
We attempted to correct for under-reporting using a three-phase modelling approach. First, we defined the expected spatial distribution of disease, based on a principal components analysis of the population-weighted probability of dengue transmission and our model-based estimates of dengue mortality. Second, we modelled the association between this expected distribution and reported incidence using mixed-effects negative binomial models, with the assumption that deviations from the expected distribution reflect deviations in completeness of reporting. And third, we calibrated the model by benchmarking these deviations against published empirical expansion factors (appendix pp 3–5). The case reports used in this analysis do not disaggregate cases by age and sex. We therefore modelled total cases by country and year and then distributed cases to age-sex groups based on the age-sex distribution of dengue cases captured by the Hospital Information System of the Brazilian Unified National Health System.
Burden estimation
We estimated three measures of burden: YLLs, YLDs, and DALYs. YLLs were estimated as the difference between the age at death and the corresponding life expectancy for those surviving to that age at death; the life expectancy was based on a theoretical composite life-table in which the target life expectancy for each age is equal to the longest recorded life expectancy among people of that age in any country.15 YLDs were calculated as the product of the number of cases having a given health state, the duration of that health state, and the disability weight for that health state. DALYs were calculated as the sum of YLLs and YLDs. To estimate YLDs, we assigned a health state and corresponding disability weight to each case. Disability weights were based on pooled results from the Global Burden of Disease 2010 Disability Weights Measurement study 17 and the more recent European disability weights study 18. We assigned each dengue case to one of two acute health states: 94∙5% of cases were assigned the disability weight for “infectious disease, acute episode, moderate” with a mean duration of 6 days; and 5∙5% were assigned the disability weight for “infectious disease, acute episode, severe” with a mean duration of 14 days.19 We derived the proportions for the split between moderate and severe states from a meta-analysis of the subset of data for case notification that presented both the total number of cases, and the number of severe cases (defined as either dengue hemorrhagic fever or dengue shock syndrome). Our definition of severe was intended to correspond to the definition of a severe acute infectious disease episode within the context of disability weights, and does not correspond to WHO’s definition of severe dengue.20 Additionally, 8∙5% of cases were assumed to include post-dengue chronic fatigue, and assigned the disability weight for “Infectious disease, post-acute consequences”, with a mean duration of 6 months.21
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
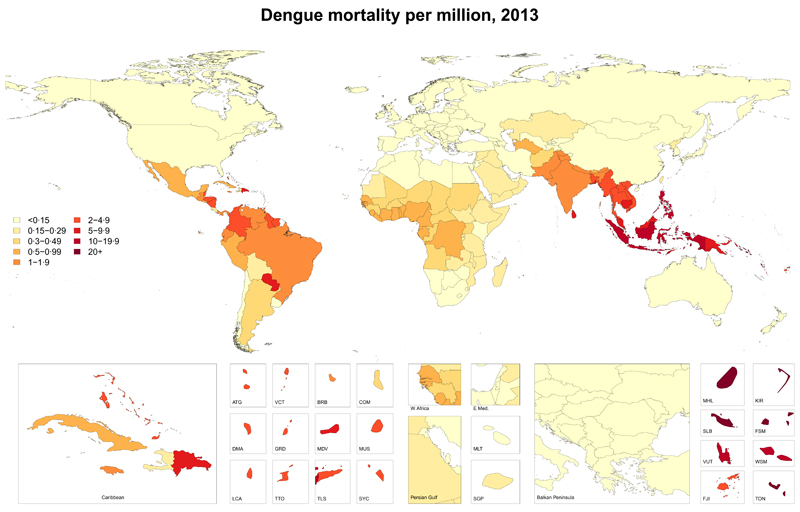
We had 1780 country-years of mortality data from 130 countries. We estimated that an average of 9,221 people died from dengue per year between 1990 and 2013, increasing from a low of 8277 (95% uncertainty interval [UI] 5353 – 10 649) in 1992 to a peak of 11 302 (6790 – 13 722) in 2010. Mortality rates were highest among patients younger than 1 year of age, decreased with age into adulthood, and increased with age older than 45 years. A slightly larger proportion of dengue deaths occurred among females (51∙5%) than males in 2013 (table 1). Most dengue deaths and the highest dengue mortality rates occurred in the southeast Asia (figure 1). We estimated a total of 576 900 (330 000 – 701 200) YLLs attributable to dengue in 2013 (table 2).
Table 1. Dengue deaths and mortality rates for 1990 and 2013.
| 1990 | 2013 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths (95% UI) | Mortality rate (95% UI; per million) | Deaths (95% UI) | Mortality rate (95% UI; per million) | ||||||||||
| Total | 8,657 | (5,484, | 10,819) | 1·64 | (1·04, | 2·04) | 9,110 | (5,630, | 10,842) | 1·27 | (0·79, | 1·52) | |
| Sex | |||||||||||||
| Male | 4,349 | (2,516, | 5,801) | 1·63 | (0·94, | 2·18) | 4,433 | (2,515, | 5,340) | 1·23 | (0·70, | 1·48) | |
| Female | 4,308 | (2,556, | 5,611) | 1·64 | (0·97, | 2·14) | 4,677 | (2,500, | 5,723) | 1·32 | (0·71, | 1·61) | |
| Age (years) | |||||||||||||
| Post Neonatal | 978 | (459, | 1,417) | 8·05 | (3·78, | 11·67) | 650 | (303, | 899) | 5·21 | (2·43, | 7·20) | |
| 1 to 4 | 3,016 | (1,400, | 4,449) | 5·97 | (2·77, | 8·81) | 1,967 | (1,047, | 2,761) | 3·75 | (2·00, | 5·27) | |
| 5 to 9 | 1,828 | (951, | 2,437) | 3·16 | (1·65, | 4·22) | 1,568 | (703, | 2,117) | 2·53 | (1·13, | 3·41) | |
| 10 to 14 | 595 | (403, | 725) | 1·13 | (0·76, | 1·37) | 711 | (387, | 862) | 1·19 | (0·65, | 1·45) | |
| 15 to 19 | 276 | (197, | 313) | 0·54 | (0·38, | 0·61) | 378 | (224, | 449) | 0·64 | (0·38, | 0·76) | |
| 20 to 24 | 273 | (210, | 326) | 0·56 | (0·43, | 0·67) | 389 | (271, | 473) | 0·63 | (0·44, | 0·77) | |
| 25 to 29 | 291 | (229, | 359) | 0·67 | (0·52, | 0·82) | 448 | (315, | 545) | 0·75 | (0·53, | 0·91) | |
| 30 to 34 | 263 | (206, | 331) | 0·68 | (0·53, | 0·86) | 420 | (300, | 506) | 0·79 | (0·57, | 0·96) | |
| 35 to 39 | 222 | (178, | 280) | 0·65 | (0·52, | 0·81) | 369 | (264, | 456) | 0·75 | (0·54, | 0·93) | |
| 40 to 44 | 187 | (156, | 244) | 0·67 | (0·56, | 0·87) | 355 | (267, | 431) | 0·75 | (0·56, | 0·91) | |
| 45 to 49 | 99 | (79, | 114) | 0·43 | (0·34, | 0·50) | 233 | (169, | 263) | 0·53 | (0·39, | 0·60) | |
| 50 to 54 | 99 | (80, | 117) | 0·47 | (0·38, | 0·55) | 232 | (163, | 261) | 0·61 | (0·43, | 0·68) | |
| 55 to 59 | 89 | (76, | 110) | 0·48 | (0·41, | 0·59) | 218 | (158, | 247) | 0·67 | (0·49, | 0·76) | |
| 60 to 64 | 96 | (83, | 123) | 0·61 | (0·52, | 0·77) | 229 | (176, | 263) | 0·82 | (0·63, | 0·94) | |
| 65 to 69 | 97 | (83, | 133) | 0·79 | (0·67, | 1·08) | 224 | (175, | 265) | 1·19 | (0·93, | 1·41) | |
| 70 to 74 | 78 | (67, | 101) | 0·9 | (0·77, | 1·16) | 199 | (147, | 237) | 1·32 | (0·97, | 1·57) | |
| 75 to 79 | 60 | (49, | 70) | 0·95 | (0·78, | 1·12) | 158 | (108, | 178) | 1·48 | (1·01, | 1·67) | |
| 80 plus | 107 | (78, | 128) | 1·89 | (1·38, | 2·26) | 364 | (252, | 443) | 3·03 | (2·10, | 3·69) | |
| Region | |||||||||||||
| Central Europe, Eastern Europe, and Central Asia | |||||||||||||
| Central Asia | 20 | (16, | 22) | 0·29 | (0·23, | 0·33) | 15 | (13, | 19) | 0·18 | (0·15, | 0·23) | |
| Central Europe | 66 | (28, | 76) | 0·53 | (0·23, | 0·62) | 12 | (11, | 19) | 0·1 | (0·09, | 0·16) | |
| Eastern Europe | 25 | (22, | 35) | 0·11 | (0·10, | 0·16) | 22 | (18, | 30) | 0·1 | (0·09, | 0·14) | |
| High-income | |||||||||||||
| Australasia | 2 | (2, | 3) | 0·11 | (0·10, | 0·15) | 3 | (2, | 3) | 0·1 | (0·07, | 0·11) | |
| High-income Asia Pacific | 22 | (20, | 27) | 0·13 | (0·12, | 0·16) | 18 | (14, | 21) | 0·1 | (0·08, | 0·12) | |
| High-income North America | 29 | (27, | 40) | 0·11 | (0·10, | 0·14) | 34 | (26, | 40) | 0·1 | (0·07, | 0·11) | |
| Southern Latin America | 33 | (26, | 37) | 0·67 | (0·53, | 0·75) | 20 | (16, | 34) | 0·33 | (0·26, | 0·55) | |
| Western Europe | 77 | (50, | 85) | 0·2 | (0·13, | 0·22) | 42 | (35, | 52) | 0·1 | (0·08, | 0·12) | |
| Latin America and Caribbean | |||||||||||||
| Andean Latin America | 55 | (47, | 68) | 1·42 | (1·22, | 1·77) | 44 | (35, | 60) | 0·77 | (0·62, | 1·05) | |
| Caribbean | 41 | (36, | 68) | 1·35 | (1·17, | 2·21) | 126 | (51, | 163) | 3·24 | (1·31, | 4·18) | |
| Central Latin America | 118 | (107, | 172) | 0·7 | (0·64, | 1·03) | 259 | (153, | 301) | 1·05 | (0·62, | 1·23) | |
| Tropical Latin America | 21 | (18, | 41) | 0·14 | (0·11, | 0·26) | 279 | (41, | 343) | 1·35 | (0·20, | 1·66) | |
| North Africa and Middle East | |||||||||||||
| North Africa and Middle East | 110 | (68, | 131) | 0·34 | (0·21, | 0·41) | 72 | (62, | 89) | 0·14 | (0·12, | 0·18) | |
| South Asia | |||||||||||||
| South Asia | 1,712 | (1,328, | 2,584) | 1·54 | (1·19, | 2·32) | 2,132 | (1,741, | 2,825) | 1·29 | (1·06, | 1·71) | |
| Southeast Asia, East Asia, and Oceania | |||||||||||||
| East Asia | 242 | (159, | 320) | 0·2 | (0·13, | 0·27) | 144 | (120, | 167) | 0·1 | (0·08, | 0·12) | |
| Oceania | 71 | (53, | 88) | 12·1 | (9·14, | 15·03) | 78 | (57, | 131) | 8·13 | (5·94, | 13·64) | |
| Southeast Asia | 5,645 | (2,727, | 7,741) | 12·4 | (5·97, | 16·95) | 5,376 | (2,494, | 6,765) | 8·49 | (3·94, | 10·68) | |
| Sub-Saharan Africa | |||||||||||||
| Central Sub-Saharan Africa | 42 | (21, | 62) | 0·81 | (0·40, | 1·18) | 62 | (39, | 89) | 0·62 | (0·38, | 0·88) | |
| Eastern Sub-Saharan Africa | 46 | (37, | 60) | 0·25 | (0·20, | 0·32) | 66 | (53, | 81) | 0·18 | (0·15, | 0·23) | |
| Southern Sub-Saharan Africa | 19 | (6, | 23) | 0·36 | (0·11, | 0·43) | 7 | (5, | 11) | 0·09 | (0·07, | 0·15) | |
| Western Sub-Saharan Africa | 260 | (129, | 374) | 1·32 | (0·65, | 1·89) | 299 | (202, | 412) | 0·82 | (0·55, | 1·13) | |
| Income category | |||||||||||||
| High income, OECD | 156 | (116, | 169) | 0.17 | (0.13, | 0.18) | 104 | (85, | 126) | 0.10 | (0.08, | 0.12) | |
| High income, nonOECD | 30 | (26, | 40) | 0.16 | (0.14, | 0.21) | 31 | (27, | 42) | 0.15 | (0.13, | 0.20) | |
| Upper middle income | 1,241 | (941, | 1,433) | 0.63 | (0.48, | 0.73) | 1,171 | (784, | 1,325) | 0.47 | (0.32, | 0.54) | |
| Lower middle income | 6,520 | (3,582, | 8,962) | 3.78 | (2.08, | 5.20) | 7,018 | (4,228, | 8,521) | 2.76 | (1.66, | 3.35) | |
| Low income | 797 | (471, | 1,026) | 1.58 | (0.93, | 2.03) | 754 | (489, | 912) | 0.87 | (0.56, | 1.05) | |
UI=uncertainty interval. OECD=Organisation for Economic Co-operation and Development.
Figure 1. Age-standardised mortality from dengue (per million person-years), in 2013.
ATG=Antigua and Barbuda. VCT=St Vincent and Grenadines. LCA=St Lucia. TTO=Trinidad and Tobago. TLS=Timor Leste. FSM=Federated States of Micronesia.
Table 2. Burden of dengue in 2013.
| Years lived with disability | Years of life lost | Disability-adjusted life-years | |||||
|---|---|---|---|---|---|---|---|
| Thousands (95% UI) | Per 100,000 person-years (95% UI) | Thousands (95% UI) | Per 100,000 person-years (95% UI) | Thousands (95% UI) | Per 100,000 person-years (95% UI) | ||
| Total | 565·9 (186·4, 1,414·6) |
7·84 (2·59, 19·61) |
576·9 (333·0, 701·2) |
7·96 (4·60, 9·68) |
1,142·7 (727·6, 1,978·2) |
15·81 (10·06, 27·38) |
|
| Sex | |||||||
| Male | 268·4 (88·3, 674·9) |
7·33 (2·41, 18·45) |
284·4 (153·4, 354·1) |
7·66 (4·13, 9·52) |
552·7 (344·9, 946·9) |
14·99 (9·34, 25·72) |
|
| Female | 297·5 (98·0, 739·7) |
8·37 (2·76, 20·81) |
292·5 (143·2, 370·1) |
8·29 (4·04, 10·49) |
590·0 (353·7, 1,042·8) |
16·66 (9·97, 29·40) |
|
| Age | |||||||
| Post Neonatal | 9·6 (3·2, 24·2) |
7·70 (2·54, 19·41) |
56·1 (26·1, 77·6) |
44·95 (20·93, 62·15) |
65·7 (36·2, 91·7) |
52·65 (28·98, 73·44) |
|
| 1 to 4 | 33·1 (10·5, 84·9) |
6·32 (2·01, 16·20) |
166·0 (88·4, 233·1) |
31·67 (16·85, 44·46) |
199·2 (123·5, 283·7) |
37·99 (23·56, 54·12) |
|
| 5 to 9 | 70·4 (22·6, 177·1) |
11·34 (3·64, 28·51) |
124·4 (55·8, 167·9) |
20·02 (8·98, 27·02) |
194·8 (109·7, 309·5) |
31·36 (17·66, 49·82) |
|
| 10 to 14 | 69·0 (22·3, 171·1) |
11·59 (3·75, 28·74) |
52·7 (28·7, 64·0) |
8·86 (4·82, 10·76) |
121·7 (72·3, 223·8) |
20·45 (12·14, 37·60) |
|
| 15 to 19 | 60·7 (20·1, 151·4) |
10·27 (3·41, 25·63) |
26·1 (15·5, 31·1) |
4·42 (2·62, 5·26) |
86·8 (46·0, 178·2) |
14·69 (7·79, 30·16) |
|
| 20 to 24 | 53·8 (18·2, 134·4) |
8·77 (2·97, 21·92) |
25·0 (17·5, 30·4) |
4·08 (2·85, 4·96) |
78·8 (42·7, 161·8) |
12·85 (6·97, 26·40) |
|
| 25 to 29 | 48·7 (16·0, 122·1) |
8·17 (2·69, 20·47) |
26·6 (18·7, 32·4) |
4·46 (3·14, 5·43) |
75·3 (42·5, 148·5) |
12·63 (7·13, 24·89) |
|
| 30 to 34 | 41·6 (13·4, 103·5) |
7·86 (2·54, 19·56) |
22·9 (16·3, 27·6) |
4·33 (3·09, 5·21) |
64·5 (36·6, 126·1) |
12·19 (6·92, 23·84) |
|
| 35 to 39 | 36·2 (11·3, 90·8) |
7·39 (2·30, 18·53) |
18·3 (13·1, 22·6) |
3·73 (2·67, 4·61) |
54·5 (30·0, 108·6) |
11·12 (6·13, 22·17) |
|
| 40 to 44 | 30·5 (9·8, 77·1) |
6·44 (2·08, 16·29) |
15·8 (11·9, 19·2) |
3·34 (2·52, 4·07) |
46·3 (25·6, 92·6) |
9·78 (5·41, 19·56) |
|
| 45 to 49 | 26·0 (8·6, 65·2) |
5·94 (1·96, 14·90) |
9·3 (6·7, 10·5) |
2·12 (1·54, 2·39) |
35·2 (17·9, 74·4) |
8·05 (4·10, 17·02) |
|
| 50 to 54 | 23·0 (7·5, 59·1) |
6·04 (1·97, 15·50) |
8·1 (5·7, 9·1) |
2·14 (1·50, 2·40) |
31·1 (15·9, 67·5) |
8·18 (4·17, 17·71) |
|
| 55 to 59 | 19·0 (6·2, 48·2) |
5·84 (1·91, 14·86) |
6·6 (4·8, 7·5) |
2·05 (1·48, 2·32) |
25·6 (12·9, 55·0) |
7·89 (3·97, 16·93) |
|
| 60 to 64 | 15·0 (4·9, 37·3) |
5·37 (1·77, 13·32) |
5·9 (4·6, 6·8) |
2·12 (1·63, 2·44) |
21·0 (11·0, 43·1) |
7·49 (3·94, 15·40) |
|
| 65 to 69 | 10·8 (3·5, 26·9) |
5·74 (1·88, 14·32) |
4·8 (3·8, 5·7) |
2·56 (2·00, 3·03) |
15·6 (8·4, 31·8) |
8·30 (4·47, 16·93) |
|
| 70 to 74 | 8·0 (2·7, 20·6) |
5·33 (1·78, 13·65) |
3·4 (2·5, 4·1) |
2·28 (1·68, 2·71) |
11·5 (6·2, 24·0) |
7·60 (4·12, 15·89) |
|
| 75 to 79 | 5·7 (1·9, 14·4) |
5·33 (1·79, 13·55) |
2·1 (1·4, 2·4) |
1·98 (1·35, 2·23) |
7·8 (4·0, 16·6) |
7·30 (3·79, 15·57) |
|
| 80 plus | 4·8 (1·6, 12·4) |
4·00 (1·31, 10·35) |
2·5 (1·8, 3·1) |
2·12 (1·48, 2·57) |
7·4 (4·1, 14·8) |
6·12 (3·42, 12·30) |
|
| Super-region/Region | |||||||
| Central Europe, Eastern Europe, and Central Asia | |||||||
| Central Asia | 0·9 (0·2, 2·8) |
1·06 (0·24, 3·23) |
1·0 (0·8, 1·3) |
1·06 (0·92, 1·39) |
1·9 (1·1, 3·7) |
2·12 (1·27, 4·28) |
|
| Central Europe | 0·0 (0·0, 0·0) |
0·00 (0·00, 0·00) |
0·6 (0·5, 0·9) |
0·58 (0·48, 1·01) |
0·6 (0·5, 0·9) |
0·58 (0·48, 1·01) |
|
| Eastern Europe | 0·0 (0·0, 0·0) |
0·00 (0·00, 0·00) |
1·0 (0·8, 1·4) |
0·55 (0·43, 0·71) |
1·0 (0·8, 1·4) |
0·55 (0·43, 0·71) |
|
| High-income | |||||||
| Australasia | <0·1 (0·0, 0·2) |
0·16 (0·02, 0·59) |
0·1 (0·1, 0·2) |
0·52 (0·33, 0·59) |
0·2 (0·1, 0·3) |
0·67 (0·44, 1·11) |
|
| High-income Asia Pacific | 0·2 (0·0, 0·5) |
0·10 (0·02, 0·34) |
0·8 (0·6, 0·9) |
0·53 (0·38, 0·61) |
0·9 (0·7, 1·3) |
0·63 (0·46, 0·90) |
|
| High-income North America | 0·5 (0·1, 2·0) |
0·15 (0·01, 0·57) |
1·7 (1·2, 1·9) |
0·52 (0·38, 0·62) |
2·2 (1·5, 3·7) |
0·67 (0·47, 1·10) |
|
| Southern Latin America | 1·5 (0·4, 4·0) |
2·35 (0·62, 6·38) |
1·1 (0·8, 1·8) |
1·86 (1·43, 3·06) |
2·5 (1·4, 5·0) |
4·20 (2·36, 8·16) |
|
| Western Europe | 0·0 (0·0, 0·0) |
0·00 (0·00, 0·00) |
1·9 (1·4, 2·1) |
0·51 (0·38, 0·58) |
1·9 (1·4, 2·1) |
0·51 (0·38, 0·58) |
|
| Latin America and Caribbean | |||||||
| Andean Latin America | 4·0 (1·3, 9·9) |
6·98 (2·32, 17·17) |
2·7 (2·1, 3·9) |
4·32 (3·45, 6·21) |
6·7 (3·9, 12·5) |
11·31 (6·45, 21·47) |
|
| Caribbean | 7·8 (2·7, 19·3) |
17·53 (6·00, 43·28) |
7·6 (2·8, 10·2) |
17·38 (6·28, 23·68) |
15·4 (8·8, 26·6) |
34·91 (19·87, 60·18) |
|
| Central Latin America | 23·3 (8·0, 57·2) |
9·33 (3·20, 23·00) |
13·3 (7·6, 16·0) |
5·28 (3·05, 6·30) |
36·6 (21·2, 70·8) |
14·61 (8·47, 28·44) |
|
| Tropical Latin America | 19·4 (6·6, 48·0) |
9·34 (3·20, 23·12) |
11·4 (1·9, 13·8) |
5·74 (0·91, 7·00) |
30·8 (15·0, 58·6) |
15·08 (7·32, 28·47) |
|
| North Africa and Middle East | |||||||
| North Africa and Middle East | 7·4 (2·2, 19·7) |
1·43 (0·42, 3·81) |
4·2 (3·6, 5·2) |
0·76 (0·66, 0·94) |
11·6 (6·3, 24·0) |
2·19 (1·18, 4·57) |
|
| South Asia | |||||||
| South Asia | 220·5 (77·9, 534·7) |
13·20 (4·68, 31·88) |
103·6 (82·6, 145·5) |
6·34 (5·10, 8·71) |
324·2 (178·8, 622·3) |
19·53 (10·87, 37·33) |
|
| Southeast Asia, East Asia, and Oceania | |||||||
| East Asia | 12·2 (3·4, 34·0) |
0·88 (0·25, 2·43) |
7·3 (6·0, 8·3) |
0·54 (0·44, 0·61) |
19·5 (10·6, 41·4) |
1·41 (0·78, 2·98) |
|
| Oceania | 1·3 (0·4, 3·1) |
11·81 (4·02, 29·12) |
3·8 (2·5, 7·3) |
37·67 (26·81, 65·23) |
5·1 (3·4, 8·9) |
49·48 (34·68, 82·81) |
|
| Southeast Asia | 212·6 (63·9, 557·9) |
33·25 (10·00, 87·25) |
384·1 (166·6, 494·0) |
58·77 (25·56, 75·58) |
596·7 (342·3, 952·0) |
92·02 (52·97, 147·61) |
|
| Sub-Saharan Africa | |||||||
| Central Sub-Saharan Africa | 5·0 (1·5, 13·0) |
4·77 (1·44, 12·53) |
4·6 (2·6, 6·7) |
2·99 (1·93, 4·24) |
9·6 (5·4, 17·0) |
7·76 (4·15, 15·22) |
|
| Eastern Sub-Saharan Africa | 10·6 (2·7, 30·6) |
2·88 (0·76, 8·28) |
3·8 (3·3, 5·0) |
1·05 (0·79, 1·28) |
14·4 (6·6, 34·1) |
3·93 (1·80, 9·21) |
|
| Southern Sub-Saharan Africa | 0·4 (0·1, 1·2) |
0·51 (0·13, 1·47) |
0·4 (0·3, 0·6) |
0·46 (0·37, 0·77) |
0·8 (0·4, 1·6) |
0·97 (0·55, 1·92) |
|
| Western Sub-Saharan Africa | 38·2 (13·6, 91·2) |
10·18 (3·64, 24·29) |
22·0 (13·5, 31·6) |
4·07 (2·81, 5·48) |
60·3 (32·9, 117·9) |
14·25 (7·29, 29·15) |
|
| Income category | |||||||
| High income, OECD | 0.6 (0.1, 2.2) |
0.05 (0.01, 0.21) |
4.8 (3.7, 5.5) |
0.45 (0.35, 0.52) |
5.3 (4.2, 7.2) |
0.50 (0.40, 0.68) |
|
| High income, nonOECD | 0.4 (0.1, 1.5) |
0.20 (0.03, 0.70) |
1.5 (1.3, 2.0) |
0.71 (0.60, 0.95) |
2.0 (1.5, 3.1) |
0.91 (0.70, 1.46) |
|
| Upper middle income | 87.4 (28.5, 221.0) |
3.53 (1.15, 8.93) |
61.5 (43.2, 71.5) |
2.49 (1.74, 2.89) |
148.9 (89.9, 282.2) |
6.02 (3.63, 11.41) |
|
| Lower middle income | 417.1 (137.5, 1,038.1) |
16.40 (5.40, 40.81) |
462.1 (252.7, 577.4) |
18.16 (9.94, 22.70) |
879.2 (560.9, 1,497.6) |
34.56 (22.05, 58.87) |
|
| Low income | 59.0 (19.6, 148.5) |
6.81 (2.26, 17.13) |
45.4 (29.8, 54.4) |
5.23 (3.43, 6.27) |
104.4 (63.5, 197.6) |
12.04 (7.32, 22.79) |
|
UI=uncertainty interval. OECD=Organisation for Economic Co-operation and Development.
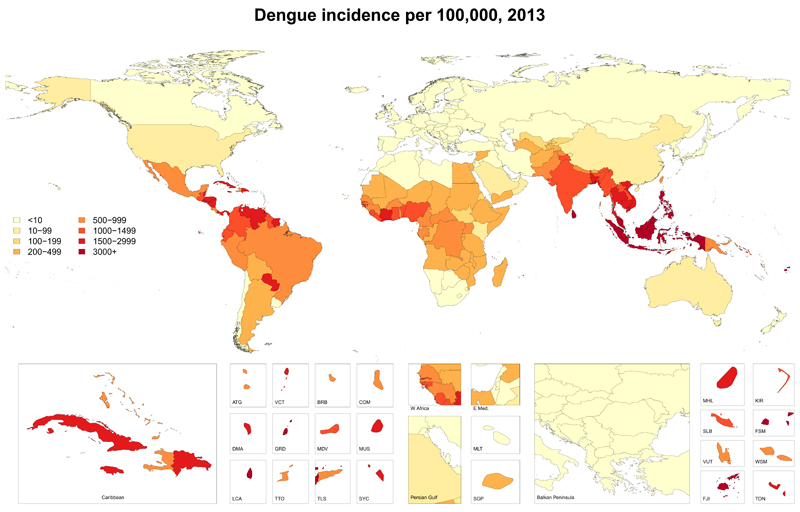
Our dataset included 1636 country-years of case reports of dengue from 76 countries, and expansion factor estimates for 14 countries. Our model suggests a substantial increase in the incidence of dengue between 1990 and 2013, with the number of apparent cases more than doubling every decade, from 8∙3 million (3∙3 million – 17∙2 million) in 1990 to 58∙4 million (23∙6 million – 121∙9 million) in 2013 (table 3). We estimated a global mean expansion factor of 12∙3 (6∙7 – 20∙8), meaning that, where we have official case reports, we believe that those reports capture an average of only 8% of symptomatic dengue infections. The highest age-standardised incidence rates occured in southeast Asia, with an annual average of 34∙3 (12∙7 – 75∙0) cases per 1000 people in the region (figure 2). A total of 566 000 (186 000 – 1,415 000) YLDs were attributable to dengue in 2013 (table 2), with post-infection chronic fatigue accounting for most of this disability: 7∙8% of YLDs were from moderate acute infection, 2∙9% were from severe acute infection, and 89∙9% were from chronic fatigue.
Table 3. Dengue cases and incidence rates for 1990 and 2013.
| 1990 | 2013 | ||||
|---|---|---|---|---|---|
| Cases (95% UI; thousands) Incidence (95% UI; | Cases (95% UI; thousands) Incidence (95% UI; | Cases (95% UI; thousands) Incidence (95% UI; | Cases (95% UI; thousands) Incidence (95% UI; | ||
| Total | 8,226 (3,297, 17,246) |
148·1 (59·4, 310·6) |
58,419 (23,611, 121,920) |
810·1 (327·4, 1,690·8) |
|
| Sex | |||||
| Male | 3,905 (1,567, 8,181) |
139·4 (56·0, 292·1) |
27,637 (11,182, 57,632) |
756·2 (306·0, 1,577·0) |
|
| Female | 4,321 (1,730, 9,065) |
157·3 (62·9, 330·1) |
30,782 (12,429, 64,287) |
865·8 (349·6, 1,808·2) |
|
| Age (years) | |||||
| Post Neonatal | 186 (75, 389) |
152·3 (61·3, 318·7) |
972 (393, 2,030) |
778·6 (314·7, 1,625·3) |
|
| 1 to 4 | 617 (248, 1,291) |
121·6 (48·9, 254·4) |
3,322 (1,342, 6,936) |
633·3 (255·9, 1,322·2) |
|
| 5 to 9 | 1,255 (504, 2,629) |
217·2 (87·2, 455·0) |
7,085 (2,857, 14,808) |
1,140·0 (459·8, 2,382·8) |
|
| 10 to 14 | 1,160 (465, 2,431) |
220·2 (88·3, 461·5) |
7,001 (2,831, 14,600) |
1,175·6 (475·5, 2,451·8) |
|
| 15 to 19 | 963 (385, 2,020) |
186·7 (74·7, 391·6) |
6,160 (2,497, 12,827) |
1,041·8 (422·2, 2,169·1) |
|
| 20 to 24 | 810 (324, 1,699) |
166·6 (66·6, 349·5) |
5,490 (2,227, 11,421) |
894·7 (363·0, 1,861·4) |
|
| 25 to 29 | 680 (272, 1,428) |
155·1 (62·0, 325·4) |
4,986 (2,018, 10,392) |
834·9 (337·9, 1,740·4) |
|
| 30 to 34 | 544 (218, 1,141) |
141·4 (56·6, 296·6) |
4,298 (1,736, 8,973) |
811·5 (327·7, 1,694·3) |
|
| 35 to 39 | 445 (179, 933) |
128·3 (51·5, 268·8) |
3,770 (1,521, 7,877) |
768·6 (310·1, 1,605·8) |
|
| 40 to 44 | 323 (130, 678) |
116·0 (46·6, 243·2) |
3,188 (1,283, 6,671) |
672·6 (270·8, 1,407·4) |
|
| 45 to 49 | 266 (107, 558) |
117·2 (47·0, 245·6) |
2,730 (1,100, 5,712) |
623·3 (251·1, 1,303·9) |
|
| 50 to 54 | 246 (99, 517) |
115·8 (46·4, 242·8) |
2,443 (985, 5,107) |
640·1 (258·2, 1,338·2) |
|
| 55 to 59 | 210 (84, 440) |
113·6 (45·5, 238·2) |
2,038 (824, 4,255) |
626·8 (253·4, 1,308·5) |
|
| 60 to 64 | 177 (71, 372) |
111·0 (44·5, 232·8) |
1,636 (662, 3,414) |
583·5 (236·1, 1,217·7) |
|
| 65 to 69 | 134 (54, 282) |
108·5 (43·5, 227·7) |
1,184 (479, 2,471) |
629·7 (254·7, 1,314·1) |
|
| 70 to 74 | 95 (38, 200) |
110·9 (44·3, 233·0) |
897 (363, 1,873) |
593·6 (240·1, 1,238·9) |
|
| 75 to 79 | 65 (26, 136) |
102·4 (40·7, 215·6) |
646 (261, 1,350) |
605·0 (244·4, 1,264·3) |
|
| 80 plus | 48 (19, 102) |
85·3 (33·9, 179·5) |
573 (231, 1,197) |
475·0 (192·0, 992·9) |
|
| Region | |||||
| Central Europe, Eastern Europe, and Central Asia | |||||
| Central Asia | 13 (3, 38) |
18·5 (4·5, 52·5) |
93 (23, 261) |
108·3 (27·0, 304·5) |
|
| Central Europe | 0 (0, 0) |
0·0 (0·0, 0·0) |
0 (0, 0) |
0·0 (0·0, 0·0) |
|
| Eastern Europe | 0 (0, 0) |
0·0 (0·0, 0·0) |
0 (0, 0) |
0·0 (0·0, 0·0) |
|
| High-income | |||||
| Australasia | 1 (0, 4) |
5·4 (0·6, 20·5) |
4 (1, 15) |
15·3 (1·9, 55·4) |
|
| High-income Asia Pacific | 4 (1, 13) |
2·5 (0·4, 8·1) |
16 (3, 50) |
10·2 (2·0, 31·4) |
|
| High-income North America | 15 (2, 56) |
5·3 (0·6, 20·1) |
51 (6, 190) |
14·6 (1·7, 54·2) |
|
| Southern Latin America | 24 (8, 57) |
49·8 (16·3, 116·1) |
151 (50, 352) |
242·5 (80·6, 563·9) |
|
| Western Europe | 0 (0, 0) |
0·0 (0·0, 0·0) |
0 (0, 0) |
0·0 (0·0, 0·0) |
|
| Latin America and Caribbean | |||||
| Andean Latin America | 58 (24, 121) |
148·1 (60·3, 307·1) |
414 (171, 855) |
726·4 (300·3, 1,497·9) |
|
| Caribbean | 136 (56, 283) |
377·0 (155·6, 782·6) |
811 (340, 1,665) |
1,823·4 (765·0, 3,742·9) |
|
| Central Latin America | 335 (141, 685) |
194·6 (82·2, 397·9) |
2,406 (1,021, 4,890) |
970·2 (411·7, 1,972·3) |
|
| Tropical Latin America | 307 (131, 621) |
196·4 (83·9, 397·5) |
2,019 (867, 4,064) |
973·7 (417·9, 1,959·8) |
|
| North Africa and Middle East | |||||
| North Africa and Middle East | 97 (34, 219) |
29·8 (10·5, 67·2) |
764 (273, 1,713) |
148·5 (53·2, 332·9) |
|
| South Asia | |||||
| South Asia | 3,218 (1,389, 6,436) |
285·3 (123·1, 570·5) |
22,851 (9,941, 45,549) |
1,379·1 (600·1, 2,748·6) |
|
| Southeast Asia, East Asia, and Oceania | |||||
| East Asia | 183 (61, 425) |
15·2 (5·1, 35·2) |
1,258 (432, 2,895) |
89·6 (30·7, 206·2) |
|
| Oceania | 18 (7, 37) |
272·2 (110·9, 563·7) |
128 (54, 264) |
1,226·2 (510·9, 2,519·8) |
|
| Southeast Asia | 3,188 (1,180, 6,958) |
682·2 (252·7, 1,488·3) |
21,841 (8,073, 47,725) |
3,432·6 (1,269·3, 7,497·8) |
|
| Sub-Saharan Africa | |||||
| Central Sub-Saharan Africa | 55 (20, 121) |
105·2 (38·6, 231·3) |
515 (190, 1,124) |
503·6 (186·4, 1,099·2) |
|
| Eastern Sub-Saharan Africa | 118 (37, 283) |
62·9 (19·9, 150·4) |
1,091 (349, 2,597) |
300·9 (96·3, 715·9) |
|
| Southern Sub-Saharan Africa | 6 (2, 16) |
11·4 (3·4, 27·6) |
42 (13, 103) |
52·8 (15·9, 127·5) |
|
| Western Sub-Saharan Africa | 448 (192, 905) |
223·7 (95·7, 451·9) |
3,963 (1,701, 7,977) |
1,071·9 (460·3, 2,157·3) |
|
| Income category | |||||
| High income, OECD | 17 (2, 67) |
1.9 (0.2, 7.3) |
60 (7, 225) |
5.7 (0.7, 21.3) |
|
| High income, nonOECD | 12/ (2, 40) |
6.3 (1.0, 21.1) |
47 (8, 150) |
22.1 (4.0, 70.2) |
|
| Upper middle income | 1,469 (589, 3,152) |
74.7 (29.9, 160.3) |
9,795 (3,958, 20,949) |
395.9 (160.0, 846.8) |
|
| Lower middle income | 6,541 (2,652, 13,955) |
379.2 (153.7, 809.0) |
46,653 (19,053, 99,229) |
1,833.9 (749.0, 3,900.7) |
|
| Low income | 851 (347, 1,814) |
168.3 (68.6, 358.8) |
6,616 (2,684, 14,150) |
763.2 (309.6, 1,632.1) |
|
UI=uncertainty interval. OECD=Organisation for Economic Co-operation and Development.
Figure 2. Age-standardised incidence rates of dengue (per 100 000 person-years), in 2013.
ATG=Antigua and Barbuda. VCT=St Vincent and Grenadines. LCA=St Lucia. TTO=Trinidad and Tobago. TLS=Timor Leste. FSM=Federated States of Micronesia.
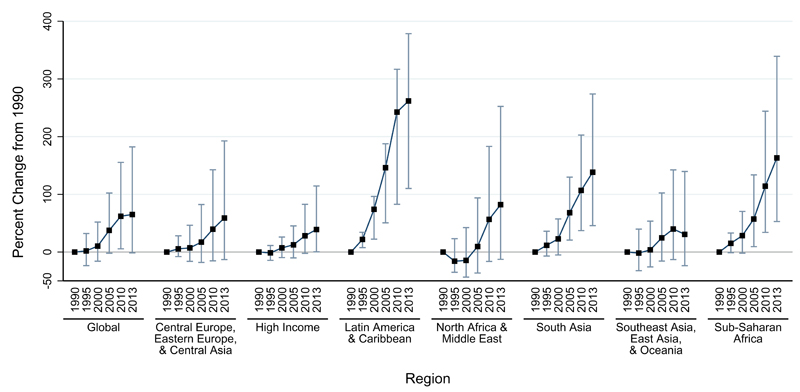
Considering both fatal and non-fatal outcomes together, dengue was responsible for 1∙14 million (0∙73 million – 1∙98 million) DALYs in 2013, representing a 58% increase from the 0∙72 million (0∙43 million – 0∙95 million) DALYs estimated for 1990 (table 2, figure 3). Given that incidence increased much more than did our mortality estimates, YLDs accounted for an increasing proportion of DALYs in later years: the proportion of DALYs from YLDs increased from 11∙1% to 49∙5% from 1990 to 2013, while the proportion from YLLs decreased from 88∙9% to 50∙5% during that period.
Figure 3. Change in disability-adjusted life-years for dengue since 1990 in dengue-endemic countries.
Dengue-endemic countries are those with a non-zero probability of dengue transmission based on Bhatt and colleagues.7
Discussion
Our results suggest that there are almost 60 million symptomatic dengue infections per year resulting in about 10 000 deaths. Our results also suggest a large increase in the incidence of dengue in the past two decades, with the number of symptomatic dengue infections more than doubling every 10 years between 1990 and 2013. In addition to this long-term secular trend, dengue occurs in both seasonal and interannual cycles that are obscured by the course temporal resolution of our estimates. The highest dengue incidence and mortality occurred in southeast Asia, where severe dengue is one of the leading causes of hospital admission and death among children.22 Overall, our findings underscore the growing disease burden of dengue on people and health systems in most tropical and subtropical countries worlwide.
Our mean estimate of dengue incidence for 2013 was lower than estimates from Bhatt and colleagues of 96 million apparent infections in 2010. However, the two estimates are not statistically significantly different (both have wide uncertainty levels) and their estimate falls within our 95% UI of 26∙6 to 121∙9 million cases. These two approaches have yielded results that bracket the commonly cited range of 50-100 million cases and offer more evidence that the true incidence probably falls within that range. Comparing regional estimates, our estimate of 5∙9 million dengue cases in 2013 in Latin America is similar to the estimate by Shepard and colleagues of 5∙6 million cases per year for 2000-07.23 However, our estimate of 21∙1 million cases in southeast Asia is notably higher than the of 2∙9 million cases per year estimated by Undurraga and colleagues for 12 countries in southeast Asia for 2001-10. 11 This difference is driven mainly by estimates for Indonesia, Malaysia, and Philippines, but the overall difference is probably caused by a combination of little available evidence at the time of the study, inadequate surveillance and substantial underreporting, and an increase in dengue incidence in the past years.
Two international vaccine trials, one in southeast Asia and one in Latin America, offer new data with which to validate our model-based estimates for nine countries, though differences in age categorization and the study years preclude a perfect comparison. Villar and colleagues24 studied Latin American children aged 9-16 years, from 2011 to 2013; we compared the incidence recorded in the control group in the trial to the Global Burden of Disease estimates for children aged 10–14 years in 2013. Our estimates were consistently lower than the incidence in the trial for all four Latin American countries, though our uncertainty intervals cover the trial estimate for Honduras. This finding is not very surprising given the diversity of dengue risk within large countries such as Mexico and Brazil, and the preference to conduct trials in the parts of a country where risk is greatest. Capeding and colleagues25 studied children aged 2-14 years in five southeast Asian countries, collecting data primarily in 2012. We compared the incidence recorded in the trial to our estimates for children aged 1–14 years in 2013. For all five countries, the uncertainty intervals overlapped; and for all countries except Thailand, our uncertainty intervals covered the point estimate from the trial.
Our estimates of the number of deaths caused by dengue each year ranged from 8365 in 1995, to 10 394 in 2010 (data not shown), lower than commonly cited figures of roughly 20 000. Our mortality estimates are also lower than those for the Global Burden of Disease Study 2010, although the differences are not statistically significant. Much of the change in mortality estimates between the 2010 and 2013 iterations of the Global Burden of Disease Study is driven by new data that suggest lower dengue mortality in some countries. For example, new detailed subnational mortality data are available from China, and with an average of 881 fewer cases per year estimated for 1990-2010, China’s estimates changed more than did those for any other country. Compared with the Global Burden of Disease Study 2010, our model estimates an average of about 2300 fewer deaths caused by dengue in non-endemic countries. This change was driven by a combination of new data and improved covariates, especially the newly added population-weighted variable for probability of dengue transmission. For 1990-2010, the total number of estimated deaths in dengue-endemic countries declined by a statistically nonsignificant amount of 250 deaths annually between the 2010 and 2013 iterations of the Global Burden of Disease Study. The important data gaps for several large, high-incidence countries (e.g. Indonesia) suggest that our mortality estimates should be interpreted with caution, and assessed in view of the totality of evidence suggesting that dengue mortality could, in fact, be substantially higher. We hope to address this limitation in future revisions of the Global Burden of Disease Study by acquiring additional data from other dengue-endemic countries.
Although our estimates of incidence and mortality overlapped geographically, the temporal trends in these two sets of estimates were quite different. Incidence has clearly increased, but the trends in mortality are more ambiguous, with a less pronounced increase. Our incidence model cannot differentiate true increases in incidence from increases in reporting. If we assume, however, that dengue has become more common, there are two possible explanations for our relatively flat mortality estimates. First, case fatality might be declining because of better clinical management of severe cases, changing numbers of average lifetime infections, or changing mortality risk due to changing age distribution of infections, which is known as endemic stability.26 Indeed the Pan American Health Organization reported a 29% decline in case fatality in the Americas between 2010 and 2013, suggesting that this explanation is plausible.27 Second, gaps in dengue mortality data – most notably in high-incidence countries in southeast Asia – might limit our ability to reliably estimate trends. It seems likely that both factors are contributing, at least to some degree, to our estimated mortality trends and this issue will require further attention in future iterations of the Global Burden of Disease Study.
The main strength of our results derives from combining multiple data sources and available evidence on dengue incidence and deaths, including the probability of dengue occurrence, adjustments for under-reporting, and a substantial refinement in our models to estimate dengue incidence and mortality rates. The main limitation relates to the limited availability and quality of surveillance data in many dengue-endemic countries, mostly including Africa and south Asia. Moreover, use made use of verbal autopsy data in our mortality estimates. Although this accounted for only 0.19% of our mortality data, it should be noted that verbal autopsy is an imperfect method for assigning deaths, especially for diseases like dengue that typically lack localising signs. There might be substantial under-reporting of fatal dengue episodes even in relatively well-funded health systems,28 and possibly misdiagnosis in countries with other predominant febrile illness such as malaria.29 Seasonal variations in transmission, dengue severity, access to healthcare, improvements in surveillance systems, and also, the fact that dengue is becoming a reportable disease in more countries, all affect the extent to which dengue is under-reported in any given country and year. New studies of the under-reporting of dengue have been published that were not included in our model, and we expect to include more evidence on our next round of estimates. Conversely, over-reporting in some areas – especially during epidemics – might also occur, and we lack adequate expansion factors to address this issue. However, we believe that if such over-reporting occurs, the overall effect should be minor.30 Finally, our DALY estimates do not capture several unique societal burdens of dengue. Its extreme year-to-year variability and potential for rapid onset of severe disease create fear in endemic areas, precautionary hospital admissions, health system congestion during outbreaks, and risk of damaging tourist economies.
We found limited age-specific data on the incidence of dengue. In the absence of better data, the age distribution that we imposed on cases represents a crude approximation. The true age distribution in a given country and year will be driven primarily by the overall force of infection and acquisition of immunity, with a higher force of infection producing an age pattern shifted toward younger age groups. With the prospect of a dengue vaccine, accurate age-specific estimates might become increasingly important for understanding the implications of recommendations for vaccine use. We hope to address this shortcoming in future revisions of the Global Burden of Disease Study. Future work will refine these expected age distributions by taking into account an area’s force of infection (as measured by age-stratified seroprevalence surveys), serotype history and different theories of acquisition of type-specific and heterologous immunity.31
Finally, the overall magnitude of dengue incidence was calibrated against expansion factor data. As we have expansion factor estimates from only 14 countries, this might represent the weakest evidential link in our modelling chain. Accordingly, we have propagated uncertainty from these expansion factors into our final estimates, and uncertainty in these expansion factors is the largest single source of uncertainty in our final incidence estimates. We believe that the resulting uncertainty intervals around our case estimates for recent years accurately reflect the uncertainty in these estimates. However, given our modelling approach, the widths of these uncertainty intervals are relative to the magnitude of point estimate, and were thus narrower for earlier years when our incidence estimates were lower. It is likely, then, that the uncertainty intervals for case estimates for earlier years underestimate the true uncertainty in those estimates.
Dengue is among the diseases with the highest increase in age-standardized incidence rates between 1990 and 2013, which counters the global trend of lessening communicable diseases. The results presented here constitute one of the most comprehensive efforts to quantify the burden of dengue in countries with evidence of ongoing dengue transmission. The methods represent a major improvement compared to those used in the Global Burden of Disease Study 2010, and we expect to keep updating and improving their accuracy. Our hope is that these improved estimates of dengue incidence and mortality, and their longer term trends, will help public health officials, scholars, and policy makers to assess and identify cost-effective control strategies to reduce the dengue transmission and disease burden.
Supplementary Material
Role of funding source
This research was supported by funding from the Bill & Melinda Gates Foundation. The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Contributors
JDS, DSS, YAH, EAU, LEC, OJB, and SIH prepared the first draft. All other authors provided critical feedback and edits to finalise the manuscript. JDS performed the final statistical analyses and prepared all tables and figures. CJLM conceived of the study and provided overall guidance. All other authors provided data, review results, provided guidance on methods, and reviewed the report.
Declaration of interests
LEC, CJLM, and JDS, have received grants from Bill & Melinda Gates Foundation, during the study; KBG received a 2011 Gustav Nossal Postgraduate Scholarship sponsored by CSL Behring. EAU and DSS have received grants from Sanofi Pasteur. The other authors declare no competing interests.
References
- 1.Brady OJ, Gething PW, Bhatt S, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DengueNet. [accessed April 24, 2015];WHO. http://www.who.int/csr/disease/dengue/denguenet/en/
- 3.World Health Organization. Vector borne and neglected tropical diseases. SEARO; [accessed June 24, 2015]. http://www.searo.who.int/entity/vector_borne_tropical_diseases/en/ [Google Scholar]
- 4.PAHO. Number of Reported Cases of Dengue and Severe Dengue in the Americas, by Country. 2014 published online June 5 http://www.paho.org/hq/index.php?option=com_topics&view=readall&cid=3273&Itemid=40734&lang=en.
- 5.WPRO. Annual Dengue Data in the Western Pacific Region. WPRO; [accessed June 24, 2015]. http://www.wpro.who.int/emerging_diseases/annual.dengue.data.wpr/en/ [Google Scholar]
- 6.Dengue. [accessed April 10, 2015];WHO. http://www.who.int/denguecontrol/en/
- 7.Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Impact of Dengue. [accessed April 24, 2015];WHO. http://www.who.int/csr/disease/dengue/impact/en/
- 9.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmons CP, Farrar JJ, van Vinh Chau N, Wills B. Dengue. N Engl J Med. 2012;366:1423–32. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 11.Undurraga EA, Halasa YA, Shepard DS. Use of Expansion Factors to Estimate the Burden of Dengue in Southeast Asia: A Systematic Analysis. PLoS Negl Trop Dis. 2013;7:e2056. doi: 10.1371/journal.pntd.0002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shepard DS, Undurraga EA, Betancourt-Cravioto M, et al. Approaches to refining estimates of global burden and economics of dengue. PLoS Negl Trop Dis. 2014;8:e3306. doi: 10.1371/journal.pntd.0003306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray Christopher JL, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015 Aug 26; doi: 10.1016/S0140-6736(15)61340-X. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vos Theo, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foreman KJ, Lozano R, Lopez AD, Murray CJ. Modeling causes of death: an integrated approach using CODEm. Popul Health Metr. 2012;10:1. doi: 10.1186/1478-7954-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salomon JA. New disability weights for the global burden of disease. Bull World Health Organ. 2010;88:879. doi: 10.2471/BLT.10.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haagsma JA, de Noordhout CM, Polinder S, et al. Assessing disability weights based on the responses of 30,660 people from four European countries. Popul Health Metr. 2015;13:10. doi: 10.1186/s12963-015-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol. 2007;5:518–28. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- 20.WHO. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control, New edition. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 21.Teixeira L de AS, Lopes JSM, da Martins AGC, Campos FAB, Miranzi S de SC, Nascentes GAN. Persistence of dengue symptoms in patients in Uberaba, Minas Gerais State, Brazil. Cad Saúde Pública. 2010;26:624–30. doi: 10.1590/s0102-311x2010000300019. [DOI] [PubMed] [Google Scholar]
- 22.Gubler DJ, Ooi EE, Vasudevan S, Farrar J, editors. Dengue and Dengue Hemorrhagic Fever. 2 edition. Wallingford, Oxfordshire; Boston, MA: CABI; 2014. [Google Scholar]
- 23.Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH. Economic Impact of Dengue Illness in the Americas. Am J Trop Med Hyg. 2011;84:200–7. doi: 10.4269/ajtmh.2011.10-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villar L, Dayan GH, Arredondo-García JL, et al. Efficacy of a Tetravalent Dengue Vaccine in Children in Latin America. New England Journal of Medicine. 2015;372:113–23. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 25.Capeding MR, Tran NH, Hadinegoro SRS, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. The Lancet. 2014;384:1358–65. doi: 10.1016/S0140-6736(14)61060-6. [DOI] [PubMed] [Google Scholar]
- 26.Egger JR, Ooi EE, Kelly DW, Woolhouse ME, Davies CR, Coleman PG. Reconstructing historical changes in the force of infection of dengue fever in Singapore: implications for surveillance and control. Bull World Health Organ. 2008;86:187–96. doi: 10.2471/BLT.07.040170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.PAHO/WHO. Five-fold increase in dengue cases in the Americas over the past decade. [accessed April 14, 2015];2014 published online May 29 http://www.paho.org/hq/index.php?option=com_content&view=article<id=9657%3Alos-casos-de-dengue-en-las-americas-se-quintuplicaron-en-diez-anos-segun-nuevos-datos-de-la-opsoms&Itemid=1926&lang=en.
- 28.Tomashek KM. The Acute Febrile Illness Surveillance Study in Puerto Rico: Findings from the First Two Years. Idsa; 2014. [accessed June 24, 2015]. https://idsa.confex.com/idsa/2014/webprogram/Paper47354.html. [Google Scholar]
- 29.Senn N, Luang-Suarkia D, Manong D, Siba PM, McBride WJH. Contribution of dengue fever to the burden of acute febrile illnesses in Papua New Guinea: an age-specific prospective study. Am J Trop Med Hyg. 2011;85:132–7. doi: 10.4269/ajtmh.2011.10-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chairulfatah A, Setiabudi D, Ridad A, Colebunders R. Clinical manifestations of dengue haemorrhagic fever in children in Bandung, Indonesia. Ann Société Belge Médecine Trop. 1995;75:291–5. [PubMed] [Google Scholar]
- 31.Imai N, Dorigatti I, Cauchemez S, Ferguson NM. Estimating Dengue Transmission Intensity from Sero-Prevalence Surveys in Multiple Countries. PLoS Negl Trop Dis. 2015;9:e0003719. doi: 10.1371/journal.pntd.0003719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.