Abstract
Promising results have been reported in preclinical stroke target validation for pharmacological principles that disrupt the N-methyl-D-aspartate receptor–post-synaptic density protein-95–neuronal nitric oxide synthase complex. However, post-synaptic density protein-95 is also coupled to potentially neuroprotective mechanisms. As post-synaptic density protein-95 inhibitors may interfere with potentially neuroprotective mechanisms and sufficient validation has often been an issue in translating basic stroke research, we wanted to close that gap by comparing post-synaptic density protein-95 inhibitors with NOS1−/− mice and a NOS inhibitor. We confirm the deleterious role of NOS1 in stroke both in vivo and in vitro, but find three pharmacological post-synaptic density protein-95 inhibitors to be therapeutically ineffective.
Keywords: Nitric oxide, stroke, excitotoxicity, experimental, free radicals
Introduction
Preclinical research has a high failure rate when it comes to clinical translation.1,2 In stroke, the third most frequent cause of death and main cause of disability, the situation is particularly desperate. Only a single drug, rt-PA, is approved, and the majority of stroke patients do not even qualify for receiving rt-PA due to numerous contraindications. With this high medical need, pharmacological inhibition of cytotoxic radical formation3 such as nitric oxide (NO) holds considerable promise. Neuronal NO synthase (NOS1) binds to and is regulated by the N-methyl-D-aspartate receptor (NMDAR) via the post-synaptic density protein-95 (PSD-95, Figure 1).4 Promising animal studies have suggested a feasible therapeutic approach by blocking the NMDAR-PSD-95-NOS1 interactions.4–8 As a major advantage in comparison with direct inhibitors of the NMDAR, which have failed clinically, the PSD-95 blocking peptide Tat-NR2B9c, its dimeric form Tat-N-dimer, or the small molecule ZL006 should specifically suppress NO-mediated neurotoxicity4 whilst leaving other vital NMDAR functions intact. These novel substances have been reported to strongly lower the stroke infarct volume (up to mean cortical infarct volume 87.0 (4.4 SE))6 and improve functional outcome after ischemic stroke when compared to control groups in rodents and non-human primates.6–12
Figure 1.
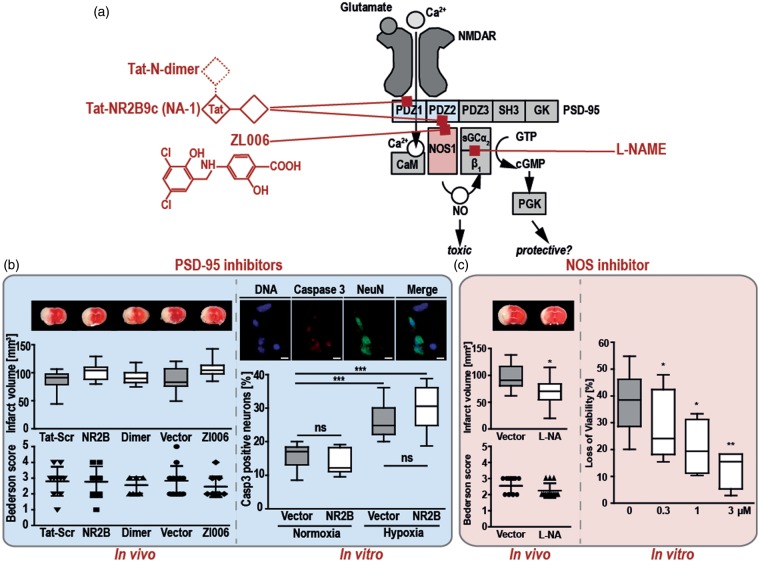
In vivo and in vitro PSD-95 inhibition fails to be neuroprotective upon ischemic damage, whilst NOS1 deletion or inhibition is neuroprotective. (a) Schematic structure of the NMDAR-PSD-95-NOS1 complex, with the binding sites of the three PSD-95i: NR2B, Dimer, and Zl006. Upon binding of glutamate, calcium enters through the transmembrane NMDAR and binds to CaM, which in turn activates NOS1. Its product, NO in low amounts activates sGC to form cGMP and activates neuroprotective mechanisms via PKG.13 All three, the NMDAR, NOS1, and sGC can co-localize at PDZ domains of PSD-95. Overproduction of NO can lead to neurotoxicity, possibly by interacting with additional reactive oxygen species formed during stroke.3 PSD-95i can thus potentially have neuroprotective (by inhibiting NOS1) or neurotoxic (by preventing neuroprotective cGMP formation) effects, or a mixture of both. (b) In vivo effects of PSD-95i, 3 nmol/g NR2B or Dimer, 1.5 mg/kg Zl006, a control peptide (Tat-Scr) or potassium chloride (vehicle), applied 60 min after tMCAO in C57BL/6 mice (left blue box). No protective effect on stroke volume, analyzed by TTC staining of brain slices (see representative pictures above the box plot of infarct volume showing the 5–95 percentile), or functional recovery (scatter dot plot with mean and SD) was observed 24 h after treatment (nTat-Src = 10, n NR2B = 9, n Dimer = 9, nVector = 12, n Zl006 = 12; Kruskal-Wallis test (p value = 0.0296 for infarct volume and p = 0.6781 for the Bederson test) with Dunn’s multiple comparison test, no group vs. the other was p < 0.05). In vitro effects of the PSD-95i, NR2B, on hippocampal neuronal cultures after OGD (right blue box). Following OGD, significantly higher numbers of apoptotic neurons were counted when both control groups were compared (***p < 0.0001; 95% CI −17.27 to −4.26). NR2B had no effect, neither under normoxic nor hypoxic conditions (***p < 0.0001; 95% CI −22.22 to −9.88) (Box plots with whiskers represent 5–95 percentile of the percentage of caspase 3 positive, i.e. apoptotic neurons, under normoxic or OGD conditions). (c) In vivo, the NOS inhibitor L-NAME (L-NA;1.5 mg/kg; n = 12) significantly lowered the infarct volumes 24 h after 1 h of tMCAO when compared with the control animals (left red box; n = 13; Unpaired t-test; *p = 0.0148; CI 5.55 to 46.22). With the Bederson test both animal groups were not significantly different (scatter dot plot showing mean and SD; Mann Whitney test, p = 0.1767). In vitro, in rat brain slices challenged with 15 min OGD and 120 minutes reoxygenation, L-NAME (0.3, 1 and 3 µM), lowered the loss of viability in a concentration-dependent manner when compared with control brain slices (right red box). Data were normalized to control slices without OGD and reoxygenation, and are presented as box plots with 5–95 percentile of n = 8 independent experiments (*p < 0.05, **p < 0.01). CaM: calmodulin, cGMP: cyclic guanosine monophosphate, Dimer: Tat-N-dimer; L-NAME: Nω-Nitro-L-arginine methyl ester hydrochloride; NMDAR: N-methyl-D aspartate receptor; NO: nitric oxide; NOS1: nitric oxidase synthase1, NR2B: Tat-NR2B9c; OGD: oxygen and glucose deprivation; PKG: cGMP-dependent protein kinase G; PSD-95: post-synaptic density protein-95; PSD-95i: PSD-95 inhibitors; sGC: soluble guanylate cyclase; tMCAO: transient middle cerebral artery occlusion; TTC: 2,3,5-triphenyltetrazolium chloride.
Based on these reports, PSD-95i have entered into clinical development for multiple indications such as acute ischemic stroke and subarachnoid hemorrhage (www.nonoinc.ca) including a phase 2 human trial using Tat-NR2B9c in patients with procedurally induced brain ischemia.14 However, mechanistically PSD-95 is also coupled to potentially neuroprotective mechanisms, such as soluble guanylate cyclase (sGC) and cGMP signaling.15 We therefore wanted to cross-validate PSD-95i with Nos1−/− mice and a NOS inhibitor.
Methods
Animal experiments
All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, the local institutional guidelines for the use of experimental animals of the University of Würzburg (Germany) approved by the local governmental authorities Regierung von Unterfranken (Würzburg, Germany), the institutional Ethics Committee of the Universidad Autónoma de Madrid (Spain), the European Guidelines for the use and care of animals for research, the European Communities Council Directive of 24 November 1986 (86/609/EEC), the Spanish Real Decreto of 10 October 2005 (RD 1201/2005), and followed the ARRIVE guidelines (http://www.nc3rs.org.uk/arrive-guidelines). Cerebral ischemia was induced by 60 min of transient middle cerebral artery occlusion (tMCAO) in mice and 90 min tMCAO in rats. Neurological deficits in the mice and the rats were scored according to Bederson and with the grip test at indicated time points. A more detailed description of the animal experiments and the exclusion criteria are provided in the Supplementary methods.
Compounds
Tat-NR2B9c monomer and Tat-N-dimer were obtained from Peps 4LS GmbH. ZL006 and the NOS inhibitor, Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME), were purchased from Sigma-Aldrich Chemie GmbH.
Immunohistochemistry
Cryo-embedded brain slices were stained overnight with an antibody against HIV1 tat (abcam, ab63957, 1:200) and a fluorescent Hoechst dye (Sigma-Aldrich, 33342, 1:500). Hippocampal neuronal cell cultures were co-labelled with an antibody against cleaved caspase-3 (Cell Signaling, #9661,1:400) and NeuN (Merck Millipore, MAB377, 1:100)
Oxygen–glucose deprivation of murine neuronal cell cultures and rat hippocampal slices and quantification of viability
Neuronal cell cultures were obtained from C57BL/6 mice embryos (E18) and Tat-NR2B9c or vehicle was added to the cell cultures 1 h prior to the induction of oxygen–glucose deprivation (OGD). Cell viability was assessed by an antibody against activated caspase-3 as described above. In vitro damage caused by OGD followed by re-oxygenation (OGD/Reox) and the protection elicited by L-NAME was studied in acutely isolated rat hippocampal slices. Hippocampal cell viability was determined through the ability of the cells to reduce MTT.
A more detailed method description and the statistical analysis are provided in the supplementary files.
Results and discussion
We could confirm the role of NOS1 in rodent (rat and mice) stroke models both in vivo (Figure 1(c) and Supplementary Figure 1(c)) and in vitro (Figure 1(c)). Conversely, in our hands, three different and structurally unrelated pharmacological PSD-95i were completely ineffective both in vivo and in vitro (Figure 1(b)). Treatment of young (8–10 weeks old) and middle aged (55 weeks old) male C57BL/6 mice with Tat-NR2B9c monomer (NR2B; 3 nmol/g, i.v. or), Tat-N-dimer (Dimer) with higher affinity (3 nmol/g, i.v.) or the small molecule inhibitor ZL006 (1.5 mg/kg, i.v.) at reperfusion after 60 min of tMCAO using the filament technique neither altered stroke volumes nor neurological deficits between 6 and 72 h post-stroke (Figure 1(b) and Supplementary Figure 2) when compared with control animals. Even a high dose of NR2B (10 nmol/g), which recently has been reported to protect mice from acute ischemic stroke,12 was ineffective in our hands (Supplementary Figure 1(a)). Blocking of PSD-95 also had no effect in female mice subjected to tMCAO or in a mouse model of permanent stroke (Supplementary Figure 2). Moreover, NR2B failed to ameliorate ischemic brain damage in a second species, i.e. rat (Supplementary Figure 1(b)). Immunohistochemistry revealed that Tat-NR2B readily crossed the blood–brain-barrier (Supplementary Figure 3).11 In line with our in vivo results, NR2B did not prevent cell death in vitro in hippocampal primary neuronal cell cultures under conditions of glucose and oxygen deprivation (Figure 1(b)), while NOS inhibition with L-NAME could prevent cell death after OGD with reoxygenation in rat hippocampal brain slices (Figure 1(c)).
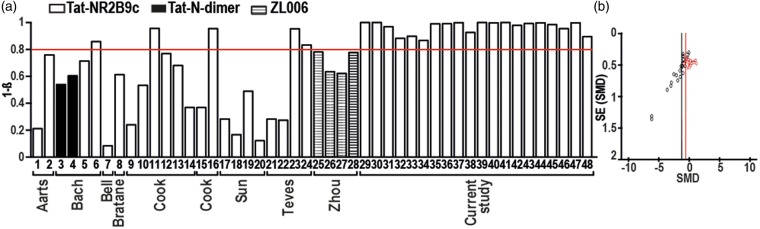
Because of the discrepancy between our results and the published protective effects of the PSD-95i, we performed a post-hoc analysis of previous animal studies on PSD-95 and stroke. It became apparent that most of the previous studies on PSD-95i lacked sufficient statistical power (Figure 2(a), Supplementary Table 2) to detect a clinically relevant 40% reduction in infarct size. Moreover, further analyses suggested a publication bias towards positive findings leaving negative data unpublished (Figure 2(b)), which is reminiscent of our recent study on a reactive oxygen generating enzyme and suggested stroke target.2
Figure 2.
Power analysis and publication bias of previous and our PSD-95i studies. (a) The bar graphs show the level of power from each study and subgroup as numbered in the first column of Supplementary Table S1. The red line indicates the acceptable statistical Power of 0.8, which is reached by only 7 of 28 published data sets and by all data sets of the present study. (b) For previous studies (black symbols) with PSD-95i in experimental stroke, a funnel plot was constructed (Y-axis representing precision; X-axis, effect size of individual studies). Based on the fact that precision in estimating the underlying treatment effect will increase as the sample size of component studies increases, the observed asymmetry suggests a publication bias. Studies showing larger infarcts in PSD-95i treated animals are missing and the mean effect size (SMD −1.19; black line) might be an overestimation of the true effect. When adding our own current data (red symbols), the overall effect shifted towards a smaller overall effect of PSD-95 inhibition (SMD −0.68; red line) and the asymmetry remained, indicating that still studies or data sets are missing. PSD-95i: PSD-95 inhibitors.
Summary/conclusions
At least two possible conclusions can be drawn from our data, both of which are equally alarming: either our negative pre-clinical rodent validation of PSD-95i in stroke is correct and predictive, then clinical development of PSD-95i should be reconsidered; or the non-human primate and early clinical data are correct and relevant, then rodent animal models can no longer be viewed as predictive for human stroke and pre-clinical research needs to fundamentally change (a conclusion that would be reminiscent of recent discussions whether inflammatory rodent models have any predictive value for human immune conditions). Both conclusions have wide implications for preclinical research in general, i.e. well beyond stroke and the clinical development of PSD-95i, and thus need an urgent cross validation and broad discussion.
Supplementary Material
Acknowledgements
The authors thank Andrea Sauer, Melanie Glaser, Barbara Reuter, Sabrina Braunschweig and Daniela Urlaub for their expert technical assistance.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study has been supported by funds from the European Research Council Advanced Investigator Grant 294682 RADMED (HHHWS) and the Deutsche Forschungsgemeinschaft (CK).
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HHHWS is co-founder of a biotech company, Vasopharm GmbH, Germany, engaged in the development of small molecule NOS inhibitors, currently in stage II/III clinical development. However, HHHWS has no operative or decision making role in the company and holds less than 1% of shares.
Authors’ contributions
CK analyzed the stroke experiments and wrote the manuscript; SM performed all animal surgeries, drug treatments and evaluations of the outcomes in a blinded manner; PWMK performed power analysis and funnel plot; MKS performed and analyzed all OGD neuronal cell culture experiments; MGL planned and analyzed the rat hippocampal slice experiments; AIC performed all experiments with rat hippocampal slices; BS revised the manuscript for the statistical guidelines, AR wrote and revised the manuscript; HHHWS planned experiments and wrote the manuscript;
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.O’Collins VE, Macleod MR, Donnan GA, et al. 1,026 Experimental treatments in acute stroke. Ann Neurol 2006; 59: 467–477. [DOI] [PubMed] [Google Scholar]
- 2.Kleikers PW, Hooijmans C, Göb E, et al. A combined pre-clinical meta-analysis and randomized confirmatory trial approach to improve data validity for therapeutic target validation. Sci Report 2015; 5: 13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleinschnitz C, Grund H, Wingler K, et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol 2010; 8: e1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Z, Huang PL, Panahian N, et al. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science 1994; 265: 1883–1885. [DOI] [PubMed] [Google Scholar]
- 5.Sattler R, Xiong Z, Lu WY, et al. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science 1999; 284: 1845–1848. [DOI] [PubMed] [Google Scholar]
- 6.Aarts M, Liu Y, Liu L, et al. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science 2002; 298: 846–850. [DOI] [PubMed] [Google Scholar]
- 7.Bach A, Clausen BH, Møller M, et al. A high-affinity, dimeric inhibitor of PSD-95 bivalently interacts with PDZ1-2 and protects against ischemic brain damage. Proc Natl Acad Sci U S A 2012; 109: 3317–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook DJ, Teves L, Tymianski M. A translational paradigm for the preclinical evaluation of the stroke neuroprotectant Tat-NR2B9c in gyrencephalic nonhuman primates. Sci Transl Med 2012; 4: 154ra33. [DOI] [PubMed] [Google Scholar]
- 9.Hajdu MA, Baumbach GL. Mechanics of large and small cerebral arteries in chronic hypertension. Am J Physiol 1994; 266: H1027–H1033. [DOI] [PubMed] [Google Scholar]
- 10.Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature 2012; 483: 213–217. [DOI] [PubMed] [Google Scholar]
- 11.Zhou L, Li F, Xu HB, et al. Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat Med 2010; 16: 1439–1443. [DOI] [PubMed] [Google Scholar]
- 12.Teves L, Cui H, Tymianski M. Efficacy of the PSD95 inhibitor Tat-NR2B9c in mice requires dose translation between species. J Cereb Blood Flow Metab 2015; 19: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding JD, Burette A, Nedvetsky PI, et al. Distribution of soluble guanylyl cyclase in the rat brain. J Comparat Neurol 2004; 472: 437–448. [DOI] [PubMed] [Google Scholar]
- 14.Hill MD, Martin RH, Mikulis D, et al. Safety and efficacy of NA-1 in patients with iatrogenic stroke after endovascular aneurysm repair (ENACT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol 2012; 11: 942–950. [DOI] [PubMed] [Google Scholar]
- 15.Russwurm M, Wittau N, Koesling D. Guanylyl cyclase/PSD-95 interaction: targeting of the nitric oxide-sensitive alpha2beta1 guanylyl cyclase to synaptic membranes. J Biol Chem 2001; 276: 44647–44652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.