Abstract
With the increased spectral resolution made possible at high fields, a second, smaller inorganic phosphate resonance can be resolved on 31P magnetic resonance spectra in the rat brain. Saturation transfer was used to estimate de novo adenosine triphosphate synthesis reaction rate. While the main inorganic phosphate pool is used by adenosine triphosphate synthase, the second pool is inactive for this reaction. Accounting for this new pool may not only help us understand 31P magnetic resonance spectroscopy metabolic profiles better but also better quantify adenosine triphosphate synthesis.
Keywords: Mathematical modelling, energy metabolism, adenosine triphosphate, pH, magnetic resonance spectroscopy
Introduction
Phosphorus (31P) magnetic resonance spectroscopy (MRS) can be used to non-invasively quantify metabolites involved in energy metabolism, such as adenosine triphosphate (ATP), phosphocreatine (PCr) and inorganic phosphate (Pi). It has been widely used to study muscles but also brain and liver (Befroy et al.1 and references herein). In addition to the metabolic profile, 31P MRS has the added benefit of allowing the non-invasive measurement of pH.2 Furthermore, saturation transfer experiments can be used to quantify reaction rates between exchanging pools3 and can be of particular interest when studying brain metabolism together with other methods.4
At high magnetic fields, there is an increase in 31P spectral resolution that can help differentiate peaks that would otherwise overlap. Previous studies in the muscle have reported resonances between 5.1 and 5.3 ppm that could be attributed to a second pool of Pi (the main pool resonating at 4.9 ppm). These studies suggested that the alkaline pool of Pi may originate from extracellular space,5 blood6 or mitochondrial space.7,8 Other studies at lower fields have been trying to deconvolve the broad Pi peak into various number of pools and attribute them to specific cellular compartments in isolated brains.9–11 A very recent study in the human brain12 found the second Pi pool resonating at 5.24 ppm to be insensitive to selective inversion of PCr and γ-ATP,13 suggesting the absence of chemical exchange with Pi and thus supporting the extra-cellular hypothesis.
The aim of this study was to combine high magnetic field (11.7 T) and spectroscopic localization to achieve good spectral resolution and test this hypothesis, i.e. characterize Pi pools (noted Pi4.9 and Pi5.3) in the rat brain, including saturation transfer experiments to estimate the participation of each pool to ATP synthesis. We also evaluated how not accounting for the existence of this smaller pool of Pi could possibly result in biased estimation of ATP synthase reaction rate (kfATPase).
Methods
Animal preparation
This study was conducted using five Sprague Dawley male rats (8–12 months old). All experiments were conducted according to the French regulation (Directive 2010/63/EU—French Act Rural Code R 214-87 to 131). The animal facility was approved by veterinarian inspectors (authorization #B 92-032-02) and complies with Standards for Humane Care and Use of Laboratory Animals of the Office of Laboratory Animal Welfare (OLAW—#A5826-01). All experimental procedures were approved by the CEA Ethic Committee (committee #44, approval #10-057). Reporting of this work complies with ARRIVE guidelines. Rats were scanned using a horizontal 11.7 T Bruker scanner (Bruker, Ettlingen, Germany) and a double tuned 1H-31P transmit-receive surface coil (Rapid Biomed GmbH, Rimpar, Germany). Rats were anesthetized with isoflurane at a concentration kept under 2% to minimize effects on metabolism,14 and fixed on a stereotaxic frame to minimize motion artifacts.
Data acquisition
Anatomical images were obtained using a standard T2 sequence and used to position a 10 × 7 × 11 mm3 voxel covering most of the brain (Figure 1). Localized shimming (∼20–25 Hz) was automatically performed after acquisition of a B0 map using the “MAPSHIM” Bruker routine.
Figure 1.
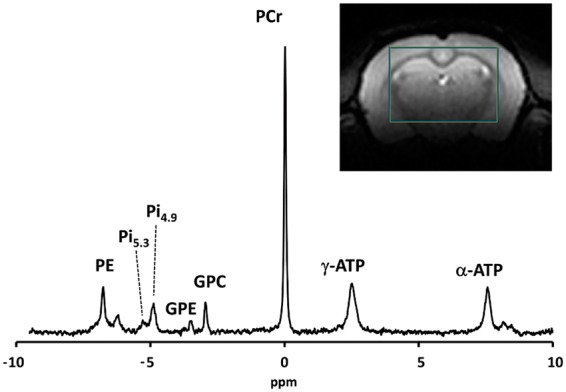
Unsaturated 31P spectra acquired in a large voxel of the rat brain, averaged over two animals, showing different resonances; phosphoethanolamine (PE), the two pools of inorganic phosphate (dominant Pi4.9, and newly resolved Pi5.3), glycerol 3-phosphorylethanolamine (GPE), glycerol 3-phosphorylcholine (GPC), phosphocreatine (PCr), and ATP resonances (terminal γ-ATP and proximal α-ATP).
Spectroscopic localization was performed using a 3D-ISIS module (TR = 8 s) with 3-ms adiabatic hyperbolic secant inversion pulses (10-kHz bandwidth), and 2-ms adiabatic half passage excitation pulse. In addition to the unsaturated spectrum (tsat = 0), spectra were acquired at five different saturation times (tsat = 0.53, 1.05, 2.10, 3.15, 4.19 s). To be less sensitive to B1 inhomogeneity, selective saturation of γ-ATP consisted of a BISTRO pulse train of hyperbolic secant pulses (50-ms each duration, 100-Hz bandwidth) with 5-ms gradient spoiling between pulses.15 Each spectrum was acquired in 34 min, keeping total examination time under 4 h. To control for direct saturation effects on the spectra, two other rats were scanned using the same protocol with the selective saturation placed symmetrical to γ-ATP resonance relative to Pi, at all tsat. No detectable signal loss was observed on the dominant Pi peak, demonstrating the absence of significant RF bleed-over effect in our experimental conditions. Hence, in the following, Pi signal attenuations will be calculated relative to the unsaturated spectrum, rather than relative to symmetrically saturated spectra. This approach allows devoting the whole experiment to acquiring “useful” saturation data, i.e. acquire data for more saturation times, and with more averaging.
Data analysis
Spectra were averaged over all five animals for each tsat to increase signal-to-noise ratio. This averaged dataset was then analyzed with LCModel16 using a simulated basis set as described in Deelchand et al.17 Both peaks were visible at the expected Pi resonances, one at 4.9 and the other at 5.3 ppm, and the basis set was built accordingly. The exact chemical shift between each Pi peak and PCr resonance was used to calculate pH in each compartment according to this equation:
Limiting shifts are δha = 3.27 (acidic species) δa = 5.68 (basic species) and deprotonation constant is pKa = 6.73.2
The decrease of Pi longitudinal magnetization during saturation transfer was modeled using Bloch equations modified to account for chemical exchange between Pi and ATP.18 Non-linear fitting was applied to estimate both T1int (the intrinsic T1, in absence of exchange, i.e. during saturation) and kfATPase and Monte-Carlo simulations over 500 repetitions was used to derive the error on estimates. Because TR was long as compared with T1, the precise value of T1mix (the T1 in presence of exchange, i.e. during sequence dead time) had only marginal impact of kfATPase, as we could verify using a complete model including T1mix.18
Results
Amount and pH of Pi pools
Figure 1 shows a localized 31P spectrum acquired in the rat brain (tsat = 0) with two separate peaks visible around 5 ppm. Each peak is identified with a CRLB of less than 6% and the correlation coefficient between them is weak (0.064). The pH found for each peak was pH4.9 = 7.06 and pH5.3 = 7.39, respectively. The signal ratio Pi5.3/Pitotal was found to be 19%.
Progressive saturation
Spectra averaged over five rats and zoomed on the Pi peaks at different saturation times are shown in Figure 2(a). There was no significant decrease of signal intensity for phosphomonoester (PE and PCho) and phosphodiester (GPE and GPC) resonances as tsat increased (not shown). The signal decrease of the largest peak at 4.9 ppm reflects the strong chemical exchange between Pi (Pi4.9) and ATP. Figure 2(b) shows the best fit for Pi4.9 signal attenuation and total Pi attenuation yielding kfATPase = 0.37 ± 0.03 s−1 and kfATPase = 0.28 ± 0.02 s−1 for Pi4.9 and Pitotal, respectively. Meanwhile, the signal of alkaline Pi was not apparently affected by progressive saturation (Figure 2(a) and (b)), demonstrating that this pool is not involved in ATP synthesis at detectable levels.
Figure 2.
(a) Signal decrease of dominant Pi when applying selective saturation on the γ-ATP pool in exchange with Pi. The weaker Pi pool remains unaffected by the downfield saturation suggesting no interaction with γ-ATP. (b) Normalized attenuation of the averaged signal (n = 5) of total Pi (♦) and intracellular Pi (◯) as a function of saturation time. The alkaline Pi5.3 (▪) was also plotted to show its independence to γ-ATP saturation. The best nonlinear fit for each Pi4.9 and Pitotal is shown in black line and the uncertainty on kfATPase is represented as lower and upper bounds in lighter shade.
Discussion
Extracellular origin of the 5.3 ppm Pi peak
As far as we know, this is the first report of two pools of Pi in the rat brain. To exclude the possibility of an alteration in metabolic profile due to the anesthetic used, e.g. as observed for isoflurane on 1H spectra (resulting in lactate increase19), we acquired control spectra on a rat anesthetised with benzodiazepine (midazolam) and medetomidine (2.5 and 0.25 mg/kg, respectively). The same peaks in similar concentrations were observed, excluding some metabolic effect due to isoflurane.
Previous studies have reported observing a second Pi peak 0.2–0.4 downfield of the main Pi4.9 peak in muscles and brain. All studies have excluded the blood contamination possibility, on the basis of small partial volume, low concentration and lack of 2,3-DPG in the spectra. Some reports assign a mitochondrial origin to the second Pi peak in the muscle, with a chemical shift actually closer to 5.1 ppm8 (and references therein), while others assign an extracellular origin in the brain.20,21 The different origin of this second Pi in brain12 compared to muscle8 is also suggested by the large difference in T1 (approximately four times longer in the brain).
The fact that the Pi5.3 peak is insensitive to γ-ATP saturation shows that this pool does not participate in de novo ATP synthesis at the time scale of the experiment. This was shown here with saturation transfer in the rat brain, and with inversion transfer techniques in the human brain.12 Using saturation transfer allowed achieving a ∼50% signal decrease for Pi4.9, i.e. a much stronger contrast compared to inversion transfer (25% maximal signal decrease). While measurement noise may have partly masked 25% signal attenuation for Pi5.3 (see Figure 6(a) in Ren et al.12), it is less likely that it may have masked a 50% Pi5.3 decrease in the present work. This strongly reinforces the idea that Pi5.3 is not in exchange with ATP, thus adding another argument in favor of the extracellular origin of Pi5.3 in the brain.
Interestingly, we estimated that the extracellular Pi represents 20% of the total Pi in the rat brain, which is close to what has been reported in Ren et al.12 (35%) and Kintner et al.20 and Du et al.21 (25%). Considering that this corresponds well to the generally accepted22 volume fraction of each of those compartments in the brain, these results suggest that Pi concentrations are similar in the intracellular and extracellular spaces.
Potential bias on kfATPase and VATP determination
The kfATPase value found when modeling only the Pi4.9 signal is ∼30% higher than when taking the total Pi signal in the voxel. This increase is expected since a higher proportion of the signal considered is in direct exchange with ATP. Remarkably, the values for ATP synthesis rates (VATP = kfATPasex[Pi]) calculated both ways (considering Pitotal or Pi4.9) are very close. This can be explained by the fact that the lower kfATPase is multiplied by a higher concentration and vice versa when considering total Pi or dominant Pi only, respectively. Using a literature value of 1.3 mM for [Pitotal], and a 1.1 g/mL brain density,18,23 ATP synthesis rate can be estimated to VATP = 23.2 ± 2.2 µmol g−1 min−1 considering only dominant Pi, and VATP = 22.0 ± 1.4 µmol g−1 min−1 considering total Pi. In other words, this means that VATP values from past studies based on total Pi attenuation may be essentially correct in practice, despite the presence of extracellular Pi in the broad peak, while the values of kfATPase may be significantly underestimated.
Note that estimated kfATPase and VATP values are slightly higher than in previous reports in awake Humans12,18,21 (10.6 µmol g−1 min−1 on average), macaques under propofol4 (7.8 µmol g−1 min−1), or rats under isoflurane24 and halothane25 (12.1 and 19.8 µmol g−1 min−1, respectively). Here, we optimized the saturation module to neglect RF bleed over and calculate signal attenuation relative to tsat = 0, rather than relative to symmetric experiment as in previous works. A small RF bleed over, below our detection capabilities, could explain this slight bias towards higher values. This does not affect the findings of the present work regarding the potential bias induced on kfATPase, and limited bias on VATP when considering total Pi.
Conclusion
In this work, we acquired localized 31P spectra in the rat brain at 11.7 T to characterize a second pool of Pi at more alkaline pH than the main Pi resonance. Based on pH measurements and saturation transfer MRS, we assigned an extracellular origin to this pool and showed that significant bias can be introduced in kfATPase measurements when not accounting for both pools. However, this bias does not strongly impact the ATP synthesis rate (VATP) estimation. Differentiating between intra and extracellular pools may help elucidate some mechanisms at stake under pathological conditions. For example, based on total Pi chemical shift at lower field, we have reported that pH increased in a rat model of Huntington’s disease as well as in patients, which we hypothesized to be of intracellular origin.26 Separating both pools could allow firmly establishing that the observed shift in Pitotal is not due to increased extracellular pH or increased Pi5.3 content, and that only intracellular pH is affected.
Acknowledgements
We thank Dr Julien Flament, Laura Mouton and Dr Vincent Lebon for the helpful discussions as well as Dr Pierre-Gilles Henry for his technical assistance on configuring LC Model, and Dr Pierrick Jego for help about experimental setup.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from Agence Nationale pour la Recherche (“HDeNERGY” project, ANR-14-CE15-0007-01). The 11.7 T MRI scanner was funded by a grant from NeurATRIS: A Translational Research Infrastructure for Biotherapies in Neurosciences (“Investissements d’Avenir,” ANR-11-INBS-0011).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
BT: Experimental design, data acquisition and analysis, data interpretation and manuscript drafting; EM: Data interpretation, critical revision of the manuscript; JV: Experimental design, data interpretation, critical revision of the manuscript and final approval.
References
- 1.Befroy DE, Rothman DL, Petersen KF, et al. 31P-Magnetization transfer magnetic resonance spectroscopy measurements of in vivo metabolism. Diabetes 2012; 61: 2669–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Graaf RA. In vivo NMR spectroscopy: Principles and techniques, 2nd ed Hoboken, NJ: John Wiley & Sons, 2007. [Google Scholar]
- 3.Brown TR, Ugurbil K, Shulman RG. 31P nuclear magnetic resonance measurements of ATPase kinetics in aerobic Escherichia coli cells. Proc Natl Acad Sci U S A 1977; 74: 5551–5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaumeil MM, Valette J, Guillermier M, et al. Multimodal neuroimaging provides a highly consistent picture of energy metabolism, validating 31P MRS for measuring brain ATP synthesis. Proc Natl Acad Sci U S A 2009; 106: 3988–3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wary C, Naulet T, Thibaud J-L, et al. Splitting of Pi and other 31P NMR anomalies of skeletal muscle metabolites in canine muscular dystrophy. NMR Biomed 2012; 25: 1160–1169. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt O, Bunse M, Dietze GJ, et al. Unveiling extracellular inorganic phosphate signals from blood in human cardiac 31P NMR spectra. J Cardiovasc Magn Reson 2001; 3: 325–329. [DOI] [PubMed] [Google Scholar]
- 7.Garlick PB, Soboll S, Bullock GR. Evidence that mitochondrial phosphate is visible in 31P NMR spectra of isolated, perfused rat hearts. NMR Biomed 1992; 5: 29–36. [DOI] [PubMed] [Google Scholar]
- 8.Kan HE, Klomp DWJ, Wong CS, et al. In vivo 31P MRS detection of an alkaline inorganic phosphate pool with short T1 in human resting skeletal muscle. NMR Biomed 2010; 23: 995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzpatrick JH, Kintner D, Anderson M, et al. NMR studies of Pi-containing extracellular and cytoplasmic compartments in brain. J Neurochem 1996; 66: 2612–2620. [DOI] [PubMed] [Google Scholar]
- 10.Gilboe DD, Kintner D, Anderson ME, et al. Inorganic phosphate compartmentation in the normal isolated canine brain. J Neurochem 1993; 60: 2192–2203. [DOI] [PubMed] [Google Scholar]
- 11.Gilboe DD, Kintner DB, Anderson ME, et al. NMR-based identification of intra- and extracellular compartments of the brain Pi peak. J Neurochem 2002; 71: 2542–2548. [DOI] [PubMed] [Google Scholar]
- 12.Ren J, Sherry AD, Malloy CR. (31)P-MRS of healthy human brain: ATP synthesis, metabolite concentrations, pH, and T1 relaxation times. NMR Biomed 2015; 28: 1455–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren J, Sherry AD, Malloy CR. Amplification of the effects of magnetization exchange by 31P band inversion for measuring adenosine triphosphate synthesis rates in human skeletal muscle. Magn Reson Med 2014; 74: 1505–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bresnen A, Duong TQ. Brain high-energy phosphates and creatine kinase synthesis rate under graded isoflurane anesthesia: an in vivo (31) P magnetization transfer study at 11.7 tesla. Magn Reson Med 2014; 73: 726–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Y, de Graaf RA, DelaBarre L, et al. BISTRO: an outer-volume suppression method that tolerates RF field inhomogeneity. Magn Reson Med 2001; 45: 1095–1102. [DOI] [PubMed] [Google Scholar]
- 16.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 2001; 14: 260–264. [DOI] [PubMed] [Google Scholar]
- 17.Deelchand DK, Nguyen T-M, Zhu X-H, et al. Quantification of in vivo31P NMR brain spectra using LCModel. NMR Biomed 2015; 28: 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei H, Ugurbil K, Chen W. Measurement of unidirectional Pi to ATP flux in human visual cortex at 7 T by using in vivo 31P magnetic resonance spectroscopy. Proc Natl Acad Sci U S A 2003; 100: 14409–14414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boretius S, Tammer R, Michaelis T, et al. Halogenated volatile anesthetics alter brain metabolism as revealed by proton magnetic resonance spectroscopy of mice in vivo. Neuroimage 2013; 69: 244–255. [DOI] [PubMed] [Google Scholar]
- 20.Kintner DB, Anderson ME, Sailor KA, et al. In vivo microdialysis of 2-deoxyglucose 6-phosphate into brain. J Neurochem 2002; 72: 405–412. [DOI] [PubMed] [Google Scholar]
- 21.Du F, Zhu X-H, Qiao H, et al. Efficient in vivo31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn Reson Med 2007; 57: 103–114. [DOI] [PubMed] [Google Scholar]
- 22.Syková E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev 2009; 88: 1277–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen JE, Drost DJ, Menon RS, et al. In vivo brain (31)P-MRS: measuring the phospholipid resonances at 4 Tesla from small voxels. NMR Biomed 2002; 15: 338–347. [DOI] [PubMed] [Google Scholar]
- 24.Du F, Zhu X-H, Zhang Y, et al. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci U S A 2008; 105: 6409–6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoubridge EA, Briggs RW, Radda GK. 31P NMR saturation transfer measurements of the steady state rates of creatine kinase and ATP synthetase in the rat brain. FEBS Lett 1982; 140: 288–292. [DOI] [PubMed] [Google Scholar]
- 26.Chaumeil MM, Valette J, Baligand C, et al. pH as a biomarker of neurodegeneration in Huntington’s disease: a translational rodent-human MRS study. J Cereb Blood Flow Metab 2012; 32: 771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]



