Abstract
A mini-review with 79 references. In this review, the most recent trends in 3D-printed microfluidic devices are discussed. In addition, a focus is given to the fabrication aspects of these devices, with the supplemental information containing detailed instructions for designing a variety of structures including: a microfluidic channel, threads to accommodate commercial fluidic fittings, a flow splitter; a well plate, a mold for PDMS channel casting; and how to combine multiple designs into a single device. The advantages and limitations of 3D-printed microfluidic devices are thoroughly discussed, as are some future directions for the field.
Introduction
Microfluidics is an ever evolving research field providing many benefits to chemical and biological research including reducing the amount of sample and reagents, decreasing experimental time, and enabling in vivo mimics.1 The first microfluidic device was a gas chromatography chip on a silicon wafer developed by Terry et al. in 1979.2 In the 1990’s, Harrison, Manz and Ramsey introduced microfluidic devices for electrophoresis-based separations.3–5 However, these devices were fabricated on hard substrates such as silicon and glass, and production was costly and time consuming. Another milestone development in the field of microfluidics was the use of poly(dimethylsiloxane) (PDMS) to fabricate devices, as first described by the Whitesides’ group.6 PDMS led to devices that were much easier to fabricate (as compared to glass) and has become the most commonly used material to fabricate microfluidic devices. Properties of PDMS that have facilitated the widespread use of the material include but are not limited to: (i) the PDMS surface is inert and not reactive with many reagents; (ii) PDMS cures at relatively low temperatures; (iii) the material is transparent, making it suitable for optical detection; (iv) it is not toxic to biological samples; and (v) PDMS is gas permeable and (vi) its surface can be modified.7, 8 PDMS microfluidic devices are commonly fabricated by soft lithography, which constructs a device by replica molding.9 The development of soft lithography facilitated the application of microfluidic devices as a powerful tool in separation and analytical sciences. For example, electrophoresis has shown great compatibility with microfluidics. With short channel networks and the ability to apply high field strengths, electrophoresis can be performed within a short time, which is of great value for (near) real time detection on small volume samples.10,11 As for the analytical sciences, in contrast to lab-scale chemical analyses, microfluidic devices with controllable flow and integration have made it possible to achieve rapid, high throughput and sensitive detection.12
Even though PDMS-based microfluidic devices have shown success, some significant disadvantages exist.13 PMDS devices are usually not rugged, which may cause flow profile problems due to leakage and/or uneven pressure.14 The fabrication efficiency of soft lithography is low, making it labor-intensive.15 Moreover, fast adjustment of device features is almost impossible without the fabrication of a brand new master. Additionally, though PDMS devices with integrated functional parts have been reported, these typically involve integration of only two discrete devices. Few examples show the combination of several working devices into a functional system. This may explain why the large body of publications in microfluidics has not led to the realization of the initial goal of building a large number of practical systems that can be utilized by researchers outside of analytical chemistry and biomedical engineering fields.
To streamline microfluidics into a higher number of laboratories, novel fabrication methods need to be developed. 3D-printing, a prototyping technology that has recently emerged as an alternative fabrication method for microfluidics, has shown the potential to address many of the problems with PDMS devices.16 Compared to the labor-intensive and multiple-step soft lithography process,9 3D-printing can potentially fabricate a microfluidic device in one step.17 Also, 3D-printing allows for quick adjustment of device features with each print by changing the design in CAD software. Additionally, 3D-printing allows for high throughput fabrication of microfluidic devices. For example, one can 3D print several identical devices for parallel experiments or multiple devices with different test parameters at one time.18 3D-printed devices also offer robust structural alternatives to conventional microfluidic devices. Some 3D-printed devices allow for flow rates as high as hundreds of μL/min.17, 19 These devices are reusable after cleaning through a chemical wash (i.e., bleach) and/or mechanical sonication, which helps minimize errors arising from using different devices.19, 20 Furthermore, by sharing the design files, researchers can easily replicate devices in different laboratories.
In this paper, the most recent trends in 3D-printed microfluidic devices will be reviewed. We will also focus on microfluidic fabrication using 3D-printing, giving detailed instructions for designing these structures. Some CAD files are also included in the supplementary information, with which the readers can print these devices directly. The advantages and limitations of 3D-printed microfluidic devices will be thoroughly discussed. At the end, ideas will be proposed to solve the problems arising from 3D-printing, as well as some future directions.
Fabrication of 3D-printed Microfluidic Devices
3D-printing, also known as additive manufacturing, builds a three-dimensional object, layer by layer.21 There are different techniques 3D-printers can utilize to fabricate 3D objects, among which the most commonly used are polyjet printing and fused deposition molding.16 Polyjet printing overlays liquid polymer layers, with each added layer cured by ultraviolet (UV) radiation. The resolution of polyjet printing can be high, but support materials are needed during the printing process that must be manually removed after printing.22 Fused deposition molding is an inexpensive technology that overlays layers of fused polymer filaments to generate a 3D structure. Supporting materials are usually not needed, but the resolution of this technique is relatively low.22 More information about commonly used 3D-printing technologies can be found in Table 1.20, 23–32
Table 1.
Summary of Different 3D-printing Technologies
| Printing Mechanism | Example Materials34–39 | Manufacturer Stated Resolution (X x Y x Z )34–39 | Example Applications & Device Dimensions (μm) |
|---|---|---|---|
|
Inkjet Printing34 Solid particles are bonded layer by layer via adhesive or UV. Support materials are needed. |
Acrylonitrile VisiJet Crystal a ABS b ThermoJet 2000c |
375 × 375 × 790 dpi 68 × 68 × 32 μm |
Evaluating printers for microfluidic fabrication 200 μm (diameter)74 |
| Microfluidic circuitry 750 μm (channel diameter)24 | |||
|
PolyJet Printing35 Liquid material is cured by UV layer by layer. Support materials are needed. |
Verowhited Full Cure 720 e Veroclear f |
600 × 600 × 1600 dpi 42 × 42 × 16 μm |
Endothelium lysis study 800 × 1000 (cross section) μm26 |
| Gradient generators and mixers within channels for biosensing 114 × 51 μm (cross section)41 | |||
| Wall-Jet electrochemical detector 375 × 375 μm (cross section)19 | |||
|
Stereolithography36, 37 Object is cured layer by layer by UV and is placed in a resin bath. Support materials are needed. |
Acrylate resin g Modified Resin |
454 × 454 × 508 dpi 56 × 56 × 50 μm |
Micromixer for pKa determination 500 × 500 μm (cross section)75 |
| immunoassay for bacteria detection 250 × 500 μm (cross section)76 | |||
| Mold to cast PDMS channels 300 × 300 μm (cross section)77 | |||
|
Fused Deposition Modeling38, 39 Fused filaments are laid layer by layer to build a 3D object. Support materials are not needed. |
Polypropelene ABS PLA h |
203 × 203 × 200 dpi 125 × 125 × 200 μm |
Biomedical Microdeivce 1000 × 500 μm (cross section)78 |
| Fluidic reactionware 800 μm (channel diameter)17 | |||
| Electrochemical detector 200 × 600 μm (cross section)79 |
Urethane acrylate oligomers (20–40 %); Ethoxylated bishenol A diacrylate (15–35%) and Tripropyleneglycol diacrylate (1.5–3%)
Acrylonitrile butadiene styrene
Thermoplastic
Acrylic monomer (<25%); Phenol, polymer with 2-propenoate (<15%); diphenyl-2,3,6-trimethylbenzoyl phosphineoxide (<2%); titanium dioxide (0.8%); Acrylic acidester (<0.3%)
Isobonyl acrylate (10–30%); Proprietary Acrylic monomer (10–30%); Proprietary acrylate oligomer (10–30%) and photoinitiator (0.1–1%)
Acrylic monomer (<30%); Isobonyl acrylate (<25%); phenol-oxirane-propenoate mix (<15%); diphenyl-2,4,6-trimethylbenzoyl phosphine oxide (<2%) and Acrylic acidester (<0.3%)
Acrylate oligomer and monomer; epoxy monomer and photoinitiator with additives.
Polylactic Acid
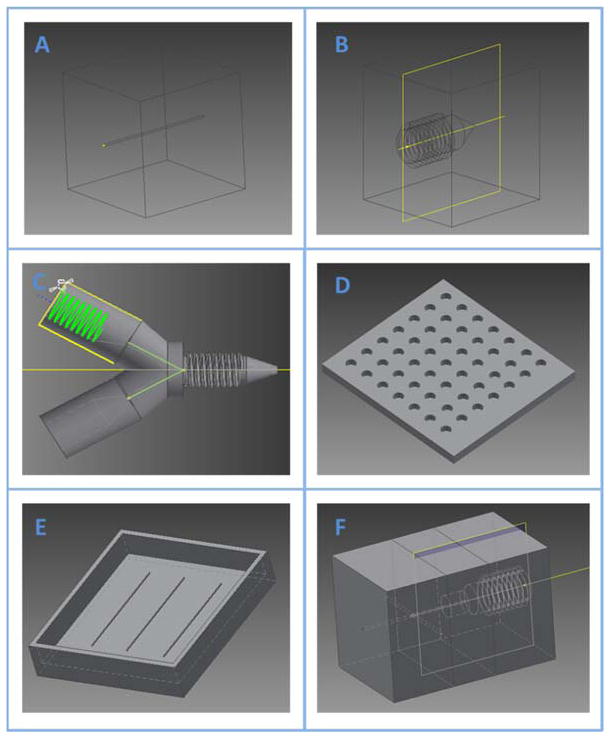
In order to 3D-print a microfluidic device, a design needs to be drawn in a CAD software package (i.e., Autodesk Inventor) in an .ipt format and then be converted to .stl format (which 3D-printers can recognize) prior to sending to a printer.33 Based on user demand, certain materials can be chosen to fabricate the device (Table 1). Figure 1 shows an example of 3D-printing an object following these steps. The design and drawing of a potential device using CAD software is key to the final structure and function of a microfluidic device. Therefore, in this review, the authors chose six examples that can be used for microfluidic devices and will introduce the detailed design and drawing process using Autodesk Inventor in the supplementary information, which include (1) a straight microfluidic channel; (2) female threads to accommodate a commercial PEEK fitting (F-120x, IDEX) and a capillary sleeve (F-230, IDEX); (3) a flow splitter; (4) holes for well inserts into a device (a well plate); (5) a mold for PDMS channel casting; and (6) combining multiple designs into a single device. The finished designs can be found in Figure 2. The .stl files of these features are included in the supplementary information as well, with which, the readers should be able to replicate these devices directly. Furthermore, the original .ipt files are also included so that the readers can edit parameters such as feature size to better fit their own research.
Figure 1.
The process to 3D-print a model. The design of a device needs to be realized in a CAD software and converted to .stl format, which can be recognized by a 3D-printer. The printed model is the exact reflection of the CAD design. This example shows the fabrication of a flow splitter using 3D-printing.
Figure 2.
Examples of useful objects for use with microfluidics designed with AutoDesk Inventor. The supporting information shows a step-by-step method for designing each of the objects shown. Listed includes (A), simple straight channel with a circlular cross-section (B), threaded object for integration with a commercial F-230 fitting (Fischer) and F-120 capillary sleeve (Fischer) (C), flow-splitter including both male and female threads for integration with fittings and object containing a channel (D), a simple well plate (E), a mold to create microchannels with soft lithography (F), an example of integrating separate object files into one
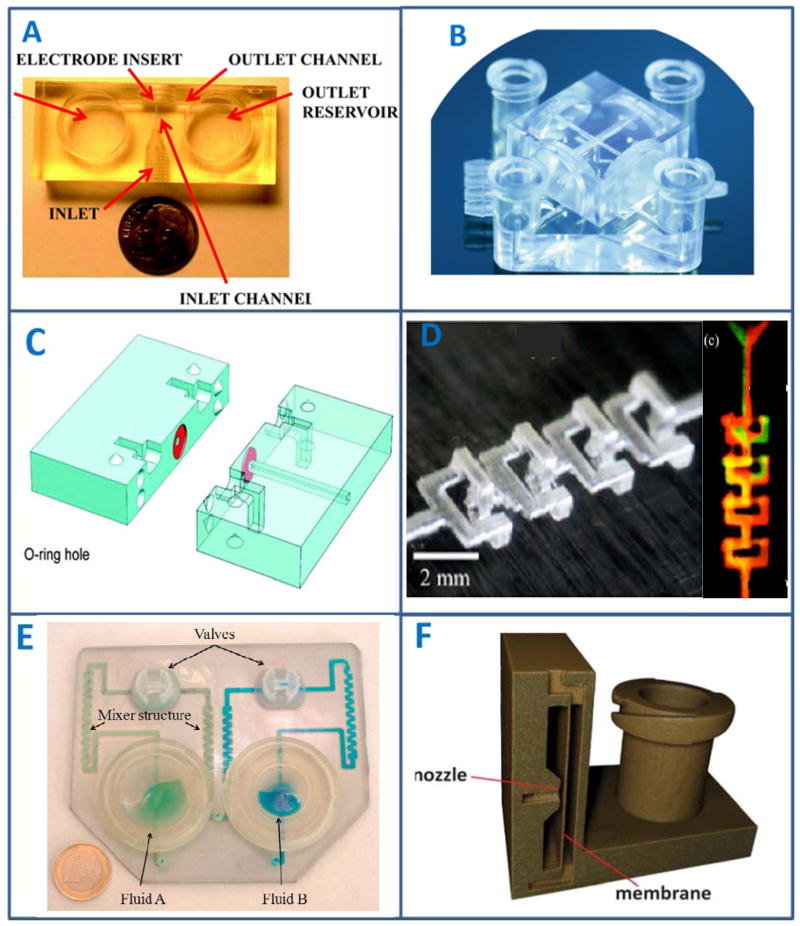
The resolution of different 3D-printing technologies and the actual sizes of example devices using the printers can be found in Table 1.34–39 As long as the CAD design of a device is within the resolution requirement of a 3D-printer, the printed part should be the exact reflection of the CAD design (Figure 1). In other words, any feature or shape drawn using a CAD software can be achieved by 3D-printing. Therefore, compared to soft lithography, 3D-printing enables the fabrication of more complicated and functional features on microfluidic devices. Figure 3 shows several published examples of unique 3D-printed functional parts that can be used for microfluidic devices.19, 40–44
Figure 3.
Examples of unique features that can be realized by 3D-printing. (A), a 3D-printed microfluidic device with wall-jet electrochemical configuration. Threaded ports that fit commercial finger tight adapters were 3D-printed on the device. A sample can be introduced through the inlet, which then hits the electrode housed in the “electrode insert” to achieve wall-jet detection. (Adapted from ref. 19 with permission from The Royal Society of Chemistry) (B), luer adapters were 3D-printed on a microfluidic device for easy connection with tubing and syringes. (Adapted from ref. 40 with permission from John Wiley & Sons, Inc) (C), O-rings can be 3D-printed with rubber-like materials on a fluidic device. In this example, the authors used the printed O-ring to connect different chips. (Adapted from ref. 41 with permission from The Royal Society of Chemistry) (D), A 3D-printed gradient generator. With 3D-printing, complicated channels were fabricated in this example. (Adapted from ref. 42 with permission from The American Chemical Society) (E), A 3D-printed microfluidic mixer. By fabricating zigzag channels and vales, two liquids can be mixed well using the device. (Adapted from ref. 44 with permission from Elsevier) (F), A 3D-printed pneumatic valve. The “membrane” is a thin layer (~200 μm) of 3D-printed structure. When proper air pressure is applied through the nozzle, the membrane can deform to open or close the channel on the right side. (Adapted from ref. 43 with permission from The Royal Society of Chemistry)
Advantageous Features of 3D-printed Microfluidic Devices
3D-printing can be an efficient method to fabricate structurally robust microfluidic devices. Furthermore, 3D-printing enables the fabrication of unique but advantageous features integrated on a microfluidic device. Although integrated components have been created with PDMS devices, it is time consuming and costly to integrate multiple functional units on one device.45 Because 3D-printing can produce any shape based on users’ demand, complex and functional 3D-printed microfluidic features have been reported.
Robust Connection Ports
Functionality of microfluidic devices can be improved by integrating chips with other devices/instrumentation with fluidic tubing. Common techniques for connecting tubing to a PDMS device include inserting tubing into punched holes or using adhesive to seal tubing onto PDMS slabs,46 although Frazier reported a locking mechanism by soft lithography that can form removable and tight connections.47 Lack of reliability of the tubing connection protocols can cause immediate device failure. For example, inserting tubing directly into punched holes is susceptible to leakage or back pressure. The use of 3D-printed devices can solve this problem by integrating connection mechanisms that cannot be achieved with soft lithography. For example, threaded ports that fit commercial finger tight adapters (Figures 1 and 3A) have been successfully integrated on 3D-printed microfluidics for convenient and reliable connection with external tubing.41 Also, with multi-material printing, O-rings can be printed on a microfluidic device to create tight seals and connections (Figure 3C).48 3D-printed customized threaded fittings, luer adapters (Figure 3F) and capillary connectors have also been reported. 43
Complex Flow Regulating Components
Precise and efficient flow control is an important factor to consider when designing a microfluidic device. On the micro scale, fluids typically exhibit laminar flow that may cause insufficient mixing between reagents if needed.49 On-chip mixing mechanisms have been integrated on PDMS devices through complex fabrication.50, 51 For example, R. F. Ismagilov group have developed multiple PDMS-based droplet microfluidic devices to enhance mixing of flowing reagents.52–54 3D-printing can also achieve complicated structures with just a few steps. For example, Lee and colleagues developed a 3D-printed channel with twists and turns, as well as a separate unit to mix the flows for alpha-fetoprotein biomarker detection. As the authors have proved, these devices were 3D-printed in a straight forward, rapid and user friendly way. 41 3D-printed valves and pumps, a common technique for fluid manipulation in PDMS devices, have also been achieved (Figure 3F).28, 43
Integration of detectors
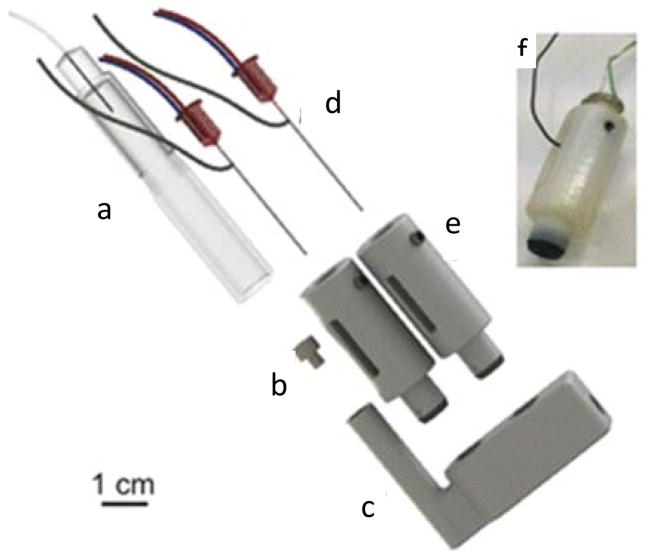
Electrochemical detection has become a commonly used technique for on-chip measurement because it enables direct and label free detection of many molecules.55 There have been many reports showing integrated electrodes in PDMS devices, but these devices often have to be discarded after electrodes foul, which can be after a single use. On a 3D-printed microfluidic device, however, electrodes can be integrated and removed easily by using connection mechanisms such as threads. Erkal reported a method to embed an electrode in a hollow plastic fitting, which can then be integrated into a threaded port in the device.20 The electrodes can be removed by unscrewing to be cleaned for reuse. With 3D-printing, more complicated electrochemical configuration can also be easily achieved. Munshi et al. reported a microfluidic wall-jet electrochemical detector with detachable and adjustable electrodes (Figure 3A), which has proven to be highly sensitive for multiple applications.19 Boutelle’s group recently described a 3D-printed integrative system that contained microdialysis probes for continuous monitoring of glucose and lactate in humans. As shown in Figure 4, the dialysis outlet (c) that collects dialysate from a human was connected to the inlet of a 3D-printed microfluidic chip (e). Electrochemistry based needle biosensors (f) were housed in two 3D-printed adapters (g), which can screw into the microfluidic channel for detection. The inset (h) is the real picture of a housed electrode and the black part was printed using a soft material for tight sealing. With this system, online monitoring of the metabolites with high temporal resolution can be achieved.56 Also, the ability of 3D-printing to mold any design enables the device shape to fit commercial detectors. For example, Chen and colleagues developed a device in the format of a standard 96-well plate that is amenable to plate readers for high throughput analysis.33 Other detection techniques such as chemiluminescence57 have also been integrated onto 3D-printed microfluidic devices.
Figure 4.
An integrated 3D-printed microfluidic system for metabolite detection. Part (a) is the outlet of a dialysis probe that collects samples from a human being. Via part (b), which is a 3D-printed adapter, (a) can be connected to the 3D-printed microfluidic device (c). Two electrodes (d) were housed in 3D-printed adapters (e), which can be screwed into the microfluidic channel with a good sealing. When a sample is collected and flowing through the channel, glucose and lactate can then be electrochemically detected. (f) is a real picture of a housed electrode and the black part was 3D-printed using a rubber-like material for tight sealing. (Adapted from ref. 56 with permission from The American Chemical Society)
On-chip Cell integration
On-chip cell culture is a significant research area in microfluidics. By controlling reagent flow, cell culture can be optimized and also integrated with on-chip analysis. Compared to static cell culture, a fluidic approach provides continuous nutrient supply and waste removal, as well as gradient control, and in vivo microenvironment mimicking (i.e., shear stress).58 Although a number of PDMS cells-on-a-chip models have been reported, 3D-printing can enhance the multiplexity, versatility and reusability of such models. Chen reported a 3D-printed system that combined pancreatic β-cells, endothelial cells and a blood flow mimic. This platform enabled multiple studies such as endocrine secretions, hormone transportation and cell-to-cell interactions. Such a device cannot be easily achieved using PDMS. Furthermore, most of the reported PDMS cells-on-a-chip systems contained only one or two cell types.59 Another important advantage includes the integration of transwell inserts for flexible cell culture on the 3D-printed device. Inserts with pre-cultured cells can be inserted and removed from the fluidic base as needed. When an insert was contaminated or the cells were not cultured correctly, the insert can be replaced rather than failure of the entire device. Though the authors tested three cell types of interest, in theory, any other cell type can be integrated on the system by this way for certain studies.
Limitations of current 3D-printed microfluidic devices
As a new, rapidly evolving technology, 3D-printing also possesses some limitations. Currently, these include but are not limited to resolution, surface properties, and compatibility issues.
Resolution
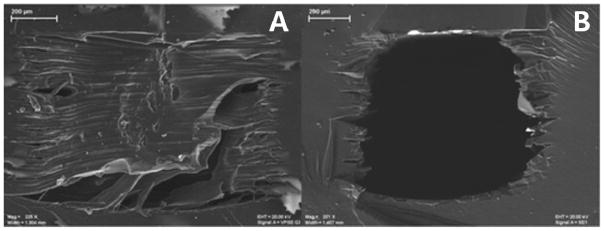
Although the resolution of many 3D-printers has been claimed to be as low as a few tens of microns, the smallest 3D-printed open channel acquired so far is approximately 200 μm wide.60 Most of currently reported 3D-printed microfluidic devices have channel sizes from hundreds of microns to a few millimeters.33, 41, 43, 59 This is partially because the printers with the highest resolution usually need supporting materials to fill the void spaces (i.e., a channel) during the printing process (Figure 5A), which typically needs to be removed manually.16 Unfortunately, it is almost impossible to remove all the waxy supporting materials from a very small channel. Even if some micron scale mechanical methods may be applied to remove the supporting materials, the removal efficiency will still be questionable. Any left-over material in a channel may adversely alter the flow pattern and/or affect the flowing reagents by absorption or even reaction. Most of the supporting materials have similar chemical composition with the construction materials,61 which makes it impossible to use harsh solvents for cleaning. Mild solvents such as isopropanol (IPA) have been tried by the authors and others, which turned out to push this situation into a dilemma: IPA treatment for a short time cannot remove much of the material; while too long treatment causes expansion of the bulk material and resulting in channel deformation or even clogging.20, 59 This limitation currently prohibits 3D-printed microfluidic devices from being used in applications that require small channels, such as cell/particle gating and electrophoresis. Moreover, most of currently reported 3D-printed microfluidic devices have straight channels, because of the difficulty to remove supporting materials from a fully closed channel with sharp bends. However, if a channel with such complicated structures as twists and turns must be fabricated, the channel may not be printed as fully closed. Instead, it can be fabricated as an engraved slab, which can then be sealed onto another substrate.44
Figure 5.
The SEM images of a 3D-printed channel before and after removing supporting material. (A), upon printed, the channel was completely filled with the supporting material, which is commonly used in high resolution 3D-printers. (B), after removal of the supporting material, rough surfaces can be seen on the inner wall of the channel. The scale bars in the images represent 200 μm. (Adapted from ref. 26 with permission from The American Chemical Society)
Surface properties
The resolution limitation of current 3D-printers can also cause rough surface profiles in a 3D-printed micro channel. Gross reported a microfluidic device fabricated using a high resolution (30 μm on x and y axes and 16 μm on z axis as said by the manufacturer) 3D-printer.26 As shown in Figure 5, after removal of the supporting material, large rough ridges can be clearly seen around the inner wall, which may cause issues such as dead volumes and inconsistent surface modifications. Absorption and adsorption are another concern about surface properties of 3D-printed devices. Though the exact composition of the 3D-printing materials is proprietary, most of them are acrylate and acrylonitrile butadiene styrene (ABS) based. These polymers absorb such molecules as proteins and lipids, which may reduce the concentration of analytes of interest, and thereby altering the detected signal.62 The unpredicted rough channel surface may even increase adsorption. To avoid the biotoxicity concern of 3D-printed devices and to reduce channel roughness, some coating protocols can be applied. For example, Gross successfully coated a layer of PDMS on the inside of a 3D-printed channel for endothelial cell culture and subsequent microscopic observation.26
Compatibility issues
Cell integration on microfluidic devices is popular. However, the biocompatibility of most of the 3D-printing resins and plastics remains unknown. 3D-printing materials are usually complex mixtures. Even though the major composition is non-toxic, other components such as commonly used photoinitiators may adversely affect cells.63 Biocompatible 3D-printing materials are being developed that are expected to be non-toxic to cells.64 However, most of these studies are still in development. Solvent compatibility is another concern in 3D-printing. Some 3D-printing materials may swell in aqueous systems, which can cause flow problems.65 Organic solvents are also commonly used in microfluidics (i.e., oils for droplet microfluidics66); but the solvent compatibility of 3D-printing materials still remains a concern.
Other issues
PDMS devices can be completely transparent for optical detection. Although “clear materials” are available for 3D-printing, they are typically translucent/semi-transparent. Labor-intensive polishing can make the outside of a device transparent and smooth; but the inner wall of a small, fully-sealed channel cannot be polished. In other words, it is almost impossible to optically observe the inside of a channel without certain treatments.67 Most of the 3D-printing materials are not gas permeable, which makes them not suitable for long-term cell culture inside a channel without additional gas exchange supporting units. In addition, 3D-printers with high resolutions (tens of microns) are relatively expensive at the moment,68 which may limit its application in many laboratories. Furthermore, routine maintenance and calibration are needed for a 3D-printer to ensure printing quality.
Future Directions
3D-printing of microfluidic devices and components has many benefits. Ho and coworkers concluded that 3D-printing can facilitate the field of microfluidics to find its “killer applications”.69 Au and colleagues predicted that 3D-printing can potentially replace soft lithography to fabricate microfluidic devices.60 However, in the authors’ opinion, due to the limitations of current 3D-printing technologies, 3D-printing is currently a complementary fabrication technique for microfluidics and it cannot replace PDMS and soft lithography at this point. For now, it is possible to consider the combination of 3D-printing and PDMS devices, where the strong points of each can be utilized. For example, one can envision a device where 3D printed connection ports are combined with PDMS-based channels.
3D-printing can also facilitate the development of modular microfluidic systems by providing robust connection mechanisms (i.e., threads, luers and O rings). Compared to an integrated single-chip device, if some part of a modular system fails, that component can be replaced without discarding the whole system.70 There have been some proof-of-concept studies regarding 3D-printed modular systems.41 High throughput chemical/biological analysis is also of great interest to the medical and pharmaceutical communities.71 Due to the ease to fabricate demand-based structures by 3D-printing, high throughput 3D-printed devices can be developed and applied in many fields. There have been initial reports of such devices that can fit plate readers to enhance detection throughput.33, 59
There is room for improvement in 3D-printing technology as well. Resolution needs to be further enhanced along with development of more user-friendly support materials. The cost of high resolution 3D-printers needs to be reduced in the future, which will eventually decrease the cost to produce a device. With this, considering the advantages of 3D-printed microfluidic devices, wider availability of robust devices can be realized, which may be a step forward to move microfluidics out of academia into real-world applications such as point-of-care diagnostics. It also takes relatively long time (hours) to fabricate an object using most 3D-printing technologies, especially for high resolution printing that requires a large number of thin layers to build a structure. Tumbleston and colleagues recently reported a new 3D-printing technology called continuous liquid interface production (CLIP), which can shorten the fabrication time to minutes by creating a “dead zone” between the polymer precursor and the solid part.72 3D-bioprinting is another emerging technique in recent years, which can additively manufacture cell-laden structures from biocompatible materials.73 Though the resolution of current 3D-bioprinting is usually at millimeter scale,32 the technique may evolve to be useful for cell integrated microfluidic device fabrication in the future.
Supplementary Material
Acknowledgments
Support for RSM from the National Institute of General Medical Sciences (Award Number R15GM084470-04) is acknowledged. This paper, as well as another paper from our group (Chen et al, Analyst, 2016, DOI: 10.1039/C6AN01282E) is dedicated to the memory of Craig Lunte, an outstanding scientist and mentor.
References
- 1.Fujii T. Microelectron Eng. 2002;61–2:907–914. [Google Scholar]
- 2.Terry SC, Jerman JH, AJB IEEE Transactions on Electron Devices. 1979;26:1880–1886. [Google Scholar]
- 3.Harrison DJ, Manz A, Fan ZH, Ludi H, Widmer HM. Anal Chem. 1992;64:1926–1932. [Google Scholar]
- 4.Manz A, Graber N, Widmer HM. Sensor Actuat B-Chem. 1990;1:244–248. [Google Scholar]
- 5.Jacobson SC, Hergenroder R, Koutny LB, Ramsey JM. Anal Chem. 1994;66:1114–1118. [Google Scholar]
- 6.Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Anal Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 7.Jo BH, Van Lerberghe LM, Motsegood KM, Beebe DJ. J Microelectromech Syst. 2000;9:76–81. [Google Scholar]
- 8.Zhou JW, Ellis AV, Voelcker NH. Electrophoresis. 2010;31:2–16. doi: 10.1002/elps.200900475. [DOI] [PubMed] [Google Scholar]
- 9.Xia YN, Whitesides GM. Angew Chem Int Edit. 1998;37:550–575. doi: 10.1002/(SICI)1521-3773(19980316)37:5<550::AID-ANIE550>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 10.Attiya S, Jemere AB, Tang T, Fitzpatrick G, Seiler K, Chiem N, Harrison DJ. Electrophoresis. 2001;22:318–327. doi: 10.1002/1522-2683(200101)22:2<318::AID-ELPS318>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 11.Metto EC, Evans K, Barney P, Culbertson AH, Gunasekara DB, Caruso G, Huvey MK, da Silva JAF, Lunte SM, Culbertson CT. Anal Chem. 2013;85:10188–10195. doi: 10.1021/ac401665u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng ZY, Soper SA, Pingle MR, Barany F, Davis LM. Anal Chem. 2010;82:9727–9735. doi: 10.1021/ac101843n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukhopadhyay R. Anal Chem. 2007;79:3248–3253. doi: 10.1021/ac071903e. [DOI] [PubMed] [Google Scholar]
- 14.Kim K, Park SW, Yang SS. Biochip Journal. 2010;4:148–154. [Google Scholar]
- 15.McDonald JC, Metallo SJ, Whitesides GM. Anal Chem. 2001;73:5645–5650. doi: 10.1021/ac010631r. [DOI] [PubMed] [Google Scholar]
- 16.Gross BC, Erkal JL, Lockwood SY, Chen CP, Spence DM. Anal Chem. 2014;86:3240–3253. doi: 10.1021/ac403397r. [DOI] [PubMed] [Google Scholar]
- 17.Kitson PJ, Rosnes MH, Sans V, Dragone V, Cronin L. Lab Chip. 2012;12:3267–3271. doi: 10.1039/c2lc40761b. [DOI] [PubMed] [Google Scholar]
- 18.Waldbaur A, Rapp H, Lange K, Rapp BE. Anal Methods. 2011;3:2681–2716. [Google Scholar]
- 19.Munshi AS, Martin RS. Analyst. 2016;141:862–869. doi: 10.1039/c5an01956g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erkal JL, Selimovic A, Gross BC, Lockwood SY, Walton EL, McNamara S, Martin RS, Spence DM. Lab Chip. 2014;14:2023–2032. doi: 10.1039/c4lc00171k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassoli E, Gatto A, Iuliano L, Violante MG. Rapid Prototyping J. 2007;13:148–155. [Google Scholar]
- 22.Ibrahim D, Broilo TL, Heitz C, de Oliveira MG, de Oliveira HW, Nobre SMW, Dos Santos JHG, Silva DN. J Cranio Maxill Surg. 2009;37:167–173. doi: 10.1016/j.jcms.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Lee JM, Zhang M, Yeong WY. Microfluid Nanofluidics. 2016;20:1–15. [Google Scholar]
- 24.Sochol RD, Sweet E, Glick CC, Venkatesh S, Avetisyan A, Ekman KF, Raulinaitis A, Tsai A, Wienkers A, Korner K, Hanson K, Long A, Hightower BJ, Slatton G, Burnett DC, Massey TL, Iwai K, Lee LP, Pister KSJ, Lin L. Lab Chip. 2016;16:668–678. doi: 10.1039/c5lc01389e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald JC, Chabinyc ML, Metallo SJ, Anderson JR, Stroock AD, Whitesides GM. Anal Chem. 2002;74:1537–1545. doi: 10.1021/ac010938q. [DOI] [PubMed] [Google Scholar]
- 26.Gross BC, Anderson KB, Meisel JE, McNitt MI, Spence DM. Anal Chem. 2015;87:6335–6341. doi: 10.1021/acs.analchem.5b01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comina G, Suska A, Filippini D. Lab Chip. 2014;14:424–430. doi: 10.1039/c3lc50956g. [DOI] [PubMed] [Google Scholar]
- 28.Rogers CI, Qaderi K, Woolley AT, Nordin GP. Biomicrofluidics. 2015;9(1):016501. doi: 10.1063/1.4905840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadimisetty K, Mosa IM, Malla S, Satterwhite-Warden JE, Kuhns TM, Faria RC, Lee NH, Rusling JF. Biosens Bioelectron. 2016;77:188–193. doi: 10.1016/j.bios.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krejcova L, Nejdl L, Rodrigo MAM, Zurek M, Matousek M, Hynek D, Zitka O, Kopel P, Adam V, Kizek R. Biosens Bioelectron. 2014;54:421–427. doi: 10.1016/j.bios.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 31.Moore JL, McCuiston A, Mittendorf I, Ottway R, Johnson RD. Microfluid Nanofluidics. 2011;10:877–888. [Google Scholar]
- 32.Cabot JM, Fuguet E, Rosés M, Smejkal P, Breadmore MC. Anal Chem. 2015;87:6165–6172. doi: 10.1021/acs.analchem.5b00845. [DOI] [PubMed] [Google Scholar]
- 33.Chen CP, Wang YM, Lockwood SY, Spence DM. Analyst. 2014;139:3219–3226. doi: 10.1039/c3an02357e. [DOI] [PubMed] [Google Scholar]
- 34.THERMOJET®. [accessed June 2016]; http://www.3dsystems.com/products/datafiles/thermojet/datasheets/TJ_Pr_DesignComm.pdf.
- 35.Proto3000. [accessed June 2016]; http://proto3000.com/eden-lineup.php.
- 36.MIICRAFT. [accessed June 2016]; http://www.miicraft.com/
- 37.B9Creator®. [accessed June 2016]; https://www.b9c.com/
- 38.Makerbot. [accessed June 2016]; http://www.makerbot.com/
- 39.3D Printing Database. [accessed June 2016]; http://www.3dprintingdatabase.org/en/services/easy-3d-maker.
- 40.Au AK, Huynh W, Horowitz LF, Folch A. Angew Chem Int Ed Engl. 2016;55:3862–3881. doi: 10.1002/anie.201504382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee KG, Park KJ, Seok S, Shin S, Kim DH, Park JY, Heo YS, Lee SJ, Lee TJ. Rsc Advances. 2014;4:32876–32880. [Google Scholar]
- 42.Shallan AI, Smejkal P, Corban M, Guijt RM, Breadmore MC. Anal Chem. 2014;86:3124–3130. doi: 10.1021/ac4041857. [DOI] [PubMed] [Google Scholar]
- 43.Au AK, Bhattacharjee N, Horowitz LF, Chang TC, Folch A. Lab Chip. 2015;15:1934–1941. doi: 10.1039/c5lc00126a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonyár Attila, Sántha Hunor, Balázs Ring MV, Kovács József Gábor, Harsányi G. Procedia Eng. 2010;5:291–294. [Google Scholar]
- 45.Ng JMK, Gitlin I, Stroock AD, Whitesides GM. Electrophoresis. 2002;23:3461–3473. doi: 10.1002/1522-2683(200210)23:20<3461::AID-ELPS3461>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 46.Beebe DJ, Mensing GA, Walker GM. Annu Rev Biomed Eng. 2002;4:261–286. doi: 10.1146/annurev.bioeng.4.112601.125916. [DOI] [PubMed] [Google Scholar]
- 47.Han KH, McConnell RD, Easley CJ, Bienvenue JM, Ferrance JP, Landers JP, Frazier AB. Sensor Actuat B-Chem. 2007;122:337–346. [Google Scholar]
- 48.Paydar OH, Paredes CN, Hwang Y, Paz J, Shah NB, Candler RN. Sensors and Actuators a-Physical. 2014;205:199–203. [Google Scholar]
- 49.Choban ER, Markoski LJ, Wieckowski A, Kenis PJA. J Power Sources. 2004;128:54–60. [Google Scholar]
- 50.Tsai JH, Lin LW. Sensors and Actuators a-Physical. 2002;97–8:665–671. [Google Scholar]
- 51.Hong CC, Choi JW, Ahn CH. Lab Chip. 2004;4:109–113. doi: 10.1039/b305892a. [DOI] [PubMed] [Google Scholar]
- 52.Song H, Tice JD, Ismagilov RF. Angew Chem Int Edit. 2003;42:768–772. doi: 10.1002/anie.200390203. [DOI] [PubMed] [Google Scholar]
- 53.Tice JD, Song H, Lyon AD, Ismagilov RF. Langmuir. 2003;19:9127–9133. [Google Scholar]
- 54.Song H, Ismagilov RF. J Am Chem Soc. 2003;125:14613–14619. doi: 10.1021/ja0354566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rossier J, Reymond F, Michel PE. Electrophoresis. 2002;23:858–867. doi: 10.1002/1522-2683(200203)23:6<858::AID-ELPS858>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 56.Gowers SAN, Curto VF, Seneci CA, Wang C, Anastasova S, Vadgama P, Yang GZ, Boutelle MG. Anal Chem. 2015;87:7763–7770. doi: 10.1021/acs.analchem.5b01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roda A, Guardigli M, Calabria D, Calabretta MM, Cevenini L, Michelini E. Analyst. 2014;139:6494–6501. doi: 10.1039/c4an01612b. [DOI] [PubMed] [Google Scholar]
- 58.Mehling M, Tay S. Curr Opin Biotech. 2014;25:95–102. doi: 10.1016/j.copbio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Liu YL, Chen CP, Summers S, Medawala W, Spence DM. Integr Bio. 2015;7:534–543. doi: 10.1039/c4ib00243a. [DOI] [PubMed] [Google Scholar]
- 60.Au AK, Huynh W, Horowitz LF, Folch A. Angew Chem Int Edit. 2016;55:3862–3881. doi: 10.1002/anie.201504382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stratasys®. [accessed June 2016]; http://www.stratasys.com/materials/material-safety-data-sheets/polyjet/support-materials.
- 62.Ma YD, Dong JL, Bhattacharjee S, Wijeratne S, Bruening ML, Baker GL. Langmuir. 2013;29:2946–2954. doi: 10.1021/la305137m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allen NS. J Photochem Photobiol a-Chemistry. 1996;100:101–107. [Google Scholar]
- 64.Bose S, Vahabzadeh S, Bandyopadhyay A. Mater Today. 2013;16:496–504. [Google Scholar]
- 65.Migneault S, Koubaa A, Erchiqui F, Chaala A, Englund K, Wolcott MP. Compos Part a. 2009;40:80–85. [Google Scholar]
- 66.Brouzes E, Medkova M, Savenelli N, Marran D, Twardowski M, Hutchison JB, Rothberg JM, Link DR, Perrimon N, Samuels ML. P Natl Acad Sci USA. 2009;106:14195–14200. doi: 10.1073/pnas.0903542106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hell S, Reiner G, Cremer C, Stelzer EHK. J Microscopy. 1993;169:391–405. [Google Scholar]
- 68.Waheed S, Cabot JM, Macdonald NP, Lewis T, Guijt RM, Paull B, Breadmore MC. Lab Chip. 2016 doi: 10.1039/c6lc00284f. [DOI] [PubMed] [Google Scholar]
- 69.Ho CMB, Ng SH, Li KHH, Yoon YJ. Lab Chip. 2015;15:3627–3637. doi: 10.1039/c5lc00685f. [DOI] [PubMed] [Google Scholar]
- 70.Shaikh KA, Ryu KS, Goluch ED, Nam JM, Liu JW, Thaxton S, Chiesl TN, Barron AE, Lu Y, Mirkin CA, Liu C. P Natl Acad Sci USA. 2005;102:9745–9750. doi: 10.1073/pnas.0504082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McClain MA, Culbertson CT, Jacobson SC, Allbritton NL, Sims CE, Ramsey JM. Anal Chem. 2003;75:5646–5655. doi: 10.1021/ac0346510. [DOI] [PubMed] [Google Scholar]
- 72.Tumbleston JR, Shirvanyants D, Ermoshkin N, Janusziewicz R, Johnson AR, Kelly D, Chen K, Pinschmidt R, Rolland JP, Ermoshkin A, Samulski ET, DeSimone JM. Science. 2015;347:1349–1352. doi: 10.1126/science.aaa2397. [DOI] [PubMed] [Google Scholar]
- 73.Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, Forgacs G. Biofabrication. 2010;2(2):022001. doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walczak R, Adamski K. J Micromech Microeng. 2015;25:085013. [Google Scholar]
- 75.Cabot JM, Fuguet E, Roses M, Smejkal P, Breadmore MC. Anal Chem. 2015;87:6165–6172. doi: 10.1021/acs.analchem.5b00845. [DOI] [PubMed] [Google Scholar]
- 76.Lee W, Kwon D, Choi W, Jung GY, Jeon S. Sci Rep. 2015;5(5):7717. doi: 10.1038/srep07717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chan HN, Chen YF, Shu YW, Chen Y, Tian Q, Wu HK. Microfluid Nanofluidics. 2015;19:9–18. [Google Scholar]
- 78.McCullough EJ, Yadavalli VK. J Mater Process Tech. 2013;213:947–954. [Google Scholar]
- 79.Bishop GW, Satterwhite JE, Bhakta S, Kadimisetty K, Gillette KM, Chen E, Rusling JF. Anal Chem. 2015;87:5437–5443. doi: 10.1021/acs.analchem.5b00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.