Abstract
Objectives
Statins partially block the production of coenzyme Q10 (CoQ10), an essential component for mitochondrial function. Reduced skeletal muscle mitochondrial oxidative capacity has been proposed to be a cause of statin myalgia and can be measured using 31phosphorus magnetic resonance spectroscopy (31P-MRS). The purpose of this study is to assess the effect of CoQ10 oral supplementation on mitochondrial function in statin users using 31P-MRS.
Design/Setting
In this randomized, double-blind, placebo-controlled pilot study, 21 adults aged 47–73 were randomized to statin+placebo (n=9) or statin+CoQ10 (n=12). Phosphocreatine (PCr) recovery kinetics of calf muscles were assessed at baseline (off statin and CoQ10) and 4 weeks after randomization to either statin+CoQ10 or statin+placebo.
Results
Baseline and post-treatment PCr recovery kinetics were assessed for 19 participants. After 4 weeks of statin+ CoQ10 or statin+placebo, the overall relative percentage change (100*(baseline−follow up)/baseline) in PCr recovery time was −15.1% compared with baseline among all participants, (p-value=0.258). Participants randomized to statin+placebo (n=9) had a relative percentage change in PCr recovery time of −18.9%, compared to −7.7% among participants (n=10) receiving statin+CoQ10 (p-value=0.448).
Conclusions
In this pilot study, there was no significant change in mitochondrial function in patients receiving 4 weeks of statin+CoQ10 oral therapy when compared to patients on statin+placebo.
Keywords: HMG-CoA reductase inhibitors, statin myalgia, coenzyme Q10, ubiquinol, MR phosphorus spectroscopy, mitochondrial oxidative metabolism
Introduction
Statin myalgia is the most commonly reported side effect of statin therapy, occurring in up to 5–10% of active users (Bruckert et al. 2005; Buettner et al. 2012). Although the etiology is unknown, one theory is that statins reduce skeletal muscle mitochondrial oxidative capacity, leading to increased reactive oxygen species (Pieczenik & Neustadt 2007) resulting in muscle aches, cramping and/or weakness (Bouitbir et al. 2011a). Mitochondrial oxidative metabolism is regulated by a large number of factors, one of which is coenzyme Q10 (CoQ10), an essential cofactor of the electron transport in the mitochondria needed to produce adenosine triphosphate (ATP) (Mas & Mori 2010). Statins partially block the production of CoQ10 by inhibiting 5-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase in the mevalonate pathway, which is the shared biosynthetic pathway for cholesterol as well as CoQ10. It is unknown whether statin inhibition of CoQ10 synthesis has clinical significance or if mitochondrial oxidation is impaired to the extent that muscle side effects occur in some but not most individuals who use statins.
Post-exercise metabolic recovery rate of phosphocreatine (PCr) in skeletal muscle is used as an index of mitochondrial oxidative capacity in vivo and can be measured quantitatively with 31P magnetic resonance spectroscopy (31P-MRS). This technique has been used to study numerous muscular disorders (Boska et al. 1999; Hug et al. 2005; Kent-Braun et al. 1994; Pipinos et al. 2000; Wu et al. 2010) and is unique in its ability to study, continuously and non-invasively, changes in PCr. During exercise, PCr is depleted in the mitochondria of skeletal muscle cells. Immediately following exercise, resynthesis of PCr depends exclusively on mitochondrial oxidative phosphorylation (Bendahan et al. 2004), and impairment of mitochondrial oxidation is reflected in prolonged (slower) post-exercise PCr recovery. We previously used 31P-MRS to show that post-exercise PCr recovery times were significantly prolonged after four weeks of statin therapy when compared to baseline measures off statins (Wu et al. 2010). The purpose of this study is to assess the effects of CoQ10 oral supplementation on calf muscle post-exercise PCr recovery kinetics in patients on statin therapy using 31P-MRS. In addition, we also explored if there are differences among those with a history of statin myalgia compared to those with a history of tolerating statins, and evaluated correlations between plasma CoQ10 levels and PCr recovery kinetics.
Materials & Methods
This study is a pilot, randomized, double-blinded, placebo-controlled, parallel arm clinical trial. All visits were conducted at Beth Israel Deaconess Medical Center (BIDMC), Boston, Massachusetts, in the Harvard-Thorndike Clinical Research Center and Radiology Department at BIDMC. All patients provided written informed consent. The study was approved by the BIDMC institutional review board (IRB) and was registered on ClinicalTrials.gov (Identifier: NCT01702987).
Study Participants
Adults ≥ 21 years who had previously been prescribed a statin by their physician were invited to participate in the study. To explore outcomes between patients with statin myalgia and patients who tolerated statins, subjects were recruited from two distinct populations and attempted to achieve equal enrollment by statin-tolerance history. We defined “statin-tolerators” by absence of a history of statin myalgia, confirmed by both the patient and the patient’s primary care physician. To ensure recruitment of individuals with a clear diagnosis of statin myalgia, patients with statin myalgia were recruited after their completion in a study running concurrently at BIDMC in which confirmation of the diagnosis of statin myalgia was based on published expert consensus and included all the following criteria: i) a history of muscle symptoms that started after initiation of statin therapy and persisted for at least two weeks of statin use, ii) improvement of muscle symptoms within two months after discontinuing statin therapy, and iii) return of muscle symptoms within 3 months during an observed statin rechallenge.
Individuals with diabetes or peripheral vascular disease/intermittent claudication were not eligible to participate in the study because these conditions may interfere with interpretation of skeletal muscle PCr recovery. Additional exclusion criteria included conditions for which a patient should not stop statin for a washout period (e.g., recent heart attack or stroke), uncontrolled medical conditions that could impair an individual’s ability to participate in the study (e.g., hypertension >170/>100, severe renal impairment (glomerular filtration rate < 30), or anemia with hemoglobin < 10, which could limit a patient’s ability to perform exercise), contraindications to MRI, creatine kinase >5 times the upper limit of normal, and aspartate aminotransferase or alanine aminotransferase >3 times normal.
Study Protocol
Participants were recruited starting in November 2012 and ending in August 2013. All study data acquisition was completed in September 2013 after the enrollment goal was reached. Interested patients meeting initial eligibility were scheduled to attend 2 study visits, a baseline visit and a follow up visit after 4 weeks of using either statin+CoQ10 or statin+placebo. Participants who were currently using a statin and/or CoQ10 supplement went through a washout period of at least 2-weeks prior to having baseline testing.
At baseline, semi-structured interviews and questionnaires were used to obtain information on demographics, medical histories, health habits, experiences using statin therapy, and past and current myalgia symptoms. Vital signs, blood testing, and 31P-MRS exercise testing were performed off statin and CoQ10. Following baseline measures, participants restarted statin therapy along with CoQ10 or placebo. All participants used a statin at a dose equivalent to simvastatin 20 mg daily or higher. Follow up testing was repeated after 4 weeks.
Study agent
Participants were randomized to receive either a CoQ10 300 mg capsule taken twice daily or a matching placebo taken twice daily. The CoQ10 supplement used was ubiquinol, the reduced form of CoQ10. Placebo and active study capsules were identical in appearance, texture, smell, and taste. Both placebo and ubiquinol capsules were manufactured in single batches by Tishcon Corp. (Westbury, NY).
Randomization
Participants were randomized using random permuted blocks, and were stratified by history of statin myalgia, with the intent of achieving 50% randomized to placebo or CoQ10. Randomization assignments were performed by the study biostatistician and were provided directly to the Head Pharmacist at the BIDMC Research Pharmacy, which dispensed the blinded study agents. Investigators and all other study staff having any participant interaction, involved in data collection or obtaining measurements/testing were blinded to treatment assignment.
Measurements
31P-MRS equipment/protocol
31P-MRS was performed on the posterior calf using a surface coil during exercise on a custom-built MRI-compatible pedal ergometer. All participants were studied before and 4 weeks after statin+CoQ10 or statin+placebo. The exercise ergometer was designed to allow the study subjects to perform plantar flexion exercise by pressing against a foot pedal while lying in the supine position. The subject’s lower extremity was secured to the MRI table with straps across the mid-thigh and mid lower leg in order to isolate usage of the posterior calf muscles. The force required to depress the pedal was supplied by a pressurized tank of nitrogen gas with an adjustable pressure regulator, which was connected to a piston cylinder attached to the foot pedal. Using their dominant foot, the maximum pressure in pounds per square inch (PSI) that could be applied to the foot pedal while the subjects could still fully depress the pedal was determined on an individual basis. To set the pressure used for each subject, a subject’s maximum calf muscle strength was determined using the one-repetition maximum test, which represents the maximum amount of weight a subject can press the pedal through the full range of motion one time. This was performed by incrementally increasing the pressure on the pedal to determine the subject’s maximum strength. 40% of the maximum pressure was then used for both the baseline and 4-week follow-up studies. The standardized exercise protocol consisted of pressing the pedal down for one second and relaxing back for one second as cued by a metronome, with subjects performing 30 plantar flexions per minute until muscle exhaustion, calf pain, or for a maximum of 7 minutes. To limit possible variability in instructing patients and overseeing the standardized exercise protocol, one radiology technician who had extensive experience in the technique performed all tests.
31P-MRS data acquisition
31P-MRS of the posterior calf muscles muscle was performed using a 3-Tesla MR system (General Electric Healthcare, Milwaukee, Wisconsin). A 7.5-cm circular 31P surface coil was centered on the maximum diameter of the calf (approximately 4 to 8 cm below the posterior knee crease). Magnetic field homogeneity was adjusted while observing the 31P signal. Spectra were acquired using a pulse-and-acquire free induction decay (FID) sequence. The surface coil accomplished localization. The 31P-MRS acquisition parameters were: sweep width, 2048 Hz; number of complex points, 1024; TR, 5 S; signal averages, 2; time per acquisition, 10 S. Subjects were studied for: (1) one minute prior to calf exercise, (2) up to 7 minutes during exercise, and (3) for 6 minutes after exercise cessation during the recovery period. 31P spectra were acquired at 10 S intervals.
Additional Measures
Laboratory evaluations were performed on venous blood samples collected at each visit. Plasma collected for CoQ10 profiles was processed immediately, frozen at −80 °C, and shipped overnight in dry ice for analysis at Cincinnati Children’s Hospital Medical Center using high-performance liquid chromatography. The method, developed by Tang, et al., has previously been described in detail (Tang et al. 2001). Serum cholesterol profiles included direct low-density lipoprotein (LDL) cholesterol and were measured by enzymatic methods to assess adherence with statins. Cardiovascular, inflammatory and endothelial factors evaluated included: C-reactive protein (CRP) measured with a high-sensitivity chemiluminescent assay (Diagnostic Products, Los Angeles, CA); interleukin-6 (IL-6) and tumar necrosis factor α (TNFα) measured using electrochemiluminescence detection (Meso Scale Diagnostics, Gaithersburg, MD); and E-selectin, vascular cell adhesion molecule-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1) assessed by enzyme-linked immunosorbent assay (R & D Systems, Minneapolis, MN). Safety measures, including creatine phosphokinase, serum alanine aminotransferase, and aspartate aminotransferase were also evaluated at baseline and week 4.
Statistical Analyses
The relative change in the PCr recovery time constant between the baseline and 4-week follow up testing for the CoQ10 versus the placebo groups was the primary outcome. The relative change was defined as 100*(baseline − follow up)/baseline. The difference between baseline and follow up was tested using the nonparametric signed rank test. The median was used as the estimator for the central tendency of the distributions of PCr recovery time. The difference between treatment groups was tested using the nonparametric test Wilcoxon rank sum. The correlation between CoQ10 and PCr recovery time was tested using the Spearman correlation coefficient. Sample size was based on our previous feasibility study using this technique (Wu et al. 2010). The power analysis was carried out based on testing the difference of two differences (i.e. the difference of pre (baseline) between treatment groups and the difference of post (follow up) between treatment groups). We assumed, for the power analysis, that the difference of pre was zero, and thus, we used the difference of post with the t-test and the means and standard deviation of the post. For the analysis, we found that these observed differences (pre and post) were non-normally distributed, and therefore, we used non-parametric tests throughout. Type-I error rate was set at 0.05. All analyses were carried out using the SAS/STAT software version 9.3.
31P-MRS data analysis
The 31P spectral data were processed using IDL software (Version 6.0. Research Systems, Inc. Boulder, CO) and SAS/STAT nonlinear estimation procedure NLIN. Spectra were acquired using a non-selective (hard) radiofrequency pulse. An exponential filter (10 Hz) was used prior to Fourier transformation. Following Fourier transformation the spectra were phased using a semi-automated phasing algorithm and the areas of the PCr peaks were calculated by integration. For each subject, we estimated this parameter using the maximum likelihood estimation method. Thus, we obtained the point estimate and standard error for the time constant parameter for each subject at each of the time points. The PCr recovery time constant was calculated using a monoexponential fit of PCr versus time, beginning at exercise completion.
Results
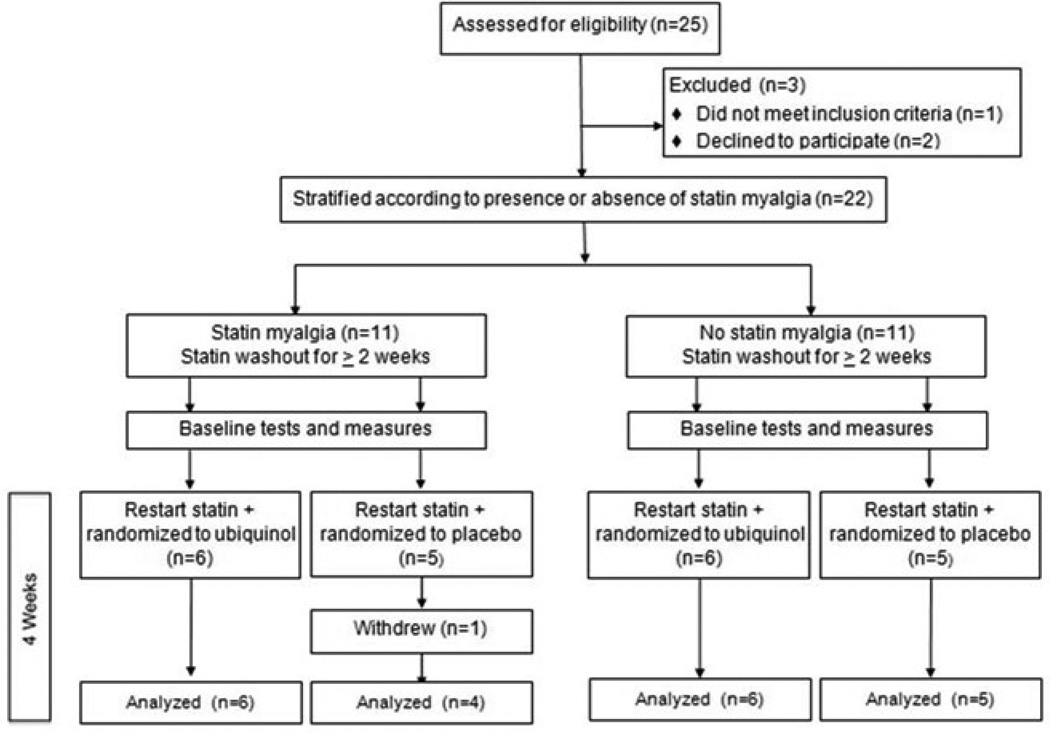
The study flow diagram for participants is shown in Figure 1. Among 25 enrolled participants, two declined randomization and one was ineligible for MRS due to cardiac hardware. Of the 22 randomized participants, 50% met criteria for having a history of statin myalgia; all others had tolerated statin therapy for at least one year (median 6 years; minimum 14 months − maximum 9 years). One participant withdrew from the study after randomization due to a viral illness. Characteristics of the 21 participants who completed the study are shown in Table 1 according to randomization to ubiquinol or placebo.
Figure 1.
Study flow diagram.
Table 1.
Characteristics of the study population according to randomization to CoQ10 or placebo.
| Characteristic | Placebo (N=9) | CoQ10 (N=12) |
|---|---|---|
| Age—yr | 60.4 ± 5.9 | 59.3 ± 7.1 |
| Female—no. (%) | 3 (33%) | 5 (42%) |
| White | 8 (89%) | 11 (92%) |
| Current/past smoking | 3 (33%) | 3 (25%) |
| Light/moderate alcohol use | 8 (89%) | 11 (92%) |
| Moderate/vigorous exercise | 6 (67%) | 6 (50%) |
| Health conditions— no. (%) | ||
| Hypertension | 5 (56%) | 5 (42%) |
| Depression | 2 (22%) | 1 (8%) |
| Pulmonary disease | 1 (11%) | 2 (17%) |
| Thyroid disease | 1 (11%) | 1 (8%) |
| Median number concurrent medications, excluding statin, (range) |
5 (1–15) | 4 (1–13) |
| Systolic blood pressure (SBP) | 135.8 ± 10.6 | 125.7 ± 10.7 |
| Body Mass Index (m/Kg2) | 28.3 ± 3.5 | 27.1 ± 3.8 |
PCr recovery kinetics
In 2 of the 21 patients who completed the study, PCr recovery kinetics from one of the 2 timepoints could not be performed due to poor effort by the patients during the study protocol and no appreciable PCr could be measured. The PCr recovery time of the 19 participants who had evaluable PCr data are shown in Table 2. The median PCr recovery of these 19 participants was 48.3 s at baseline and 55.6 s after 4 weeks of statin therapy with a relative percentage difference of −15.1%; however, this difference was not statistically significant, (p-value=0.258). A negative value for percentage change indicates that PCr recovery post-treatment was slower than baseline indicating worsening mitochondrial function as expected due to the statin therapy. Patients randomized to statin+placebo (n=9) had a PCr recovery percentage change of −18.9% (IQR: 137.7); and patients randomized to statin+CoQ10 (n=10) had a PCr recovery percentage change of −7.7% (IQR: 38.7). This difference between groups was also not significant, (p-value=0.448).
Table 2.
PCr recovery pre- and post- therapy according to randomization and history of statin myalgia
| ID | Age (years) |
Sex | Statin Dose (mg/d) | Relative Change in PCr Recovery Time (%)* |
Myalgia pain after 4 weeks (0–10) |
Time of exercise (max 420s) |
|---|---|---|---|---|---|---|
| 1 | 62 | M | Rosuvastatin (10) | −26.0 | 0 | 190 |
| 2 | 63 | M | Simvastatin (20) | 25.4 | 0 | 420 |
| 3 | 56 | M | Simvastatin (20) | −222.6 | 0 | 230 |
| 4 | 62 | F | Simvastatin (20) | 1.5 | 0 | 420 |
| 5 | 65 | M | Atorvastatin (10) | −148.6 | 0 | 420 |
| Subgroup Median [IQR] | −26.0 [150.0] | |||||
| 6 | 69 | F | Simvastatin (20) | −115.2 | 3 | 420 |
| 7 | 52 | F | Atorvastatin (20) | 22.5 | 0 | 420 |
| 8 | 63 | M | Atorvastatin (20) | −18.9 | 3 | 300 |
| 9 | 52 | M | Simvastatin (20) | 29.1 | 0 | 340 |
| Subgroup Median [IQR] | 1.8 [92.8] | |||||
| Placebo Median [IQR] | −18.9 [137.7] | |||||
| 12 | 47 | F | Simvastatin (20) | −16.6 | 0 | 420 |
| 13 | 58 | M | Simvastatin (20) | −0.3 | 0 | 420 |
| 14 | 60 | F | Simvastatin (40) | −15.0 | 0 | 420 |
| 15 | 55 | M | Atorvastatin (10) | −105.5 | 0 | 420 |
| Subgroup Median [IQR] | −15.8 [53.4] | |||||
| 16 | 73 | M | Atorvastatin (10) | 85.6 | 0 | 420 |
| 17 | 70 | F | Rosuvastatin (5) | 42.1 | 0 | 420 |
| 18 | 52 | M | Simvastatin (20) | −25.1 | 5 | 420 |
| 19 | 58 | F | Rosuvastatin (5) | −93.7 | 3 | 390 |
| 20 | 61 | M | Rosuvastatin (20) | 13.6 | 3 | 420 |
| 21 | 56 | M | Atorvastatin (80) | 3.9 | 0 | 130 |
| Subgroup Median [IQR] | 8.8 [67.2] | |||||
|
| ||||||
| CoQ10 Median [IQR] | −7.7 [38.7] | |||||
The relative change in PCr recovery time was defined as 100*(baseline − follow up)/baseline).
Serum LDL and plasma CoQ10 as measures of adherence
Serum LDL and plasma CoQ10 measures for all 21 participants are shown in Table 3 at baseline and 4 weeks following randomization. LDL cholesterol was significantly reduced after 4 weeks, One participant (ID 8) assigned to statin+placebo demonstrated a substantial increase in oxidized CoQ10, suggesting possible exogenous CoQ10 (ubiquinone or oxidized CoQ10) supplementation, while two participants (IDs 20 and 21) assigned to statin+CoQ10 had a reduction or negligible increase in plasma CoQ10, suggesting non-adherence with CoQ10 use. Based on pill counts and participant report, 2 participants used <75% of their assigned study capsules (CoQ10 or placebo), including ID 7, who returned 40%, and ID 21, who returned 50%, of their study capsules.
Table 3.
LDL and plasma CoQ10 reduced, oxidized levels pre- and 4 weeks post- statin therapy.
| ID | LDL* (mmol/ L) Pre- Statin |
LDL* (mmol/L) Post- Statin |
% Decrease LDL |
CoQ10 Reduced Pre- Statin |
CoQ10 Reduced Post- Statin |
% Decrease CoQ10 Reduced |
CoQ10 Reduced /LDL Pre- Statin |
CoQ10 Reduced /LDL Post- Statin |
% Decrease CoQ10 Reduced /LDL |
CoQ10 Oxidized Pre- Statin |
CoQ10 Oxidized Post- Statin |
% Decrease CoQ10 Oxidized |
CoQ10 Oxidized /LDL Pre- Statin |
CoQ10 Oxidized /LDL Post- Statin |
% Decrease CoQ10 Oxidized /LDL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup 1. No History of Myalgia−Placebo+Statin | |||||||||||||||
| 1 | 4.09 | 2.53 | 38.0 | 1.04 | 0.58 | 44.5 | 0.25 | 0.23 | 10.6 | 0.098 | 0.041 | 58.2 | 0.024 | 0.016 | 32.5 |
| 2 | 4.76 | 3.36 | 29.3 | 1.13 | 0.92 | 18.5 | 0.24 | 0.27 | −15.4 | 0.113 | 0.032 | 71.7 | 0.024 | 0.010 | 59.9 |
| 3 | 4.19 | 1.99 | 52.5 | 0.83 | 0.49 | 41.0 | 0.20 | 0.24 | −24.0 | 0.202 | 0.034 | 83.2 | 0.048 | 0.017 | 64.6 |
| 4 | 4.37 | 2.95 | 32.5 | 0.96 | 0.83 | 13.5 | 0.22 | 0.28 | −28.2 | 0.065 | 0.046 | 29.2 | 0.015 | 0.016 | −4.9 |
| 5 | 4.09 | 2.59 | 36.7 | 0.76 | 0.54 | 28.7 | 0.19 | 0.21 | −12.6 | 0.062 | 0.030 | 51.6 | 0.015 | 0.012 | 23.5 |
| Subgroup 2. History of Myalgia−Placebo+Statin | |||||||||||||||
| 6 | 5.79 | 3.75 | 35.3 | 1.58 | 0.82 | 47.9 | 0.27 | 0.22 | 19.5 | 0.152 | 0.068 | 55.3 | 0.026 | 0.018 | 30.9 |
| 7 | 5.90 | 2.84 | 51.8 | 0.93 | 0.44 | 53.0 | 0.16 | 0.15 | 2.5 | 0.063 | 0.036 | 42.9 | 0.011 | 0.013 | −18.4 |
| 8 | 5.20 | 3.96 | 23.9 | 1.47 | 1.05 | 28.5 | 0.28 | 0.27 | 6.1 | 0.052 | 0.176 | −238 | 0.010 | 0.044 | −345 |
| 9 | 3.18 | 2.59 | 18.7 | 1.28 | 1.14 | 11.0 | 0.40 | 0.44 | −9.5 | 0.078 | 0.064 | 17.9 | 0.025 | 0.025 | −0.9 |
| Subgroup 3. No History of Myalgia−Ubiquinol+Statin | |||||||||||||||
| 10 | 6.03 | 4.53 | 24.9 | 0.90 | 3.97 | −340 | 0.15 | 0.88 | −486 | 0.076 | 0.152 | −100 | 0.013 | 0.034 | −166 |
| 11 | 3.52 | 2.59 | 26.5 | 0.76 | 4.32 | −467 | 0.22 | 1.67 | −671 | 0.123 | 0.213 | −73.2 | 0.035 | 0.082 | −136 |
| 12 | 4.32 | 3.52 | 18.6 | 0.99 | 4.91 | −395 | 0.23 | 1.39 | −508 | 0.045 | 0.171 | −280 | 0.010 | 0.049 | −367 |
| 13 | 2.20 | 1.47 | 32.9 | 0.78 | 6.04 | −674 | 0.35 | 4.10 | −1054 | 0.074 | 0.296 | −300 | 0.034 | 0.201 | −497 |
| 14 | 4.84 | 2.59 | 46.5 | 1.12 | 7.01 | −523 | 0.23 | 2.71 | −1065 | 0.113 | 0.143 | −26.5 | 0.023 | 0.055 | −137 |
| 15 | 4.19 | 2.59 | 38.3 | 0.75 | 7.05 | −839 | 0.18 | 2.72 | −1422 | 0.035 | 0.163 | −366 | 0.008 | 0.063 | −655 |
| Subgroup 4. History of Myalgia−Ubiquinol+Statin | |||||||||||||||
| 16 | 4.19 | 2.72 | 35.2 | 1.46 | 4.90 | −234 | 0.35 | 1.80 | −416 | 0.124 | 0.180 | −45.2 | 0.030 | 0.066 | −124 |
| 17 | 4.63 | 3.08 | 33.5 | 0.57 | 5.49 | −863 | 0.12 | 1.78 | −1348 | 0.110 | 0.136 | −23.6 | 0.024 | 0.044 | −86 |
| 18 | 6.03 | 3.57 | 40.8 | 1.09 | 6.51 | −497 | 0.18 | 1.83 | −908 | 0.070 | 0.086 | −22.9 | 0.012 | 0.024 | −107 |
| 19 | 3.96 | 2.28 | 42.5 | 0.72 | 4.45 | −518 | 0.18 | 1.96 | −974 | 0.040 | 0.202 | −405 | 0.010 | 0.089 | −778 |
| 20 | 3.21 | 2.40 | 25.0 | 3.78 | 2.16 | 42.9 | 1.18 | 0.90 | 23.9 | 0.179 | 0.052 | 70.9 | 0.056 | 0.022 | 61.3 |
| 21 | 2.66 | 2.87 | −7.8 | 0.58 | 0.68 | −16.3 | 0.22 | 0.24 | −8.0 | 0.032 | 0.032 | 0 | 0.012 | 0.011 | 7.2 |
To convert LDL (low density lipoprotein) from SI units (mmol/L) to mg/dL multiply by 38.67.
Plasma CoQ10 and PCr recovery kinetics
Because CoQ10 is carried on LDL particles in blood, LDL adjusted measures for CoQ10 were calculated and are presented in Table 3. Among participants receiving statin+placebo (subgroups 1 and 2) no significant decreases in plasma CoQ10 concentrations adjusted for LDL were observed. No meaningful correlations between PCr recovery kinetics and plasma total CoQ10 (r= −0.06, p=0.81), reduced CoQ10 (r= −0.01, p=0.95), or oxidized CoQ10 (r=0.00, p=1.00) were revealed among the 19 participants analyzed using Spearman rank correlation.
Participants with a history of Statin Myalgia Compared to Statin Tolerators
Among 10 participants with a history of statin myalgia, 4 were randomized to statin+placebo and 6 were randomized to statin+CoQ10. In each group 50% experienced a return of myalgia symptoms during the 4-week study.
Inflammatory and Vascular Biomarkers and Safety Measures
The effects of CoQ10 supplementation on cardiovascular, inflammatory and vascular factors, as well as liver and muscle enzymes were evaluated for 21 participants. We did not find significant differences between participants assigned to CoQ10 or placebo for any measure. One adverse event leading to the participant electing to withdraw from the study occurred in the placebo group. This participant developed gastrointestinal symptoms attributed to viral gastroenteritis, resulting in her inability to consume a normal diet or use the study agent for several weeks. No adverse events occurred in the CoQ10 group.
Discussion
In this study, we examined post-exercise PCr recovery time in calf muscles using 31P-MRS at baseline and after 4 weeks of statin+CoQ10 or statin+placebo therapy. Among the 19 subjects with complete data, the relative percentage change in PCr recovery time was −15.1%. Although this change between baseline and 4 weeks of therapy was not statistically significant, the magnitude and direction of change is similar to a prior study showing a decrease in mitochondrial function after one-month of statin use (Wu et al. 2010).
As for the effect of CoQ10 oral supplementation on counteracting the negative effects of statins on mitochondrial function, our results did not show a statistically significant benefit. The group randomized to statin+placebo showed a relative percentage change in the PCr recovery time of −18.9%, while those randomized to statin+CoQ10 had a change of −7.7%. Although the subjects on statin+CoQ10 did have less decrease in their mitochondrial function when compared to statin+placebo, the between-group difference was not statistically significant. It is unclear if increasing the length of therapy could reveal a significant effect.
In regards to correlation of the various serum cardiovascular, inflammatory, and vascular biomarkers, we found no significant correlation between any of the markers with PCr recovery. However, the use of ubiquinol 300 mg twice daily was well tolerated and was sufficient to significantly increase plasma total and reduced CoQ10 levels to demarcate participants who were and were not adherent with the study medication.
As a pilot study the sample size for this trial was likely not large enough to detect small changes in PCr recovery time, and we observed greater variability in PCr testing than we had previously observed in our prior study (Wu et al. 2010). We reviewed the results of our prior study to consider reasons for greater variability seen in this study. Compared to our prior study, we note that this study involved older patients (the median age of patients in this study was 60 years compared to 49 years in our prior study). Along with older age comes an increased number of co-morbidities, and wider ranges in both health status and levels of physical activity. In this study, the majority of patients were on >3 concurrent medications, up to 15. While no participant was using medications contraindicated with statin therapy, little is known about the contributions of different medications to PCr recovery kinetics. Moreover, in this study, subjects varied in habitual/routine exercise, a known significant confounder of mitochondrial capacity (Phielix et al. 2008), as well as a confounder of effects of statins on muscle (Bouitbir et al. 2011b; Dirks & Jones 2006; Mikus et al. 2013; Sirvent et al. 2005; Sirvent et al. 2008). Moderate or vigorous exercise was performed by the majority of statin tolerators, compared to those with a history of myalgia, the majority of who were sedentary.
In addition to limitations related to small sample size and greater variability in PCr recovery times, additional limitations of this study include the 4-week duration of the oral medication. It is possible that early changes may differ from changes over a longer time period, particularly with respect to the return of myalgia symptoms, which may take 3 months or longer to reoccur. Moreover, larger differences in mitochondrial function may occur between treatment groups with increase length of therapy. Finally, although this non-invasive technique was used as an alternative to invasive muscle biopsy, lack of comparison to CoQ10 from muscle biopsy in the same sample is a limitation in judging whether 31P-MRS testing might aid in differentiating between patients who experience statin myalgia and patients who tolerate statin therapy.
Despite limitations in the study, our findings indicate no significant effect of ubiquinol supplementation on skeletal muscle mitochondrial function in individuals using a statin. Although, our sample size is small, these findings seem consistent with a recent larger study showing lack of improvement using CoQ10 supplementation in patients with confirmed statin myalgia (Taylor et al. 2015), and suggest that other mechanisms unrelated to mitochondrial capacity should continue to be explored as causes of statin myalgia.
Acknowledgments
This study was funded by the Kaneka Corporation (Osaka, Japan) and Kaneka North America L.L.C. (Pasadena, TX) and was conducted with support from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170-05 and financial contributions from Harvard University and its affiliated academic health care centers. Dr. Buettner’s work was also supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K23AR055664. The content is solely the responsibility of the authors and does not necessarily represent the official views of Kaneka Corporation, Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations & Units
- (CoQ10)
coenzyme Q10
- (31P-MRS)
31phosphorus magnetic resonance spectroscopy
- (PCr)
phosphocreatine
- (ATP)
adenosine triphosphate
- (HMG-CoA)
5-hydroxy-3-methylglutaryl-coenzyme A
- (BIDMC)
Beth Israel Deaconess Medical Center
- (IRB)
institutional review board
- (PSI)
pounds per square inch
- (FID)
free induction decay
- (CRP)
C-reactive protein
- (IQR)
interquartile range
- (LDL)
low-density lipoprotein
- (IL-6)
interleukin-6
- (TNFα)
tumor necrosis factor α
- (VCAM-1)
vascular cell adhesion molecule-1
- (ICAM-1)
intercellular adhesion molecule-1
References
- 1.Bendahan D, Giannesini B, Cozzone PJ. Functional investigations of exercising muscle: a noninvasive magnetic resonance spectroscopy-magnetic resonance imaging approach. Cellular and molecular life sciences : CMLS. 2004;61:1001–1015. doi: 10.1007/s00018-004-3345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boska MD, Nelson JA, Sripathi N, Pipinos Ii, Shepard AD, Welch KM. 31P MRS studies of exercising human muscle at high temporal resolution. Magn Reson Med. 1999;41:1145–1151. doi: 10.1002/(sici)1522-2594(199906)41:6<1145::aid-mrm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Bouitbir J, Charles AL, Echaniz-Laguna A, Kindo M, Daussin F, Auwerx J, Piquard F, Geny B, et al. Opposite effects of statins on mitochondria of cardiac and skeletal muscles: a 'mitohormesis' mechanism involving reactive oxygen species and PGC-1. Eur Heart J. 2011a;33:1397–1407. doi: 10.1093/eurheartj/ehr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouitbir J, Charles AL, Rasseneur L, Dufour S, Piquard F, Geny B, Zoll J. Atorvastatin treatment reduces exercise capacities in rats: involvement of mitochondrial impairments and oxidative stress. Journal of applied physiology. 2011b;111:1477–1483. doi: 10.1152/japplphysiol.00107.2011. [DOI] [PubMed] [Google Scholar]
- 5.Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 6.Buettner C, Rippberger MJ, Smith JK, Leveille SG, Davis RB, Mittleman MA. Statin use and musculoskeletal pain among adults with and without arthritis. Am J Med. 2012;125:176–182. doi: 10.1016/j.amjmed.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dirks AJ, Jones KM. Statin-induced apoptosis and skeletal myopathy. Am J Physiol Cell Physiol. 2006 doi: 10.1152/ajpcell.00226.2006. [DOI] [PubMed] [Google Scholar]
- 8.Hug F, Bendahan D, Le Fur Y, Cozzone PJ, Grelot L. Metabolic recovery in professional road cyclists: a 31P-MRS study. Med Sci Sports Exerc. 2005;37:846–852. doi: 10.1249/01.mss.0000162616.20085.b4. [DOI] [PubMed] [Google Scholar]
- 9.Kent-Braun JA, Sharma KR, Miller RG, Weiner MW. Postexercise phosphocreatine resynthesis is slowed in multiple sclerosis. Muscle Nerve. 1994;17:835–841. doi: 10.1002/mus.880170802. [DOI] [PubMed] [Google Scholar]
- 10.Mas E, Mori TA. Coenzyme Q(10) and statin myalgia: what is the evidence? Curr Atheroscler Rep. 2010;12:407–413. doi: 10.1007/s11883-010-0134-3. [DOI] [PubMed] [Google Scholar]
- 11.Mikus CR, Boyle LJ, Borengasser SJ, Oberlin DJ, Naples SP, Fletcher J, Meers GM, Ruebel M, et al. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol. 2013;62:709–714. doi: 10.1016/j.jacc.2013.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phielix E, Schrauwen-Hinderling VB, Mensink M, Lenaers E, Meex R, Hoeks J, Kooi ME, Moonen-Kornips E, et al. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57:2943–2949. doi: 10.2337/db08-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Experimental and molecular pathology. 2007;83:84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Pipinos Ii, Shepard AD, Anagnostopoulos PV, Katsamouris A, Boska MD. Phosphorus 31 nuclear magnetic resonance spectroscopy suggests a mitochondrial defect in claudicating skeletal muscle. J Vasc Surg. 2000;31:944–952. doi: 10.1067/mva.2000.106421. [DOI] [PubMed] [Google Scholar]
- 15.Sirvent P, Bordenave S, Vermaelen M, Roels B, Vassort G, Mercier J, Raynaud E, Lacampagne A. Simvastatin induces impairment in skeletal muscle while heart is protected. Biochemical and biophysical research communications. 2005;338:1426–1434. doi: 10.1016/j.bbrc.2005.10.108. [DOI] [PubMed] [Google Scholar]
- 16.Sirvent P, Mercier J, Lacampagne A. New insights into mechanisms of statin-associated myotoxicity. Curr Opin Pharmacol. 2008;8:333–338. doi: 10.1016/j.coph.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Tang PH, Miles MV, Degrauw A, Hershey A, Pesce A. HPLC analysis of reduced and oxidized coenzyme Q(10) in human plasma. Clinical chemistry. 2001;47:256–265. [PubMed] [Google Scholar]
- 18.Taylor BA, Lorson L, White CM, Thompson PD. A randomized trial of coenzyme Q10 in patients with confirmed statin myopathy. Atherosclerosis. 2015;238:329–335. doi: 10.1016/j.atherosclerosis.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu JS, Buettner C, Smithline H, Ngo LH, Greenman RL. Evaluation of skeletal muscle during calf exercise by 31-phosphorus magnetic resonance spectroscopy in patients on statin medications. Muscle Nerve. 2010;43:76–81. doi: 10.1002/mus.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]