Abstract
Liver fibrosis is the final stage of liver diseases that lead to liver failure and cancer. While various diagnostic methods, including the use of serum marker, have been established, no standard therapy has been developed. The objective of this study was to assess the approach of overexpressing matrix metalloproteinase-13 gene (MMP13) in rat liver to prevent liver fibrosis progression. A rat liver fibrosis model was established by ligating the bile duct, followed by liver-targeted hydrodynamic gene delivery of a MMP13 expression vector, containing a CAG promoter-MMP13-IRES-tdTomato-polyA cassette. After 14 days, the serum level of MMP13 peaked at 71.7 pg/ml in MMP13-treated group, whereas the nontreated group only showed a level of ~5 pg/ml (P < 0.001). These levels were sustained for the next 60 days. The statistically lower level of the hyaluronic acids in treated group versus the nontreated group (P < 0.05) reveals the therapeutic effect of MMP13 overexpression. Quantitative analysis of tissue stained with sirius red showed a statistically larger volume of fibrotic tissue in the nontreated group compared to that of MMP13-treated rats (P < 0.05). These results suggest that the liver-targeted hydrodynamic delivery of MMP13 gene could be effective in the prevention of liver fibrosis.
Keywords: gene therapy, hydrodynamic gene delivery, liver cirrhosis, MMP13, nucleic acids delivery
Introduction
Liver fibrosis is the final stage of all chronic liver disease and a leading cause of premature morbidity and mortality worldwide. The etiologies are varied, including viral hepatitis (e.g., hepatitis B virus, hepatitis C virus), alcohol overdose–induced liver injury, and autoimmune diseases (primary biliary cirrhosis and autoimmune hepatitis and nonalcoholic steatohepatitis).1 Severity of liver diseases depends on the hepatic reserve functions and the portal hypertension associated with various symptoms including jaundice, ascites, varices, and encephalopathy. In addition, hepatocellular carcinomas are found in 3–5% of liver cirrhosis patients on an annual basis in worldwide.1 Although various diagnostic methods have been established for this disease, no standard therapy for liver fibrosis has been developed. Efforts have been made in recent years to establish cell therapies and gene therapies for liver fibrosis patients in order to reduce fibrotic tissue, recover liver function, and decrease the occurrence of hepatocellular carcinomas.2,3,4,5,6,7,8
Liver fibrosis is characterized by the replacement of normal liver tissues with extracellular matrix (ECM), consisting of fibrillary collagen, fibronectin, and laminin. These fibrotic tissues are mainly produced by the activated hepatic stellate cells (HSCs) and are activated by continuous liver injury where the cells differentiate into myofibroblast-like cells and produce significant quantities of ECM. HSCs not only contribute to the progression of liver fibrosis2 but also play a pivotal role in resolving fibrosis by producing various enzymes, such as matrix metalloproteinases (MMPs). Severity of liver fibrosis depends on the balance between MMPs released from HSCs and tissue inhibitors of metalloproteinase (TIMP). TIMP is an inhibitor of MMPs released from Kupffer cells and other interstitial cells.1 Pathologically, liver fibrosis represents an imbalance between production and dissolution of ECM due to liver injury. Consequently, in terms of a therapeutic target, there are two logical strategies to manipulate the levels of MMPs and TIMPs, resulting in upregulation of collagenase activity in fibrotic livers.
Of the various MMPs, Quinn et al.9 reported that MMP13 was a major collagenase and a key factor for ECM resolution in liver fibrosis in rats. MMP13 is activated by the inflammatory cytokine and hepatocyte growth factor and demonstrates collagenase activity through induction of other collagenases, including MMP2 and MMP9 (ref. 10). Watanabe et al.11 reported that MMP13 gene expression was inhibited in the advanced stage of liver fibrosis, while transient but prominent MMP13 gene expression occurs during the early phase of recovery from experimental rat liver fibrosis. Therefore, it is reasonable to consider that upregulation of MMP13 gene expression may lead to a consequent increase in collagenase activity in fibrotic livers and offer an approach to antifibrotic therapy in the prevention of liver fibrosis. Since complete regression from advanced cirrhosis is both questionable and controversial, it is more feasible to develop a safe and efficient way of preventing the progression of fibrosis in liver disease. With regard to the transfer of the MMP gene into the liver, it is possible to selectively inject naked DNA into the liver via either the portal vein or the hepatic artery. Gene delivery possesses safe and efficient therapeutic potential because the target livers are already infected with HBV and/or HCV, or by other inflammatory diseases. It is currently not ideal to deliver genes by viral vectors into healthy livers.
In this study, we have examined the antifibrotic effect of MMP13 using the liver-targeted hydrodynamic gene delivery procedure in a rat liver fibrosis model.
Our results show that the overexpression of MMP13 in hepatocytes has significant preventive effect against liver fibrosis, suggesting the clinical applicability of this procedure for antifibrotic liver therapy.
Results
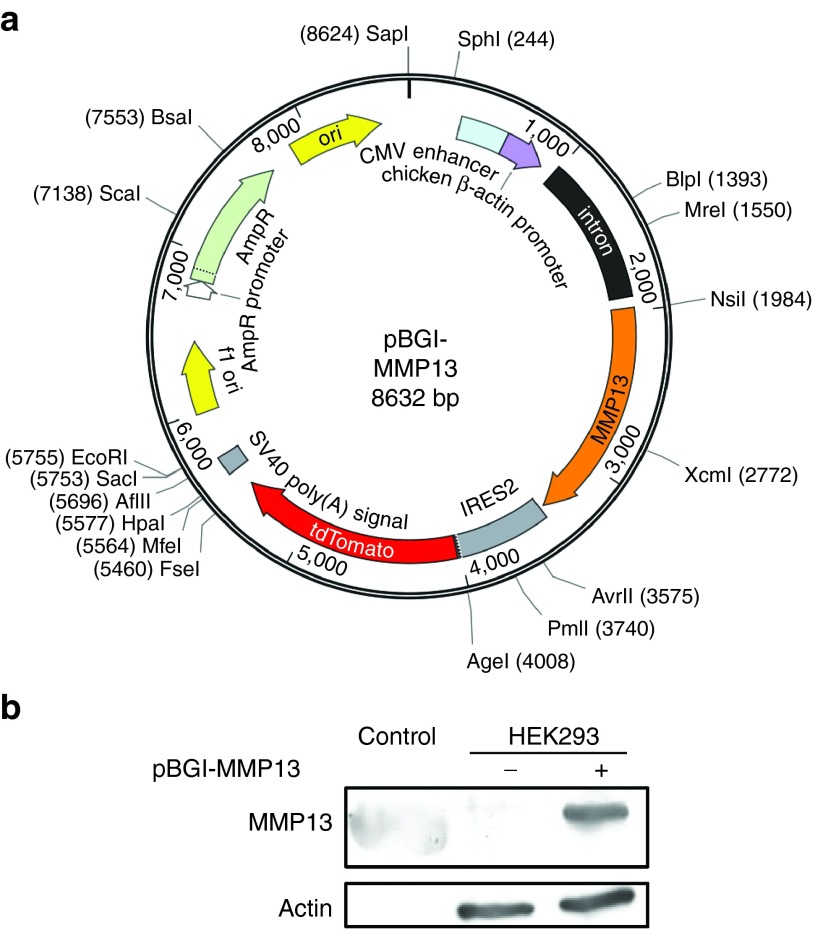
Development of MMP13-expressing plasmid
The complementary DNA of MMP13 was inserted into the pIRES2-tdTomato plasmid vector containing an internal ribosome entry site (IRES) and tdTomato protein sequences. The expression of MMP13 was under the control of a CAG promoter (chicken β-actin promoter and cytomegalovirus enhancer). The chicken beta-actin intron sequence was also inserted for long-term gene expression. The plasmid was named as pBGI-MMP13 and had a size of 8,632 bp (Figure 1a). The expression of MMP13 protein in mammalian cells was examined by transfecting pBGI-MMP13 plasmid into HEK293 and HLE liver cancer cells by lipofection. The transfected HEK293 cells and HLE cells showed efficient expression of MMP13 (Figure 1b) and tdTomato protein (Supplementary Figure S1) in vitro with no cytotoxicity.
Figure 1.
Development of MMP13-expressing plasmid. (a) The MMP13 expression vector, containing CAG promoter-MMP13-IRES-tdTomato-polyA cassette, was generated through a multistep and ligation-based cloning (pBGI-MMP13). (b) The expression efficiency of MMP13 from the pBGI-MMP13 was confirmed in vitro by western blotting. Control, standard recombinant MMP13 protein.
Liver-targeted hydrodynamic gene delivery of pBGI-MMP13
The pBGI-MMP13 plasmid was hydrodynamically delivered into the rat liver using liver-targeted hydrodynamic gene delivery method. Briefly, the procedure involves insertion of a catheter into the inferior vena cava (IVC) between temporal occlusions at the supra- and infra-hepatic IVC, followed by hydrodynamic injection of 5% body weight volume of pBGI-MMP13 solution.12 By comparing the liver extract of injected rats using the liver-targeted hydrodynamic gene delivery versus the liver extract using saline injection, we confirmed efficient expression of MMP13 (Figure 2a). No expression of MMP13 was found in the other organs including the heart, lungs, and kidneys (Supplementary Figure S2), indicating the site-specific gene delivery efficiency of the procedure. The expression of tdTomato protein was also confirmed in the cytoplasm of hepatocytes of the injected liver (Figure 2b), whereas we could not detect tdTomato in saline-injected rat livers. This would indicate that tdTomato can be used as an efficient surrogate biomarker for confirming MMP13 expression in rat liver hepatocytes. Biochemical markers were assessed to examine the safety of plasmid delivery. Transient increases in serum level of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) were seen immediately after hydrodynamic injection however they recovered to the normal ranges within 3-4 days, as previously described.12 Furthermore, there was no sustained increase of these markers or total bilirubin in the injected animals during long-term observation (Figure 2c) compared to the results obtained from the rats injected with human factor IX–expressing plasmid (Supplementary Figure S3). These results suggest that liver-targeted hydrodynamic gene delivery of pBGI-MMP13 plasmid to rat livers is safe and efficient.
Figure 2.
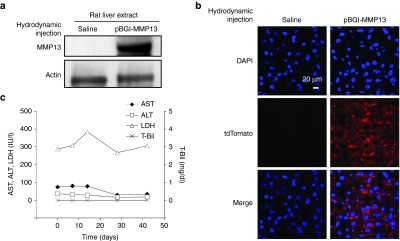
Efficiency and safety of liver-targeted pBGI-MMP13 hydrodynamic delivery. (a) Efficiency of the liver-targeted hydrodynamic gene delivery of pBGI-MMP13 in rats. Western blotting of the liver extract. (b) Detection of tdTomato protein in the liver. Bar = 20 μm. (c) Serum concentrations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), and total bilirubin (T-Bil). The values represent mean data from two rats.
Expression of MMP13 to prevent liver fibrosis
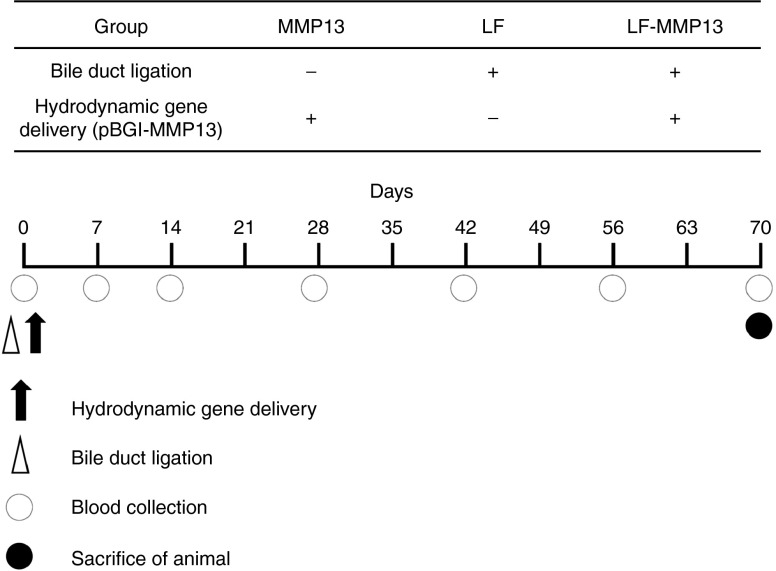
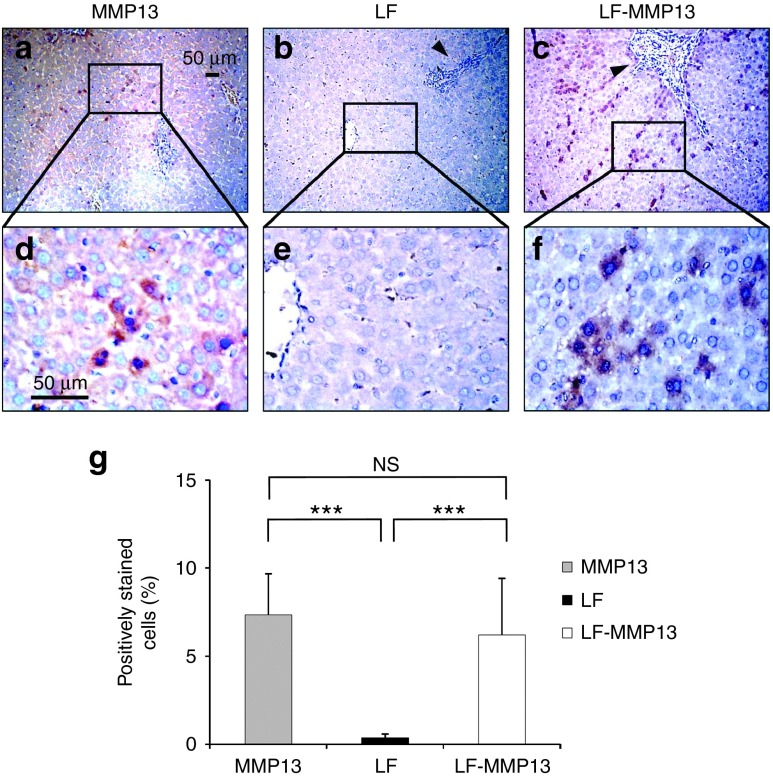
To examine the preventive effect of the MMP13 gene transfer against liver fibrosis, the liver-directed hydrodynamic gene delivery of MMP13 was performed in the rat liver fibrosis model. After the liver-targeted hydrodynamic gene delivery procedure, the gene expression from the plasmid peaked 24 hours after delivery and was sustained for 2 months.12 We performed, simultaneously, the hydrodynamic gene delivery of pBGI-MMP13 in liver fibrosis model established using bile duct ligation procedure (Figure 3). Three groups of rats were established: MMP13-injected (MMP13); liver fibrosis-induced (LF); and liver fibrosis-induced rats treated with MMP13 (LF-MMP13). The animals were carefully monitored for 70 days with blood samples being collected at appropriate time points and organs collected at the end of the study period (Figure 3). To confirm the expression of MMP13 in the hepatocytes and determine the distribution of the MMP13-expressing hepatocytes, immunohistochemical analysis was performed on the liver sections (Figure 4a–f). Compared with the control group, MMP13 showed a 7.35 ± 2.33% of positively stained hepatocytes (Figure 4a,d), whereas LF-MMP13 showed 6.21 ± 3.21% (Figure 4c,f) and LF showed 0.35 ± 0.21% (Figure 4b,e). The control group of injecting saline to LF rats showed no change to LF group. The latter was statistically lower than the MMP13 and LF-MMP13 groups (Figure 4g). These outcomes indicate efficient gene expression of MMP13 in the rat liver fibrosis model.
Figure 3.
Animal models and experimental protocol. The rat liver fibrosis model was developed by the bile duct ligation (BDL). Three groups of MMP13: pBGI-MMP13-injected normal rats; LF, liver fibrosis: BDL alone; and LF-MMP13: BDL simultaneously followed by hydrodynamic delivery of pBGI-MMP13 were developed. Each model was analyzed for the severity of the liver fibrosis at appropriate time points using serum marker of hyaluronic acid, hepatobiliary enzymes, and histological analysis including sirius red staining. MMP13 expression was confirmed by enzyme-linked immunosorbent assay (ELISA) using the serum.
Figure 4.
Immunohistochemical staining of MMP13 on rat liver fibrosis model. (a–f) Liver tissues were collected 70 days after the injection, and IHC was performed with rabbit anti-MMP13 polyclonal antibody. Bar = 50 μm. Black arrowheads represent fibrotic tissue. A quantitative analysis was performed using ImageJ software (version 1.6.0_20, National Institutes of Health). The values represent mean ± SD. Total of 100 sections from each three rats were analyzed.) ***P < 0.001, and NS, no statistical significance between the level in LF and LF-MMP13 groups. One-way analysis of variance followed by Bonferroni's multiple comparison test. IHC, immunohistochemistry.
Serum level of MMP13 after liver-targeted hydrodynamic gene delivery
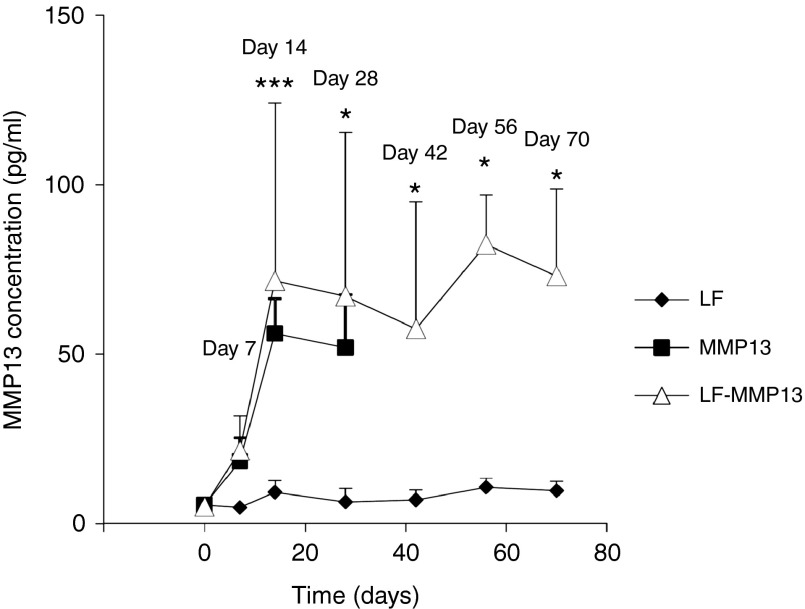
The level of serum MMP13 was determined by enzyme-linked immunosorbent assay to assess the efficiency of serum excretion of MMP13 in the rat liver after the liver-targeted hydrodynamic gene delivery. Serum MMP13 showed a peak level of 56.1 ± 10.3 and 71.7 ± 52.5 pg/ml in the control MMP13 group and the LF-MMP13 group 14 days after the hydrodynamic gene delivery procedure, and these levels were sustained for 70 days (Figure 5). These levels were statistically higher than the levels in the LF group, which did not receive the hydrodynamic delivery of MMP13 gene (P < 0.05). The LF group showed mild increase in serum MMP13 from 5.50 ± 0.80 to 9.24 ± 3.47 pg/ml in 14 days; however, this returned to background levels of 5–6 pg/ml within the next 14 days. Overall, these results suggest that efficient gene delivery of MMP13 into the liver contributed to an increase of serum MMP13 protein, which was sustained for 70 days using our MMP13-expressing plasmid.
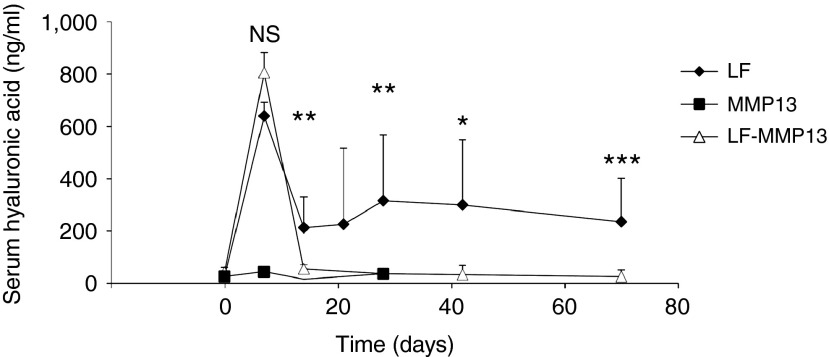
Figure 5.
Time-dependent course of serum MMP13 concentration in the rats. Serum concentration of MMP13 was quantified by ELISA. The values represent mean ± SD. (n = 3–5 for each group) *P < 0.05, ***P < 0.001, and NS, no statistical significance between the level in LF and LF-MMP13 groups. Two-way factor repeated-measures analysis of variance followed by Bonferroni's multiple comparison test.
Antifibrotic effect of hydrodynamic MMP13 gene delivery
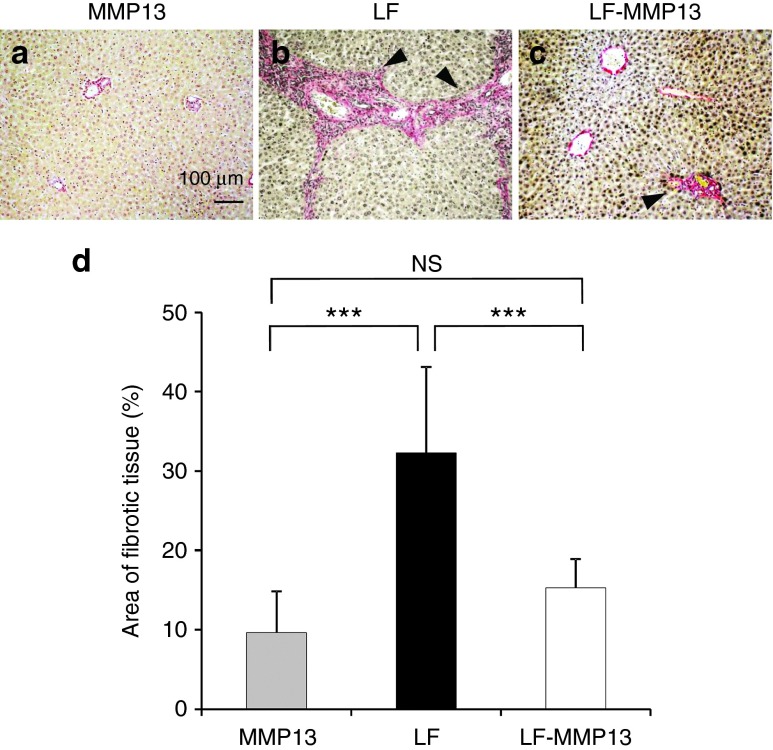
To examine the therapeutic effect of liver-targeted hydrodynamic gene delivery of pBGI-MMP13 in rats, we analyzed serum level of fibrotic markers (hyaluronic acid and type 4 collagen) (Figure 6) and performed the histological examination using sirius red staining which is useful to specifically detect the collagen fibrils in the tissues (Figure 7). The hyaluronic acid showed the highest level of 635.2 ± 54.8 ng/ml in the LF group 7 days after the procedure, which then decreased to the level of 208.6 ± 118.8 ng/ml during the next 7 days, and then sustained for the remainder of the study. The peak observed in first 7 days may have been due to the acute damage mediated by bile juice congestion, and the sustained level of hyaluronic acid may reflect the level of fibrotic change in the liver (Figure 6). The LF-MMP13 group showed a peak level of 802.0 ± 75.6 ng/ml, reflecting acute liver damage in 7 days; however, the level returned to 50.8 ± 17.0 ng/ml in next 7 days, which was statistically lower than that of the LF group (P < 0.05) but was not statistically significantly different compared with the level obtained in the MMP13-injected group (Figure 6). This level was sustained for the remainder of the study. A type 4 collagen, assessed as another serum fibrotic marker, showed 8.24 ± 4.47 ng/ml in the LF group, which was twofold higher than that in the LF-MMP group 70 days after the treatment (P < 0.05). These results suggest that the efficient increase in serum MMP13 concentration contributed to reduced production of serum fibrosis markers. The histological analysis was performed using sirius red staining to examine the effect of MMP13 gene delivery on the prevention of accumulation of fibrotic tissues in rat livers. Quantitative analysis showed that 70 days after the induction of bile duct ligation, ~32.3 ± 10.8% of tissue was fibrotic in the LF group. This was statistically higher than 9.63 ± 5.20% and 15.3 ± 3.67% in MMP13 and LF-MMP13 groups, respectively (P < 0.05) (Figure 7). These results provide direct evidence in support of efficient antifibrotic effect of the liver-targeted MMP13 hydrodynamic gene delivery.
Figure 6.
Time-dependent course of serum hyaluronic acid concentration in the rats. Serum concentration of hyaluronic acid was quantified. The values represent mean ± SD. (n = 3–5 for each group) *P < 0.05, **P < 0.01, ***P < 0.001, and NS, no statistical significance between the level in LF and LF-MMP13 groups. Two-way factor repeated-measures analysis of variance followed by Bonferroni's multiple comparison test.
Figure 7.
Comparison of the fibrotic tissue in the liver. (a-c) The standard sirius red staining was performed to evaluate the fibrotic tissues in the rat livers in each group. The liver tissues were collected 10 weeks after the treatment. Bar = 100 μm. Black arrowheads indicate the fibrotic tissue. (d) A quantitative analysis of fibrotic tissue positively stained was performed using Image J software (version 1.6.0_20, National Institutes of Health) as previously reported. The values represent mean ± SD. (Total of 250 sections from each three rats were analyzed.) ***P < 0.001 and NS, no statistical significance between the level in LF and LF-MMP13 groups. One-way analysis of variance followed by Bonferroni's multiple comparison test.
Discussion
Although various precipitants can cause liver fibrosis resulting in hepatic failure and hepatocarcinogenesis, there are no standard therapeutic options to prevent and ameliorate fibrotic changes. Therefore, the establishment of an effective antifibrotic therapy for liver fibrosis remains an unmet clinical requirement. The current focus of basic research is to define a therapeutic target for liver fibrosis, which encompasses suppression of inflammation,1 control of the HSC activity and proliferation,8,13,14,15 bone marrow transplantation as an antifibrotic therapy,8,16 and enhancement of ECM degradation.2,3,10,17,18,19
The concept of degrading the matrix to prevent liver fibrosis emanates from the molecular mechanism of liver fibrosis development, i.e., the increase in ECM caused by an imbalance between a low level of MMPs and a high level of TIMPs. Therefore, one possible approach for matrix degradation is the overexpression of interstitial MMPs in liver. To date, MMP1 (ref. 17), MMP8 (ref. 3), MMP13 (ref. 10), transferred by adenoviral vector, and MMP13 (ref. 18) transferred by chemical method, have resulted in amelioration of fibrotic tissue in CCl4-induced liver fibrosis and bile duct ligation in rats and mice. In this study, we have examined whether liver-targeted hydrodynamic delivery of MMP13 gene can contribute to block the progression of liver fibrosis induced by bile duct ligation. MMP13 has been reported to be the major collagenase in rodents and is a key factor for ECM resolution in rat liver fibrosis.9 Transient but prominent MMP13 gene expression during the early phase of recovery from experimental rat liver fibrosis has also been observed.11 Further reports state an increase in MMP13 with the progression of liver cirrhotic stages as a physiological response20 that was also seen in our study, although it was insufficient level. These results strongly suggest that the sufficient MMP13 expression can inhibit the progression of fibrotic changes. Various methods of gene transfer, including chemical methods18 and viral vectors,10 have been tested in vivo. Kim et al.18 reported that a newly designed hyaluronic acid–shielded polyethylenimine complex efficiently delivered MMP13-expressing plasmids in a mouse liver cirrhosis model and showed a decrease in collagen deposition in the liver. Endo et al.10 reported the therapeutic effect of recombinant adenovirus-mediated human MMP13 gene transfer in a rat liver cirrhosis model. Administration of vascular endothelial growth factor-neutralizing antibodies also showed prevention of fibrosis through production of chemokine ligand 9 and MMP13 (ref. 21). Among various MMPs, MMP13 has been shown to play pivotal roles in cleavage and remodeling of ECM and decreased after the development of liver fibrosis.2,11 Therefore, it is reasonable to specifically deliver MMP13 into the liver to induce fibrolysis in the fibrotic liver, including liver cirrhosis and to prevent the progression of fibrosis due to the diseases including congenital liver fibrosis and primary biliary cirrhosis. In taking advantage of site-specific gene deliver by the procedure of liver-targeted hydrodynamic gene delivery that we have recently established, we have examined the antifibrotic effect of overexpression of MMP13 gene on liver fibrosis in rat model. Hydrodynamic gene delivery was originally reported as a simple and easy in vivo gene transfer method by mice tail-vein injection with high speed and large volume.22,23 Since then, it has been used as an experimental tool for functional analysis of genetic elements, therapeutic effect of oligonucleotides, and other research objectives.24,25,26 To apply this method for gene therapy, Kamimura et al. have reported a site-specific gene delivery procedure in small and large animals and showed safe and efficient gene delivery of the liver-targeted hydrodynamic gene delivery procedure12,27,28,29,30 as well as muscle-targeted gene delivery.31 The major advantage of the method is an efficient gene expression in the targeted area, and its safety without the need for chemicals and/or viral vectors both of which can be a potential hazard of carcinogenesis.
We utilized the bile duct ligation to induce liver fibrosis since this method has been used in similar types of studies.4,14,17,21,32,33 In addition, this model is the only one allowed due to the regulations of our animal facility restricting the usage of CCl4. Importantly, Tag et al.34 reported time-dependent progression of liver fibrosis by bile duct ligation.
Our results showed an increase in serum concentration of MMP13 concentration that was significantly higher than the noninjected LF group and was similar to MMP13-injected normal rats after the delivery. Slightly higher levels of MMP13 in the LF group may be due to the endogenous production of MMP13 with progression of fibrosis,20 although bile duct ligation–induced liver fibrosis has shown less influence of endogenous MMP13 levels compared to that of CCl4-induced liver fibrosis.11,21,35 The elevated serum level of MMP13 is responsible for the antifibrotic effect in the rat liver fibrosis model. The serum level of hyaluronic acid and the volume of fibrotic tissue in the sections collected 10 weeks after the treatment showed significantly lower levels in the LF group than that of LF group. No marked changes to the conditions of the rats were observed in the treated group. Our results showed that the liver-targeted hydrodynamic delivery of MMP13 effectively and safely blocked the progression of liver fibrosis. Further studies are needed to examine whether overexpression of MMP13, at a different time points after bile duct ligation, can both prevent and also revert liver fibrosis at later and more severe stages of this disease as MMP13 plays a crucial role to degrade developed collagen and is involved in the degradation of newly formed collagen fibril.2,11 For this purpose, we need to modify the hydrodynamic procedure using a recently developed computer-controlled injector to control precisely the flow of plasmid solution into the fibrotic, hardened liver12 and examine the usefulness of various plasmids including the plasmid with a liver-specific promoter to restrict MMP13 expression in hepatocytes.36 In addition, the preclinical trial in the large animals should be carefully conducted to assess the safety and effectiveness of MMP13 gene therapy using our computer-controlled device.12
In summary, we have developed an antifibrotic gene therapy for liver fibrosis and confirmed effective prevention of fibrosis in this study. While the study is preliminary in its nature, i.e., use of an animal model, it still stands as proof of concept for the efficacy of MMP13 overexpression in the liver in treating liver fibrosis. Combined with the hydrodynamic procedure that we have developed in the studies conducted on large animals, we can expect MMP13 gene therapy to be used for clinical application. Further studies are necessary to develop the method to reverse advanced liver fibrosis, and this translational research will encourage hepatologists to treat patients with liver fibrosis.
Materials and methods
Materials. The MMP13-expressing plasmid (pBGI-MMP13) was constructed by subcloning full-length MMP13 complementary DNA (transOMIC Technologies Huntsville, AZ) into the pIRES2-tdTomato expression vector encoding tdTomato under the control of CAG promoter (chicken β-actin promoter and cytomegalovirus enhancer). A circular plasmid map was generated using the SnapGene Viewer (http://www.snapgene.com/products/snapgene_viewer). Plasmid was purified by Plasmid Mega Kit (Qiagen, Hilde, Germany). The purity of the plasmid preparation was checked by absorbency at 260 and 280 nm and 1% agarose gel electrophoresis. Wistar rats (female, 200–250 g) were purchased from Japan SLC (Hamamatsu, Shizuoka, Japan).
In vitro pBGI-MMP13 plasmid transfection. Human embryonic kidney 293 (HEK293) cultured in Dulbecco's Modified Eagle medium (Life Technologies, Carlsbad, CA) containing 10% fetal bovine serum and 100 IU/ml penicillin and streptomycin. Cells were incubated in a 5% CO2 humidified incubator at 37 °C. These cells were transfected with the pBGI-MMP13 plasmid using FuGENE6 (Promega, Madison, WI) following the instruction supplied.
Hydrodynamic injection into rat liver. All animal experiments were conducted in full compliance with regulation and approved by the Institutional Animal Care and Committee at the Niigata University, Niigata, Japan. Liver-targeted hydrodynamic gene delivery to the rats was performed as previously described.12 In summary, a midline skin incision was made on the rats under the general anesthesia using isoflurane and 2,2,2-tribromoethanol (concentration, 0.016 g/ml in 0.9 % saline; dose, 1.25 ml/100 g body weight). An injection catheter (SURFLO 22 gauge, Terumo, Shibuya-ku, Tokyo, Japan) was inserted to the IVC, and its tip was placed at the junction of the IVC and hepatic veins. Saline containing pBGI-MMP13 plasmid DNA (5 μg/ml) was hydrodynamically injected into the liver via the catheter with temporal blood flow occlusions at the supra- and infra-hepatic IVC.12 Injection volume and flow rate were fixed at 5% body weight and 1 ml/second, respectively. The abdominal median incision was sutured after the procedure.
Western blot analysis. A tissue lysate was prepared by homogenization in an equal volume of lysis buffer, 0.125 mol/l Tris–HCL (pH 6.8), 10% sucrose, 10% sodium dodecyl sulfate, 10% 2-mercaptoethanol, and 0.004% bromophenolblue. The extract was electrophoresed in 4–15% Mini-Protean TGX Gel (BIO-RAD, Hercules, CA) and blotted onto Clear Blot Membrane-p (AE-6660, ATTO, Bunkyo-ku, Tokyo, Japan). The membrane was blocked with blocking buffer (5% nonfat milk in phosphate-buffered saline–Tween buffer) for 1 hour at room temperature and probed with primary antibodies of either MMP13 (SC-30073, Santa Cruz Biotechnology, Santa Cruz, CA) or β-actin (Sigma-aldrich, St Louis, MO) for overnight at 4 °C. Then, the membrane was incubated with secondary antibodies of antirabbit (NA9340V, GE Healthcare, Little Chalfont, Buckinghamshire, UK) and antimouse (NA9310V, GE Healthcare) antibody conjugated with horseradish peroxidase. Protein bands were visualized using Amersham ECL Prime Western Blotting Detection Reagent (GE Healthcare).
Immunohistochemical staining. Tissue samples for immunohitsochemical staining were collected 10 weeks after the gene delivery. Four different liver sections from three rats in each group were immunohistochemically stained with anti-MMP13 antibody. Tissues were fixed in 10% formalin upon the collection and embedded in paraffin. Sections (10 µm) were made, and standard immunohistochemistry was performed with a rabbit anti-MMP13 polyclonal antibody (sc-30073, 1:250 dilution; Santa Cruz Biotechnology), Vecstain Elite ABC Rabbit IgG kit (PK-6101; Vector Laboratories, Burlingame, CA), and DAB chromogen tablet (Muto Pure Chemicals, Bunkyo-ku, Tokyo, Japan) for MMP13 staining.
Fluorescence image. Cell lines were cultured at chamber slides (SCS-022, Matsunami Glass, Kishiwada, Osaka, Japan) for fluorescence images. Frozen liver embedded in OCT compound (Sakura Finetek, Torrance, CA). Nuclei were counterstained with DAPI. The tdTomato protein expressed by pBGI-MMP13 plasmid DNA was visualized using excitation filter of 543 nm length. All fluorescence images were obtained with Zeiss Axiovert 200M microscope.
Serum MMP13 concentration. Blood samples were collected at appropriate time points, and serum was used to analyze the serum level of MMP13 by enzyme-linked immunosorbent assay using Human MMP13 ELISA Kit (ELH-MMP13, RayBiothech Norcross, GA).
Serum biochemical analysis. Serum biochemical markers including aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, total bilirubin, and hyaluronic acid were analyzed using serum collected at an appropriate time points.
Development of liver fibrosis model and analysis of fibrotic changes. Rat liver fibrosis model was developed by the bile duct ligation as previously reported.4,14,17,21,32,33 In summary, the common bile duct was exposed under general anesthesia and ligated. To determine the amount of fibrotic tissue in the liver, liver tissue were collected at the appropriate time points and collected liver tissue were fixed, embedded, and cut at 10-μm-thick sections. Then, the standard hematoxyline and eosin staining and sirius red staining were performed on the tissues.
Images were captured from each section randomly, and a quantitative analysis of fibrotic area was performed using the ImageJ software (version 1.6.0_20, National Institutes of Health, Bethesda, MD) as previously reported.37
Statistical methods. The data of serum MMP, hyaluronic acid, enzyme-linked immunosorbant assay, and histological analyses were statistically evaluated by analysis of variance followed by Bonferroni's multiple comparison test.
SUPPLEMENTARY MATERIAL Figure S1. Transfection of pBGI-MMP13 plasmid into HLE liver cancer cells. (a) Nucelar staining by SYTOX Green. (b) Detection of tdTomato protein in the liver cells. Scale bar represents 20 μm. (c) Merged image of (a) and (b). Figure S2. Western blotting of the extracts from other organs. Figure S3. Serum concentrations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), and total bilirubin (T-Bil) in two rats injected with human factor IX expressing plasmid. The values represent mean data. Materials and Methods. Western blotting and serum biochemical analysis were performed as described in the manuscript. HLE liver cancer cell lines were cultured as described in the manuscript. These cells were transfected with the pBGI-MMP13 plasmid using FuGENE6 (Promega, Madison, WI, USA) and the nucleus was stained with SYTOX Green (Thermo Fisher Scientific, Waltham, MA).
Acknowledgments
The authors thank Minoru Nomoto and Takao Tsuchida in the Division of Gastroenterology and Hepatology at the Niigata University and Miki Obata in the Department of Molecular Genetics at the Niigata University for the excellent assistance in histological analysis. The authors also thank Nobuyoshi Fujisawa, Yoshitaka Maeda, Toshikuni Sasaoka, and all staff members in Division of Laboratory Animal Resources in Niigata University. The authors also thank Enago for the critical reading of the manuscript and English language review. The research in the authors' laboratory has been supported in part by Grant-in-Aid for Scientific Research from Japanese Society for the Promotion of Sciences 26293175 to T.S., 24592084 to T.M., and 22890064, 23790595, 26860354, and Takara Bio Award from JSGT to K.K. The authors declare that they have no conflict of interest.
Supplementary Material
Transfection of pBGI-MMP13 plasmid into HLE liver cancer cells. (a) Nucelar staining by SYTOX Green. (b) Detection of tdTomato protein in the liver cells. Scale bar represents 20 μm. (c) Merged image of (a) and (b).
Western blotting of the extracts from other organs.
Serum concentrations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), and total bilirubin (T-Bil) in two rats injected with human factor IX expressing plasmid. The values represent mean data.
References
- Nusrat, S, Khan, MS, Fazili, J and Madhoun, MF (2014). Cirrhosis and its complications: evidence based treatment. World J Gastroenterol 20: 5442–5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iimuro Yuji, Brenner David A (2008). Matrix metalloproteinase gene delivery for liver fibrosis. Pharm Res 25: 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller-López, F, Sandoval, A, Salgado, S, Salazar, A, Bueno, M, Garcia, J et al. (2004). Treatment with human metalloproteinase-8 gene delivery ameliorates experimental rat liver cirrhosis. Gastroenterology 126: 1122–33; discussion 949. [DOI] [PubMed] [Google Scholar]
- Sato, Y, Murase, K, Kato, J, Kobune, M, Sato, T, Kawano, Y et al. (2008). Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol 26: 431–442. [DOI] [PubMed] [Google Scholar]
- Adrian, JE, Poelstra, K, Scherphof, GL, Meijer, DK, van Loenen-Weemaes, AM, Reker-Smit, C et al. (2007). Effects of a new bioactive lipid-based drug carrier on cultured hepatic stellate cells and liver fibrosis in bile duct-ligated rats. J Pharmacol Exp Ther 321: 536–543. [DOI] [PubMed] [Google Scholar]
- Sobrevals, L, Enguita, M, Rodriguez, C, Gonzalez-Rojas, J, Alzaguren, P, Razquin, N et al. (2012). AAV vectors transduce hepatocytes in vivo as efficiently in cirrhotic as in healthy rat livers. Gene Ther 19: 411–417. [DOI] [PubMed] [Google Scholar]
- Abe H, Kamimura K, Yokoo T, Suda T, Kobayashi Y, Aoyagi Y (2014). Gene therapy for liver fibrosis. JSM Gastroenterol Hepatol 2: 1028. [Google Scholar]
- Terai, S, Tanimoto, H, Maeda, M, Zaitsu, J, Hisanaga, T, Iwamoto, T et al. (2012). Timeline for development of autologous bone marrow infusion (ABMi) therapy and perspective for future stem cell therapy. J Gastroenterol 47: 491–497. [DOI] [PubMed] [Google Scholar]
- Quinn, CO, Scott, DK, Brinckerhoff, CE, Matrisian, LM, Jeffrey, JJ and Partridge, NC (1990). Rat collagenase. Cloning, amino acid sequence comparison, and parathyroid hormone regulation in osteoblastic cells. J Biol Chem 265: 22342–22347. [PubMed] [Google Scholar]
- Endo, H, Niioka, M, Sugioka, Y, Itoh, J, Kameyama, K, Okazaki, I et al. (2011). Matrix metalloproteinase-13 promotes recovery from experimental liver cirrhosis in rats. Pathobiology 78: 239–252. [DOI] [PubMed] [Google Scholar]
- Watanabe, T, Niioka, M, Hozawa, S, Kameyama, K, Hayashi, T, Arai, M et al. (2000). Gene expression of interstitial collagenase in both progressive and recovery phase of rat liver fibrosis induced by carbon tetrachloride. J Hepatol 33: 224–235. [DOI] [PubMed] [Google Scholar]
- Yokoo, T, Kamimura, K, Suda, T, Kanefuji, T, Oda, M, Zhang, G et al. (2013). Novel electric power-driven hydrodynamic injection system for gene delivery: safety and efficacy of human factor IX delivery in rats. Gene Ther 20: 816–823. [DOI] [PubMed] [Google Scholar]
- Bataller, R and Brenner, DA (2001). Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis 21: 437–451. [DOI] [PubMed] [Google Scholar]
- Granzow, M, Schierwagen, R, Klein, S, Kowallick, B, Huss, S, Linhart, M et al. (2014). Angiotensin-II type 1 receptor-mediated Janus kinase 2 activation induces liver fibrosis. Hepatology 60: 334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y, Zhang, QQ, Guo, XH, Zhang, HY and Liu, LX (2014). IGFBPrP1 induces liver fibrosis by inducing hepatic stellate cell activation and hepatocyte apoptosis via Smad2/3 signaling. World J Gastroenterol 20: 6523–6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaida, I, Terai, S, Yamamoto, N, Aoyama, K, Ishikawa, T, Nishina, H et al. (2004). Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology 40: 1304–1311. [DOI] [PubMed] [Google Scholar]
- Iimuro, Y, Nishio, T, Morimoto, T, Nitta, T, Stefanovic, B, Choi, SK et al. (2003). Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology 124: 445–458. [DOI] [PubMed] [Google Scholar]
- Kim, EJ, Cho, HJ, Park, D, Kim, JY, Kim, YB, Park, TG et al. (2011). Antifibrotic effect of MMP13-encoding plasmid DNA delivered using polyethylenimine shielded with hyaluronic acid. Mol Ther 19: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki, T, Kaneda, Y, Tsutsui, H, Nakanishi, K, Sawa, Y, Morishita, R et al. (1999). Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med 5: 226–230. [DOI] [PubMed] [Google Scholar]
- Patel, K, Remlinger, KS, Walker, TG, Leitner, P, Lucas, JE, Gardner, SD et al. (2014). Multiplex protein analysis to determine fibrosis stage and progression in patients with chronic hepatitis C. Clin Gastroenterol Hepatol 12: 2113–20.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L, Kwon, J, Popov, Y, Gajdos, GB, Ordog, T, Brekken, RA et al. (2014). Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology 146: 1339–50.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, F, Song, Y and Liu, D (1999). Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther 6: 1258–1266. [DOI] [PubMed] [Google Scholar]
- Zhang, G, Budker, V and Wolff, JA (1999). High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther 10: 1735–1737. [DOI] [PubMed] [Google Scholar]
- Zhang, G, Song, YK and Liu, D (2000). Long-term expression of human alpha1-antitrypsin gene in mouse liver achieved by intravenous administration of plasmid DNA using a hydrodynamics-based procedure. Gene Ther 7: 1344–1349. [DOI] [PubMed] [Google Scholar]
- Kamimura, K and Liu, D (2008). Physical approaches for nucleic acid delivery to liver. AAPS J 10: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura, K, Suda, T, Zhang, G and Liu, D (2011). Advances in gene delivery systems. Pharmaceut Med 25: 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura, K, Suda, T, Xu, W, Zhang, G and Liu, D (2009). Image-guided, lobe-specific hydrodynamic gene delivery to swine liver. Mol Ther 17: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura, K, Suda, T, Zhang, G, Aoyagi, Y and Liu, D (2013). Parameters affecting image-guided, hydrodynamic gene delivery to swine liver. Mol Ther Nucleic Acids 2: e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura, K, Kanefuji, T, Yokoo, T, Abe, H, Suda, T, Kobayashi, Y et al. (2014). Safety assessment of liver-targeted hydrodynamic gene delivery in dogs. PLoS One 9: e107203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura K, Abe H, Suda T, Aoyagi Y, Liu D (2013). Liver-directed gene therapy. JSM Gastroenterol Hepatol 1: 1005. [Google Scholar]
- Kamimura, K, Zhang, G and Liu, D (2010). Image-guided, intravascular hydrodynamic gene delivery to skeletal muscle in pigs. Mol Ther 18: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, SW, Zhang, XR, Wang, CZ, Chen, WZ, Xie, WF and Chen, YX (2008). RNA interference targeting the platelet-derived growth factor receptor beta subunit ameliorates experimental hepatic fibrosis in rats. Liver Int 28: 1446–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, SL, Pan, H, Lu, WY, Wang, J, Wu, J and Wang, JY (2007). Cyclic Arg-Gly-Asp peptide-labeled liposomes for targeting drug therapy of hepatic fibrosis in rats. J Pharmacol Exp Ther 322: 560–568. [DOI] [PubMed] [Google Scholar]
- Tag CG, Sauer-Lehnen S, Weiskirchen S, Borkham-Kamphorst E, Tolba RH, Tacke F, et al. (2015). Bile duct ligation in mice: induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J Vis Exp 96: 52438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama, R, Inagaki, Y, Hong, YY, Kushida, M, Nakao, S, Niioka, M et al. (2007). Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology 45: 213–222. [DOI] [PubMed] [Google Scholar]
- Wolff, LJ, Wolff, JA and Sebestyén, MG (2009). Effect of tissue-specific promoters and microRNA recognition elements on stability of transgene expression after hydrodynamic naked plasmid DNA delivery. Hum Gene Ther 20: 374–388. [DOI] [PubMed] [Google Scholar]
- Vrekoussis, T, Chaniotis, V, Navrozoglou, I, Dousias, V, Pavlakis, K, Stathopoulos, EN et al. (2009). Image analysis of breast cancer immunohistochemistry-stained sections using ImageJ: an RGB-based model. Anticancer Res 29: 4995–4998. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transfection of pBGI-MMP13 plasmid into HLE liver cancer cells. (a) Nucelar staining by SYTOX Green. (b) Detection of tdTomato protein in the liver cells. Scale bar represents 20 μm. (c) Merged image of (a) and (b).
Western blotting of the extracts from other organs.
Serum concentrations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), and total bilirubin (T-Bil) in two rats injected with human factor IX expressing plasmid. The values represent mean data.