Abstract
Background
Patients with chronic obstructive pulmonary disease (COPD) experience respiratory symptoms, which impair quality of life. This pooled analysis of two Phase III studies assessed the impact of aclidinium/formoterol on patients with COPD categorized by symptom status.
Methods
Data were pooled from two 24-week, randomized, placebo-controlled studies of twice-daily aclidinium/formoterol 400/12 µg in moderate-to-severe COPD (ACLIFORM [NCT01462942] and AUGMENT [NCT01437397]). These post hoc analyses evaluated the efficacy of aclidinium/formoterol versus placebo or monotherapies in patients defined as less/more symptomatic by a) Evaluating Respiratory Symptoms (E-RS™) score ≥10/<10 and b) Baseline Dyspnea Index score <7/≥7. Endpoints included trough and 1-hour morning postdose forced expiratory volume in 1 second (FEV1), Transition Dyspnea Index, E-RS total score, early-morning and nighttime symptom severity, early-morning limitation of activities, and exacerbation rate.
Results
Data for 3,394 patients were analyzed (mean age: 63.5 years; 60.5% male). In both definitions of less and more symptomatic patients, aclidinium/formoterol improved 1-hour morning postdose FEV1 from baseline at week 24 versus placebo (P<0.001) and both monotherapies (P<0.05). Aclidinium/formoterol improved trough FEV1 from baseline in both groups versus placebo (P<0.05) and formoterol (P<0.05); improvements were greater in more symptomatic patients. Improvements versus aclidinium were also observed in more symptomatic patients (P<0.05). Aclidinium/formoterol improved dyspnea, early-morning symptom severity, and limitation of activities versus placebo in both less and more symptomatic patients (P<0.001). In more symptomatic patients, aclidinium/formoterol also improved E-RS total score and severity of nighttime symptoms from baseline versus placebo and one or both monotherapies (P<0.05). The rate of moderate/severe exacerbations was reduced with aclidinium/formoterol versus placebo in more symptomatic patients.
Conclusion
Aclidinium/formoterol 400/12 µg provided consistent improvements in bronchodilation and symptoms versus monotherapies and reduced exacerbations versus placebo in more symptomatic patients with moderate-to-severe COPD, regardless of the definition used. Furthermore, patients with a low symptom burden achieved benefits with aclidinium/formoterol versus monotherapies in postdose FEV1, dyspnea, and early-morning symptoms.
Keywords: aclidinium, formoterol, COPD, lung function, dyspnea, symptoms
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by progressive loss of lung function and a high symptom burden, including shortness of breath, chronic cough, wheezing, and sputum production, which can impair patients’ quality of life.1 Bronchodilators are regarded as initial or foundation therapy of COPD and the current Global initiative for chronic Obstructive Lung Disease (GOLD) report recommends selecting and escalating bronchodilator therapy based upon reported patient symptom burden. Patients with minimal symptoms may initially receive treatment with short-acting agents or long-acting monotherapy, while patients with greater symptom burden may receive dual therapy with two long-acting bronchodilators in combination – a long-acting muscarinic antagonist (LAMA) and a long-acting β2-agonist (LABA).2 However, this stratagem has not been examined by assessing the effectiveness of dual bronchodilation in patients stratified according to reported symptom burden.
ACLIFORM and AUGMENT were two large 24-week, randomized, placebo-controlled Phase III trials evaluating the efficacy of dual therapy with aclidinium bromide 400 µg (a LAMA) and formoterol fumarate 12 µg (a LABA) twice daily versus placebo or monotherapies in moderate-to-severe COPD. As these studies did not restrict the inclusion of patients based on symptoms, the populations had a broad spectrum of symptom burden.3,4 Accordingly, the identification of patients with more symptomatic disease in these trials allows for a more comparable population when considering other trials of LABA/LAMAs in which patients were required to be symptomatic.5–7
Several validated tools are available to assess COPD symptoms. For daily clinical practice, GOLD recommends the use of the modified Medical Research Council (mMRC) tool, the clinical COPD questionnaire, and the COPD Assessment Test (CAT) tools as they are quick and easy to administer. However, in a clinical study environment, more detailed and complex instruments can be used for a more comprehensive assessment. Symptom burden in clinical trials is commonly assessed using the St George’s Respiratory Questionnaire and/or the CAT to measure health status. However, as COPD is a multifactorial and highly symptomatic disease, categorization according to broad health status may not adequately identify patients who have a high or low symptom burden, and evidence of treatment efficacy according to stratified symptom levels in clinical trials is lacking.2 Therefore, in ACLIFORM and AUGMENT, symptoms were assessed using the Baseline Dyspnea Index (BDI) and the Evaluating Respiratory Symptoms (E-RS) in COPD diary (E-RS™: COPD; formerly EXAcerbations of Chronic pulmonary disease Tool [EXACT™] − Respiratory Symptoms) (The EXACT and E-RS are owned by Evidera. Permission to use these instruments may be obtained from Evidera [exactpro@evidera.com]). The E-RS is a derivative of EXACT and was developed to assess the efficacy of treatment on symptoms in clinical trials.8,9 The E-RS, which uses the eleven respiratory symptom items from the 14-item EXACT daily diary, provides a more comprehensive assessment of the burden of respiratory symptoms in COPD than standard patient-reported outcomes and has shown consistency with the classification of less symptomatic and more symptomatic patients by GOLD groups.10
The BDI is a fully validated, interviewer-administered measure of breathlessness, assessing functional impairment, the effect on activity, and the degree of effort required to perform daily tasks.11 The interviewer asks open-ended questions concerning the patient’s breathlessness and then selects a grade reflecting the degree of impairment related to dyspnea in each category. Similar to mMRC, BDI reports on dyspnea; however, BDI is a more comprehensive measurement of breathlessness, and together with the Transition Dyspnea Index (TDI), it is more suited to the clinical trial environment as it is specifically designed to measure change in breathlessness over time.11
The aim of this pooled post hoc analysis of ACLIFORM and AUGMENT was to evaluate the efficacy of aclidinium/formoterol 400/12 µg in patients with COPD who were identified as being less symptomatic or more symptomatic using two different measures of symptoms, baseline E-RS total score and BDI.
Methods
Study design
The study designs of ACLIFORM (NCT01462942) and AUGMENT (NCT01437397), two 24-week, double-blind, randomized, parallel-group, active- and placebo-controlled multicenter Phase III studies of twice-daily aclidinium/formoterol 400/12 µg in moderate-to-severe COPD, have been reported previously.3,4 Following screening and a 2- to 3-week run-in period, patients were randomized to twice-daily aclidinium/formoterol 400/12 µg, aclidinium/formoterol 400/6 µg, aclidinium 400 µg, formoterol 12 µg, or placebo (ACLIFORM 2:2:2:2:1; AUGMENT 1:1:1:1:1). Only the aclidinium/formoterol 400/12 µg dose was investigated in this pooled post hoc analysis as this is the dose developed for clinical use and currently approved for use in the European Union and several other countries worldwide, including Australia and Canada.12 All treatments were administered using the same multidose dry powder inhaler (Genuair™/Pressair®) for 24 weeks (Registered trademark of AstraZeneca group of companies; for use within the US as Pressair® and as Genuair™ within all other licensed territories). Two doses of aclidinium/formoterol were studied; however, only the approved 400/12 µg dose was investigated in this pooled post hoc analysis.
The studies were approved by an independent ethics committee at each site (Table S1 and S2) and were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation, and Good Clinical Practice.3,4 All patients provided written informed consent.
Patients
Inclusion and exclusion criteria have been previously reported for both studies.3,4 Briefly, male and female patients aged ≥40 years old, current or former smokers (history of ≥10 pack-years) diagnosed with moderate-to-severe stable COPD, a post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity ratio of <70%, and a post-bronchodilator FEV1 ≥30% and <80% predicted were included. Patients with a history or current diagnosis of asthma, a respiratory tract infection or COPD exacerbation within 6 weeks (<3 months if hospitalized) prior to screening, or clinically significant respiratory or cardiovascular conditions other than COPD were excluded. Use of inhaled salbutamol was permitted as relief medication and discontinued 6 hours prior to study visits. Concomitant corticosteroids (inhaled, oral, or parenteral at doses equivalent to 10 mg prednisone/day or 20 mg every other day), oral sustained-release theophylline, and oxygen therapy <15 h/day were permitted if dosage was stable >4 weeks before screening.
Post hoc analysis of more or less symptomatic patients
In this post hoc subgroup analysis of pooled data from ACLIFORM and AUGMENT, all patients in the pooled population were identified as being less symptomatic or more symptomatic by applying thresholds to two baseline symptom scales, E-RS and BDI. For the E-RS definition, more symptomatic patients were defined as those patients with an E-RS baseline score ≥10 units. This threshold was selected based on evidence suggesting that an E-RS score ≥10 units could distinguish between less symptomatic (GOLD groups A and C) and more symptomatic (GOLD groups B and D) patients.10 For the BDI definition, a score of <7 was used to identify more symptomatic patients, while a score of ≥7 was used to identify less symptomatic patients. The rationale for this threshold is based on a previously reported study, which showed that a BDI score ≥7 and <7 corresponds with the mMRC thresholds used by GOLD to identify less symptomatic and more symptomatic patients, respectively.13 All patients with baseline E-RS and BDI data were included in this post hoc analysis.
Assessments and endpoints
The efficacy of aclidinium/formoterol 400/12 µg versus monotherapies and placebo was assessed for both definitions of less symptomatic and more symptomatic patients. Lung function was assessed by standardized spirometric techniques at each study visit. Coprimary lung function endpoints were changed from baseline in 1-hour morning postdose FEV1 and changed from baseline in morning predose (trough) FEV1 at week 24. Dyspnea was assessed using the TDI to evaluate improvements in focal score at week 24.11 Daily COPD symptoms were evaluated using the E-RS questionnaire (eleven respiratory items from the 14-item EXACT questionnaire assessing breathlessness, cough and sputum, and chest symptoms) via electronic diaries, and change from baseline in E-RS total score was assessed at 24 weeks. The severities of early-morning and nighttime symptoms as well as limitation of morning activities were assessed using a COPD symptoms questionnaire, which was completed by patients each morning via electronic diaries (early-morning and nighttime symptoms: 5-point scale: 1= “did not experience symptoms”, 5= “very severe”; limitation of morning activities: 5-point scale: 1= “not at all”, 5= “a very good deal”). The rate of moderate-to-severe COPD exacerbations (defined as a worsening of symptoms for at least two consecutive days requiring treatment with antibiotics, systemic corticosteroids, or hospitalization) per patient per year was also assessed. Change from baseline in rescue medication use at week 24 was evaluated using an electronic diary.
Statistical analysis
Statistical analyses were performed using SAS® version 9.4 (SAS Institute Inc., Cary, NC, USA). All efficacy endpoints were analyzed using the pooled intent-to-treat (ITT) population, defined as all randomized patients who received ≥1 dose of study medication and had a baseline and at least one post-baseline FEV1 assessment. Exacerbations were analyzed using the pooled ITT-exacerbation population, defined as all patients who received ≥1 dose of study medication and had a baseline FEV1 assessment. All endpoints were analyzed using a mixed model for repeated measures with treatment group, sex, smoking status, visit, subgroup, and treatment-group-by-subgroup, treatment-group-by-visit and treatment-group-by-visit-by-subgroup interactions as fixed-effect factors, corresponding baseline values and age as covariates, and pre- and post-bronchodilator FEV1 as a covariate for FEV1 endpoints. Rate of exacerbations was analyzed by means of a log-linear model with age as a covariate, and treatment group, study, sex, baseline COPD severity, smoking status, subgroup, and treatment-by-subgroup as factors. The log of the total exposure time in years for a patient was included as an offset.
Results
Patient disposition and baseline characteristics
The pooled ITT population consisted of 3,394 patients and the pooled ITT-exacerbation population comprised 3,398 patients. Of those patients, 3,336 had E-RS baseline scores recorded and 3,297 had BDI scores recorded. Using the E-RS definition, there were 1,172 less symptomatic patients (E-RS <10; 34.5% of pooled ITT population) and 2,164 more symptomatic patients (E-RS ≥10; 63.8% of pooled ITT population). According to the BDI definition, there were 1,519 less symptomatic patients (BDI ≥7; 44.8% of pooled ITT population) and 1,778 more symptomatic patients (BDI <7; 52.4% of pooled ITT population). The proportions of patients in each treatment arm of this post hoc analysis were similar to those in the overall pooled population for both definitions of less symptomatic and more symptomatic patients. Demographics and baseline characteristics of less symptomatic and more symptomatic patients for each definition are shown in Table 1. The overall demographics and baseline characteristics for each treatment group have been reported previously and were similar across treatment groups.14 The baseline demographics for each treatment arm, stratified by BDI and E-RS, are presented in Table S3. A higher proportion of patients were classified as GOLD stage III (severe) in the more symptomatic subgroups. Baseline exacerbation rates were also higher in more symptomatic patients. Furthermore, there were more current smokers in the more symptomatic subgroup according to the E-RS definition and marginally lower baseline FEV1 in the more symptomatic subgroup according to the BDI definition. Other demographic parameters were similar across all groups, with the exception of the intrinsic differences in baseline E-RS total scores and BDI scores between less symptomatic and more symptomatic patients.
Table 1.
Baseline demographics and patient characteristics of less symptomatic and more symptomatic patients according to baseline E-RS and BDI
| Demographic parameter | E-RS
|
BDI
|
||
|---|---|---|---|---|
| Less symptomatic (E-RS <10) n=1,172 |
More symptomatic (E-RS ≥10) n=2,164 |
Less symptomatic (BDI ≥7) n=1,519 |
More symptomatic (BDI <7) n=1,778 |
|
| Age, mean (SD) | 64.2 (8.3) | 63.1 (8.5) | 63.5 (8.3) | 63.5 (8.6) |
| Sex, male, % | 59.1 | 61.2 | 61.2 | 60.1 |
| COPD severity (GOLD stages) (%) | ||||
| Stage II (moderate) | 68.5 | 53.1 | 66.8 | 51.4 |
| Stage III (severe) | 31.1 | 46.2 | 32.5 | 48.1 |
| Current smoker, % | 44.4 | 52.0 | 49.5 | 49.3 |
| Smoking history, pack-years, mean (SD) | 47.2 (24.4) | 45.8 (24.2) | 45.5 (23.7) | 47.0 (24.8) |
| No exacerbations in previous | 0.3 (0.6) | 0.5 (0.9) | 0.3 (0.7) | 0.5 (0.9) |
| 12 months, mean (SD) | ||||
| Baseline FEV1, L, mean (SD) | 1.4 (0.5) | 1.4 (0.5) | 1.5 (0.5) | 1.3 (0.5) |
| Baseline FVC, L, mean (SD) | 2.9 (0.9) | 2.8 (0.9) | 3.0 (0.9) | 2.8 (0.8) |
| FEV1/FVC, %, mean (SD) | 48.8 (10.4) | 47.3 (10.9) | 49.2 (10.5) | 46.3 (10.9) |
| E-RS total score at baseline (SD) | 5.7 (2.8) | 16.3 (4.6) | 10.3 (5.9) | 14.5 (6.4) |
| BDI score at baseline (SD) | 7.5 (2.1) | 5.9 (2.0) | 8.2 (1.4) | 4.9 (1.4) |
Abbreviations: BDI, Baseline Dyspnea Index; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global initiative for chronic Obstructive Lung Disease; SD, standard deviation.
Efficacy
Lung function
Aclidinium/formoterol 400/12 µg provided improvements of 286–302 mL in 1-hour morning postdose FEV1 versus placebo at week 24 in both less and more symptomatic patients, regardless of the definition used (P<0.001 for all comparisons; Figure 1). Furthermore, aclidinium/formoterol dual therapy provided greater improvements in 1-hour postdose FEV1 at week 24 than either monotherapy in both definitions of less symptomatic patients (E-RS <10: 120 mL vs aclidinium and 90 mL vs formoterol; BDI ≥7: 115 mL vs aclidinium and 109 mL vs formoterol; P<0.001 for all comparisons; Figure 1). Improvements of a similar magnitude were demonstrated for aclidinium/formoterol dual therapy versus both monotherapies in more symptomatic patients (E-RS ≥10: 120 mL vs aclidinium and 130 mL vs formoterol; BDI <7: 127 mL vs aclidinium and 126 mL vs formoterol; P<0.001 for all comparisons; Figure 1).
Figure 1.
One-hour morning postdose FEV1 change from baseline in less symptomatic and more symptomatic patients with COPD at week 24.
Notes: Data are LS mean ± SE for the pooled ITT population; ***P<0.0001.
Abbreviations: AB, aclidinium bromide 400 µg; BDI, Baseline Dyspnea Index; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms; FEV1, forced expiratory volume in 1 second; FF, formoterol fumarate 12 µg; LS, least squares; ITT, intent-to-treat; SE, standard error.
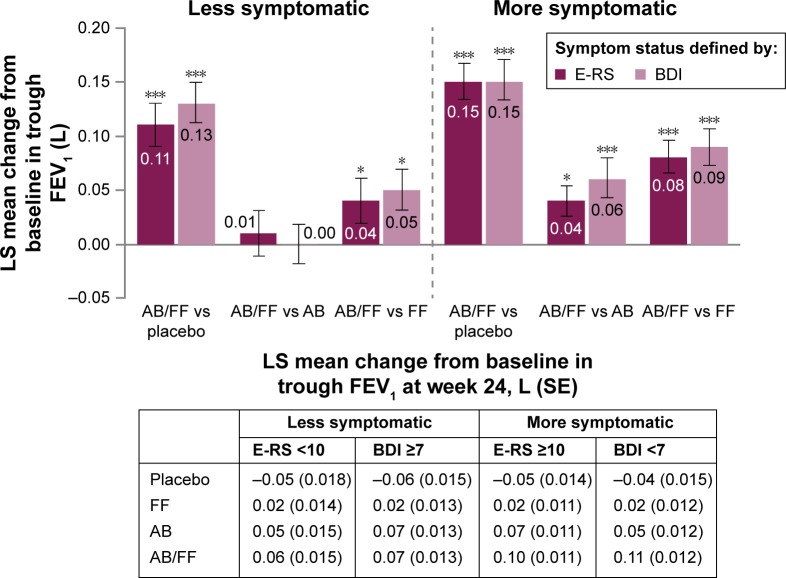
Aclidinium/formoterol improved trough FEV1 from baseline at week 24 compared with placebo in both less and more symptomatic patients, irrespective of the definition used. However, increases in trough FEV1 with aclidinium/formoterol versus placebo were greater among more symptomatic patients (E-RS ≥10: 150 mL; BDI <7: 152 mL; P<0.001 for both; Figure 2) compared with less symptomatic patients (E-RS <10: 110 mL; BDI ≥7: 132 mL; P<0.001 for both; Figure 2). Both monotherapies improved trough FEV1 from baseline versus placebo for both definitions of less and more symptomatic patients (P<0.01 for all comparisons). Aclidinium/formoterol resulted in greater improvements in trough FEV1 versus both monotherapies in more symptomatic patients at week 24 (40 mL for E-RS ≥10 and 60 mL for BDI <7 vs aclidinium and 80 mL for E-RS ≥10 and 90 mL for BDI <7 vs formoterol; P<0.05 for all; Figure 2). However, improvements for aclidinium/formoterol versus monotherapies were ameliorated in less symptomatic patients (Figure 2).
Figure 2.
Trough FEV1 (1-hour morning predose) change from baseline in less symptomatic and more and symptomatic patients with COPD at week 24.
Notes: Data are LS mean ± SE for the pooled ITT population; *P<0.05; ***P<0.001.
Abbreviations: AB, aclidinium bromide 400 µg; BDI, Baseline Dyspnea Index; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms; FEV1, forced expiratory volume in 1 second; FF, formoterol fumarate 12 µg; ITT, intent-to-treat; LS, least squares; SE, standard error.
Dyspnea
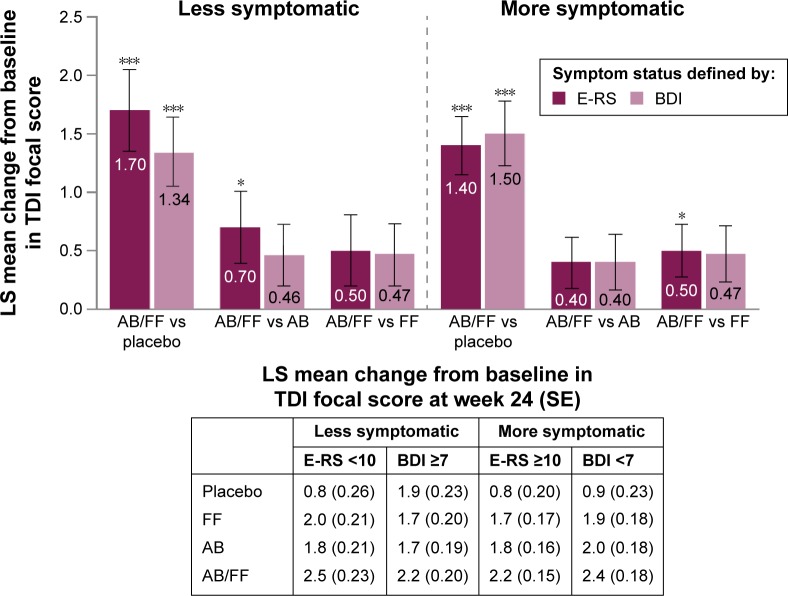
For both definitions of less and more symptomatic patients, aclidinium/formoterol demonstrated a similar magnitude of improvements in TDI focal score compared with placebo at week 24 (all P<0.001; Figure 3). Aclidinium and formoterol monotherapies also improved TDI focal score at week 24 compared with placebo for both definitions of less and more symptomatic patients (all P<0.01). In both less symptomatic and more symptomatic patients, all active treatments achieved the minimum clinically important difference of 1-unit improvement from baseline versus placebo at week 24, regardless of the definition used. Improvements in TDI with aclidinium/formoterol versus monotherapies were comparable in both less symptomatic and more symptomatic patients, with the exception of a greater effect of dual therapy versus aclidinium in less symptomatic patients with E-RS <10 (P<0.05; Figure 3).
Figure 3.
TDI focal score change from baseline in less symptomatic and more symptomatic patients with COPD at week 24.
Notes: Data are LS mean ± SE for the pooled ITT population; *P<0.05; ***P<0.001.
Abbreviations: AB, aclidinium bromide 400 µg; BDI, Baseline Dyspnea Index; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms; FF, formoterol fumarate 12 µg; ITT, intent-to-treat; LS, least squares; SE, standard error; TDI, Transition Dyspnea Index.
Daytime and nighttime respiratory symptoms
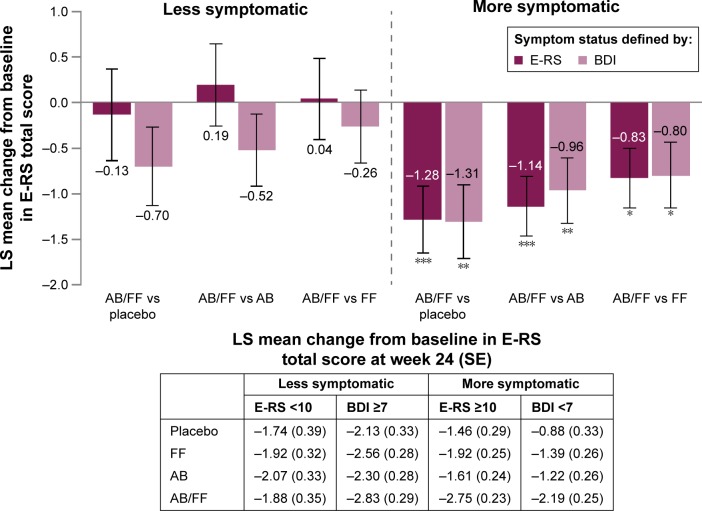
Aclidinium/formoterol improved E-RS total score from baseline at 24 weeks versus placebo and both monotherapies in more symptomatic patients for both E-RS and BDI definitions (P<0.05 for all comparisons; Figure 4). There were no clear trends among E-RS-defined less symptomatic patients, and only moderate improvements among less symptomatic patients defined by BDI were observed (Figure 4).
Figure 4.
E-RS total score change from baseline in less symptomatic and more symptomatic patients with COPD at week 24.
Notes: Data are LS mean ± SE for the pooled ITT population; *P<0.05; **P<0.01; ***P<0.001.
Abbreviations: AB, aclidinium bromide 400 µg; BDI, Baseline Dyspnea Index; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms; FF, formoterol fumarate 12 µg; ITT, intent-to-treat; LS, least squares; SE, standard error.
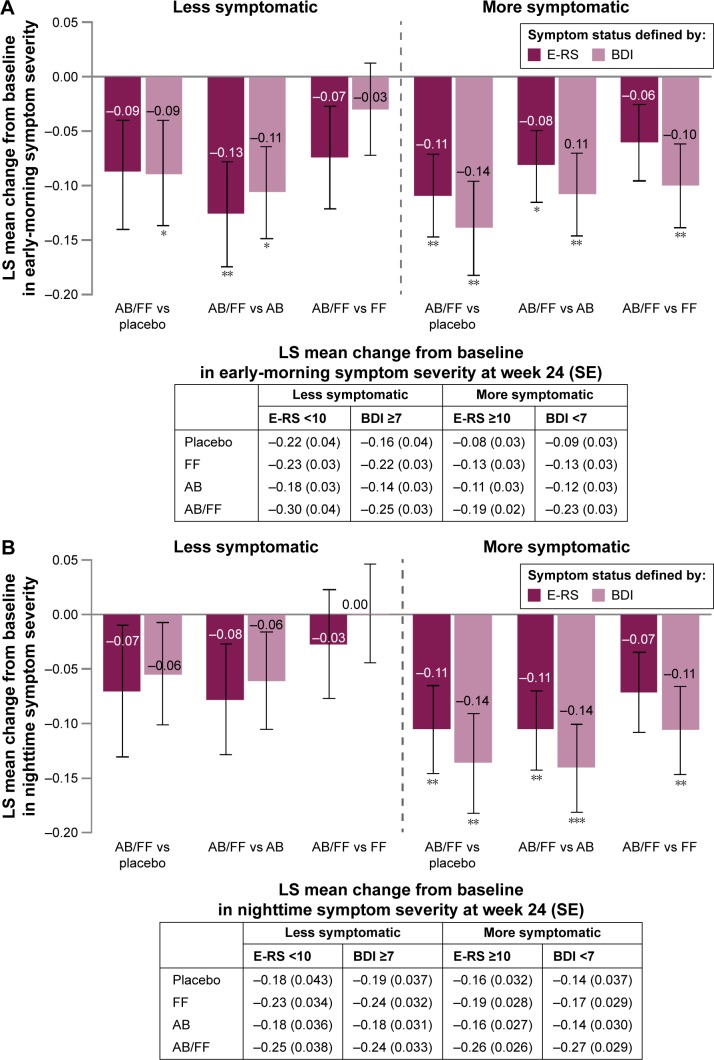
Aclidinium/formoterol reduced early-morning symptom severity from baseline in more symptomatic patients (E-RS ≥10 or BDI <7) at week 24 compared with placebo and aclidinium (all P<0.05; Figure 5A). Reductions in early-morning symptom severity were also observed for aclidinium/formoterol versus formoterol in BDI-defined more symptomatic patients (P<0.01). For both definitions of less symptomatic patients, reductions in early-morning symptom severity were demonstrated for aclidinium/formoterol versus aclidinium (P<0.05) and versus placebo in the BDI ≥7 subgroup (P<0.05) (Figure 5A).
Figure 5.
Change from baseline in (A) early-morning and (B) nighttime symptom severity in less symptomatic and more symptomatic patients with COPD at week 24.
Notes: Data are LS mean ± SE for the pooled ITT population; *P<0.05; **P≤0.01; ***P<0.001.
Abbreviations: AB, aclidinium bromide 400 µg; BDI, Baseline Dyspnea Index; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms; FF, formoterol fumarate 12 µg; ITT, intent-to-treat; LS, least squares; SE, standard error.
Nighttime symptom severity decreased from baseline in more symptomatic patients at week 24 with aclidinium/formoterol versus placebo (both definitions P<0.05) and these improvements were greater than in less symptomatic patients. Similarly, the magnitude of improvements with aclidinium/formoterol versus monotherapies was greater in more symptomatic patients than in less symptomatic patients (Figure 5B).
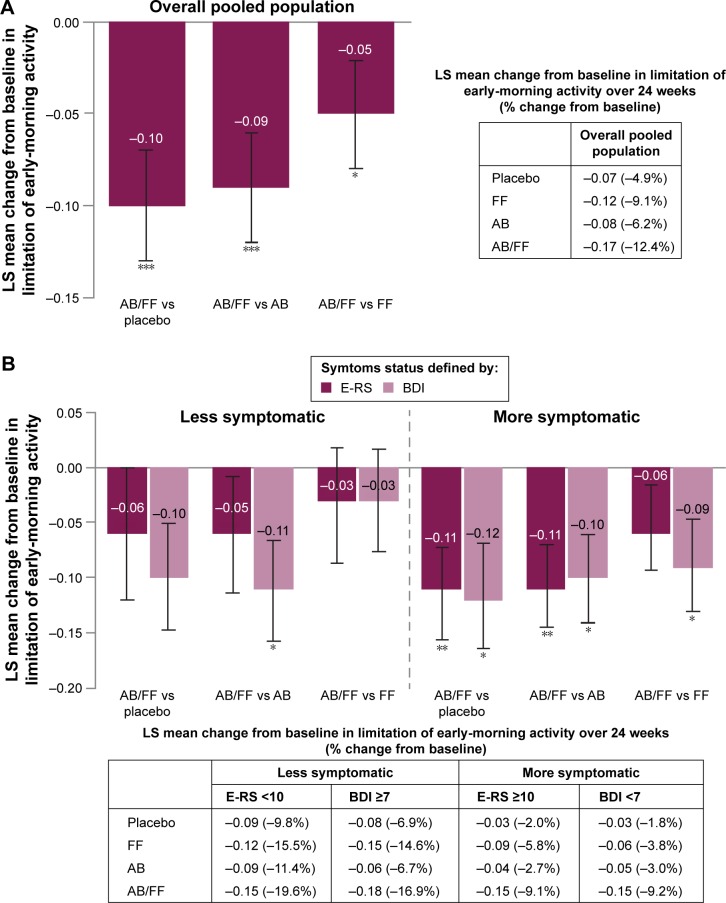
Limitation of early-morning activity
In addition to investigating the efficacy of aclidinium/formoterol on respiratory symptoms, the impact of dual therapy on limitation of early-morning activity was assessed. In the total pooled population, an improvement with aclidinium/formoterol versus placebo and monotherapies was observed (P<0.05; Figure 6A). When patients were stratified according to symptom burden, aclidinium/formoterol reduced early-morning limitation of activity compared with placebo and aclidinium in more symptomatic patients (E-RS ≥10: P<0.01 for both; BDI <7: P<0.05 for both; Figure 6B). Additionally, in less symptomatic patients according to the BDI definition, early-morning limitation of activity was reduced with aclidinium/formoterol compared with aclidinium (P<0.05; Figure 6B).
Figure 6.
Change from baseline in early-morning limitation of activity in (A) the overall pooled population and (B) less symptomatic and more symptomatic patients with COPD.
Notes: Data are LS mean ± SE for the pooled ITT population; *P<0.05; **P<0.01; ***P<0.001.
Abbreviations: AB, aclidinium bromide 400 µg; BDI, Baseline Dyspnea Index; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms; FF, formoterol fumarate 12 µg; ITT, intent-to-treat; LS, least squares; SE, standard error.
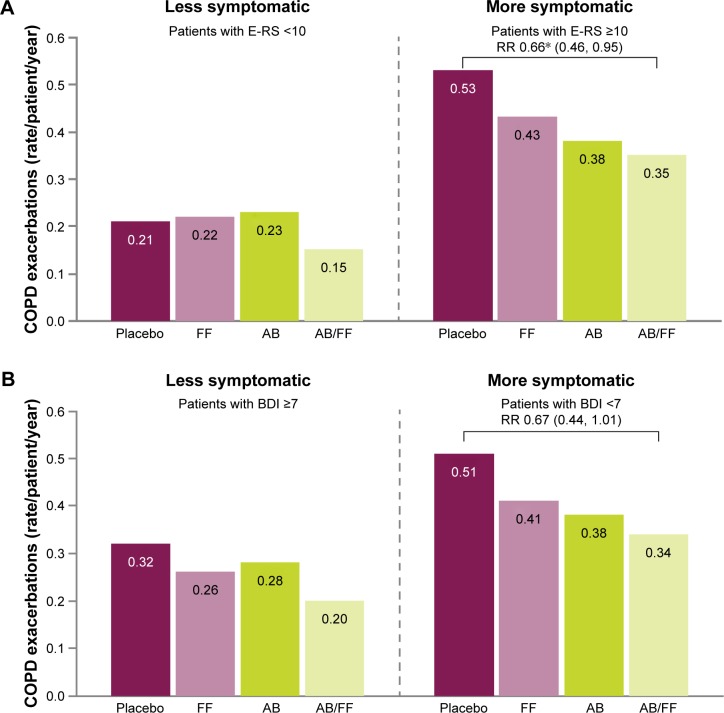
Exacerbations
For both definitions of more symptomatic patients, consistently higher rates of moderate or severe health care resource utilization exacerbations were observed across all treatment arms compared with less symptomatic patients (Figure 7). There was a reduction in the rate of exacerbations with aclidinium/formoterol compared with placebo in more symptomatic patients with E-RS ≥10 (P<0.05) and a consistent trend toward a reduction in exacerbation rate with aclidinium/formoterol versus placebo in more symptomatic patients with BDI <7 (P=0.057) (Figure 7).
Figure 7.
Rate of moderate or severe COPD exacerbations (HCRU criteria) in less symptomatic and more symptomatic patients at Week 24, (A) less symptomatic and more symptomatic defined by E-RS score, (B) less symptomatic and more symptomatic defined by BDI score.
Note: *P≤0.05; P=0.057 vs placebo in more symptomatic patients with BDI <7; Moderate or severe exacerbations are defined as exacerbations that require treatment with antibiotics or corticosteroids and those which result in hospitalization, respectively; Exacerbations data are RR (95% CI).
Abbreviations: AB, aclidinium bromide 400 µg; BDI, Baseline Dyspnea Index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms; FF, formoterol fumarate 12 µg; HCRU, healthcare resource utilization; RR, rate ratio.
Rescue medication
Aclidinium/formoterol reduced the use of daily rescue medication from baseline to week 24 for both less symptomatic and more symptomatic patients versus placebo (E-RS <10: −0.74. BDI ≥7: −0.81. E-RS ≥10: −0.89. BDI <7: −0.94; P<0.001 for all comparisons). Aclidinium/formoterol reduced daily rescue medication use from baseline versus both monotherapies in more symptomatic patients with E-RS ≥10 (−0.60 vs aclidinium and −0.45 vs formoterol; P<0.01 for both) and versus aclidinium in more symptomatic patients with BDI <7 (−0.57; P<0.01).
Discussion
Dual bronchodilation with LAMA/LABA combination therapy is recommended for the treatment of COPD in patients with respiratory symptoms; however, there are limited data available showing the effectiveness of dual bronchodilation in patients stratified according to symptom levels.2 In contrast to other LAMA/LABA Phase III studies,5–7 ACLIFORM and AUGMENT recruited patients with a broad spectrum of symptom burden.3,4 This pooled post hoc analysis of these two Phase III clinical trials assessed the efficacy of aclidinium/formoterol 400/12 µg versus placebo and monotherapies in less symptomatic (low symptom burden) or more symptomatic (high symptom burden) patients with moderate-to-severe COPD using baseline E-RS total score and BDI score to define symptomatic status.
For routine clinical practice, GOLD recommends the mMRC or CAT to categorize the impact of symptoms on a patient. These are validated instruments that have the benefit of being very quick to administer and simple to interpret. However, stratifying patients by E-RS total score and BDI score in a clinical trial environment allows for a more rigorous assessment of COPD symptoms than is achieved with common clinical tools, such as the mMRC or CAT. E-RS and BDI are sophisticated, sensitive, validated tools to investigate and quantify the symptoms of COPD in a clinical trial setting. They are not intended to replace those measures proposed by GOLD in clinical practice. BDI and E-RS correspond well with mMRC and CAT in terms of the assessments made, yet the mMRC and CAT are more practical tools for use in everyday clinical practice.
An interesting outcome to have emerged from this current post hoc analysis was that even those patients who did not present with significant respiratory symptoms at baseline achieved meaningful improvements in lung function with dual bronchodilator therapy. Improvements in postdose FEV1 with aclidinium/formoterol compared with monotherapies were of a similar magnitude for both less symptomatic and more symptomatic patients. However, when considering trough FEV1, aclidinium/formoterol demonstrated greater improvements from baseline in more symptomatic patients compared with less symptomatic patients, suggesting that dual therapy becomes increasingly beneficial with worsening symptom severity.
All active treatments provided clinically meaningful improvements in dyspnea (TDI reduced ≥1 unit) compared with placebo in both less symptomatic and more symptomatic patients, which suggests that patients with minimal symptoms at baseline can also benefit from dual bronchodilator therapy. This finding may be particularly noteworthy as other Phase III LABA/LAMA trials required patients to have a baseline mMRC dyspnea scale grade of at least 2,6,7 meaning that data on the efficacy in patients with a lower symptom burden have hitherto been lacking.
Using several measures of COPD symptoms (E-RS total score, early-morning and nighttime symptom severity, and early-morning limitation of activity) and two different ways of stratifying more symptomatic patients, the results from this post hoc analysis demonstrate that more symptomatic patients receive substantial benefits from aclidinium/formoterol dual therapy. The picture is less clear in less symptomatic patients, as there was no strong evidence of improvement or benefit according to the E-RS tool. Similarly, there were no clear improvements in nighttime symptoms in less symptomatic patients. This is understandable, as improvements in symptoms would not be expected in patients considered to be symptom-free. However, early morning is often reported by patients to be the most troubling part of the day for COPD symptoms,15,16 and the results from this study demonstrate that even patients reporting fewer symptoms showed some improvements with aclidinium/formoterol in early-morning symptom severity and limitation of early-morning activities.
In more symptomatic patients, dual bronchodilator therapy with aclidinium/formoterol reduced the risk of exacerbations compared with placebo, and there was also a trend toward a lower rate of exacerbations compared with monotherapies. The higher rate of exacerbations observed in the more symptomatic subgroups, which is not unexpected and has been reported previously,17 is likely to be why these reductions were detected in more symptomatic patients, but not in less symptomatic patients.
Taken together, the results of this study clearly demonstrate the benefits of dual bronchodilator therapy with aclidinium/formoterol in more symptomatic patients in terms of lung function, breathlessness, early-morning and nighttime symptoms, and exacerbations, compared with placebo or monotherapies. Notably, in less symptomatic patients, the improvements observed in postdose FEV1, breathlessness, and early-morning symptoms demonstrate that even patients judged to have fewer symptoms benefited from dual bronchodilator therapy. This is particularly pertinent given that it has been shown that patients often underestimate or underreport the severity of their COPD symptoms and that there can be a disparity in the perception of symptoms by patients and physicians.18,19
A potential constraint of this post hoc analysis was the method used to distinguish between less symptomatic and more symptomatic patients. The use of dyspnea tools to identify less symptomatic and more symptomatic patient subgroups is well established2 and the BDI threshold has been shown previously to correlate with established mMRC cut-offs.13 However, the threshold used to determine less symptomatic and more symptomatic patients for the E-RS definition was selected based on historical score distributions and further validation of the tool is required.10 Overall, results for less symptomatic and more symptomatic patients were largely consistent between the E-RS and BDI definitions, suggesting that the thresholds selected were appropriate. A further limitation of this post hoc analysis is the low patient numbers in some subgroups, which may account for why some improvements reached statistical significance, while others of a similar magnitude did not.
Conclusion
In this post hoc analysis, we have shown that aclidinium/formoterol 400/12 µg provides consistent improvements in bronchodilation and symptoms compared with monotherapies and a reduction in exacerbations versus placebo in more symptomatic patients with moderate-to-severe COPD, regardless of the definition used. Furthermore, patients with a low symptom burden achieved some benefit from dual bronchodilator therapy versus monotherapies, as demonstrated by improvements in postdose FEV1, dyspnea, early-morning symptoms, and limitation of activities.
Acknowledgments
The authors would like to thank all of the patients and their families, the team of investigators, research nurses, and operations staff involved in these studies. This study was funded by Almirall SA, Barcelona, Spain. The authors would also like to thank Jennifer Higginson, PhD, of Complete Medical Communications, who provided medical writing support under the direction of the authors, funded by AstraZeneca.
Footnotes
Disclosure
MM has received compensation for speaking/consultancy from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Gebro Pharma, GlaxoSmithKline, MedImmune, Menarini, Nycomed, Novartis, Pfizer, Talecris-Grifols, and Takeda. KRC has received grants from Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Forest Laboratories, Genentech, GlaxoSmithKline, Grifols, Novartis, and Roche.
In the past 3 years, KRC has received compensation for consulting with AstraZeneca, Baxter, Boehringer Ingelheim, CSL Behring, GlaxoSmithKline, Grifols, Kamada, Novartis, Nycomed, Roche, and Telacris; has undertaken research funded by Amgen, AstraZeneca, Baxter, Boehringer Ingelheim, CSL Behring, Forest Labs, GlaxoSmithKline, Grifols, Novartis, Roche, and Takeda; and has participated in continuing medical education activities sponsored in whole or in part by AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Grifols, Merck Frosst, Novartis, Pfizer, and Takeda. He is participating in research funded by the Canadian Institutes of Health Research operating grant entitled: Canadian Cohort Obstructive Lung Disease (CanCOLD). KRC holds the GSK-CIHR Research Chair in Respiratory Health Care Delivery at the University Health Network, Toronto, Canada.
FC, AR, and EGG are employees of AstraZeneca.
The authors report no other conflicts of interest in this work.
References
- 1.Tsiligianni I, Kocks J, Tzanakis N, Siafakas N, van der Molen T. Factors that influence disease-specific quality of life or health status in patients with COPD: a review and meta-analysis of Pearson correlations. Prim Care Respir J. 2011;20(3):257–268. doi: 10.4104/pcrj.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. [Accessed April 28, 2016]. [updated 2016]. Available from: http://goldcopd.org/gold-reports/
- 3.D’Urzo AD, Rennard SI, Kerwin EM, Mergel V, Leselbaum AR, Caracta CF. Efficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD study. Respir Res. 2014;15(1):123–141. doi: 10.1186/s12931-014-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh D, Jones PW, Bateman ED, et al. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised study. BMC Pulm Med. 2014;14(1):178–189. doi: 10.1186/1471-2466-14-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42(6):1484–1494. doi: 10.1183/09031936.00200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donohue JF, Maleki-Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107(10):1538–1546. doi: 10.1016/j.rmed.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Mahler DA, Decramer M, D’Urzo A, et al. Dual bronchodilation with QVA149 reduces patient-reported dyspnoea in COPD: the BLAZE study. Eur Respir J. 2014;43(6):1599–1609. doi: 10.1183/09031936.00124013. [DOI] [PubMed] [Google Scholar]
- 8.Leidy NK, Sexton CC, Jones PW, et al. Measuring respiratory symptoms in clinical trials of COPD: reliability and validity of a daily diary. Thorax. 2014;69(5):443–449. doi: 10.1136/thoraxjnl-2013-204428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leidy NK, Murray LT, Monz BU, et al. Measuring respiratory symptoms of COPD: performance of the EXACT-Respiratory Symptoms Tool (E-RS) in three clinical trials. Respir Res. 2014;15(1):124. doi: 10.1186/s12931-014-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones PW, Leidy NK, Hareendran A, Lamarca R, Chuecos F, Garcia Gil E. The effect of aclidinium bromide on daily respiratory symptoms of COPD, measured using the evaluating respiratory symptoms in COPD (E-RS: COPD) diary: pooled analysis of two 6-month Phase III studies. Respir Res. 2016;17(1):61. doi: 10.1186/s12931-016-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85(6):751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 12.European Medicines Agency Duaklir Genuair. [Accessed July 11, 2016]. [updated June 21, 2016]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003745/human_med_001811.jsp&mid=WC0b01ac058001d124.
- 13.Mahler DA, Kerstjens HA, Donohue JF, Buhl R, Lawrence D, Altman P. Indacaterol vs tiotropium in COPD patients classified as GOLD A and B. Respir Med. 2015;109(8):1031–1039. doi: 10.1016/j.rmed.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Bateman ED, Chapman KR, Singh D, et al. Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multi-centre, randomised studies (ACLIFORM and AUGMENT) Respir Res. 2015;16:92. doi: 10.1186/s12931-015-0250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miravitlles M, Worth H, Soler Cataluna JJ, et al. Observational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: results from the ASSESS study. Respir Res. 2014;15:122. doi: 10.1186/s12931-014-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin. 2009;25(8):2043–2048. doi: 10.1185/03007990903103006. [DOI] [PubMed] [Google Scholar]
- 17.Miravitlles M, Worth H, Soler-Cataluna JJ, et al. The relationship between 24-hour symptoms and COPD exacerbations and healthcare resource use: results from an observational study (ASSESS) COPD. 2016:1–8. doi: 10.3109/15412555.2016.1150447. [DOI] [PubMed] [Google Scholar]
- 18.Miravitlles M, Ferrer J, Baro E, Lleonart M, Galera J. Differences between physician and patient in the perception of symptoms and their severity in COPD. Respir Med. 2013;107(12):1977–1985. doi: 10.1016/j.rmed.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Rennard S, Decramer M, Calverley PM, et al. Impact of COPD in North America and Europe in 2000: subjects’ perspective of confronting COPD International Survey. Eur Respir J. 2002;20(4):799–805. doi: 10.1183/09031936.02.03242002. [DOI] [PubMed] [Google Scholar]