Abstract
Thermoregulatory ability and behavior influence organismal responses to their environment. By measuring thermal preferences, researchers can better understand the effects that temperature tolerances have on ecological and physiological responses to both biotic and abiotic stressors. However, because of funding limitations and confounders, measuring thermoregulation can often be difficult. Here, we provide an effective, affordable (∼$50 USD per unit), easy to construct, and validated apparatus for measuring the long-term thermal preferences of animals. In tests, the apparatus spanned temperatures from 9.29 to 33.94 °C, and we provide methods to further increase this range. Additionally, we provide simple methods to non-invasively measure animal and substrate temperatures and to prevent temperature preferences of the focal organisms from being confounded with temperature preferences of its prey and its humidity preferences. To validate the apparatus, we show that it was capable of detecting individual-level consistency and among individual-level variation in the preferred body temperatures of Southern toads (Anaxyrus terrestris) and Cuban tree frogs (Osteopilus septentrionalis) over three-weeks. Nearly every aspect of our design is adaptable to meet the needs of a multitude of study systems, including various terrestrial amphibious, and aquatic organisms. The apparatus and methods described here can be used to quantify behavioral thermal preferences, which can be critical for determining temperature tolerances across species and thus the resiliency of species to current and impending climate change.
Keywords: Thermoregulation, Thermal preference, Thermoregulatory behavior, Experimental design
1. Introduction
Quantifying thermoregulatory behavior in ectotherms can elucidate fundamental aspects of organismal physiology, behavior, and ecology (Huey and Stevenson, 1979; Bauwens et al., 1990; Hutchison and Dupré, 1992; Hertz et al., 1993; Blouin-Demers and Weatherhead, 2001; Angilletta et al., 2010). Specifically, determining thermal preferences and tolerances is critical for understanding how individuals mediate both biotic and abiotic stressors. Indeed, thermoregulatory behavior has been observed in almost all ectothermic taxa, including reptiles (Monagas and Gatten, 1983; Burns et al., 1996), amphibians (Kluger, 1977; Hutchison and Murphy, 1985), bony fishes (Reynolds et al., 1976, 1977), and invertebrates (Bicego et al., 2007). By seeking external sources of heat or refuge in cool places, ectotherms can regulate their metabolism to facilitate feeding and digestion (Ayers and Shine, 1997), reproduction (Christiansen and Bakke, 1968), growth (Lillywhite et al., 1973; Sinervo and Adolph, 1989; Calsbeek and Sinervo, 2002a), immune function and disease resistance (Blanford and Thomas, 1999; Mondal and Rai, 2001; Rohr et al., 2013), territory selection and defense (Calsbeek and Sinervo, 2002b), mate search and mating (Calsbeek and Sinervo, 2002b), and many other physiological functions (Bennett, 1980). Hence, measuring ther-moregulatory behaviors and temperature preferences is important to understanding many aspects of the fundamental biology of ectotherms.
Quantification of thermal preferences can also inform issues relevant to applied biology. For instance, many anthropogenic factors can alter the thermal environment posing threats to the performance of organisms. Global climate change is the most obvious (Deutsch et al., 2008; Seebacher and Post, 2015), but there are other examples as well. For instance, deforestation, or more generally the loss of shading caused by habitat destruction, can greatly increase the temperatures to which organisms are exposed (Gordon, 2003). Moreover, infectious diseases, many of which are introduced or exacerbated by humans, often induce behavioral fevers (preference of warmer temperatures in response to pathogen exposure) in ectotherms (Blanford and Thomas, 1999) that can be important for resisting infections and reducing the adverse consequences on hosts (Pörtner, 2002; Raffel et al., 2006; Lafferty, 2009; Rohr and Raffel, 2010; Rohr et al., 2011a, 2013). Hence, determining the bounds of thermoregulatory abilities among ectothermic populations will be critical for predicting the impacts of widespread anthropogenic change (Addo-Bediako et al., 2000; Deutsch et al., 2008; Seebacher and Post, 2015).
Although thermal preferences are an important determinant of many aspects of temperature-dependent physiology of ectotherms, experimentally determining thermal preferences can be challenging and expensive. A standard method for determining thermal preferences is to place focal animals in a thermal gradient chamber and monitor body temperatures over some length of time (Row and Blouin-Demers, 2006; Weatherhead et al., 2012). In the absence of other stimuli (e.g. food, conspecifics, etc.), the assumption is that the focal animal will spend the majority of time within their preferred temperature range (Hertz et al., 1993). However, there can be many confounding factors that can make the results of these trials difficult to interpret, particularly when the duration of these trials exceed the food deprivation limits of the focal organism. For example, because environmental temperature is correlated positively with evaporative water loss, temperature gradients are often confounded with moisture gradients (Malvin and Wood, 1991; Rohr and Palmer, 2013). Given that organisms must maintain moisture in addition to preferred temperatures (Bellis, 1962; Rohr and Madison, 2003; Rohr and Palmer, 2005), confounding moisture and temperature gradients makes it challenging to assess whether true temperature preferences are being quantified.
Additionally, longer-term trials that require feeding the test animal can pose additional confounders. Ectotherms often prefer warmer temperatures during digestion (Lillywhite et al., 1973; Greenwald and Kanter, 1979) making it challenging to discriminate baseline and digestion-related temperature preferences. Live prey provided as a food source might prefer temperatures outside of the preferred temperature range of the focal organism, confounding the temperature preference of the prey and test organism. Finally, invasive temperature measurement techniques, such as dermally attached loggers, brain implants, or thermometer probes, are used commonly in longer-term experiments, but they can alter behavior and thus can compromise measurements of true temperature preferences (Rowley and Alford, 2007).
Here, we provide an inexpensive, efficient, and validated method for measuring thermoregulatory behavior in the laboratory for extended periods of time while controlling for humidity, disturbance, and other confounders. Using supplies found in most hardware stores, we constructed thermal gradient apparatuses for less than $50 USD, spanning temperatures from 9.29 to 33.94 °C (for supply list see Table S1). The methodological details and results presented here demonstrate that our apparatus and methods (1) maintain consistent high humidity across the entire temperature gradient, (2) allow for long-term maintenance of animals in the temperature gradient, (3) do not confound temperature preference of the prey with the focal organism, (4) measure temperature using minimally invasive techniques, and (5) can detect consistency in the thermal preferences within individuals but differences in preferences among individuals and species, a prerequisite for quantifying temperature preferences and behavioral thermoregulation. At a time when measuring temperature tolerance across species is critical to assess the ability of organisms to respond to climate change and other stressors, our method provides an affordable, easy to implement, effective way to measure thermal responses across a wide range of species of varying sizes.
2. Materials and methods
2.1. Apparatus
We constructed 28 thermal gradient apparatuses using 274 × 8 × 12 cm aluminum downspout gutters cut in half along the longest and widest sides yielding final internal dimensions of 137 × 8 × 6 cm (Fig. 1a and b). Each apparatus was insulated using foam windowsill insulation with holes cut where the metal meets a heat or cooling source (Fig. 1c and d) and was capped at the ends using Styrofoam and silicon sealant (Fig. 1b). The top of each apparatus was sealed using five 27 × 10 cm Plexiglas® windows resting on window weather-stripping. These Plexiglas® windows were held in place by small duct tape hinges (Fig. 1b). The windows allow ambient light to pass through and give the experimenter access to each section of the apparatus with minimal disturbance to the organism. Each window was secured to prevent animals from escaping using twine and cord locks (Fig. 1b).
Fig. 1.

Temperature gradient apparatuses. (a) The entire set up of thermoregulatory apparatus, showing insulating 2 × 4's and large Plexiglas covers. (b) View of the sphagnum moss interior and small Plexiglas window sealing each apparatus. (c) Heat tape gradient and bottom of apparatus showing the space where the aluminum meets the heat tape. (d) Ice packs and bottom of apparatus showing the space where the aluminum meets the ice packs.
2.2. Maintaining temperature and humidity
The warmer ends of the apparatuses rested on a gradient of 7.62 cm 10W heat tape (Flexwatt Industrial Sales®, Maryville, TN) controlled by a bulb-and-capillary thermostat (Selco Products Co., Orange, CA). The temperature gradient was created by adhering six 132 cm strips of heat tape to a piece of plywood (60.96 × 132.08 cm) at increasing distances from the end of the apparatus (Fig. 1c). The cooler end of each apparatus rests on a frozen (−80 °C) gel pack (32 oz No-Sweat, Temperatsure Inc., Reno, NV) (Fig. 1d). Ice packs sat on windowsill insulation and were replaced every 12 h. Plywood height was adjusted to the height of the ice packs to level the apparatuses. Two pieces of wood (5.08 × 10.16 × 137 cm) rested against the outside of the outermost apparatuses to prevent heat loss (Fig. 1a). Each shelf of apparatuses was covered by two large sheets of Plexiglas to further insulate the apparatuses while maintaining the desired photo-period (Fig. 1a). Organic sphagnum moss substrate, kept saturated with artificial spring water (Cohen et al., 1980), was used to maintain constant high humidity throughout each apparatus.
2.3. Maintaining animals and taking temperature measurements
In separate trials, we housed a total of 36 Southern toads (Anaxyrus terrestris, mean mass: 0.61 g; ± 0.01 SE), and 25 Cuban tree frogs (Osteopilus septentrionalis, mean mass: 7.67 g; ± 0.35 SE) in the apparatuses (one animal per apparatus) for three weeks during the trials. For both species, we used an ecologically relevant temperature gradient of 12–33 °C (US Climate Data, 2016). All animals were fed 10 live crickets twice a week in containment to prevent crickets from moving freely within the apparatuses. The feeding containers were constructed of quart-size zip-top bags with plastic coated paper clips adhered to the outside for structure (Fig. S1). Feeding containers were placed in the thermal gradient apparatuses at the location each individual was found prior to feeding. After seven hours of confinement, quantity of crickets eaten was recorded for each individual to monitor feeding success throughout the duration of the experiment. No temperature measurements were taken on feeding days because of the limited movement allowed by focal animals and their prey during feeding.
Temperature measurements were taken with an Extech® High Temperature Infrared Thermometer (accuracy: ± 2% of rdg <932°F), which uses a laser to non-invasively measure temperatures and minimize disturbance to the animal. In amphibians, this method is comparable to cloacal measurements taken via thermally sensitive radio-transmitters (Rowley and Alford, 2007). At each temperature measurement, we located the individual opened the appropriate Plexiglas window section directly above the animal, and then measured the body temperature of the animal and the temperature of the substrate as close as possible to where the animal was found with the infrared thermometer. Given that some animals can move regularly, measuring both the temperature of the substrate and animals offers insight into whether the animal has been at a given location long enough for it body temperature to conform to environmental temperature. The animals did not respond to the infrared laser in any observable manner. Temperature measurements were taken every four hours, four times a day between 1000 h and 2200 h, five days a week, for three weeks totaling 100 body temperature measurements per individual. The four hour time intervals within a day and a 12 h gap between days was chosen to allow ample opportunity for organisms to move between measurements within and among days. Measurements were averaged within a day to meet the assumptions of normality (i.e. central limit theorem).
2.4. Validating temperature and humidity
To determine the relationship between location in the gutters and temperature, time of day and temperature, and humidity and temperature, we monitored the substrate surface temperature and humidity of seven randomly selected apparatuses over time using five equally spaced Thermochron iButtons® (Maxim Integrated Products, Inc.) and five equally spaced Xintiandi™ Hygrometers (accuracy ± 5%) while no animals were in the apparatuses.
2.5. Statistical analyses
To test for a temperature and humidity gradient across the gutters, we regressed spatial location of the iButtons against the associated temperature and humidity measurements (using the lm function in R). To assure that shelf location and any associated variation in access to light did not influence results, we included shelf as a predictor in all analyses. To test for individual consistency in temperature variation within individuals and variation in body temperature preferences among individuals, we conducted a one-way repeated measures ANOVA blocking by shelf (using Statistica, Statsoft, Tulsa, OK). This analysis tested whether temperature preferences of individuals varied significantly across days (main effect of day) and whether temperature preferences varied among individuals (within-individual variance, s2). Additionally, we calculated repeatability (Lessells and Boag, 1987), the proportion of the variance explained by the individual (Falconer and Mackay, 1995). For each analysis, residuals were normally distributed and met the assumptions of the analyses. Results are presented as mean ± 1 SE.
3. Results
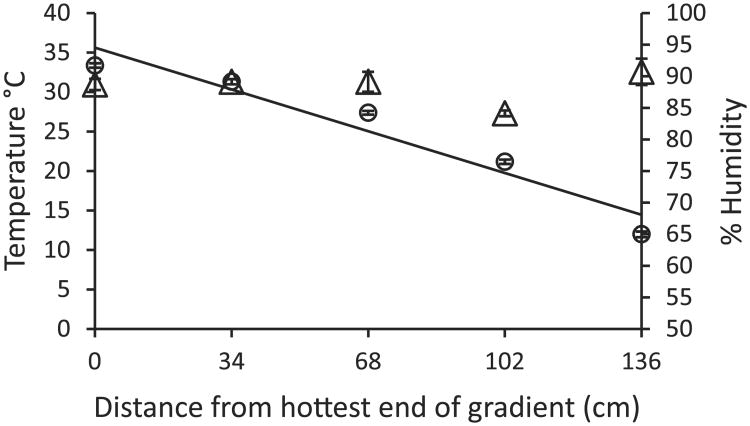
Our apparatuses maintained an average thermal gradient between 12.0 and 33.4 °C (±0.36 and 0.28 °C) (Fig. 2) across 135 cm with a mean daily range of 9.29–33.94 °C (±0.08 and 0.01 °C) (mean room temperature: 21.16±0.07 °C). While change in temperature was slightly more pronounced in the warmest 27 cm of the apparatus, the temperature gradient was generally even across the remaining length (Fig. 2). The saturated moss maintained humidity between 84.1% and 90.7% (±0.65%) throughout the apparatuses (Fig. 2), essentially functioning as a wick, drawing moisture from the cool to warm end to maintain the constant humidity. In fact, although temperature significantly declined across the five iButton locations (χ2=408.56, df=1, p≤0.001), humidity did not significantly change across this temperature gradient (χ2=0.16, df=1, p=0.69), nor was there a significant impact of location on humidity (χ2=0.01, df=1, p=0.91; Fig. 2).
Fig. 2.
Substrate surface temperature (circles and trend line) and humidity (triangles) gradients as a function of the distance from the warmest end of the apparatus. At the five equidistant locations receiving iButtons and hygrometers, average temperature ranged from 12.0 to 33.4 °C (±0.36 and 0.28 °C SE), whereas average humidity ranged from 84.1% to 90.7% (±0.65% SE). Temperature declined significantly as distance from the warmest end of the apparatus increased (χ2 = 408.56, df=1, p ≤ 0.001), but, there was no significant change in humidity across this temperature gradient (χ2 = 0.16, df=1, p = 0.69) nor was there a significant impact of location on humidity (χ2 = 0.01, df = 1, p=0.91). Points indicate means ± 1 standard error of seven replicates.
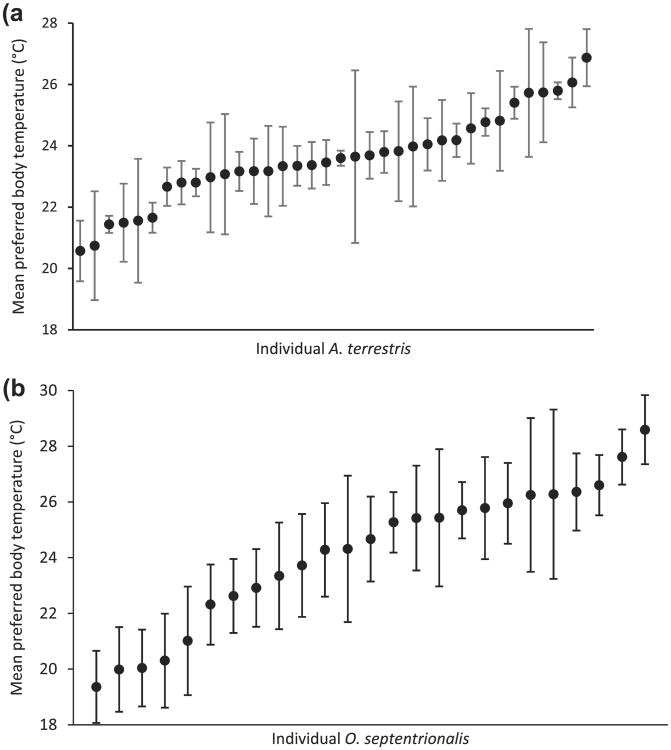
Over the course of a 12-h period, melting ice packs only moderately altered the temperature of the coldest third of the apparatuses (they experienced an average 4.12±0.31 °C shift in temperature twice daily; Fig. 3a). These daily temperature fluctuations likely did not affect temperature preferences as the remaining two thirds of the apparatuses were not impacted and the thawing only altered temperatures for a very short amount of time. Both within and across days, temperature fluctuations in the apparatuses were minimal (Fig. 3a and b). Mean temperature preferences of individual A. terrestris ranged from 22 to 27 °C with a mean (±SE) overall preference of 23.8 °C (±0.17 °C; Fig. 4a) and O. septentrionalis ranged from 19 to 27 °C with a mean (±SE) overall preference of 22.8 °C (±0.50 °C; Fig. 4b). Importantly, using our apparatuses, we were able to detect consistency in the temperature preference of individual A. terrestris and O. septentrionalis (A. terrestris main effect of day: F=1.32, df=3, p=0.27; O. septentrionalis main effect of day: F=0.26, df=3, p=0.86) but variation in temperature preferences among A. terrestris (within-individual variance: s2=17.5; repeatability: r=.99; Fig. 4a) and O. septentrionalis (within-individual variance: s2=9.5 repeatability: r=0.99; Fig. 4b). We also determined that there was no main effect (F=0.782, df=2, p=0.466) or interacting effect of shelf location on body temperature preference over time (F=0.357, df=5, p=0.838), therefore, we dropped shelf from the model.
Fig. 3.
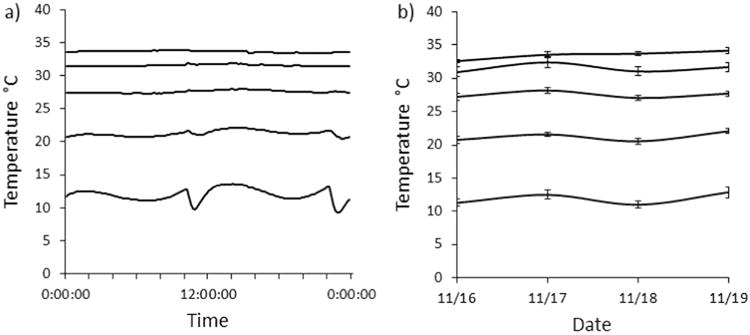
Variation in substrate surface temperature within and across gutters and within and across days. (a) Mean substrate surface temperature for five equally spaced positions in the apparatuses (n=7) over 24 h. The variation in the curves in the coldest section of the apparatuses is a product of the ice packs melting and being replaced every 12 h, which shifted temperature 4.12 °C (±0.31 °C SE) twice daily. (b) Mean (±SE) temperature for five equally spaced positions in the apparatuses (n = 7) over 4 days, showing the consistency in measurements at each location. There was no significant effect of date on temperature across the gradient (F = 0.03, df = 3, p = 0.88).
Fig. 4.
Plot of individual (a) Anaxyrus terrestris (n = 36) and (b) Osteopilus septentrionalis (n = 25) preferred body temperature. A. terrestris and O. septentrionalis mean preferred body temperatures were 23.8 °C (±0.17 °C) and 22.8 °C (±0.50 °C), respectively. Individuals exhibited consistency in their preferred body temperature (main effect of day: F = 1.32, df = 3, p = 0.27 and F = 0.26, df = 3, p = 0.86) but there was significant variation among individuals in preferred body temperature (within-individual variance and repeatability: s2 = 17.5, r = 0.99 and s2 = 9.5, r = 0.99).
4. Discussion
Here we offer a validated, inexpensive, and efficient way to quantify the long-term thermal preferences of animals while avoiding moisture and feeding confounders. We tested A. terrestris and O. septentrionalis preferred body temperatures using a novel design for a thermal gradient apparatus. We found that the apparatus functioned well, with apparatus temperatures, temperature variation, and humidity being relatively uniform over time. As further evidence for apparatus functioning, variation in the preferred body temperatures of A. terrestris and O. septentrionalis within individuals was minimal compared to variation among individuals. Being able to quantify individual consistency and variation among individuals in preferred body temperatures is a pre-requisite for quantifying temperature preferences and behavioral thermoregulation. Additionally, variation among individuals within a population is the raw material on which selection acts, thus, measuring this variation is critical for predicting how populations might adapt to climate change (Rowley and Alford, 2013). The design detailed here could be used for a variety of thermal ecology applications.
Nearly every aspect of our design can be easily modified to meet the needs of individual researchers. Temperature range of the apparatuses can be shifted or expanded by altering the heating and cooling sources. The thermostat used in these experiments can decrease the temperature of the heat tape down to room temperature and, with alternative thermostats, Flexwatt heat tape can reach temperatures over 40 °C. Additionally, the cooler end of the gradient could be maintained at a more stable temperature using a cold water cooling system or by replacing the ice packs more frequently to maintain temperatures closer to our minimal temperature of 9.3 °C. Because our apparatuses are set up on shelves, there may be slight differences in light intensity across shelves. Any differences in light intensity can be dealt with by adding additional lighting to each shelf or by randomly distributing individuals across shelves and including shelf as a block in any subsequent statistical analyses. The most substantial drawback to this design is the labor intensive nature of the temperature measurements, given that an infrared heat gun is used to noninvasively record each measurement. More invasive forms of temperature measurements that log body temperature continuously (e.g. surgically implanted monitors) might be more appropriate for some studies and our design can also accommodate these forms of measurements.
Depending on the physiology of the focal animals, apparatus humidity can easily be modified. Although we kept humidity relatively high to accommodate the needs of amphibians, alternative substrates can easily be used to accommodate a wide variety of focal taxa (e.g. cotton, sand, soil, mulch, or paper towels), such as arthropods, lizards, snakes, and even small mammals. Because of the duration of the trials, we found it necessary to feed our test animals. The feeding containment bag design we employed is simple and easy to replicate. All of the A. terrestris and O. septentrionalis in our study fed successfully in their feeding containers, finishing 78.9% (±0.33% SE) and 75.7% (±0.02% SE) of their crickets within the seven-hour feeding period. If needed, the containers could be easily cleaned and bleached for reuse, thereby facilitating use in studies testing for behavioral fever in response to infections (Blanford and Thomas, 1999).
The expansive application potential of our thermal gradient apparatuses, coupled with the straightforward, effective, and affordable design, makes them ideal for measuring thermo-regulatory behavior. Unlike most thermal gradient apparatuses used in thermal biology studies (Klein et al., 1992; Burns et al., 1996; Zdanovich, 2006; Lourdais et al., 2013; Lara-Reséndiz et al., 2015), we were able to create a broad gradient representing an ecologically relevant temperature range, control for humidity, avoid feeding confounders, avoid invasive temperature measurement techniques, and maintain animals for substantial time periods. While we did not control for spatial preferences within the apparatuses in our validation trials, testing for temperature preferences while controlling for spatial distribution within each apparatus could easily be done by alternating the direction of the temperature gradient on each shelf or by including identical apparatuses held at a constant temperature. The overall cost of each thermal preference apparatus was $48.20 USD (see Table S1 for a list and cost breakdown of all the supplies). Additionally, we were able to capture individual-level preferred body temperatures of 36 A. terrestris and 25 O. septentrionalis over the course of three weeks and have used the apparatuses for additional studies, successfully detecting host behavioral fever responses to pathogens (unpublished).
Body temperature is remarkably influential to almost every facet of physiological performance in both endothermic and ectothermic organisms (Huey and Kingsolver, 1989; Angilletta et al., 2010). The importance of understanding thermoregulation and thermal biology will only increase as organisms face new anthropogenic stressors and threats, such as climate and land use change (Rohr et al., 2011b; Rohr and Palmer, 2013). Hence, we believe our method for measuring thermoregulation will facilitate future research in the continually expanding field of thermal biology.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Science Foundation (EF-1241889), National Institutes of Health (R01GM109499 and R01TW010286), US Department of Agriculture (NRI 2006-01370 and 2009-35102-0543), and US Environmental Protection Agency (CAREER 83518801) to J.R.R.
Footnotes
Appendix A. Supplementary material: Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jtherbio.2016.07.016.
References
- Addo-Bediako A, Chown SL, Gaston KJ. Thermal tolerance, climatic variability and latitude. Proc R Soc Lond Ser B: Biol Sci. 2000;267:739–745. doi: 10.1098/rspb.2000.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angilletta M, Cooper BS, Schuler MS, Boyles JG. The evolution of thermal physiology in endotherms. Front Biosci E. 2010;2:861–881. doi: 10.2741/e148. [DOI] [PubMed] [Google Scholar]
- Ayers D, Shine R. Thermal influences on foraging ability: body size, posture and cooling rate of an ambush predator, the python Morelia spilota. Funct Ecol. 1997;11:342–347. [Google Scholar]
- Bauwens D, Castilla AM, Van Damme R, Verheyen RF. Field body temperatures and thermoregulatory behavior of the high altitude lizard, Lacerta bedriagae. J Herpetol. 1990;24:88–91. [Google Scholar]
- Bellis ED. The influence of humidity on wood frog activity. Am Midland Nat. 1962;68:139–148. [Google Scholar]
- Bennett AF. The thermal dependence of lizard behaviour. Anim Behav. 1980;28:752–762. [Google Scholar]
- Bicego KC, Barros RCH, Branco LGS. Physiology of temperature regulation: comparative aspects. Comp Biochem Physiol A: Mol Integr Physiol. 2007;147:616–639. doi: 10.1016/j.cbpa.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Blanford S, Thomas MB. Host thermal biology: the key to understanding host–pathogen interactions and microbial pest control? Agric For Entomol. 1999;1:195–202. [Google Scholar]
- Blouin-Demers G, Weatherhead PJ. Thermal ecology of black rat snakes (Elaphe obsoleta) in a thermally challenging environment. Ecology. 2001;82:3025–3043. [Google Scholar]
- Burns G, Ramos A, Muchlinski A. Fever response in North American snakes. J Herpetol. 1996;30:133–139. [Google Scholar]
- Calsbeek R, Sinervo B. An experimental test of the ideal despotic distribution. J Anim Ecol. 2002a;71:513–523. [Google Scholar]
- Calsbeek R, Sinervo B. Uncoupling direct and indirect components of female choice in the wild. Proc Natl Acad Sci. 2002b;99:14897–14902. doi: 10.1073/pnas.242645199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen E, Bakke A. Temperature preference in adults of Hylobius abietis L. (Coleoptera: Curculionidae) during feeding and oviposition. Z Angew Èntomol. 1968;62:83–89. [Google Scholar]
- Cohen LM, Neimark H, Eveland L. Schistosoma mansoni: response of cercariae to a thermal gradient. J Parasitol. 1980;66:362–364. [PubMed] [Google Scholar]
- Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA. 2008;105:6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer D, Mackay T. Introduction to Quantitative Genetics. Vol. 19. Harlow: Longman Group Ltd; 1995. p. 1. [Google Scholar]
- Gordon CJ. Role of environmental stress in the physiological response to chemical toxicants. Environ Res. 2003;92:1–7. doi: 10.1016/s0013-9351(02)00008-7. [DOI] [PubMed] [Google Scholar]
- Greenwald O, Kanter ME. The effects of temperature and behavioral thermoregulation on digestive efficiency and rate in corn snakes (Elaphe guttata guttata) Physiol Zool. 1979:398–408. [Google Scholar]
- Hertz PE, Huey RB, Stevenson R. Evaluating temperature regulation by field-active ectotherms: the fallacy of the inappropriate question. Am Nat. 1993:796–818. doi: 10.1086/285573. [DOI] [PubMed] [Google Scholar]
- Huey RB, Kingsolver JG. Evolution of thermal sensitivity of ectotherm performance. Trends Ecol Evol. 1989;4:131–135. doi: 10.1016/0169-5347(89)90211-5. [DOI] [PubMed] [Google Scholar]
- Huey RB, Stevenson RD. Integrating thermal physiology and ecology of ectotherms: a discussion of approaches. Am Zool. 1979;19:357–366. [Google Scholar]
- Hutchison VH, Dupré K. Thermoregulation. In: Feder ME, Burggren WW, editors. Environmental Physiology of the Amphibians. The University of Chicago Press; Chicago, Illinois: 1992. pp. 206–249. [Google Scholar]
- Hutchison VH, Murphy K. Behavioral thermoregulation in the salamander Necturus maculosus after heat-shock. Comp Biochem Physiol A: Physiol. 1985;82:391–394. doi: 10.1016/0742-8413(86)90074-5. [DOI] [PubMed] [Google Scholar]
- Klein MS, Conn CA, Kluger MJ. Behavioral thermoregulation in mice inoculated with influenza virus. Physiol Behav. 1992;52:1133–1139. doi: 10.1016/0031-9384(92)90472-e. [DOI] [PubMed] [Google Scholar]
- Kluger MJ. Fever in frog Hyla cinerea. J Therm Biol. 1977;2:79–81. [Google Scholar]
- Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- Lara-Reséndiz RA, Gadsden H, Rosen PC, Sinervo B, Méndez-De la Cruz FR. Thermoregulation of two sympatric species of horned lizards in the Chihuahuan Desert and their local extinction risk. J Therm Biol. 2015;48:1–10. doi: 10.1016/j.jtherbio.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Lessells C, Boag PT. Unrepeatable repeatabilities: a common mistake. The Auk. 1987:116–121. [Google Scholar]
- Lillywhite HB, Licht P, Chelgren P. The role of behavioral thermoregulation in the growth energetics of the Toad, Bufo boreas. Ecology. 1973;54:375–383. [Google Scholar]
- Lourdais O, Guillon M, DeNardo D, Blouin-Demers G. Cold climate specialization: adaptive covariation between metabolic rate and thermoregulation in pregnant vipers. Physiol Behav. 2013;119:149–155. doi: 10.1016/j.physbeh.2013.05.041. [DOI] [PubMed] [Google Scholar]
- Malvin GM, Wood SC. Behavioral thermoregulation of the Toad, Bufo marinus: effects of air humidity. J Exp Zool. 1991;258:322–326. doi: 10.1002/jez.1402580307. [DOI] [PubMed] [Google Scholar]
- Monagas WR, Gatten RE. Behavioral fever in the turtles Terrapene carolina and Chrysemys picta. J Therm Biol. 1983;8:285–288. [Google Scholar]
- Mondal S, Rai U. In vitro effect of temperature on phagocytic and cytotoxic activities of splenic phagocytes of the wall lizard, Hemidactylus flaviviridis. Comp Biochem Physiol Part A: Mol Integr Physiol. 2001;129:391–398. doi: 10.1016/s1095-6433(00)00356-1. [DOI] [PubMed] [Google Scholar]
- Pörtner HO. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol Part A: Mol Integr Physiol. 2002;132:739–761. doi: 10.1016/s1095-6433(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Raffel TR, Rohr JR, Kiesecker JM, Hudson PJ. Negative effects of changing temperature on amphibian immunity under field conditions. Funct Ecol. 2006;20:819–828. [Google Scholar]
- Raffel TR, Romansic JM, Halstead NT, McMahon TA, Venesky MD, Rohr JR. Disease and thermal acclimation in a more variable and unpredictable climate. Nat Clim Change. 2013;3:146–151. [Google Scholar]
- Reynolds WW, Casterlin ME, Covert JB. Behavioral fever in teleost fishes. Nature. 1976;259:41–42. doi: 10.1038/259041a0. [DOI] [PubMed] [Google Scholar]
- Reynolds WW, Casterlin ME, Covert JB. Febrile responses of aquatic ectotherms to bacterial pyrogens. Am Zool. 1977;17:903. [Google Scholar]
- Rohr JR, Dobson AP, Johnson PTJ, Kilpatrick AM, Paull SH, Raffel TR, Ruiz-Moreno D, Thomas MB. Frontiers in climate change–disease research. Trends Ecol Evol. 2011a;26:270–277. doi: 10.1016/j.tree.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Madison DM. Dryness increases predation risk in efts: support for an amphibian decline hypothesis. Oecologia. 2003;135:657–664. doi: 10.1007/s00442-003-1206-7. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Palmer BD. Aquatic herbicide exposure increases salamander desiccation risk eight months later in a terrestrial environment. Environ Toxicol Chem. 2005;24:1253–1258. doi: 10.1897/04-448r.1. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Palmer BD. Climate change, multiple stressors, and the decline of ectotherms. Conserv Biol. 2013;27:741–751. doi: 10.1111/cobi.12086. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR. Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proc Natl Acad Sci. 2010;107:8269–8274. doi: 10.1073/pnas.0912883107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR, Blaustein AR, Johnson PTJ, Paull SH, Young S. Using physiology to understand climate-driven changes in disease and their implications for conservation. Conserv Physiol. 2013;1 doi: 10.1093/conphys/cot022. http://dx.doi.org/10.1093/con-phys/cot1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Sesterhenn TM, Stieha C. Will climate change reduce the effects of a pesticide on amphibians? Partitioning effects on exposure and susceptibility to pollution. Glob Change Biol. 2011b;17:657–666. [Google Scholar]
- Row JR, Blouin-Demers G. Thermal quality influences effectiveness of thermoregulation, habitat use, and behaviour in milk snakes. Oecologia. 2006;148:1–11. doi: 10.1007/s00442-005-0350-7. [DOI] [PubMed] [Google Scholar]
- Rowley JJL, Alford RA. Non-contact infrared thermometers can accurately measure amphibian body temperatures. Herpetol Rev. 2007;38:308–311. [Google Scholar]
- Rowley JJL, Alford RA. Hot bodies protect amphibians against chytrid infection in nature. Sci Rep. 2013;3:1515. doi: 10.1038/srep01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebacher F, Post E. Climate change impacts on animal migration. Clim Change Responses. 2015;2:1–2. [Google Scholar]
- Sinervo B, Adolph S. Thermal sensitivity of growth rate in hatchling Sceloporus lizards: environmental, behavioral and genetic aspects. Oecologia. 1989;78:411–419. doi: 10.1007/BF00379118. [DOI] [PubMed] [Google Scholar]
- US Climate Data. 2016 from http://www.usclimatedata.com.
- Weatherhead PJ, Sperry JH, Carfagno GL, Blouin-Demers G. Latitudinal variation in thermal ecology of North American ratsnakes and its implications for the effect of climate warming on snakes. J Therm Biol. 2012;37:273–281. [Google Scholar]
- Zdanovich V. Alteration of thermoregulation behavior in juvenile fish in relation to satiation level. J Ichthyol. 2006;46:S188–S193. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.