Abstract
Red deer (Cervus elaphus) are hosts of liver fluke (Fasciola hepatica); yet, prevalence is rarely quantified in wild populations. Testing fresh samples from remote regions by faecal examination (FE) can be logistically challenging; hence, we appraise frozen storage and the use of a coproantigen ELISA (cELISA) for F. hepatica surveillance. We also present cELISA surveillance data for red deer from the Highlands of Scotland. Diagnoses in faecal samples (207 frozen, 146 fresh) were compared using a cELISA and by FE. For each storage method (frozen or fresh), agreement between the two diagnostics was estimated at individual and population levels, where population prevalence was stratified into cohorts (e.g., by sampling location). To approximate sensitivity and specificity, 65 post-slaughter whole liver examinations were used as a reference. At the individual level, FE and cELISA diagnoses agreed moderately (κfrozen = 0.46; κfresh = 0.51), a likely reflection of their underlying principles. At the population level, FE and cELISA cohort prevalence correlated strongly (Pearson’s R = 0.89, p < 0.0001), reflecting good agreement on relative differences between cohort prevalence. In frozen samples, prevalence by cELISA exceeded FE overall (42.8% vs. 25.8%) and in 9/12 cohorts, alluding to differences in sensitivity; though, in fresh samples, no significant difference was found. In 959 deer tested by cELISA across the Scottish Highlands, infection prevalence ranged from 9.6% to 53% by sampling location. We highlight two key advantages of cELISA over FE: i) the ability to store samples long term (frozen) without apparent loss in diagnostic power; and ii) reduced labour and the ability to process large batches. Further evaluation of cELISA sensitivity in red deer, where a range of fluke burdens can be obtained, is desirable. In the interim, the cELISA is a practicable diagnostic for F. hepatica surveillance in red deer, and its application here has revealed considerable geographic, temporal, sex and age related differences in F. hepatica prevalence in wild Scottish Highland red deer.
Introduction
Red deer (Cervus elaphus) are a recognised host of liver fluke (Fasciola hepatica) throughout Europe [1–5]. In contrast to domestic ruminants, in which disease monitoring is routine, apart from a handful of species, (namely, coypu (Myocastor coypus) [6,7], European hare (Lepus europaeus) [8,9], European rabbit (Oryctolagus cuniculus) [8], wild boar (Sus scrofa) [10] and roe deer (Capreolus capreolus) [11]), data concerning F. hepatica in wildlife is limited to incidental observations, which is the case for red deer in the Scottish Highlands [3]. In the UK as a whole, annual diagnostic rates of fasciolosis, the parasitic disease caused by Fasciola spp., have increased significantly in domestic sheep and cattle since the late 1990s [12] and are predicted to continue rising [13]; hence, surveillance of wild red deer in the Scottish Highlands at this moment, is desirable.
The role of wildlife as F. hepatica reservoirs (a population that harbours macro- or microparasites that can result in infection of other species or populations) is still the subject of some discussion. Typically, species are implicated following discovery of at least two of the following: i) high F. hepatica prevalence, ii) confirmation of viable eggs in host excreta, iii) close genetic relatedness between flukes collected from the suspected reservoir and another population; and, consideration of the maintenance of the F. hepatica life cycle. For example, in the northern Netherlands, F. hepatica is present in up to 41% of hares (L. europaeus); from whom, recovered fluke specimens belong to the same genetic clades as fluke recovered from cattle in the same region [9]. Similarly, F. hepatica prevalence of 11% and 40–90% egg viability, implicate wild boar (S. scrofa) as a likely reservoir to cattle in NW Spain [10].
The prevalence of F. hepatica in Scottish Highland red deer has not been objectively quantified. The most recent parasitological survey of Scottish deer (formalin archived tissue samples collected during 1991–1997) identified “non-specific parasite related changes” in the liver tissue of 10% of the population [14], perhaps an indication of F. hepatica prevalence; and, pertinent to the present study, significantly more animals with “other non-specific” signs of liver disease (though potentially also fluke related) were found in the Highlands than in any other region [14]. Moreover, within the Highland region, environmental variation is marked in terms of topography, geochemistry, climate and land use; all of which are associated with F. hepatica infection risk [15,16]. Therefore, owing to the lack of contemporary data for deer in the region and their unknown epidemiological role (potentially associated with hill sheep), one aim of this study was to quantify F. hepatica prevalence in wild red deer within the Highlands at the sub-regional hunting estate scale.
The presence of F. hepatica, established by faecal examination (FE) (often extended to faecal egg counts; FEC), requires samples to be processed fresh (i.e., prior to freezing or desiccation which may damage/deform eggs), sedimentation time, sample staining and slide preparation, and is therefore largely impractical to apply to wild deer in remote regions such as the Scottish Highlands. Alternatively, the F. hepatica coproantigen enzyme-linked immunosorbent assay (cELISA;[17]) may be more feasible, as it is based on detection of fluke excretory-secretory antigens, which remain stable when frozen [18,19]. The cELISA is also advantageous because it requires less processing time and facilitates batch testing. To date, the performance of the cELISA has shown considerable potential for sheep and cattle diagnostics [18,20–23], and in wild boar (S. scrofa) [10]; whereas in horses, the cELISA has been attributed only 9% sensitivity and has therefore been deemed unsuitable [22]. Here, the performance of the F. hepatica cELISA is compared with FE and whole liver examination (for fluke presence) in Scottish Highland red deer, considering both epidemiological and practical characteristics of the tests. In addition, we provide new data on the prevalence and distribution of liver fluke in red deer (n = 959) from Scottish Highland hunting estates.
Materials and Methods
Sampling took place between August 2012 and February 2014. All samples were collected by deer stalkers/gamekeepers on privately owned Scottish hunting estates (Aline, Alladale, Altnaharra, Applecross Trust, Ardnamurchan, Badanloch, Ben Loyal, Conaglen, North Harris Trust and Strathconon). The owners of these estates legally delegate their right to kill deer on their land to their employee deer stalkers/gamekeepers under the Deer Act 1991 and the Deer (Scotland) Act 1996; hence, permission to sample and indeed instruction to kill wild deer during this study was given by the land owner(s) to the head deer stalker on each estate—as such, no individual deer was killed specifically for the purposes of this research. The culling of deer is carried out during routine deer management; hence, samples constituted a by-product of this activity so did not require a licence under the Animals (Scientific Procedures) Act 1986. No animals were specifically killed for this study—as such, University ethics approval was also not required. Each deer was killed by shooting in accordance with the Deer (Firearms etc.)(Scotland) Order1985 and current “Best Practice Guidance” developed within Scotland’s deer management sector.
Faecal samples from red deer were collected into 50ml centrifuge tubes (Fisher Scientific, UK). Samples were collected directly from the rectum of 959 wild red deer that had been culled by deer stalkers working on nine estates within the Scottish Highlands; and, owing to constraints on sample volumes, 353 of these samples were used for the diagnostic method evaluation aspect of this study. Samples were taken during the annual red deer cull, which runs from 1st July to 20th October for males, and 21st November to 15th February for females. A subset of these 353 samples (n = 146) were immediately refrigerated following collection (not frozen) and analysed by FEC (within seven days)—the data from which were later converted to binary FE positives and negatives. During FEC, supernatants were also collected and frozen for subsequent cELISA analysis. The remaining samples (n = 207) were frozen at -20°C on the day of collection, and then defrosted prior to FEC analysis and supernatant preparation at a later date. In addition to faecal samples, whole livers were collected from 65 of the culled individuals on two estates (49 females, 17 from Badanloch, 32 from Altnaharra; and 16 males, all from Badanloch) and stored frozen; faecal samples from these 65 individuals form part of the aforementioned subset that were stored fresh. All faecal samples were accompanied by a datasheet containing information regarding sex, age category and spatial and temporal data. However, on 32 sampling occasions, datasheets were either not provided or did not include a date of cull; these represent 19 females (9 positive diagnoses by cELISA; 6 by FE; 11 positives in total) and 13 males (7 positive by cELISA; 4 by FE; 7 positives in total). Where temporal data were available, samples were categorised by month, year, sex and sampling estate.
Coprological methods
Coproantigen ELISA kits (specific to F. hepatica) were used to analyse faecal samples in accordance with the manufacturer’s guidelines for sheep faeces (BioX Diagnostics, Belgium). For supernatant preparation, faecal samples were homogenised with a stainless steel spatula and 0.50 ± 0.03g sub-samples were weighed into 12ml centrifuge tubes (round-bottomed, Greiner bio-one CELLSTAR, UK) to which 2ml of the kit’s dilution buffer was added. Each tube was then vortex mixed for 3s prior to centrifuging for 10 minutes at 1000g. Approximately 1ml of supernatant was extracted by pipette from each centrifuge tube. This was stored at -20°C in 2ml microfuge tubes (Eppendorf, Germany) until a cELISA kit plate could be filled. Eggs of F. hepatica were counted in faeces using a sedimentation technique [20].
Whole liver examination
Whole livers were defrosted for 24 hours prior to full visual examination. All livers were sliced into 1-2cm parallel strips with a scalpel [2]. Sliced liver was then gently squeezed to encourage fluke to slide out of bile ducts and parenchyma. In each burdened animal, there was a tendency for fluke to reside in groups within pockets of pale-coloured scarred tissue, rather than in the bile ducts. These pockets were found to contain between 1 and 13 fluke and a grey-coloured viscous fluid. Fluke from each liver were removed, rinsed with MilliQ® water and frozen at -20°C or preserved in 70% ethanol for future reference. Numbers of fluke heads (noted by the presence of a ventral sucker; S1 Fig) counted in each liver were recorded and animals were considered infected if at least one fluke was found.
Sensitivity and specificity
Whole liver examination was used as a concurrent reference standard against which to estimate sensitivity and specificity of the cELISA and FE methods. However, it is acknowledged that liver examination may be imperfect (i.e., not 100% sensitive) owing to potential losses of fluke specimens at the deer larder during carcass evisceration, or simply because fluke are missed during visual inspection. As such, our estimates of specificity are inherently negatively biased owing to apparent false (FE or cELISA) positives.
Nevertheless, liver examination is 100% specific; hence our estimates of cELISA and FE sensitivity are not biased—though we recognise that FE sensitivity estimates are related to patent infection only. Confidence intervals for sensitivity and specificity (i.e., proportions) (95% CI) were calculated using Eq 1.
| (1) |
For completeness, we estimate the sensitivity of the cELISA to patent infection (from FE positives) in frozen and fresh (where livers were not always available) samples. We do not use the FE negative animals for calculation of specificity, because the cELISA is designed to detect pre-patent as well as patent infection (even if debatable under field conditions [20]).
Statistical analyses
All statistical analyses were carried out using R [24] and RStudio [25]. At the population level, the correlation between estimated cohort (e.g., sex and location specific) prevalence of infection by the cELISA and the FE methods was examined using Pearson’s R coefficient using the R{cor.test} function [24]. Owing to repeated measures/correlated binary outcomes (i.e., a single subject is diagnosed by two or more of the diagnostics), a one-sided McNemar’s χ2 test for paired proportions (using the R {mcnemar.test} function) was used to identify whether differences in diagnostic outcomes of the FE and cELISA (as collated in 2 × 2 contingency tables) were significant.
The agreement between the three diagnostic tests was analysed separately for fresh and frozen samples at the individual and population levels. At the individual level, Cohen’s kappa (κ) was chosen to quantify the agreement between tests [26] (see S1 Text for further detail). In addition to provision of the kappa statistic, a bias effect (i.e., the difference between methods in terms of percentage of positive/negative diagnoses; apparent relative sensitivities) and a prevalence effect (i.e., the percentage of the sampled population diagnosed as infected) were quantified [27,28] (see S1 Text for further detail). For completeness, we also quote the maximum attainable kappa, proportion of observed agreement, po, and the proportion of observed positive ppos, and negative pneg, agreement. Also at the individual level, Spearman’s ρ coefficient was used to quantify the association between FEC eggs per gram (epg) and cELISA titres (ELISA units (EU) equivalent to % of positive reference standard titre).
Chi-square tests of independence were used to examine the associations between prevalence (estimated by cELISA alone) of F. hepatica infection and sampling season, sex and age class; whereby the {chisq.test} function in R [24] was used. Furthermore, significant differences at the 5% level in prevalence of F. hepatica infection between months, estates and age groups were identified using post hoc Tukey contrasts using the {glht} function in the {multcomp} package [29] applied to binomial (logit link) generalised linear models {glm} in R.
Results
Diagnostic agreement at the population and individual level
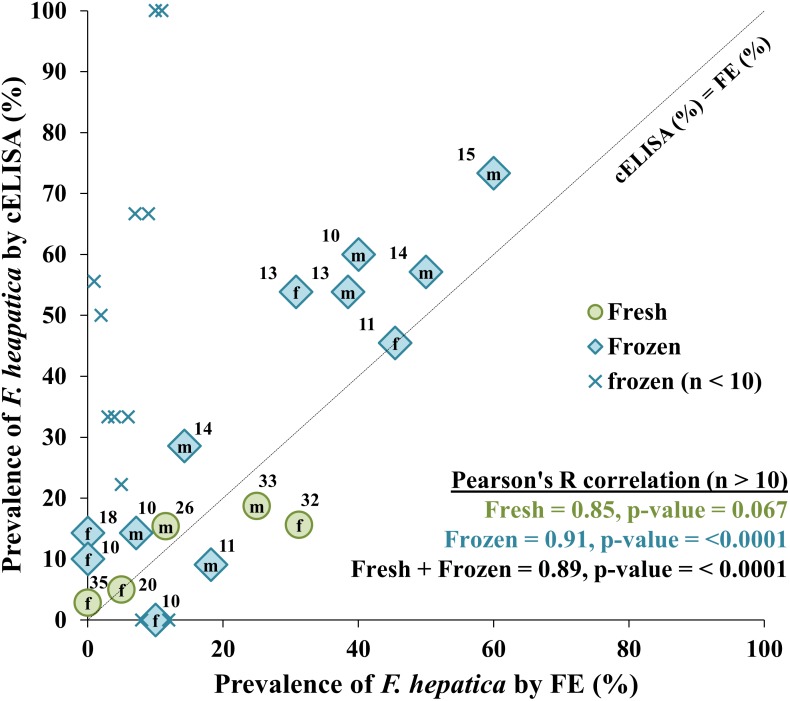
In 146 fresh faecal samples collected during 2013–14, prevalence of F. hepatica estimated by FE (12.3%) and cELISA (9.6%) did not differ significantly (McNemar’s χ2 = 0.64, df = 1, p-value = 0.42) (S1 Table); whereas in 159 frozen samples collected during 2012–13, prevalence estimated by cELISA (42.8%) was significantly greater than FE (25.8%) (McNemar’s χ2 = 16.5, df = 1, p-value <0.001) (S1 Table).When stratified by year, sex and month (S1 Table; where n > 10 per cohort), prevalence estimated by cELISA in frozen samples exceeded FE in 4/6 cohorts; whereas in fresh samples, prevalence estimated by cELISA only exceeded FE in 1/7 cohorts. When stratified by year, sex and estate (S2 Table; where n > 10 per cohort), prevalence in frozen samples estimated by cELISA exceeded FE in 9/12 cohorts, and estimates of prevalence by cELISA and FE were strongly correlated (Pearson’s R = 0.89, p < 0.0001) (Fig 1); whereas in fresh samples, prevalence estimated by cELISA (all 2013–14) only exceeded FE in 2/5 and the correlation between the cELISA and FE was not significant (Pearson’s R = 0.85, p < 0.067).
Fig 1. FE and cELISA estimated prevalence of F. hepatica infection (percentage of specific cohorts infected by sex (male, m; female, f) and sample storage method).
Cohorts were from nine wild red deer populations during two stalking seasons (2012–13 and 2013–14); cohort sample sizes are shown next to each point. See S2 Table for details of sampling sites and seasons to which these data relate.
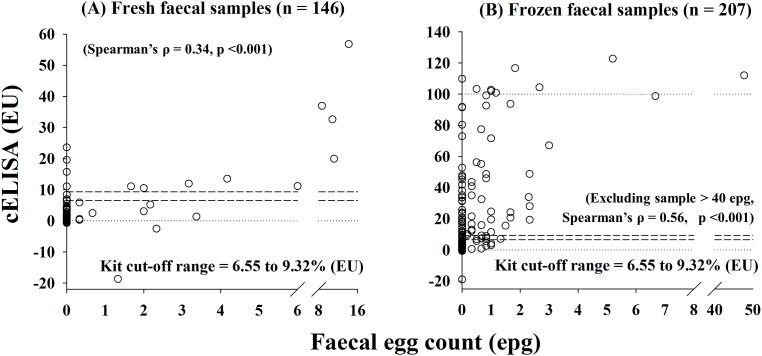
Agreement between the cELISA and the FE at the individual level was moderate (κfrozen = 0.46, κfresh = 0.51; Table 1) [30]). Of the 146 fresh faecal samples analysed, 14 were positive by the cELISA and 18 were positive by the FE method (23 positives in total)—in nine of these cases the techniques were in agreement (Table 1). Of the 207 frozen faecal samples analysed, 80 were positive by the cELISA and 52 were positive by the FE method (91 positives in total)—in 41 samples the techniques were in agreement (Table 1). In both fresh and frozen samples, there were significant correlations between FEC (epg) and cELISA (EU) (Spearman’s ρ = 0.34, p < 0.0001 for fresh; Spearman’s ρ = 0.56, p < 0.0001 for frozen) (Fig 2).
Table 1. Cross-tabulated comparison between the diagnostic outcomes of a cELISA (BioX, Belgium), FE and post mortem whole liver examination for F. hepatica infection using faecal samples collected from wild Scottish red deer (n = 353), stored fresh and frozen.
| Fresh faeces (n = 146) | Frozen faeces (n = 207) | ||||||
| FE | FE | ||||||
| Positive | Negative | Total | Positive | Negative | Total | ||
| cELISA | Positive | 9 (14) | 5 (0) | 14 | 41 (52) | 39 (28) | 80 |
| Negative | 9 (4) | 123 (128) | 132 | 11 (0) | 116 (127) | 127 | |
| Total | 18 | 128 | 146 | 52 | 155 | 207 | |
| Agreement | 9 | 123 | 132 | 41 | 116 | 157 | |
| Exp. by chance (nearest whole number) | 2 | 116 | 118 | 20 | 95 | 115 | |
| Kappa (max attainable) | 0.51 | (0.86) | 0.46 | (0.70) | |||
| Prop. agree (ppos, pneg) | 0.90 | (0.56, 0.95) | 0.76 | (0.62, 0.82) | |||
| Bias Index, BI | 0.03 | 0.14 | |||||
| Prevalence Index, PI | 0.78 | 0.36 | |||||
| Fresh faces and whole liver examination (n = 65) | |||||||
| cELISA | FE | ||||||
| Positive | Negative | Total | Positive | Negative | Total | ||
| Liver exam. | Positive | 6 (8) | 12 (10) | 18 | 9 (11) | 9 (7) | 18 |
| Negative | 2 (0) | 45 (47) | 47 | 2 (0) | 45 (47) | 47 | |
| Total | 8 | 57 | 65 | 11 | 54 | 65 | |
| Agreement | 6 | 45 | 51 | 9 | 45 | 54 | |
| Exp. by chance (nearest whole number) | 2 | 41 | 43 | 3 | 39 | 42 | |
| Kappa (max attainable) | 0.35 | (0.54) | 0.52 | (0.69) | |||
| Prop. agree (ppos, pneg) | 0.78 | (0.46, 0.87) | 0.83 | (0.62, 0.89) | |||
| Bias Index, BI | 0.15 | 0.11 | |||||
| Prevalence Index, PI | 0.60 | 0.55 | |||||
Maximum attainable kappa is obtained using the italicised numbers in parentheses (i.e., those numbers that would secure maximum agreement between the tests given the (fixed) marginal totals) [28].
Fig 2. Scatter plots comparing results of FEC and a commercial cELISA (Bio X, Belgium) for F. hepatica infection in faecal samples that were collected from wild Scottish red deer between 2012 and 2014 (n = 353).
Assays were carried out on samples that had been stored in two ways: (A) fresh (unfrozen) from the time of collection until time of testing (n = 146), and (B) frozen immediately after being collected from culled deer (n = 207). For the FEC test, results are recorded in eggs per gram of faeces (epg). For the cELISA, results are expressed in ELISA units (EU). Positive diagnosis by the cELISA was recorded for samples where results fell above a cut off derived using a positive reference standard.
Concurrent diagnoses by FE, cELISA and whole liver examination
Prior to slicing into 1-2cm parallel strips, initial visual inspection revealed obvious scarring in fluke infested tissue of the heaviest burdened livers (i.e., 4 or more fluke) and enabled targeted removal of several specimens. Eighteen of 65 examined livers were found to contain at least one mature (categorised by size; S1 Fig) liver fluke (range: one to 13; only one seemingly immature fluke was found and this was found alongside mature fluke); six were positive by cELISA, and nine were positive by FE (22 positives between the three tests, 14 positives between FE and cELISA, 20 positives between FE and liver, and 20 positives between cELISA and liver) (Table 1; see S3 Table for data stratified by month, sex and estate). Two cELISA positives (both FE negatives) were fluke-free using visual liver inspection, and, two FE positives (both cELISA negatives) were also fluke-free by visual liver inspection (Table 1). Liver examination diagnosed significantly more positives than the cELISA (McNemar’s χ2 = 5.79, df = 1, p-value = 0.016), but not more than FE (McNemar’s χ2 = 3.27, df = 1, p-value = 0.070)), and differences between prevalence estimated by FE and cELISA were not significant (McNemar’s χ2 = 0.44, df = 1, p-value = 0.51).
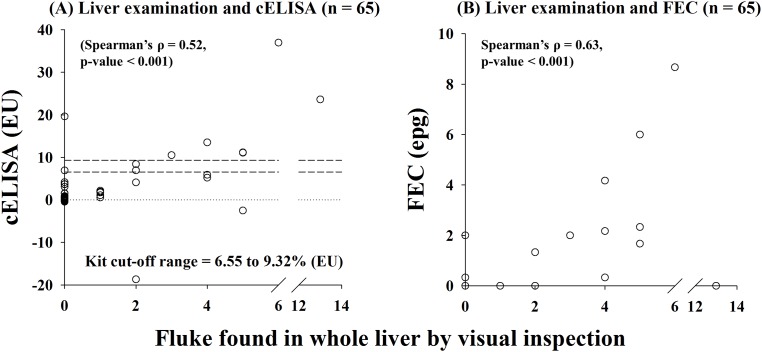
At the individual level, agreement between liver examination and cELISA was low (κcELISA = 0.35), and agreement between liver examination and FE was moderate (κFE = 0.52) (Table 1); whereas correlation between cELISA (EU) vs. number of fluke (Spearman’s ρ = 0.52, p < 0.001) was similar to the correlation between FEC (epg) vs. number of fluke (Spearman’s ρ = 0.63, p < 0.001) (Fig 3), and the correlation between cELISA and FEC was markedly weaker (Spearman’s ρ = 0.30, p = 0.014; S2 Fig).
Fig 3. Scatter plots comparing results of FEC and cELISA (BioX, Belgium) diagnostic tests for F. hepatica infection against concurrent visual inspection of whole livers from red deer for fluke.
Faecal samples and livers were collected from carcasses of wild Scottish red deer culled between 2012 and 2014 (n = 65). Livers were sliced and visually inspected for flukes. For the FEC test, results are recorded in eggs per gram of faeces (epg). For the cELISA, results are expressed in ELISA units (EU). Positive diagnosis by the cELISA was recorded for samples where results fell above a cut off derived using a positive reference standard.
Using the 18 known positives classified by liver examination, the sensitivity of the cELISA and FE were 33% (95% CI: 11%–55%) and 50% (95% CI: 32%–78%), respectively; and, there was no significant difference between them (McNemar’s χ2 = 0.8, df = 1, p-value = 0.37). Using the 47 apparent negatives, the specificity of the cELISA and the FE were both 96% (95% CI: 90%–100%).
Where whole livers were not available, 52 frozen faecal samples were identified as positive by FE, and 41 of these were positive by cELISA, indicating cELISA sensitivity to patent infection of 79% (95% CI 73%–84%). Similarly, of 18 fresh faecal samples (7 of which did not have an accompanying whole liver) found positive by FE, 9 were positive by cELISA, indicating cELISA sensitivity of 50% (95% CI: 32%–78%).
Application of the cELISA to quantify F. hepatica prevalence in Scottish Highland deer
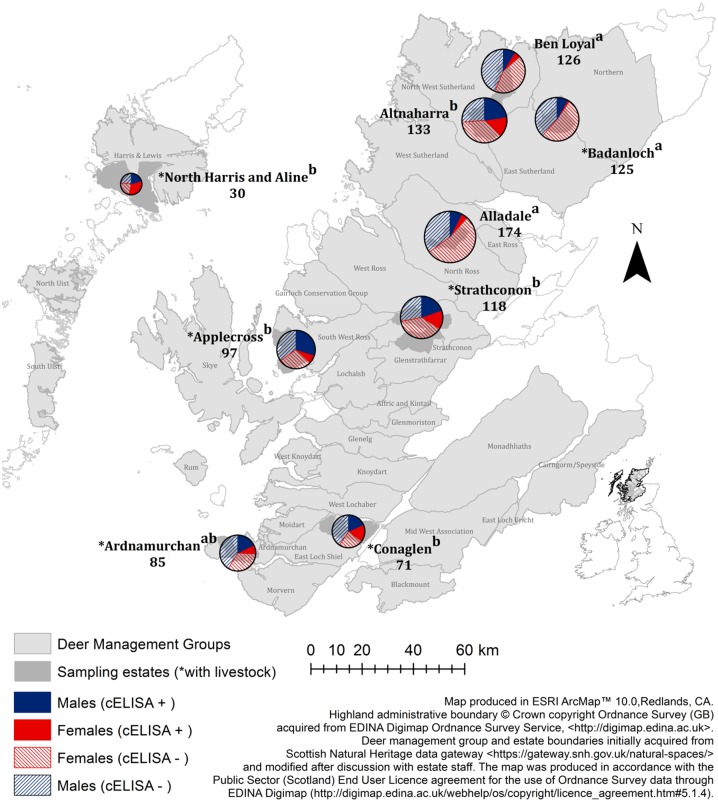
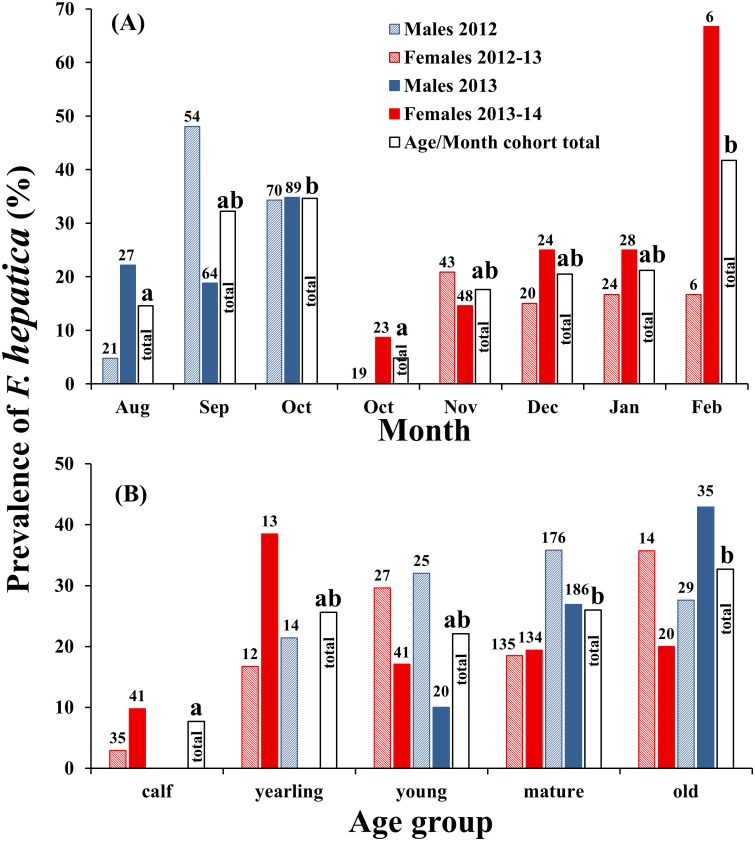
Marked geographic differences in F. hepatica prevalence were found between estates (Fig 4 and S4 Table). Furthermore, overall prevalence in males was greater than in females (male prevalence 30.8%, female prevalence 18.4%; χ2 = 19.049, df = 1, p-value < 0.0001), and prevalence of F. hepatica increased with increasing deer age class and throughout the stalking (sampling) seasons (Fig 5).
Fig 4. Prevalence of F. hepatica in red deer in the Scottish Highlands (n = 959).
Pie charts illustrate the proportions of infected and non-infected male and female deer in each estate. The size of each pie chart correlates with the number of samples collected on each estate. Overall differences in prevalence between estates are denoted by compact letter descriptors (calculated using Tukey Contrasts in the {glht} function in R), whereby estates that share at least one letter do not have significant differences (at the 5% level) in prevalence. See S4 Table for prevalence stratified by sex and sampling seasons. Livestock presence (*) is noted for estates that contained sheep and/or cattle. All (*) denoted estates had hill sheep, and Applecross, Conaglen and Strathconon also had cattle.
Fig 5. Prevalence of F. hepatica estimated by cELISA in red deer culled during the 2012–13 and 2013–14 stalking seasons in relation to: (A) Month (mature animals only; nmale = 325; nfemale = 241; ntotal = 566); and (B) Age group (nmale = 485; nfemale = 472; ntotal = 957).
Significant differences at the 5% level (calculated using Tukey Contrasts in the {glht} function in R) are shown by compact letter descriptors; months/ages sharing a letter did not have significantly different prevalences. For clarity, data for two male calves (one positive in 2012; one negative in 2013) are not illustrated in (B), but were included in the statistical analyses.
Discussion
This study has demonstrated that the F. hepatica cELISA is ostensibly more practicable for surveillance in wild red deer than the alternative, FE; and, though cELISA sensitivity to pre-patent infection remains unclear, we have found similar sensitivity to patent infection to that reported in sheep and cattle [17,22], and negligible uncertainty surrounding its well-recognised high specificity [17,31]. Our data has further revealed marked geographic, temporal and age related variation in the prevalence of F. hepatica in wild red deer in the Scottish Highlands.
Diagnostic evaluation
Practicality of sample handling is a key consideration when gathering wildlife surveillance data; hence, freezing faecal samples was our chief approach because it was straightforward for gamekeepers in remote areas of the Highlands to collect and store deer faeces. However, the standard diagnostic for F. hepatica in deer, the FE, is intended for fresh faeces; thus, fresh samples were collected during 2013–14 for comparison. Freezing of faeces destined for FE is advised against because it significantly reduces egg detectability for nematodes such as Haemonchus contortus in sheep [32] and in deer [33], though it does not necessarily reduce detectability for all trematodes (e.g., Dicrocoelium dendriticum in sheep [34]). Here, we were able to identify recognisable F. hepatica eggs in frozen samples (S3 Fig as an example), though egg collapse (and resultant reduction in detection) may well have contributed to disparities in prevalence estimates between cELISA and FE diagnoses (S1 and S2 Tables). In addition, F. hepatica excretory-secretory antigens (when frozen on the day of collection) appear to remain stable for at least a year in frozen red deer faeces, as reported (though for a shorter timespan) in domestic ruminants [18,19]; however, it is unclear for how long antigens remain stable in fresh samples. Owing to our sampling design, up to seven days post-sampling/pre-shipping delay (for fresh samples) left potential for antigen degradation—perhaps reducing detectability of already low burdens (Fig 3), as reported in human faeces [35].
To assess the agreement between the cELISA and the FE, we examined individual level paired diagnoses, and population level prevalence estimates. At the individual level, we noted a high proportion of agreement between the cELISA and FE, but the low underlying prevalence in fresh samples reduced the potential for agreement beyond chance, resulting in moderate kappa (Table 1). In frozen samples, underlying prevalence was greater, indicating that the moderate agreement between the cELISA and FE was more likely a consequence of true disparities in diagnoses—perhaps caused by egg collapse. To our knowledge, agreement (estimated by kappa) has not been reported in the peer-reviewed literature for the F. hepatica cELISA and FE, and instead diagnostic performance is compared in terms of population prevalence; e.g., percent positive samples based on FE, serum-antibody and copro-antigen ELISAs [36]. Nevertheless, we can refer to publically available data for FE and cELISA in sheep naturally exposed to F. hepatica metacercariae [37]. These data show stronger agreement (κ = 0.83) along with generally higher egg counts (mean 12.5 epg) than observed in our deer study. Moreover, given the median FEC that corresponded with positive cELISA diagnoses in the sheep was 11.8 epg, strong agreement is not surprising. In contrast, we observed excess of 6 epg in only five of 146 fresh and two of 207 frozen deer faecal samples, likely reducing the potential for concurrent positive cELISA diagnoses (at least in fresh samples; Fig 2).
In terms of population level agreement, cELISA and FE prevalence were strongly correlated (Fig 1), but the cELISA regularly diagnosed greater prevalence than the FE, which (assuming both tests are highly specific) alluded to greater sensitivity of the cELISA. To further explore these diagnostic parameters, we estimated sensitivity and specificity of both tests against our liver examination reference standard (limited to fresh faecal samples); and, indeed, the specificity of both methods was very high, but there was no difference in sensitivity.
In frozen samples, we had no concurrent liver reference standard against which to estimate cELISA and FE sensitivity and specificity; nevertheless, as we were concerned primarily with the suitability of the cELISA for surveillance, its sensitivity based on patent infection was similar to that in sheep and cattle with 1–2 fluke [17,22], underlining its suitability to wild deer surveillance based on frozen samples. If indeed an improvement on this sensitivity were desired, it may be worth investigating the use of a customised cut-off of the cELISA (though it would be challenging at best to obtain known non-infected wild red deer), or (more realistically) modifying laboratory procedures (e.g., increasing sample incubation time), or both [18,22].
Finally, in terms of diagnostic evaluation, we considered latent class models (LCM) as described by Hui and Walter [38], but our data did not meet the assumption of independent errors; i.e., the FE, liver examination and cELISA all work on the principal of detecting parasites, thus the higher the fluke burden, the more detectable they become in faeces (higher antigen concentrations, more eggs [36]) and livers (Figs 2 and 3). An alternative approach of Bayesian LCM (where the independent errors assumption may be relaxed [39]) was also considered, but was deemed inappropriate because we had insufficient knowledge of FE or liver examination sensitivity or underlying population prevalence in the Highland deer population; hence, we could not specify enough parameter distributions (independent of our data) a priori, which is required for such an approach. Lastly, we note that the cELISA is reported to detect immature fluke [17] (pre-patent infection) (though this has not been demonstrated in the field [20]), whereas FE cannot. If positive cELISA diagnoses in deer are indeed attributed to pre-patent infection, then the diagnostics are not designed to detect the same disease state, which itself is incompatible with the assumption specified by Hui and Walter [38]—that the tests should be conditionally independent given the true (but latent) disease state.
We note some peculiarities of our results and potential limitations of the diagnostics. Firstly, the two cELISA positives (both FE negatives) that were fluke-free by visual liver inspection and two FE positives (both cELISA negatives) also fluke-free by visual liver inspection are potentially a consequence of imperfect visual liver inspection, or, loss of fluke during larder collection of whole livers (Table 1 and Fig 3). Indeed, fluke can be lost in sheep at post-mortem as a consequence of removal of the gall bladder (which provides a physical obstacle to escaping fluke); red deer on the other hand do not have a gall bladder, so removal of whole liver may lead to loss of mature fluke directly out of the large bile duct. Alternatively, the FE disparities could be explained by egg ingestion and subsequent passage in the faeces of non-infected deer—it is worth noting that these two FE positives (liver examination negatives) were females, and female herds typically “heft” to the same areas in which they are born—perhaps making them more likely to ingest eggs. In addition, there is potential for misidentification of rumen fluke (Calicophoron daubneyi) eggs as F. hepatica eggs, though rumen fluke infection in wild deer has only recently been reported in the Republic of Ireland [4], and there have been no reported cases in wild Scottish red deer. In terms of the cELISA, the positive value that falls just above the 6.55% (EU) cut-off line (where no flukes were found) could be a consequence of the (unknown) variation of cELISA titres for samples collected from uninfected red deer; i.e., it may be a false positive. This may be a consequence of the manufacturer’s cut-off value, which is calculated as a percentage of the positive reference antigen supplied with the kit, so does not take titre variation of truly negative samples into account. The positive cELISA titre that falls at 20 EU (where no flukes were found) is perhaps a consequence of cross-contamination during sample handling and/or cross-reactivity with other molecules or antigens present in faeces, though this has not been observed where deliberately explored with other fluke species; e.g., Dicrocoelium dentriticum and Paramphistomum cervi [17], and Calicophoron daubneyi [31].
A peculiarity of F. hepatica in deer, which may explain the relatively low sensitivities of cELISA and FE to the observed burdens, relates to the way in which fluke inhabit deer livers. Here, it was not clear whether the “pockets” containing fluke ran unobstructed to the bile ducts; if obstructed, there would be an incomplete path for eggs and antigens to enter the digestive system, which would impair detection. Finally, red deer have no gall bladder (unlike sheep, for which the kit is designed), so eggs and antigens theoretically form a constant trickle into the digestive system and do not pool in an intermittently evacuated reservoir from which antigen concentration may (in sheep) be amplified; thus, only infection with burdens in excess of six fluke may reliably detected by cELISA.
F. hepatica prevalence in wild Scottish red deer
To our knowledge, this is the first Scottish Highland regional survey of F. hepatica in wild red deer, though our estimates of prevalence fall with the range of sub-regional European studies; e.g., in Spain (34% by serum ELISA and 7% at necropsy) [2] and in Belarus (33% at necropsy) [5].
Greater F. hepatica prevalence was observed in males than in female; reflecting male-biased parasitism observed in other ungulates (e.g., gastrointestinal worm burden attributed to stress hormones in chamois (Rupicapra rupicapra rupicapra) [40], and rumen fluke prevalence in cattle [41]). We eliminated the potentially confounding factor of age by considering only mature animals, though the observed sex bias remains entangled with temporal effects, as males are harvested earlier (August—October) than females (October—February). We speculate that males in our study would have had higher daily food intake owing to the disparity in size between the sexes; hence, males would have a higher probability of eating infective metacercariae than females.
Prevalence of F. hepatica also increased as both male and female stalking seasons progressed (Fig 5). Such a temporal signal is inherently related to the parasite life cycle, but it is difficult to disentangle this signal from the unknown longevity of F. hepatica in red deer. With this in mind we looked for an overall age-related increase in prevalence and a temporal increase in prevalence in mature deer only. Interestingly, the age related increase in prevalence was not significant between yearling, young, mature and old animals (Fig 5), perhaps suggesting that the longevity of fluke in deer may not be similar to sheep (5 + years) [42] and goats (11 + years) [43]; indeed, the marked increase in prevalence between the beginning and end of both the male and female stalking seasons suggests that fluke prevalence is indicative of annual reinfection and points to a perhaps shorter and more similar longevity to that in cattle (26 + months) [44]. The liver tissue response in red deer associated with F. hepatica has not been extensively documented; however, where reported, it is characterised by thickened cyst (i.e., “pockets” as observed in our study) walls, though there is not the calcification that is typically observed in cattle [45]. Further complicating matters, if fluke survive for more than a year inside the final deer host, and considering that wild red deer, unlike livestock, are not treated with flukicides, infections detected early in the stalking season could represent persistent infections from the previous calendar years, or new infection sustained in the current calendar year.
This study is the first to quantify the extent of geographic variation in F. hepatica prevalence in wild deer in the Scottish Highland region. Here, we have observed low F. hepatica prevalence where livestock are not present and high prevalence in the presence of hill sheep (and three estates with small numbers of cattle) (Fig 4). Interestingly, there are two notable exceptions to this: i) low prevalence was observed at Badanloch, where there is an extensive hill sheep farm, and ii) high prevalence was observed in Altnaharra, where no livestock were present. In addition, the marked disparity in prevalence between Altnaharra and its neighbouring estate, Ben Loyal, reflects the sub-regional (post code scale) variation in prevalence that has been observed in livestock in England and Wales [46]. With these observations in mind, sub-estate scale (i.e., deer home-range) environmental variation associated with probability of infection remains to be explored.
Conclusions
We have highlighted two advantages of cELISA over FE in terms of F. hepatica surveillance in red deer in a remote region: i) owing to its propensity to diagnose greater prevalence than FE in frozen sample cohorts (and its apparent high specificity), the cELISA has seemingly greater sensitivity; and ii) reduced labour and the ability to process large batches. We acknowledge that the lack of true (known) negative individuals studied here (typical of a study involving wild populations) and the lack of a gold-standard diagnostic test made traditional evaluation of the cELISA’s sensitivity and specificity imperfect, but, emphasise that even our conservative estimates of test parameters support the use of the cELISA for wild red deer surveillance. For completeness, a larger, focussed evaluation of cELISA sensitivity in red deer is desirable. In the meantime, our observation of geographic variation in particular highlights that further research into F. hepatica and the factors associated with wild deer infection is warranted. We advocate further application of cELISA to wild red deer, and are intrigued as to its potential application to other potentially important wild liver fluke hosts such as other cervids or even leporids.
Supporting Information
Note that based on size (scale centimetres), these fluke were considered mature, and therefore evidence of patent infection. Where fluke segments were found during liver examination, only heads were counted; identified by the presence of ventral suckers as highlighted.
(TIF)
Samples and livers were collected from carcasses of wild Scottish red deer culled between 2012 and 2014. For the FEC test, results are recorded in eggs per gram of faeces (epg). For the cELISA, results are expressed in ELISA units (EU). Positive diagnosis by the cELISA was recorded for samples where results fell above a cut off derived using a positive reference standard.
(TIF)
The other faecal matter visible in this image is counter-stained with 1% methylene blue.
(TIF)
Fresh samples in bold.
(DOCX)
Fresh samples are highlighted in bold.
(DOCX)
Significant differences (identified using Chi-square test of independence and further explored using Tukey contrasts) are indicated by compact letter descriptors; diagnostic methods sharing a letter were not significantly different from each other.
(DOCX)
Significant differences (at the 5% significance level, identified using Chi-square test of independence and further explored using Tukey contrasts) between estates are indicated by compact letter descriptors, whereby estates that share a letter did not have significantly different prevalences.
(DOCX)
An explanation of the kappa statistic and its associated equations and interpretational parameters.
(DOCX)
Acknowledgments
We would like to thank the game-keeping department of North Highland College and the deer stalkers of: Alladale, Altnaharra, Aline, Applecross, Ardnamurchan, Badanloch, Ben Loyal, Conaglen, North Harris Trust and Strathconon estates for their diligent sample collection, without whom, this study would not have been possible. We would also like to thank Daniel Haydon and Kenny Black for their constructive comments on the study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
ASF’s studentship was funded by the Highlands and Islands Scotland European Social Fund 2007-2013 programme. Funding for contributions by GM, PJS and RNZ was obtained from the Scottish Government's Rural and Environment Science and Analytical Services Division. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alasaad S, Huang CQ, Li QY, Granados JE, García-Romero C, Pérez JM, et al. Characterization of Fasciola samples from different host species and geographical localities in Spain by sequences of internal transcribed spacers of rDNA. Parasitol Res. 2007;101: 1245–1250. 10.1007/s00436-007-0628-2 [DOI] [PubMed] [Google Scholar]
- 2.Arias MS, Martínez-Carrasco C, León-Vizcaíno L, Paz-Silva A, Díez-Baños P, Morrondo P, et al. Detection of Antibodies In Wild Ruminants To Evaluate Exposure To Liver Trematodes. J Parasitol. 2012;98: 754–759. 10.1645/GE-2804.1 [DOI] [PubMed] [Google Scholar]
- 3.Mitchell B, McCowan D, Parish T. Performance and population dynamics in relation to management of red deer Cervus elaphus at Glenfeshie, inverness-shire, Scotland. Biol Conserv. 1986;37: 237–267. 10.1016/0006-3207(86)90084-4 [DOI] [Google Scholar]
- 4.O’Toole A, Browne JA, Hogan S, Bassière T, DeWaal T, Mulcahy G, et al. Identity of rumen fluke in deer. Parasitol Res. 2014; 10.1007/s00436-014-4078-3 [DOI] [PubMed] [Google Scholar]
- 5.Shimalov VV, Shimalov VT. Findings of Fasciola hepatica Linnaeus 1758, in wild animals in Belorussian Polesye. Parasitol Res. 2000;86: 342–342. 10.1007/s004360050056 [DOI] [PubMed] [Google Scholar]
- 6.Issia L, Pietrokovsky S, Sousa-Figueiredo J, Stothard JR, Wisnivesky-Colli C. Fasciola hepatica infections in livestock flock, guanacos and coypus in two wildlife reserves in Argentina. Vet Parasitol. 2009;165: 341–344. 10.1016/j.vetpar.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 7.Ménard A, Agoulon A, L’Hostis M, Rondelaud D, Collard S, Chauvin A. Myocastor coypus as a reservoir host of Fasciola hepatica in France. Vet Res. 2001;32: 499–508. [DOI] [PubMed] [Google Scholar]
- 8.Rondelaud D, Vignoles P, Abrous M, Dreyfuss G. The definitive and intermediate hosts of Fasciola hepatica in the natural watercress beds in central France. Parasitol Res. 2001;87: 475–478. 10.1007/s004360100385 [DOI] [PubMed] [Google Scholar]
- 9.Walker SM, Johnston C, Hoey EM, Fairweather I, Borgsteede FHM, Gaasenbeek CPH, et al. Potential role of hares in the spread of liver fluke in the Netherlands. Vet Parasitol. 2011;177: 179–181. 10.1016/j.vetpar.2010.11.043 [DOI] [PubMed] [Google Scholar]
- 10.Mezo M, González-Warleta M, Castro-Hermida JA, Manga-González MY, Peixoto R, Mas-Coma S, et al. The wild boar (Sus scrofa Linnaeus, 1758) as secondary reservoir of Fasciola hepatica in Galicia (NW Spain). Vet Parasitol. 2013;198: 274–283. 10.1016/j.vetpar.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 11.Arias MS, Piñeiro P, Sánchez-Andrade R, Suárez JL, Hillyer GV, Díez-Baños P, et al. Relationship between exposure to Fasciola hepatica in roe deer (Capreolus capreolus) and cattle extensively reared in an endemic area. Res Vet Sci. 2013;95: 1031–1035. 10.1016/j.rvsc.2013.07.027 [DOI] [PubMed] [Google Scholar]
- 12.van Dijk J, Sargison ND, Kenyon F, Skuce PJ. Climate change and infectious disease: helminthological challenges to farmed ruminants in temperate regions. animal. 2010;4: 377 10.1017/S1751731109990991 [DOI] [PubMed] [Google Scholar]
- 13.Fox NJ, White PCL, McClean CJ, Marion G, Evans A, Hutchings MR. Predicting Impacts of Climate Change on Fasciola hepatica Risk. PLoS ONE. 2011;6: e16126 10.1371/journal.pone.0016126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Böhm M, White PCL, Daniels MJ, Allcroft DJ, Munro R, Hutchings MR. The health of wild red and sika deer in Scotland: An analysis of key endoparasites and recommendations for monitoring disease. Vet J. 2006;171: 287–294. 10.1016/j.tvjl.2004.10.020 [DOI] [PubMed] [Google Scholar]
- 15.Bennema SC, Ducheyne E, Vercruysse J, Claerebout E, Hendrickx G, Charlier J. Relative importance of management, meteorological and environmental factors in the spatial distribution of Fasciola hepatica in dairy cattle in a temperate climate zone. Int J Parasitol. 2011;41: 225–233. 10.1016/j.ijpara.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 16.McCann CM, Baylis M, Williams DJL. The development of linear regression models using environmental variables to explain the spatial distribution of Fasciola hepatica infection in dairy herds in England and Wales. Int J Parasitol. 2010;40: 1021–1028. 10.1016/j.ijpara.2010.02.009 [DOI] [PubMed] [Google Scholar]
- 17.Mezo M, González-Warleta M, Carro C, Ubeira FM. An ultrasensitive capture elisa for detection of fasciola hepatica coproantigens in sheep and cattle using a new monoclonal antibody (mm3). J Parasitol. 2004;90: 845–852. 10.1645/GE-192R [DOI] [PubMed] [Google Scholar]
- 18.Brockwell YM, Spithill TW, Anderson GR, Grillo V, Sangster NC. Comparative kinetics of serological and coproantigen ELISA and faecal egg count in cattle experimentally infected with Fasciola hepatica and following treatment with triclabendazole. Vet Parasitol. 2013;196: 417–426. 10.1016/j.vetpar.2013.04.012 [DOI] [PubMed] [Google Scholar]
- 19.Flanagan AM, Edgar HWJ, Forster F, Gordon A, Hanna REB, McCoy M, et al. Standardisation of a coproantigen reduction test (CRT) protocol for the diagnosis of resistance to triclabendazole in Fasciola hepatica. Vet Parasitol. 2011;176: 34–42. 10.1016/j.vetpar.2010.10.037 [DOI] [PubMed] [Google Scholar]
- 20.Gordon DK, Zadoks RN, Stevenson H, Sargison ND, Skuce PJ. On farm evaluation of the coproantigen ELISA and coproantigen reduction test in Scottish sheep naturally infected with Fasciola hepatica. Vet Parasitol. 2012;187: 436–444. 10.1016/j.vetpar.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 21.Mezo M, González-Warleta M, Ubeira FM. The use of mm3 monoclonal antibodies for the early immunodiagnosis of ovine fascioliasis. J Parasitol. 2007;93: 65–72. 10.1645/GE-925R.1 [DOI] [PubMed] [Google Scholar]
- 22.Palmer D, Lyon J, Palmer M, Forshaw D. Evaluation of a copro-antigen ELISA to detect Fasciola hepatica infection in sheep, cattle and horses. Aust Vet J. 2014;92: 357–361. 10.1111/avj.12224 [DOI] [PubMed] [Google Scholar]
- 23.Valero MA, Ubeira FM, Khoubbane M, Artigas P, Muiño L, Mezo M, et al. MM3-ELISA evaluation of coproantigen release and serum antibody production in sheep experimentally infected with Fasciola hepatica and F. gigantica. Vet Parasitol. 2009;159: 77–81. 10.1016/j.vetpar.2008.10.014 [DOI] [PubMed] [Google Scholar]
- 24.R Core Team. R: A language and environment for statistical computing. [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2015. Available: https://www.R-project.org/ [Google Scholar]
- 25.RStudio Team. RStudio: Integrated Development for R. [Internet]. Boston, MA; 2015. Available: http://www.rstudio.com/ [Google Scholar]
- 26.Cohen J. A Coefficient of Agreement for Nominal Scales. Educ Psychol Meas. 1960;20: 37–46. 10.1177/001316446002000104 [DOI] [Google Scholar]
- 27.Byrt T, Bishop J, Carlin JB. Bias, prevalence and kappa. J Clin Epidemiol. 1993;46: 423–429. 10.1016/0895-4356(93)90018-V [DOI] [PubMed] [Google Scholar]
- 28.Sim J, Wright CC. The Kappa Statistic in Reliability Studies: Use, Interpretation, and Sample Size Requirements. Phys Ther. 2005;85: 257–268. [PubMed] [Google Scholar]
- 29.Hothorn T, Bretz F, Westfall P. Simultaneous Inference in General Parametric Models. Biom J. 2008;50: 346–363. 10.1002/bimj.200810425 [DOI] [PubMed] [Google Scholar]
- 30.Landis JR, Koch GG. The Measurement of Observer Agreement for Categorical Data. Biometrics. 1977;33: 159–174. 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 31.Gordon DK, Roberts LCP, Lean N, Zadoks RN, Sargison ND, Skuce PJ. Identification of the rumen fluke, Calicophoron daubneyi, in GB livestock: possible implications for liver fluke diagnosis. Vet Parasitol. 2013;195: 65–71. 10.1016/j.vetpar.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 32.Van Wyk JA, Van Wyk L. Freezing of sheep faeces invalidates Haemonchus contortus faecal egg counts by the McMaster technique. Onderstepoort J Vet Res. 2002;69: 299–304. [PubMed] [Google Scholar]
- 33.Foreyt WJ. Recovery of nematode eggs and larvae in deer: evaluation of fecal preservation methods. J Am Vet Med Assoc. 1986;189: 1065–1067. [PubMed] [Google Scholar]
- 34.Rinaldi L, Coles GC, Maurelli MP, Musella V, Cringoli G. Calibration and diagnostic accuracy of simple flotation, McMaster and FLOTAC for parasite egg counts in sheep. Vet Parasitol. 2011;177: 345–352. 10.1016/j.vetpar.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 35.Ubeira FM, Muiño L, Valero MA, Periago MV, Pérez-Crespo I, Mezo M, et al. MM3-ELISA detection of Fasciola hepatica coproantigens in preserved human stool samples. Am J Trop Med Hyg. 2009;81: 156–162. [PubMed] [Google Scholar]
- 36.Mezo M, González-Warleta M, Castro-Hermida JA, Muiño L, Ubeira FM. Field evaluation of the MM3-SERO ELISA for detection of anti-Fasciola IgG antibodies in milk samples from individual cows and bulk milk tanks. Parasitol Int. 2010;59: 610–615. 10.1016/j.parint.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 37.Gordon-Gibbs D. Fasciola hepatica infection in sheep: Current and novel diagnostic tests [Internet]. Doctoral Thesis, University of Edinburgh. 2014. Available: https://www.era.lib.ed.ac.uk/bitstream/handle/1842/15875/Gordon-Gibbs2015.pdf?sequence=2&isAllowed=y
- 38.Hui SL, Walter SD. Estimating the Error Rates of Diagnostic Tests. Biometrics. 1980;36: 167–171. 10.2307/2530508 [DOI] [PubMed] [Google Scholar]
- 39.Branscum AJ, Gardner IA, Johnson WO. Estimation of diagnostic-test sensitivity and specificity through Bayesian modeling. Prev Vet Med. 2005;68: 145–163. 10.1016/j.prevetmed.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 40.Hoby S, Schwarzenberger F, Doherr MG, Robert N, Walzer C. Steroid hormone related male biased parasitism in chamois, Rupicapra rupicapra rupicapra. Vet Parasitol. 2006;138: 337–348. 10.1016/j.vetpar.2006.01.028 [DOI] [PubMed] [Google Scholar]
- 41.Toolan DP, Mitchell G, Searle K, Sheehan M, Skuce PJ, Zadoks RN. Bovine and ovine rumen fluke in Ireland—Prevalence, risk factors and species identity based on passive veterinary surveillance and abattoir findings. Vet Parasitol. 2015;212: 168–174. 10.1016/j.vetpar.2015.07.040 [DOI] [PubMed] [Google Scholar]
- 42.Durbin CG. Longevity of the liver fluke, Fasciola sp. in sheep. Proc Helminthol Soc Wash. 1952;19: 120. [Google Scholar]
- 43.Leiper JWG. The longevity of Fasciola hepatica. J Helminthol. 1938;16: 173–176. 10.1017/S0022149X00018551 [DOI] [Google Scholar]
- 44.Ross JG. The life span of Fasciola hepatica in cattle. Vet Rec. 1968;82: 587–589. [Google Scholar]
- 45.Barth D, Schaich K. Untersuchungen zur experimentellen Fasciologse bei Reh-(Capreolus capreolus) und Rotwild (Cervus elaphus). Z Für Jagdwiss. 1973;19: 183–197. 10.1007/BF01904174 [DOI] [Google Scholar]
- 46.McCann CM, Baylis M, Williams DJL. Seroprevalence and spatial distribution of Fasciola hepatica-infected dairy herds in England and Wales. Vet Rec. 2010;166: 612–617. 10.1136/vr.b4836 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note that based on size (scale centimetres), these fluke were considered mature, and therefore evidence of patent infection. Where fluke segments were found during liver examination, only heads were counted; identified by the presence of ventral suckers as highlighted.
(TIF)
Samples and livers were collected from carcasses of wild Scottish red deer culled between 2012 and 2014. For the FEC test, results are recorded in eggs per gram of faeces (epg). For the cELISA, results are expressed in ELISA units (EU). Positive diagnosis by the cELISA was recorded for samples where results fell above a cut off derived using a positive reference standard.
(TIF)
The other faecal matter visible in this image is counter-stained with 1% methylene blue.
(TIF)
Fresh samples in bold.
(DOCX)
Fresh samples are highlighted in bold.
(DOCX)
Significant differences (identified using Chi-square test of independence and further explored using Tukey contrasts) are indicated by compact letter descriptors; diagnostic methods sharing a letter were not significantly different from each other.
(DOCX)
Significant differences (at the 5% significance level, identified using Chi-square test of independence and further explored using Tukey contrasts) between estates are indicated by compact letter descriptors, whereby estates that share a letter did not have significantly different prevalences.
(DOCX)
An explanation of the kappa statistic and its associated equations and interpretational parameters.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.