Abstract
Purpose
The purpose of this randomized trial was to evaluate the efficacy of the Mindfulness-Based Stress Reduction for Breast Cancer (MBSR[BC]) program in improving psychological and physical symptoms and quality of life among breast cancer survivors (BCSs) who completed treatment. Outcomes were assessed immediately after 6 weeks of MBSR(BC) training and 6 weeks later to test efficacy over an extended timeframe.
Patients and Methods
A total of 322 BCSs were randomly assigned to either a 6-week MBSR(BC) program (n = 155) or a usual care group (n = 167). Psychological (depression, anxiety, stress, and fear of recurrence) and physical symptoms (fatigue and pain) and quality of life (as related to health) were assessed at baseline and at 6 and 12 weeks. Linear mixed models were used to assess MBSR(BC) effects over time, and participant characteristics at baseline were also tested as moderators of MBSR(BC) effects.
Results
Results demonstrated extended improvement for the MBSR(BC) group compared with usual care in both psychological symptoms of anxiety, fear of recurrence overall, and fear of recurrence problems and physical symptoms of fatigue severity and fatigue interference (P < .01). Overall effect sizes were largest for fear of recurrence problems (d = 0.35) and fatigue severity (d = 0.27). Moderation effects showed BCSs with the highest levels of stress at baseline experienced the greatest benefit from MBSR(BC).
Conclusion
The MBSR(BC) program significantly improved a broad range of symptoms among BCSs up to 6 weeks after MBSR(BC) training, with generally small to moderate overall effect sizes.
INTRODUCTION
Approximately 14.5 million cancer survivors are living in the United States, with an estimated increase to 19 million by 2024,1 and 41% are breast cancer (BC) survivors (BCSs).1 Treatments have increased survival rates up to 100% for those with stage 0 to I, 93% for those with stage II, and 72% for those with stage III disease.2
Although BCSs are living longer, they often experience late effects from either the disease and/or treatment, which may affect daily functioning and quality of life (QOL).3,4 BCSs are often unprepared for the emotional trauma associated with diagnosis and treatment; the immediate and long-term sequalae often resulting in depression, anxiety, sleep disturbances,5-8 and fears of recurrence (FORs); or the physical problems of pain and fatigue.6,7,9-14 During the period when BCSs transition to treatment completion, worries and FORs often accompany physical symptoms,15 placing BCSs at higher risk for anxiety and depression secondary to multiple other stressors.16 Frequently, psychological and physical symptoms overlap, exacerbating one another and affecting QOL for many years after treatment.5,10-13,17,18
Considering the multiple symptoms BCSs endure, there is a need for large clinical trials to test the efficacy of effective interventions that can assist these survivors.19 Mindfulness-Based Stress Reduction (MBSR) has been found to be an effective intervention for BCSs in decreasing depression, anxiety, stress, FORs,5,20-24 and QOL.25 Preliminary evidence exists for the efficacy of MBSR in decreasing fatigue, pain, and sleep disturbances among BCSs.22,24,26,27 MBSR continues to demonstrate varied effectiveness in reducing symptoms during the transitional phase after treatment and thereafter.21-24,28 Despite promising results, there is limited evidence from large clinical trials regarding the effects of MBSR on QOL among BCSs.22,23 A recent trial among 229 BCSs no longer receiving treatment demonstrated greater improvement in QOL at both 8- and 12-week follow-up compared with controls.22 Meditative training in mindfulness (attention to the breath and body sensations) facilitates self-regulation of emotions by acceptance and nonreactive awareness of internal and external experiences.29,30 We postulate that this technique reduces rumination and reactions to emotional and physical triggers.
In summary, although evidence exists to support the use of MBSR among BCSs to alleviate symptom burden, few studies have tested the efficacy of MBSR in improvement of multiple psychological and physical symptoms along with QOL in a methodologic, rigorous large trial.30,31 The major aim of this large randomized controlled trial (RCT) was to evaluate the efficacy of the MBSR for BC (MBSR[BC]) program in improving psychological and physical symptoms and QOL among BCSs recently completing treatment. We hypothesized that compared with usual care (UC), participants randomly assigned to MBSR(BC) would experience greater improvements in psychological symptoms (depression, anxiety, perceived stress, and FORs), physical symptoms (pain and fatigue), and QOL, with improvements immediately after MBSR(BC) training and 6 weeks later. Additionally, we expected BCSs with the most distress at baseline would experience the greatest benefits. To test this possibility, perceived stress was examined at baseline to determine if it moderated treatment effects of MBSR(BC).
PATIENTS AND METHODS
Participants
From April 2009 through March 2013, 322 BCSs age 21 years or older with a diagnosis of stage 0 to III BC who had completed treatment from 2 weeks to 2 years before were recruited from the Moffitt Cancer Center, Carol and Frank Morsani Center for Advanced Healthcare, and Life Hope Medical Group, located in Tampa, Florida. Exclusion criteria included a diagnosis of stage IV BC, severe mental disorder, and/or BC recurrence.
Procedures
Study design and randomization.
Participants were randomly assigned at a one-to-one ratio to MBSR(BC) or UC with waitlisted MBSR(BC). An SPSS macro (version 17.0; SPSS, Chicago, IL) was used to create a stratified block randomization scheme, stratifying patients by type of surgery (lumpectomy v mastectomy), BC treatment (chemotherapy with or without radiotherapy v radiotherapy alone), and BC stage (stage 0 to I v II to III). This, along with the blocking mechanism, was performed to ensure balanced distributions of baseline factors between the two study groups (eg, pre-existing levels of anxiety). The sample size was calculated to compare adjusted mean outcome scores separately at 6 and 12 weeks of follow-up between the two groups. Allowing for 10% loss to follow-up of the sample, a sample of 300 or more would provide 90% power (type I error rate, 0.01) to detect an effect size (between groups) of (d) 0.45.
Recruitment and data collection procedures.
The trial protocol was approved by the institutional review board at the University of South Florida and the Moffitt Cancer Center Scientific Review Committee. BCSs who met inclusion criteria were invited to an orientation session during which informed consent was obtained along with baseline assessments followed by random assignment to either MBSR(BC) or UC.
MBSR(BC) intervention.
Participants randomly assigned to MBSR(BC) attended 2-hour sessions once per week for 6 weeks conducted by a clinical psychologist trained in MBSR and were provided training manuals and CDs to guide their practice. MBSR(BC) was adapted from the 8-week program of Kabat-Zinn et al32,33 to address the specific needs, concerns, and symptomology of BCSs and consists of educational materials, practice sessions of four meditative techniques(ie, sitting meditation, walking meditation, body scan, and gentle Hatha yoga33), and group processes related to barriers to the practice of meditation, application in daily situations, and supportive interaction among group members.33 BCSs were also taught informal techniques of integrating mindfulness into their daily activities. Compliance was assessed by the number of classes attended, completion of diaries, and minutes practiced. Indicators of MBSR(BC) compliance were established as at least 75% attendance at the MBSR(BC) sessions and completion of at least 75% of the homework assigned (ie, 15 to 45 minutes practiced each day).
Fidelity.
A structured observational method was used to evaluate instructors’ adherence to the intervention protocol. Participant diaries were evaluated monthly for fidelity to the MBSR(BC) intervention.
UC.
Participants randomly assigned to UC attended standard post-treatment clinic visits and were offered the MBSR(BC) program on study completion.
Measurements
Physical symptoms.
The Brief Pain Inventory, a 15-item questionnaire, was used to assess pain severity and pain interference in daily living, with higher scores indicative of greater pain severity and interference. The Fatigue Symptom Inventory was used to measure severity and perceived interference with QOL; higher scores are demonstrative of higher fatigue severity and interference with QOL.34
Psychological symptoms.
The Center for Epidemiologic Studies Depression Scale was used to evaluate depression; greater scores are associated with greater depressive symptomology.35 The State-Trait Anxiety Inventory–State, a subscale of the State-Trait Anxiety Inventory, was used to measure situational anxiety; higher scores are indicative of greater anxiety. The Perceived Stress Scale, a 14-item questionnaire, was used to measure stress; higher scores are characteristic of greater stress. The Concerns About Recurrence Scale was used to measure overall FOR and problems related to FORs; higher scores are indicative of greater overall fear and worry.36
QOL.
The Medical Outcomes Study Short Form was used to assess mental and physical health as related to QOL; higher scores are demonstrative of better mental and physical health.
Demographic data and clinical history.
Socioeconomic and demographic data were collected on age, sex, ethnicity, education, marital status, income, and employment status, along with clinical history data on cancer diagnosis and treatment. All demographic and clinical history data were completed at baseline and updated at 6 and 12 weeks.
Statistical Methods
The intent-to-treat principle was used, and all outcomes were considered of equal importance (ie, no secondary outcomes). There were two post-MBSR(BC) outcome assessment periods: one at 6 weeks immediately on completion of the program and one at 12 weeks (an additional 6 weeks after program completion). This design, coupled with initial baseline assessment, resulted in two separate assessment time points, allowing for the opportunity for repeated measures analysis. Linear mixed models were implemented to assess the interaction between participant assignment (MBSR[BC] v UC) and time (baseline, 6 weeks, and 12 weeks) in relation to symptom outcomes, testing whether the rate of symptom change varied by study assignment. Linear mixed models were also used to account for baseline differences and allow for inclusion and analysis of participants with some missing data (the number of whom was minimal). These linear mixed models assumed a compound symmetry correlation structure, and a two-sided P value of less than .01 was used to define statistical significance for all analyses (to test the sensitivity of effects, a parallel set of tests assumed an unstructured correlation structure). Model estimation was conducted in Mplus (version 7.1; Muthén and Muthén, Los Angeles, CA) using full-information maximum-likelihood estimation to benefit from available information in the data. Participants with missing information were included in the analyses under the assumption of missing at random (ie, missing data conditional on observed variables). Because BCSs who were most distressed at study enrollment seemed to benefit the most from MBSR(BC), participant stress (Perceived Stress Scale) was selected as a moderator. Moderator effects were explored using analysis of covariance and tested as interaction effects in the context of a series of linear mixed models. Characteristics at baseline were tested as moderators of the most robust main effects on symptom improvement (FOR as a psychological symptom and fatigue as a physical symptom).
RESULTS
Participant Characteristics
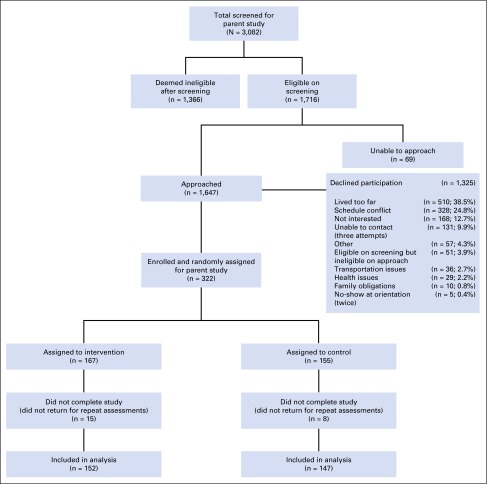
Of the 322 BCSs enrolled in the study, 299 completed the baseline and 6- and 12-week assessments, yielding a 9% attrition rate. The CONSORT diagram in Figure 1 identifies the number of patients screened, randomly assigned, and retained. Table 1 lists demographic characteristics by random assignment, identifying no statistically significant differences between groups at baseline and no significant differences in clinical characteristics, except in the use of anxiety medications (Table 2). Nearly 20% of BCSs had at least one first-degree relative with BC, and 10.9% of those first-degree relatives were diagnosed at age 50 years or younger.
Fig 1.
CONSORT flow chart showing recruitment and enrollment of 322 participants in the parent study; 152 participants in the intervention group and 147 in the usual care group completed the study and were included in outcome analyses.
Table 1.
Demographic Characteristics by Random Assignment to MBSR(BC) or UC
| Characteristic | No. (%) | P | ||
|---|---|---|---|---|
| All Patients (N = 322) | UC (n = 155) | MBSR(BC) (n = 167) | ||
| Age, years | .80 | |||
| Mean | 56.6 | 57.6 | 56.5 | |
| SD | 9.7 | 9.2 | 10.2 | |
| Race/ethnicity | .88 | |||
| White non-Hispanic | 69.4 (222) | 71.9 (110) | 67.1 (112) | |
| Black non-Hispanic | 11.6 (37) | 10.5 (16) | 12.6 (21) | |
| Hispanic | 10.3 (33) | 9.1 (14) | 11.4 (19) | |
| Other single race/ethnicity | 3.7 (12) | 3.3 (5) | 4.2 (7) | |
| More than one race/ethnicity reported | 5.0 (16) | 5.2 (8) | 4.8 (8) | |
| Marital status | .65 | |||
| Married | 64.4 (206) | 66.7 (102) | 62.3 (104) | |
| Single | 9.4 (30) | 9.1 (14) | 9.6 (16) | |
| Widowed | 11.2 (36) | 11.8 (18) | 10.8 (18) | |
| Divorced | 15.0 (48) | 12.4 (19) | 17.4 (29) | |
| Highest level of education | .78* | |||
| High school or less | 17.8 (57) | 17.5 (27) | 18.0 (30) | |
| Some college or vocational | 38.6 (124) | 40.3 (62) | 37.1 (62) | |
| College graduate and above | 43.6 (140) | 42.2 (65) | 44.9 (75) | |
| Current employment status, hours worked per week | .64 | |||
| ≥ 32 | 26.3 (84) | 28.8 (44) | 23.9 (40) | |
| < 32 | 11.9 (38) | 13.7 (21) | 10.2 (17) | |
| Retired | 27.8 (89) | 26.1 (40) | 29.3 (49) | |
| Medical leave or disabled | 10.3 (33) | 9.8 (15) | 10.8 (18) | |
| Other | 23.7 (76) | 21.6 (33) | 25.8 (43) | |
| Annual income | .45* | |||
| < $10,000 | 15.6 (49) | 15.2 (23) | 16.0 (26) | |
| $10,000 to < $20,000 | 16.2 (51) | 13.9 (21) | 18.4 (30) | |
| $20,000 to < $40,000 | 22.9 (72) | 24.5 (37) | 21.5 (35) | |
| $40,000 to < $80,000 | 24.2 (76) | 23.8 (36) | 24.5 (40) | |
| $80,000 to < $100,000 | 8.9 (28) | 9.3 (14) | 8.6 (14) | |
| ≥ $100,000 | 12.1 (38) | 13.3 (20) | 11.0 (18) | |
Abbreviations: MBSR(BC), Mindfulness-Based Stress Reduction for Breast Cancer; SD, standard deviation; UC, usual care.
P value for linear trend.
Table 2.
Clinical Characteristics by Random Assignment to MBSR(BC) or UC
| Characteristic | No. (%) | P | ||
|---|---|---|---|---|
| All Patients (N = 322) | UC (n = 155) | MBSR(BC) (n = 167) | ||
| Days since cancer treatment at baseline | .34 | |||
| Mean | 231 | 221 | 241 | |
| SD | 180 | 176 | 185 | |
| Stage of disease | .55* | |||
| 0 | 12.4 (40) | 12.3 (19) | 12.6 (21) | |
| I | 33.8 (109) | 36.1 (56) | 31.7 (53) | |
| II | 35.7 (115) | 34.8 (54) | 36.5 (61) | |
| III | 18.0 (58) | 16.8 (26) | 19.2 (32) | |
| Type of surgery | .62 | |||
| Lumpectomy | 46.6 (150) | 45.2 (70) | 47.9 (80) | |
| Mastectomy | 53.4 (172) | 54.8 (85) | 52.1 (87) | |
| Type of treatment received | .83 | |||
| Chemotherapy | 13.0 (42) | 14.8 (23) | 11.4 (19) | |
| Radiotherapy | 29.2 (94) | 28.4 (44) | 29.9 (50) | |
| Chemotherapy and radiotherapy | 35.7 (115) | 35.5 (55) | 35.9 (60) | |
| No chemotherapy or radiotherapy | 22.1 (71) | 21.3 (33) | 22.8 (38) | |
| Endocrine treatment | 55.9 (180) | 51.6 (80) | 59.9 (100) | .14 |
| Anastrozole | 17.7 (57) | 16.1 (25) | 19.2 (32) | .48 |
| Letrozole | 13.4 (43) | 10.3 (16) | 16.2 (27) | .12 |
| Levothyroxine | 12.1 (39) | 12.6 (21) | 11.6 (18) | .79 |
| Tamoxifen | 16.1 (52) | 15.5 (24) | 16.8 (28) | .76 |
| Other | 1.9 (6) | 1.8 (3) | 1.9 (3) | .93 |
| Multiple | 7.5 (24) | 7.1 (11) | 7.8 (13) | .81 |
| Pain medication | 24 (77) | 25 (38) | 23 (39) | .81 |
| Anxiolytic medication | 14 (45) | 10 (15) | 18 (30) | .03 |
| Antidepressant medication | 12 (38) | 9 (14) | 14 (24) | .14 |
Abbreviations: MBSR(BC), Mindfulness-Based Stress Reduction for Breast Cancer; SD, standard deviation; UC, usual care.
P value for linear trend.
Efficacy of MBSR(BC) on Outcomes
Linear mixed models were used to test the efficacy of MBSR(BC) on each outcome. Descriptive data for each outcome are summarized in Tables 3 and 4.
Table 3.
Psychological and QOL Outcomes at Baseline and 6 and 12 Weeks for MBSR(BC) and UC Groups and Estimates of Treatment Effects
| Psychological Outcome Measure | Experimental Group (MBSR[BC]) | Control Group (UC) | Between-Groups Effect Size Adjusting for Baseline | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | No. | Mean | SD | No. | d | 95% CI | |
| Depression (CES-D) | ||||||||
| T1 baseline | 10.87 | 6.89 | 167 | 10.04 | 6.46 | 155 | ||
| T2 week 6 | 8.12 | 5.45 | 154 | 8.82 | 6.05 | 146 | 0.20 | 0.00 to 0.40 |
| T3 week 12 | 8.66 | 6.26 | 155 | 8.95 | 6.8 | 148 | 0.16 | −0.05 to 0.36 |
| P* | .06 | |||||||
| Anxiety (STAI-S) | ||||||||
| T1 baseline | 38.62 | 12.3 | 167 | 35.86 | 11.29 | 155 | ||
| T2 week 6 | 30.62 | 12.8 | 159 | 31.76 | 13.2 | 152 | 0.26 | 0.06 to 0.46 |
| T3 week 12 | 31.82 | 12.1 | 155 | 32.99 | 13.4 | 148 | 0.27 | 0.06 to 0.47 |
| P* | .007 | |||||||
| FOR—overall (CARS) | ||||||||
| T1 baseline | 12.29 | 5.64 | 167 | 10.75 | 5.57 | 155 | ||
| T2 week 6 | 9.77 | 5.34 | 154 | 9.85 | 5.4 | 146 | 0.30 | 0.10 to 0.50 |
| T3 week 12 | 9.25 | 5.28 | 161 | 9.61 | 5.77 | 155 | 0.28 | 0.08 to 0.48 |
| P* | .001 | |||||||
| FOR—problems (CARS) | ||||||||
| T1 baseline | 39.43 | 24.4 | 167 | 31.84 | 24.5 | 155 | ||
| T2 week 6 | 28.51 | 24.04 | 152 | 29.87 | 25.7 | 145 | 0.46 | 0.25 to 0.66 |
| T3 week 12 | 28.36 | 24.54 | 153 | 28.01 | 26.01 | 146 | 0.35 | 0.15 to 0.56 |
| P* | .001 | |||||||
| QOL—general health (MOS SF-36) | ||||||||
| T1 baseline | 62.63 | 21.24 | 167 | 66.29 | 20.76 | 155 | ||
| T2 week 6 | 67.93 | 19.87 | 152 | 68.28 | 21.28 | 145 | 0.16 | −0.04 to 0.37 |
| T3 week 12 | 68.76 | 21.50 | 153 | 67.85 | 21.99 | 146 | 0.21 | 0.01 to 0.42 |
| P* | .05 | |||||||
Abbreviations: CARS, Concerns About Recurrence Scale; CES-D, Center for Epidemiologic Studies Depression Scale; FOR, fear of recurrence; MBSR(BC), Mindfulness-Based Stress Reduction for Breast Cancer; MOS SF-36, Medical Outcomes Study Short Form; QOL, quality of life; SD, standard deviation; STAI-S, State-Trait Anxiety Inventory–State; T, time point; UC, usual care.
Linear mixed-model interaction: time point by treatment group assignment.
Table 4.
Physical Outcomes at Baseline and 6 and 12 Weeks for MBSR(BC) and UC Groups and Estimates of Treatment Effects
| Physical Outcome Measure | Experimental Group (MBSR[BC]) | Control Group (UC) | Between-Groups Effect Size Adjusted for Baseline | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | No. | Mean | SD | No. | d | 95% CI | |
| Fatigue—severity (FSI) | ||||||||
| T1 baseline | 16.38 | 8.79 | 167 | 14.48 | 8.36 | 155 | ||
| T2 week 6 | 12.33 | 7.59 | 152 | 13.38 | 8.45 | 145 | 0.33 | 0.13 to 0.54 |
| T3 week 12 | 12.2 | 8.56 | 152 | 13.27 | 8.71 | 147 | 0.27 | 0.07 to 0.47 |
| P* | .002 | |||||||
| Fatigue—interference (FSI) | ||||||||
| T1 baseline | 30.27 | 21.78 | 167 | 25.51 | 19.93 | 155 | ||
| T2 week 6 | 20.25 | 16.35 | 154 | 21.45 | 18.2 | 146 | 0.30 | 0.10 to 0.51 |
| T3 week 12 | 21.98 | 20.91 | 152 | 22.49 | 19.52 | 147 | 0.23 | 0.02 to 0.43 |
| P* | .006 | |||||||
| Pain—severity (BPI) | ||||||||
| T1 baseline | 11.42 | 10.12 | 167 | 9.69 | 8.6 | 155 | ||
| T2 week 6 | 9.59 | 9.44 | 152 | 8.28 | 8.16 | 151 | 0.02 | −0.18 to 0.22 |
| T3 week 12 | 8.46 | 9.41 | 153 | 8.66 | 8.4 | 155 | 0.19 | −0.01 to 0.39 |
| P* | .08 | |||||||
| Pain—interference (BPI) | ||||||||
| T1 baseline | 18.04 | 18.85 | 167 | 15.13 | 16.51 | 155 | ||
| T2 week 6 | 14.16 | 16.55 | 152 | 12.52 | 15.31 | 145 | 0.03 | −0.17 to 0.24 |
| T3 week 12 | 17.89 | 27.16 | 153 | 20 | 28.69 | 146 | 0.17 | −0.04 to 0.37 |
| P* | .12 | |||||||
Abbreviations: BPI, Brief Pain Inventory; FSI, Fatigue Symptom Inventory; MBSR(BC), Mindfulness-Based Stress Reduction for Breast Cancer; SD, standard deviation; T, time point; UC, usual care.
Linear mixed-model interaction: time point by treatment group assignment.
Psychological symptoms.
When examining effect sizes, the largest MBSR(BC)-related improvements occurred during the first 6 weeks, and most were maintained at 12 weeks. Statistically significant psychological symptom improvements were observed for BCSs in the MBSR(BC) program through the 12-week period. Participants randomly assigned to MBSR(BC) showed significantly greater improvements in anxiety (P = .007) and FORs (overall and problems; P < .01). Twelve-week effect sizes (Cohen’s d) were between 0.27 and 0.35. Results for depression were consistent with the pattern observed for the other psychological measures. Participants assigned to MBSR(BC) tended to experience greater improvement than those assigned to UC; however, this trend did not reach statistical significance (P = .06). Table 3 includes P values for the condition-by-time improvement of psychological symptoms, along with the effect sizes at 6 and 12 weeks. Because use of anxiety medications differed between experimental groups at baseline, anxiety medication was included as a covariate in the mixed models testing both anxiety and FOR effects, which did not alter the patterns of statistical significance.
Physical symptoms.
BCSs randomly assigned to MBSR(BC) demonstrated greater symptom improvement in fatigue (severity and interference; P < .01). Effect sizes (Cohen’s d) were between 0.27 and 0.23. Similar to improvement in psychological symptoms, a majority of improvements in fatigue occurred during the MBSR(BC) training, with little change occurring during the follow-up period (6 to 12 weeks). Groups did not differ statistically in pain outcomes. Table 4 includes P values for the condition-by-time improvement of physical symptoms, along with the effect sizes at 6 and 12 weeks.
QOL.
Although a trend for QOL was observed (P < .05), with an effect size (d) of 0.21, the difference was not statistically significant (defined as P < .01). Table 3 includes P values for the condition-by-time improvement of QOL, along with the effect sizes at 6 and 12 weeks.
Moderators.
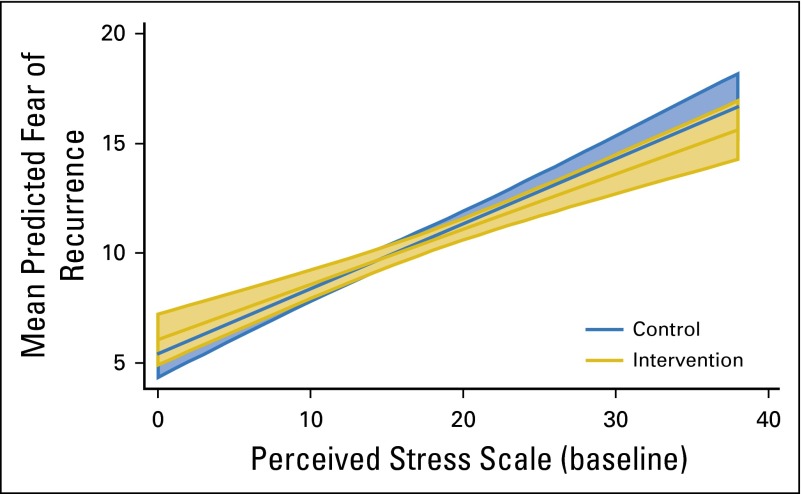
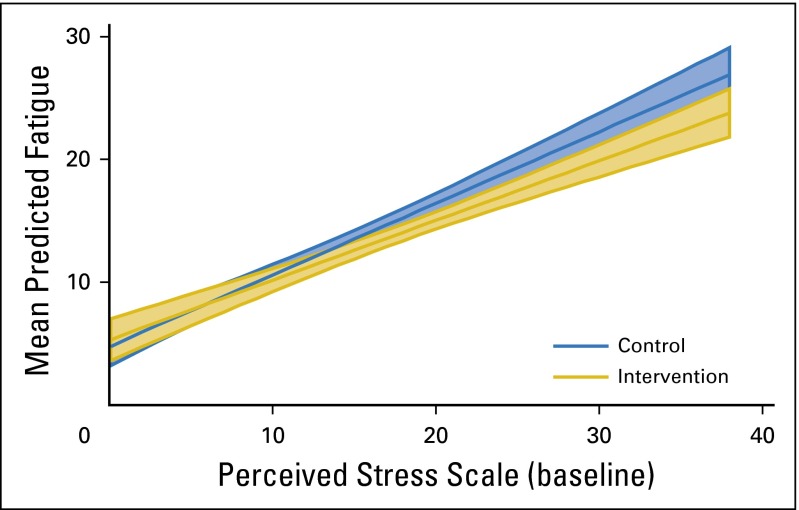
Baseline stress was tested as a moderator of the most robust main effect symptom improvements (FORs and fatigue), and a consistent pattern was observed across both tests. BCSs with the most stress at baseline experienced the most improvement from MBSR(BC) in both fatigue and FORs (both P < .001; Figs 2 and 3 show moderation effects).
Fig 2.
Significant moderation effects (P < .001) of perceived stress on treatment as predictors of fear of recurrence. The plot depicts mean predicted fear of recurrence by perceived stress at baseline for each treatment group. Shaded areas indicate 95% CIs.
Fig 3.
Significant moderation effects (P < .001) of perceived stress on treatment as predictors of fatigue. The plot depicts mean predicted levels of fatigue by perceived stress at baseline for each treatment group. Shaded areas indicate 95% CIs.
Sensitivity analysis.
Each mixed model was tested assuming a compound symmetry correlation structure. To test whether effects were robust to different correlation structures, all models were also tested with an unstructured correlation matrix. All differences remained statistically significant except for anxiety, which went from a P value of .007 to .018.
Compliance.
A majority of MBSR(BC) participants were classified as compliant. There was no evidence of effect modification by compliance with respect to the outcome of anxiety, FORs, or fatigue (P > .05).
DISCUSSION
This clinical trial further supports the empirically established benefits of MBSR(BC) and shows added efficacy over an extended timeframe for wide-ranging symptoms. The major benefit of MBSR(BC) is that it provides evidence of alleviation of multiple symptoms concurrently. This RCT is the largest clinical trial to our knowledge testing the effects of MBSR(BC) among BCSs with a 12-week follow-up examining the immediate and sustained treatment effects on physical (fatigue and pain) and psychological symptoms (anxiety, depression, stress, and FORs) and QOL. Methodologically, this study met the standards for a large, rigorous RCT with sufficient power to detect treatment effects. It is important to note that a majority of symptom improvements occurred during the MBSR(BC) training (baseline to 6 weeks) and were sustained during the follow-up period. We find it promising that participants assigned to MBSR(BC) experienced immediate improvements in fatigue and also psychological symptoms during the MBSR(BC) training period.
This trial provides evidence to support MBSR(BC) as clinical treatment with sustained effects for the symptoms associated with BC treatment. Our work is similar to a study of MBSR among 229 BCSs no longer receiving treatment, which found significant improvements in total mood at 8 and 12 weeks, subscales of anxiety and depression at 8 weeks only, and anger at 12 weeks only.22 Psychological improvements in stress, anxiety, and depression among BCSs in clinical trials have been validated in earlier work by our team23 and others.20,21 Although FOR is a chronic problem for BCS and is associated with heightened stress, anxiety, and depression, it is not often examined as an outcome of MBSR. FOR is triggered by antecedents such as physical symptoms or by perceived risk, resulting in psychological distress,37,38 and contributes indirectly to fatigue.39,40 Previous research has shown that MBSR(BC) reduces FOR and distal psychological and physical symptoms.41
This is the only large clinical trial to our knowledge to examine and validate the effects of MBSR(BC) on FOR among BCSs unique showing immediate (6 weeks) and sustained (12 weeks) improvements for reducing FOR. The improvement in FOR is consistent with that seen in our previous small R21 National Cancer Institute trial23 and our pilot study testing the feasibility of the MBSR(BC) intervention.42 Although a sensitivity analysis decreased the P value of the effect of MBSR on anxiety, numerous replications of MBSR-related anxiety reduction make us confident that this was not a type I error.
A second major finding provides evidence that MBSR(BC) had significant effects on fatigue severity and interference up to 6 weeks after cessation of MBSR(BC) training. In comparison, Hoffman et al22 found significant improvements at 8 weeks in fatigue, vigor, and confusion. Our results were also similar to those of Carlson et al.43 The moderation analysis suggests patients who were experiencing the greatest stress at baseline gained the greatest benefit from MBSR(BC) in decreasing their fatigue and FOR.
Relatively small effect sizes may have resulted from low symptom levels at baseline (floor effects), particularly because this study did not screen for high levels distress, anxiety, and/or depression before enrollment. Future studies may consider screening for distress or other moderating factors. Floor effects may also be the reason that little further improvement was observed in symptoms during the follow-up period.
This large clinical trial provides support for MBSR(BC) as clinical treatment for psychological and physical symptoms experienced by BCSs as they transition away from treatment and move forward into long-term survivorship. Although in 2014, the National Comprehensive Cancer Network indicated that MBSR had a high level of evidence, there have been few large randomized trials reporting significant effects.5,22,28 Our large RCT adds additional scientific evidence for the extended clinical effectiveness of MBSR(BC) to alleviate multiple existing psychological and physical symptoms in BCSs. Major benefits of the MBSR(BC) program include that it can be easily learned and does not require a great deal of physical effort when practiced. MBSR(BC) can become a beneficial way of life, if BCSs cultivate decreased reactions to stress through their formal or informal meditation practice.
A practical limitation in widespread clinical translation of MBSR(BC) is that the current delivery approach requires in-person sessions over a minimum of 6 weeks. This warrants the development and testing of technology-based methods that can deliver the MBSR(BC) intervention remotely in an efficacious manner, such as by the use of video telecommunications or mobile devices, for the wide adoption of this evidence-based program.
A limitation of this study was the trial design. Although participants were randomly assigned to either MBSR(BC) or UC, an ideal control condition would have been an active attention comparison treatment, thereby controlling for expectancy effects and time and attention. Our sample was largely middle class with some diversity; however, we did not include non–English speaking BCSs. Blinding to group assignment after the baseline assessment by the assessors was not possible with use of the waitlisted control design. Although participants were randomly assigned, this trial enrolled BCSs interested in the trial (ie, motivated), possibly creating some selection bias. Finally, related to data in Figure 1, the participation rate among screened eligible BCSs (ie, those who ultimately enrolled) was low; however, this does not indicate a lack of generalizability per se, because the primary reasons for nonenrollment were logistic in nature (eg, distance too far to travel), taking into consideration that many of the patients traveled to the cancer center from a distance.
In conclusion, this study provides evidence to support the benefits of MBSR(BC) as a clinical nonpharmacologic intervention targeting extended relief for BCSs experiencing distressing psychological and physical symptoms. Major advantages of this intervention are that MBSR(BC) targets multiple distressing symptoms concurrently and that it is most beneficial to those who are most distressed. Future technology-based approaches should be developed and tested to widely deliver this intervention in an efficacious manner.
Supplementary Material
Footnotes
Supported by Award No. 1R01CA131080-01A2 from the National Cancer Institute and in part by the Survey Methods Core Facility at the H. Lee Moffitt Cancer Center and Research Institute.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. This study protocol was approved by the institutional review board at the University of South Florida to ensure the ethical treatment of participants.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01177124.
AUTHOR CONTRIBUTIONS
Conception and design: Cecile A. Lengacher, Richard R. Reich, Versie Johnson-Mallard, Manolete Moscoso, Paul B. Jacobsen, Matthew Goodman, Kevin E. Kip
Administrative support: Charles E. Cox
Provision of study materials or patients: Charles E. Cox, Matthew Goodman
Collection and assembly of data: Carly L. Paterson, Sophia Ramesar, Jong Y. Park, Pinky Budhrani-Shani
Data analysis and interpretation: Cecile A. Lengacher, Richard R. Reich, Sophia Ramesar, Jong Y. Park, Carissa Alinat, Branko Miladinovic, Charles E. Cox, Kevin E. Kip
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Examination of Broad Symptom Improvement Resulting From Mindfulness-Based Stress Reduction for Breast Cancer Survivors: A Randomized Controlled Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Cecile A. Lengacher
No relationship to disclose
Richard R. Reich
No relationship to disclose
Carly L. Paterson
No relationship to disclose
Sophia Ramesar
No relationship to disclose
Jong Y. Park
No relationship to disclose
Carissa Alinat
No relationship to disclose
Versie Johnson-Mallard
No relationship to disclose
Manolete Moscoso
No relationship to disclose
Pinky Budhrani-Shani
No relationship to disclose
Branko Miladinovic
No relationship to disclose
Paul B. Jacobsen
Consulting or Advisory Role: Onyx Pharmaceuticals, Carevive
Research Funding: Pfizer, Carevive
Charles E. Cox
Honoraria: Cianna Medical, Agendia, Genentech
Consulting or Advisory Role: Cianna Medical, Agendia
Speakers’ Bureau: Cianna Medical, Agendia, Genentech
Research Funding: Cianna Medical, Agendia
Patents, Royalties, Other Intellectual Property: Patent #8114006 USF (Inst)
Travel, Accommodations, Expenses: Cianna Medical, Agendia
Matthew Goodman
No relationship to disclose
Kevin E. Kip
No relationship to disclose
REFERENCES
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society . Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- 3.Kenyon M, Mayer DK, Owens AK. Late and long-term effects of breast cancer treatment and surveillance management for the general practitioner. J Obstet Gynecol Neonatal Nurs. 2014;43:382–398. doi: 10.1111/1552-6909.12300. [DOI] [PubMed] [Google Scholar]
- 4.Wu HS, Harden JK. Symptom Burden and Quality of Life in Survivorship: A Review of the Literature. Cancer Nurs. 2014;38:E29–E54. doi: 10.1097/NCC.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 5.Lengacher C, Reich R, Post-White J, et al. Mindfulness based stress reduction in post-treatment breast cancer patients: An examination of symptoms and symptom clusters. J Behav Med. 2012;35:86–94. doi: 10.1007/s10865-011-9346-4. [DOI] [PubMed] [Google Scholar]
- 6.Byar KL, Berger AM, Bakken SL, et al. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol Nurs Forum. 2006;33:E18–E26. doi: 10.1188/06.ONF.E18-E26. [DOI] [PubMed] [Google Scholar]
- 7.Kenefick AL. Patterns of symptom distress in older women after surgical treatment for breast cancer. Oncol Nurs Forum. 2006;33:327–335. doi: 10.1188/06.ONF.327-335. [DOI] [PubMed] [Google Scholar]
- 8.Dodd MJ, Cho MH, Cooper BA, et al. The effect of symptom clusters on functional status and quality of life in women with breast cancer. Eur J Oncol Nurs. 2010;14:101–110. doi: 10.1016/j.ejon.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger AM, Visovsky C, Hertzog M, et al. Usual and worst symptom severity and interference with function in breast cancer survivors. J Support Oncol. 2012;10:112–118. doi: 10.1016/j.suponc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Ganz PA, Desmond KA, Leedham B, et al. Quality of life in long-term, disease-free survivors of breast cancer: A follow-up study. J Natl Cancer Inst. 2002;94:39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 11.Ganz PA, Guadagnoli E, Landrum MB, et al. Breast cancer in older women: Quality of life and psychosocial adjustment in the 15 months after diagnosis. J Clin Oncol. 2003;21:4027–4033. doi: 10.1200/JCO.2003.08.097. [DOI] [PubMed] [Google Scholar]
- 12.Lee K, Cho M, Miaskowski C, et al. Impaired sleep and rhythms in persons with cancer. Sleep Med Rev. 2004;8:199–212. doi: 10.1016/j.smrv.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Serlin RC, Mendoza TR, Nakamura Y, et al. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 14.Salz T, Oeffinger KC, McCabe MS, et al. Survivorship care plans in research and practice. CA Cancer J Clin. 2012;62:101–117. doi: 10.3322/caac.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passik SD, Kirsh KL, Rosenfeld B, et al. The changeable nature of patients’ fears regarding chemotherapy: Implications for palliative care. J Pain Symptom Manage. 2001;21:113–120. doi: 10.1016/s0885-3924(00)00249-9. [DOI] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology: Survivorship. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 17.Dodd MJ, Miaskowski C, Lee KA. Occurrence of symptom clusters. J Natl Cancer Inst Monogr. 2004;32:76–78. doi: 10.1093/jncimonographs/lgh008. [DOI] [PubMed] [Google Scholar]
- 18.Esper P, Heidrich D. Symptom clusters in advanced illness. Semin Oncol Nurs. 2005;21:20–28. doi: 10.1053/j.soncn.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Macmillan Cancer Support . National Cancer Survivorship Initiative; http://www.ncsi.org.uk. [Google Scholar]
- 20.Würtzen H, Dalton SO, Elsass P, et al. Mindfulness significantly reduces self-reported levels of anxiety and depression: Results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur J Cancer. 2013;49:1365–1373. doi: 10.1016/j.ejca.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Henderson VP, Clemow L, Massion AO, et al. The effects of mindfulness-based stress reduction on psychosocial outcomes and quality of life in early-stage breast cancer patients: A randomized trial. Breast Cancer Res Treat. 2012;131:99–109. doi: 10.1007/s10549-011-1738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman C, Ersser S, Hopkinson J, et al. Effectiveness of Mindfulness-Based Stress Reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0-III breast cancer: A randomized controlled trial. J Clin Oncol. 2012;30:1335–1342. doi: 10.1200/JCO.2010.34.0331. [DOI] [PubMed] [Google Scholar]
- 23.Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18:1261–1272. doi: 10.1002/pon.1529. [DOI] [PubMed] [Google Scholar]
- 24.Johns SA, Brown LF, Beck-Coon K, et al. Randomized controlled pilot study of mindfulness-based stress reduction for persistently fatigued cancer survivors. Psychooncology. 2015;24:885–893. doi: 10.1002/pon.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlson LE, Doll R, Stephen J, et al. Randomized controlled trial of mindfulness-based cancer recovery versus supportive expressive group therapy for distressed survivors of breast cancer. J Clin Oncol. 2013;31:3119–3126. doi: 10.1200/JCO.2012.47.5210. [DOI] [PubMed] [Google Scholar]
- 26.Lengacher CA, Reich RR, Paterson CL, et al. The effects of mindfulness-based stress reduction on objective and subjective sleep parameters in women with breast cancer: A randomized controlled trial. Psychooncology. 2015;24:424–432. doi: 10.1002/pon.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garland SN, Carlson LE, Stephens AJ, et al. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: A randomized, partially blinded, noninferiority trial. J Clin Oncol. 2014;32:449–457. doi: 10.1200/JCO.2012.47.7265. [DOI] [PubMed] [Google Scholar]
- 28.Andersen SR, Würtzen H, Steding-Jessen M, et al. Effect of mindfulness-based stress reduction on sleep quality: results of a randomized trial among Danish breast cancer patients. Acta Oncol. 2013;52:336–344. doi: 10.3109/0284186X.2012.745948. [DOI] [PubMed] [Google Scholar]
- 29.Baer RA. Mindfulness training as a clinical intervention: A conceptual and empirical review. Clin Psychol Sci Pract. 2003;10:125–143. [Google Scholar]
- 30.Bishop SR, Lau M, Shapiro S, et al. Mindfulness: A proposed operational definition. Clin Psychol Sci Pract. 2004;11:230–241. [Google Scholar]
- 31.Lerman R, Jarski R, Rea H, et al. Improving symptoms and quality of life of female cancer survivors: A randomized controlled study. Ann Surg Oncol. 2012;19:373–378. doi: 10.1245/s10434-011-2051-2. [DOI] [PubMed] [Google Scholar]
- 32.Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8:163–190. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- 33.Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149:936–943. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- 34.Hann DM, Jacobsen P, Martin S, et al. Fatigue and quality of life following radiotherapy for breast cancer: A comparative study. J Clin Psychol Med Settings. 1998;5:19–33. [Google Scholar]
- 35.Radloff L. The CES-D scale: A self-report depression scale for researching the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 36.Vickberg SM. The Concerns About Recurrence Scale (CARS): A systematic measure of women’s fears about the possibility of breast cancer recurrence. Ann Behav Med. 2003;25:16–24. doi: 10.1207/S15324796ABM2501_03. [DOI] [PubMed] [Google Scholar]
- 37.Simard S, Savard J, Ivers H. Fear of cancer recurrence: Specific profiles and nature of intrusive thoughts. J Cancer Surviv. 2010;4:361–371. doi: 10.1007/s11764-010-0136-8. [DOI] [PubMed] [Google Scholar]
- 38.van den Beuken-van Everdingen MH, Peters ML, de Rijke JM, et al. Concerns of former breast cancer patients about disease recurrence: A validation and prevalence study. Psychooncology. 2008;17:1137–1145. doi: 10.1002/pon.1340. [DOI] [PubMed] [Google Scholar]
- 39.Minton O, Stone P. How common is fatigue in disease-free breast cancer survivors? A systematic review of the literature. Breast Cancer Res Treat. 2008;112:5–13. doi: 10.1007/s10549-007-9831-1. [DOI] [PubMed] [Google Scholar]
- 40.Young KE, White CA. The prevalence and moderators of fatigue in people who have been successfully treated for cancer. J Psychosom Res. 2006;60:29–38. doi: 10.1016/j.jpsychores.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Lengacher CA, Shelton MM, Reich RR, et al. Mindfulness based stress reduction (MBSR(BC)) in breast cancer: Evaluating fear of recurrence (FOR) as a mediator of psychological and physical symptoms in a randomized control trial (RCT) J Behav Med. 2014;37:185–195. doi: 10.1007/s10865-012-9473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lengacher CA, Johnson-Mallard V, Barta M, et al. Feasibility of a mindfulness-based stress reduction program for early-stage breast cancer survivors. J Holist Nurs. 2011;29:107–117. doi: 10.1177/0898010110385938. [DOI] [PubMed] [Google Scholar]
- 43.Carlson LE, Ursuliak Z, Goodey E, et al. The effects of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients: 6-month follow-up. Support Care Cancer. 2001;9:112–123. doi: 10.1007/s005200000206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.