Abstract
Purpose
Health providers’ implicit racial bias negatively affects communication and patient reactions to many medical interactions. However, its effects on racially discordant oncology interactions are largely unknown. Thus, we examined whether oncologist implicit racial bias has similar effects in oncology interactions. We further investigated whether oncologist implicit bias negatively affects patients’ perceptions of recommended treatments (i.e., degree of confidence, expected difficulty). We predicted oncologist implicit bias would negatively affect communication, patient reactions to interactions, and, indirectly, patient perceptions of recommended treatments.
Methods
Participants were 18 non-black medical oncologists and 112 black patients. Oncologists completed an implicit racial bias measure several weeks before video-recorded treatment discussions with new patients. Observers rated oncologist communication and recorded interaction length of time and amount of time oncologists and patients spoke. Following interactions, patients answered questions about oncologists’ patient-centeredness and difficulty remembering contents of the interaction, distress, trust, and treatment perceptions.
Results
As predicted, oncologists higher in implicit racial bias had shorter interactions, and patients and observers rated these oncologists’ communication as less patient-centered and supportive. Higher implicit bias also was associated with more patient difficulty remembering contents of the interaction. In addition, oncologist implicit bias indirectly predicted less patient confidence in recommended treatments, and greater perceived difficulty completing them, through its impact on oncologists’ communication (as rated by both patients and observers).
Conclusion
Oncologist implicit racial bias is negatively associated with oncologist communication, patients’ reactions to racially discordant oncology interactions, and patient perceptions of recommended treatments. These perceptions could subsequently directly affect patient-treatment decisions. Thus, implicit racial bias is a likely source of racial treatment disparities and must be addressed in oncology training and practice.
INTRODUCTION
Black patients generally receive lower quality medical treatment than white patients. This disparity occurs across a wide variety of diseases1-3 but is especially well-documented in cancer treatment.4,5 Although tumor type/stage, comorbidities, and health-care system influence treatment, cancer treatment disparities persist after these factors are controlled.6-15
Communication difficulties during medical interactions have been linked to poorer health-care outcomes.16 Relative to racially concordant medical interactions, communication is poorer in racially discordant interactions (i.e., black patient and non-black physician),9,17,18 which make up about 80% of black patients’ medical interactions.19,20 We investigated one potential cause of communication difficulties in racially discordant oncology interactions: oncologist racial bias.
Racial bias can involve explicit or implicit negative thoughts and feelings about blacks. Explicit racial biases are deliberative and operate at the conscious level; their expression can be deliberately controlled.21,22 Because expression of explicit racial bias among physicians runs counter to personal, social, and professional norms,23-25 physicians generally exhibit relatively low levels of explicit bias. Consequently, physician explicit bias has limited impact on racially discordant medical interactions.26 In contrast, implicit racial bias is automatically activated and operates at a nonconscious level.21,24,25,27 Non-black (i.e., white, Asian, and Hispanic/Latino) health-care providers display substantial implicit racial bias toward blacks at levels comparable to the general public.23,24,27,28
Two lines of research have explored the influence of providers’ implicit racial bias on health care received by black patients. The first uses hypothetical vignettes29-33 to examine the impact of implicit bias on providers’ treatment decisions. This research has not found a consistent pattern of association between providers’ implicit bias and their treatment decisions.28 The other line of research focuses on the influence of providers’ racial bias on their communication and patients’ reactions in actual health-care interactions. These studies found that primary care physicians’ implicit racial bias negatively affects their communication and/or black patients’ reactions to them.28,34-39 Similar effects occur in interactions involving physicians treating patients with spinal cord injury40 and interactions with genetic counselors.41
The effects of implicit racial bias in racially discordant oncology interactions are, however, largely unknown. Thus, we investigated whether oncologist implicit bias has effects on communication and patient perceptions in racially discordant oncology interactions similar to those in other medical interactions. Furthermore, we extended the investigation of bias effects to patient perceptions of recommended treatments. Finding significant associations among implicit racial bias and communication, patient reactions, and patient treatment perceptions in oncology interactions would substantially expand the scope of recognition and understanding of the negative influence of provider racial bias on racially discordant medical interactions and their outcomes.
From a clinical perspective, as already noted,19,20 most black patients with cancer will experience racially discordant oncology interactions. Empirical evidence of negative effects of oncologist implicit bias in racially discordant oncologic interactions therefore would have significant implications for the quality of care received by large numbers of black patients with cancer.
This study’s first purpose was to examine the impact of oncologist implicit racial bias on communications with patients and patients’ reactions to them and the interaction. We examined outcomes that prior research had found were affected by provider implicit racial bias, including interaction length,35 verbal dominance,35,36 extent of patient involvement in treatment decisions,34 patient perceptions of provider patient-centeredness,34,36,37,41 and patients’ trust in their physician.39 Additionally, we examined three aspects of medical interactions not previously studied: observers’ ratings of oncologist communication with patients, patient reports of difficulty remembering contents of the interactions, and patient reports of distress. We predicted that oncologist implicit racial bias would negatively affect all of these outcomes.
The second purpose was to investigate potential effects of oncologist implicit racial bias on patients’ perceptions of treatments recommended by their oncologist. Specifically, we examined patients’ degree of confidence in the efficacy of the recommended treatments and their perceptions of the difficulty in completing recommended treatments. These outcomes were chosen because of their possible direct influence on patients’ treatment decisions. We predicted that oncologist implicit bias would decrease patients’ confidence in treatments and increase their perception of the difficulty of completing treatment. However, we expected this to be an indirect process. We propose a model in which oncologist implicit bias would be negatively associated with quality of oncologists’ communication, which would then negatively affect patients’ degree of confidence in and perceptions of the difficulty of completing recommended treatments.
METHODS
Design and Participants
Data were collected between April 2012 and December 2014 at two cancer hospitals in Detroit, Michigan, as part of a larger study designed to improve communication during racially discordant oncology interactions. Medical oncologists were eligible if they did not self-identify as black or African American and if they treated cancer patients at either hospital. After oncologists consented, their eligible patients were informed about the study by clinical staff or via oncologist opt-out letters. Patients were eligible if they self-identified as black or African American; were between 30 and 85 years old; comprehended English well enough to provide informed consent; had a confirmed diagnosis of breast, colorectal, or lung cancer (any stage); and had an appointment to see a participating oncologist within 1 week for an initial discussion of treatment options. Institutional review boards at both hospitals and Wayne State University approved study procedures. Oncologists received $50 gift cards for study participation; patients received $60 gift cards.
Participants were 18 oncologists (90%) and 112 (98%) patients from the larger study. Oncologists were included if they completed the measure of implicit racial bias and at least one of the study outcome measures. Patients were included if they completed at least one of the study outcome measures.
Procedures
Participants read and signed informed consent forms that described all study procedures. Within 2 weeks of consenting but several weeks before any interactions with study patients, oncologists completed a baseline questionnaire via an online platform (Qualtrics, Provo, UT). This baseline assessment included the measure of implicit racial bias. Immediately after consenting, patients completed a baseline questionnaire also via the online platform or on paper and were then randomly assigned to one of three study arms: (1) control (usual care); (2) receiving a “question prompt list” containing questions patients might ask their oncologist,42 or (3) receiving the question prompt list and meeting with a “coach” who reviewed questions with them.
Within 1 week of completing the baseline questionnaire, patients had a clinical interaction with an oncologist. The interaction was an initial discussion of treatment of a current cancer; patients had not previously met with the oncologist to discuss any treatments. Ninety-six of 112 interactions were video recorded; 16 interactions were not recorded because of logistical problems. Prior research43,44 has shown that cameras have no discernable impact on participants’ verbal or nonverbal behavior in oncology interactions.
Immediately after the interactions, oncologists answered a question about patient participation in treatment decisions. Separately, and out of their oncologist’s view, patients answered questions about their perceptions of the oncologist and the interaction. Patients also reported their perceptions of the recommended treatment. One week later, patients participated in a follow-up telephone interview that included questions about perceptions of the interaction and trust in their oncologist.
Measures
Oncologists.
The Implicit Association Test (IAT)22,45 was used to assess oncologist implicit racial bias.27 The IAT is the most widely used measure of implicit bias and is extensively validated.22,46,47 Standard procedures46 were followed for IAT administration and scoring (Data Supplement.) The IAT yields a standardized difference score (d) for each respondent, which represents the relative strength of a respondent’s pro-white/anti-black implicit racial bias. More positive scores indicate more implicit pro-white/anti-black bias.22,45
Four research staff (two black, two white), blind to study hypotheses, study arm, and oncologists’ level of implicit bias, viewed the 96 video recordings. Observers used a five-point rating scale to rate oncologists on a communication measure48 with three four-item subscales: (1) informativeness (eg, “doctor was very informative about patient’s health”); (2) supportiveness (eg, “doctor made patient feel completely at ease”); and (3) partnership building (eg, “doctor asked for patient’s thoughts about his/her health”). At least two observers were randomly assigned to view each interaction and separately responded to individual scale items. Individual item ratings were averaged across observers who viewed the same interaction. Each subscale’s total score for an interaction was the average of the four average item ratings in that subscale. Total score was the average of the three subscale averages. Intraclass correlation coefficients for observers’ ratings ranged from 0.57 to 0.74 (P < .05). Coefficient α values were informativeness, 0.91; supportiveness, 0.91; partnership building, 0.77; and total scale, 0.88.
One observer used observational coding software (Studiocode; studiocodegroup.com, Lincoln, NE) to record interaction length (i.e., length of time patients and oncologists were both in room). Two observers recorded the amount of time each participant spoke while in the room together (79.9% agreement). To assess verbal dominance, the ratio of oncologist talk time to patient talk time was computed and log-transformed.35
Immediately after interactions, oncologists who recommended treatment used a five-point rating scale to indicate how much they involved patients in treatment decisions.
Patients.
Immediately after interactions, patients completed the perceived patient-centeredness measure.49 They used a four-point scale to rate the extent to which they perceived their oncologist had displayed each of 14 patient-centered behaviors, such as “showed respect” and “was concerned about me as a person” (α = .83). Scores were averaged across the 14 behaviors. Patients then used a single five-point scale to rate their difficulty remembering the contents of the interaction and an 11-point scale to rate their current level of distress.50 Patients used separate five-point rating scales to report their degree of confidence in the recommended treatment and perceptions of the difficulty of completing it.
In follow-up telephone interviews, patients again rated their difficulty remembering conversation content; and used five-point rating scales to answer five questions about their trust in their oncologist (α =.79).51
Statistical Analyses
Bivariate associations.
Multilevel models, with patients nested within oncologists, were used to test all hypotheses about bivariate associations. Study arm was a covariate in the analyses. Preliminary analyses disclosed no significant interactions between study arm and implicit bias; therefore, interaction terms were not included in final models. Models used standardized predictors and outcomes (i.e., z-transformed); model regression weights, thus, represent standardized estimates of effect size.
Preliminary analysis of data structure disclosed unequal variance among oncologists in distributions of how implicit bias affected some measures. Therefore, for each regression analysis, two models were estimated and tested: One assumed equal variance among oncologists; another assumed unequal variance. Fit of the two models was compared using Akaike's Information Criterion (AIC),52 the Bayesian Information Criterion,53 and a χ2 increment in a model-fit test; results are reported for best fitting models. Sample size permitted detection of medium-effect sizes with 80% power and 5% type I error rate.
Oncologists’ demographic and professional characteristics and patients’ demographic and medical characteristics (i.e., cancer site, stage) were explored in bivariate regression analyses as possible covariates of study outcomes. Patient income positively covaried with involving patients in treatment decisions; oncologist age positively covaried with the oncologist supportive communication subscale. These covariates were included in appropriate analyses.
Indirect effects.
The same multilevel, patient-nested models were used in tests of indirect effects on patient confidence in and perceived difficulty of recommended treatments. Tests of indirect paths were conducted by testing the product of (1) the regression coefficient for the mediator (i.e., patient-centered communication) regressed onto the independent variable (implicit bias) multiplied by (2) the regression coefficient for the dependent variable (confidence or difficulty) regressed onto both the mediator and independent variable. Nonparametric bootstrap resampling54 (5,000 samples) of regression coefficients was used to obtain confidence intervals, which were used to interpret significance of the indirect path.54-56 For all significance tests, α was two-tailed at .05.
RESULTS
Participant Characteristics
Table 1 presents the professional characteristics of the oncologists. Most were men (56%), and had been in practice, on average, for about 7 years. Table 2 presents patients’ personal and medical characteristics. Most patients were women (91%) who had been diagnosed with cancer 3 months or less before study entry. The most frequent cancer type was breast (84%); all disease stages were represented. Data on oncologists’ and patients’ ratings and responses to questions are presented in Table 3.
Table 1.
Oncologist Characteristics (N = 18)
| Characteristic | Value |
|---|---|
| Sex, no. (%) | |
| Male | 10 (56) |
| Age, years, mean (SD) | 46.44 (10.38) |
| Ethnicity (self-reported), no. (%) | |
| White | 9 (50) |
| Asian | 4 (22) |
| Arab/Middle Eastern | 5 (28) |
| Position, no. (%) | |
| Fellow | 3 (17) |
| Attending | 15 (83) |
| Years in practice (postfellowship), mean (SD), median | 7.21 (10.31), 2.91 |
| Implicit bias (IAT score), mean (SD), median | 0.18 (0.51), 0.35 |
| d (range) | 0.26 (−0.99 to 0.76) |
Abbreviations: d, standardized difference score; IAT, Implicit Association Test; SD, standard deviation.
Table 2.
Patient Characteristics (N = 112)*
| Characteristic | Value |
|---|---|
| Sex | |
| Female | 102 (91) |
| Age, years, mean (SD) | 58.69 (10.38) |
| Highest level of education | |
| Did not graduate high school | 26 (23) |
| Graduated high school | 13 (12) |
| Some college | 37 (33) |
| Graduated college | 21 (19) |
| Postgraduate | 15 (13) |
| Income (US$) | |
| 0-19,999 | 44 (42) |
| 20,000-39,999 | 33 (31) |
| 40,000-59,999 | 9 (9) |
| 60,000-79,999 | 10 (9) |
| > 80,000 | 9 (9) |
| Primary tumor site | |
| Breast | 92 (84) |
| Colorectal | 8 (7) |
| Lung | 12 (9) |
| Stage | |
| 0 | 3 (3) |
| I | 40 (36) |
| II | 37 (33) |
| III | 23 (21) |
| IV | 7 (6) |
| Unknown | 2 (2) |
Data given as no. (%) unless otherwise indicated. Abbreviation: SD, standard deviation.
Because of omissions in patient records and/or failure of patients/oncologists to respond to a question, the sum of the numbers by category may not equal the total number of patients or oncologists.
Table 3.
Oncologists’ and Patients’ Ratings and Responses to Questions
| Ratings and Responses by Group | Mean (SD) |
|---|---|
| Oncologists | |
| Communication with patient (1: strongly disagree, to 5: strongly agree) | |
| Informativeness (n = 96) | 3.92 (0.60) |
| Supportiveness (n = 96) | 3.58 (0.52) |
| Partnership building (n = 96) | 3.28 (0.52) |
| Patient-centered communication (average) (n = 95) | 3.59 (0.52) |
| Length of time oncologist and patient both in room (n = 96) | 30.07 (13.57) |
| Oncologist-to-patient talk time ratio (n = 95) | 3.82 (2.66) |
| Involving patient in treatment decision (1: completely, to 5: not at all) (n = 88) | 2.88 (1.16) |
| Patients | |
| Immediately after visit | |
| Perceived patient-centeredness (1: not at all, to 4: completely) (n = 105) | 3.65 (0.34) |
| Distress after visit (1: none, to 11: extreme) (n = 102) | 4.75 (3.01) |
| Difficulty remembering what was said (1: very easy, to 5: very difficult) (n = 103) | 1.83 (0.96) |
| Follow-up interview | |
| Difficulty remembering what was said (1: very easy, to 5: very difficult) (n = 107) | 2.07 (0.91) |
| Oncologist trustworthy (1: strongly disagree, to 5: strongly agree) (n = 98) | 4.19 (0.61) |
| Treatment expectations | |
| Confidence in recommended treatment (1: extremely unsure, to 5: extremely sure) (n = 88) | 4.68 (0.94) |
| Difficulty of completing treatment (1: extremely easy, to 5: extremely hard) (n = 70) | 2.46 (0.96) |
| Severity of treatment side effects (1: extremely mild, to 5: extremely serious) (n = 68) | 3.24 (1.13) |
Abbreviation: SD, standard deviation.
Oncologist implicit racial bias.
Oncologists’ mean and median IAT d-scores were statistically significant (P < .01) but had a small to moderate level (d = .26) of implicit racial bias. This level is lower than national norms for physicians24 but consistent with IAT data from physicians in the same geographic region.34 Bivariate relationships between oncologist implicit bias and outcome measures are shown in Table 4.
Table 4.
Bivariate Associations With Oncologist Implicit Bias
| Measure | No. | β | SE | CI | P |
|---|---|---|---|---|---|
| Oncologists | |||||
| Informative style | 96 | −0.21 | 0.14 | −0.65 to 0.20 | .18 |
| Supportive style | 96 | −0.42 | 0.17 | −0.77 to −0.06 | <.01* |
| Partnership style | 96 | −0.11 | 0.15 | −0.44 to 0.22 | .48 |
| Patient-centered style | 96 | −0.19 | 0.17 | −0.55 to 0.17 | .28 |
| Length of interaction | 96 | −0.37 | 0.14 | −0.67 to −0.08 | .02 |
| Talk-time ratio | 96 | −0.04 | 0.03 | −0.10 to 0.05 | .27 |
| Involving patient | 88 | 0.27 | 0.14 | −0.02 to 0.57 | .22* |
| Patients | |||||
| Post-visit reactions | |||||
| Patient centeredness | 105 | −0.25 | 0.09 | −0.51 to −0.05 | .01 |
| Difficulty remembering | 103 | 0.15 | 0.04 | 0.05 to 0.36 | .01 |
| Distress | 102 | 0.06 | 0.11 | −0.16 to 0.29 | .57 |
| Follow-up reactions | |||||
| Difficulty remembering | 107 | 0.20 | 0.09 | −0.39 to 0.01 | .06 |
| Trust | 98 | −0.11 | 0.06 | −0.23 to 0.01 | .08 |
| Treatment perceptions | |||||
| Confidence | 88 | −0.13 | 0.10 | −0.35 to 0.07 | .19 |
| Difficulty | 70 | 0.08 | 0.12 | −0.17 to 0.33 | .51 |
With covariate.
Oncologists
Communication, patient involvement, and talk time.
There was a significant negative association between oncologist implicit racial bias and observers’ ratings of oncologists’ supportive communication (P < .01), after controlling for physician age. There was also a significant negative association between oncologist implicit bias and interaction length (P = .02). Associations between oncologist implicit bias and the extent to which oncologists involved their patients in treatment decisions (controlling for patient income) and talk-time ratio were not significant (P > .22).
Patients
Reactions to oncologists and interactions.
Patients who interacted with oncologists with higher implicit racial bias perceived their communication as less patient-centered (P = .01) and reported greater difficulty remembering conversation contents (P < .01) immediately after interactions. Higher oncologist implicit bias was not associated with patients’ immediate post-visit distress, with continued difficulty remembering conversation contents, or with trust of their oncologist assessed 1 week later (P > .05).
Perceptions of recommended treatments.
There were no significant direct associations between implicit bias and patient treatment perceptions (P > .20). However, as predicted, there were significant indirect effects of oncologist implicit racial bias on patients’ degree of confidence in recommended treatments and perceptions of difficulty in completing them.
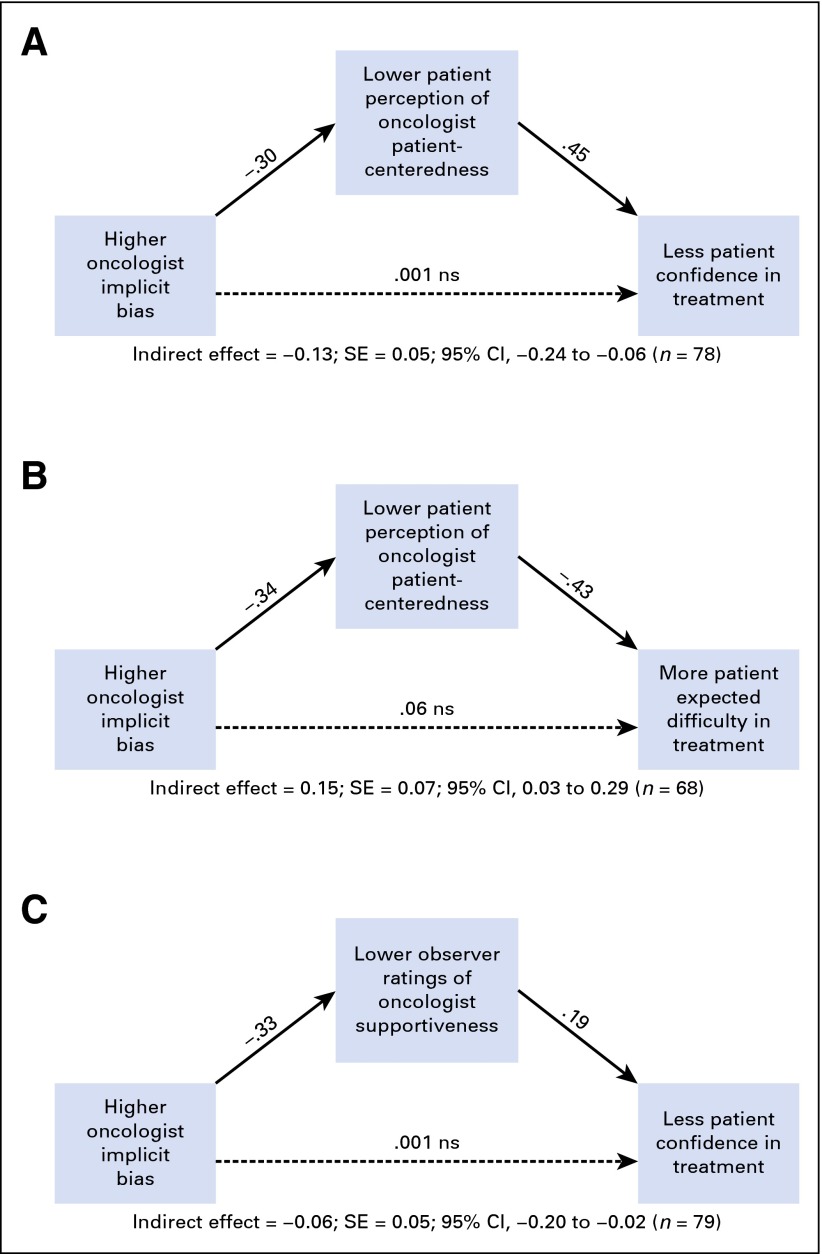
Figure 1A shows the significant indirect path from oncologist implicit racial bias to patients’ perceptions of oncologist patient-centeredness to patient confidence in recommended treatments (95% CI, −0.24 to −0.06). Higher levels of oncologist implicit bias were associated with patients perceiving the oncologist as less patient-centered, which, in turn, was associated with less patient confidence in recommended treatments.
Fig 1.
Indirect effects of oncologist implicit bias on patient treatment expectations. (A) Patients’ perceptions of oncologist patient centeredness and confidence in treatment. (B) Patients’ perceptions of oncologist patient centeredness and expected difficulty in treatment. (C) Observers’ ratings of oncologist supportiveness and patient confidence in treatment.
Figure 1B shows the significant indirect path from oncologist implicit bias to patients’ perceptions of difficulty completing treatment (95% CI, 0.03 to 0.29). Higher levels of oncologist implicit bias were associated with patients perceiving the oncologist as less patient-centered, which, in turn, was associated with patients perceiving greater difficulty in completing treatments.
Figure 1C shows another significant indirect path from oncologist implicit bias to patients’ confidence in recommended treatments (95% CI, −0.19 to −0.01). Higher levels of implicit bias were associated with lower observer ratings of oncologist supportive communication, which, in turn, were associated with less patient confidence in recommended treatments.
DISCUSSION
Tests of the first set of hypotheses replicated prior findings of associations between physician implicit racial bias and their communication and patient reactions,26,36,37 and identified new associations as well. Of special note, we showed that independent observers of the interactions, like the black patients, perceived lower-quality communication among oncologists who had higher levels of implicit bias. Perhaps even more importantly, tests of the second set of hypotheses showed for the first time a significant link between non-black oncologist implicit bias and black patients’ perceptions of the treatments recommended to them.
Limitations
Because data came only from racially discordant interactions, we could not demonstrate that the effects of oncologist implicit racial bias were unique to racially discordant interactions. However, four prior studies of provider implicit bias included white patients36,37,40,41; all found that implicit bias predicted more positive provider behavior toward and reactions from white patients. It seems likely that had we included white patients, implicit bias would have undermined only the quality of interactions involving black patients.
Because of a priori predictions, analyses were not adjusted for multiple testing, which is a potential limitation. Another limitation is small effect sizes (0.10 to 0.15) for indirect paths affecting patient treatment perceptions.57 Many factors (eg, type of proposed treatment) can affect patients’ treatment perceptions. Finding consistent and convergent effects of oncologist implicit bias on patient perceptions, despite these other factors, suggests the reliability and potency of the effects of implicit bias. Moreover, even relatively small statistical effects can have substantial practical impact when they occur frequently. Perhaps the best example of this is aspirin’s impact on the incidence of myocardial infarctions (MIs). The original randomized study of aspirin’s effects58 found that, over 5 years, 139 of 10,898 physicians who took aspirin daily had an MI, whereas 239 of 10,795 physicians who took a placebo had an MI, which was significant (P < .001). Rosenthal59 recommended casting these data in a 2 (aspirin v placebo) × 2 (MIs present v absent) table and calculating the actual effect size for the findings (a ϕ coefficient); the ϕ was .03, a small significant effect.57 Yet daily use of aspirin has dramatic health benefits for men older than age 50 years. Indeed, over a 5-year period, using aspirin daily would result in at least 400,000 fewer MIs (Data Supplement).59,60 Relating this to the current findings, about 190,000 blacks are diagnosed annually with cancer.61 An effect size of 0.10 on treatment decisions that is due to oncologist implicit racial bias could affect a substantial number of patient-treatment decisions.
Implications
The common goal of oncology interactions is to discuss treatments for potentially life-threatening diseases. One might suppose that in such high-stakes interactions, factors such as oncologist racial bias and patient race would have minimal roles in how an interaction proceeds and its outcomes. However, our findings provide rather persuasive empirical evidence that contradicts this supposition. Oncologist implicit racial bias was negatively associated with important aspects of what transpired between non-black oncologists and black patients.
We acknowledge it is unlikely racial bias alone is the major source of the well-documented, widespread racial disparities in cancer treatment. Factors such as patient socioeconomic status, limited access to high-quality health care, and patient health-related attitudes also contribute to racial disparities in cancer treatment.1,17 However, our data suggest that oncologist implicit racial bias may uniquely contribute to these disparities and should be further explored. Greater understanding of how oncologist implicit bias affects the quality of care received by black patients with cancer may enable researchers to identify which of many proposed interventions39,62,63 may hold the greatest promise for the critical task of reducing the impact of implicit racial bias on racial disparities in cancer treatment.
Supplementary Material
Footnotes
Listen to the podcast by Dr Fiscella at www.jco.org/podcasts
Supported by Grant Nos. 1U54CA153606-01 and P30CA022453 from the National Cancer Institute, and by the Southeast Michigan Partners Against Cancer Research Advisory Committee.
Presented at the Medical Interactions First Annual Well-Med Conference, Alexandroupolis, Greece, May 28-June 1, 2014.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Louis A. Penner, John F. Dovidio, Terrance L. Albrecht, Robert Chapman, Anthony F. Shields, Susan Eggly
Collection and assembly of data: Louis A. Penner, Tanina Foster, Nao Hagiwara, Lauren M. Hamel, Anthony F. Shields, Shirish Gadgeel, Michael S. Simon
Data analysis and interpretation: Louis A. Penner, Richard Gonzalez, Tanina Foster, Felicity W.K. Harper, Jennifer J. Griggs
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The Effects of Oncologist Implicit Racial Bias in Racially Discordant Oncology Interactions
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Louis A. Penner
No relationship to disclose
John F. Dovidio
No relationship to disclose
Richard Gonzalez
No relationship to disclose
Terrance L. Albrecht
Honoraria: Eli Lilly and Company
Consulting or Advisory Role: Eli Lilly and Company
Travel, Accommodations, Expenses: Eli Lilly and Company
Other Relationship: Albrecht Pharmaceutical Consulting (I)
Robert Chapman
No relationship to disclose
Tanina Foster
No relationship to disclose
Felicity W.K. Harper
No relationship to disclose
Nao Hagiwara
No relationship to disclose
Lauren M. Hamel
No relationship to disclose
Anthony F Shields
Consulting or Advisory Role: GE Healthcare
Speakers' Bureau: GE Healthcare
Research Funding: Karyopharm Therapeutics
Travel, Accommodations, Expenses: GE Healthcare
Shirish Gadgeel
Consulting or Advisory Role: Pfizer, Novartis, Boehringer Ingelheim, Genentech/Roche, AstraZeneca/MedImmune, Bristol-Myers Squibb, ARIAD
Speakers' Bureau: Genentech/Roche, AstraZeneca
Michael S. Simon
No relationship to disclose
Jennifer J. Griggs
No relationship to disclose
Susan Eggly
No relationship to disclose
REFERENCES
- 1.Smedley BD, Stith AY, Nelson AR. Unequal treatment: Confronting racial and ethnic disparities in health care. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 2. Agency for Healthcare Research and Quality. 2014 National Healthcare Quality and Disparities Report. Rockville, MD, Agency for Healthcare Research and Quality, 2015. [Google Scholar]
- 3. Mead H, Cartwright-Smith L, Jones K, et al. Racial and Ethnic Disparities in U.S. Healthcare: A Chartbook. New York, NY, Commonwealth Fund, 2008. [Google Scholar]
- 4.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 5.Tehranifar P, Neugut AI, Phelan JC, et al. Medical advances and racial/ethnic disparities in cancer survival. Cancer Epidemiol Biomarkers Prev. 2009;18:2701–2708. doi: 10.1158/1055-9965.EPI-09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65:221–238. doi: 10.3322/caac.21271. [DOI] [PubMed] [Google Scholar]
- 7.Hayn MH, Orom H, Shavers VL, et al. Racial/ethnic differences in receipt of pelvic lymph node dissection among men with localized/regional prostate cancer. Cancer. 2011;117:4651–4658. doi: 10.1002/cncr.26103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griggs JJ, Sorbero ME, Stark AT, et al. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003;81:21–31. doi: 10.1023/A:1025481505537. [DOI] [PubMed] [Google Scholar]
- 9.Penner LA, Eggly S, Griggs JJ, et al. Life-threatening disparities: The treatment of black and white cancer patients. J Soc Issues. 2012;68:328–357. doi: 10.1111/j.1540-4560.2012.01751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griggs JJ, Culakova E, Sorbero ME, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007;25:2522–2527. doi: 10.1200/JCO.2006.10.2749. [DOI] [PubMed] [Google Scholar]
- 11.Phelan JC, Link BG. Is racism a fundamental cause of inequalities in health? Ann. Rev Sociol. 2015;41:311–330. [Google Scholar]
- 12.Murphy CC, Harlan LC, Warren JL, et al. Race and insurance differences in the receipt of adjuvant chemotherapy among patients with stage III colon cancer. J Clin Oncol. 2015;33:2530–2536. doi: 10.1200/JCO.2015.61.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meghani SH, Kang Y, Chittams J, et al. African Americans with cancer pain are more likely to receive an analgesic with toxic metabolite despite clinical risks: A mediation analysis study. J Clin Oncol. 2014;32:2773–2779. doi: 10.1200/JCO.2013.54.7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel MI, Ma Y, Mitchell B, et al. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24:344–349. doi: 10.1158/1055-9965.EPI-14-0963. [DOI] [PubMed] [Google Scholar]
- 15.Hassett MJ, Schymura MJ, Chen K, et al. Variation in breast cancer care quality in New York and California based on race/ethnicity and Medicaid enrollment. Cancer. 2016;122:420–431. doi: 10.1002/cncr.29777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein RM, Street RL., Jr . Patient-Centered Communication in Cancer Care: Promoting healing and reducing suffering. National Cancer Institute, Bethesda, MD. 2007. [Google Scholar]
- 17.Penner L, Albrecht TL, Coleman DK, et al. Interpersonal perspectives on Black-White health disparities: social policy implications. Soc Issues Policy Rev. 2007;1:63–98. [Google Scholar]
- 18.Dovidio JF, Penner LA, Albrecht TL, et al. Disparities and distrust: The implications of psychological processes for understanding racial disparities in health and health care. Soc Sci Med. 2008;67:478–486. doi: 10.1016/j.socscimed.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Hamel LM, Chapman R, Malloy M, et al. Critical shortage of African American medical oncologists in the United States. J Clin Oncol. 2015;33:3697–3700. doi: 10.1200/JCO.2014.59.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laveist TA, Nuru-Jeter A. Is doctor-patient race concordance associated with greater satisfaction with care? J Health Soc Behav. 2002;43:296–306. [PubMed] [Google Scholar]
- 21. Blair IV, Dasgupta N, Glaser J: Implicit attitudes, in Mikulincer M, Shaver PR (eds): APA Handbook of Personality and Social Psychology. Washington, DC, American Psychological Association, 2015, pp 665691. [Google Scholar]
- 22.Greenwald AG, Poehlman TA, Uhlmann EL, et al. Understanding and using the Implicit Association Test: III. Meta-analysis of predictive validity. J Pers Soc Psychol. 2009;97:17–41. doi: 10.1037/a0015575. [DOI] [PubMed] [Google Scholar]
- 23.Blair IV, Havranek EP, Price DW, et al. Assessment of biases against Latinos and African Americans among primary care providers and community members. Am J Public Health. 2013;103:92–98. doi: 10.2105/AJPH.2012.300812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabin J, Nosek BA, Greenwald A, et al. Physicians’ implicit and explicit attitudes about race by MD race, ethnicity, and gender. J Health Care Poor U. 2009;20:896–913. doi: 10.1353/hpu.0.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sritharan R, Gawronski B. Changing implicit and explicit prejudice insights from the associative-propositional evaluation model. Soc Psychol. 2010;41:113–123. [Google Scholar]
- 26.Penner LA, Blair IV, Albrecht TL, et al. Reducing racial health care disparities: A social psychological analysis. Policy Insights Behav Brain Sci. 2014;1:204–212. doi: 10.1177/2372732214548430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Godsil RD, Tropp LR, Goff PA, et al. Addressing Implicit Bias, Racial Anxiety, and Stereotype Threat in Education and Health Care. Berkeley, CA, Perception Institute, 2014. [Google Scholar]
- 28.Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: A systematic review. Am J Public Health. 2015;105:e60–e76. doi: 10.2105/AJPH.2015.302903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabin JA, Rivara FP, Greenwald AG. Physician implicit attitudes and stereotypes about race and quality of medical care. Med Care. 2008;46:678–685. doi: 10.1097/MLR.0b013e3181653d58. [DOI] [PubMed] [Google Scholar]
- 30.Haider AH, Sexton J, Sriram N, et al. Association of unconscious race and social class bias with vignette-based clinical assessments by medical students. JAMA. 2011;306:942–951. doi: 10.1001/jama.2011.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haider AH, Schneider EB, Sriram N, et al. Unconscious race and class bias: Its association with decision making by trauma and acute care surgeons. J Trauma Acute Care Surg. 2014;77:409–416. doi: 10.1097/TA.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 32.Oliver MN, Wells KM, Joy-Gaba JA, et al. Do physicians’ implicit views of African Americans affect clinical decision making? J Am Board Fam Med. 2014;27:177–188. doi: 10.3122/jabfm.2014.02.120314. [DOI] [PubMed] [Google Scholar]
- 33.Hirsh AT, Hollingshead NA, Ashburn-Nardo L, et al. The interaction of patient race, provider bias, and clinical ambiguity on pain management decisions. J Pain. 2015;16:558–568. doi: 10.1016/j.jpain.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penner LA, Dovidio JF, West TV, et al. Aversive racism and medical interactions with Black patients: A field study. J Exp Soc Psychol. 2010;46:436–440. doi: 10.1016/j.jesp.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hagiwara N, Penner LA, Gonzalez R, et al. Racial attitudes, physician-patient talk time ratio, and adherence in racially discordant medical interactions. Soc Sci Med. 2013;87:123–131. doi: 10.1016/j.socscimed.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102:979–987. doi: 10.2105/AJPH.2011.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blair IV, Steiner JF, Fairclough DL, et al. Clinicians’ implicit ethnic/racial bias and perceptions of care among Black and Latino patients. Ann Fam Med. 2013;11:43–52. doi: 10.1370/afm.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagiwara N, Dovidio JF, Eggly S, et al. The effects of racial attitudes on affect and engagement in racially discordant medical interactions between non-Black physicians and Black patients. Group Process Interg. doi: 10.1177/1368430216641306. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penner LA, Gaertner S, Dovidio JF, et al. A social psychological approach to improving the outcomes of racially discordant medical interactions. J Gen Intern Med. 2013;28:1143–1149. doi: 10.1007/s11606-013-2339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hausmann LR, Myaskovsky L, Niyonkuru C, et al. Examining implicit bias of physicians who care for individuals with spinal cord injury: A pilot study and future directions. J Spinal Cord Med. 2015;38:102–110. doi: 10.1179/2045772313Y.0000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaa KL, Roter DL, Biesecker BB, et al. Genetic counselors’ implicit racial attitudes and their relationship to communication. Health Psychol. 2015;34:111–119. doi: 10.1037/hea0000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eggly S, Tkatch R, Penner LA, et al. Development of a question prompt list as a communication intervention to reduce racial disparities in cancer treatment. J Cancer Educ. 2013;28:282–289. doi: 10.1007/s13187-013-0456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riddle DL, Albrecht TL, Coovert MD, et al. Differences in audiotaped versus videotaped physician-patient interactions. J Nonverbal Behav. 2002;26:219–239. [Google Scholar]
- 44. Penner LA, Orom H, Albrecht TL, et al: Camera-related behaviors during video recorded medical interactions. J Nonverbal Behav 31:99-117, 2007 doi: 10.1007/s10919-007-0024-8. [Google Scholar]
- 45.Greenwald AG, Nosek BA, Banaji MR. Understanding and using the implicit association test: I. An improved scoring algorithm. J Pers Soc Psychol. 2003;85:197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- 46.Gawronski B. What does the implicit association test measure? A test of the convergent and discriminant validity of prejudice-related IATs. Exp Psychol. 2002;49:171–180. doi: 10.1026//1618-3169.49.3.171. [DOI] [PubMed] [Google Scholar]
- 47. Nosek BA, Greenwald A, Banaji MR: The Implicit Association Test at age 7: A methodological and conceptual review, in Bargh JA (ed): Social Psychology and the Unconscious: The Automaticity of Higher Mental Processes, New York, NY, Psychology Press, 2007, pp. 265-292.
- 48.Street RL, Jr, Gordon H, Haidet P. Physicians’ communication and perceptions of patients: Is it how they look, how they talk, or is it just the doctor? Soc Sci Med. 2007;65:586–598. doi: 10.1016/j.socscimed.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49:796–804. [PubMed] [Google Scholar]
- 50.Jacobsen PB. Screening for psychological distress in cancer patients: Challenges and opportunities. J Clin Oncol. 2007;25:4526–4527. doi: 10.1200/JCO.2007.13.1367. [DOI] [PubMed] [Google Scholar]
- 51.Dugan E, Trachtenberg F, Hall MA. Development of abbreviated measures to assess patient trust in a physician, a health insurer, and the medical profession. BMC Health Serv Res. 2005;5:64. doi: 10.1186/1472-6963-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 53.Posada D, Buckley TR. Model selection and model averaging in phylogenetics: Advantages of akaike information criterion and bayesian approaches over likelihood ratio tests. Syst Biol. 2004;53:793–808. doi: 10.1080/10635150490522304. [DOI] [PubMed] [Google Scholar]
- 54.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 55.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- 56.Mackinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behav Res. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 58. doi: 10.1056/NEJM198907203210301. Steering Committee of the Physicians’ Health Study Research Group: Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 321:129-135, 1989 . [DOI] [PubMed] [Google Scholar]
- 59.Rosenthal R. How are we doing in soft psychology? Am Psychol. 1990;45:775–777. [Google Scholar]
- 60.Greenwald AG, Banaji MR, Nosek BA. Statistically small effects of the Implicit Association Test can have societally large effects. J Pers Soc Psychol. 2015;108:553–561. doi: 10.1037/pspa0000016. [DOI] [PubMed] [Google Scholar]
- 61. American Cancer Society: Cancer facts and figures for African Americans 2016-2018. Atlanta, GA, American Cancer Society, 2016.
- 62.Burgess D, van Ryn M, Dovidio J, et al. Reducing racial bias among health care providers: Lessons from social-cognitive psychology. J Gen Intern Med. 2007;22:882–887. doi: 10.1007/s11606-007-0160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burgess D J, Fu SS, van Ryn M. Why do providers contribute to disparities and what can be done about it? J Gen Intern Med. 2004;19:1154–1159. doi: 10.1111/j.1525-1497.2004.30227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.