Abstract
Purpose
The optimal frequency of monitoring patients with metastatic breast cancer (MBC) is unknown; however, data suggest that intensive monitoring does not improve outcomes. We performed a population-based analysis to evaluate patterns and predictors of extreme use of disease-monitoring tests (serum tumor markers [STMs] and radiographic imaging) among women with MBC.
Methods
The SEER-Medicare database was used to identify women with MBC diagnosed from 2002 to 2011 who underwent disease monitoring. Billing dates of STMs (carcinoembryonic antigen and/or cancer antigen 15-3/cancer antigen 27.29) and imaging tests (computed tomography and/or positron emission tomography) were recorded; if more than one STM or imaging test were completed on the same day, they were counted once. We defined extreme use as > 12 STM and/or more than four radiographic imaging tests in a 12-month period. Multivariable analysis was used to identify factors associated with extreme use. In extreme users, total health care costs and end-of-life health care utilization were compared with the rest of the study population.
Results
We identified 2,460 eligible patients. Of these, 924 (37.6%) were extreme users of disease-monitoring tests. Factors significantly associated with extreme use were hormone receptor–negative MBC (odds ratio [OR], 1.63; 95% CI, 1.27 to 2.08), history of a positron emission tomography scan (OR, 2.92; 95% CI, 2.40 to 3.55), and more frequent oncology office visits (OR, 3.14; 95% CI, 2.49 to 3.96). Medical costs per year were 59.2% higher in extreme users. Extreme users were more likely to use emergency department and hospice services at the end of life.
Conclusion
Despite an unknown clinical benefit, approximately one third of elderly women with MBC were extreme users of disease-monitoring tests. Higher use of disease-monitoring tests was associated with higher total health care costs. Efforts to understand the optimal frequency of monitoring are needed to inform clinical practice.
INTRODUCTION
The distribution of health care expenditures in the United States is skewed, with a small proportion of patients consuming a disproportionately high proportion of health care resources. In 2010, 1% of the total population accounted for > 20% of total health care costs, and the top 50% accounted for 97.2% of overall health care expenditures.1 In the Medicare population, diagnostic testing, including radiographic imaging, is the most rapidly growing sector of reimbursed services and represents almost one quarter of ambulatory health care costs.2 Among Medicare beneficiaries with cancer, imaging costs have risen at a rate that outpaces total health care costs.3 A population-based study of Medicare patients with metastatic cancer demonstrated a > 50% increase in imaging tests per patient per month from 1995 to 2006.4 This is particularly salient because cancer care costs are rising rapidly and are highest in patients with advanced cancer.5
In patients with advanced cancer, the optimal frequency and modality of disease monitoring are unknown. A potential advantage of more frequent testing may be earlier detection of disease progression that would result in a switch to alternate therapies. Clinical guidelines, such as those of the National Comprehensive Cancer Center Network, suggest that patients with metastatic disease should be monitored routinely while undergoing systemic therapy to continue treatments that control disease while avoiding toxicities from nonefficacious therapies. These guidelines do not specify the optimal frequency or the modality (radiographic imaging, serum tumor markers [STMs]).6 Although no prospective studies have evaluated whether frequent testing is associated with better outcomes, some data have suggested that earlier detection of disease progression is not associated with improved outcomes.7 Disease monitoring contributes significantly to health care costs for patients with metastatic breast cancer (MBC).8-14
A subset of the general population uses a disproportionately high percentage of health care services.15 Previous reports have shown that higher use of imaging studies is associated with patient age, ethnicity, and socioeconomic status.16,17 The objectives of this study were to identify patterns and predictors of use and extreme use of disease-monitoring tests (ie, STMs, radiographic imaging studies) among women with MBC.
METHODS
Data Source
Data from the SEER-Medicare database were analyzed.18 SEER data provide tumor characteristics and represent 28% of the US population.19 Linkage with the Medicare database allows longitudinal evaluation of cancer care and characterizes inpatient, outpatient, and physician-billed services, including diagnoses and health care costs.20
Cohort Selection
We identified all women age ≥ 65 years with pathologically confirmed breast cancer diagnosed between January 1, 2002, and December 31, 2011. To evaluate patients monitored with both STMs and imaging, the study population was restricted to patients with stage IV breast cancer who had at least two claims for STM tests and at least two claims for a computed tomography (CT) and/or positron emission tomography (PET) scan after the date of diagnosis.
We excluded patients who were enrolled in a non-Medicare health maintenance organization or who were not continuously enrolled by Medicare Parts A and B for a period of 12 months before diagnosis through death or end of the study period. Patients who were enrolled in Medicare due to end-stage renal disease as well as patients with other primary cancers were excluded.
Disease-Monitoring Testing and Extreme Use
Breast cancer STM tests included carcinoembryonic antigen (CEA), cancer antigen (CA) 27.29, and CA 15-3.21-23 CEA testing (Healthcare Common Procedure Coding System [HCPCS] code 82378) was identified uniquely; however, CA 15-3 and CA 27.29 testing could not be separated because one code is used for both tests (HCPCS 86300).24 The date of each STM test was recorded from the time of diagnosis until death or end of the study period. To avoid overcounting, each CEA and CA 27.29/15-3 claim was counted as a single STM test if performed on the same day.
Claims for imaging tests were identified with HCPCS, International Classification of Diseases, Ninth Revision (ICD-9), and Current Procedural Terminology (CPT) codes.4 CT scans of the chest, abdomen, and pelvis performed on the same day were counted as one imaging test. Each PET-CT scan (claims for PET and CT scans on the same day) was counted as one PET claim. We eliminated duplicate claims within each file by matching patient identifier, date of procedure, CPT, HCPCS, or ICD-9 code. Because outpatient claims often do not include date of service and may be submitted according to a billing cycle rather than to the date of service, we extended the date criteria for this match to 7 days; > 98% of matches occurred on the same day.4 For duplicate claims, we preferentially retained the claim billed with CPT or HCPCS codes over ICD-9 codes.4 To avoid duplicate counting, a cap of two imaging tests per week was applied.
On the basis of our prior work and monitoring patterns in clinical trials, we defined extreme users of disease-monitoring testing as any patients who had > 12 STMs and/or more than four radiographic imaging tests in any 1-year interval from diagnosis until death or end of the study period.9,25,26
Covariates
Demographic covariates included age at diagnosis (65 to 69, 70 to 74, 75 to 79, ≥ 80 years), year of diagnosis, marital status (married, single, unknown), ethnicity (white, other), geographic area classified (metropolitan, other), region, Charlson comorbidity score (0, 1, ≥ 2), and socioeconomic status. Tumors were categorized as hormone receptor (estrogen receptor [ER] and/or progesterone receptor [PR]) positive, negative, or unknown.
Other covariates were history of PET scan after diagnosis of breast cancer (yes, no), maximum number of imaging tests in any 1-year period (two or fewer, three, four, five or more), and maximum number of medical oncology office visits in a 1-year time frame (low, medium, high). To define medical oncology office visits, physician files were used to determine provider specialty and date of office visit. Medical oncologists were identified as physicians with a listed specialty of medical oncology or hematology/oncology. Visits were identified through HCPCS codes for office new visits, office established visits, and office consultations.
Costs of Care
Costs of care were calculated from Medicare reimbursement claims from physician, hospital, outpatient, durable medical equipment, and hospice filings between the date of diagnosis and the date of death or end of the study period. Costs were categorized as total costs in the first year, total costs in the last year of life, and total costs per year alive.
End-of-Life Care
To further understand the relationship between disease monitoring and health care costs at the end of life, end-of-life quality-of-care indicators were evaluated in the last month of life.27,28 Quality-of-care indicators evaluated in the last month of life were more than one emergency department visit, more than one hospital admission, more than 14 days hospitalized, admission to the intensive care unit (ICU), and admission to hospice within 3 days before death.27,28 ICU admissions in the last month of life were identified by using ICD-9 codes (96.7x) and diagnosis-related group codes (475 or 483) for mechanical ventilation and the ICU indicator variable in the Medicare inpatient file.29,30 Hospice admissions were identified through billing claims for hospice in the Medicare hospice files during the last month of life.
Statistical Analysis
We calculated the per-patient-per-year STM testing rate and per-patient-per-year radiographic imaging rate. Univariable analyses comparing characteristics of patients and the care they received were performed with t tests for continuous variables and χ2 tests for categorical variables. We developed logistic regression models to determine the association between clinical, demographic, and treatment factors and extreme use of disease-monitoring testing.31,32
A linear regression model was used to estimate the association between extreme use of disease monitoring and total cost of care. Total cost was approximately log-normally distributed, and log-transformed cost of care was analyzed as a continuous response variable. To display results as percent changes in cost of care, parameter estimates from the regression model were exponentiated.24 We performed a sensitivity analysis by removing the costs of STM and radiographic imaging tests for each subject from the regression models. Costs of each STM and imaging modality were calculated from Medicare reimbursement rates.33,34 The effect extreme use of disease monitoring on overall survival (OS) was evaluated by using a Cox proportional hazard model. All analyses were conducted with SAS version 9.4 software (SAS Institute, Cary, NC). All statistical tests were two sided, with α = .05.
RESULTS
We identified 6,038 women with de novo MBC between 2002 and 2011 of whom 2,460 (40.7%) were eligible for the analysis. A total of 3,548 (58.8%) were excluded because they were not monitored with STMs. The cohort was predominantly white (85.4%), single (60.3%), and without comorbidities (57.3%; Table 1). The majority (1,784 [72.5%]) had hormone receptor–positive MBC. The majority (85.7%) were alive > 12 months from time of diagnosis during the study period. Among the 2,460 included patients, 924 (37.6%) were classified as extreme users, 222 (9.0%) were extreme users of STM tests, and 807 (32.8%) were extreme users of radiographic imaging. Additionally, the results of a sensitivity analysis of the proportion of extreme users with a cap of one imaging test per week were similar. Extreme users were more likely to be younger (age ≥ 80 years, 18.8% v 32.6%; P < .001), to have ER/PR-negative cancer (18.4% v 10.7%; P < .001), to have had at least one PET scan (73.5% v 47.6%; P < .001), and to have more oncology visits (42.8% v 20.4%; P < .001).
Table 1.
Demographics of Study Population and Associations With Extreme Use of Disease-Monitoring Testing
| Total Population | Extreme Users | OR* | 95% CI | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| No. of patients | 2,460 | 100 | 924 | 37.6 | ||
| Age, years | ||||||
| 65-69 | 581 | 23.6 | 260 | 28.2 | 0.97 | 0.76 to 1.23 |
| 70-74 | 655 | 26.6 | 285 | 30.8 | Reference | |
| 75-79 | 550 | 22.4 | 205 | 22.2 | 0.85 | 0.68 to 1.12 |
| ≥ 80 | 674 | 27.4 | 174 | 18.8 | 0.58† | 0.45 to 0.75 |
| Diagnosis year | ||||||
| 2002-2004 | 649 | 26.4 | 241 | 26.1 | Reference | |
| 2005-2007 | 796 | 32.4 | 316 | 34.2 | 0.94 | 0.74 to 1.19 |
| 2008-2011 | 1,015 | 41.3 | 367 | 39.7 | 0.75† | 0.59 to 0.95 |
| Ethnicity | ||||||
| White | 2,200 | 85.4 | 791 | 85.6 | Reference | |
| Other/unknown | 360 | 14.6 | 133 | 14.4 | 1.02 | 0.79 to 1.33 |
| Marital status | ||||||
| Married | 870 | 35.4 | 380 | 41.1 | Reference | |
| Single | 1,483 | 60.3 | 500 | 54.1 | 0.77† | 0.63 to 0.93 |
| Geographic area | ||||||
| Large metropolitan | 1,460 | 59.3 | 526 | 56.9 | 0.83 | 0.69 to 1.00 |
| Other | 1,000 | 40.7 | 398 | 43.1 | Reference | |
| Charlson comorbidity score | ||||||
| 0 | 1,408 | 57.3 | 526 | 57.0 | Reference | |
| 1 | 632 | 25.7 | 232 | 25.1 | 1.15 | 0.93 to 1.43 |
| ≥ 2 | 417 | 17.0 | 165 | 17.9 | 1.43† | 1.12 to 1.84 |
| Region | ||||||
| East | 757 | 30.8 | 273 | 29.5 | Reference | |
| Midwest | 832 | 33.8 | 327 | 35.4 | 1.04 | 0.83 to 1.32 |
| West | 871 | 35.4 | 324 | 35.1 | 0.89 | 0.71 to 1.11 |
| Socioeconomic status | ||||||
| Low | 1,112 | 45.2 | 417 | 45.2 | Reference | |
| Medium | 620 | 25.2 | 216 | 23.4 | 0.92 | 0.73 to 1.16 |
| High | 726 | 29.6 | 289 | 31.4 | 1.15 | 0.92 to 1.45 |
| ER/PR status | ||||||
| Positive | 1,784 | 72.5 | 644 | 69.7 | Reference | |
| Negative | 335 | 13.6 | 170 | 18.4 | 1.63† | 1.27 to 2.08 |
| Unknown | 341 | 13.9 | 110 | 11.9 | 0.97 | 0.74 to 1.25 |
| PET use | ||||||
| No | 1,050 | 42.7 | 245 | 26.5 | Reference | |
| Yes | 1,410 | 57.3 | 679 | 73.5 | 2.92† | 2.40 to 3.55 |
| Maximum oncology office visit volume in any 1 year | ||||||
| Low | 792 | 32.2 | 205 | 22.2 | Reference | |
| Medium | 799 | 32.5 | 277 | 30.0 | 1.42† | 1.13 to 1.78 |
| High | 709 | 28.8 | 395 | 42.7 | 3.14† | 2.49 to 3.96 |
| Maximum No. of imaging tests in any 1 year | 1.31 | 0.88 to 1.95 | ||||
| ≤ 2 | 623 | 25.3 | ||||
| 3 | 546 | 22.2 | ||||
| 4 | 484 | 19.7 | ||||
| ≥ 5 | 807 | 32.8 | ||||
| More than 12 STM tests in any 1 year | ||||||
| Yes | 222 | 9.0 | ||||
| No | 2,238 | 91.0 | ||||
Abbreviations: ER/PR, estrogen receptor/progesterone receptor; OR, odds ratio; PET, positron emission tomography; STM, serum tumor marker.
ORs were derived from multivariable analysis, and models were adjusted for all other factors listed in the table.
P < .05.
In a multivariable model (Table 1), extreme use of disease-monitoring tests was associated with a Charlson comorbidity score ≥ 2 (odds ratio [OR], 1.43; 95% CI, 1.12 to 1.84), an ER/PR-negative cancer (OR, 1.63; 95% CI, 1.27 to 2.08), and a history of at least one PET scan (OR, 2.92; 95% CI, 2.40 to 3.55). Patients who had a higher number of oncology visits were more likely to have frequent testing (OR, 3.14; 95% CI, 2.49 to 3.96). Patients ≥ 80 years old (OR, 0.58; 95% CI, 0.45 to 0.75), patients diagnosed in later years (2008 to 2011), and patients who were single (OR, 0.77; 95% CI, 0.63 to 0.93) were less likely to be extreme users.
Increased use of STM tests was associated with having a higher socioeconomic status, having at least one PET scan (OR, 2.02; 95% CI, 1.42 to 2.88), and a higher frequency of office visits (OR, 1.72; 95% CI, 1.10 to 2.68; Table 2). Women with ER/PR-negative MBC (OR, 0.59; 95% CI, 0.37 to 0.95) were less likely to be extreme users of STM tests. Similar associations were seen with extreme use of radiographic imaging; however, women with ER/PR-negative MBC had a higher odds of extreme imaging (OR, 1.93; 95% CI, 1.50 to 2.49; Table 2). We found no difference in OS for patients who were extreme users of disease-monitoring testing (hazard ratio, 0.93; 95% CI, 0.86 to 1.02).
Table 2.
Demographics and Associations of Extreme Users of STM Tests and Radiographic Imaging
| STM Test | Radiographic Imaging | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | % | OR* | 95% CI | No. | % | OR* | 95% CI | |
| No. of patients | 222 | 9.0 | 807 | 32.8 | ||||
| Age, years | ||||||||
| 65-69 | 66 | 29.7 | 0.98 | 0.68 to 1.42 | 232 | 28.7 | 1.07 | 0.84 to 1.37 |
| 70-74 | 75 | 33.8 | Reference | 239 | 29.6 | Reference | ||
| 75-79 | 48 | 21.6 | 0.84 | 0.56 to 1.25 | 182 | 22.6 | 0.99 | 0.77 to 1.28 |
| ≥ 80 | 33 | 14.9 | 0.57† | 0.37 to 0.90 | 154 | 19.1 | 0.67† | 0.52 to 0.87 |
| Diagnosis year | ||||||||
| 2002-2004 | 68 | 30.6 | Reference | 198 | 24.5 | Reference | ||
| 2005-2007 | 74 | 33.3 | 0.83 | 0.56 to 1.21 | 275 | 34.1 | 1.01 | 0.79 to 1.29 |
| 2008-2011 | 80 | 36.1 | 0.67† | 0.46 to 0.98 | 334 | 41.4 | 0.89 | 0.70 to 1.13 |
| Ethnicity | ||||||||
| White | 202 | 91.0 | Reference | 683 | 84.6 | Reference | ||
| Other/unknown | 20 | 9.0 | 0.69 | 0.42 to 1.14 | 124 | 15.4 | 1.11 | 0.85 to 1.45 |
| Marital status | ||||||||
| Married | 100 | 45.1 | Reference | 334 | 41.4 | Reference | ||
| Single | 112 | 50.4 | 0.80 | 0.59 to 1.08 | 437 | 54.1 | 0.76† | 0.63 to 0.93 |
| Geographic location | ||||||||
| Large metropolitan | 134 | 60.4 | 0.85 | 0.62 to 1.16 | 458 | 56.7 | 0.88 | 0.72 to 1.06 |
| Other | 88 | 39.6 | Reference | 349 | 43.3 | Reference | ||
| Charlson comorbidity score | ||||||||
| 0 | 142 | 64.0 | Reference | 447 | 55.5 | Reference | ||
| 1 | 52 | 23.4 | 1.00 | 0.70 to 1.42 | 204 | 25.3 | 1.19 | 0.96 to 1.48 |
| ≥ 2 | 28 | 12.6 | 0.87 | 0.56 to 1.36 | 155 | 19.2 | 1.61† | 1.25 to 2.08 |
| Region | ||||||||
| East | 70 | 31.5 | Reference | 237 | 29.4 | Reference | ||
| Midwest | 42 | 18.9 | 0.58† | 0.38 to 0.89 | 305 | 37.8 | 1.16 | 0.91 to 1.48 |
| West | 110 | 49.6 | 1.51† | 1.07 to 2.13 | 265 | 32.8 | 0.77† | 0.61 to 0.97 |
| Socioeconomic status | ||||||||
| Low | 71 | 32.0 | Reference | 382 | 47.4 | Reference | ||
| Medium | 67 | 30.2 | 1.53† | 1.05 to 2.23 | 184 | 22.9 | 0.84 | 0.66 to 1.06 |
| High | 84 | 37.8 | 1.56† | 1.07 to 2.27 | 239 | 29.7 | 0.98 | 0.77 to 1.24 |
| ER/PR status | ||||||||
| Positive | 174 | 77.0 | Reference | 557 | 69.0 | Reference | ||
| Negative | 23 | 10.2 | 0.59† | 0.37 to 0.95 | 159 | 19.7 | 1.93† | 1.50 to 2.49 |
| Unknown | 29 | 12.8 | 1.02 | 0.65 to 1.59 | 91 | 11.3 | 0.95 | 0.72 to 1.26 |
| PET use | ||||||||
| No | 53 | 23.9 | Reference | 208 | 25.8 | Reference | ||
| Yes | 169 | 76.1 | 2.02† | 1.42 to 2.88 | 599 | 74.2 | 2.77† | 2.26 to 3.39 |
| Maximum oncology office visit volume in any 1 year | ||||||||
| Low | 43 | 19.4 | Reference | 177 | 21.9 | Reference | ||
| Medium | 52 | 23.4 | 1.06 | 0.70 to 1.63 | 245 | 30.4 | 1.46† | 1.15 to 1.85 |
| High | 121 | 54.5 | 2.58† | 1.75 to 3.80 | 343 | 42.5 | 2.84† | 2.23 to 3.61 |
| Maximum No. of imaging tests in any 1 year | ||||||||
| ≤ 2 | 33 | 14.9 | Reference | |||||
| 3 | 32 | 14.4 | 0.95 | 0.57 to 1.59 | ||||
| 4 | 52 | 23.4 | 1.60 | 0.99 to 2.59 | ||||
| ≥ 5 | 105 | 47.3 | 1.72† | 1.10 to 2.68 | ||||
| More than 12 STM tests in any 1 year | 105 | 13.0 | ||||||
| No | 702 | 87.0 | Reference | |||||
| Yes | 105 | 13.0 | 1.44† | 1.06 to 1.96 | ||||
Abbreviations: ER/PR, estrogen receptor/progesterone receptor; OR, odds ratio; PET, positron emission tomography; STM, serum tumor marker.
ORs were derived from multivariable analysis, and models were adjusted for all other factors listed in the table.
P < .05.
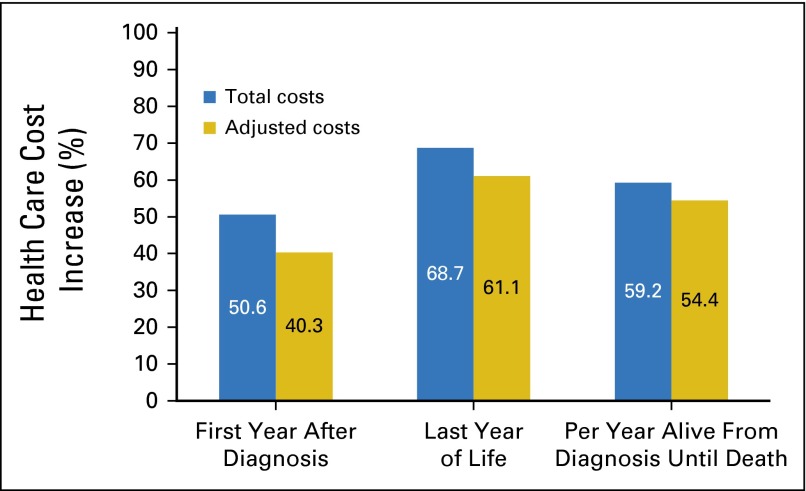
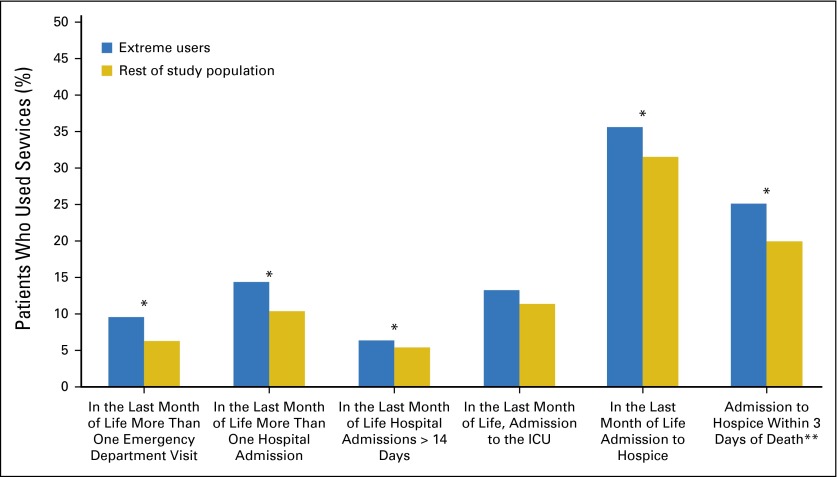
Total costs of care were higher for patients categorized as extreme users of disease-monitoring testing (Fig 1). For extreme users in the first year after diagnosis, costs were 50.6% higher (95% CI, 40.7% to 61.1%), and mean cost of care was $56,249 compared with $37,121 for the rest of the study population (P < .001). In the last year of life, costs were 68.7% higher (95% CI, 54.2% to 84.6%) in extreme users, and mean cost of care was $63,697 compared with $39,843 in the rest of the study population (P < .001). Total costs per year after diagnosis were also 59.2% (95% CI, 49.8% to 69.1%) higher in extreme users, and mean cost per year was $54,211 compared with $35,038 for the rest of the study population (P < .001). The results were similar after removing the total costs from disease-monitoring testing. With the exception of ICU admissions, extreme users were more likely to use health care services at the end of life compared with the rest of the study population (Fig 2). Extreme users were also more likely to be admitted to hospice closer to death.
Fig 1.
Percentage increase in total and adjusted costs of care from breast cancer diagnosis until death for extreme users of disease-monitoring testing. Adjusted for costs of disease-monitoring testing, including serum tumor-marker tests and radiographic imaging and analyzed by using natural log transformation by multivariable linear regression adjusted for characteristics displayed in Table 1 (20 participants not included due to no cost data). P < .001 for all values.
Fig 2.
Relationship between aggressive end-of-life care and extreme use of disease-monitoring testing. *P < .03. **Percentage of patients admitted to hospice. ICU, intensive care unit.
DISCUSSION
The findings suggest that approximately 40% of women older than age 65 years with MBC are monitored with both STMs and radiographic imaging. Of these patients, approximately one third undergo frequent disease monitoring. Women who have more appointments with their medical oncologist are more likely to undergo frequent testing as are those who undergo more expensive imaging tests (ie, PET scans). Total health care costs are approximately 50% higher in patients who have more frequent disease-monitoring testing, even after accounting for the individual costs of the tests. Additionally, extreme users of disease-monitoring testing are more likely to have increased health care service utilization near the end of life. Finally, there seems to be no association between disease monitoring and OS.
In the United States, an estimated 6% to 10% of new breast cancer cases are initially diagnosed as stage IV, and 20% to 30% of all cases become metastatic over time.35-37 Clinical guidelines suggest that patients with MBC should be actively monitored for disease progression to continue effective therapies and to avoid toxicities from therapies that are no longer effective.6 The National Comprehensive Cancer Network guidelines do not specifically recommend which tests to use and at what frequency.6 Currently in the metastatic setting, there is no evidence to suggest that more frequent testing is associated with better outcomes. A clinical trial in women with MBC with persistently increased circulating tumor cells suggested that changing treatment early was not associated with improvement in survival.7 Furthermore, in women with early-stage breast cancer, surveillance testing, which can result in earlier treatment initiation, does not affect survival.38,39
In addition to cost, frequent disease monitoring can be associated with emotional harm. The prevalence of anxiety and depression among patients with advanced cancers is estimated to be 25% to 65%.40 Previous work has shown that depression and anxiety can increase over time in patients with metastatic solid tumors and has been attributed to multiple factors, including fear of death and fear of disease progression.41,42 A study of 154 women with ovarian cancer found that on average, most women were moderately preoccupied with their CA-125 levels and that degree of preoccupation was associated with increased emotional distress.43 In a study that assessed distress in women during the surveillance period, women with more frequent testing had higher levels of anxiety without survival benefit.44
Despite potential emotional harms and unclear benefits, patients with other advanced solid tumors undergo frequent disease monitoring. Recently, we retrospectively evaluated 928 patients with advanced solid tumors and found almost one quarter had three or more individual STM tests within a 1-month period.9 To determine the rationale for STM evaluation, medical records of the top 10% of STMs were reviewed. Only 2% of patients had a change in treatment as a result of rising STMs after confirmation with radiographic imaging. The majority of oncologists reported that STM tests were ordered by copying the previous order.9
In patients with asymptomatic early-stage breast cancer, strong evidence exists against the use of surveillance testing to detect early recurrence.23,45-47 However, despite this evidence, there are still high rates of testing in this population. Recently, a population-based study demonstrated that 42.0% of elderly patients had STM tests despite guidelines against their use, and this testing was associated with increased Medicare expenditures.24 Another study demonstrated that 77% of women received at least one tumor marker test and that 57% received at least one nonrecommended imaging test.48
Another setting of high health care utilization in patients with cancer is during the end of life. Despite recommendations against aggressive care at the end of life, which represents poor quality of care, a high percentage of patients receive aggressive end-of-life care.27,28 A population-based study of elderly patients with advanced cancers found that approximately 10% of patients had aggressive use of hospital resources, including the emergency department, hospital admissions, and the ICU, in the last month of life.27 Also similar was a high proportion of patients admitted to hospice within the last 3 days of life.27 The same study found use of health care resources at the end of life is associated with receipt of other aggressive care measures. These results are similar to the present findings that extreme users of disease-monitoring testing were more likely to use other health care services, including more-aggressive end-of-life care.
The changing of physician behavior is challenging. Physicians are motivated by both extrinsic (ie, financial reimbursement) and intrinsic (ie, altruism) factors.49 Additionally, physicians who profit from monitoring tests should be mindful of potential conflicts of interest. A successful strategy may be to change policy for reimbursement. For example, this approach helped to curb inappropriate use of erythropoiesis-stimulating agents. After initial US Food and Drug Administration approval, uptake was high (27%) in patients with cancer who received chemotherapy.50-53 After changes in reimbursement, there was a rapid decline in the proportion of patients with cancer patients treated with these agents,54 which demonstrates that changes in reimbursement policy could curb overuse.
The present work has several important limitations. The SEER-Medicare database only includes patients who are 65 years or older with Medicare insurance and may not be generalizable to all patient populations. Almost 50% of women with MBC were excluded from the analysis due to having fewer than two claims for STM tests likely because of normal STM values. Because the analysis was done by using claims data, we do not know the reason for the diagnostic tests. Patients in clinical trials may undergo more frequent evaluation, and we were unable to account for that; however, < 2% of patients with cancer participate in trials, and rates are significantly lower in elderly patients, so we believe that this did not have a significant impact on the findings.55 The cost estimates did not include costs associated with oral therapies and, therefore, may be an underestimate of total cancer costs. Because there are no prospective studies about optimal frequency and modality of disease-monitoring testing, it is currently unknown whether patients who have more frequent disease-monitoring testing have better clinical outcomes; however, we found no association of frequent monitoring with OS. In the absence of prospective studies, our definition of extreme use is conservative on the basis of clinical practice and not defined by specific guidelines; future studies are necessary to define optimal timing of disease-monitoring testing.
In summary, we found that approximately one third of elderly women with MBC monitored with both STMs and imaging were extreme users of disease-monitoring testing despite its unproven benefit and higher health care costs. Extreme use may reflect both patient and physician factors, and these should be targeted for interventions to curb spending, including potential health policy changes. In addition, better evidence is needed with regard to the benefits and harms of frequent disease-monitoring testing to inform guidelines. Future research should determine the most cost-effective strategy to monitor patients with MBC.
Acknowledgment
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute, the Office of Research, Development, and Information, Centers for Medicare & Medicaid Services; Information Management Services, Inc.; and the SEER program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Processed as a Rapid Communication manuscript.
M.K.A. is a recipient of a postdoctoral fellowship (R25 CA094061-12) from the National Cancer Institute and a Young Investigator Award from the Conquer Cancer Foundation of ASCO. J.D.W. (R01CA169121) and D.L.H. (R01 CA186084) are recipients of grants from the National Cancer Institute. D.L.H. is a recipient of a grant from the ASCO/Breast Cancer Research Foundation.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
See accompanying editorial on page 2807
AUTHOR CONTRIBUTIONS
Conception and design: Melissa K. Accordino, Jason D. Wright, Dawn L. Hershman
Financial support: Dawn L. Hershman
Collection and assembly of data: Melissa K. Accordino, Jason D. Wright, Sowmya Vasan, Dawn L. Hershman
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Use and Costs of Disease Monitoring in Women With Metastatic Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Melissa K. Accordino
No relationship to disclose
Jason D. Wright
No relationship to disclose
Sowmya Vasan
No relationship to disclose
Alfred I. Neugut
Consulting or Advisory Role: Pfizer, TEVA Pharmaceuticals Industries, Otsuka, United Biosource Corporation, EHE International
Grace C. Hillyer
No relationship to disclose
Jim C. Hu
No relationship to disclose
Dawn L. Hershman
No relationship to disclose
REFERENCES
- 1. Cohen S, Uberoi N: Differentials in the Concentration in the Level of Health Expenditures Across Population Subgroups in the U.S., 2010. Rockville, MD, Agency for Healthcare Research and Quality, Statistical brief 421, 2013. [PubMed] [Google Scholar]
- 2. Medpac. A Data Book: Health Care Spending and the Medicare Program, June 2014. http://www.medpac.gov/documents/publications/jun14databookentirereport.pdf.
- 3. Dinan MA, Curtis LH, Hammill BG, et al: Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999-2006. JAMA 303:1625-1631, 2010. [DOI] [PubMed]
- 4.Hu YY, Kwok AC, Jiang W, et al. High-cost imaging in elderly patients with stage IV cancer. J Natl Cancer Inst. 2012;104:1164–1172. doi: 10.1093/jnci/djs286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) - Breast Cancer Version 1.2016. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 7. Smerage JB, Barlow WE, Hortobagyi GN, et al: Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol 32:3483-3489, 2014. [DOI] [PMC free article] [PubMed]
- 8. Gold LS, Lee CI, Devine B, et al: Imaging Techniques for Treatment Evaluation for Metastatic Breast Cancer. Rockville, MD, Agency for Healthcare Research and Quality, 2014. [Google Scholar]
- 9. doi: 10.1200/JOP.2015.005660. Accordino MK, Wright JD, Vasan S, et al: ReCAP: Serum tumor marker use in patients with advanced solid tumors. J Oncol Pract 12:65-66, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson TL, Rinehart DJ, Durfee J, et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff (Millwood) 2015;34:1312–1319. doi: 10.1377/hlthaff.2014.1186. [DOI] [PubMed] [Google Scholar]
- 11. Ottenbacher KJ, Karmarkar A, Graham JE, et al: Thirty-day hospital readmission following discharge from postacute rehabilitation in fee-for-service Medicare patients. JAMA 311:604-614, 2014. [DOI] [PMC free article] [PubMed]
- 12.Singh S, Lin YL, Kuo YF, et al. Variation in the risk of readmission among hospitals: The relative contribution of patient, hospital and inpatient provider characteristics. J Gen Intern Med. 2014;29:572–578. doi: 10.1007/s11606-013-2723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park L, Andrade D, Mastey A, et al. Institution specific risk factors for 30 day readmission at a community hospital: A retrospective observational study. BMC Health Serv Res. 2014;14:40. doi: 10.1186/1472-6963-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang HJ, Weiss AJ, Barrett ML, et al: Characteristics of Hospital Stays for Super-Utilizers by Payer Rockville, MD, Agency for Healthcare Policy and Research, Statistical brief 190, 2006. [PubMed] [Google Scholar]
- 15. Robert Wood Johnson Foundation: Super-Utilizer Summit: Common Themes From Innovative Complex Care Management Programs, 2013. http://www.rwjf.org/content/dam/farm/reports/reports/2013/rwjf407990.
- 16.Prasad SM, Gu X, Lipsitz SR, et al. Inappropriate utilization of radiographic imaging in men with newly diagnosed prostate cancer in the United States. Cancer. 2012;118:1260–1267. doi: 10.1002/cncr.26416. [DOI] [PubMed] [Google Scholar]
- 17.Bhargavan M, Sunshine JH. Utilization of radiology services in the United States: Levels and trends in modalities, regions, and populations. Radiology. 2005;234:824–832. doi: 10.1148/radiol.2343031536. [DOI] [PubMed] [Google Scholar]
- 18.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 19.National Cancer Institute Overivew of the SEER Program. http://seer.cancer.gov/about/overview.html.
- 20. National Cancer Institute: SEER-Medicare Linked Database: http://healthcaredelivery.cancer.gov/seermedicare.
- 21. Hayes DF, Zurawski VR Jr, Kufe DW: Comparison of circulating CA15-3 and carcinoembryonic antigen levels in patients with breast cancer. J Clin Oncol 4:1542-1550, 1986. [DOI] [PubMed]
- 22.Tondini C, Hayes DF, Gelman R, et al. Comparison of CA15-3 and carcinoembryonic antigen in monitoring the clinical course of patients with metastatic breast cancer. Cancer Res. 1988;48:4107–4112. [PubMed] [Google Scholar]
- 23. Harris L, Fritsche H, Mennel R, et al: American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25:5287-5312; 2007. [DOI] [PubMed]
- 24. Ramsey SD, Henry NL, Gralow JR, et al: Tumor marker usage and medical care costs among older early-stage breast cancer survivors. J Clin Oncol 33:149-155, 2015. [DOI] [PMC free article] [PubMed]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 27. Earle CC, Neville BA, Landrum MB, et al: Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 22:315-321, 2004. [DOI] [PubMed]
- 28. Earle CC, Park ER, Lai B, et al: Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol 21:1133-1138, 2003. [DOI] [PubMed]
- 29.Cooke CR, Feemster LC, Wiener RS, et al. Aggressiveness of intensive care use among patients with lung cancer in the Surveillance, Epidemiology, and End Results-Medicare registry. Chest. 2014;146:916–923. doi: 10.1378/chest.14-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th Revision, clinical modification administrative data. Med Care. 2004;42:801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 31.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 33. Centers for Medicare & Medicaid Services. Fee Schedule. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab.html.
- 34.Centers for Medicare & Medicaid Services Physician Fee Schedule Look-Up Tool. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PFSlookup.
- 35. National Cancer Institute: SEER Stat Fact Sheets: Female Breast Cancer. http://seer.cancer.gov/statfacts/html/breast.html.
- 36.American Cancer Society . Breast Cancer Facts & Figures 2013-2014. Atlanta, GA: American Cancer Society, Inc; 2013. [Google Scholar]
- 37.O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10(suppl 3):20–29. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- 38.Merimsky O, Kovner F, Inbar M, et al. Tamoxifen for disease-negative but MCA-positive breast cancer patients. Oncol Rep. 1997;4:843–847. doi: 10.3892/or.4.4.843. [DOI] [PubMed] [Google Scholar]
- 39.Mathew J, Prinsloo P, Agrawal A, et al. Pilot randomised study of early intervention based on tumour markers in the follow-up of patients with primary breast cancer. Breast. 2014;23:567–572. doi: 10.1016/j.breast.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Salvo N, Zeng L, Zhang L, et al. Frequency of reporting and predictive factors for anxiety and depression in patients with advanced cancer. Clin Oncol (R Coll Radiol) 2012;24:139–148. doi: 10.1016/j.clon.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Couper JW, Love AW, Duchesne GM, et al. Predictors of psychosocial distress 12 months after diagnosis with early and advanced prostate cancer. Med J Aust. 2010;193(suppl 5):S58–S61. doi: 10.5694/j.1326-5377.2010.tb03930.x. [DOI] [PubMed] [Google Scholar]
- 42.Vilhauer RP. A qualitative study of the experiences of women with metastatic breast cancer. Palliat Support Care. 2008;6:249–258. doi: 10.1017/S1478951508000382. [DOI] [PubMed] [Google Scholar]
- 43.Parker PA, Kudelka A, Basen-Engquist K, et al. The associations between knowledge, CA125 preoccupation, and distress in women with epithelial ovarian cancer. Gynecol Oncol. 2006;100:495–500. doi: 10.1016/j.ygyno.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 44.Rustin GJ, van der Burg ME, Griffin CL, et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): A randomised trial. Lancet. 2010;376:1155–1163. doi: 10.1016/S0140-6736(10)61268-8. [DOI] [PubMed] [Google Scholar]
- 45.Carlson RW, Allred DC, Anderson BO, et al. Invasive breast cancer. J Natl Compr Canc Netw. 2011;9:136–222. doi: 10.6004/jnccn.2011.0016. [DOI] [PubMed] [Google Scholar]
- 46. Khatcheressian JL, Hurley P, Bantug E, et al: Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 31:961-965, 2013. [DOI] [PubMed]
- 47. Schnipper LE, Smith TJ, Raghavan D, et al: American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol 30:1715-1724, 2012. [DOI] [PubMed]
- 48.Hahn EE, Hays RD, Kahn KL, et al. Use of imaging and biomarker tests for posttreatment care of early-stage breast cancer survivors. Cancer. 2013;119:4316–4324. doi: 10.1002/cncr.28363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Judson TJ, Volpp KG, Detsky AS. Harnessing the right combination of extrinsic and intrinsic motivation to change physician behavior. JAMA 314:2233-2234, 2015. [DOI] [PubMed]
- 50.Hershman DL, Buono DL, Malin J, et al. Patterns of use and risks associated with erythropoiesis-stimulating agents among Medicare patients with cancer. J Natl Cancer Inst. 2009;101:1633–1641. doi: 10.1093/jnci/djp387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leyland-Jones B, Semiglazov V, Pawlicki M, et al: Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: A survival study. J Clin Oncol 23:5960-5972, 2005. [DOI] [PubMed]
- 52.Henke M, Laszig R, Rübe C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 53. US Food and Drug Administration: Epogen (Epoetin Alfa) for Injection, 2008. http://www.fda.gov/Safety/MedWatch/SafetyInformation/Safety-RelatedDrugLabelingChanges/ucm116177.htm.
- 54.Hershman DL, Neugut AI, Shim JJ, et al. Erythropoiesis-stimulating agent use after changes in Medicare reimbursement policies. J Oncol Pract. 2014;10:264–269. doi: 10.1200/JOP.2013.001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]