Abstract
The intestine is a common site for a variety of pathogenic infections. Helminth infections continue to be major causes of disease worldwide, and are a significant burden on health care systems. Lysine methyltransferases are part of a family of novel attractive targets for drug discovery. SETD7 is a member of the Suppressor of variegation 3-9-Enhancer of zeste-Trithorax (SET) domain-containing family of lysine methyltransferases, and has been shown to methylate and alter the function of a wide variety of proteins in vitro. A few of these putative methylation targets have been shown to be important in resistance against pathogens. We therefore sought to study the role of SETD7 during parasitic infections. We find that Setd7 -/- mice display increased resistance to infection with the helminth Trichuris muris but not Heligmosomoides polygyrus bakeri. Resistance to T. muris relies on an appropriate type 2 immune response that in turn prompts intestinal epithelial cells (IECs) to alter differentiation and proliferation kinetics. Here we show that SETD7 does not affect immune cell responses during infection. Instead, we found that IEC-specific deletion of Setd7 renders mice resistant to T. muris by controlling IEC turnover, an important aspect of anti-helminth immune responses. We further show that SETD7 controls IEC turnover by modulating developmental signaling pathways such as Hippo/YAP and Wnt/β-Catenin. We show that the Hippo pathway specifically is relevant during T. muris infection as verteporfin (a YAP inhibitor) treated mice became susceptible to T. muris. We conclude that SETD7 plays an important role in IEC biology during infection.
Author Summary
The gastrointestinal tract is a common site for infection by a variety of pathogens. For example, gut-dwelling parasitic worms currently infect over a billion people, mostly in developing nations. Deworming strategies have been shown to improve physical and intellectual development of infected children, but current therapies do not offer a sustainable solution. We still have too little insight into how these pathogens are causing disease and how immunity to them is regulated. In this study we show that SETD7, an enzyme that modifies the function of other proteins by methylation, plays an important role in the development of intestinal immunity to the helminth parasite Trichuris muris but not Heligmosomoides polygyrus bakeri. Specifically, we show that SETD7 affects intestinal epithelial turnover, a key mechanism through which T. muris worms are extruded from the body. Our studies identify pathways that are important for immunity to infection, that were previously believed to be involved primarily during embryonic development.
Introduction
The gastrointestinal tract is responsible for absorption of nutrients and water, but at the same time it has an important role in acting as a barrier to the external environment [1]. This barrier function is further complicated by the requirement to respond appropriately to pathogens, but remain tolerant to innocuous antigens like commensal organisms and food. Understanding the molecular pathways that control intestinal homeostasis is critical for promoting immunity and limiting inflammation.
Intestinal homeostasis is the result of a complex interplay between the environment, intestinal epithelial cells (IECs), mesenchymal cells, vascular endothelial cells, and cells of the innate and adaptive immune systems. This interconnected system relies on a multitude of signaling pathways in the various cell types, and aberrant signaling is a key feature in chronic intestinal inflammatory diseases [2]. However, for immunity against certain pathogens, a temporally controlled high level of immune activation is required, including strong inflammatory cues that may lead to significant tissue damage [3–6]. A repair process is then initiated that is essential to regain barrier function and prevent sustained inflammation. IECs play an important role in many of these processes as they can act as the first sensor of pathogens [7], they execute immune responses by responding to specific cues [8], and initiate repair processes that require intestinal stem cells (ISCs) [9–11]. Despite their importance, the molecular pathways that regulate IEC function in immunity, inflammation and repair remain poorly described.
IECs have a remarkable turnover of around 3–5 days [12]. During homeostasis this turnover is driven by ISCs that reside at the bottom of crypts and divide every day [13]. Upon division ISCs leave the stem cell zone to become progenitors for either enterocytes or one of the secretory lineages such as goblet cells and Paneth cells [12]. A variety of signal transduction pathways, including Wnt, Notch, and Hippo are important regulators of ISC and IEC biology. Although several studies have emerged identifying the importance of IECs in immunity to pathogens [6,8,14,15], the molecular pathways that control IEC dynamics during infection remain unknown.
Lysine methyltransferases represent part of a family of novel druggable targets that are currently being investigated for a variety of diseases [16]. SETD7 is a member of the Suppressor of variegation 3-9-Enhancer of zeste-Trithorax (SET) domain-containing family of lysine methyltransferases, and has been shown to methylate and alter the function of a wide variety of proteins in vitro [17]. SETD7 has been shown to have in vitro effects on a wide variety of signaling intermediates including NF-κB and STAT3 [18,19] that are crucial for immunity to pathogens [6,8]. We have recently found that there is an interplay between SETD7 and the Hippo and Wnt pathways, which are evolutionarily conserved signaling pathways that are important for IEC homeostasis, regeneration and tumorigenesis [20–22]. In this study we identify a critical role for IEC-intrinsic expression of Setd7 in immunity to helminth infection.
Results
Setd7 -/- mice are more resistant to T. muris infection
Immunity to infection with the intestinal helminth parasite T. muris is mediated by a complex interplay between IECs and the innate and adaptive immune systems [5,8,23]. Commonly, resistance relies on the development of an adaptive TH2 cell response as opposed to a non-protective TH1 cell response that leads to susceptibility. This TH2 cell response is mediated by a wide variety of innate and adaptive immune cells that require appropriate signaling [5].
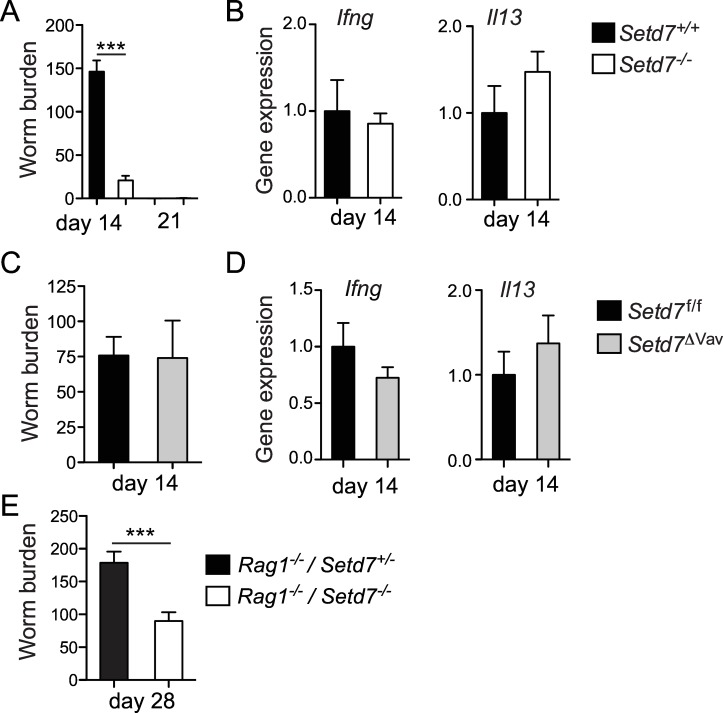
To test whether Setd7 plays a role in the development of intestinal immunity, we infected Setd7 +/+ and Setd7 -/- mice with 200 embryonated T. muris eggs [24]. Setd7 -/- mice display no overt developmental phenotypes and we did not observe compensational expression of related lysine methyltransferases in the intestine upon deletion of Setd7 (S1 Fig). At day 21 post infection both wild type and knock out mice had fully cleared the infection, indicating that complete loss of Setd7 does not render mice susceptible to T. muris infection (Fig 1A). In contrast, at day 14 we found that although Setd7 +/+ mice were in the midst of expelling the worms, Setd7 -/- mice had already cleared most of their worm burden (Fig 1A), suggesting that Setd7 -/- mice were more resistant to T. muris infection. Enhanced resistance to infection with T. muris was not due to an intrinsic difference in microbiota-mediated hatching [25], as fecal contents from either strain of mice were equally able to induce egg hatching in vitro (S2A Fig) and we detected equal worm burdens at day 12 post infection (S2B Fig). Despite the increased immunity to infection in Setd7 -/- mice, we did not detect any significant differences in expression of TH1 cell- or TH2 cell-mediated cytokine genes such as Ifng and Il13 in the gut by qPCR (Figs 1B and S2C). These results suggest that the enhanced resistance to infection in SETD7-deficient mice was neither due to an increased protective type 2 nor a decreased non-protective type 1 immune response. Consistent with this hypothesis, infection of mice with a hematopoietic cell-intrinsic deletion of Setd7 (Setd7 ΔVav mice, generated by crossing Setd7 f/f mice with Vav-Cre mice) revealed that SETD7-deficiency in immune cells had no effect on resistance to infection. At day 14 post-infection, both Setd7 f/f and Setd7 ΔVav mice had equal worm burdens (Fig 1C) and similar gene expression levels of Ifng and Il13 in the intestine (Fig 1D), suggesting that SETD7 expression in hematopoietic cells was not responsible for the increased immunity to T. muris. Importantly, Setd7 -/- mice that lack an adaptive immune system (Rag1 -/- / Setd7 -/- mice, generated by crossing Setd7 -/- mice with Rag1 -/- mice) also displayed increased resistance to infection with T. muris compared to Rag1 -/- / Setd7 +/- littermate controls, with a significant reduction in worm burden at day 28 post-infection (Fig 1E). Thus, in the absence of SETD7, an adaptive immune system is dispensable for the development of immunity to T. muris, suggesting that SETD7 expression in non-hematopoietic cells is a critical component of the response to T. muris infection.
Fig 1. Deletion of Setd7 renders mice resistant to T. muris.
(A & C) Indicated mice were infected with 200 T. muris eggs and killed on day 14 or day 21 after infection. Worm burdens were determined microscopically. (n = 5, *** P<0.001). (B & D) Ifng and Il13 mRNA expression of proximal colon tissue was assessed by qPCR at day 14 post infection. Gene expression is relative to infected control mice (Setd7 +/+ and Setd7 f/f for B and D respectively) (n = 5) (E) Indicated mice were infected with 200 T. muris eggs and killed on day 28 post infection. Worm burdens were determined microscopically. (n≥9, *** P<0.001).
IEC-intrinsic deletion of Setd7 in mice leads to increased resistance to T. muris infection
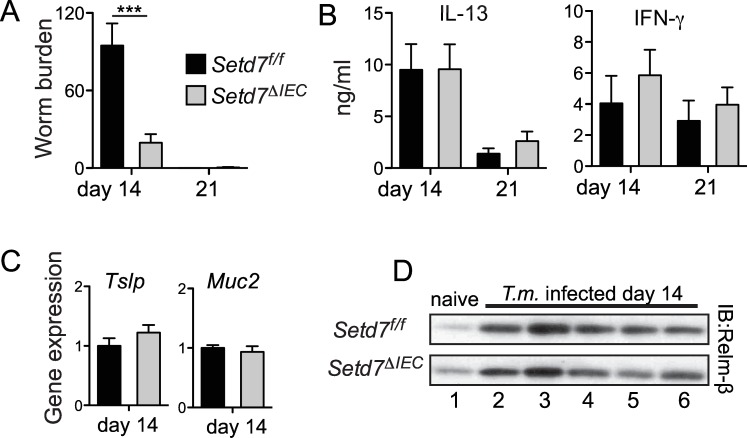
Several studies have shown that immunity to T. muris is associated with a variety of IEC responses, including goblet cell hyperplasia and mucin production, expression of secreted molecules such as thymic stromal lymphopoietin (TSLP) and resistin-like molecule-β (RELMβ), and increased proliferation and turnover [8,15,26–28]. We next directly tested whether IEC-intrinsic deletion of Setd7 would lead to increased resistance to T. muris infection. We infected mice with an IEC-specific deletion of Setd7 (Setd7 ΔIEC mice) [20] with T. muris and found that similar to Setd7 -/- mice, Setd7 ΔIEC mice displayed enhanced resistance to infection, with reduced worm burden at day 14 post-infection (Fig 2A). We failed to observe any differences in levels of IFN-γ or IL-13 produced by restimulated mesenteric lymph node (mLN) cells (Fig 2B) or in gene expression by qPCR of intestinal tissues (S3A Fig) between Setd7 f/f and Setd7 ΔIEC mice. Further, we detected equal worm burdens and Ifng and Il13 expression between infected Setd7 f/f and Setd7 ΔIEC mice at day 12 post infection (S3B and S3C Fig). Thus, IEC-intrinsic expression of SETD7 negatively regulates resistance to infection with T. muris.
Fig 2. Deletion of Setd7 specifically in IECs renders mice resistant to T. muris.
(A) Setd7 f/f (black bars) and Setd7 ΔIEC (grey bars) mice were infected with 200 T. muris eggs and worm burdens were determined at day 14 or day 21 post infection (n≥9 for day 14, n = 6 for day 21, *** P<0.001). (B) Mesenteric lymph node cells from infected mice were re-stimulated for 72 h. IL-13 and IFN-γ concentrations in supernatants was determined by ELISA. (n≥4). (C) Expression of Tslp and Muc2 in proximal colon at day 14 post infection with T. muris was assessed by qPCR. Expression is relative to infected control (Setd7 f/f) mice. (n≥8). (D) Protein levels of RELMβ that was secreted into the gut lumen of naïve and T. muris infected mice evaluated by Western blot.
IECs have been shown to differentiate into goblet cells following infection with T. muris in response to TH2 cell-derived cytokines and produce effector molecules such as the mucins Muc5AC [28] and Muc2 [27], cytokines such as TSLP [29] as well as the small protein RELMβ [26]. Consistent with our results demonstrating equivalent TH2 cell responses, we did not observe any differences in expression of Muc5ac (S3D Fig), Muc2 or Tslp (Fig 2C) or secretion of RELMβ into the intestinal lumen (Fig 2D) between Setd7 f/f and Setd7 ΔIEC mice following T. muris infection. Thus, the increased resistance to T. muris in Setd7 ΔIEC mice is not due to the enhanced production of effector molecules by IECs.
Crypt length and IEC turnover dictate resistance to T. muris in Setd7 ΔIEC mice
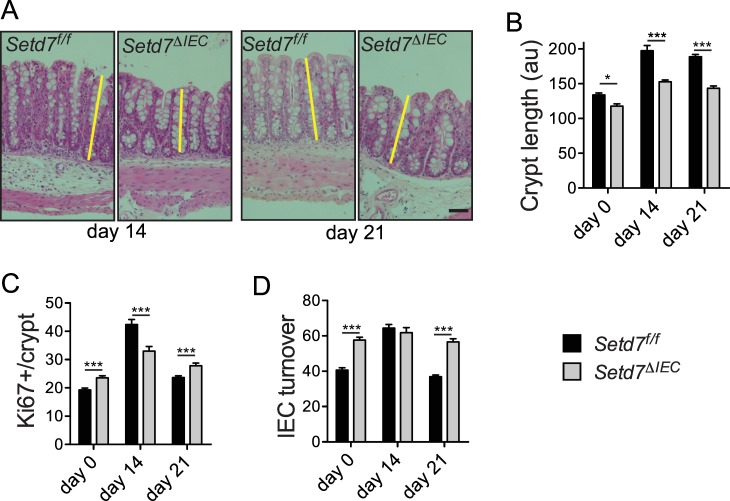
In addition to IEC differentiation, it has been shown that IEC turnover is important for resistance to infection with T. muris [15]. IEC turnover can be defined by combining crypt length (or cells per crypt) with the number of proliferating cells [15,28]. For example, short crypts with many proliferating cells have high turnover whereas long crypts with few proliferating cells have slow turnover. In resistant mice, IEC turnover is induced to clear T. muris, whereas in susceptible mice this increased turnover does not occur [15]. First, we carefully analyzed crypt length and found that Setd7 ΔIEC mice have shorter crypts compared to Setd7 f/f mice before and throughout T. muris infection (Fig 3A and 3B) [20]. Crypt length was highly correlated with the number of cells per crypt as counted by DAPI (S4A–S4C Fig). Next, we analyzed the number of IECs that are proliferating by enumerating Ki67+ cells per crypt (Fig 3C and S4A Fig). We found that prior to infection (day 0) an increased number of IECs from Setd7 ΔIEC mice stained positive for Ki67, suggesting increased proliferation, consistent with our previous study [20]. To measure IEC turnover we calculated the frequency of cells proliferating by dividing the number of Ki67+ cells by the DAPI+ (total number) cell numbers (Fig 3D). Combining proliferation with crypt length (or number of cells per crypt) is essential because increased proliferation does not always correlate with turnover, i.e. it may just lead to longer crypts. We found that under homeostatic conditions, Setd7 ΔIEC mice display faster IEC turnover compared to Setd7 f/f mice (Fig 3D). Following infection with T. muris, Setd7 f/f mice increase IEC turnover (Fig 3D)[15], suggesting that this is the peak turnover that is required for parasite expulsion. Our results suggest that the turnover induced by T. muris in Setd7 f/f mice that is associated with worm expulsion is already present during homeostasis in naive Setd7 ΔIEC mice, resulting in enhanced worm expulsion. Thus, loss of SETD7 is associated with increased IEC turnover that promotes resistance to T. muris.
Fig 3. Setd7 regulates IEC turnover responses after T. muris infection.
(A) H&E stained caecal sections of Setd7 f/f and Setd7 ΔIEC mice at day 14 and day 21 post infection with T. muris. Yellow lines indicate crypt length. Original magnification is 100X. Bar = 50 μm (B) Crypt length (au = arbitrary units) of caecums of naïve (day 0) and T. muris infected mice (day 14 and day 21 post infection). (n≥30 of at least 4 mice, * P<0.05, *** P<0.001). (C) Proliferation as measured by counting Ki67+ cells per crypt from images such as in S4A. (n≥20 of at least 4 mice, *** P<0.001). (D) IEC turnover was determined by dividing Ki67+ cells by total DAPI+ cells from images such as shown in S4A. Of note, crypt length correlated with total DAPI+ cells per crypt at all time points (see S4C). (n≥20 of at least 4 mice, *** P<0.001).
IEC-specific deletion of Setd7 renders mice resistant to chronic T. muris infection
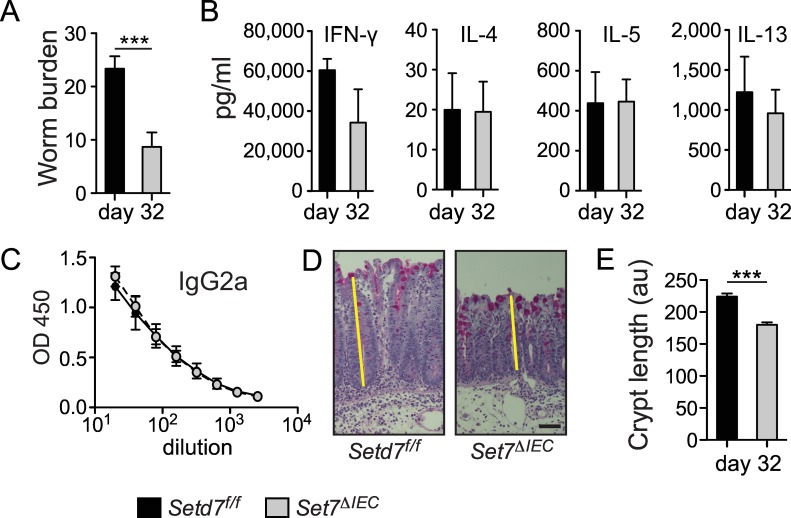
Although a high-dose T. muris infection (>100 eggs) is very suitable for studying various immunological processes in mice, it is arguably not the perfect model for studying chronic helminth infections that dramatically affect human lives, mainly in developing countries [30]. In contrast, a low-dose (<50 eggs) T. muris infection leads to a persistent infection with a chronic worm burden [31]. To test if SETD7 played a role in the development of chronic helminth infection, we infected mice with a low-dose (~30 eggs) of T. muris infection. We found that Setd7 ΔIEC mice were also more resistant to chronic infection with T. muris compared to Setd7 f/f mice at day 32 post infection (Fig 4A). At day 21 post infection we observed equivalent infection rates between Setd7 f/f and Setd7 ΔIEC mice, which was associated with equivalent expression of Ifng and Il13 in the intestine (S5A and S5B Fig). Further, we did not observe differences in adaptive immune responses as measured by cytokine secretion following polyclonal restimulation of mLN cells, serum levels of T. muris-specific IgG2a and IgG1, as well as cytokine gene expression in the intestinal tissue at day 32 post infection (Fig 4B and 4C and S5C and S5D Fig). However, consistent with our results with high-dose infection, we did observe differences in crypt length between Setd7 f/f and Setd7 ΔIEC mice, albeit with equal robust reduction of goblet cells (Fig 4D and 4E). Thus, loss of SETD7 in IECs leads to increased resistance to chronic helminth infection independent of the immune response, identifying a potential new therapeutic target to treat persistent helminth infections.
Fig 4. Setd7 ΔIEC mice resistant to low dose T. muris infection.
(A) Setd7 f/f and Setd7 ΔIEC mice were infected with ~35 T. muris eggs (low dose) and worm burdens were determined at day 32 post infection (n = 9, pooled from 2 independent experiments, *** P<0.001). (B) Cytokine production of mesentyric lymph node cells that were re-stimulated for 72 h was determined by ELISA. (n≥4). (C) Serially diluted serum of infected mice was analyzed by ELISA to measure T. muris-specific IgG2a. (n≥4). (D) Periodic acid-Schiff stained caecal sections show a robust depletion of goblet cells in both Setd7 f/f and Setd7 ΔIEC mice at day 32 post infection. Yellow lines indicate crypt length. Original magnification is 100X. Bar = 50 μm (E) Crypt length (au = arbitrary units) in caecums of mice at day 32 post infection. (n≥60 of 9 mice in each group, *** P<0.001). Setd7 f/f black bars, Setd7 ΔIEC grey bars.
IEC-specific deletion of Setd7 does not modify resistance to Heligmosomoides polygyrus bakeri
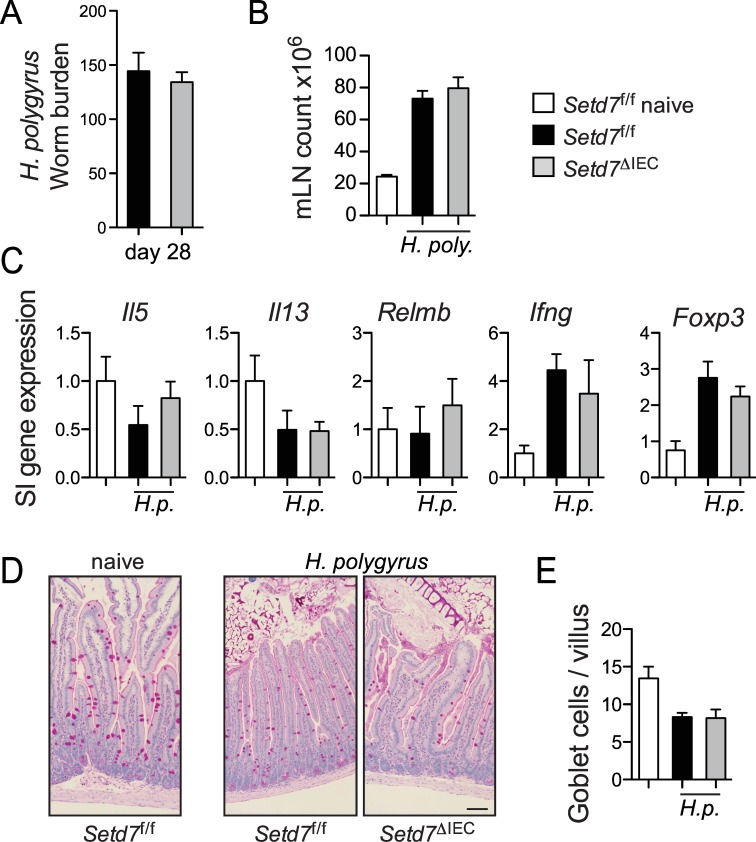
T. muris resides in the caecum embedded in the intestinal epithelium in close association with IECs [32]. IEC proliferation and turnover has been shown to play a role in expulsion via an ‘epithelial escalator’ [15]. In contrast, larvae of Heligmosomoides polygyrus bakeri penetrate the epithelium into the submucosa, undergo two molts, and the worms resurface in the lumen wrapped around small intestinal villi [33]. Resistance to H. p. bakeri is accomplished by a TH2-cell-mediated ‘weep and sweep’ response, which consists of goblet-cell-mediated secretion of mucins (weep) and muscle contraction (sweep) [34]. To test whether Setd7 ΔIEC mice also have altered resistance against H. p. bakeri, we infected Setd7 f/f and Setd7 ΔIEC mice with ~200 larvae. At 28 days post infection we found no difference in worm burden or induction of mesenteric lymph node cell numbers (Fig 5A and 5B). There was an upregulation of expression of indicators of TH1 (Ifng) and Treg (Foxp3) cell responses at this time point (Fig 5C). However, we failed to observe any differences in expression of Il5, Il13, Relmb, Ifng, Foxp3 between infected Setd7 f/f and Setd7 ΔIEC mice (Fig 5C). We observed a reduction of periodic acid-Schiff (PAS) positive cells in infected animals compared to naïve controls, but this too was equivalent between Setd7 f/f and Setd7 ΔIEC mice (Fig 5D and 5E). Thus, in contrast to T. muris, IEC-intrinsic deletion of Setd7 does not affect immunity against H. p. bakeri.
Fig 5. Setd7 ΔIEC mice do not have increased resistance against infection with H. p. bakeri.
(A) Setd7 f/f and Setd7 ΔIEC mice were infected with ~ 200 H. p. bakeri eggs and were killed day 28 post infection. Worm burdens were determined microscopically from the small intestine. (B) Mesenteric lymph node (mLN) cell counts from naïve and H. p. bakeri infected mice (day 28 post infection). (C) Gene expression of indicated genes in small intestine (SI) that was adjacent to infection site at day 28 post infection with H. p. bakeri. Expression is relative to naïve mice (white bars). (D) Periodic acid-Schiff (PAS) staining of small intestinal tissue sections of indicated mice at day 28 post infection. Bar = 100 μm. (E) PAS+ cells per villus were counted from images as shown in (D). (A-E) n = 3 (naives), n = 8 (infected) from 2 independent experiments. Setd7 f/f black bars, Setd7 ΔIEC grey bars.
SETD7 regulates Wnt and Hippo signaling during T. muris infection
We have previously shown that increased IEC proliferation and turnover in the absence of SETD7 during homeostasis correlated with dysregulated signaling by the Hippo pathway [20], a pathway well known to control IEC proliferation [35–38]. SETD7 is required for the proper subcellular localization of the Hippo pathway transducer YAP [20]. In the absence of SETD7, YAP is enriched in the nucleus where it interacts with the TEAD family of transcription factors, resulting in heightened YAP/TEAD-dependent gene expression. Recent studies have also linked Hippo/YAP signaling with Wnt/β-Catenin signaling in the control of IEC proliferation and turnover [38–41]. Indeed, we have recently shown that SETD7-dependent methylation of YAP is required for optimal Wnt/β-Catenin signaling during intestinal regeneration and tumorigenesis [22]. We therefore analyzed Wnt and Hippo activity in IECs during T. muris infection by measuring specific target genes of each pathway. We found that both Wnt (Lgr5 and Axin2) and Hippo (Ctgf and Gli2) target genes are upregulated in IECs during T. muris infection of control Setd7 f/f mice compared to naïve mice (Fig 6A and S6 Fig). In the absence of SETD7, we found that Wnt target gene expression is reduced while Hippo target gene expression is increased in IECs following infection (Fig 6A and S6 Fig). Thus, IEC-intrinsic expression of SETD7 regulates IEC turnover during T. muris infection, possibly by influencing Wnt and Hippo signaling.
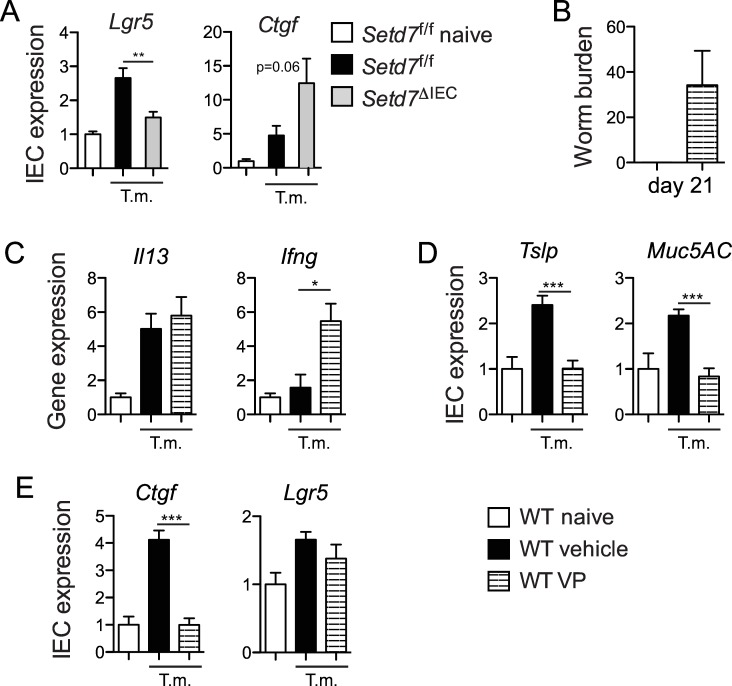
Fig 6. Inhibition of YAP-TEAD interactions in vivo in resistance to T. muris.
(A) IEC-specific expression of Wnt (Lgr5) and Hippo (Ctgf) target genes in T. muris infected Setd7 f/f (black bars) and Setd7 ΔIEC (grey bars) mice. Expression is relative to naïve mice (white bars). (n≥8, ** P<0.01). (B) Worm burdens at day 21 post infection of wild type (WT) mice. Mice were either vehicle treated (black bars) or vertepofin (VP) treated (striped bars) (n≥6). (C) Gene expression of Il13 and Ifng from proximal colon at day 21 post infection. Expression is relative to uninfected, untreated WT mice (white bars, WT naive). (n≥6, * P<0.05) (D & E) IEC-specific expression of effector (Tslp and Muc5AC) and Hippo and Wnt target (Ctgf and Lgr5) genes. Expression is relative to naïve mice (white bars). (n≥6, *** P<0.001).
YAP inhibitor verteporfin renders mice susceptible to high-dose T. muris infection
We next tested whether blocking YAP transcriptional activity would affect resistance to infection with T. muris. To do this, we treated wild type mice with liposome-encapsulated verteporfin (VP), a recently discovered inhibitor of YAP-TEAD interactions [42]. Following infection with T. muris, we found that VP-treated mice failed to fully clear their infection by day 21 in comparison to vehicle-treated mice (Fig 6B). Consistent with the increased susceptibility to infection, we observed a significant increase in gene expression of the TH1 cell-associated cytokine Ifng in the intestine of VP-treated mice, even though Il13 levels were equal (Fig 6C). Further, induction of IEC-specific genes associated with resistance to infection such as Muc5ac and Tslp was abrogated by VP treatment (Fig 6D). As expected, VP treatment abolished T. muris-mediated upregulation of YAP target gene Ctgf (Fig 6E), but we did not observe a striking effect on Wnt/β-Catenin target genes such as Lgr5 (Fig 6E). Together, these experiments show that YAP-TEAD interactions are important for the development of immunity to T. muris.
Discussion
In this study we describe the role of SETD7 during helminth infection. We find that deletion of Setd7 in IECs renders mice resistant to the helminth T. muris but does not affect H. p. bakeri infection. Interestingly, Setd7 does not affect T helper cell responses or goblet cell differentiation, both of which are deemed very important for resistance against T. muris [27,28,43,44]. Instead, we find that epithelial turnover is affected by the lack of Setd7, and therefore, IEC turnover can be dominant over adaptive immune responses in terms of importance for resistance to T. muris. In Setd7 ΔIEC mice we observed higher turnover during homeostasis compared to Setd7 f/f mice, demonstrating that SETD7 regulates IEC turnover independently of infection-induced immune cell cues. In contrast, control Setd7 f/f mice relied on type 2 immune responses to increase its IEC turnover to expel T. muris worms. We quantified turnover by examining crypt length, number of cells per crypt, and number of cells proliferating. However, we did not directly measure the migration of cells up the crypt, for example by pulse-chase experiments, nor did we quantify cell shedding into the lumen, both of which are also important elements of turnover [15,45]. IECs originate from stem cells at the bottom of crypts, divide several times in the transit-amplifying zone (bottom half of crypts), after which they differentiate and finally are shed into the lumen due to crowding [13,45]. Nevertheless, Setd7 deletion provides resistance to infection even in mice that completely lack an adaptive immune system. Thus, these results uncover an additional pathway to target in the design of therapies to treat helminth infection.
We also provide evidence that the developmental Hippo/YAP and Wnt/β-Catenin signaling pathways are important components of resistance to T. muris infection. Both Hippo and Wnt gene expression programs are induced upon infection and are mediated by SETD7. It would be particularly interesting to identify what cues drive these pathways during helminth infection, and whether the previously identified regulator of epithelial turnover, CXCL10, plays any role [15]. A potential alternative pathway that merges inflammation with regeneration is the recently described gp130-Src-YAP axis that is induced during intestinal inflammation [46]. Although this study did not identify a link with Wnt/β-Catenin, it remains to be seen if intestinal infection relies on this pathway. Nevertheless, our results show that SETD7-dependent regulation of Hippo and Wnt signaling plays a critical role in the development of resistance to intestinal helminth infection.
We used VP to test our hypothesis that YAP-TEAD mediated gene expression programs are important for T. muris resistance. Indeed, we find that VP treated animals become susceptible to T. muris compared to vehicle treated mice. However, we observed that VP treated animals also had increased TH1 cell-associated cytokines (IFN-γ) and reduced epithelial markers for resistance (TSLP and Muc5AC), indicating that the Hippo pathway may play a wider role. This is in line with a recent study identifying a role for YAP in goblet cell function [40]. In addition, it suggests that a YAP-TEAD mediated regenerative gene expression program is required to avoid a shift towards a TH1 response upon infection with T. muris.
In summary, we have identified a SETD7-dependent regulatory pathway in IECs that regulates immunity in the intestine. Modulation of SETD7 activity may provide a therapeutic strategy to improve anti-helminthic treatments independently of the innate and adaptive immune systems.
Materials and Methods
Mice and infection
Villin-Cre and Rag1 -/- mice were obtained from Jackson Laboratories. Vav-Cre mice were obtained from T. Graf (Centre for Genomic Regulation, Barcelona, Spain). Setd7 -/- and Setd7 f/f mice were described previously [20,47]. We did not observe any physiological effects from Cre expression during homeostasis or infection. Animals were maintained in a specific-pathogen-free environment and tested negative for pathogens in routine screening. All experiments were carried out at the University of British Columbia following institutional guidelines. We used both males and females that were littermates and age matched (ranging from 7–15 weeks old) for all experiments. Isolation of T. muris eggs was carried out as described previously [24]. Mice were infected on day 0 with high dose (~200) or low dose (~35) of embryonated eggs by oral gavage, and parasite burdens were assessed microscopically on days 14, 21, 28, or 32 post-infection. Mice were infected with 200 H. polygyrus bakeri L3 larvae by oral gavage and parasite burdens were assessed microscopically on day 28. Liposome-encapsulated verteporfin (VP, Visudyne) was a kind gift by Novartis and was used at 50 mg/kg [42] by intraperitoneal injection at days -3, 0, 4, 7, 10, 13, 17, 20. Control mice were injected with the same volume of vehicle with the same schedule.
Ethics statement
All experiments were performed according to protocols (A11-0290, A11-0329, A13-0010) approved by the University of British Columbia's Animal Care Committee and in direct accordance with The Canadian Council on Animal Care (CCAC) guidelines.
Analysis of T. muris induced immunity
Mesenteric lymph node cells from T. muris-treated mice were isolated and single-cell suspensions were plated at 4 × 106 per ml in the medium or in the presence of antibodies against CD3 (145-2C11) and CD28 (37.51); 1 μg ml−1 each; (eBioscience, San Diego, CA) or T. muris antigen (50 μg ml-1) for 72 h. Cytokine production from cell-free supernatants was determined by standard sandwich enzyme-linked immunosorbent assay (ELISA) using commercially available antibodies (eBioscience). T. muris-specific serum IgG1 and IgG2a levels were determined by ELISA on plates coated with T. muris Ag (5 μg ml-1). Total protein was isolated from fecal samples, resolved by sodium dodecyl sulfate- polyacrylamide gel electrophoresis, and immunoblotted using a rabbit anti-mouse RELM-β antibody (PeproTech, Rocky Hill, NJ).
RNA extraction and qPCR
RNA was purified from whole intestine using mechanical disruption followed by TRIzol according to the manufacturer’s instructions, or from isolated IECs using RNeasy isolation kit (Qiagen). Reverse transcription using High Capacity cDNA Reverse Transcription kit (Applied Biosystems) was used to generate cDNA and qPCR was performed using SYBR green with primers from the Primer Bank (http://pga.mgh.harvard.edu/primerbank) using SYBR green chemistry on an ABI 7900 real-time PCR system (Applied Biosystems). Samples were normalized against Actb or Gapdh and are presented as fold over wild type, naïve, or relative to housekeeping gene as is indicated in figure legends.
Tissue staining
Tissues were fixed in formalin and paraffin-embedded. Sections (5 μm) were stained with hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS). Slides were analyzed on a Zeiss Axioplan2 microscope and images captured using a Qimaging Retiga EX CCD camera and Openlab 4.0.4 software (PerkinElmer). For immunofluorescence, 5 μm sections of paraformaldehyde-fixed, paraffin-embedded tissues were incubated with anti-Ki67 (SP6 clone, Sigma)) followed by Alexa568-conjugated goat-anti-rabbit and DAPI.
IEC turnover was calculated by Ki67+ cells / total cells (DAPI+) per crypt X 100. Of note, crypt length was under all conditions equally associated with total cells per crypt.
Statistical analysis
Results represent the mean ± s.e.m. Statistical significance was determined by Student’s t-test or 1-way ANOVA with subsequent post hoc test.
Supporting Information
IECs were isolated from Setd7 +/- and Setd7 -/- mice and gene expression of indicated genes relative to the housekeeping gene Actb was determined by qPCR. n≥4.
(TIF)
(A) Egg hatching was done in fecal extracts from indicated mice. n = 3. (B) Worm burden at day 12 post infection of indicated mice. Of note, at this point worms are very small and hard to distinguish, which leads to low numbers detectable. n = 5. (C) Infg and Il13 expression in the proximal colon. Mice were killed at day 12 post infection. Gene expression is relative to infected control (Setd7 +/-) mice. n = 7.
(TIF)
(A) Gut gene expression of indicated genes and indicated mice at day 14 and day 21 post infection. Gene expression was calculated as fold over naive. n≥4. (B) Worm burden at day 12 post infection of indicated mice. Of note, at this point worms are very small and hard to distinguish, which leads to low numbers detectable. n = 7. (C) Expression of indicated genes and mice 12 days post infection. Gene expression is relative to infected control (Setd7 f/f) mice. n = 7 (D) IEC Muc5ac gene expression of naïve and T. muris infected mice at day 21 post infection. Fold over naive mice is shown. n≥5
(TIF)
(A) Ki67 and DAPI staining of ceacal sections after 14 and 21 days of T. muris infection. (B) Counts of number of nuclei per crypt as counted using DAPI staining as shown in (A). (C) Ratio between DAPI numbers (B) and crypt length (Fig 3B) showing that the ratio of those two parameters is equal, and independent of infection.
(TIF)
(A) Worm burden at day 21 post infection with low dose (~35 eggs) T. muris of indicated mice. n = 4. (B) Gut gene expression of indicated genes and mice 21 days post infection. n = 4 (C) Gut gene expression of indicated genes and mice at 32 days post infection. (D) Serially diluted serum of infected mice was analyzed by ELISA to measure T. muris-specific IgG1.
(TIF)
IEC gene expression of indicated genes of indicated mice after T. muris (T.m.) infection. n≥8 from at least 2 independent experiments.
(TIF)
Acknowledgments
We thank the BRC Animal Care facility; Taka Murakami of the BRC Genotyping facility; Les Rollins, Rupinder Dhesi and Michael Williams of the BRC core staff; Ingrid Barta of the BRC histology facility and Novartis for providing Verteporfin (Visudyne).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding for this study is from the Canadian Institutes of Health Research (www.cihr.ca, Fellowships to MJO and FA and Project grants MOP-89773 and MOP-106623 to CZ), Michael Smith Foundation for Health Research (www.msfhr.org, Fellowships to MJO and Career Investigator Award to CZ), Banting Postdoctoral Fellowship (banting.fellowships-bourses.gc.ca, to MJO) and veski (www.veski.org, Fellowship VIF24 to CZ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu. Rev. Pathol. 2010;5: 119–144. 10.1146/annurev.pathol.4.110807.092135 [DOI] [PubMed] [Google Scholar]
- 2. Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474: 298–306. 10.1038/nature10208 [DOI] [PubMed] [Google Scholar]
- 3. Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011;32: 256–264. 10.1016/j.it.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 4. Asquith M, Powrie F. An innately dangerous balancing act: intestinal homeostasis, inflammation, and colitis-associated cancer. J. Exp. Med. 2010;207: 1573–1577. 10.1084/jem.20101330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Artis D, Grencis RK. The intestinal epithelium: sensors to effectors in nematode infection. Mucosal Immunol. 2008;1: 252–264. 10.1038/mi.2008.21 [DOI] [PubMed] [Google Scholar]
- 6. Collins JW, Keeney KM, Crepin VF, Rathinam VAK, Fitzgerald KA, Finlay BB, et al. Citrobacter rodentium: infection, inflammation and the microbiota. Nat. Rev. Micro. 2014;12: 612–623. 10.1038/nrmicro3315 [DOI] [PubMed] [Google Scholar]
- 7. Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014;14: 141–153. 10.1038/nri3608 [DOI] [PubMed] [Google Scholar]
- 8. Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446: 552–556. 10.1038/nature05590 [DOI] [PubMed] [Google Scholar]
- 9. Hanash AM, Dudakov JA, Hua G, O'Connor MH, Young LF, Singer NV, et al. Interleukin-22 Protects Intestinal Stem Cells from Immune-Mediated Tissue Damage and Regulates Sensitivity to Graft versus Host Disease. Immunity. 2012;37: 339–350. 10.1016/j.immuni.2012.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lindemans CA, Calafiore M, Mertelsmann AM, O'Connor MH, Dudakov JA, Jenq RR, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528: 560–564. 10.1038/nature16460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14: 149–159. 10.1016/j.stem.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 12. van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009;71: 241–260. 10.1146/annurev.physiol.010908.163145 [DOI] [PubMed] [Google Scholar]
- 13. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449: 1003–1007. 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- 14. Bergstrom KSB, Morampudi V, Chan JM, Bhinder G, Lau J, Yang H, et al. Goblet Cell Derived RELM-β Recruits CD4+ T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation. PLoS Pathog. 2015;11: e1005108 10.1371/journal.ppat.1005108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308: 1463–1465. 10.1126/science.1108661 [DOI] [PubMed] [Google Scholar]
- 16. Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nat. Rev. Drug Disc. 2012;11: 384–400. 10.1038/nrd3674 [DOI] [PubMed] [Google Scholar]
- 17. Herz H-M, Garruss A, Shilatifard A. SET for life: biochemical activities and biological functions of SET domain-containing proteins. Trends Biochem. Sci. 2013;38: 621–639. 10.1016/j.tibs.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang J, Huang J, Dasgupta M, Sears N, Miyagi M, Wang B, et al. Reversible methylation of promoter-bound STAT3 by histone-modifying enzymes. Proc. Natl. Acad. Sci. U.S.A. 2010;107: 21499–21504. 10.1073/pnas.1016147107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang X-D, Huang B, Li M, Lamb A, Kelleher NL, Chen L-F. Negative regulation of NF-kappaB action by Set9-mediated lysine methylation of the RelA subunit. EMBO J. 2009 ed. 2009;28: 1055–1066. 10.1038/emboj.2009.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oudhoff MJ, Freeman SA, Couzens AL, Antignano F, Kuznetsova E, Min PH, et al. Control of the hippo pathway by Set7-dependent methylation of Yap. Dev. Cell. 2013;26: 188–194. 10.1016/j.devcel.2013.05.025 [DOI] [PubMed] [Google Scholar]
- 21. Barsyte-Lovejoy D, Li F, Oudhoff MJ, Tatlock JH, Dong A, Zeng H, et al. (R)-PFI-2 is a potent and selective inhibitor of SETD7 methyltransferase activity in cells. Proc. Natl. Acad. Sci. U.S.A. 2014;111: 12853–12858. 10.1073/pnas.1407358111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oudhoff MJ, Braam MJS, Freeman SA, Wong D, Rattray DG, Wang J, et al. SETD7 Controls Intestinal Regeneration and Tumorigenesis by Regulating Wnt/β-Catenin and Hippo/YAP Signaling. Dev. Cell. 2016;37: 47–57. 10.1016/j.devcel.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 23. Klementowicz JE, Travis MA, Grencis RK. Trichuris muris: a model of gastrointestinal parasite infection. Semin. Immunopathol. 2012;34: 815–828. 10.1007/s00281-012-0348-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Antignano F, Mullaly SC, Burrows K, Zaph C. Trichuris muris infection: a model of type 2 immunity and inflammation in the gut. J. Vis. Exp. 2011; 51: pii:2774 10.3791/2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hayes KS, Bancroft AJ, Goldrick M, Portsmouth C, Roberts IS, Grencis RK. Exploitation of the Intestinal Microflora by the Parasitic Nematode Trichuris muris. Science. 2010;328: 1391–1394. 10.1126/science.1187703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, et al. RELMβ/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc. Natl. Acad. Sci. U.S.A. 2004;101: 13596–13600. 10.1073/pnas.0404034101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hasnain SZ, Wang H, Ghia JE, Haq N, Deng Y, Velcich A, et al. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology. 2010;138: 1763–1771. 10.1053/j.gastro.2010.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, et al. Muc5ac: a critical component mediating the rejection of enteric nematodes. J. Exp. Med. 2011;208: 893–900. 10.1084/jem.20102057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, Comeau MR, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 2009;206: 655–667. 10.1084/jem.20081499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367: 1521–1532. 10.1016/S0140-6736(06)68653-4 [DOI] [PubMed] [Google Scholar]
- 31. Chenery AL, Antignano F, Burrows K, Scheer S, Perona-Wright G, Zaph C. Low-Dose Intestinal Trichuris muris Infection Alters the Lung Immune Microenvironment and Can Suppress Allergic Airway Inflammation. Infect. Immun. 2016;84: 491–501. 10.1128/IAI.01240-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tilney LG, Connelly PS, Guild GM, Vranich KA, Artis D. Adaptation of a nematode parasite to living within the mammalian epithelium. J. Exp. Zool. A Comp. Exp. Biol. 2005;303: 927–945. 10.1002/jez.a.214 [DOI] [PubMed] [Google Scholar]
- 33. Reynolds LA, Filbey KJ, Maizels RM. Immunity to the model intestinal helminth parasite Heligmosomoides polygyrus. Semin. Immunopathol. 2012;34: 829–846. 10.1007/s00281-012-0347-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anthony RM, Rutitzky LI, Urban JF, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 2007;7: 975–987. 10.1038/nri2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24: 2383–2388. 10.1101/gad.1978810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 2007;17: 2054–2060. 10.1016/j.cub.2007.10.039 [DOI] [PubMed] [Google Scholar]
- 37. Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, et al. YAP/TAZ Incorporation in the β-Catenin Destruction Complex Orchestrates the Wnt Response. Cell. 2014;158: 157–170. 10.1016/j.cell.2014.06.013 [DOI] [PubMed] [Google Scholar]
- 38. Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526: 715–718. 10.1038/nature15382 [DOI] [PubMed] [Google Scholar]
- 39. van Es JH, Haegebarth A, Kujala P, Itzkovitz S, Koo B-K, Boj SF, et al. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol. Cell Biol. 2012;32: 1918–1927. 10.1128/MCB.06288-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Imajo M, Ebisuya M, Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat. Cell Biol. 2014;17: 7–19. 10.1038/ncb3084 [DOI] [PubMed] [Google Scholar]
- 41. Barry ER, Morikawa T, Butler BL, Shrestha K, la Rosa de R, Yan KS, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493: 106–110. 10.1038/nature11693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee S-J, Anders RA, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26: 1300–1305. 10.1101/gad.192856.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cliffe LJ, Grencis RK. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv. Parasitol. 2004;57: 255–307. 10.1016/S0065-308X(04)57004-5 [DOI] [PubMed] [Google Scholar]
- 44. Hasnain SZ, Thornton DJ, Grencis RK. Changes in the mucosal barrier during acute and chronic Trichuris muris infection. Parasite Immunol. 2011;33: 45–55. 10.1111/j.1365-3024.2010.01258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien C-B, Morcos PA, et al. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484: 546–549. 10.1038/nature10999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taniguchi K, Wu L-W, Grivennikov SI, de Jong PR, Lian I, Yu F-X, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519: 57–62. 10.1038/nature14228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lehnertz B, Rogalski JC, Schulze FM, Yi L, Lin S, Kast J, et al. p53-Dependent Transcription and Tumor Suppression Are Not Affected in Set7/9-Deficient Mice. Mol. Cell. 2011;43: 673–680. 10.1016/j.molcel.2011.08.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IECs were isolated from Setd7 +/- and Setd7 -/- mice and gene expression of indicated genes relative to the housekeeping gene Actb was determined by qPCR. n≥4.
(TIF)
(A) Egg hatching was done in fecal extracts from indicated mice. n = 3. (B) Worm burden at day 12 post infection of indicated mice. Of note, at this point worms are very small and hard to distinguish, which leads to low numbers detectable. n = 5. (C) Infg and Il13 expression in the proximal colon. Mice were killed at day 12 post infection. Gene expression is relative to infected control (Setd7 +/-) mice. n = 7.
(TIF)
(A) Gut gene expression of indicated genes and indicated mice at day 14 and day 21 post infection. Gene expression was calculated as fold over naive. n≥4. (B) Worm burden at day 12 post infection of indicated mice. Of note, at this point worms are very small and hard to distinguish, which leads to low numbers detectable. n = 7. (C) Expression of indicated genes and mice 12 days post infection. Gene expression is relative to infected control (Setd7 f/f) mice. n = 7 (D) IEC Muc5ac gene expression of naïve and T. muris infected mice at day 21 post infection. Fold over naive mice is shown. n≥5
(TIF)
(A) Ki67 and DAPI staining of ceacal sections after 14 and 21 days of T. muris infection. (B) Counts of number of nuclei per crypt as counted using DAPI staining as shown in (A). (C) Ratio between DAPI numbers (B) and crypt length (Fig 3B) showing that the ratio of those two parameters is equal, and independent of infection.
(TIF)
(A) Worm burden at day 21 post infection with low dose (~35 eggs) T. muris of indicated mice. n = 4. (B) Gut gene expression of indicated genes and mice 21 days post infection. n = 4 (C) Gut gene expression of indicated genes and mice at 32 days post infection. (D) Serially diluted serum of infected mice was analyzed by ELISA to measure T. muris-specific IgG1.
(TIF)
IEC gene expression of indicated genes of indicated mice after T. muris (T.m.) infection. n≥8 from at least 2 independent experiments.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.