Abstract
Extra-intestinal pathogenic Escherichia coli (ExPEC) are responsible for diverse infections including meningitis, sepsis and urinary tract infections. The alarming rise in anti-microbial resistance amongst ExPEC complicates treatment and has highlighted the need for alternative preventive measures. SslE is a lipoprotein secreted by a dedicated type II secretion system in E. coli that was first identified as a potential vaccine candidate using reverse genetics. Although the function and protective efficacy of SslE has been studied, the molecular mechanisms that regulate SslE expression remain to be fully elucidated. Here, we show that while the expression of SslE can be detected in E. coli culture supernatants, different strains express and secrete different amounts of SslE when grown under the same conditions. While the histone-like transcriptional regulator H-NS strongly represses sslE at ambient temperatures, the variation in SslE expression at human physiological temperature suggested a more complex mode of regulation. Using a genetic screen to identify novel regulators of sslE in the high SslE-expressing strain UTI89, we defined a new role for the nucleoid-associated regulator Fis and the ribosome-binding GTPase TypA as positive regulators of sslE transcription. We also showed that Fis-mediated enhancement of sslE transcription is dependent on a putative Fis-binding sequence located upstream of the -35 sequence in the core promoter element, and provide evidence to suggest that Fis may work in complex with H-NS to control SslE expression. Overall, this study has defined a new mechanism for sslE regulation and increases our understanding of this broadly conserved E. coli vaccine antigen.
Introduction
Escherichia coli are highly diverse bacteria ranging from harmless gut commensal organisms to specialized pathogens capable of causing a variety of infections in humans and animals [1]. Extra-intestinal pathogenic E. coli (ExPEC) cause infections outside the intestinal tract, including sepsis, neonatal meningitis and urinary tract infection (UTI). Among these infections, UTIs represent the most substantial burden to healthcare systems worldwide [2, 3].
Uropathogenic E. coli (UPEC), the primary cause of UTI [4], is the largest and most clinically significant ExPEC pathotype [1, 5]. On a global scale, the prevalence of UPEC (and other Enterobacteriaceae) resistant to multiple classes of antibiotics is increasing rapidly [6, 7]. This alarming trend has led to an enhanced rate of UTI treatment failure with conventional antibiotics and increased dependence on last-line therapies such as carbapenems, further promoting the emergence of drug resistant strains [8–11]. Among the potential new approaches currently being investigated for the prevention of UTI, vaccination represents a viable alternative in some patient groups [12, 13]. Strategies for vaccination have included immunization against virulence factors such as adhesins and iron acquisition receptor proteins; as well as the use of lysates of inactivated uropathogens and live attenuated strains [14–19]. Despite these efforts, there is still no vaccine currently available for the prevention of UTI [12, 13].
SslE (also known as YghJ or ECOK1_3385) is a secreted and surface exposed lipoprotein of E. coli identified by reverse vaccinology as a strongly immunogenic vaccine antigen against ExPEC in a murine sepsis model [20]. SslE is secreted by a dedicated type II secretion system (T2SS) [20, 21], a conserved, multicomponent structure used by Gram-negative bacteria to export a variety of proteins including many virulence factors [22–26]. SslE contributes to biofilm maturation and virulence in enteropathogenic E. coli (EPEC) [21], although a similar role in atypical EPEC has not been demonstrated [27]. An important function of SslE is its ability to actively degrade intestinal mucins including Muc2, Muc3 and bovine submaxillary mucin, which facilitates E. coli penetration of mucus and enhances access to apical epithelial cells [28–30]. Immunization with SslE protects mice against both UTI and intestinal infection, suggesting it may be effective as a broadly protective E. coli vaccine antigen [28]. This was further corroborated by the identification of SslE as an immunogenic antigen in patients infected with enterotoxigenic E. coli (ETEC) [31, 32].
The molecular mechanisms governing the regulation and expression of SslE by E. coli strains from different pathotypes remain to be properly elucidated. In ETEC, the sslE gene is transcribed as a polycistronic mRNA together with 13 downstream genes encoding its cognate T2SS (sslE-aspS-pppA-gspC-M) [33, 34]. The operon is repressed in a temperature-dependent manner by the nucleoid-associated proteins H-NS and StpA, with stronger expression at human physiological temperature (37°C) compared to ambient temperature (22°C) [33]. Both H-NS and StpA bind within the regulatory region of the sslE promoter and block transcription initiation by inhibiting promoter open complex formation. StpA binds with higher affinity than H-NS to this region, possibly via the formation of heteromeric complexes with H-NS that enhance its stability [33]. The regulatory role of H-NS in sslE transcription in ETEC is consistent with its function as a transcriptional repressor of many virulence genes in UPEC, including genes encoding autotransporter proteins (e.g. UpaC, UpaH, UpaG) [35–37], fimbriae (e.g. F9) [38], and toxins (e.g. α-hemolysin) [39]. In the non-pathogenic E. coli strain W, SslE expression is influenced by both temperature and nutrients, with stronger expression observed at 37°C in rich medium [40].
In this study, we examined the expression and secretion of SslE in different ExPEC strains. We showed that some strains, including the well-characterized UPEC strain UTI89 and the neonatal meningitis-associated E. coli (NMEC) strain IHE3034 produce high levels of SslE, suggesting alleviation of H-NS repression at physiological temperature. Consistent with this observation, a genetic screen using the high SslE-expressing strain UTI89 identified the nucleoid associated global transcriptional regulator Fis and the ribosome binding GTPase TypA as positive regulators of sslE transcription. Evidence for the function of Fis as an activator of SslE was demonstrated by the generation and analysis of specific mutant and complemented strains and reporter constructs.
Materials and Methods
Bacterial strains, plasmids and culture conditions
All strains and plasmids used in this study are listed in Table 1. The UPEC isolates examined for SslE expression were obtained from our in-house clinical collection [41]. E. coli strains were routinely cultured at 37°C on solid or in liquid Luria-Bertani (LB) media supplemented with appropriate antibiotics (100 μg/ml ampicillin, 100 μg/ml kanamycin, 30 μg/ml chloramphenicol). Where necessary, gene expression was induced with 1mM isopropyl β-D-1-thiogalactopyranoside (IPTG).
Table 1. Bacterial strains and plasmids used in this study.
| Strain or plasmid | Relevant characteristics | Reference |
|---|---|---|
| E. coli K12 strains | ||
| MG1655 | K-12 reference strain | [42, 43] |
| BL21 (DE3) | F–ompT hsdSB(rB–, mB–) gal dcm (DE3) | Stratagene |
| Clinical UPEC collection | 18 strains randomly selected from a collection of isolates from patients with urosepsis | This study |
| ExPEC strains | ||
| 536 | UPEC pyelonephritis isolate | [5, 44] |
| 536fis | 536fis::kan; Kanr | This study |
| 536fis(pFis) | 536fis::kan + pFis; Kanr Cmr | This study |
| EC958 | ST131 UPEC isolate | [45–47] |
| EC958hns | EC958hns::cm; Cmr | This study |
| EC958hns(pHNS) | EC958hns pHNS; Cmr | This study |
| EC958fis | EC958fis::cm; Cmr | This study |
| EC958fis(pFis) | EC958fis pFis; Cmr | This study |
| IHE3034 | NMEC isolate | [20, 48] |
| IHE3034sslE | IHE3034sslE::kan; Kanr | This study |
| IHE3034hns | IHE3034hns::kan; Kanr | This study |
| IHE3034hns(pHNS) | IHE3034hns::kan pHNS; Kanr Ampr | This study |
| IHE3034fis | IHE3034fis::kan; Kanr | This study |
| IHE3034fis(pFis) | IHE3034fis::kan pFis; Kanr Cmr | This study |
| UTI89 | UPEC cystitis isolate | [49, 50] |
| UTI89sslE | UTI89sslE::kan; Kanr | This study |
| UTI89hns | UTI89hns::kan; Kanr | This study |
| UTI89hns(pHNS) | UTI89hns::kan pHNS; Kanr Ampr | This study |
| UTI89fis | UTI89fis::kan; Kanr | This study |
| UTI89fis(pFis) | UTI89fis::kan pFis; Kanr Cmr | This study |
| UTI89typA | UTI89typA::kan; Kanr | This study |
| UTI89typA(pTypA) | UTI89typA::kan pTypA; Kanr Cmr | This study |
| UTI89lacI-Z | UTI89lacI-Z::gfp | This study |
| UTI89lacI-Z sslE::lacZ | UTI89lacI-Z::gfp sslE::lacZ | This study |
| UTI89lacI-Z fis | UTI89lacI-Z::gfp fis::kan; Kanr | This study |
| Plasmids | ||
| pKD3 | Template plasmid for cm gene amplification | [51] |
| pKD4 | Template plasmid for kan gene amplification | [51] |
| pKD46 | λ-red recombinase expressing plasmid | [51] |
| pCP20 | FLP expressing plasmid | [52] |
| pMCSG7 | Ligation-independent 6xHis tag cloning vector | [53] |
| pHNS | pBR322 cloned with hns gene | [35] |
| pSU2718 | pACYC184-derived cloning plasmid | [54] |
| pHNS-cm | pSU2718 cloned with hns gene | This study |
| pTW26 | pSU2718 modified with a xhoI site inserted at original HincII site | This study |
| pFis | dusB-fis operon cloned in pTW26 | This study |
| pTypA | typA gene cloned in pTW26 | This study |
| pQF50 | Promoterless lacZ reporter plasmid | [55] |
| pQF50-sslE | sslE promoter region cloned in pQF50 | This study |
| pQF50-sslEM1 | pQF50-sslE with 2 nucleotide mutations in F1 | This study |
| pQF50-sslEM2 | pQF50-sslE with 3 nucleotide mutations in F2 | This study |
| pQF50-sslEM3 | pQF50-sslE with 5 nucleotide mutations in F1 and F2 | This study |
DNA manipulation and genetic techniques
Oligonucleotides were synthesized by Integrated DNA Technologies. A list of primers used in this study is provided in S1 Table. Plasmid DNA was isolated using the QIAprep Spin Miniprep kit (Qiagen). Chromosomal DNA was purified using the Wizard® Genomic DNA Purification Kit (Promega). PCR amplification was performed using OneTaq® DNA Polymerase (New England Biolabs) or KAPA HiFi™ polymerase (Kapa Biosystems) where high-fidelity amplification was required. PCR products and other DNA fragments were purified using the QIAquick PCR Purification kit or the QIAquick Gel Extraction kit (Qiagen). Restriction endonucleases, T4 polynucleotide kinase, T4 ligase and T4 polymerase treatment were performed following manufacturer’s recommendations (New England Biolabs). Nucleic acid quantification was performed using a Nano-Drop 2000 Spectrophotometer (Thermo Scientific). DNA sequencing was performed using Big Dye Terminator Sequencing v3.1 Cycle Sequencing (Applied Biosystems) at the Australian Equine Genomic Research Centre (AEGRC), University of Queensland.
Construction of plasmids
Plasmids pFis and pTypA were constructed by PCR amplification of dusB-fis (primers 4539 and 4538) and typA (primers 4535 and 4536), respectively, from UTI89. PCR products were digested with XhoI (forward primer) and HindIII (reverse primer) and ligated into a similarly digested plasmid pTW26 (modified pSU2718 with xhoI) [54]. Two plasmids containing hns were used. One contained hns cloned in plasmid pBR322 (pHNS) [36]. The second plasmid was constructed by PCR amplification of hns (primers 4588 and 4589) from EC958 and ligated into BamHI-HindIII digested plasmid pSU2718 (pHNS-cm). Plasmid pQF50-sslE was constructed by PCR amplification of the sslE promoter region from the chromosome of UTI89 using primers 5570 and 5571. PCR products were digested with BamHI (forward primer) and HindIII (reverse primer) and ligated into BamHI-HindIII digested plasmid pQF50 [55]. Plasmids pQF50-sslEM1, pQF50-sslEM2 and pQF30-sslEM3 were synthesized by Epoch Life Science Inc.
Construction of mutants
All mutants were generated using λ-Red recombinase mediated homologous recombination as previously described [51]. Mutant strains IHE3034sslE, UTI89sslE, UTI89fis and UTI89typA were constructed using primers with 50-bp homology extensions to amplify the kanamycin (kan) resistance cassette with FRT sites from pKD4. The following primers were used. IHE3034sslE and UTI89sslE: 2886 and 2887; UTI89fis: 4411 and 4412; UTI89typA: 4399 and 4400. To generate hns deletion mutants in strains IHE3034, UTI89 and 536, the kan resistance cassette along with 500 bp homology region was amplified from a previously constructed CFT073hns mutant [35] using primers 2361 and 2363, generating the strains IHE3034hns, UTI89hns and 536hns. To generate fis deletion mutants in IHE3034 and 536, the kan resistance cassette with a 250bp homology region was amplified from the UTI89fis mutant using primers 6044 and 6043, and λ-Red recombination was used to generate the strains IHE3034fis and 536fis. To generate deletion mutants in EC958, a three-way PCR procedure was employed to amplify a chloramphenicol (cm) resistance cassette with 500bp homology region from pKD3 [46]. EC958hns was generated using primers 3915, 3916, 2247, 2248, 3917 and 3918. EC958fis was generated using primers 6218, 6219, 6220, 6221, 6222 and 6223. UTI89fis hns was generated by mutating hns in the UTI89fis mutant using a cm cassette with 500bp homology region as described above. In order to generate an sslE-lacZ reporter construct, the lacI-Z genes from UTI89 were initially deleted with a chloramphenicol-gfp cassette essentially as previously described [56]; with the exception that the lacI-Z genes were deleted instead of the entire lac operon; using primers 3997 and 3998. The chloramphenicol resistance cassette was subsequently removed using pCP20 [51], and the sslE::lacZ promoter fusion strain was generated as previously described [36], with the exception that a chloramphenicol resistance cassette was used instead of zeocin (Primers 4052 and 4061). The chloramphenicol cassette was subsequently removed using pCP20 to generate the strain UTI89lacI-Z sslE::lacZ.
Growth assays
All growth assays were performed using the FLUOstar OPTIMA Microplate Reader (BMG LABTECH). Strains were assayed in triplicate in sterile 96-well plates using LB broth as growth media and a total volume of 200 μl. Each starting culture was standardized to OD600 = 0.05. Plates were incubated at 37°C with shaking; OD600 readings were taken at 15 min intervals.
Generation of SslE polyclonal antibodies
A segment of the sslE gene encoding a 282 amino-acid N-terminal fragment of the protein (minus the predicted signal peptide) was PCR amplified with primers 2673 and 2674 from IHE3034 and cloned as an N-terminal 6xHis fusion in plasmid pMCSG7 via ligation-independent cloning [53]. E. coli BL21 (DE3) was transformed with this plasmid and cells were grown in LB medium containing IPTG to induce the expression of recombinant SslE. His-tagged recombinant SslE (SslEʹ) was purified using the nickel-nitrilotriacetic acid (Ni-NTA) affinity chromatography (Qiagen) following the manufacturer’s instructions. Purified SslE’ was quantified using the Bicinchoninic Acid Protein Assay Kit (Sigma) and assessed for purity via SDS-PAGE. Polyclonal antibodies were generated in rabbits at the Institute of Medical and Veterinary Science (IMVS), South Australia. The antiserum was adsorbed against a crude protein extract of UTI89sslE prior to use.
Protein preparation and immunoblotting
Strains were cultured in LB to an optical density at 600nm (OD600nm) ~3.0. Whole cell lysates were prepared by centrifuging 1 ml of cultures standardized to OD600nm = 1.0. Cell pellets were resuspended in 50 μl water and 50 μl 2×SDS loading buffer (100mM Tris-HCl, 4% w/v SDS, 20% w/v glycerol, 0.2% w/v bromophenol blue, pH 6.8). Supernatant proteins were prepared following a standard procedure, which involved centrifugation of an OD600nm ~3.0 culture, filtration of the supernatant fraction (0.22μm filter) and precipitation with 10% trichloroacetic acid (TCA; 7.2 ml filtered supernatant, 0.8 ml 100% TCA). Precipitated proteins were pelleted by centrifugation, washed twice in 100% ethanol and air-dried. Proteins were resuspended in 50 μl of resuspension buffer (50 mM Ammonium Bicarbonate, 3 M Urea, 5 mM DTT) and an equal volume of 2×SDS loading buffer and samples were boiled for 10 min prior to electrophoresis; a volume of 10 μl was routinely analyzed. SDS-PAGE and transfer of proteins to a PVDF membrane for western blot analysis was performed as previously described [57]. SslE polyclonal antibodies were used as the primary antibody, and alkaline phosphatase conjugated anti-rabbit antibodies (Sigma Aldrich) were used as the secondary antibody. SIGMAFAST™ BCIP®/NBT (Sigma-Aldrich) was used as the substrate for detection. Western blots were scanned using the Bio-Rad GS-800™ calibrated imaging densitometer.
Transposon mutagenesis
Transposon mutagenesis of UTI89lacI-Z sslE::lacZ was performed using the Epicentre EZ::Tn5 custom transposome construction kit. A miniTn5-Cm transposon was generated by PCR using plasmid pKD3 as template DNA with primers 2279 and 2280 that contain Tn5 mosaic ends. Purified Tn5-Cm DNA was phosphorylated and incubated with the Transposase (1μg DNA with 4U Transposase). Transposomes were transformed into competent UTI89lacI-Z sslE::lacZ cells via electroporation and transposon mutants were plated on LB agar supplemented with chloramphenicol and 5-bromo-4-chloro-3-indolyl β-D-galactopyranoside (X-Gal). Transposon mutants with altered β-galactosidase activity (determined via blue-white screening) were selected and confirmed by repeat subculture. The insertion site of the transposon mutants was identified via a 2-step arbitrary PCR as previously described [58], with the following primers specific to the chloramphenicol cassette on the transposon: 2209 (round 1) and 3340 (round 2).
5′ Rapid amplification of cDNA ends (5′ RACE)
The transcription start site of sslE was determined using 5′ RACE (Version 2.0; Invitrogen) [59]. Exponentially growing cells (OD600nm = 0.6) were stabilized with two-volumes of RNAprotect Bacteria Reagent (Qiagen) prior to RNA extraction using the RNeasy Mini Kit (Qiagen) with optional on-column DNase digestion. First-strand cDNA was synthesized and PCR amplified using the following gene specific primers: 4207, 4208 and 4209 following manufacturer’s specification. Amplified cDNA ends were sequenced to determine the transcription start site.
Quantitative reverse transcription PCR (qRT-PCR)
Total RNA extraction was performed as described above. Purified RNA samples were further treated with rDNase I (Ambion) to ensure the complete removal of contaminating DNA, and re-purified using the RNeasy Mini Kit (Qiagen) RNA cleanup protocol. First-strand cDNA synthesis was performed using the SuperScript® III First-Strand Synthesis System (Invitrogen) as per manufacturer’s recommendation. Real-time PCR was performed using SYBR® Green PCR Master Mix (Applied Biosystems) on a ViiA™ 7 Real-Time PCR System (Applied Biosystems), using the following primers for sslE, primers 4205 and 4206. Transcript levels of each gene were normalized to gapA as the endogenous gene control (primers 820 and 821). Gene expression levels were determined using the 2-ΔΔCT method [60], with relative mRNA fold-difference expressed against the respective wild-type strains. All experiments were performed as three independent replicates, with all samples analyzed in triplicate. Statistical analysis of fold differences from wild-type, mutant and complemented strains was performed using an unpaired, two tailed student’s t-test.
β-galactosidase assay
β-galactosidase assays were performed essentially as previously described [61]. Briefly, strains to be assessed were grown overnight in LB broth supplemented with the necessary antibiotics. Cultures were diluted in Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 50 mM β-mercaptoethanol, 10 mM KCl, 1mM MgSO4, pH 7) with 0.004% SDS and chloroform added. Samples were vortexed and incubated at 28°C to permeabilize the cells. The substrate o-nitrophenyl- β-D-galactopyranoside (ONPG) was added to initiate the reaction which was subsequently stopped with sodium bicarbonate. β-galactosidase activity was assessed in quadruplicate for each strain by measuring the absorbance at 420 nm. All experiments were performed as three independent replicates. Statistical analysis of β-galactosidase levels between each wild-type and fis mutant strains carrying the different pQF50 constructs was performed using an unpaired, two tailed student’s t-test.
Results
SslE is expressed at different levels by diverse ExPEC strains
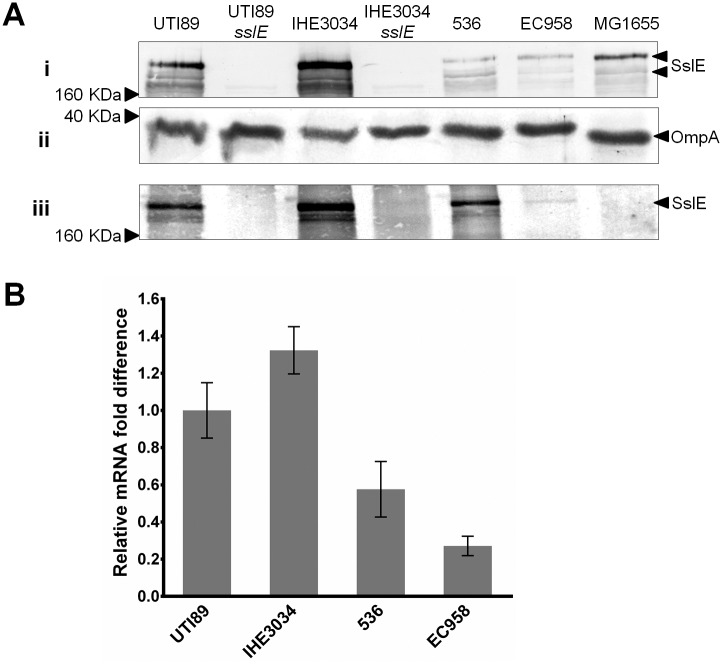
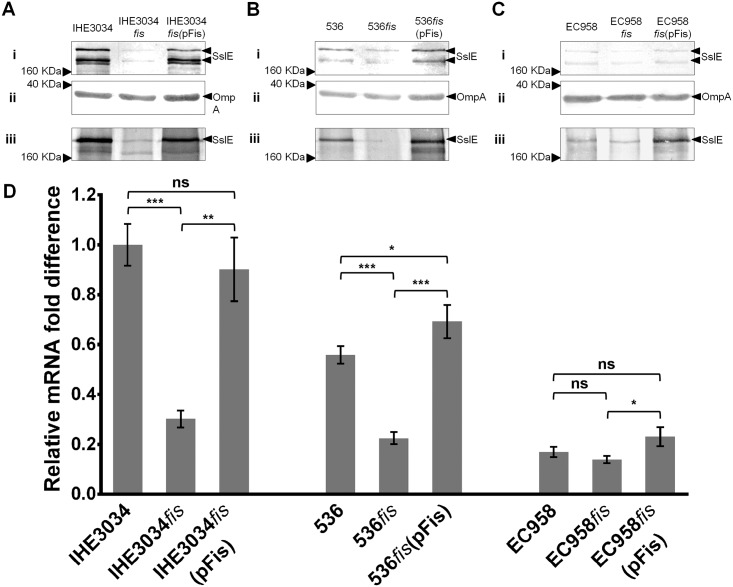
The expression of SslE was assessed by western blot analysis using an SslE-specific antibody. SslE expression was examined in the whole-cell lysate and supernatant fractions prepared from cultures of four well-characterized reference ExPEC strains grown at 37°C: UTI89, EC958 (cystitis isolates), IHE3034 (NMEC isolate), and 536 (pyelonephritis isolate) (Table 1; Fig 1A). A band corresponding to the size of SslE was detected in both whole-cell and supernatant fractions prepared from all four strains. However, different levels of SslE expression were observed based on calibration with an OmpA loading control, with the strongest expression detected in IHE3034 and UTI89. To confirm the identity of the SslE protein band, the sslE gene was deleted in UTI89 and IHE3034 to generate the mutant strains UTI89sslE and IHE3034sslE; protein preparations from both mutants did not react with the SslE antibody. SslE is a large ~167 kDa protein and we consistently observed a band of this size as well as smaller cross-reacting bands in our western blot analysis; these smaller bands are likely to represent SslE breakdown products as they were absent from UTI89sslE and IHE3034sslE. SslE expression was also examined in the K-12 strain MG1655, which possesses an intact sslE gene but a mutated downstream T2SS locus. Consistent with previous reports [20], SslE expression was detected in the whole-cell lysate of MG1655 but not in the secreted fraction. Based on the western blot analysis of both whole-cell and supernatant fractions, we observed an overall correlation between the level of SslE synthesis and secretion, a finding consistent with the fact that the sslE gene is co-transcribed as a single operon with its cognate T2SS encoding genes [33]. We also examined SslE secretion in 18 UPEC strains from our clinical isolate collection that were positive for the sslE and T2SS-encoding genes. SslE was detected in the supernatant fraction of all 18 isolates, however consistent with our results for UTI89, EC958, IHE3034 and 536, different levels of SslE secretion were observed among these strains (S1 Fig).
Fig 1. Western blot and qRT-PCR analysis to examine sslE expression in ExPEC strains.
(A) Western blot analysis of SslE from (i) whole-cell lysates and (iii) supernatant fractions prepared from UTI89, UTI89sslE, IHE3034, IHE3034sslE, 536, EC958 and MG1655. SslE has a predicted size of approximately 167 kDa. Two major cross-reacting bands of this size were detected (indicated by arrows), possibly representing full-length and processed SslE. No cross-reacting bands were detected in samples prepared from UTI89sslE and IHE3034sslE, demonstrating the specificity of antibody. (ii) Loading control for whole cell lysate samples. The same samples used above were examined by western blot using an OmpA antibody. Similar levels of OmpA were detected in all samples, indicating equivalent loading of total protein. (B) Relative fold-difference of sslE transcript levels of ExPEC strains UTI89, IHE3034, 536, EC958 as determined by qRT-PCR. All mRNA levels were calculated relative to the level of UTI89 sslE mRNA. The relative sslE mRNA levels were consistent with the data observed from the western blot analysis. The data was obtained from three independent experiments; error bars indicate standard deviation.
SslE expression is regulated at the level of transcription
The transcript abundance of sslE was assessed in UTI89, IHE3034, EC958 and 536 via qRT-PCR (Fig 1B). Compared to UTI89, sslE transcript level was higher in IHE3034 (~1.3 fold increase), and lower in 536 (~1.7-fold decrease) and in EC958 (~3.7-fold decrease), respectively. Overall, the difference in relative mRNA transcript level of sslE for each strain corresponded with the expression pattern observed by western blot analysis. Taken together, the results suggest that the expression of SslE is regulated at the transcriptional level.
H-NS is not a strong repressor of sslE transcription in UTI89 at 37°C
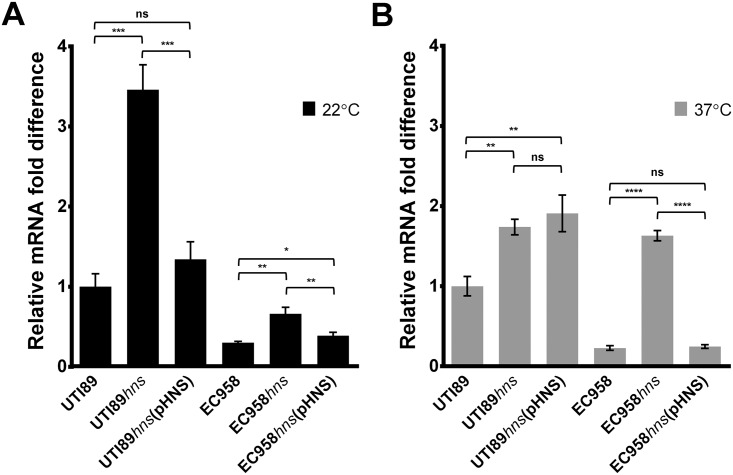
In ETEC, H-NS mediates temperature-dependent repression of sslE transcription [33]. To investigate the impact of H-NS on SslE expression in UPEC, the hns gene was mutated in the high SslE-expressing strain UTI89 (UTI89hns) and the low SslE-expressing strain EC958 (EC958hns). In both strains, the mutation of hns had a similar effect and resulted in slightly reduced growth (S2 Fig). The deletion mutants were complemented with a recombinant plasmid containing the hns gene (pHNS or pHNS-cm, respectively). The wild-type, hns mutant and complemented strains were grown at 22°C and 37°C, and the level of sslE transcription was assessed by qRT-PCR (Fig 2). Consistent with previous data from ETEC H10407 [33], the transcript level of sslE was strongly increased in UTI89hns (~3.5-fold) and EC958hns (~2.2-fold) at 22°C and was reduced to wild-type levels in the complemented strains UTI89hns(pHNS) and EC958hns(pHNS-cm). At 37°C, the sslE transcript level in EC958hns increased ~7.2-fold and was restored to wild-type level in EC958hns(pHNS-cm). In contrast, however, the increase in sslE transcript level in UTI89hns at 37°C was smaller (~1.7-fold), and the effect of H-NS could not be complemented in UTI89hns(pHNS). A similar difference in SslE expression in UTI89 (compared to EC958) was also observed by western blot analysis (S3 Fig). Overall, the phenotype of the UTI89hns and UTI89hns(pHNS) strains, together with the high expression of SslE in UTI89 at 37°C (Fig 1), led us to investigate the regulation of sslE at this temperature as outlined in the experiments described below.
Fig 2. Effect of hns deletion on sslE transcription in UTI89 and EC958 at 22°C and 37°C via qRT-PCR analysis.
Relative fold-difference of sslE transcript levels of UTI89 and EC958 with their respective hns mutant and complemented strains grown at (A) 22°C (black bars) and (B) 37°C (grey bars). All mRNA levels were calculated relative to the level of UTI89 sslE mRNA at the respective temperature. The data was obtained from three independent experiments; error bars indicate standard deviation. Statistical analysis was performed using an unpaired, two-tailed t-test.
Identification of genes involved in the positive regulation of sslE
To investigate the genetic basis of strong sslE transcription in UTI89, we generated a chromosomal sslE promoter-lacZ reporter fusion construct. We first mapped the transcription start site and promoter of sslE in UTI89, which was in agreement to the promoter mapped in ETEC H10407 [33]. A lacZ reporter was inserted as a transcriptional fusion to the sslE promoter on the chromosome of a UTI89lacI-Z strain to generate the strain UTI89lacI-Z sslE::lacZ. When grown on LB agar in the presence of X-gal at 37°C, all UTI89lacI-Z sslE::lacZ colonies were dark blue, indicating strong activity of the sslE promoter. In order to identify transcriptional regulators of sslE in UTI89, the reporter strain UTI89lacI-Z sslE::lacZ was subjected to transposon mutagenesis using a mini-Tn5 cassette. Approximately 25,000 transposon mutants (~5-fold coverage) were generated and screened on LB agar supplemented with chloramphenicol and X-gal. In this screen, 13 mutants were identified that grew as white/pale-blue colonies. The β-galactosidase activity of the 13 transposon mutants was measured (S2 Table) and the transposon insertion site determined by arbitrary PCR. Overall, Tn5 insertions were identified within six different genes, three of which contained multiple independent insertions; two mutants contained insertions in the lacZ gene (Table 2). The three genes with more than one independent Tn5 insertions were: (i) fis, which encodes a nucleoid associated protein that acts as a global transcriptional regulator [62–66], (ii) typA (also known as bipA) which encodes a ribosome associated GTPase that has been associated with post-transcriptional regulation in EPEC [67, 68], and (iii) nusA which encodes a co-factor of Rho-dependent transcriptional termination [69, 70]. In addition, two mutants containing a unique Tn5 insertion in the tandemly arranged ygaZ-ygaH genes, respectively, were identified; the function of these genes is not known. Finally, one mutant contained a Tn5 insertion in dusB (previously known as yhdG) [71, 72], which encodes for a tRNA-dihydrouridine synthase [73]. The dusB gene is located immediately upstream of fis; the two genes are co-transcribed as a bicistronic operon [74, 75] and DusB is absolutely required for efficient translation of fis mRNA [72, 76]. Based on these findings, we selected Fis and TypA for further analysis as potential regulators of sslE transcription.
Table 2. List of genes identified in the transposon mutagenesis screen.
| Gene | Description | Number independent insertions |
|---|---|---|
| typA | Ribosome associated GTPase | 4 |
| fisa | Nucleoid-associated transcriptional factor | 2 |
| nusA | Rho-dependent transcriptional termination co-factor | 2 |
| ygaZb | Hypothetical protein of unknown function | 1 |
| ygaHb | Hypothetical protein of unknown function | 1 |
| dusBa | tRNA-dihydrouridine synthase | 1 |
a,bTandemly arranged genes
The nucleoid associated protein Fis and ribosome binding GTPase TypA are positive regulators of SslE expression
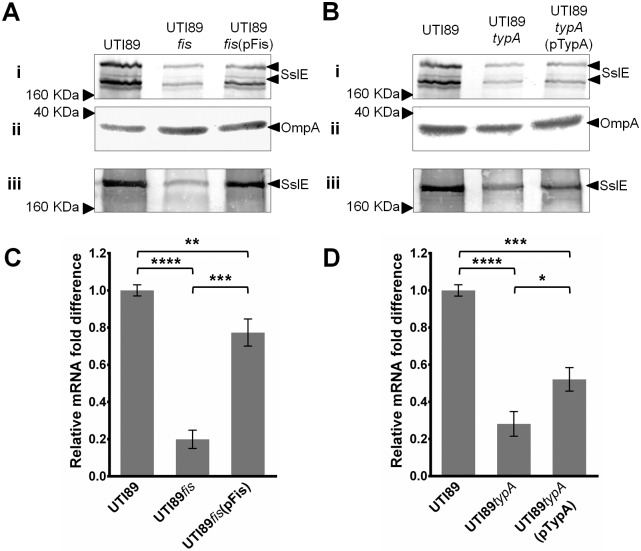
The fis and typA genes were mutated in UTI89 to generate strains UTI89fis and UTI89typA. The expression of SslE was then examined by western blot analysis of whole-cell lysates and supernatant fractions prepared from wild-type UTI89, UTI89fis and UTI89typA following growth in LB broth at 37°C (Fig 3A and 3B). A strong reduction in the level of SslE was detected in preparations from UTI89fis. A reduction in SslE expression was also observed in UTI89typA, albeit not as strong. To confirm the role of fis and typA in the regulation of sslE, the mutants were complemented with a plasmid containing each respective gene (i.e. pFis and pTypA). Complementation of UTI89fis with plasmid pFis restored the amount of SslE detected in whole-cell lysates and supernatant fractions to wild-type level (Fig 3A). In the case of the UTI89typA mutant, transformation with plasmid pTypA resulted in partial complementation of SslE expression (Fig 3B). Taken together, these results confirm a role for Fis and TypA in sslE regulation.
Fig 3. Effect of fis and typA deletion in UTI89 on sslE expression determined via western blot and qRT-PCR analysis.
(A) Western blot analysis of SslE using (i) whole-cell lysates and (iii) supernatant fractions prepared from UTI89, UTI89fis and UTI89fis(pFis). (ii) Western blot loading control for whole cell lysate samples using an OmpA antibody. (B) Western blot analysis of SslE using whole-cell lysates and supernatant fractions prepared from UTI89, UTI89typA and UTI89typA(pTypA). (ii) Western blot loading control for whole cell lysate samples using an OmpA antibody. (C) Relative fold-difference of sslE transcript levels of UTI89, UTI89fis and UTI89fis(pFis); mRNA levels were calculated relative to the level of UTI89 sslE mRNA. (D) Relative fold-difference of sslE transcript levels of UTI89, UTI89typA and UTI89typA(pTypA); mRNA levels were calculated relative to the level of UTI89 sslE mRNA. The relative sslE mRNA levels were consistent with the data observed from the western blot analysis. The data was obtained from three independent experiments; error bars indicate standard deviation. Statistical analysis was performed using an unpaired, two-tailed t-test.
Fis and TypA affect the transcription of sslE
We sought to investigate if the regulatory effect of Fis and TypA occurs at the transcriptional level. Relative mRNA transcript levels of sslE were determined via qRT-PCR using wild-type UTI89 and the respective fis and typA mutant and complemented strains following growth in LB broth at 37°C (Fig 3C). The transcript level of sslE was reduced ~5-fold compared to wild-type UTI89. In the complemented UTI89fis(pFis) strain, the sslE transcript level was restored to almost wild-type level. The transcript level of sslE was also decreased in UTI89typA (~3.6-fold) and partially complemented in UTI89typA(pTypA); this pattern was similar to that observed by western blot analysis. Taken together, the agreement of the qRT-PCR analysis findings with the levels of SslE protein expression detected by western blot analysis indicates that both Fis and TypA affect sslE transcription. Fis is global transcriptional factor that regulates genes involved in multiple processes including virulence, metabolism and DNA replication [62, 64–66, 77]. TypA, on the other hand, is a ribosome binding GTPase that has been shown to contribute to the regulation of several virulence genes [68, 78–80]. The precise mechanism by which TypA exerts its regulatory effect remains unclear, however current evidence suggests this occurs at the level of translation [67, 68, 81, 82]. The finding that TypA affected the transcript levels of sslE suggests that its regulatory effect might be indirect. The remainder of our study focused on the characterization of Fis as a transcriptional activator of sslE in UTI89.
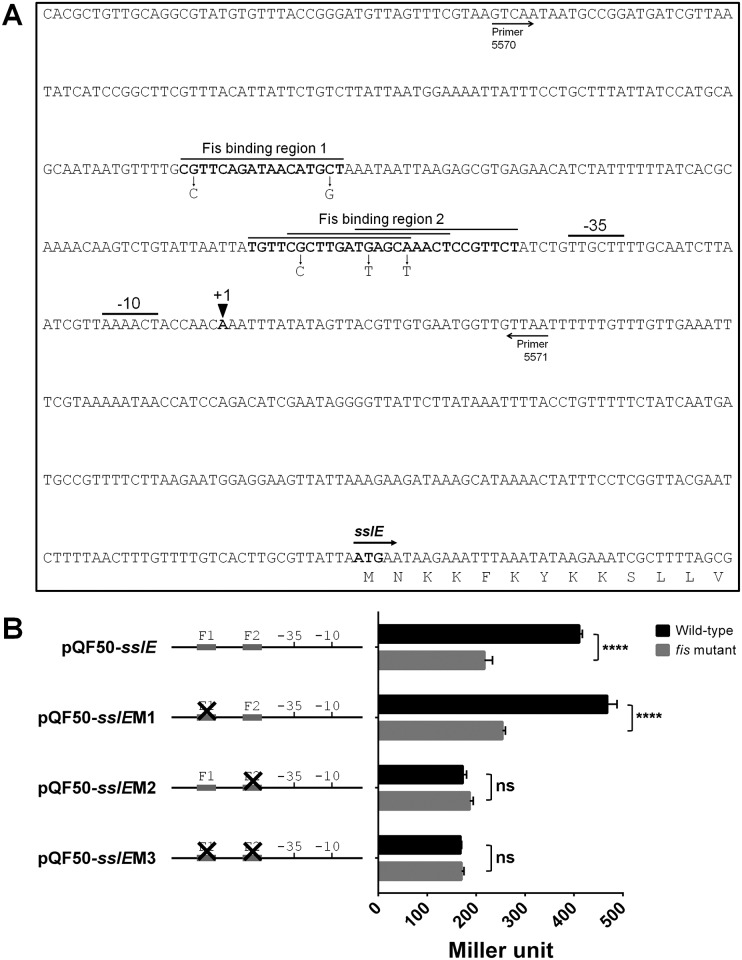
Mutation of a putative Fis-binding site within the sslE promoter alters its activity
The binding dynamics of Fis to DNA and its role as a transcriptional regulator have been extensively studied [83, 84]. The sslE promoter element contains two regions that contain putative Fis-binding consensus sequences based on a 15 bp sequence inclusion/exclusion rule for Fis binding as determined by previous studies [83, 84]. The first putative Fis-binding site is centered at position -138 (Fis-binding region 1; F1), while the second region consists of three overlapping putative Fis-binding sequences proximal to the -35 element, between positions -41 to -68 (Fis-binding region 2; F2) (Fig 4A). To examine the role of these putative Fis-binding sites on sslE promoter activity, the sslE promoter region was cloned into the lacZ reporter plasmid pQF50 to generate pQF50-sslE. Three additional constructs were created to introduce point mutations in key nucleotides within the putative Fis-binding sites (Fig 4A). The first construct, pQF50-sslEM1, contained two nucleotide changes that disrupted F1; the second construct, pQF50-sslEM2, contained three nucleotide changes that disrupted all three overlapping putative Fis binding sequences within F2; while the last construct, pQF50-sslEM3, contained both sets of mutations. All four plasmid constructs were transformed into UTI89lacI-Z (wild-type) and UTI89lacI-Z fis (fis mutant) and the promoter activity in each strain was quantified by the measurement of β-galactosidase activity (Fig 4B). The β-galactosidase activity measured from UTI89lacI-Z containing pQF50-sslE (410.8±6.3 Miller units) was approximately double the activity measured from UTI89lacI-Z fis containing pQF50-sslE (217±16.2 Miller units), confirming that Fis positively regulates the activity of the sslE promoter. The β-galactosidase activity derived from plasmid pQF50-sslEM1 (contains mutations in F1) was similar to that derived from pQF50-sslE in both strain backgrounds, indicating that F1 is not required for Fis activation of the sslE promoter. In contrast, the β-galactosidase activity derived from plasmid pQF50-sslEM2 (contains mutations in F2) was significantly reduced in UTI89lacI-Z (172.7±7.8 Miller units), and similar to that obtained in UTI89lacI-Z fis (189.7±8 Miller units). The β-galactosidase activity derived from plasmid pQF50-sslEM3 (contains mutations in F1 and F2) was similar to that derived from pQF50-sslEM2 in wild-type and fis mutant backgrounds. Overall, these data suggest that F2 is important for activation of the sslE promoter by Fis, and that disruption of this putative binding region is sufficient to abolish Fis activation of the sslE promoter.
Fig 4. Promoter region of sslE in UTI89 and analysis of the effect of putative Fis-binding sequences on sslE promoter activity.
(A) Promoter region of sslE in UTI89. The transcription start site as determined via 5'RACE is indicated as +1. Putative -10 and -35 promoter sites and the ATG translational start site are indicated accordingly. The region cloned into the promoter-less lacZ reporter plasmid pQF50 is indicated by arrows showing the binding positions of the primers used to amplify the fragment. Putative Fis-binding sites are indicated in bold and overlined. Fis-binding region 1 (F1) consists of one Fis consensus binding sequence. Fis-binding region 2 (F2) consists of three over-lapping consensus sequences. The nucleotide changes introduced to disrupt the Fis-binding sites in the constructs are indicated by an arrow depicting the nucleotide change. (B) β-galactosidase activity represented as Miller units measured for the various sslE promoter-lacZ reporter plasmid constructs in UTI89lacI-Z (wild-type) and UTI89lacI-Z fis:kan (fis mutant) strains. Plasmid pQF50-sslE containing the intact sslE promoter resulted in approximately double the β-galactosidase activity in the wild-type compared to the fis mutant (p < 0.05; t-test). Plasmid pQF50-sslEM1 containing mutations in F1 resulted in similar levels of β-galactosidase activity to pQF50-sslE in both strains. Plasmids pQF50-sslEM2 and pQF50-sslEM3, which were disrupted in F2 and F1/F2, respectively, resulted in a similarly reduced β-galactosidase activity in both the wild-type and the fis mutant strains (not significant; ns), indicating that F2 is important for Fis activation of the sslE promoter.
Fis positively regulates sslE transcription and expression in the other ExPEC strains
To examine the effect of Fis on SslE expression in IHE3034, 536 and EC958, the fis gene was mutated in all three strains and similarly complemented with the plasmid pFis. SslE expression and transcription was examined via western blot analysis and qRT-PCR (Fig 5). For IHE3034, the phenotype observed in the fis mutant and complemented strains was similar to that observed for UTI89 (Fig 5A). In 536, SslE expression and secretion was reduced in 536fis, and complemented to levels higher than wild-type in 536fis(pFis) (Fig 5B). In EC958, due to the lower level of SslE expression, the effect of Fis was not as clear. However, as observed in 536, over-expression of Fis (from plasmid pFis) increased SslE expression in EC958 (Fig 5C). In all cases, relative sslE transcript levels as determined via qRT-PCR showed an overall pattern consistent with data from the western blot analysis (Fig 5D). Taken together, our data demonstrates a role for Fis in the activation of sslE transcription in multiple E. coli strains.
Fig 5. Effect of fis deletion on sslE expression in strains IHE3034, 536 and EC958 determined via western blot and qRT-PCR analysis.
Western blot analysis of SslE using preparations from (A) IHE3034, (B) 536 and (C) EC958; each with their respective fis mutant and complemented strains. In each analysis, (i) whole-cell lysates and (iii) supernatant fractions were examined. In addition, (ii) a control for whole cell lysate samples was performed using an OmpA antibody. (D) Relative fold-difference of sslE transcript levels of IHE3034, 536 and EC958, and their respective fis mutant and complemented strains. All mRNA levels were calculated relative to the level of IHE3034 sslE mRNA. The relative sslE mRNA levels were consistent with the data observed from the western blot analysis. The data was obtained from three independent experiments; error bars indicate standard deviation. Statistical analysis was performed using an unpaired, two-tailed t-test.
Fis and H-NS have contrasting roles in sslE regulation
To investigate whether the effect of Fis on SslE expression in UTI89 is dependent on H-NS, a fis-hns double mutant was generated (UTI89fis hns), and SslE expression following growth at 37°C was determined via western blot analysis (Fig 6A). As UTI89 already expresses a high level of SslE, mutation of hns did not lead to a detectable increase in protein expression by western blot analysis. Mutation of fis, on the other hand, significantly reduced SslE expression. In the UTI89fis hns double mutant, we detected a reduction in the level of SslE associated with the cell fraction (but not the supernatant fraction), suggesting that overall SslE expression is slightly reduced in this background compared to wild-type UTI89 (but increased compared to UTI89fis). Relative sslE transcript levels as determined via qRT-PCR showed a similar pattern, although the relative sslE transcript level detected in UTI89fis hns was similar to that of wild-type UTI89 (Fig 6B). Thus, in UTI89 at 37°C, Fis and H-NS play contrasting roles in SslE regulation; Fis strong activation and H-NS weak-moderate repression.
Fig 6. Effect of fis and hns double deletion on sslE expression in UTI89 determined via western blot and qRT-PCR analysis.
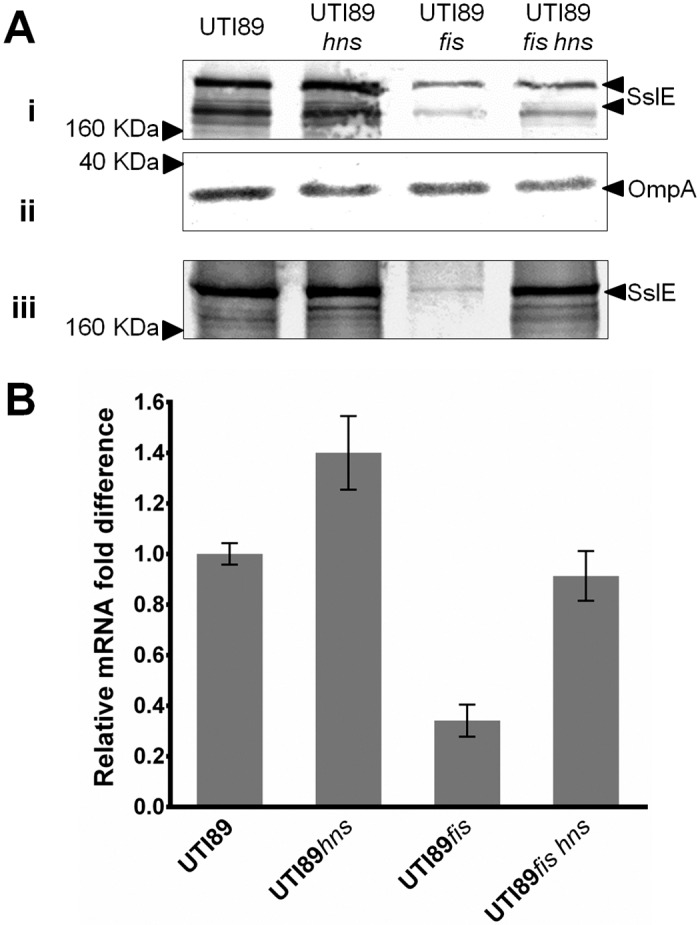
(A) Western blot analysis of SslE using (i) whole-cell lysates and (iii) supernatant fractions prepared from UTI89, UTI89hns, UTI89fis and UTI89fis hns. (ii) Western blot loading control for whole cell lysate samples using an OmpA antibody. The overall level of SslE was reduced in UTI89fis hns compared to wild-type UTI89. (B) Relative fold-difference of sslE transcript levels of UTI89, UTI89hns, UTI89fis and UTI89fis hns. All mRNA levels were calculated relative to the level of UTI89 sslE mRNA. The relative sslE mRNA levels were consistent with the data observed from the western blot analysis. The data was obtained from three independent experiments; error bars indicate standard deviation.
Discussion
Since its identification as a vaccine candidate, the lipoprotein SslE has been characterized with respect to its function and immunogenicity. Immunization of mice with SslE provides protection in sepsis, intestinal and UTI models [20, 28], and SslE has also been identified as an immunogenic antigen following ETEC infection [31, 32]. SslE is secreted via a dedicated T2SS, but also remains partially attached to the cell surface [20, 21], thus explaining its role in the maturation of EPEC biofilms [21]. More recently, SslE has been shown to degrade mucin and this property is linked to the presence of a conserved M60-like zinc-metalloprotease domain [28–30, 85]. These findings, along with the high prevalence of the sslE gene in all E. coli pathotypes, suggest that SslE may contribute to long-term colonization of various mucosal sites [28, 29].
In this study, we observed that SslE expression is variable among different UPEC strains. The reference strains UTI89 and IHE3034 expressed and secreted high levels of SslE following growth in LB broth at 37°C. In contrast, the ST131 strain EC958 produced and secreted significantly less SslE under the same conditions. Further examination of UTI89 and EC958 revealed that at the transcriptional level, sslE is regulated by H-NS in a temperature-dependent manner, consistent with many other H-NS regulated genes in E. coli [86–89]. Both strains exhibited strong H-NS repression of sslE transcription at 22°C, a finding similar to that reported previously for ETEC H10407 [33]. At 37°C, however, strong transcription of sslE was observed in UTI89, and mutation of hns had only a minor effect on total SslE production. This finding was in contrast to that observed in EC958, where H-NS strongly repressed sslE expression at 37°C. These observations led us to investigate the genetic basis of sslE regulation at 37°C in UTI89 using transposon mutagenesis in combination with an sslE promoter-lacZ-reporter transcriptional fusion construct. Through this approach, we identified six genes associated with increased sslE promoter activity—fis, typA, nusA, ygaZ, ygaH and dusB. The nusA gene encodes a multi-functional transcription factor that plays an essential role in transcription elongation, pausing, termination and anti-termination [90–95]. Despite repeated attempts, we were unable to generate a specific nusA mutation in UTI89 and confirm its role in sslE regulation. We attribute this to the importance of nusA in viability [70, 96–98], and the observation that nusA is essential in E. coli K-12 [99, 100]. We note that a mutant containing a transposon insertion in the 3'-end of nusA (corresponding to residue 417 out of 495 amino acids) has been reported [101]. Our Tn5 insertions in nusA corresponded to nucleotide position 1131 and 1279 out of 1488 bp (amino acids 377 and 427), respectively, suggesting that mutations that disrupt the 3'-end of nusA are tolerated, while mutation of the entire nusA gene is lethal. It is also possible that the identification of nusA in our screen was due to its requirement for β-galactosidase synthesis as previously reported [102, 103]. However, the role of NusA in promoting factor-dependent transcription termination [104], together with the contribution of H-NS and StpA to this process [105], lends support to its role in sslE regulation, although this remains to be experimentally proven. One Tn5 insertion mutant was also identified in each of the tandemly arranged hypothetical ygaZ-ygaH genes, respectively. However, we were unable to demonstrate a role for either gene in sslE regulation in subsequently constructed specific mutants, either in tandem or individually. The dusB gene is co-transcribed with fis [75], and therefore its identification is most likely linked to disruption of this operon. The fis and typA genes, which were identified in several independent Tn5 mutants, were thus selected for study in greater detail.
We showed that the ribosomal binding GTPase TypA enhances SslE expression. Mutation of typA in UTI89 resulted in reduced SslE expression. This effect was only partially complemented by re-introduction of the typA gene, a result possibly linked to the use of a plasmid based system. TypA belongs to a ribosomal binding GTPase superfamily and is widely prevalent among different bacteria [78]. In E. coli, TypA contributes to the regulation of a range of virulence phenotypes, including flagella-mediated motility, resistance to antimicrobial peptides and the production of virulence determinants encoded by the locus of enterocyte effacement (LEE) pathogenicity island [67, 68, 106]; as well as K5 capsule production and growth at low temperatures [80, 107]. Our data adds SslE to the growing number of surface factors regulated by TypA in E. coli, although like many previous reports, the precise molecular mechanism by which TypA exerts its regulatory affect remains unclear [67, 68, 80]. TypA has not been shown to interact directly with DNA, with current evidence suggesting that TypA mediated regulation may be indirect and elicited at the translational level [68, 80–82, 108]. Further support of our results comes from a report that identified TypA as a regulator of the LEE-encoded type III secretion system in the EPEC strain E2348/69 [68]. Here, Grant et al. showed that in addition to controlling the LEE-encoded regulator Ler, TypA also regulates a ~170kDa secreted protein independent of the Type III secretion system. E2348/69 has been completely sequenced, and our analysis of its genome identified five genes that could encode proteins 160-180kDa in size: sslE (yghJ), gltB (encoding a glutamate synthase), mukB (encoding a chromosome partitioning protein), yfaS and yfhM (encoding hypothetical proteins). Of these, sslE is the only gene encoding a protein predicted to be secreted, suggesting that TypA also regulates SslE in E2348/69.
Several lines of evidence demonstrated a role for Fis in the regulation of sslE transcription. First, Tn5 insertions were identified in both fis and dusB, which are co-transcribed as a single mRNA. Thus, independent Tn5 insertions that either mutate fis or disrupt its transcription were identified. Second, mutation of fis in UTI89 led to a dramatic decrease in SslE expression and this could be complemented with a plasmid encoding the fis gene. Third, the mRNA transcript levels of sslE were reduced in a fis mutant and restored to wild-type level upon complementation. Fourth, cloning the sslE promoter region into a promoterless-lacZ reporter plasmid revealed that sslE promoter activity was approximately halved in a fis mutant background compared to wild-type. Finally, mutation of a putative consensus Fis-binding site in the sslE promoter region significantly reduced the activity of the sslE promoter. Fis is also known to activate the transcription of several other genes in E. coli, including the LEE transcriptional regulatory gene ler [66], and the enteroaggregative E. coli (EAEC) autotransporter toxin gene pet [65]. Fis also upregulates a wide variety of genes important during exponential growth including those involved in translation, metabolism, motility and nutrient transport [63]. Fis binding to DNA requires contact points to specific nucleotides, where explicit inclusion and exclusion rules at defined nucleotide positions necessary for Fis contact have been established [83, 84]. Our bioinformatic analysis identified two putative Fis-binding regions proximal to the sslE promoter that fulfilled these nucleotide inclusion/exclusion requirements. We further experimentally established that the putative F2 binding site, which is located immediately upstream of the -35 promoter element, is necessary for Fis activation of the sslE promoter. Disruption of F2 reduced the sslE promoter activity to a level similar to that observed in a fis mutant background. The F2 site consists of three overlapping Fis-binding consensus sequences [83, 84], and thus we chose to alter nucleotides that would disrupt all three binding sites simultaneously. The proximity of the F2 site to the sslE promoter suggests a mechanism typical of transcriptional activators: binding of Fis upstream of the sslE -35 promoter element and recruitment of RNA polymerase. Notably, our data does not rule out a role for the putative F1 binding site, as the function of upstream binding sites in Fis regulation can mediate subtle changes to local DNA topology [76]. Further experiments using purified Fis protein are now required to demonstrate its direct binding to the sslE promoter region.
The regulation of SslE by Fis was also demonstrated in strains IHE3034 and 536, and to a lesser extent in the low SslE-expressing strain EC958. A nucleotide alignment of the sslE promoter region from UTI89, IHE3034, 536 and EC958 revealed several nucleotide changes unique to both 536 and EC958 (S4 Fig). Notably, one of these changes occurs in the F2 Fis binding region, although the change is not in a key conserved nucleotide defined within the Fis-binding inclusion/exclusion rules. Furthermore, the higher level of SslE expression in 536 compared to EC958 suggests that this sequence change alone cannot explain the differences in SslE expression. Further analysis of the sslE promoter region in 87 completely sequenced E. coli genomes available on the NCBI database that were positive for sslE (S3 Table) revealed that there are no other nucleotide sequence changes in the F2 Fis binding sequence. In contrast to Fis, a stringent consensus DNA binding sequence for H-NS has not been defined, and it is generally accepted that H-NS binds to AT-rich and highly curved DNA [109–113]. Yang et al. previously reported that the ETEC H10407 sslE promoter region contains such features and binds specifically to purified H-NS protein [33], and we confirmed the AT-rich and curved DNA topology of the sslE promoter region in our strains using BEND-IT (http://hydra.icgeb.trieste.it/dna/bend_it.html). H-NS was not identified in our mutagenesis screen, which was designed to identify positive regulators of the sslE promoter. However, we showed that both Fis and H-NS alter the activity of the sslE promoter in a mutually antagonistic fashion. Fis and H-NS have been shown to co-regulate other genes in E. coli; for example dps and nir are repressed by the cooperative action of Fis and H-NS [114, 115], and both proteins also regulate transcription from the ribosomal RNA promoter (rrnB P1) as well as the hns promoter itself [116–118]. In Shigella and enteroinvasive E. coli, the virF gene is repressed by H-NS at 30°C and activated by Fis at 37°C [88]. In our experiments in UTI89, we showed that at 37°C mutation of fis leads to strong attenuation of SslE expression, and that this affect is partly overcome by the subsequent mutation of hns. This suggests an interplay between Fis and H-NS that may involve several mechanisms, including the direct competition for overlapping binding sites and temperature-mediated changes in DNA conformation that alter the binding affinity of both proteins. Both of these overlapping mechanisms could explain the molecular interactions that underpin Fis and H-NS regulation of sslE in UTI89.
SslE is a highly prevalent secreted and surface associated colonization factor of E. coli. In addition, SslE represents a promising vaccine antigen that provides broad protection from infection by multiple E. coli pathotypes. Overall, this work has identified differences in SslE expression by different UPEC strains at core body temperature, and provides evidence to demonstrate a role for Fis in the regulatory control of the sslE gene.
Supporting Information
Western blot analysis of SslE using supernatant fractions from UTI89, UTI89sslE and 18 clinical UPEC isolates from our laboratory collection. SslE secretion varied among the different UPEC isolates.
(TIF)
Growth assays performed at 37°C under shaking conditions for (A) UTI89 and UTI89hns, (B) EC958 and EC958hns. In both strains, hns deletion mutants were attenuated in growth compared to their respective wild-type strains.
(TIF)
Western blot analysis of SslE using preparations from (A) UTI89 and (B) EC958; each with their respective hns mutant and complemented strains. In each analysis, (i) whole-cell lysates and (iii) supernatant fractions were examined. In addition, (ii) a control for whole cell lysate samples was performed using an OmpA antibody.
(TIF)
The translation start site, transcription start site and promoter elements (-10 and -35 sequences) are indicated accordingly. The predicted Fis binding region (based on results from Fig 4) is boxed, with key nucleotide residues important for Fis binding indicated with a red overline. Nucleotide differences from UTI89 are highlighted.
(TIF)
(XLSX)
Insertion site of the 13 Tn5 mutants analyzed and the corresponding β-galactosidase activity for each mutant along with the control strains UTI89lacI-Z sslE::lacZ and UTI89lacI-Z.
(XLSX)
The 87 completely sequenced E. coli strains are listed, along with associated isolate information, accession number and reference where available. All isolate information was derived from the NCBI database or corresponding reference.
(DOCX)
Acknowledgments
We thank Dr David Looke, Dr Joan Faoagali and other members of the Microbiology Lab, Princess Alexandra Hospital, for the collection of urosepsis strains, and Barbara Johnson for the collection of patient clinical data.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the Australian National Health and Medical Research Council (NHMRC; GNT1042651 and GNT1067455). MAS is supported by an NHMRC Senior Research Fellowship (GNT1106930), MT is supported by an ARC Discovery Early Career Researcher Award (DE130101169) and SAB is supported by an NHMRC Career Development Fellowship (GNT1090456). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Russo TA, Johnson JR. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J Infect Dis. 2000;181(5):1753–4. Epub 2000/05/24. 10.1086/315418 . [DOI] [PubMed] [Google Scholar]
- 2.Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509–15. Epub 2000/12/19. . [DOI] [PubMed] [Google Scholar]
- 3.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113 Suppl 1A:5S–13S. Epub 2002/07/13. . [DOI] [PubMed] [Google Scholar]
- 4.Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med. 2002;113 Suppl 1A:14S–9S. Epub 2002/07/13. . [DOI] [PubMed] [Google Scholar]
- 5.Brzuszkiewicz E, Bruggemann H, Liesegang H, Emmerth M, Olschlager T, Nagy G, et al. How to become a uropathogen: comparative genomic analysis of extraintestinal pathogenic Escherichia coli strains. Proc Natl Acad Sci U S A. 2006;103(34):12879–84. Epub 2006/08/17. 10.1073/pnas.0603038103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta K. Addressing antibiotic resistance. Am J Med. 2002;113 Suppl 1A:29S–34S. Epub 2002/07/13. . [DOI] [PubMed] [Google Scholar]
- 7.Kahlmeter G. An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: the ECO.SENS Project. J Antimicrob Chemother. 2003;51(1):69–76. Epub 2002/12/21. . [DOI] [PubMed] [Google Scholar]
- 8.Manges AR, Johnson JR, Foxman B, O'Bryan TT, Fullerton KE, Riley LW. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. N Engl J Med. 2001;345(14):1007–13. Epub 2001/10/06. 10.1056/NEJMoa011265 . [DOI] [PubMed] [Google Scholar]
- 9.Petty NK, Ben Zakour NL, Stanton-Cook M, Skippington E, Totsika M, Forde BM, et al. Global dissemination of a multidrug resistant Escherichia coli clone. Proc Natl Acad Sci U S A. 2014;111(15):5694–9. Epub 2014/04/08. 10.1073/pnas.1322678111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peirano G, Schreckenberger PC, Pitout JD. Characteristics of NDM-1-producing Escherichia coli isolates that belong to the successful and virulent clone ST131. Antimicrob Agents Chemother. 2011;55(6):2986–8. Epub 2011/03/30. 10.1128/aac.01763-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tangden T, Giske CG. Global dissemination of extensively drug-resistant carbapenemase-producing Enterobacteriaceae: clinical perspectives on detection, treatment and infection control. J Intern Med. 2015;277(5):501–12. Epub 2015/01/06. 10.1111/joim.12342 [DOI] [PubMed] [Google Scholar]
- 12.Brumbaugh AR, Mobley HL. Preventing urinary tract infection: progress toward an effective Escherichia coli vaccine. Expert Rev Vaccines. 2012;11(6):663–76. Epub 2012/08/10. 10.1586/erv.12.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moriel DG, Schembri MA. Vaccination approaches for the prevention of urinary tract infection. Curr Pharm Biotechnol. 2014;14(11):967–74. Epub 2014/01/01. . [DOI] [PubMed] [Google Scholar]
- 14.Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner JS, Burlein J, et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276(5312):607–11. Epub 1997/04/25. . [DOI] [PubMed] [Google Scholar]
- 15.Langermann S, Mollby R, Burlein JE, Palaszynski SR, Auguste CG, DeFusco A, et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis. 2000;181(2):774–8. Epub 2000/02/11. 10.1086/315258 . [DOI] [PubMed] [Google Scholar]
- 16.Russo TA, McFadden CD, Carlino-MacDonald UB, Beanan JM, Olson R, Wilding GE. The Siderophore receptor IroN of extraintestinal pathogenic Escherichia coli is a potential vaccine candidate. Infect Immun. 2003;71(12):7164–9. Epub 2003/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brumbaugh AR, Smith SN, Mobley HL. Immunization with the yersiniabactin receptor, FyuA, protects against pyelonephritis in a murine model of urinary tract infection. Infect Immun. 2013;81(9):3309–16. Epub 2013/06/27. 10.1128/iai.00470-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer HW, Rahlfs VW, Lauener PA, Blessmann GS. Prevention of recurrent urinary tract infections with immuno-active E. coli fractions: a meta-analysis of five placebo-controlled double-blind studies. Int J Antimicrob Agents. 2002;19(6):451–6. Epub 2002/07/24. . [DOI] [PubMed] [Google Scholar]
- 19.Bauer HW, Alloussi S, Egger G, Blumlein HM, Cozma G, Schulman CC. A long-term, multicenter, double-blind study of an Escherichia coli extract (OM-89) in female patients with recurrent urinary tract infections. Eur Urol. 2005;47(4):542–8; discussion 8. Epub 2005/03/19. 10.1016/j.eururo.2004.12.009 . [DOI] [PubMed] [Google Scholar]
- 20.Moriel DG, Bertoldi I, Spagnuolo A, Marchi S, Rosini R, Nesta B, et al. Identification of protective and broadly conserved vaccine antigens from the genome of extraintestinal pathogenic Escherichia coli. Proc Natl Acad Sci U S A. 2010;107(20):9072–7. Epub 2010/05/05. 10.1073/pnas.0915077107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldi DL, Higginson EE, Hocking DM, Praszkier J, Cavaliere R, James CE, et al. The type II secretion system and its ubiquitous lipoprotein substrate, SslE, are required for biofilm formation and virulence of enteropathogenic Escherichia coli. Infect Immun. 2012;80(6):2042–52. Epub 2012/03/28. 10.1128/iai.06160-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filloux A, Bally M, Ball G, Akrim M, Tommassen J, Lazdunski A. Protein secretion in gram-negative bacteria: transport across the outer membrane involves common mechanisms in different bacteria. EMBO J. 1990;9(13):4323–9. Epub 1990/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandkvist M, Michel LO, Hough LP, Morales VM, Bagdasarian M, Koomey M, et al. General secretion pathway (eps) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J Bacteriol. 1997;179(22):6994–7003. Epub 1997/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horstman AL, Kuehn MJ. Bacterial surface association of heat-labile enterotoxin through lipopolysaccharide after secretion via the general secretory pathway. J Biol Chem. 2002;277(36):32538–45. Epub 2002/06/28. 10.1074/jbc.M203740200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwobi A, Heesemann J, Garcia E, Igwe E, Noelting C, Rakin A. Novel virulence-associated type II secretion system unique to high-pathogenicity Yersinia enterocolitica. Infect Immun. 2003;71(4):1872–9. Epub 2003/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zalewska-Piatek B, Bury K, Piatek R, Bruzdziak P, Kur J. Type II secretory pathway for surface secretion of DraD invasin from the uropathogenic Escherichia coli Dr+ strain. J Bacteriol. 2008;190(14):5044–56. Epub 2008/05/27. 10.1128/jb.00224-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernandes RT, De la Cruz MA, Yamamoto D, Giron JA, Gomes TA. Dissection of the role of pili and type 2 and 3 secretion systems in adherence and biofilm formation of an atypical enteropathogenic Escherichia coli strain. Infect Immun. 2013;81(10):3793–802. Epub 2013/07/31. 10.1128/iai.00620-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nesta B, Valeri M, Spagnuolo A, Rosini R, Mora M, Donato P, et al. SslE elicits functional antibodies that impair in vitro mucinase activity and in vivo colonization by both intestinal and extraintestinal Escherichia coli strains. PLoS Pathog. 2014;10(5):e1004124 Epub 2014/05/09. 10.1371/journal.ppat.1004124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valeri M, Rossi Paccani S, Kasendra M, Nesta B, Serino L, Pizza M, et al. Pathogenic E. coli exploits SslE mucinase activity to translocate through the mucosal barrier and get access to host cells. PLoS One. 2015;10(3):e0117486 Epub 2015/03/20. 10.1371/journal.pone.0117486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo Q, Kumar P, Vickers TJ, Sheikh A, Lewis WG, Rasko DA, et al. Enterotoxigenic Escherichia coli secretes a highly conserved mucin-degrading metalloprotease to effectively engage intestinal epithelial cells. Infect Immun. 2014;82(2):509–21. Epub 2014/01/31. 10.1128/iai.01106-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy K, Bartels S, Qadri F, Fleckenstein JM. Enterotoxigenic Escherichia coli elicits immune responses to multiple surface proteins. Infect Immun. 2010;78(7):3027–35. Epub 2010/05/12. 10.1128/iai.00264-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo Q, Qadri F, Kansal R, Rasko DA, Sheikh A, Fleckenstein JM. Conservation and immunogenicity of novel antigens in diverse isolates of enterotoxigenic Escherichia coli. PLoS Negl Trop Dis. 2015;9(1):e0003446 Epub 2015/01/30. 10.1371/journal.pntd.0003446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Baldi DL, Tauschek M, Strugnell RA, Robins-Browne RM. Transcriptional regulation of the yghJ-pppA-yghG-gspCDEFGHIJKLM cluster, encoding the type II secretion pathway in enterotoxigenic Escherichia coli. J Bacteriol. 2007;189(1):142–50. Epub 2006/11/07. 10.1128/jb.01115-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunstan RA, Heinz E, Wijeyewickrema LC, Pike RN, Purcell AW, Evans TJ, et al. Assembly of the type II secretion system such as found in Vibrio cholerae depends on the novel Pilotin AspS. PLoS Pathog. 2013;9(1):e1003117 Epub 2013/01/18. 10.1371/journal.ppat.1003117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allsopp LP, Beloin C, Ulett GC, Valle J, Totsika M, Sherlock O, et al. Molecular characterization of UpaB and UpaC, two new autotransporter proteins of uropathogenic Escherichia coli CFT073. Infect Immun. 2012;80(1):321–32. Epub 2011/09/21. 10.1128/iai.05322-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allsopp LP, Beloin C, Moriel DG, Totsika M, Ghigo JM, Schembri MA. Functional heterogeneity of the UpaH autotransporter protein from uropathogenic Escherichia coli. J Bacteriol. 2012;194(21):5769–82. Epub 2012/08/21. 10.1128/jb.01264-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Totsika M, Wells TJ, Beloin C, Valle J, Allsopp LP, King NP, et al. Molecular characterization of the EhaG and UpaG trimeric autotransporter proteins from pathogenic Escherichia coli. Appl Environ Microbiol. 2012;78(7):2179–89. Epub 2012/01/31. 10.1128/aem.06680-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wurpel DJ, Totsika M, Allsopp LP, Hartley-Tassell LE, Day CJ, Peters KM, et al. F9 fimbriae of uropathogenic Escherichia coli are expressed at low temperature and recognise Galβ1-3GlcNAc-containing glycans. PLoS One. 2014;9(3):e93177 Epub 2014/03/29. 10.1371/journal.pone.0093177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller CM, Dobrindt U, Nagy G, Emody L, Uhlin BE, Hacker J. Role of histone-like proteins H-NS and StpA in expression of virulence determinants of uropathogenic Escherichia coli. J Bacteriol. 2006;188(15):5428–38. Epub 2006/07/21. 10.1128/jb.01956-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Decanio MS, Landick R, Haft RJ. The non-pathogenic Escherichia coli strain W secretes SslE via the virulence-associated type II secretion system beta. BMC Microbiol. 2013;13:130 Epub 2013/06/14. 10.1186/1471-2180-13-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nichols KB, Totsika M, Moriel DG, Lo AW, Yang J, Wurpel DJ, et al. Molecular characterisation of the Vacuolating Autotransporter Toxin in Uropathogenic Escherichia coli. J Bacteriol. 2016. Epub 2016/02/10. 10.1128/jb.00791-15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bachmann BJ. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972;36(4):525–57. Epub 1972/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blattner FR, Plunkett G 3rd, Bloch CA, Perna NT, Burland V, Riley M, et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277(5331):1453–62. Epub 1997/09/05. . [DOI] [PubMed] [Google Scholar]
- 44.Hacker J, Knapp S, Goebel W. Spontaneous deletions and flanking regions of the chromosomally inherited hemolysin determinant of an Escherichia coli O6 strain. J Bacteriol. 1983;154(3):1145–52. Epub 1983/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lau SH, Kaufmann ME, Livermore DM, Woodford N, Willshaw GA, Cheasty T, et al. UK epidemic Escherichia coli strains A-E, with CTX-M-15 β-lactamase, all belong to the international O25:H4-ST131 clone. J Antimicrob Chemother. 2008;62(6):1241–4. Epub 2008/09/10. 10.1093/jac/dkn380 . [DOI] [PubMed] [Google Scholar]
- 46.Totsika M, Beatson SA, Sarkar S, Phan MD, Petty NK, Bachmann N, et al. Insights into a multidrug resistant Escherichia coli pathogen of the globally disseminated ST131 lineage: genome analysis and virulence mechanisms. PLoS One. 2011;6(10):e26578 Epub 2011/11/05. 10.1371/journal.pone.0026578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forde BM, Ben Zakour NL, Stanton-Cook M, Phan MD, Totsika M, Peters KM, et al. The complete genome sequence of Escherichia coli EC958: a high quality reference sequence for the globally disseminated multidrug resistant E. coli O25b:H4-ST131 clone. PLoS One. 2014;9(8):e104400 Epub 2014/08/16. 10.1371/journal.pone.0104400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Achtman M, Mercer A, Kusecek B, Pohl A, Heuzenroeder M, Aaronson W, et al. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983;39(1):315–35. Epub 1983/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69(7):4572–9. Epub 2001/06/13. 10.1128/iai.69.7.4572-4579.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen SL, Hung CS, Xu J, Reigstad CS, Magrini V, Sabo A, et al. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc Natl Acad Sci U S A. 2006;103(15):5977–82. Epub 2006/04/06. 10.1073/pnas.0600938103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97(12):6640–5. Epub 2000/06/01. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cherepanov PP, Wackernagel W. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene. 1995;158(1):9–14. Epub 1995/05/26. . [DOI] [PubMed] [Google Scholar]
- 53.Eschenfeldt WH, Lucy S, Millard CS, Joachimiak A, Mark ID. A family of LIC vectors for high-throughput cloning and purification of proteins. Methods Mol Biol. 2009;498:105–15. Epub 2008/11/07. 10.1007/978-1-59745-196-3_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez E, Bartolome B, de la Cruz F. pACYC184-derived cloning vectors containing the multiple cloning site and lacZα reporter gene of pUC8/9 and pUC18/19 plasmids. Gene. 1988;68(1):159–62. Epub 1988/08/15. . [DOI] [PubMed] [Google Scholar]
- 55.Farinha MA, Kropinski AM. Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J Bacteriol. 1990;172(6):3496–9. Epub 1990/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allsopp LP, Totsika M, Tree JJ, Ulett GC, Mabbett AN, Wells TJ, et al. UpaH is a newly identified autotransporter protein that contributes to biofilm formation and bladder colonization by uropathogenic Escherichia coli CFT073. Infect Immun. 2010;78(4):1659–69. Epub 2010/02/11. 10.1128/iai.01010-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulett GC, Webb RI, Schembri MA. Antigen-43-mediated autoaggregation impairs motility in Escherichia coli. Microbiology. 2006;152(Pt 7):2101–10. Epub 2006/06/29. 10.1099/mic.0.28607-0 . [DOI] [PubMed] [Google Scholar]
- 58.O'Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol. 1998;28(3):449–61. Epub 1998/06/19. . [DOI] [PubMed] [Google Scholar]
- 59.Rapid amplification of 5' complementary DNA ends (5' RACE). Nat Methods. 2005;2(8):629–30. Epub 2005/09/09. . [DOI] [PubMed] [Google Scholar]
- 60.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25(4):402–8. Epub 2002/02/16. 10.1006/meth.2001.1262 . [DOI] [PubMed] [Google Scholar]
- 61.Miller JH. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 62.Filutowicz M, Ross W, Wild J, Gourse RL. Involvement of Fis protein in replication of the Escherichia coli chromosome. J Bacteriol. 1992;174(2):398–407. Epub 1992/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bradley MD, Beach MB, de Koning AP, Pratt TS, Osuna R. Effects of Fis on Escherichia coli gene expression during different growth stages. Microbiology. 2007;153(Pt 9):2922–40. Epub 2007/09/05. 10.1099/mic.0.2007/008565-0 . [DOI] [PubMed] [Google Scholar]
- 64.Bosch L, Nilsson L, Vijgenboom E, Verbeek H. FIS-dependent trans-activation of tRNA and rRNA operons of Escherichia coli. Biochim Biophys Acta. 1990;1050(1–3):293–301. Epub 1990/08/27. . [DOI] [PubMed] [Google Scholar]
- 65.Rossiter AE, Browning DF, Leyton DL, Johnson MD, Godfrey RE, Wardius CA, et al. Transcription of the plasmid-encoded toxin gene from enteroaggregative Escherichia coli is regulated by a novel co-activation mechanism involving CRP and Fis. Mol Microbiol. 2011;81(1):179–91. Epub 2011/05/06. 10.1111/j.1365-2958.2011.07685.x . [DOI] [PubMed] [Google Scholar]
- 66.Goldberg MD, Johnson M, Hinton JC, Williams PH. Role of the nucleoid-associated protein Fis in the regulation of virulence properties of enteropathogenic Escherichia coli. Mol Microbiol. 2001;41(3):549–59. Epub 2001/09/05. . [DOI] [PubMed] [Google Scholar]
- 67.Farris M, Grant A, Richardson TB, O'Connor CD. BipA: a tyrosine-phosphorylated GTPase that mediates interactions between enteropathogenic Escherichia coli (EPEC) and epithelial cells. Mol Microbiol. 1998;28(2):265–79. 10.1046/j.1365-2958.1998.00793.x [DOI] [PubMed] [Google Scholar]
- 68.Grant AJ, Farris M, Alefounder P, Williams PH, Woodward MJ, O'Connor CD. Co-ordination of pathogenicity island expression by the BipA GTPase in enteropathogenic Escherichia coli (EPEC). Mol Microbiol. 2003;48(2):507–21. Epub 2003/04/05. . [DOI] [PubMed] [Google Scholar]
- 69.Kainz M, Gourse RL. The C-terminal domain of the alpha subunit of Escherichia coli RNA polymerase is required for efficient rho-dependent transcription termination. J Mol Biol. 1998;284(5):1379–90. Epub 1999/01/08. 10.1006/jmbi.1998.2272 . [DOI] [PubMed] [Google Scholar]
- 70.Nakamura Y, Mizusawa S, Court DL, Tsugawa A. Regulatory defects of a conditionally lethal nusAts mutant of Escherichia coli. Positive and negative modulator roles of NusA protein in vivo. J Mol Biol. 1986;189(1):103–11. Epub 1986/05/05. . [DOI] [PubMed] [Google Scholar]
- 71.Xing F, Martzen MR, Phizicky EM. A conserved family of Saccharomyces cerevisiae synthases effects dihydrouridine modification of tRNA. RNA. 2002;8(3):370–81. Epub 2002/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nafissi M, Chau J, Xu J, Johnson RC. Robust translation of the nucleoid protein Fis requires a remote upstream AU element and is enhanced by RNA secondary structure. J Bacteriol. 2012;194(10):2458–69. Epub 2012/03/06. 10.1128/jb.00053-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bishop AC, Xu J, Johnson RC, Schimmel P, de Crecy-Lagard V. Identification of the tRNA-dihydrouridine synthase family. J Biol Chem. 2002;277(28):25090–5. Epub 2002/05/02. 10.1074/jbc.M203208200 . [DOI] [PubMed] [Google Scholar]
- 74.Ninnemann O, Koch C, Kahmann R. The E. coli fis promoter is subject to stringent control and autoregulation. EMBO J. 1992;11(3):1075–83. Epub 1992/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beach MB, Osuna R. Identification and characterization of the fis operon in enteric bacteria. J Bacteriol. 1998;180(22):5932–46. Epub 1998/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerganova V, Maurer S, Stoliar L, Japaridze A, Dietler G, Nasser W, et al. Upstream binding of idling RNA polymerase modulates transcription initiation from a nearby promoter. J Biol Chem. 2015;290(13):8095–109. Epub 2015/02/05. 10.1074/jbc.M114.628131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ball CA, Osuna R, Ferguson KC, Johnson RC. Dramatic changes in Fis levels upon nutrient upshift in Escherichia coli. J Bacteriol. 1992;174(24):8043–56. Epub 1992/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scott K, Diggle MA, Clarke SC. TypA is a virulence regulator and is present in many pathogenic bacteria. Br J Biomed Sci. 2003;60(3):168–70. Epub 2003/10/17. . [DOI] [PubMed] [Google Scholar]
- 79.Neidig A, Yeung AT, Rosay T, Tettmann B, Strempel N, Rueger M, et al. TypA is involved in virulence, antimicrobial resistance and biofilm formation in Pseudomonas aeruginosa. BMC Microbiol. 2013;13:77 Epub 2013/04/11. 10.1186/1471-2180-13-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rowe S, Hodson N, Griffiths G, Roberts IS. Regulation of the Escherichia coli K5 capsule gene cluster: evidence for the roles of H-NS, BipA, and integration host factor in regulation of group 2 capsule gene clusters in pathogenic E. coli. J Bacteriol. 2000;182(10):2741–5. Epub 2000/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choudhury P, Flower AM. Efficient assembly of ribosomes is inhibited by deletion of bipA in Escherichia coli. J Bacteriol. 2015;197(10):1819–27. Epub 2015/03/18. 10.1128/jb.00023-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fan H, Hahm J, Diggs S, Perry JJ, Blaha G. Structural and Functional Analysis of BipA, a Regulator of Virulence in enteropathogenic Escherichia coli. J Biol Chem. 2015. Epub 2015/07/15. 10.1074/jbc.M115.659136 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shao Y, Feldman-Cohen LS, Osuna R. Functional characterization of the Escherichia coli Fis-DNA binding sequence. J Mol Biol. 2008;376(3):771–85. Epub 2008/01/08. 10.1016/j.jmb.2007.11.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nowak-Lovato K, Alexandrov LB, Banisadr A, Bauer AL, Bishop AR, Usheva A, et al. Binding of nucleoid-associated protein fis to DNA is regulated by DNA breathing dynamics. PLoS Comput Biol. 2013;9(1):e1002881 Epub 2013/01/24. 10.1371/journal.pcbi.1002881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakjang S, Ndeh DA, Wipat A, Bolam DN, Hirt RP. A novel extracellular metallopeptidase domain shared by animal host-associated mutualistic and pathogenic microbes. PLoS One. 2012;7(1):e30287 Epub 2012/02/03. 10.1371/journal.pone.0030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dorman CJ. Nucleoid-associated proteins and bacterial physiology. Adv Appl Microbiol. 2009;67:47–64. Epub 2009/02/28. 10.1016/s0065-2164(08)01002-2 . [DOI] [PubMed] [Google Scholar]
- 87.Madrid C, Nieto JM, Paytubi S, Falconi M, Gualerzi CO, Juarez A. Temperature- and H-NS-dependent regulation of a plasmid-encoded virulence operon expressing Escherichia coli hemolysin. J Bacteriol. 2002;184(18):5058–66. Epub 2002/08/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Falconi M, Prosseda G, Giangrossi M, Beghetto E, Colonna B. Involvement of FIS in the H-NS-mediated regulation of virF gene of Shigella and enteroinvasive Escherichia coli. Mol Microbiol. 2001;42(2):439–52. Epub 2001/11/13. . [DOI] [PubMed] [Google Scholar]
- 89.Falconi M, Colonna B, Prosseda G, Micheli G, Gualerzi CO. Thermoregulation of Shigella and Escherichia coli EIEC pathogenicity. A temperature-dependent structural transition of DNA modulates accessibility of virF promoter to transcriptional repressor H-NS. EMBO J. 1998;17(23):7033–43. Epub 1998/12/08. 10.1093/emboj/17.23.7033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Friedman DI, Baron LS. Genetic characterization of a bacterial locus involved in the activity of the N function of phage λ. Virology. 1974;58(1):141–8. Epub 1974/03/01. . [DOI] [PubMed] [Google Scholar]
- 91.Yang X, Molimau S, Doherty GP, Johnston EB, Marles-Wright J, Rothnagel R, et al. The structure of bacterial RNA polymerase in complex with the essential transcription elongation factor NusA. EMBO Rep. 2009;10(9):997–1002. Epub 2009/08/15. 10.1038/embor.2009.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yakhnin AV, Babitzke P. NusA-stimulated RNA polymerase pausing and termination participates in the Bacillus subtilis trp operon attenuation mechanism in vitro. Proc Natl Acad Sci U S A. 2002;99(17):11067–72. Epub 2002/08/06. 10.1073/pnas.162373299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou J, Ha KS, La Porta A, Landick R, Block SM. Applied force provides insight into transcriptional pausing and its modulation by transcription factor NusA. Mol Cell. 2011;44(4):635–46. Epub 2011/11/22. 10.1016/j.molcel.2011.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmidt MC, Chamberlin MJ. nusA protein of Escherichia coli is an efficient transcription termination factor for certain terminator sites. J Mol Biol. 1987;195(4):809–18. Epub 1987/06/20. . [DOI] [PubMed] [Google Scholar]
- 95.Vogel U, Jensen KF. NusA is required for ribosomal antitermination and for modulation of the transcription elongation rate of both antiterminated RNA and mRNA. J Biol Chem. 1997;272(19):12265–71. Epub 1997/05/09. . [DOI] [PubMed] [Google Scholar]
- 96.Nakamura Y, Uchida H. Isolation of conditionally lethal amber mutations affecting synthesis of the NusA protein of Escherichia coli. Mol Gen Genet. 1983;190(2):196–203. Epub 1983/01/01. . [DOI] [PubMed] [Google Scholar]
- 97.Schauer AT, Carver DL, Bigelow B, Baron LS, Friedman DI. λ N antitermination system: functional analysis of phage interactions with the host NusA protein. J Mol Biol. 1987;194(4):679–90. Epub 1987/04/20. . [DOI] [PubMed] [Google Scholar]
- 98.Zheng C, Friedman DI. Reduced Rho-dependent transcription termination permits NusA-independent growth of Escherichia coli. Proc Natl Acad Sci U S A. 1994;91(16):7543–7. Epub 1994/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hashimoto M, Ichimura T, Mizoguchi H, Tanaka K, Fujimitsu K, Keyamura K, et al. Cell size and nucleoid organization of engineered Escherichia coli cells with a reduced genome. Mol Microbiol. 2005;55(1):137–49. Epub 2004/12/23. 10.1111/j.1365-2958.2004.04386.x . [DOI] [PubMed] [Google Scholar]
- 100.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2:2006 0008. Epub 2006/06/02. 10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kang Y, Durfee T, Glasner JD, Qiu Y, Frisch D, Winterberg KM, et al. Systematic mutagenesis of the Escherichia coli genome. J Bacteriol. 2004;186(15):4921–30. Epub 2004/07/21. 10.1128/jb.186.15.4921-4930.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kung H, Spears C, Weissbach H. Purification and properties of a soluble factor required for the deoxyribonucleic acid-directed in vitro synthesis of β-galactosidase. J Biol Chem. 1975;250(4):1556–62. Epub 1975/02/25. . [PubMed] [Google Scholar]
- 103.Greenblatt J, Li J, Adhya S, Friedman DI, Baron LS, Redfield B, et al. L factor that is required for β-galactosidase synthesis is the nusA gene product involved in transcription termination. Proc Natl Acad Sci U S A. 1980;77(4):1991–4. Epub 1980/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saxena S, Gowrishankar J. Modulation of Rho-dependent transcription termination in Escherichia coli by the H-NS family of proteins. J Bacteriol. 2011;193(15):3832–41. Epub 2011/05/24. 10.1128/jb.00220-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kotlajich MV, Hron DR, Boudreau BA, Sun Z, Lyubchenko YL, Landick R. Bridged filaments of histone-like nucleoid structuring protein pause RNA polymerase and aid termination in bacteria. Elife. 2015;4 Epub 2015/01/17. 10.7554/eLife.04970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barker HC, Kinsella N, Jaspe A, Friedrich T, O'Connor CD. Formate protects stationary-phase Escherichia coli and Salmonella cells from killing by a cationic antimicrobial peptide. Mol Microbiol. 2000;35(6):1518–29. Epub 2000/04/12. . [DOI] [PubMed] [Google Scholar]
- 107.Pfennig PL, Flower AM. BipA is required for growth of Escherichia coli K-12 at low temperature. Mol Genet Genomics. 2001;266(2):313–7. Epub 2001/10/31. . [DOI] [PubMed] [Google Scholar]
- 108.deLivron MA, Makanji HS, Lane MC, Robinson VL. A novel domain in translational GTPase BipA mediates interaction with the 70S ribosome and influences GTP hydrolysis. Biochemistry. 2009;48(44):10533–41. Epub 2009/10/07. 10.1021/bi901026z . [DOI] [PubMed] [Google Scholar]
- 109.Yamada H, Yoshida T, Tanaka K, Sasakawa C, Mizuno T. Molecular analysis of the Escherichia coli hns gene encoding a DNA-binding protein, which preferentially recognizes curved DNA sequences. Mol Gen Genet. 1991;230(1–2):332–6. Epub 1991/11/01. . [DOI] [PubMed] [Google Scholar]
- 110.Spurio R, Falconi M, Brandi A, Pon CL, Gualerzi CO. The oligomeric structure of nucleoid protein H-NS is necessary for recognition of intrinsically curved DNA and for DNA bending. EMBO J. 1997;16(7):1795–805. Epub 1997/04/01. 10.1093/emboj/16.7.1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Navarre WW, Porwollik S, Wang Y, McClelland M, Rosen H, Libby SJ, et al. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science. 2006;313(5784):236–8. Epub 2006/06/10. 10.1126/science.1128794 . [DOI] [PubMed] [Google Scholar]
- 112.Bouffartigues E, Buckle M, Badaut C, Travers A, Rimsky S. H-NS cooperative binding to high-affinity sites in a regulatory element results in transcriptional silencing. Nat Struct Mol Biol. 2007;14(5):441–8. Epub 2007/04/17. 10.1038/nsmb1233 . [DOI] [PubMed] [Google Scholar]
- 113.Fang FC, Rimsky S. New insights into transcriptional regulation by H-NS. Curr Opin Microbiol. 2008;11(2):113–20. Epub 2008/04/05. 10.1016/j.mib.2008.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grainger DC, Goldberg MD, Lee DJ, Busby SJ. Selective repression by Fis and H-NS at the Escherichia coli dps promoter. Mol Microbiol. 2008;68(6):1366–77. Epub 2008/05/03. 10.1111/j.1365-2958.2008.06253.x . [DOI] [PubMed] [Google Scholar]
- 115.Browning DF, Cole JA, Busby SJ. Suppression of FNR-dependent transcription activation at the Escherichia coli nir promoter by Fis, IHF and H-NS: modulation of transcription initiation by a complex nucleo-protein assembly. Mol Microbiol. 2000;37(5):1258–69. Epub 2000/09/06. . [DOI] [PubMed] [Google Scholar]
- 116.Tippner D, Afflerbach H, Bradaczek C, Wagner R. Evidence for a regulatory function of the histone-like Escherichia coli protein H-NS in ribosomal RNA synthesis. Mol Microbiol. 1994;11(3):589–604. Epub 1994/02/01. . [DOI] [PubMed] [Google Scholar]
- 117.Afflerbach H, Schroder O, Wagner R. Conformational changes of the upstream DNA mediated by H-NS and FIS regulate E. coli RrnB P1 promoter activity. J Mol Biol. 1999;286(2):339–53. Epub 1999/02/12. 10.1006/jmbi.1998.2494 . [DOI] [PubMed] [Google Scholar]
- 118.Falconi M, Brandi A, La Teana A, Gualerzi CO, Pon CL. Antagonistic involvement of FIS and H-NS proteins in the transcriptional control of hns expression. Mol Microbiol. 1996;19(5):965–75. Epub 1996/03/01. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western blot analysis of SslE using supernatant fractions from UTI89, UTI89sslE and 18 clinical UPEC isolates from our laboratory collection. SslE secretion varied among the different UPEC isolates.
(TIF)
Growth assays performed at 37°C under shaking conditions for (A) UTI89 and UTI89hns, (B) EC958 and EC958hns. In both strains, hns deletion mutants were attenuated in growth compared to their respective wild-type strains.
(TIF)
Western blot analysis of SslE using preparations from (A) UTI89 and (B) EC958; each with their respective hns mutant and complemented strains. In each analysis, (i) whole-cell lysates and (iii) supernatant fractions were examined. In addition, (ii) a control for whole cell lysate samples was performed using an OmpA antibody.
(TIF)
The translation start site, transcription start site and promoter elements (-10 and -35 sequences) are indicated accordingly. The predicted Fis binding region (based on results from Fig 4) is boxed, with key nucleotide residues important for Fis binding indicated with a red overline. Nucleotide differences from UTI89 are highlighted.
(TIF)
(XLSX)
Insertion site of the 13 Tn5 mutants analyzed and the corresponding β-galactosidase activity for each mutant along with the control strains UTI89lacI-Z sslE::lacZ and UTI89lacI-Z.
(XLSX)
The 87 completely sequenced E. coli strains are listed, along with associated isolate information, accession number and reference where available. All isolate information was derived from the NCBI database or corresponding reference.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.