Abstract
Purpose
To investigate whether anti–vascular endothelial growth factor therapy with bevacizumab prolongs progression-free survival (PFS) when added to first-line letrozole as treatment of hormone receptor–positive metastatic breast cancer (MBC).
Patients and Methods
Women with hormone receptor–positive MBC were randomly assigned 1:1 in a multicenter, open-label, phase III trial of letrozole (2.5 mg orally per day) with or without bevacizumab (15 mg/kg intravenously once every 3 weeks) within strata defined by measurable disease and disease-free interval. This trial had 90% power to detect a 50% improvement in median PFS from 6 to 9 months. Using a one-sided α = .025, a target sample size of 352 patients was planned.
Results
From May 2008 to November 2011, 350 women were recruited; 343 received treatment and were observed for efficacy and safety. Median age was 58 years (range, 25 to 87 years). Sixty-two percent had measurable disease, and 45% had de novo MBC. At a median follow-up of 39 months, the addition of bevacizumab resulted in a significant reduction in the hazard of progression (hazard ratio, 0.75; 95% CI, 0.59 to 0.96; P = .016) and a prolongation in median PFS from 15.6 months with letrozole to 20.2 months with letrozole plus bevacizumab. There was no significant difference in overall survival (hazard ratio, 0.87; 95% CI, 0.65 to 1.18; P = .188), with median overall survival of 43.9 months with letrozole versus 47.2 months with letrozole plus bevacizumab. The largest increases in incidence of grade 3 to 4 treatment-related toxicities with the addition of bevacizumab were hypertension (24% v 2%) and proteinuria (11% v 0%).
Conclusion
The addition of bevacizumab to letrozole improved PFS in hormone receptor–positive MBC, but this benefit was associated with a markedly increased risk of grade 3 to 4 toxicities. Research on predictive markers will be required to clarify the role of bevacizumab in this setting.
INTRODUCTION
Endocrine therapy (ET) is a relatively well-tolerated treatment for hormone receptor–positive breast cancer. Molecular diversity within this subgroup can influence response to therapy.1-4 Specific interactions between the estrogen receptor (ER) and cell cycle survival5,6 and/or growth factor signaling pathways7,8 may influence sensitivity to antiestrogen therapy. Angiogenesis is a well-described hallmark of malignancy.9,10 Furthermore, angiogenesis and its vascular endothelial growth factor receptor (VEGFR) signaling pathway are potential mechanisms of resistance to ET that can be targeted with selected newer agents.11,12
Angiogenesis is influenced by estrogen under both physiologic and pathologic conditions. Cyclical neovascularization of the premenopausal female reproductive tract occurs in response to changing levels of estradiol and other sex steroids.13 In vitro and in vivo models demonstrate a link between estradiol and endothelial cell proliferation,14-16 and these interactions are regulated by vascular endothelial growth factor (VEGF).17,18 This stimulatory effect on angiogenesis is also observed in preclinical models of breast cancer.19,20 Furthermore, induction of VEGF is implicated as a mechanism for the emergence of ET resistance.12 After the initial vascular regression that follows hormone ablation therapy, increasing VEGF levels have been associated with a wave of angiogenesis and tumor neovascularization that supports tumor regrowth.11
In patients with breast cancer, retrospective studies demonstrate that increased tumor VEGF levels are associated with decreased responsiveness to antiestrogen therapy and worse outcome in all stages of this disease.21-24 Therefore, VEGF and the VEGFR pathway may be an important target for treatment of hormone receptor–positive breast cancer. Together, these observations suggest that antiangiogenic agents could be more effective in a low-estrogen environment and that some of the proven efficacy of antiestrogen therapy may be mediated via inhibition of angiogenesis.
Bevacizumab, a humanized monoclonal antibody to VEGFA, has activity in combination with chemotherapy in metastatic breast cancer (MBC).25-29 Several trials have demonstrated the safety and feasibility of combining ET with bevacizumab,30-33 but its impact on outcomes remains uncertain. We conducted Cancer and Leukemia Group B (CALGB, now known as Alliance) 40503, a randomized phase III trial, to determine whether bevacizumab can prolong progression-free survival (PFS) when added to first-line ET with letrozole for hormone receptor–positive advanced-stage breast cancer.
PATIENTS AND METHODS
Study Design
CALGB 40503 was initiated in 2008 as two parallel randomized trials to compare ET alone with ET plus bevacizumab as first-line endocrine therapy for hormone receptor–positive advanced-stage breast cancer: a phase III study with letrozole as ET with a primary end point of PFS and a phase II study with tamoxifen as ET with toxicity as the primary end point (results will be reported separately). The study was supported by the National Cancer Institute (NCI) and CALGB/Alliance. This report provides the results from the phase III study of letrozole with or without bevacizumab.
Patients
Eligible patients included women age 18 years or older with locally advanced, unresectable, or metastatic breast cancer who were postmenopausal (or receiving ovarian suppression with a leuteinizing hormone-releasing hormone agonist). Patients could have either measurable or nonmeasurable disease defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0. Tumors were required to be positive for the ER and/or progesterone receptor defined as ≥ 1%. Human epidermal growth factor receptor 2 (HER2) status could be either positive or negative. Patients may have received (neo)adjuvant chemotherapy (≥ 12 months since completion of chemotherapy) and any duration of adjuvant ET (≤ 4 weeks of ET for MBC before trial registration). At study initiation, no prior chemotherapy for MBC was permitted; an amendment in May 2010 allowed no more than one prior chemotherapy treatment for MBC. Patients were required to have Eastern Cooperative Oncology Group performance status ≤ 1 and adequate bone marrow, hepatic, and renal function, including a urine protein dipstick grade of ≤ 1+ or urine protein:creatinine (UPC) ratio of < 1. Patients were excluded if they had any of the following: prior anti-VEGF or VEGFR tyrosine kinase therapy; major (within 28 days) or minor (within 7 days) surgical procedures; known brain or leptomeningeal metastasis; ongoing uncontrolled hypertension (blood pressure: systolic > 150 mmHg and/or diastolic > 90 mmHg); New York Heart Association grade ≥ 2 congestive heart failure; history of hypertensive crisis or encephalopathy; uncontrolled seizures despite standard medication; history (within the past 6 months) of myocardial infarction, unstable angina, stroke, abdominal fistula or abscess, or significant bleeding episode; history of GI perforation within 12 months; any nonhealing wound or fracture; or life expectancy ≤ 12 weeks. Each participant signed an institutional review board approved, protocol-specific informed consent form in accordance with federal and institutional guidelines.
Treatments and Dose Modifications
At activation in May 2008, patients were randomly assigned 1:1 in double-blinded fashion to letrozole plus bevacizumab or letrozole plus placebo. Patients were stratified by measurable disease (no/yes) and disease-free interval (≤ 24 months/> 24 months). In May 2010, the study was amended to include an open-label design with the intention of increasing accrual. In August 2010, all patients who had started treatment during the placebo-control design were unblinded, and accrual to the open-label trial continued. Letrozole was administered at 2.5 mg orally once per day and bevacizumab was administered at 15 mg/kg intravenously once every 3 weeks (± 5 days) until disease progression or unacceptable toxicity. One cycle was equivalent to 3 weeks. Restaging scans were performed every three cycles for the first 18 cycles and then every four cycles until first disease progression. The follow-up schedule was the same for both treatment arms.
No dose reductions were permitted for letrozole or bevacizumab. Letrozole was held for grade > 3 hepatic dysfunction and resumed when grade ≤ 2 was reached. Bevacizumab was held for blood pressure > 160/100 mmHg, urine protein ≥ 2 g per 24 hours or UPC ≥ 2 (could be resumed upon reaching < 2 g per 24 hours or UPC < 2), grade 3 to 4 venous thromboembolic events (resumed once stable on anticoagulation), and for patients who required surgery while on study. Bevacizumab was permanently discontinued for grade ≥ 4 hypertension; nephrotic syndrome; reversible posterior leukoencephalopathy syndrome; grade ≥ 3 hemorrhage/congestive heart failure; grade ≥ 2 arterial thromboembolic events; any grade GI perforation, leak, or fistula; wound dehiscence requiring intervention; or grade ≥ 3 or 4 unspecified bevacizumab-related adverse events (AEs). Bevacizumab was discontinued if held for toxicity for > 8 weeks, but patients could continue on letrozole alone.
Study End Points
The primary efficacy end point was investigator-determined PFS measured from study entry until first disease progression or death without progression. Those who discontinued treatment before progression were observed until first disease progression. Event-free patients were censored at last clinical assessment. Secondary end points included objective response and clinical benefit, PFS at 6 and 12 months, overall survival (OS), and toxicity. For patients with measurable disease, objective response rate (ORR) was defined as either complete response or partial response without any requirement for confirmatory scans; clinical benefit rate (CBR) was defined as complete response, partial response, or stable disease for at least 24 weeks. Tumor assessments of response and progression were defined according to RECIST v1.0. OS was measured from study entry until death as a result of any cause or last contact. Toxicity was graded according to NCI Common Toxicity Criteria version 3 and was reported for grade 3 to 5 toxicities considered possibly, probably, or definitely treatment related.
Statistical Analysis
A target enrollment of 352 patients and a final analysis at 274 PFS events was planned to yield 90% power to detect an improvement in median PFS from 6 months in the control arm (letrozole alone) to 9 months (hazard ratio [HR], 0.66) in the experimental arm (letrozole plus bevacizumab) using a one-sided α = .025. The study design assumed 22 months of accrual (16 patients per month) with 7 months of additional follow-up to final analysis. The study was monitored biannually by a data and safety monitoring board (DSMB) in accordance with NCI guidelines. Interim analyses were preplanned to start at 137 PFS events (50% information) and to consider stopping early only for futility. Nonbinding futility boundaries were defined according to Freidlin and Korn34 using a one-sided α = .005.
Efficacy analyses followed protocol and used a modified intention-to-treat approach that included all patients who began protocol therapy. Time-to-event distributions were estimated by the Kaplan-Meier method. The primary analysis of PFS and OS used a log-rank test stratified by measurable disease and disease-free interval. HRs for letrozole plus bevacizumab compared to letrozole alone and 95% CIs were taken from corresponding Cox proportional hazard models. Subgroup analyses were exploratory and hypothesis generating, using univariate Cox models to obtain HR estimates and 95% CIs without hypothesis testing. An arm effect on ORR and CBR was tested in univariate logistic regression models using a two-sided α = .05.
Study data were reviewed by the Alliance study data coordinator. Data quality was confirmed by study chair review following CALGB policies. Statistical analyses conducted by the Alliance Statistics and Data Center were performed by using SAS v9.2 (SAS Institute, Cary, NC) and R 3.1.1.35
RESULTS
Between May 2008 and November 2011, 350 patients were enrolled in the phase III study of letrozole versus letrozole plus bevacizumab. The first interim analysis was reported to the DSMB in June 2012 after 57% of the required events had occurred. After a sixth interim analysis was reported in November 2014 (94% of events), the DSMB released the study results. The results presented here are based upon data available in the Alliance database as of April 9, 2015, representing 42 months of follow-up after the enrollment period and 264 PFS events (96% of required events).
Of the 350 patients enrolled, 136 registered before the May 2010 amendment, and 214 registered after the amendment. Two patients were not randomly assigned because of an error. The remaining 348 patients were randomly assigned to letrozole plus bevacizumab (n = 174; 67 before the amendment and 107 after the amendment) or letrozole (n = 174; 69 before the amendment and 105 after the amendment). One patient on the letrozole plus bevacizumab arm and four patients on the letrozole arm were randomly assigned but did not receive protocol treatment (Fig 1). This left 343 patients (98%) who received treatment, 173 on the letrozole plus bevacizumab arm and 170 on the letrozole arm; those patients constituted the per protocol modified intention-to-treat population for evaluating efficacy and safety (Fig 1). The test of heterogeneity in PFS distributions between pre- and postamendment cohorts was not significant, so all analyses were performed on the overall study population. Overall, 171 patients (50%) died, 12 (3%) withdrew consent for follow-up for survival, 40 (12%) remained on protocol treatment, and 120 (35%) were alive.
Fig 1.
CONSORT diagram.
Patient Characteristics
Patient demographics and clinical and pathologic tumor characteristics were well balanced between the two treatment arms (Table 1). Nearly all tumors (98%) were ER positive; 4% were HER2 positive. Median age was 58 years (range, 25 to 87 years), 62% had measurable disease, and 25% had bone-only metastases. Forty-five percent of patients had de novo metastatic disease, and 44% had a disease-free interval longer than 2 years; these two cohorts made up the majority of study patients. Nearly half the patients (48%) received prior hormone therapy such as an aromatase inhibitor (23%) or tamoxifen (36%), and 40% received prior chemotherapy.
Table 1.
Patient Demographic and Tumor Characteristics
| Characteristic | Letrozole + Bevacizumab | Letrozole | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| No. randomly assigned and treated | 173 | 100 | 170 | 100 |
| Patient and clinical factors | ||||
| Disease measurability | ||||
| Nonmeasurable | 67 | 39 | 63 | 37 |
| Measurable | 106 | 61 | 107 | 63 |
| Race/ethnicity | ||||
| White | 154 | 89 | 155 | 91 |
| Black | 9 | 5 | 12 | 7 |
| Asian | 2 | 1 | 3 | 2 |
| All other, including multiracial | 8 | 5 | 0 | 0 |
| Age, years | ||||
| ≤ 30 | 1 | 1 | 1 | 1 |
| 31-40 | 17 | 10 | 13 | 8 |
| 41-50 | 35 | 20 | 25 | 15 |
| 51-60 | 45 | 26 | 53 | 31 |
| 61-70 | 53 | 31 | 54 | 32 |
| 71-80 | 17 | 10 | 16 | 9 |
| 80+ | 5 | 3 | 8 | 5 |
| Median (range) | 56 (25-85) | 59 (29-87) | ||
| ECOG performance score | ||||
| 0 | 105 | 61 | 101 | 59 |
| 1 | 64 | 37 | 64 | 38 |
| 2 | 1 | 1 | 2 | 1 |
| Missing | 3 | 2 | 3 | 2 |
| Disease-free interval, years | ||||
| De novo | 74 | 43 | 81 | 48 |
| ≤ 1 | 11 | 6 | 2 | 1 |
| > 1 to ≤ 2 | 10 | 6 | 7 | 4 |
| > 2 | 75 | 43 | 77 | 45 |
| Missing | 3 | 2 | 3 | 2 |
| No. of metastatic sites | ||||
| 1 | 55 | 32 | 56 | 33 |
| 2 | 56 | 32 | 60 | 35 |
| 3 | 41 | 24 | 28 | 16 |
| 4 | 14 | 8 | 14 | 8 |
| 5-6 | 4 | 2 | 9 | 5 |
| Missing | 3 | 2 | 3 | 2 |
| Location of metastatic site | ||||
| Bone only | 41 | 24 | 43 | 25 |
| Visceral only | 41 | 24 | 41 | 24 |
| Bone and visceral | 88 | 51 | 83 | 49 |
| Tumor features | ||||
| ER status | ||||
| Negative | 0 | 0 | 1 | 1 |
| Positive | 170 | 98 | 166 | 98 |
| Missing | 3 | 2 | 3 | 2 |
| PgR status | ||||
| Negative | 41 | 24 | 31 | 18 |
| Positive | 129 | 75 | 133 | 78 |
| Missing | 3 | 2 | 6 | 3 |
| HER2 status | ||||
| Negative | 159 | 92 | 152 | 89 |
| Positive | 5 | 3 | 9 | 5 |
| Missing | 9 | 5 | 9 | 5 |
| Prior endocrine therapy | ||||
| Any | 82 | 47 | 83 | 49 |
| Tamoxifen | 61 | 35 | 61 | 36 |
| Aromatase inhibitor | 36 | 21 | 43 | 25 |
| Prior chemotherapy | 72 | 42 | 65 | 38 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PgR, progesterone receptor.
Efficacy Analysis
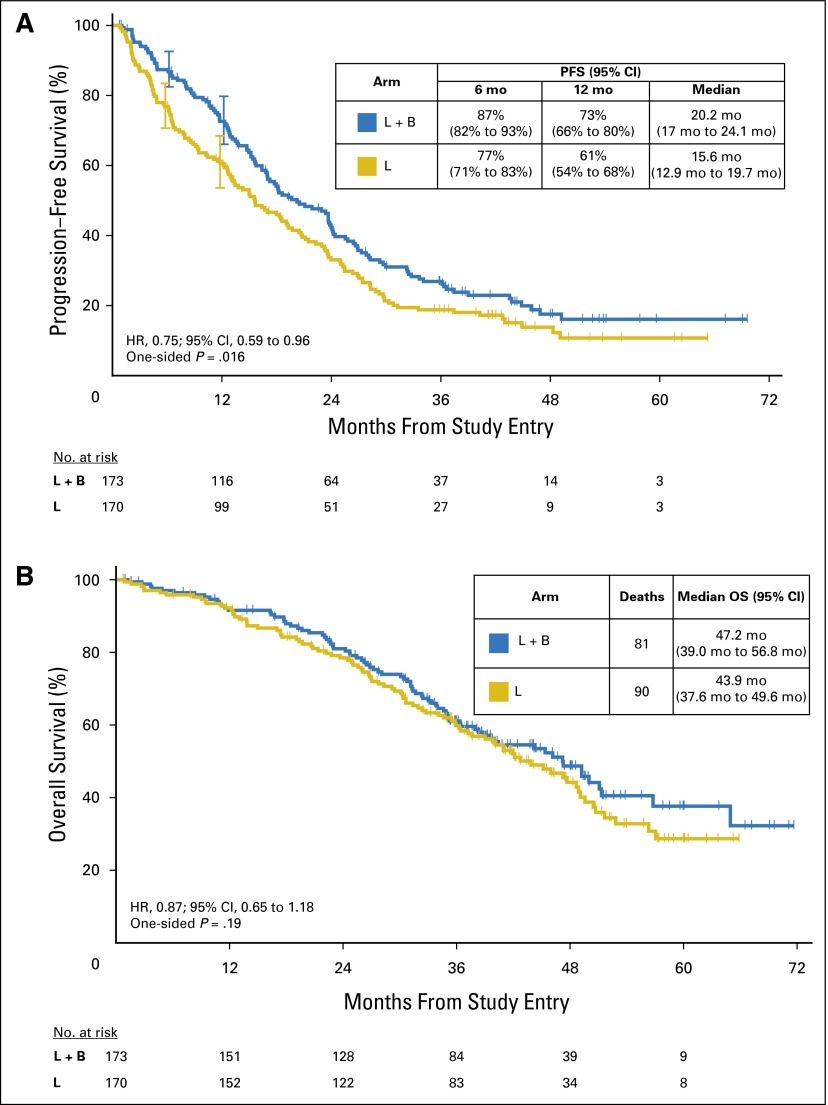
There were 264 PFS events (letrozole, 138; letrozole plus bevacizumab, 126), with a median follow-up for clinical assessment of 39 months and a maximum follow-up of 70 months. Results of the per-protocol primary stratified analysis showed an observed HR of 0.75 (95% CI, 0.59 to 0.96), indicating that the addition of bevacizumab resulted in a statistically significant improvement in PFS over letrozole (one-sided P = .016; Fig 2A and Appendix Table A1, online only). The addition of bevacizumab to letrozole showed a 4.6-month prolongation in median PFS (letrozole: 15.6 months [95% CI, 12.9 to 19.7 months]; letrozole plus bevacizumab: 20.2 months [95% CI, 17.0 to 24.1 months]).
Fig 2.
Kaplan-Meier time-to-event curves. (A) Progression-free survival (PFS); (B) Overall survival (OS). HR, hazard ratio. L, letrozole; B, bevacizumab.
The observed medians of both arms were considerably longer than anticipated in the initial study design. The proportion of patients who were progression free at 6 months was 87% (95% CI, 82% to 93%) on the letrozole plus bevacizumab arm versus 77% (95% CI, 71% to 83%) on the letrozole arm. At 12 months, 73% (95% CI, 66% to 80%) versus 61% (95% CI, 54% to 68%), respectively, were progression free. In exploratory subgroup analyses, improvement in PFS with letrozole plus bevacizumab versus letrozole did not vary substantially by age, de novo versus recurrent disease, bone-only versus other sites of metastases, or number of metastatic sites (Fig 3).
Fig 3.
Exploratory subgroup analyses of improvement in progression-free survival (PFS) in the L + B arm over the L arm. HR, hazard ratio. L, letrozole; B, bevacizumab.
Secondary Analyses
Overall survival.
At a median follow-up of 42 months (maximum follow-up of 90 months), there were 171 deaths, 81 on the letrozole plus bevacizumab arm and 90 on the letrozole arm. OS did not differ significantly by arm (observed HR for letrozole plus bevacizumab:letrozole, 0.87; 95% CI, 0.65 to 1.18; one-sided P = .188). The median survival for the letrozole plus bevacizumab arm was 47.2 months compared with 43.9 months for the letrozole arm (Fig 2B and Appendix Table A1).
Tumor response.
Of the 213 patients with measurable disease, 197 had baseline and sufficient repeat scans to assess objective response (OR). The incidence of OR was significantly higher for the letrozole plus bevacizumab arm compared with the letrozole arm (69% v 49%; P = .004). Similarly, the incidence of clinical benefit was higher for the letrozole plus bevacizumab arm compared with the letrozole arm (80% v 62%; P < .001; Appendix Table A2, online only).
AEs.
At time of reporting, 303 patients (88%) had ended protocol treatment, 150 on the letrozole plus bevacizumab arm and 153 on the letrozole arm. Of these, a higher proportion on the letrozole plus bevacizumab arm ended therapy because of an AE than on the letrozole arm (21 patients [14%] v two patients [1%], respectively; Fig 1). Among all patients, including those still being treated, the median number of cycles was 22 (maximum, 100) for the letrozole plus bevacizumab arm and 17 (maximum, 88) for the letrozole arm. Of patients randomly assigned to letrozole plus bevacizumab, 76 (46%) had at least one cycle of bevacizumab delayed because of toxicity.
Approximately 47% of patients receiving letrozole plus bevacizumab compared with 14% receiving letrozole had at least one treatment-related AE of grade ≥ 3 (Table 2). Of these AEs, the most common were hypertension (24% v 2%) and proteinuria (11% v 0%; Table 3). Most events occurred with < 3% frequency, including thromboembolic events and hemorrhage. There were two treatment-related deaths, one in each arm. One patient randomly assigned to letrozole plus bevacizumab died after a CNS hemorrhage; the other, who was randomly assigned to letrozole, died as a result of pneumonia.
Table 2.
Maximum AEs Grade 3 or Higher With Treatment Attribution
| AE | Letrozole + Bevacizumab (n = 173) | Letrozole (n = 170) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Any AE | ||||
| Grade 3 | 71 | 42 | 21 | 13 |
| Grade 4 | 7 | 4.2 | 1 | 0.6 |
| Grade 5 | 1 | 0.6 | 1 | 0.6 |
| Nonhematologic events | ||||
| Grade 3 | 68 | 41 | 21 | 13 |
| Grade 4 | 7 | 4.2 | 1 | 0.6 |
| Grade 5 | 1 | 0.6 | 1 | 0.6 |
| Hematologic events | ||||
| Grade 3 | 5 | 3.0 | 0 | |
| Grade 4 | 0 | 0 | ||
| Grade 5 | 0 | 0 | ||
| Treatment-related death | 1* | 0.6 | 1† | 0.6 |
NOTE. Reported adverse events (AEs) were graded by using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3 with attribution possibly, probably, or definitely related to treatment.
CNS hemorrhage.
Pneumonia.
Table 3.
Treatment-Related AEs Grade 3 or Higher of Special Interest
| AE | Letrozole + Bevacizumab (%) (n = 173) | Letrozole (%) (n = 170) |
|---|---|---|
| Hypertension | 24 | 2 |
| Proteinuria | 11 | 0 |
| Head or headache pain | 5 | 1 |
| Joint pain | 10 | 0 |
| Left ventricular systolic dysfunction | 2 | 0 |
| Cardiopulmonary arrest | 0 | 1 |
| Cardiac ischemia or infarction | 1 | 0 |
| Thrombosis or embolism | 2 | 1 |
| Wound complications | 1 | 0 |
| CNS hemorrhage | 1 | 1 |
| GI hemorrhage | 1 | 1 |
| CNS cerebrovascular ischemia | 0 | 1 |
NOTE. Reported adverse events (AEs) were graded using National Cancer Institute Common Terminology Criteria for Adverse Events version 3 with attribution possibly, probably, or definitely related to treatment.
DISCUSSION
For women with hormone receptor–positive MBC, CALGB 40503 demonstrates that bevacizumab, an anti–VEGF A monoclonal antibody, prolongs PFS from a median of 15.6 months with letrozole alone to 20.2 months, representing a 25% reduction in the hazard of progression. Although the proportions of patients with an objective response and clinical benefit were greater with letrozole plus bevacizumab compared with letrozole alone, there was no statistically significant difference in OS. These results are consistent with both preclinical models and earlier clinical trial results for bevacizumab with various chemotherapy agents.25-28
Toxicity for bevacizumab was also consistent with prior experience.36 More patients in the bevacizumab-containing arm experienced grade ≥ 3 AEs and discontinuation because of AEs, but there were no unexpected or unusual toxicities seen in combination with letrozole.
These results are similar to those reported in the Letrozole/Fulvestrant and Avastin Study (LEA), which was the first multicenter, open-label phase III trial to demonstrate an increase in PFS (from 14.4 to 19.3 months) with the addition of bevacizumab to first-line ET.32 However, in the LEA study, the difference in PFS did not reach statistical significance (log-rank P = .126). In both trials, similar benefits were observed with respect to ORR and CBR, but neither trial showed a difference in OS. Both the CALGB 40503 and the LEA trials showed bevacizumab-related toxicities, mainly hypertension and proteinuria. In the LEA trial, eight patients died during therapy or within 30 days of completing therapy. In CALGB 40503, almost half the patients on letrozole plus bevacizumab had at least one treatment-related grade ≥ 3 AE; however, there was no difference in treatment-related deaths. The difference in treatment-related deaths may be secondary, in part because of the higher proportion of patients older than age 70 years in the LEA trial. Given the increased bevacizumab-related AEs, the value of the PFS benefit seen in CALGB 40503 must be carefully weighed against the added bevacizumab expense, toxicity, and inconvenience of intravenous administration as well as additional monitoring for hypertension and proteinuria.
Our observation of a PFS improvement without an OS benefit is similar to results from prior chemotherapy-based studies testing bevacizumab in MBC.25-28,37 CALGB 40503 was activated in 2008, soon after the US Food and Drug Administration granted accelerated approval of bevacizumab for the treatment of HER2-negative MBC in combination with first-line chemotherapy. In 2011, the Food and Drug Administration rescinded this approval.38 Since then, several phase III (neo)adjuvant trials have been negative in patients with differing pathologic and molecular subtypes of early-stage breast cancer, including those with HER2-negative,39,40 triple-negative,41 or HER2-positive disease.42 One exception is NSABP B40 (Chemotherapy With or Without Bevacizumab in Treating Women With Stage I, Stage II, or Stage IIIA Breast Cancer That Can Be Removed By Surgery), which demonstrated an increase in OS with the addition of neoadjuvant bevacizumab.43 To date, a biomarker that defines a subset of patients most likely to benefit from bevacizumab has not been identified.
Broadly, these data challenge the assumption that PFS is a surrogate for OS in MBC or that PFS alone can be used in all settings to identify superior treatment options. Given the extent of data for bevacizumab across multiple stages and settings in breast cancer, it is clear that the statistically significant improvement in PFS that our trial was designed to detect is not, in itself, sufficient to change standards of care. Were it associated with improved survival, a different (and lesser) toxicity profile, or different (and lesser) costs, then perhaps our assessment would change.
It is noteworthy that the median PFS for the CALGB 40503 control arm (letrozole alone, 15.6 months) substantially exceeded the projection based on literature available when the study was designed.44-47 Almost half the patients achieved an OR to therapy. These results are consistent with other recently reported phase III first-line ET trials32,48 that enrolled similar proportions of hormone receptor–positive patients with MBC with de novo and/or ET-naïve metastatic disease. The favorable outcome for these endocrine-sensitive subgroups should have an impact on PFS event rate estimates and future first-line ET clinical trial design. Given continued efforts to develop therapies that delay the emergence of endocrine resistance in hormone receptor–positive MBC,49-51 this observation has immediate relevance. With added inconvenience and toxicities that can be a burden for some, it is important for us to define hormone receptor–positive populations that are more likely to benefit from these newer targeted agents and to identify those patients who may do well with ET alone.
Given the positive results of CALGB 40503 on our primary end point, we will refine subgroup selection by using patient and tumor characteristics to potentially identify factors in both the control and experimental arms that are predictive of benefit from ET alone or with bevacizumab. Results may inform the design of new trials to test bevacizumab or other classes of targeted therapies added to ET.
Supplementary Material
Acknowledgment
We thank the patients who participated in this trial, the investigators who treated patients on this trial, and all other Alliance members and local research staff who supported this study.
Appendix
Table A1.
Observed Effect of Treatment Arm on PFS and OS
| Parameter | PFS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Letrozole + Bevacizumab | Letrozole | Letrozole + Bevacizumab v Letrozole | Letrozole + Bevacizumab | Letrozole | Letrozole + Bevacizumab v Letrozole | |||||
| HR | 95% CI | P* | HR | 95% CI | P* | |||||
| No. of patients | 173 | 170 | 173 | 170 | ||||||
| No. of events | 126 | 138 | 81 | 90 | ||||||
| Median, months | 20.2 | 15.6 | 47.2 | 43.9 | ||||||
| 0.75 | 0.59 to 0.96 | .016 | 0.87 | 0.65 to .18 | .188 | |||||
Abbreviations: HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
One-sided.
Table A2.
Incidence of OR and CB by Arm for Measurable Tumors
| Characteristic | Letrozole + Bevacizumab | Letrozole | ||||
|---|---|---|---|---|---|---|
| No. | % | 95% CI | No. | % | 95% CI | |
| No. with measurable disease | 106 | 100 | 107 | 100 | ||
| No. assessable for response* | 98 | 92 | 99 | 93 | ||
| No. with measurable disease and assessable for response | 98 | 100 | 99 | 100 | ||
| Complete response | 4 | 4 | 7 | 7 | ||
| Partial response | 64 | 65 | 42 | 42 | ||
| Stable disease, weeks | 22 | 22 | 34 | 34 | ||
| < 24 | 12 | 22 | ||||
| ≥ 24 | 10 | 12 | ||||
| Progression | 8 | 8 | 16 | 16 | ||
| Objective tumor response† | 68 | 69 | 60 to 78 | 49 | 49 | 40 to 59 |
| CB‡ | 78 | 80 | 71 to 86 | 61 | 62 | 52 to 71 |
Abbreviations: CB, clinical benefit; ECOG, Eastern Cooperative Oncology Group; OR, objective response.
Sufficient tumor information was available to assess response.
Objective tumor response is defined as complete response or partial response.
Clinical benefit is defined as complete response, partial response or stable disease for at least 24 weeks.
The following institutions (along with the principal investigator and grant number) participated in this study: Colorado Cancer Research Program (National Cancer Institute Community Oncology Research Program [NCORP]), Denver, CO, Keren Sturtz (UG1CA189805); Dana-Farber/Partners Cancer Care, Boston, MA, Harold J. Burstein (U10CA180867); Delaware/Christiana Care NCORP, Newark, DE, Stephen S. Grubbs (UG1CA189819); Essentia Health NCORP, Duluth, MN, Bret E. Friday (UG1CA189812); Heartland Cancer Research NCORP, Decatur, IL, James L. Wade III (UG1CA189830); Hematology-Oncology Associates of Central New York Community Clinical Oncology Program, Syracuse, NY, Jeffrey Kirshner (CA45389); Iowa-Wide Oncology Research Coalition NCORP, Des Moines, IA, Robert J. Behrens (UG1CA189816); Johns Hopkins University, Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD, Julie R. Brahmer (U10CA180802); Mayo Clinic, Rochester, MN, Steven R. Alberts (U10CA180790); Memorial Sloan Kettering Cancer Center, New York, NY, Carol Aghajanian (U10CA180791); Michigan Cancer Research Consortium NCORP, Ann Arbor, MI, Philip J. Stella (UG1CA189971); Mount Sinai Medical Center, Miami, FL, Michael A. Schwartz (CA45564); New Hampshire Oncology-Hematology PA, Concord, NH, Douglas J. Weckstein; Sanford NCORP of the North Central Plains, Sioux Falls, SD, Preston D. Steen (UG1CA189825); The Ohio State University, Columbus, OH, Richard M. Goldberg (U10CA180850); University of California at San Francisco, San Francisco, CA, Charles J. Ryan (CA138561); University of Chicago, Chicago, IL, Hedy L. Kindler (U10CA180836); University of Illinois Minority-Based Community Clinical Oncology Program, Chicago, IL, David J. Peace (U10CA074811); University of Iowa, Iowa City, IA, Daniel A. Vaena (CA47642); University of Nebraska Medical Center, Omaha, NE, Apar Ganti (CA77298); University of North Carolina, Chapel Hill, NC, Lisa A. Carey (U10CA180838); Washington University School of Medicine, St Louis, MO, Nancy L. Bartlett (U10CA180833); Wisconsin NCORP, Marshfield, WI, Douglas J. Reding (UG1CA189956).
Footnotes
Supported by National Cancer Institute Awards No. U10CA031946, U10CA033601, U10CA180821, and U10CA180882 (to the Alliance for Clinical Trials in Oncology); and in part by Grants No. U10CA138561, U10CA180791, U10CA180795, U10CA180820, U10CA180838, U10CA180858, U10CA180867, and U10CA180888; by Genentech (research funding and bevacizumab) and Novartis (letrozole); and by Core Grant No. P30 CA008748 from Memorial Sloan Kettering Cancer Center.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Presented in part at the 51st Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 29-June 2, 2015.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. contributions are found at the end of this article.
Clinical Trial information: NCT00601900
AUTHOR CONTRIBUTIONS
Conception and design: Maura N. Dickler, William T. Barry, Matthew J. Ellis, Mary Ellen Moynahan, Federico Innocenti, Arti Hurria, Hope S. Rugo, Olwen Hahn, Lisa A. Carey, Eric P. Winer, Clifford A. Hudis
Administrative support: Olwen Hahn, Eric P. Winer, Clifford A. Hudis
Provision of study materials or patients: Maura N. Dickler, Hope S. Rugo, Diana E. Lake, Olwen Hahn, Bryan P. Schneider, Debasish Tripathy
Collection and assembly of data: William T. Barry, Constance T. Cirrincione, Diana E. Lake, Bryan P. Schneider, Debasish Tripathy, Lisa A. Carey, Eric P. Winer, Clifford A. Hudis
Data analysis and interpretation: William T. Barry, Constance T. Cirrincione, Mary Ellen Moynahan, Federico Innocenti, Arti Hurria, Hope S. Rugo, Olwen Hahn, Lisa A. Carey, Eric P. Winer, Clifford A. Hudis
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase III Trial Evaluating Letrozole As First-Line Endocrine Therapy With or Without Bevacizumab for the Treatment of Postmenopausal Women With Hormone Receptor–Positive Advanced-Stage Breast Cancer: CALGB 40503 (Alliance)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Maura N. Dickler
Consulting or Advisory Role: Bristol-Myers Squibb, AstraZeneca, Novartis, Merrimack Pharmaceuticals, Genentech, Pfizer, Neovacs
Research Funding: Novartis (Inst), Eli Lilly (Inst), Genentech (Inst)
William T. Barry
Research Funding: Pfizer (Inst)
Constance T. Cirrincione
No relationship to disclose
Matthew J. Ellis
Employment: Bioclassifier
Leadership: Bioclassifier
Stock or Other Ownership: Bioclassifier
Consulting or Advisory Role: Novartis, Pfizer, Celgene, AstraZeneca, NanoString Technologies
Patents, Royalties, Other Intellectual Property: Patent on PAM50, which generates royalties on the license to Bioclassifier/Prosigna/NanoString Technologies
Travel, Accommodations, Expenses: Pfizer, AstraZeneca
Mary Ellen Moynahan
Consulting or Advisory Role: Novartis
Research Funding: Novartis (Inst)
Federico Innocenti
No relationship to disclose
Arti Hurria
Consulting or Advisory Role: GTx, Seattle Genetics, Boehringer Ingelheim, On Q Health, Sanofi
Speakers’ Bureau: Sanofi
Research Funding: GlaxoSmithKline (Inst), Celgene (Inst)
Hope S. Rugo
Honoraria: Genomic Health
Speakers’ Bureau: Genomic Health
Research Funding: Plexxikon (Inst), MacroGenics (Inst), OBI Pharma (Inst), Eisai (Inst), Pfizer (Inst), Novartis (Inst), Eli Lilly (Inst), GlaxoSmithKline (Inst), Genentech (Inst), Celsion (Inst), Nektar Therapeutics (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Novartis, Genentech, OBI Pharma, Mylan, Pfizer
Diana E. Lake
No relationship to disclose
Olwen Hahn
Honoraria: Cardinal Health (I)
Travel, Accommodations, Expenses: Cardinal Health (I)
Bryan P. Schneider
No relationship to disclose
Debasish Tripathy
Consulting or Advisory Role: Merck, Novartis, Nektar Therapeutics, Puma Biotechnology
Research Funding: Genentech (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Novartis
Lisa A. Carey
Research Funding: GlaxoSmithKline, Genentech
Eric P. Winer
Consulting or Advisory Role: Novartis, Genentech, Verastem
Research Funding: Genentech (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Genentech
Clifford A. Hudis
Consulting or Advisory Role: Pfizer, Genentech, Novartis, Merck, Eli Lilly
Other Relationship: Breast Cancer Research Foundation
REFERENCES
- 1.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toy W, Shen Y, Won H, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45:1439–1445. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oesterreich S, Davidson NE. The search for ESR1 mutations in breast cancer. Nat Genet. 2013;45:1415–1416. doi: 10.1038/ng.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asghar U, Witkiewicz AK, Turner NC, et al. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thangavel C, Dean JL, Ertel A, et al. Therapeutically activating RB: Reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer. 2011;18:333–345. doi: 10.1530/ERC-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massarweh S, Schiff R. Unraveling the mechanisms of endocrine resistance in breast cancer: New therapeutic opportunities. Clin Cancer Res. 2007;13:1950–1954. doi: 10.1158/1078-0432.CCR-06-2540. [DOI] [PubMed] [Google Scholar]
- 8.Zardavas D, Baselga J, Piccart M. Emerging targeted agents in metastatic breast cancer. Nat Rev Clin Oncol. 2013;10:191–210. doi: 10.1038/nrclinonc.2013.29. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain RK, Safabakhsh N, Sckell A, et al. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: Role of vascular endothelial growth factor. Proc Natl Acad Sci USA. 1998;95:10820–10825. doi: 10.1073/pnas.95.18.10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu Z, Van Ginkel S, Roy AM, et al. Vascular endothelial growth factor reduces tamoxifen efficacy and promotes metastatic colonization and desmoplasia in breast tumors. Cancer Res. 2008;68:6232–6240. doi: 10.1158/0008-5472.CAN-07-5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Losordo DW, Isner JM. Estrogen and angiogenesis: A review. Arterioscler Thromb Vasc Biol. 2001;21:6–12. doi: 10.1161/01.atv.21.1.6. [DOI] [PubMed] [Google Scholar]
- 14.Morales DE, McGowan KA, Grant DS, et al. Estrogen promotes angiogenic activity in human umbilical vein endothelial cells in vitro and in a murine model. Circulation. 1995;91:755–763. doi: 10.1161/01.cir.91.3.755. [DOI] [PubMed] [Google Scholar]
- 15.Gagliardi AR, Hennig B, Collins DC. Antiestrogens inhibit endothelial cell growth stimulated by angiogenic growth factors. Anticancer Res. 1996;16:1101–1106. [PubMed] [Google Scholar]
- 16.Hyder SM, Stancel GM, Chiappetta C, et al. Uterine expression of vascular endothelial growth factor is increased by estradiol and tamoxifen. Cancer Res. 1996;56:3954–3960. [PubMed] [Google Scholar]
- 17.Kazi AA, Jones JM, Koos RD. Chromatin immunoprecipitation analysis of gene expression in the rat uterus in vivo: Estrogen-induced recruitment of both estrogen receptor alpha and hypoxia-inducible factor 1 to the vascular endothelial growth factor promoter. Mol Endocrinol. 2005;19:2006–2019. doi: 10.1210/me.2004-0388. [DOI] [PubMed] [Google Scholar]
- 18.Kazi AA, Koos RD. Estrogen-induced activation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor expression, and edema in the uterus are mediated by the phosphatidylinositol 3-kinase/Akt pathway. Endocrinology. 2007;148:2363–2374. doi: 10.1210/en.2006-1394. [DOI] [PubMed] [Google Scholar]
- 19.Takei H, Lee ES, Jordan VC. In vitro regulation of vascular endothelial growth factor by estrogens and antiestrogens in estrogen-receptor positive breast cancer. Breast Cancer. 2002;9:39–42. doi: 10.1007/BF02967545. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura J, Savinov A, Lu Q, et al. Estrogen regulates vascular endothelial growth/permeability factor expression in 7,12-dimethylbenz(a)anthracene-induced rat mammary tumors. Endocrinology. 1996;137:5589–5596. doi: 10.1210/endo.137.12.8940388. [DOI] [PubMed] [Google Scholar]
- 21.Manders P, Beex LV, Tjan-Heijnen VC, et al. Vascular endothelial growth factor is associated with the efficacy of endocrine therapy in patients with advanced breast carcinoma. Cancer. 2003;98:2125–2132. doi: 10.1002/cncr.11764. [DOI] [PubMed] [Google Scholar]
- 22.Linderholm B, Grankvist K, Wilking N, et al. Correlation of vascular endothelial growth factor content with recurrences, survival, and first relapse site in primary node-positive breast carcinoma after adjuvant treatment. J Clin Oncol. 2000;18:1423–1431. doi: 10.1200/JCO.2000.18.7.1423. [DOI] [PubMed] [Google Scholar]
- 23.Linderholm BK, Lindh B, Beckman L, et al. Prognostic correlation of basic fibroblast growth factor and vascular endothelial growth factor in 1307 primary breast cancers. Clin Breast Cancer. 2003;4:340–347. doi: 10.3816/cbc.2003.n.039. [DOI] [PubMed] [Google Scholar]
- 24.Foekens JA, Peters HA, Grebenchtchikov N, et al. High tumor levels of vascular endothelial growth factor predict poor response to systemic therapy in advanced breast cancer. Cancer Res. 2001;61:5407–5414. [PubMed] [Google Scholar]
- 25.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 26.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 27.Robert NJ, Diéras V, Glaspy J, et al. RIBBON-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 28.Brufsky AM, Hurvitz S, Perez E, et al. RIBBON-2: A randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2011;29:4286–4293. doi: 10.1200/JCO.2010.34.1255. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter D, Kesselheim AS, Joffe S. Reputation and precedent in the bevacizumab decision. N Engl J Med. 2011;365:e3. doi: 10.1056/NEJMp1107201. [DOI] [PubMed] [Google Scholar]
- 30.Traina TA, Rugo HS, Caravelli JF, et al. Feasibility trial of letrozole in combination with bevacizumab in patients with metastatic breast cancer. J Clin Oncol. 2010;28:628–633. doi: 10.1200/JCO.2009.21.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yardley DA, Burris HA, III, Clark BL, et al. Hormonal therapy plus bevacizumab in postmenopausal patients who have hormone receptor-positive metastatic breast cancer: A phase II Trial of the Sarah Cannon Oncology Research Consortium. Clin Breast Cancer. 2011;11:146–152. doi: 10.1016/j.clbc.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 32. Martín M, Loibl S, von Minckwitz G, et al: Phase III trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer: The Letrozole/Fulvestrant and Avastin (LEA) study. J Clin Oncol 33:1045-1052, 2015) [DOI] [PubMed]
- 33.Forero-Torres A, Saleh MN, Galleshaw JA, et al. Pilot trial of preoperative (neoadjuvant) letrozole in combination with bevacizumab in postmenopausal women with newly diagnosed estrogen receptor- or progesterone receptor-positive breast cancer. Clin Breast Cancer. 2010;10:275–280. doi: 10.3816/CBC.2010.n.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freidlin B, Korn EL. A comment on futility monitoring. Control Clin Trials. 2002;23:355–366. doi: 10.1016/s0197-2456(02)00218-0. [DOI] [PubMed] [Google Scholar]
- 35. R Foundation for Statistical Computing: R: A language and environment for statistical computing. Vienna, Austria, 2014. [Google Scholar]
- 36.Cobleigh MA, Langmuir VK, Sledge GW, et al. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol. 2003;30:117–124. doi: 10.1053/j.seminoncol.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 37. O’Shaughnessy J, Miles D, Gray RJ, et al: A meta-analysis of overall survival data from three randomized trials of bevacizumab (BV) and first-line chemotherapy as treatment for patients with metastatic breast cancer (MBC). J Clin Oncol 28:15s, 2010 (suppl; abstr 1005)
- 38.D’Agostino RB., Sr Changing end points in breast-cancer drug approval: The Avastin story. N Engl J Med. 2011;365:e2. doi: 10.1056/NEJMp1106984. [DOI] [PubMed] [Google Scholar]
- 39. Miller K, O’Neill AM, Dang CT, et al: Bevacizumab (Bv) in the adjuvant treatment of HER2-negative breast cancer: Final results from Eastern Cooperative Oncology Group E5103. J Clin Oncol 32, 2014 (suppl 5s; abstr 500) [Google Scholar]
- 40.von Minckwitz G, Eidtmann H, Rezai M, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012;366:299–309. doi: 10.1056/NEJMoa1111065. [DOI] [PubMed] [Google Scholar]
- 41.Cameron D, Brown J, Dent R, et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): Primary results of a randomised, phase 3 trial. Lancet Oncol. 2013;14:933–942. doi: 10.1016/S1470-2045(13)70335-8. [DOI] [PubMed] [Google Scholar]
- 42. Slamon DJ, Swain SM, Buyse M, et al: Primary results from BETH, a phase 3 controlled study of adjuvant chemotherapy and trastuzumab ± bevacizumab in patients with HER2-positive, node-positive or high risk node-negative breast cancer. Cancer Res 73, 2013 (suppl; abstr S1-03) [Google Scholar]
- 43.Bear HD, Tang G, Rastogi P, et al. Neoadjuvant plus adjuvant bevacizumab in early breast cancer (NSABP B-40 [NRG Oncology]): Secondary outcomes of a phase 3, randomised controlled trial. Lancet Oncol. 2015;16:1037–1048. doi: 10.1016/S1470-2045(15)00041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mouridsen H, Gershanovich M, Sun Y, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: Results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19:2596–2606. doi: 10.1200/JCO.2001.19.10.2596. [DOI] [PubMed] [Google Scholar]
- 45.Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: Results of a North American multicenter randomized trial. J Clin Oncol. 2000;18:3758–3767. doi: 10.1200/JCO.2000.18.22.3758. [DOI] [PubMed] [Google Scholar]
- 46.Howell A, Robertson JF, Abram P, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: A multinational, double-blind, randomized trial. J Clin Oncol. 2004;22:1605–1613. doi: 10.1200/JCO.2004.02.112. [DOI] [PubMed] [Google Scholar]
- 47.Bonneterre J, Buzdar A, Nabholtz JM, et al. Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer. 2001;92:2247–2258. doi: 10.1002/1097-0142(20011101)92:9<2247::aid-cncr1570>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 48.Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012;367:435–444. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 50.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.