Abstract
Purpose
Patients with bladder cancer with clinical lymph node involvement (cN+) are at high risk for distant metastases, but are potentially curable. Such patients are excluded from neoadjuvant chemotherapy trials and pooled with patients with distant metastases in first-line chemotherapy trials not suited to define the role of combined-modality therapy. To address this evidence void, we performed a comparative effectiveness analysis.
Methods
We included cTanyN1-3M0 bladder cancer patients from the National Cancer Data Base (2003-2012) treated with chemotherapy and/or cystectomy. We used multistate survival analysis, allowing for delayed entry, to assess overall survival (OS) according to various treatment strategies. Effectiveness was estimated with multivariable adjustment for tumor-, patient-, and facility-level characteristics.
Results
Among 1,739 patients (cN1, 48%; cN2, 45%; cN3, 7%), 1,104 underwent cystectomy and 635 were treated with chemotherapy alone. Of the cystectomy patients, 363 received preoperative and 328 received adjuvant chemotherapy. The crude 5-year OS for chemotherapy alone, cystectomy alone, preoperative chemotherapy followed by cystectomy, and cystectomy followed by adjuvant chemotherapy was 14% (95% CI, 11% to 17%), 19% (95% CI, 15% to 24%), 31% (95% CI, 25% to 38%), and 26% (95% CI, 21% to 34%), respectively. Compared with cystectomy alone, preoperative chemotherapy was associated with a significant improvement in OS (hazard ratio, 0.80; 95% CI, 0.66 to 0.97). Adjuvant chemotherapy was also associated with a significant improvement in survival compared with cystectomy alone. The survival of patients treated with chemotherapy alone was worse than those treated with cystectomy alone.
Conclusion
A subset of patients with cN+ bladder cancer achieves long-term survival. Combined-modality therapy, with chemotherapy and cystectomy, is associated with the best outcomes.
BACKGROUND
Muscle-invasive urothelial cancer of the bladder is an aggressive malignancy with a high propensity for metastatic dissemination. Nonetheless, in the absence of radiographic evidence of metastatic disease, surgical removal of the bladder or radiation therapy may be curative in a large proportion of patients.1 Two randomized clinical trials and a meta-analysis demonstrated a further survival advantage with the administration of neoadjuvant cisplatin-based combination chemotherapy before definitive local therapy.2-4 Clinical trials exploring adjuvant chemotherapy have been flawed and/or underpowered, but accumulating evidence also supports an improvement in outcomes with this approach.5-7 Unfortunately, once bladder cancer has visibly metastasized to distant sites, although systemic chemotherapy may impart therapeutic benefit, patients rarely achieve cure, and median survival is only approximately 14 months.8
The standard management of patients with clinically localized and distant metastatic bladder cancer has been defined through a series of prospective randomized controlled trials. However, evidence guiding the optimal management of patients with clinical evidence of metastases limited to regional lymph nodes (ie, cN+) is strikingly lacking. Refining treatment strategies for patients with cN+ bladder cancer is critical, because such patients are at high risk for distant metastatic spread, although still potentially curable. Yet, patients with cN+ disease have historically been excluded from neoadjuvant chemotherapy trials and pooled with patients with distant metastatic disease in first-line chemotherapy trials not suited to define the potential role of combined-modality therapy.9,10 A few retrospective series, mostly from single institutions and/or academic centers, have explored the outcomes of patients with cN+ bladder cancer.11-14 In an early analysis in 1999, Dodd et al11 reported that among nine patients with radiographic pelvic lymph node involvement undergoing postchemotherapy cystectomy, three patients were alive at 5 years. This single-center experience was expanded by Herr et al12 in 2001, demonstrating durable disease control in a subset of patients, particularly those achieving a partial or complete radiographic response to chemotherapy. Notably, only one of 12 patients who responded to chemotherapy but declined postchemotherapy surgery achieved long-term survival in that series. More recently, single-center and multicenter series from academic institutions, comprising 55 to 304 patients who were treated with initial chemotherapy followed by cystectomy, reported similar results.13,14 Although these data suggest that outcomes may be optimized with combined-modality therapy, interpretation is hampered by a lack of consideration of various treatment approaches and sequences, methodological heterogeneity, and limited generalizability. To address this major evidence gap, we performed a comparative effectiveness analysis using a large observational cohort.
METHODS
Data Source
Data for this study were derived from the National Cancer Data Base (NCDB). The NCDB, a joint program of the Commission on Cancer of the American College of Surgeons and the American Cancer Society, is a registry comprising data from more than 1,500 hospitals in the United States and includes approximately 70% of all newly diagnosed cases of cancer. The NCDB has previously been described in detail.15 This study used deidentified data and was exempt from institutional review board approval.
Study Population
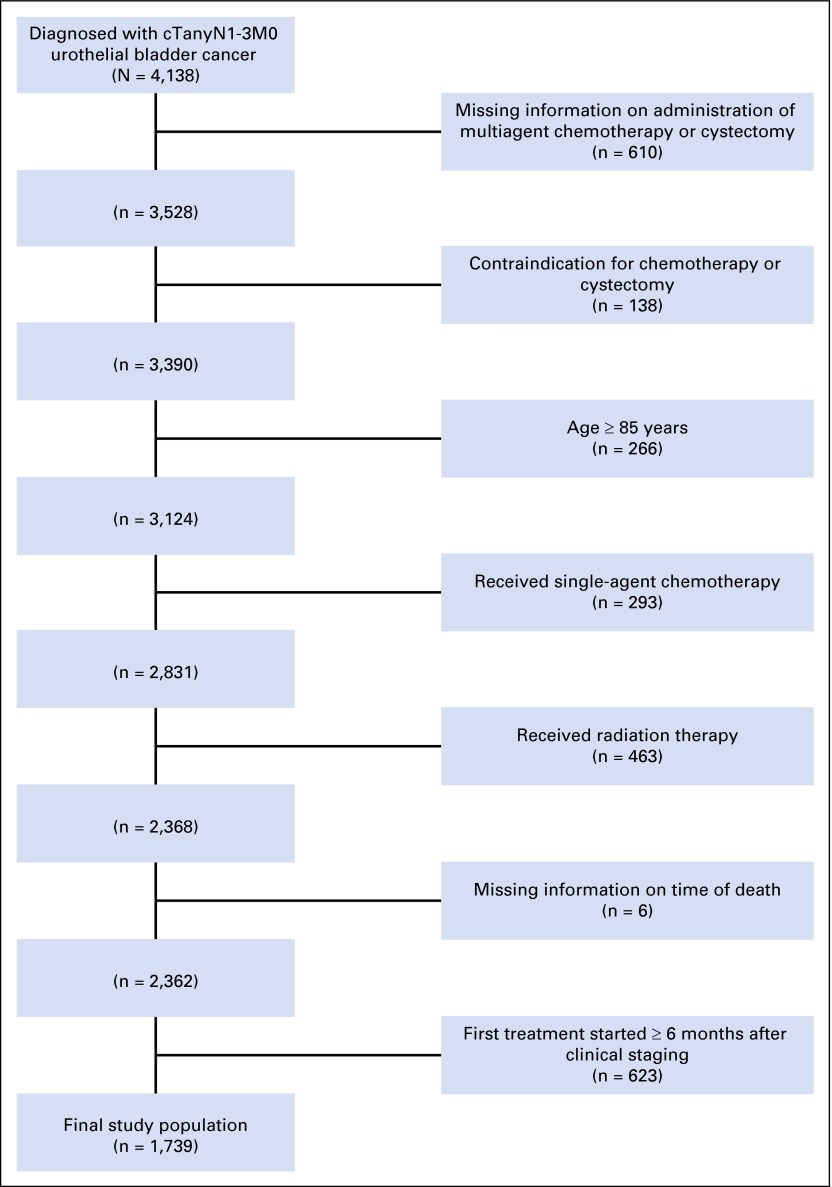
Using American Joint Committee on Cancer (AJCC) TNM staging data from 2003 through 2011, we identified 4,138 patients who were diagnosed with cTanyN1-3M0 urothelial cancer of the bladder. Before January 1, 2010, staging was based on the sixth edition of the AJCC Cancer Staging Manual, and for cancers diagnosed on or after this date, the seventh edition was used; cN1-2 in the seventh edition encompasses all nodal metastases previously classified as cN1-3 in the sixth edition (Appendix Table A1, online only).16 After excluding patients based on missing data regarding the timing or administration of chemotherapy, contraindications to chemotherapy due to patient comorbidities (defined by NCDB coding), receipt of single-agent chemotherapy or radiation, initiation of first treatment ≥ 6 months after clinical staging, or age ≥ 85 years, the final study cohort comprised 1,739 patients (Fig 1).
Fig 1.
Patients included in the analysis and reasons for patient exclusions.
Treatment Definitions
For the analyses of treatment effectiveness, we defined preoperative chemotherapy as initiating multiagent chemotherapy within 6 months before the time of cystectomy. Adjuvant chemotherapy was defined as initiating multiagent chemotherapy within 4 months after cystectomy. NCDB coding does not reliably permit identification of patients who received adjuvant chemotherapy after having previously received preoperative chemotherapy; the preoperative chemotherapy group and the adjuvant chemotherapy groups in this analysis are mutually exclusive.
Confounding Variables
To estimate treatment effects on overall survival, we aimed to adjust for confounding by indication. Therefore, we included several baseline patient-, tumor-, and facility-level variables within multivariable analyses. Patient-level variables included age, ethnicity/race, education level, income, insurance status, and Charlson/Deyo Comorbidity Index (categorized by the NCDB into 0, 1, and ≥ 2). Tumor-level variables included clinical T and N stage. Facility-level variables included the type of facility, as assigned by the Commission on Cancer, setting and location of the facility, and number of cystectomies performed by the facility in the year preceding clinical staging.
Missing Confounding Variables
Missing values were imputed 20 times with a flexible additive model including status variables for death, cystectomy, preoperative and adjuvant therapy, and the Nelson-Aalen estimators of the cumulative hazard for death, cystectomy, and preoperative and adjuvant chemotherapy.17 Rubin’s rules were used to summarize the effect estimates and variances of the 20 different analyses across multiple imputed datasets.
Statistical Analyses
Baseline variables were compared using the Kruskal-Wallis test and χ2 test and, in case of low numbers, Fisher’s exact test. We used multivariable logistic regression including the previously mentioned confounders in the subset of cystectomy-treated patients (n = 1,104) to address the effect of preoperative chemotherapy versus initial cystectomy on achieving a complete pathologic response in the regional lymph nodes (ie, pN0).
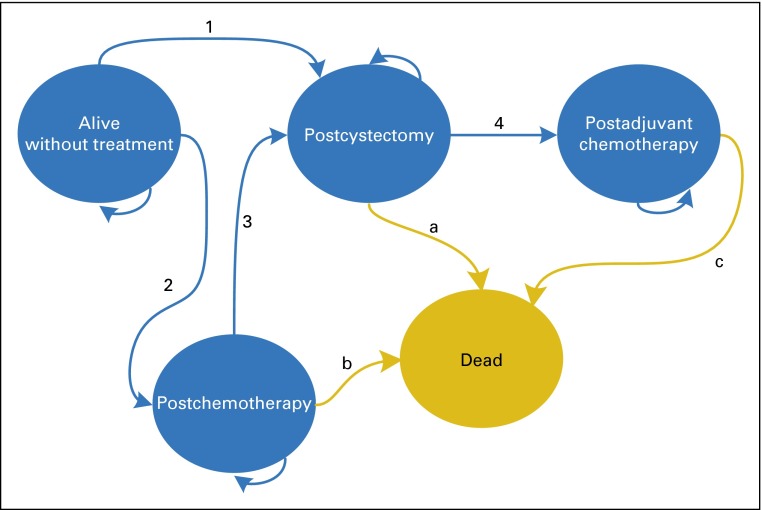
Univariable and multivariable Cox proportional hazards regression analysis with time since clinical staging as the timescale was performed to evaluate the effect of chemotherapy alone and preoperative and adjuvant chemotherapy on all-cause mortality, with cystectomy as the reference. To address differences in the timing of treatment initiation, we used time-dependent treatment covariates, allowing patients to be included in the analysis at the time of treatment initiation calculated since clinical staging (delayed entry). Therefore, we created a multistate model comprising five health states: (1) alive without treatment, (2) postchemotherapy, (3) postcystectomy, (4) postadjuvant chemotherapy, and (5) deceased (Fig 2). All patients (N = 1,739) entered the analysis in the alive-without-treatment state and then could move to either the postchemotherapy state or the postcystectomy state. If chemotherapy was followed by cystectomy, patients would move from the postchemotherapy state to the postcystectomy state and were then considered to be treated with preoperative chemotherapy. Patients treated by adjuvant chemotherapy had moved from the alive-without-treatment state to the postcystectomy state and to the postadjuvant chemotherapy state. Thus, within the time-dependent survival analysis, patients contributed to the at-risk cohorts across follow-up time, with membership at each time point based on the last received treatment. Patients’ time of death was right censored at 5 years or at the time of loss to follow-up.
Fig 2.
Multistate model of treatment strategies. This bubble diagram demonstrates how patients were traced over time within the multistate model according to treatment and survival history. Each bubble represents a possible health state. The included health states were mutually exclusive, meaning that at each time point, a patient could reside in only one health state. Arrows labeled with a number or letter refer to a transition or change in health states as time evolves, that is, treatment initiation or death. All patients started in the alive-without-treatment state at the time of clinical staging and subsequently moved to the applicable post-treatment state at the time that particular treatment was initiated. Patients who died moved to the dead state, an absorbing health state, at the time of death, and patients who survived remained in their post-treatment state until the end of the study or lost to follow-up (right censored). The arrows that connect to the same health state from which they arise indicate that patients could remain at risk and stay within the same health state if they had not been right censored, changed treatment status, or experienced death. Transitions indicated by arrows a, b, and c were used for the estimation of effectiveness of treatment strategies on all-cause mortality: a, used for estimation of the effect of preoperative chemotherapy (with and without censoring for transition 4), also used for estimation of the death rate in the reference group, cystectomy; b, used for estimation of the effect of chemotherapy alone; c, used for estimation of the effect of adjuvant chemotherapy, also used for estimation of the death rate in the reference group, cystectomy. Transitions 1, 2, 3, and 4 were used for classifying patients according to treatment strategy: 1, used to classify patients as having cystectomy alone; 2, used to classify patients as having chemotherapy alone; 3, used to classify patients as having preoperative chemotherapy; 4, used to classify patients as having adjuvant chemotherapy. For more details on the multistate model, see the Methods section.
For preoperative chemotherapy, the time-dependent covariate was defined at the time of cystectomy. Because cystectomy patients who did not receive preoperative chemotherapy were able to receive adjuvant chemotherapy, we estimated the effect of preoperative chemotherapy versus cystectomy alone by censoring those who initiated adjuvant chemotherapy. To evaluate the extent to which the protective effect of preoperative chemotherapy was associated with tumor downstaging, we adjusted for pathologic stage and surgical margin status. The effect of adjuvant chemotherapy was estimated in the subset of patients who underwent cystectomy, while adjusting for preoperative chemotherapy, pathologic stage, and surgical margin status. Because the vast majority of bladder cancer deaths in patients with locally advanced/metastatic disease occur within 5 years of diagnosis, and only overall mortality data are available in the NCDB, we censored time for patients being alive at a maximal follow-up time of 5 years.1
Violation of the proportional hazards assumption was evaluated by plotting scaled Schoenfeld residuals as a function of time. To analyze treatment variables that demonstrated violation, we split follow-up time into different periods in an additional analysis. Age, distance to facility, and procedural volume were winsorized at their 1st and 99th percentiles to limit the influence of extreme values. Nonlinear terms for these continuous variables were considered using restricted cubic spline functions with three and four knots. Decisions about the functional form of continuous predictors were determined by Akaike’s information criterion. Statistical significance of interactions of treatment approach with clinical nodal stage was tested by the likelihood ratio test, modified for multiple imputations, with a significance level of .05.18 Interactions were tested in analyses with cystectomy alone as the reference. All analyses were performed using R version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Population
Among the 1,739 patients included in the analysis, (cN1, 48%; cN2, 45%; cN3, 7%), 36% received treatment with chemotherapy alone, 24% underwent cystectomy alone, 21% received preoperative chemotherapy followed by cystectomy, and 19% underwent cystectomy followed by adjuvant chemotherapy. Baseline patient, facility, and tumor characteristics are listed in Tables 1 and 2.
Table 1.
Patient and Facility Characteristics
| Characteristic | Chemotherapy Only (n = 635) | Cystectomy Only (n = 413) | Cystectomy + Preoperative Chemotherapy (n = 363) | Cystectomy + Adjuvant Chemotherapy (n = 328) | P |
|---|---|---|---|---|---|
| Age, years | 65.6 ± 11.0 | 68.6 ± 10.1 | 61.1 ± 11.0 | 64.1 ± 10.7 | < .001 |
| Male | 478 (75) | 297 (72) | 267 (74) | 250 (76) | .51 |
| Race/ethnicity | .07 | ||||
| White | 503 (79) | 339 (82) | 315 (87) | 264 (80) | |
| Black | 49 (8) | 26 (6) | 18 (5) | 16 (5) | |
| Hispanic | 17 (3) | 6 (2) | 5 (1) | 5 (2) | |
| Other | 66 (10) | 42 (10) | 25 (7) | 43 (13) | |
| Insurance status | <.001 | ||||
| Medicaid | 56 (9) | 21 (5) | 29 (8) | 22 (7) | |
| Medicare | 324 (51) | 244 (59) | 143 (39) | 143 (44) | |
| Not insured | 30 (5) | 16 (4) | 12 (3) | 18 (5) | |
| Other government | 5 (1) | 2 (0) | 4 (1) | 4 (1) | |
| Private | 210 (33) | 112 (30) | 172 (47) | 136 (41) | |
| Missing | 10 (2) | 8 (2) | 3 (1) | 5 (2) | |
| Median income, $ | .21 | ||||
| < 30,000 | 102 (16) | 73 (18) | 68 (19) | 68 (21) | |
| 30,000-34,999 | 179 (28) | 119 (29) | 100 (28) | 90 (27) | |
| 35,000-45,999 | 100 (16) | 52 (13) | 37 (10) | 32 (10) | |
| ≥ 46,000 | 226 (36) | 149 (36) | 135 (37) | 126 (38) | |
| Missing | 28 (4) | 20 (5) | 23 (6) | 12 (4) | |
| Without HS education* | 0.63 | ||||
| > 29 | 99 (16) | 72 (17) | 49 (14) | 43 (13) | |
| 20-28.9 | 150 (24) | 106 (26) | 80 (22) | 76 (23) | |
| 14-19.9 | 147 (23) | 96 (23) | 90 (25) | 83 (25) | |
| < 14 | 211 (33) | 119 (29) | 121 (33) | 114 (35) | |
| Missing | 28 (4) | 20 (5) | 23 (6) | 12 (4) | |
| Charlson Comorbidity Index | .03 | ||||
| 0 | 489 (77) | 292 (71) | 291 (80) | 244 (74) | |
| 1 | 111 (17) | 88 (21) | 60 (17) | 68 (21) | |
| 2+ | 35 (6) | 33 (8) | 12 (3) | 16 (5) | |
| Year of diagnosis | < .001 | ||||
| 2003 | 33 (5) | 22 (5) | 8 (2) | 14 (4) | |
| 2004 | 51 (8) | 33 (8) | 12 (3) | 19 (6) | |
| 2005 | 48 (8) | 32 (8) | 13 (4) | 28 (9) | |
| 2006 | 53 (8) | 46 (11) | 15 (4) | 36 (11) | |
| 2007 | 53 (8) | 33 (8) | 45 (12) | 32 (10) | |
| 2008 | 79 (13) | 62 (15) | 62 (17) | 51 (16) | |
| 2009 | 97 (16) | 76 (18) | 55 (15) | 54 (16) | |
| 2010 | 96 (15) | 57 (13) | 76 (21) | 57 (17) | |
| 2011 | 123 (19) | 52 (13) | 77 (21) | 37 (11) | |
| Facility type | < .001 | ||||
| Community Cancer Program | 69 (11) | 22 (5) | 20 (6) | 28 (9) | |
| Comp Comm Cancer Ctr | 304 (48) | 183 (44) | 102 (28) | 146 (45) | |
| Academic/research | 262 (41) | 208 (50) | 239 (66) | 151 (46) | |
| Other | 0 (0) | 0 (0) | 2 (1) | 3 (1) | |
| Facility location | .01 | ||||
| New England | 60 (9) | 22 (5) | 22 (6) | 14 (4) | |
| Middle Atlantic | 90 (14) | 57 (14) | 40 (11) | 53 (16) | |
| South Atlantic | 161 (25) | 88 (21) | 68 (19) | 62 (19) | |
| East North Central | 106 (17) | 75 (18) | 81 (22) | 78 (24) | |
| East South Central | 49 (8) | 31 (8) | 27 (7) | 26 (8) | |
| West North Central | 44 (7) | 30 (7) | 36 (10) | 31 (9) | |
| West South Central | 47 (7) | 29 (7) | 29 (8) | 17 (5) | |
| Mountain | 21 (3) | 20 (5) | 14 (4) | 11 (3) | |
| Pacific | 57 (9) | 61 (15) | 46 (13) | 36 (11) | |
| Facility setting | .15 | ||||
| Rural | 23 (4) | 10 (2) | 8 (2) | 7 (2) | |
| Urban | 97 (15) | 67 (16) | 77 (21) | 64 (20) | |
| Metropolitan | 484 (76) | 315 (76) | 259 (71) | 242 (74) | |
| Missing | 31 (5) | 21 (5) | 19 (5) | 15 (5) | |
| Median distance to facility, miles | 9.9 (4.2-27.6) | 13.8 (5.2-38.1) | 22.5 (7.0-62.4) | 12.9 (5.8-27.0) | < .001 |
| Missing | 18 (3) | 11 (3) | 16 (4) | 7 (2) | |
| Median annual cystectomy volume | 6 (2-16) | 8 (3-16) | 17 (5-33) | 7 (3-14) | < .001 |
| Missing | 5 (1) | 4 (1) | 2 (1) | 2 (1) |
NOTE: Data are presented as mean ± SD or median (interquartile range) for continuous variables and as frequencies (%) for categorical data.
Abbreviations: Comp Comm Cancer Ctr, Comprehensive Community Cancer Center; HS, high school; SD, standard deviation.
Percentage of persons with less than a high-school education within the patient’s census tract of residence.
Table 2.
Tumor Characteristics
| Characteristic | Chemotherapy Only (n = 635) | Cystectomy Only (n = 413) | Cystectomy + Preoperative Chemotherapy (n = 363) | Cystectomy + Adjuvant Chemotherapy (n = 328) | P |
|---|---|---|---|---|---|
| Clinical T stage | < .001 | ||||
| T0 | 1 (0) | 1 (0) | 1 (0) | 0 (0) | |
| T1 | 62 (10) | 13 (3) | 20 (6) | 8 (2) | |
| T2 | 243 (38) | 110 (26) | 131 (36) | 80 (24) | |
| T3 | 112 (18) | 133 (32) | 86 (24) | 107 (33) | |
| T4 | 137 (22) | 109 (26) | 65 (18) | 83 (25) | |
| Tx | 39 (6) | 12 (3) | 13 (4) | 14 (4) | |
| Not applicable | 41 (6) | 35 (8) | 47 (13) | 36 (11) | |
| Clinical N stage | < .001 | ||||
| N1 | 262 (41) | 218 (53) | 200 (55) | 153 (47) | |
| N2 | 309 (49) | 181 (44) | 139 (38) | 161 (49) | |
| N3 | 64 (10) | 14 (4) | 24 (7) | 14 (4) | |
| Pathologic T stage | < .001 | ||||
| T0 | 3 (0) | 3 (1) | 46 (13) | 1 (0) | |
| T1 | 15 (2) | 9 (2) | 15 (4) | 4 (1) | |
| T2 | 73 (11) | 77 (19) | 70 (19) | 49 (15) | |
| T3 | 23 (4) | 188 (46) | 123 (34) | 162 (49) | |
| T4 | 17 (3) | 112 (27) | 55 (15) | 88 (27) | |
| Tx | 427 (67) | 20 (5) | 41 (11) | 18 (5) | |
| Not applicable | 77 (12) | 4 (1) | 13 (4) | 6 (2) | |
| Pathologic N stage | < .001 | ||||
| N0 | 12 (2) | 28 (7) | 133 (37) | 10 (3) | |
| N1 | 31 (5) | 140 (34) | 66 (18) | 109 (33) | |
| N2 | 45 (7) | 194 (47) | 90 (25) | 163 (50) | |
| N3 | 6 (1) | 15 (4) | 23 (6) | 15 (5) | |
| Nx | 465 (73) | 32 (8%) | 38 (10%) | 25 (8%) | |
| Not applicable | 76 (12%) | 4 (1%) | 13 (4%) | 6 (2%) | |
| Surgical margin status | < .001 | ||||
| Negative | 74 (12%) | 305 (74%) | 293 (81%) | 250 (76%) | |
| Positive | 120 (19%) | 87 (21%) | 50 (14%) | 62 (19%) | |
| Not evaluable | 288 (45%) | 9 (2%) | 12 (3%) | 8 (2%) | |
| No surgical procedure | 91 (14%) | 0 (0) | 0 (0) | 0 (0) | |
| Missing | 62 (10%) | 12 (3%) | 8 (2%) | 8 (2%) | |
| Nodes examined | < .001 | ||||
| 0 | 493 (78%) | 13 (3%) | 31 (9%) | 12 (0.04) | |
| 1+ | 85 (13%) | 388 (94%) | 318 (87%) | 311 (0.95) | |
| Median | 0 (0-0) | 9 (4-18) | 15 (6-24) | 10 (5-19) | < .001 |
| By aspiration | 36 (6%) | 0 (0) | 3 (1%) | 0 (0) | |
| Unknown | 21 (3%) | 12 (3%) | 11 (3%) | 5 (0.02) | |
| Median time to cystectomy, months | — | 1.6 (1.0-2.5) | 5.4 (4.3-6.3) | 1.4 (0.8-2.0) | < .001 |
NOTE: Data are presented as median (interquartile range) for continuous variables and as frequencies (%) for categorical data.
Pathologic Response in Patients Treated With Preoperative Chemotherapy
The pathologic N stage was lower in patients undergoing preoperative chemotherapy than in patients undergoing initial cystectomy (Table 2). Among 363 patients treated with preoperative chemotherapy followed by cystectomy, 133 (37%) were pathologic N0 compared with 38 of 741 patients (5%) undergoing initial cystectomy. The multivariable-adjusted odds ratio of achieving pN0 status with preoperative chemotherapy versus initial cystectomy was 11.21 (95% CI, 7.08 to 17.74). A complete pathologic response (ie, pT0N0) was observed in 33 of 363 patients (9%) undergoing preoperative chemotherapy and in 1 patient of 741 (0.1%) treated with initial cystectomy.
Treatment Effectiveness
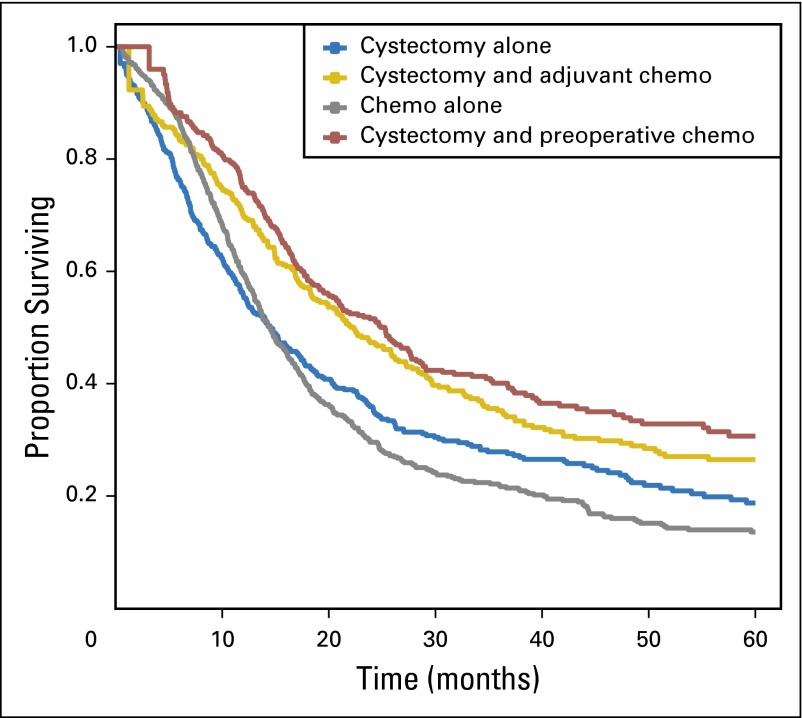
The median follow-up period was 16.6 months (interquartile range, 9.0-31.2 months); 70.3% of the cohort had died within 5 years. The crude 5-year overall survival (Fig 3) for chemotherapy alone, cystectomy alone, preoperative chemotherapy followed by cystectomy, and cystectomy followed by adjuvant chemotherapy was 14% (95% CI, 11% to 17%), 19% (95% CI, 15% to 24%), 31% (95% CI, 25% to 8%), and 26% (95% CI, 21% to 34%), respectively. Multivariable multistate models (Table 3), censored for start of adjuvant chemotherapy, demonstrated that compared with cystectomy, preoperative chemotherapy was associated with an improvement in overall survival (hazard ratio [HR], 0.80; 95% CI, 0.66 to 0.97). The survival benefit with preoperative chemotherapy was associated with pathologic downstaging, because the effect disappeared after adjusting for pathologic stage. Adjuvant chemotherapy was also associated with a significant improvement in survival compared with cystectomy alone (Table 3). Overall survival for patients treated with chemotherapy alone was slightly worse than for patients treated with cystectomy alone: multivariable HR, 1.14 (95% CI, 0.97 to 1.33). This HR, however, should be interpreted as an average HR across time, because the effect appeared to change over time, violating the proportional hazards assumption until approximately 12 months of follow-up (Fig 2). The HR was 0.92 (95% CI, 0.75 to 1.13) until 12 months and 1.54 (95% CI, 1.21 to 1.96) from 12 months onward.
Fig 3.
Overall survival according to treatment scenario. Chemo, chemotherapy.
Table 3.
Multivariable Adjusted HRs for Overall Survival (Reference: Cystectomy With or Without Adjuvant Chemotherapy)
| Analysis | Treatment | Univariable HR (95% CI) | Multivariable HR (95% CI)* |
|---|---|---|---|
| Overall, uncensored | Chemotherapy alone | 1.29 (1.14 to 1.47) | 1.33 (1.17 to 1.53) |
| Preoperative chemotherapy | 0.78 (0.67 to 0.92) | 0.94 (0.79 to 1.12) | |
| Overall, censored for adjuvant chemotherapy | Chemotherapy alone | 1.10 (0.95 to 1.26) | 1.14 (0.97 to 1.33) |
| Preoperative chemotherapy | 0.66 (0.55 to 0.79) | 0.80 (0.66 to 0.97) | |
| Overall, censored for adjuvant chemotherapy, adjusted for pathologic stage and surgical margin status | Preoperative chemotherapy | 0.84 (0.69 to 1.03) | 0.99 (0.80 to 1.22) |
| Cystectomy only, adjusted for pathologic stage and surgical margin status | Adjuvant chemotherapy | 0.65 (0.54 to 0.78) | 0.68 (0.56 to 0.83) |
Abbreviation: HR, hazard ratio.
Multivariable models included age, gender, race, insurance status, median income, education, Charlson Comorbidity Index, year of diagnosis, facility type, facility location, facility setting, distance to facility, annual cystectomy volume, clinical T stage, and clinical N stage.
Interactions of treatment approach with clinical N stage were not significant: P = .18 for preoperative chemotherapy, P = .11 for chemotherapy alone, and P = .28 for adjuvant chemotherapy. Because of changes to the definition of N3 disease with the seventh edition of AJCC staging manual, we repeated our analysis excluding N3 patients diagnosed from 2010 through 2011 (n = 21); removing these patients had no overall impact on the results (Appendix Table A2, online only). Additionally, repeating the analyses in patients with complete data on all confounders (n = 1,614) did not change our findings (Appendix Table A3, online only).
DISCUSSION
After decades with modest progress, there has recently been an explosion of knowledge regarding the biology and treatment of advanced bladder cancer, with identification of distinct subtypes of the disease, recurrent somatic alterations representing novel therapeutic targets, and immune checkpoint blockade as a viable treatment strategy.19-22 Despite such advances, there remains a critical need to refine the use of currently available tools to optimize patient care, particularly in potentially curative settings. In this large observational cohort of real-world patients, we demonstrated that durable disease control is possible in a subset of patients with cN+ disease. Furthermore, we demonstrated that combined-modality therapy, with systemic chemotherapy and surgery, is associated with the best outcomes.
There are several strengths to our study. To our knowledge, this is the largest cohort used to assess the outcomes of patients with cN+ bladder cancer. The NCBD includes data from approximately 70% of newly diagnosed cancer patients in the United States, suggesting a high level of generalizability. Because failure to allow for delayed entry can lead to biased estimates of the effect of preoperative chemotherapy, we used multistate survival analysis allowing various treatment sequences and delayed entry, with time since clinical staging as the timescale.23 We adjusted for a large number of patient-, tumor-, and facility-level variables to further account for differences in survival due to bias by indication.
There are potential limitations to our analysis. First, as with any retrospective study, despite our attempts to comprehensively address sources of bias, the results may be subject to residual confounding. Although propensity scores have been advocated to address confounding by indication for treatment, this method was not used for our analysis, because our study sample included a large number of events relative to the number of confounders. In such a situation, multivariable adjustment is less biased, especially when the strength of the association of the treatment is low, whereas the precision of effect size estimation is similar.24 In addition, we evaluated more than two treatment strategies, complicating the use of propensity scores.25 Although comorbidity index data were available, data regarding other potential confounders, particularly performance status, are not captured by the NCDB and could have biased the results in favor of combined-modality therapy. However, prior studies have demonstrated that performance status does not add independent prognostic information beyond comorbidity indices, and in a study of patients undergoing cystectomy, prognostic models incorporating comorbidity index outperformed models incorporating performance status in predicting survival.26,27 Furthermore, the finding that the survival benefit conferred by preoperative chemotherapy was associated with pathologic downstaging (ie, establishing a mechanistic link), coupled with the finding that chemotherapy was associated with improved survival regardless of the treatment sequence (in a group of patients who were all sufficiently fit to undergo cystectomy), suggests that the results are unlikely to predominantly reflect the impact of unmeasured confounders.
Second, only limited data are available regarding the proportion of patients with cN1-3 disease who underwent biopsy confirmation of regional nodal metastases. However, this does represent a real-world cohort, and among the 741 patients treated with initial cystectomy, only 5% had pN0 disease, suggesting a relatively low frequency of misclassification. Third, although single-agent versus multiagent chemotherapy is recorded in the NCBD, specific chemotherapy regimens, doses, and treatment durations are not included. Fourth, we did not include patients treated with radiation in our analysis because the administration of concurrent chemotherapy with radiation cannot be definitively ascertained on the basis of NCDB coding; radiation could also potentially play a role in the combined-modality treatment of patients with cN+ bladder cancer. Finally, cancer recurrence and cancer-specific survival data are not captured in the NCDB, precluding assessment of these end points.
Other studies, using different datasets and methodologies, have also suggested a benefit with combined-modality therapy for patients with bladder cancer and clinical evidence of regional nodal involvement.11-14 The current analysis adds to this literature, using, to our knowledge, the largest cohort to compare the effectiveness of various treatment strategies for cN+ disease derived from a registry representing the majority of incident cancer diagnoses in the United States, captured across the continuum of practice settings and employing strategies to address confounding.
Although the current study supports combined-modality therapy for patients with cN+ disease, the optimal sequence and modalities remain incompletely defined. Even though our results suggest a survival benefit with either chemotherapy used before cystectomy or in the adjuvant setting, the analysis was not designed to compare these approaches directly. Initial chemotherapy does offer practical advantages, including the potential to spare patients with rapid disease progression and limited longevity the morbidity of cystectomy. In fact, the poor outcomes observed with chemotherapy alone likely reflect, at least in part, inclusion of a mix of patients in whom chemotherapy alone was the recommended treatment approach and patients who did not achieve a radiographic response to chemotherapy and were therefore not considered for surgical consolidation. Whether patients should be offered cystectomy, regardless of response to initial chemotherapy, cannot be determined by our analysis.
The mechanistic basis for improved survival with surgical resection of the primary tumor, in the context of metastatic disease, is unclear, although several hypotheses have been proposed. These mechanisms include (1) preventing local recurrence, (2) removing the major reservoir of ongoing waves of metastases, (3) disrupting tumor self-seeding28, and (4) restoring antitumor immunity through reducing tumor burden.29 Indeed, there are compelling data in model systems, as well as data in patients, lending support to each of these potential mechanisms, and they are unlikely to be mutually exclusive.28,29
Evidence to guide the care of patients with cancer is ideally generated in the setting of prospective randomized controlled trials. Unfortunately, conducting prospective trials to address every important scenario in the care of patients may be impractical, especially for less common malignancies, and observational analyses may help address critical knowledge gaps.30 Neoadjuvant chemotherapy trials in patients with muscle-invasive bladder cancer have historically excluded patients with cN+ disease, with the exception of a few small phase II trials.31,32 Rather, patients with cN+ disease have been included in trials exploring first-line chemotherapy regimens for patients with distant metastatic disease. Although this is a pragmatic approach, such trials are not designed specifically to address the optimal treatment of patients with cN+ disease and simultaneously affect the overall interpretation of these trials by driving the tail on the survival curve. Multidisciplinary collaboration is needed to explore the feasibility of prospective trials solely for cN+ patients and/or to define the optimal setting in which to further study this important disease state.
In summary, in this large observational study, a subset of patients with cN+ bladder cancer achieved long-term survival, and combined-modality therapy was associated with the best outcomes. Collaborative efforts are needed to refine the care of patients with cN+ bladder cancer, a disease state heretofore lacking a solid evidence base to guide management. In the meantime, our results lend support to the use of combined-modality therapy to optimize the likelihood of cure.
Appendix
Table A1.
American Joint Committee on Cancer and International Union for Cancer Control Regional Nodal Staging of Bladder Cancer
| Sixth Edition | Seventh Edition | |
|---|---|---|
| Location | Lymph nodes in the true pelvis | Lymph nodes in the true pelvis or common iliacs |
| Clinical N1 | Single lymph node ≥ 2 cm | Single lymph node metastasis in the true pelvis |
| Clinical N2 | Single lymph node 2-5 cm or multiple lymph nodes < 5 cm | Multiple lymph node metastases in the true pelvis |
| Clinical N3 | Lymph node > 5 cm | Metastases to the common iliac lymph nodes |
Table A2.
Multivariable Adjusted HRs for Overall Survival (Reference: Cystectomy With or Without Adjuvant Chemotherapy) Excluding N3 Patients (n = 21) Diagnosed From 2010 Through 2011 (Total N = 1,718)
| Analysis | Treatment | Univariable HR (95% CI) | Multivariable HR (95% CI)* |
|---|---|---|---|
| Overall, uncensored | Chemotherapy alone | 1.30 (1.15 to 1.48) | 1.34 (1.17 to 1.54) |
| Preoperative chemotherapy | 0.79 (0.67 to 0.93) | 0.95 (0.79 to 1.13) | |
| Overall, censored for adjuvant chemotherapy | Chemotherapy alone | 1.10 (0.95 to 1.27) | 1.15 (0.98 to 1.34) |
| Preoperative chemotherapy | 0.66 (0.55 to 0.80) | 0.80 (0.66 to 0.98) | |
| Overall, censored for adjuvant chemotherapy, adjusted for pathologic stage and surgical margin status | Preoperative chemotherapy | 0.84 (0.69 to 1.03) | 1.00 (0.81 to 1.23) |
| Cystectomy only, adjusted for pathologic stage and surgical margin status | Adjuvant chemotherapy | 0.64 (0.54 to 0.78) | 0.68 (0.56 to 0.83) |
Abbreviation: HR, hazard ratio.
Multivariable models included age, gender, race, insurance status, median income, education, Charlson Comorbidity Index, year of diagnosis, facility type, facility location, facility setting, distance to facility, annual cystectomy volume, clinical T stage, and clinical N stage.
Table A3.
Multivariable HRs for Overall Survival (Reference: Cystectomy With or Without Adjuvant Chemotherapy) in Complete Cases (Total N = 1,614)
| Analysis | Treatment | Univariable HR (95% CI) | Multivariable HR (95% CI)* |
|---|---|---|---|
| Overall, uncensored | Chemotherapy alone | 1.29 (1.14 to 1.47) | 1.37 (1.20 to 1.58) |
| Preoperative chemotherapy | 0.78 (0.67 to 0.92) | 0.94 (0.78 to 1.13) | |
| Overall, censored for adjuvant chemotherapy | Chemotherapy alone | 1.10 (0.95 to 1.26) | 1.19 (1.01 to 1.40) |
| Preoperative chemotherapy | 0.66 (0.55 to 0.79) | 0.81 (0.66 to 1.00) | |
| Overall, censored for adjuvant chemotherapy, adjusted for pathologic stage and surgical margin status | Preoperative chemotherapy | 0.84 (0.69 to 1.03) | 1.01 (0.81 to 1.27) |
| Cystectomy only, adjusted for pathologic stage and surgical margin status | Adjuvant chemotherapy | 0.65 (0.54 to 0.78) | 0.71 (0.58 to 0.87) |
Abbreviation: HR, hazard ratio.
Multivariable models included age, gender, race, insurance status, median income, education, Charlson Comorbidity Index, year of diagnosis, facility type, facility location, facility setting, distance to facility, annual cystectomy volume, clinical T stage, and clinical N stage.
Footnotes
Supported by Grant No. 1R21CA176551-01A1 (M.D.G., M.D., N.M., and B.F.); National Cancer Institute Cancer Center Support Grant P30 CA196521-01 (B.F. and M.M.); and Biostatistics Shared Resource Facility, Icahn School of Medicine at Mount Sinai.
Presented at the 52nd ASCO Annual Meeting, held June 4-6, 2016, in Chicago, IL.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
The data used in the study are derived from a deidentified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the investigator.
AUTHOR CONTRIBUTIONS
Conception and design: Matthew D. Galsky, Bart Ferket
Financial support: Matthew D. Galsky
Administrative support: Matthew D. Galsky
Provision of study materials or patients: Matthew D. Galsky
Collection and assembly of data: Matthew D. Galsky, Kristian Stensland, Bart Ferket
Data analysis and interpretation: Matthew D. Galsky, John P. Sfakianos, Reza Mehrazin, Michael Diefenbach, Nihal Mohamed, Che-Kai Tsao, Paolo Boffetta, Peter Wiklund, William K. Oh, Madhu Mazumdar, Bart Ferket
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Comparative Effectiveness of Treatment Strategies for Bladder Cancer With Clinical Evidence of Regional Lymph Node Involvement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Matthew D. Galsky
Stock or Other Ownership: Dual Therapeutics
Consulting or Advisory Role: BioMotiv, Janssen Pharmaceuticals, Dendreon, Merck, GlaxoSmithKline, Eli Lilly, Astellas Pharma, Genentech
Research Funding: Janssen Oncology (Inst), Dendreon (Inst), Novartis (Inst), Bristol-Myers Squibb (Inst), Merck (Inst)
Patents, Royalties, Other Intellectual Property: Methods and Compositions for Treating Cancer and Related Methods (Inst)
Travel, Accommodations, Expenses: BioMotiv, Merck, Dendreon, Astellas Pharma
Kristian Stensland
No relationship to disclose
John P. Sfakianos
No relationship to disclose
Reza Mehrazin
No relationship to disclose
Michael Diefenbach
No relationship to disclose
Nihal Mohamed
No relationship to disclose
Che-Kai Tsao
Consulting or Advisory Role: Genentech
Paolo Boffetta
No relationship to disclose
Peter Wiklund
Honoraria: Intuitive Surgical
Research Funding: Intuitive Surgical (Inst)
Travel, Accommodations, Expenses: Intuitive Surgical
William K. Oh
No relationship to disclose
Madhu Mazumdar
No relationship to disclose
Bart Ferket
No relationship to disclose
REFERENCES
- 1. Bochner BH, Kattan MW, Vora KC: Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol 24:3967-3972, 2006. [DOI] [PubMed]
- 2.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 3.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 48:202–205, 2005; discussion 205-206. doi: 10.1016/j.eururo.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 4. Griffiths G, Hall R, Sylvester R, et al: International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Long-term results of the BA06 30894 trial. J Clin Oncol 29:2171-2177, 2011. [DOI] [PMC free article] [PubMed]
- 5.Galsky MD, Stensland KD, Moshier E, et al. Effectiveness of adjuvant chemotherapy for locally advanced bladder cancer. J Clin Oncol. 2016;34:825–832. doi: 10.1200/JCO.2015.64.1076. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): An intergroup, open-label, randomised phase 3 trial. Lancet Oncol. 2015;16:76–86. doi: 10.1016/S1470-2045(14)71160-X. [DOI] [PubMed] [Google Scholar]
- 7. Advanced Bladder Cancer (ABC) Meta-analysis Collaboration: Adjuvant chemotherapy in invasive bladder cancer: A systematic review and meta-analysis of individual patient data Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur Urol 48:189-201, 2005. [DOI] [PubMed]
- 8. Von der Maase H, Sengelov L, Roberts JT, et al: Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23:4602-4608, 2005. [DOI] [PubMed]
- 9.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 10. Bellmunt J, von der Maase H, Mead GM, et al: Randomized phase III study comparing paclitaxel/cisplatin/ gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol 30:1107-1113, 2012. [DOI] [PMC free article] [PubMed]
- 11.Dodd PM, McCaffrey JA, Herr H, et al. Outcome of postchemotherapy surgery after treatment with methotrexate, vinblastine, doxorubicin, and cisplatin in patients with unresectable or metastatic transitional cell carcinoma. J Clin Oncol. 1999;17:2546–2552. doi: 10.1200/JCO.1999.17.8.2546. [DOI] [PubMed] [Google Scholar]
- 12.Herr HW, Donat SM, Bajorin DF. Post-chemotherapy surgery in patients with unresectable or regionally metastatic bladder cancer. J Urol. 2001;165:811–814. [PubMed] [Google Scholar]
- 13.Ho PL, Willis DL, Patil J, et al. Outcome of patients with clinically node-positive bladder cancer undergoing consolidative surgery after preoperative chemotherapy: The M.D. Anderson Cancer Center Experience. Urol Oncol. 2016;34:59.e1–59.e8. doi: 10.1016/j.urolonc.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Zargar-Shoshtari K, Zargar H, Lotan Y, et al. A multi-institutional analysis of outcomes of patients with clinically node positive urothelial bladder cancer treated with induction chemotherapy and radical cystectomy. J Urol. 2016;195:53–59. doi: 10.1016/j.juro.2015.07.085. [DOI] [PubMed] [Google Scholar]
- 15.Steele GD, Jr, Winchester DP, Menck HR. The National Cancer Data Base. A mechanism for assessment of patient care. Cancer. 1994;73:499–504. doi: 10.1002/1097-0142(19940115)73:2<499::aid-cncr2820730241>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 16. Edge SB, Compton CC: The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–1474, 2010. [DOI] [PubMed] [Google Scholar]
- 17.White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28:1982–1998. doi: 10.1002/sim.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng X-L Rubin DB. Performing likelihood ratio tests with multiply-imputed data sets. Biometrika. 1992;79:103–111. [Google Scholar]
- 19.Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–165. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci USA. 2014;111:3110–3115. doi: 10.1073/pnas.1318376111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. doi: 10.1038/nature12965. The Cancer Genome Atlas Research Network: Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507:315-322, 2014 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 23.Wolkewitz M, Allignol A, Harbarth S, et al. Time-dependent study entries and exposures in cohort studies can easily be sources of different and avoidable types of bias. J Clin Epidemiol. 2012;65:1171–1180. doi: 10.1016/j.jclinepi.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Cepeda MS, Boston R, Farrar JT, et al. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol. 2003;158:280–287. doi: 10.1093/aje/kwg115. [DOI] [PubMed] [Google Scholar]
- 25.McCaffrey DF, Griffin BA, Almirall D, et al. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32:3388–3414. doi: 10.1002/sim.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobbins TA, Badgery-Parker T, Currow DC, et al. Assessing measures of comorbidity and functional status for risk adjustment to compare hospital performance for colorectal cancer surgery: A retrospective data-linkage study. BMC Med Inform Decis Mak. 2015;15:55. doi: 10.1186/s12911-015-0175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boorjian SA, Kim SP, Tollefson MK, et al. Comparative performance of comorbidity indices for estimating perioperative and 5-year all cause mortality following radical cystectomy for bladder cancer. J Urol. 2013;190:55–60. doi: 10.1016/j.juro.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Comen E, Norton L, Massagué J. Clinical implications of cancer self-seeding. Nat Rev Clin Oncol. 2011;8:369–377. doi: 10.1038/nrclinonc.2011.64. [DOI] [PubMed] [Google Scholar]
- 29.Danna EA, Sinha P, Gilbert M, et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205–2211. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- 30.Booth CM, Tannock IF. Randomised controlled trials and population-based observational research: Partners in the evolution of medical evidence. Br J Cancer. 2014;110:551–555. doi: 10.1038/bjc.2013.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choueiri TK, Jacobus S, Bellmunt J, et al. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: Pathologic, radiologic, and biomarker correlates. J Clin Oncol. 2014;32:1889–1894. doi: 10.1200/JCO.2013.52.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grivas PD, Hussain M, Hafez K, et al. A phase II trial of neoadjuvant nab-paclitaxel, carboplatin, and gemcitabine (ACaG) in patients with locally advanced carcinoma of the bladder. Urology. 2013;82:111–117. doi: 10.1016/j.urology.2013.03.044. [DOI] [PubMed] [Google Scholar]