Abstract
Purpose
Obesity after a diagnosis of specific cancers has been associated with worse prognosis. We examined the trend in obesity prevalence among cancer survivors in the United States in the past two decades and compared trends with those of adults without a history of cancer.
Patients and Methods
This was a population-based nationally representative sample of 538,969 noninstitutionalized US adults 18 to 85 years old with and without a history of cancer who participated in annual cross-sectional National Health Interview Surveys from 1997 to 2014. Obesity was defined as body mass index ≥ 30 kg/m2 for non-Asians and body mass index ≥ 27.5 kg/m2 for Asians.
Results
Among 32,447 cancer survivors identified, the most common cancer diagnoses were breast (n = 6,948), prostate (n = 3,984), and colorectal (n = 2,546). From 1997 to 2014, the prevalence of obesity increased from 22.4% to 31.7% in cancer survivors and from 20.9% to 29.5% in adults without a history of cancer (P for trend < .001, both groups). Over this period, the estimated rate of annual increase in obesity prevalence was higher in women and men with a history of cancer compared with those without a history of cancer (all P for interaction < .001). The estimated rate of annual increase in obesity prevalence was 3.1% in female and 3.7% in male colorectal cancer survivors, 3.0% in breast cancer survivors, and 2.1% in prostate cancer survivors (all P < .001). In subgroup analyses, populations with the highest rates of increasing obesity burden were colorectal cancer survivors, breast cancer survivors, and non-Hispanic blacks.
Conclusion
From 1997 to 2014, obesity increased more rapidly among adult cancer survivors compared with the general population. Colorectal and breast cancer survivors and non-Hispanic blacks were identified as being at the highest risk for obesity.
INTRODUCTION
Obesity is a major public health issue associated with increased morbidity and mortality in the United States and globally.1-3 According to the most recent US obesity trends analysis, the prevalence of obesity escalated from 1980 to 2000 and continued to increase among non-Hispanic black and Mexican-American women from 1999 to 2010.4 By 2030, the continued increase in obesity in the United States and the United Kingdom is projected to cause an additional 6 to 8.5 million new diagnoses of diabetes and 5.7 to 7.3 million new diagnoses of heart disease and stroke, and a loss of 26 to 55 million quality-adjusted life-years.5
Obesity has also been associated with cancer incidence, progression, and survival, especially in hormonally and metabolically driven cancers such as breast and colorectal cancer.6-9 The projected increases in population-based obesity rates likely pose additional health hazards for cancer survivors. For example, recent studies suggest that weight at the time of breast cancer diagnosis and weight gain after diagnosis are both associated with higher cancer mortality and all-cause mortality.10-12 Although patients with cancer and cancer survivors are subject to similar energy-balance–related causes of weight gain as is the general population of the United States, they are also at risk for increased weight gain caused by specific cancer treatments, including chemotherapy, steroid medications, and hormonal therapy.13,14 Several national organizations recommend weight control for cancer survivors,15,16 and a recent ASCO position statement encouraged oncology care providers to address weight management among their patients.9 Whether US cancer survivors have experienced different rates of changes in obesity burden compared with adults without a history of cancer has not been evaluated. Large population-based studies are needed to evaluate whether a distinct obesity pattern and trajectory exist in cancer survivors.
To better inform future research on obesity and cancer survival and to inform the planning and implementation of weight-loss interventions in cancer survivors,17 we compared trends from 1997 to 2014 in obesity prevalence in US adults with and without a history of cancer. We hypothesized that the prevalence of obesity would increase over time among adults both with and without a history of cancer, and that the amount of annual increase would be greater in adults with a history of cancer. To identify populations of cancer survivors at the highest risk for obesity, we examined trends in obesity prevalence among cancer survivors by cancer type, age, race/ethnicity, and time since diagnosis.
PATIENTS AND METHODS
Study Participants
The analysis used data from adult participants 18 to 85 years of age in the annual 1997 to 2014 waves of the National Health Interview Survey (NHIS). The NHIS is an ongoing cross-sectional survey of the health status, health care access, and behaviors of the US civilian noninstitutionalized population conducted by the National Center for Health Statistics. Details on the NHIS sample design are published elsewhere.18 Briefly, each year, the NHIS uses multistage probability sampling to select 428 primary sampling units from approximately 1,900 geographically defined primary sampling units covering the 50 states and the District of Columbia. Approximately 41,000 households and 107,000 individuals are selected each year. The NHIS has used two sets of questionnaires since 1997: (1) a set of basic health and demographic items (ie, the Core questionnaire) for all individuals living in a house and (2) one or more sets of questions on current health topics (ie, the Supplements) for one adult and one child, each randomly selected from each house. The Cancer Screening questionnaire has been included as a supplement every year since 1997. The NHIS annual response rate is approximately 80%. In this analysis, we used data from adults who responded to both the Core questionnaire and the Cancer Screening supplement. All NHIS activities undergo human subject oversight, and participants provide informed consent.
Cancer History
Interviewers asked participants if a physician or other health professional had ever informed them that they had cancer, and if the answer was yes, in what year the cancer diagnosis occurred. Cancer survivors included individuals who reported a cancer diagnosis at any age and excluded those who reported only nonmelanoma skin cancers.
Obesity
Self-reported height and weight were used to compute body mass index (BMI, kg/m2). BMI was categorized as underweight (BMI < 18.5 kg/m2), normal weight (BMI, 18.5 to < 25 kg/m2), overweight (BMI, 25 to < 30 kg/m2), class I obesity (BMI, 30 to < 35 kg/m2), and class II/III obesity (BMI ≥ 35 kg/m2).19 For Asians, the WHO Asian cut points were applied for underweight (BMI < 23 kg/m2), overweight (BMI, 23 to < 27.5 kg/m2), and obese (class I, BMI ≥ 27.5 kg/m2 and class II/III, BMI ≥ 32.5 kg/m2) participants.20 In analyses of obesity prevalence, we dichotomized BMI as nonobese and obese (BMI < 30 kg/m2 v ≥ 30 kg/m2 for non-Asians; BMI < 27.5 kg/m2 v ≥ 27.5 kg/m2 for Asians).
Demographic and Behavioral Information
Participants provided self-reported information on age, sex, race/ethnicity, education, employment, family income, health insurance coverage, nation of birth, current smoking behavior, current alcohol use, physical activity, and noncancer chronic conditions.
Statistical Analyses
NHIS-constructed survey weights were applied to account for the NHIS complex stratified sampling methods and to make estimates representative of the US civilian noninstitutionalized adult population.18 To compare population characteristics over time, survey participants were grouped into six consecutive 3-year samples (1997 to 1999, 2000 to 2002, 2003 to 2005, 2006 to 2008, 2009 to 2011, and 2012 to 2014). In descriptive analyses for each sample, Pearson’s χ2 tests were used to compare demographic and health characteristics of cancer survivors and cancer-free participants; a Bonferroni correction was used to account for multiple comparisons.21 To examine the differences in obesity prevalence between cancer survivors and cancer-free participants, the age-standardized prevalence of obesity was calculated for each sample using the 2000 US population for appropriate comparisons between the survey years.22 Cochrane-Armitage trend tests were used to determine whether demographic and health characteristics of the population changed over time for each group. Changes from the first to the last survey were compared between the two groups using Z-tests. To visually illustrate changes in obesity prevalence over time, we calculated the age-standardized prevalence of obesity for each survey year by sex and cancer status and displayed the results as smoothed lines using locally weighted scatterplot smoothing.23 The same method was used to display the prevalence of adults with one or more chronic condition(s) by cancer and BMI category.
Individual-level data on obesity status were aggregated by survey year, sex, age group, and cancer status, accounting for survey weights. These data included the estimated number of obese and nonobese adults by survey year, sex, age group, and cancer status. The aggregate data were used to analyze the prevalence of obesity over time, and the unit of analysis was the count of obese and nonobese individuals in the population for each year-sex-age-cancer group. Poisson regression was used to estimate the rate ratio because the distribution of the primary outcome (ie, the number of obese individuals in a specific population) is a discrete frequency distribution that counts the number of events occurring in a fixed period of time. We estimated the relative increase in obesity prevalence per year using Poisson regression adjusting for age, and fit models separately by sex and cancer type. We tested the interaction between year and cancer status to test for differential annual increases in obesity prevalence between all cancer survivors compared with participants without a history of cancer. We also separately tested the interaction between year and cancer status for survivors of each of the most common cancers, including colorectal, breast, and prostate cancer. Subgroup analyses were conducted by age group, race/ethnicity, and length of cancer survivorship.
All tests of significance were two tailed, with α = .05. Analyses were performed using R (version 3.2.4; https://cran.r-project.org/). All analyses used the R survey package24,25 to apply the complex survey design.
RESULTS
Participant Characteristics
The characteristics of 32,447 adult cancer survivors and 506,522 adults without a history of cancer are summarized in Table 1 by NHIS participation time period and cancer history status. Over the 18-year period, the proportion of US adults with a history of cancer increased steadily (5.1% between 1997 and 1999, 5.3% between 2000 and 2002, 5.5% between 2003 and 2005, 5.6% between 2006 and 2008, 6.2% between 2009 and 2011, and 6.2% between 2012 and 2014). In almost all periods, compared with adults without a history of cancer, cancer survivors were more likely to be female, non-Hispanic white, have been born in the United States, have higher education attainment, not currently employed, have lower incomes, have health insurance, be a heavy smoker, a light-to-moderate drinker, and have more than one chronic condition (all P values < .001).
Table 1.
Sample Characteristics by Year
| Characteristic | 1997-1999a | 2000-2002 | 2003-2005 | 2006-2008 | 2009-2011 | 2012-2014 | Difference Between 2012-2014 and 1997-1999 (95% CI)b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer | Noncancer | Cancer | Noncancer | Cancer | Noncancer | Cancer | Noncancer | Cancer | Noncancer | Cancer | Noncancer | Cancer | Noncancer | |
| Sample size | 5,230 | 91,981 | 5,194 | 89,464 | 5,389 | 85,963 | 4,027 | 63,639 | 5,530 | 79,945 | 7,077 | 95,530 | ||
| Projected US population, 1,000sc | 29,664 | 552,872 | 31,600 | 569,699 | 35,010 | 599,114 | 36,982 | 618,169 | 41,576 | 632,498 | 43,437 | 652,916 | ||
| Female, % | 63.1 | 47.1 | 66.4 | 47.1 | 64.6 | 46.6 | 64.2 | 46.3 | 65.2 | 47.2 | 62.2 | 47.1 | −0.92 (−0.95 to −0.90) | 0.01 (0.01 to 0.02) |
| Race/ethnicity, % | ||||||||||||||
| Non-Hispanic white | 87.3 | 78.1 | 89.2 | 76.1 | 86.6 | 74.2 | 83.9 | 72.4 | 82.1 | 70.8 | 80.7 | 69.0 | −6.56 (−6.57 to −6.54) | −9.12 (−9.12 to −9.11) |
| Non-Hispanic black | 7.1 | 10.5 | 5.8 | 11.5 | 5.7 | 11.2 | 8.7 | 11.3 | 8.8 | 11.4 | 6.4 | 11.4 | −0.60 (−0.62 to −0.59) | 0.90 (0.90 to 0.90) |
| Hispanic | 4.9 | 9.7 | 4.7 | 10.4 | 6.8 | 12.4 | 5.6 | 13.5 | 8.2 | 14.6 | 10.7 | 16.1 | 5.83 (5.82 to 5.85) | 6.41 (6.41 to 6.41) |
| Asian | 0.8 | 1.8 | 0.3 | 2.1 | 0.8 | 2.2 | 1.8 | 2.7 | 1.0 | 3.2 | 2.1 | 3.6 | 1.33 (1.33 to 1.34) | 1.81 (1.81 to 1.81) |
| Education, % | ||||||||||||||
| High school graduate or less | 42.3 | 44.7 | 43.1 | 43.7 | 39.0 | 41.6 | 37.0 | 41.3 | 32.6 | 37.2 | 32.7 | 34.8 | −9.52 (−9.54 to −9.49) | −9.89 (−9.89 to −9.88) |
| Associate's degree/some college | 33.8 | 30.7 | 34.5 | 30.9 | 33.3 | 31.1 | 32.9 | 31.1 | 37.8 | 32.5 | 36.0 | 32.7 | 2.23 (2.21 to 2.26) | 2.00 (2.00 to 2.01) |
| Bachelor's degree and above | 24.0 | 24.7 | 22.5 | 25.4 | 27.8 | 27.3 | 30.1 | 27.7 | 29.6 | 30.3 | 31.2 | 32.6 | 7.28 (7.26 to 7.30) | 7.89 (7.88 to 7.89) |
| Employment, % | ||||||||||||||
| Unemployed | 86.3 | 89.4 | 80.9 | 87.2 | 81.2 | 87.0 | 79.7 | 86.7 | 78.5 | 84.1 | 80.8 | 85.7 | −5.50 (−5.52 to −5.48) | −3.67 (−3.67 to −3.67) |
| Employed | 0.4 | 0.2 | 2.2 | 2.4 | 4.5 | 3.3 | 3.6 | 3.0 | 6.3 | 5.7 | 5.0 | 4.4 | 4.70 (4.69 to 4.71) | 4.21 (4.21 to 4.21) |
| Not in labor force | 13.4 | 10.4 | 16.9 | 10.4 | 14.3 | 9.7 | 16.7 | 10.3 | 15.2 | 10.1 | 14.2 | 9.9 | 0.81 (0.79 to 0.83) | −0.55 (−0.55 to −0.54) |
| Income < $20,000, %d | 50.9 | 45.5 | 49.5 | 41.0 | 41.8 | 37.6 | 42.3 | 35.9 | 41.2 | 35.3 | 37.6 | 33.3 | −13.22 (−13.24 to −13.20) | −12.14 (−12.14 to −12.13) |
| No health insurance, %e | 8.6 | 9.3 | 12.0 | 12.0 | 12.6 | 13.7 | 14.0 | 15.0 | 15.3 | 16.5 | 13.9 | 15.2 | 5.26 (5.25 to 5.28) | 5.89 (5.88 to 5.89) |
| US born, % | 95.0 | 89.2 | 94.9 | 87.6 | 93.9 | 85.7 | 94.5 | 85.3 | 91.6 | 83.9 | 91.4 | 82.4 | −3.57 (−3.58 to −3.55) | −6.82 (−6.83 to −6.82) |
| Smoking history, %f | ||||||||||||||
| Current, heavy smoking | 23.1 | 20.5 | 28.0 | 20.0 | 23.8 | 18.0 | 24.3 | 17.7 | 19.3 | 15.8 | 17.1 | 13.8 | −5.97 (−5.99 to −5.95) | −6.63 (−6.63 to −6.62) |
| Current, light-moderate smoking | 3.1 | 4.5 | 4.8 | 4.2 | 5.8 | 4.4 | 3.9 | 4.5 | 3.2 | 4.7 | 4.4 | 4.0 | 1.30 (1.29 to 1.31) | −0.48 (−0.49 to −0.48) |
| Former smoker | 29.4 | 24.5 | 23.4 | 23.7 | 26.8 | 22.3 | 27.7 | 21.8 | 26.8 | 21.7 | 26.1 | 21.9 | −3.30 (−3.32 to −3.28) | −2.54 (−2.54 to −2.53) |
| Never-smoker | 44.5 | 50.6 | 43.8 | 52.0 | 43.6 | 55.3 | 44.1 | 56.0 | 50.7 | 57.8 | 52.4 | 60.2 | 7.97 (7.94 to 7.99) | 9.65 (9.64 to 9.65) |
| Alcohol use, %g | ||||||||||||||
| Current, heavy drinking | 19.4 | 23.4 | 20.0 | 22.3 | 21.0 | 22.8 | 25.0 | 25.0 | 21.2 | 26.4 | 27.2 | 28.3 | 7.75 (7.73 to 7.77) | 4.89 (4.89 to 4.90) |
| Current, light-moderate drinking | 49.5 | 45.6 | 45.8 | 45.5 | 48.1 | 45.3 | 47.4 | 44.5 | 49.0 | 45.7 | 47.0 | 44.0 | −2.49 (−2.51 to −2.46) | −1.59 (−1.60 to −1.59) |
| Former drinker | 17.7 | 13.9 | 18.5 | 14.0 | 14.6 | 13.0 | 14.7 | 13.5 | 16.4 | 12.3 | 15.7 | 11.5 | −2.08 (−2.09 to −2.06) | −2.42 (−2.42 to −2.42) |
| Never-drinker | 13.3 | 17.1 | 15.7 | 18.3 | 16.2 | 18.9 | 12.9 | 17.0 | 13.5 | 15.6 | 10.1 | 16.2 | −3.19 (−3.21 to −3.18) | −0.89 (−0.89 to −0.88) |
| Meets physical activity guidelines, %h | 31.5 | 30.1 | 29.8 | 31.4 | 29.0 | 30.3 | 29.2 | 30.6 | 37.7 | 35.3 | 36.1 | 38.3 | 4.60 (4.57 to 4.62) | 8.12 (8.12 to 8.13) |
| No. chronic conditionsi | ||||||||||||||
| 0 | 66.4 | 75.6 | 54.0 | 67.6 | 60.6 | 72.7 | 52.4 | 66.1 | 54.5 | 69.9 | 55.1 | 71.8 | −11.33 (−11.36 to −11.31) | −3.81 (−3.81 to −3.80) |
| 1 | 18.7 | 15.6 | 24.8 | 20.1 | 22.5 | 17.2 | 23.6 | 21.1 | 24.6 | 19.0 | 24.9 | 18.6 | 6.14 (6.13 to 6.16) | 2.99 (2.98 to 2.99) |
| ≥ 2 | 14.9 | 8.8 | 21.2 | 12.3 | 16.9 | 10.1 | 23.9 | 12.9 | 21.0 | 11.1 | 20.1 | 9.7 | 5.19 (5.17 to 5.20) | 0.82 (0.82 to 0.82) |
| BMI (kg/m2), % | ||||||||||||||
| 18.5 | 8.2 | 6.0 | 8.8 | 5.5 | 6.3 | 5.2 | 7.0 | 5.1 | 6.6 | 4.9 | 7.0 | 4.7 | −1.14 (−1.15 to −1.13) | −1.36 (−1.36 to −1.36) |
| 18.5-24.9 | 33.9 | 36.4 | 32.1 | 34.0 | 35.4 | 32.4 | 31.5 | 31.0 | 32.4 | 30.3 | 30.6 | 29.9 | −3.31 (−3.33 to −3.29) | −6.56 (−6.56 to −6.55) |
| 25-29.9 | 35.5 | 36.7 | 35.2 | 36.9 | 31.4 | 37.3 | 32.7 | 36.7 | 31.9 | 36.3 | 30.6 | 36.0 | −4.90 (−4.92 to −4.87) | −0.68 (−0.68 to −0.67) |
| ≥ 30 | 22.4 | 20.9 | 24.0 | 23.6 | 26.9 | 25.2 | 28.8 | 27.3 | 29.0 | 28.5 | 31.7 | 29.5 | 9.35 (9.33 to 9.37) | 8.59 (8.58 to 8.59) |
Abbreviation: BMI, body mass index.
Pearson’s χ2 test results between adults with and without cancer within each period were statistically significant (all P < .001 after Bonferroni correction) for sex, race/ethnicity, education, employment, income, insurance, nativity, current smoking, and current drinking.
Trend test for proportions over time showed statistically significant trends in all sample characteristics (all P < .001 after Bonferroni correction). Z-tests were used to compare changes in characteristics between populations with and without cancer for the first (1997-1999) and last (2012-2014) periods; there were statistically significant differences for all characteristics (all P < .001 after Bonferroni correction), expect for the proportion of employed adults.
The projected size of populations with and without cancer were estimated using National Health Interview Survey complex survey design and cancer survey weights.
Individuals’ total incomes in the previous year, including hourly wages, salaries, tips, and commissions.
Health insurance included self-reported Medicaid, Medicare, private insurance, insurance offered at workplace, military health care/Veterans Affairs insurance, Medi-Gap, and Indian Health Service insurance.
Smoking: current smoking was defined as self-reported smoking at the time of interview, heavy smoking was defined as smoking every day, and light-moderate smoking was defined as currently smoking less than every day.
Alcohol use: current alcohol use was defined as having at least 12 drinks of any type of alcoholic beverage in the past year, heavy drinking was defined as having at least 1 day with five or more drinks during the past year, and light-moderate drinking was defined as any current alcohol use that was less than heavy drinking.
Physical activity: adults classified as partaking in regular leisure-time activity reported at least three sessions per week of vigorous leisure-time physical activity lasting at least 20 minutes in duration or at least five sessions per week of light/moderate physical activity lasting at least 30 minutes in duration.26
Self-reported noncancer chronic conditions included angina pectoris, cerebral palsy, coronary heart disease, congenital heart disease, noncongenital heart condition, heart attack, stroke, other heart condition, asthma, chronic bronchitis, emphysema, chronic obstructive pulmonary disease, asthma attack, arthritis, diabetes, hepatitis, liver condition, and weak/failing kidney. The number of chronic conditions other than cancer was categorized into 0, 1, and ≥ 2.27
Obesity Trends
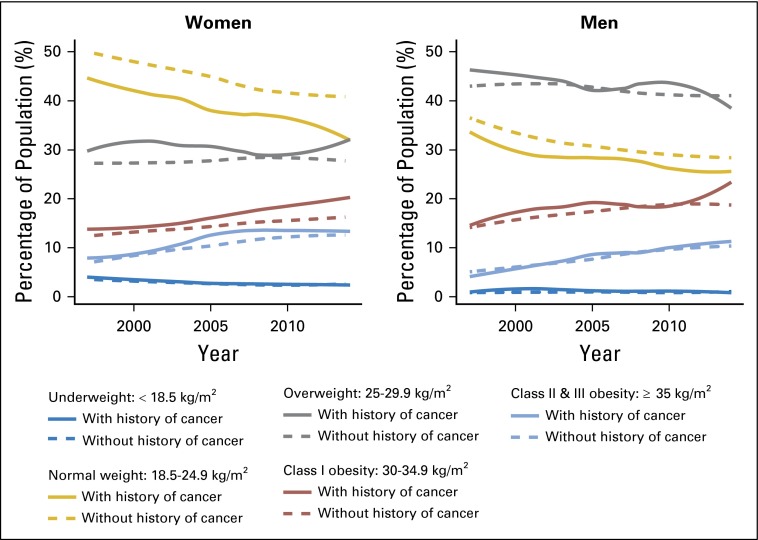
The prevalence of obesity consistently increased from 22.4% in 1997 to 31.7% in 2014 in cancer survivors and from 20.9% to 29.5%, respectively, in adults without a history of cancer (P for trend < .001 for both groups; Fig 1; Table 1). Meanwhile, there was a decreasing prevalence of normal weight (BMI, 18.5 to < 25 kg/m2) and underweight (BMI < 18.5 kg/m2) individuals and no consistent change in overweight individuals in adults with and without a history of cancer (Fig 1; Table 1). Across the time periods, changes in obesity prevalence were similar in women (21.2% to 35.0% in cancer survivors v 19.2% to 29.4% in adults without a history of cancer) and men (17.0% to 34.9% in cancer survivors v 19.1% to 29.2% in adults without a history of cancer). Of particular note, among women, across all time periods, the prevalence of class I obesity was consistently higher in cancer survivors compared with women without a history of cancer (13.6% to 21.0% in female cancer survivors v 12.2% to 16.6% in women without a history of cancer), and the differences grew larger over time (Fig 1).
Fig 1.
Changes in BMI categories from 1997 to 2014 in US adults with and without a history of a cancer diagnosis. Survey-weighted and age-standardized prevalence of five BMI categories were estimated for men and women with and without a history of cancer at each survey year. BMI was categorized according to National Heart, Lung, and Blood Institute guidelines as underweight (BMI < 18.5 kg/m2), normal weight (BMI, 18.5 to < 25 kg/m2), overweight (BMI, 25 to < 30 kg/m2), class I obesity (BMI, 30 to < 35 kg/m2), and class II/III obesity (BMI ≥ 35 kg/m2). For Asians, the WHO Asian cut points were applied for underweight (BMI < 23 kg/m2), overweight (BMI, 23 to < 27.5 kg/m2), and obesity (class I, BMI ≥ 27.5 kg/m2 and class II/III, BMI ≥ 32.5 kg/m2). Data were plotted as smoothed lines using locally weighted scatterplot smoothing. BMI, body mass index.
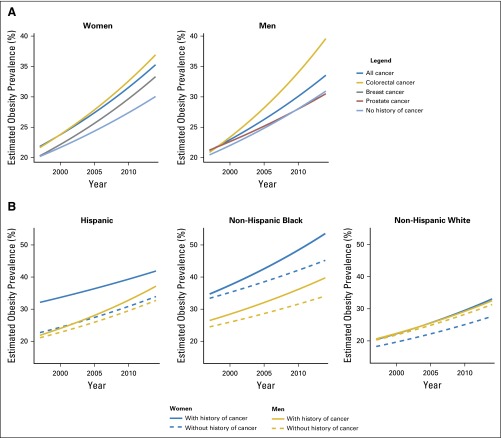
Over the study period, the prevalence of obesity was estimated to increase by 2.9% and 2.8% each year for women and men, respectively, with a history of cancer, and by 2.3% and 2.4%, respectively, for women and men without a history of cancer (Table 2; Fig 2). The annual increase in the rate of obesity was significantly greater in women and men with a history of cancer compared with those without a history of cancer (all P for interaction < .001). The elevated annual increase in rates of obesity was even higher in colorectal (3.1% in women and 3.7% in men) and breast (3.0%) cancer survivors compared with adults without a history of cancer but was lower in prostate (2.1%) cancer survivors (all P for interaction < .001).
Table 2.
Estimated Annual Increase Rates in Obesity Prevalence From 1997 to 2014 in US Adults With and Without a History of Cancer
| Population | Women | P for Interaction† | Men | P for Interaction† | ||
|---|---|---|---|---|---|---|
| Annual Increase in Obesity Prevalence (%)* | 95% CI | Annual Increase in Obesity Prevalence (%)* | 95% CI | |||
| No history of cancer | 2.31 | 2.31 to 2.31 | 2.38 | 2.38 to 2.38 | ||
| History of cancer | ||||||
| All cancers | 2.88 | 2.87 to 2.88 | < .001 | 2.77 | 2.77 to 2.78 | < .001 |
| Colorectal cancer | 3.09 | 3.07 to 3.12 | < .001 | 3.70 | 3.68 to 3.73 | < .001 |
| Breast cancer | 3.01 | 3.00 to 3.02 | < .001 | — | — | — |
| Prostate cancer | — | — | — | 2.06 | 2.04 to 2.07 | < .001 |
Annual increases were estimated using Poisson regressions adjusted for age and stratified by sex and cancer type.
P value for the interaction term between cancer status (yes/no) and time.
Fig 2.
Trends in obesity prevalence in US adults from 1997 to 2014, by sex, cancer history and race/ethnicity. (A) Obesity trends by sex and cancer history. Obesity (defined as body mass index ≥ 30 kg/m2) prevalence was estimated for men and women with a history of any cancer, colorectal, breast, and prostate cancers, and those without a history of cancer. Separate Poisson regression models were fit for each group to estimate the annual increase rates in obesity prevalence during this period. Each line represents the trend of predicted obesity prevalence over the specific period. Tests of differential increased rates showed that the increased rates were significantly higher among female cancer survivors compared with women without a history of cancer, and significantly higher among survivors of all cancer and colorectal cancer in men (all P for interaction < .001). (B) Obesity trends by race/ethnicity. Subgroup analyses by sex and race/ethnicity were conducted for all cancer survivors. The difference in increased rates of obesity prevalence was greater in non-Hispanic blacks compared with non-Hispanic whites and Hispanics.
Subgroup Analyses of Obesity Trends by Age, Race/Ethnicity, and Years Since Diagnosis, by Cancer Type
The prevalence of obesity among the most common cancers (colorectal, breast, and prostate) was examined by age, race/ethnicity, and years since diagnosis (Table 3). The prevalence of obesity grew more rapidly in nearly every subgroup of colorectal and breast cancer survivors compared with the corresponding groups of adults without a history of cancer (all P for interaction < .001). Among colorectal cancer survivors, women 18 to 44 years of age, non-Hispanic blacks, and those 2 to 9 years from diagnosis had the highest increasing rates of obesity. Among female breast cancer survivors, women 18 to 44 years of age, non-Hispanic whites, and those ≤ 1 year from diagnosis were the groups with the highest increasing rates of obesity (all P for interaction < .001). The patterns were different among men. Among male colorectal cancer survivors, the increasing rates of obesity were highest among men 65 to 85 years of age, non-Hispanic blacks, and those ≥ 10 years from diagnosis. Among prostate cancer survivors, men 18 to 44 years of age, non-Hispanic whites, and those 2 to 9 years from diagnosis had the highest increasing rates of obesity (all P for interaction < .001).
Table 3.
Annual Percent Changes in Obesity Prevalence From 1997 to 2014 in US Adults, by History of Breast, Colorectal, and Prostate Cancer
| Population Characteristic | History of Cancer | No History of Cancer | ||
|---|---|---|---|---|
| Annual Change in Obesity Prevalence (%)* | 95% CI | Annual Change in Obesity Prevalence (%)* | 95% CI | |
| Colorectal cancer, women | ||||
| Age at survey, years | ||||
| 18-44 | 7.95 | 7.84 to 8.06 | 2.65 | 2.65 to 2.65 |
| 45-64 | 1.44 | 1.40 to 1.49 | 1.72 | 1.71 to 1.72 |
| 65-85 | 3.12 | 3.09 to 3.16 | 2.33 | 2.32 to 2.33 |
| Race/ethnicity | ||||
| Non-Hispanic white | 3.00 | 2.97 to 3.04 | 2.39 | 2.39 to 2.39 |
| Black | 3.85 | 3.79 to 3.92 | 1.72 | 1.72 to 1.73 |
| Hispanic | −3.29 | −3.39 to −3.18 | 2.31 | 2.30 to 2.31 |
| Years since diagnosis† | ||||
| Never | 2.31 | 2.31 to 2.32 | ||
| ≤ 1 | 2.98 | 2.89 to 3.08 | ||
| 2-9 | 3.32 | 3.29 to 3.36 | ||
| ≥ 10 | 1.48 | 1.43 to 1.53 | ||
| Breast cancer, women | ||||
| Age at survey, years | ||||
| 18-44 | 5.31 | 5.26 to 5.35 | 2.65 | 2.65 to 2.65 |
| 45-64 | 2.18 | 2.16 to 2.20 | 1.72 | 1.71 to 1.72 |
| 65-85 | 3.09 | 3.08 to 3.11 | 2.33 | 2.32 to 2.33 |
| Race/ethnicity | ||||
| Non-Hispanic white | 3.13 | 3.12 to 3.14 | 2.39 | 2.39 to 2.39 |
| Black | 2.06 | 2.03 to 2.10 | 1.72 | 1.72 to 1.73 |
| Hispanic | 0.29 | 0.25 to 0.33 | 2.31 | 2.30 to 2.31 |
| Years since diagnosis† | ||||
| Never | 2.31 | 2.31 to 2.32 | ||
| ≤ 1 | 5.86 | 5.82 to 5.91 | ||
| 2-9 | 2.71 | 2.69 to 2.73 | ||
| ≥ 10 | 3.28 | 3.26 to 3.30 | ||
| Colorectal cancer, men | ||||
| Age at survey, years | ||||
| 18-44 | 0.70 | 0.60 to 0.80 | 2.36 | 2.35 to 2.36 |
| 45-64 | 3.09 | 3.05 to 3.13 | 2.14 | 2.14 to 2.15 |
| 65-85 | 3.78 | 3.75 to 3.81 | 3.06 | 3.05 to 3.07 |
| Race/ethnicity | ||||
| Non-Hispanic white | 3.37 | 3.34 to 3.40 | 2.49 | 2.49 to 2.49 |
| Black | 8.44 | 8.33 to 8.54 | 1.92 | 1.92 to 1.93 |
| Hispanic | −2.25 | −2.36 to −2.14 | 2.54 | 2.53 to 2.54 |
| Years since diagnosis† | ||||
| Never | 2.38 | 2.38 to 2.39 | ||
| ≤ 1 | −0.18 | −0.26 to −0.11 | ||
| 2-9 | 4.88 | 4.85 to 4.92 | ||
| ≥ 10 | 5.33 | 5.28 to 5.38 | ||
| Prostate cancer, men | ||||
| Age at survey, years | ||||
| 18-44 | 8.21 | 7.86 to 8.56 | 2.36 | 2.35 to 2.36 |
| 45-64 | 0.74 | 0.71 to 0.77 | 2.14 | 2.14 to 2.15 |
| 65-85 | 2.56 | 2.54 to 2.58 | 3.06 | 3.05 to 3.07 |
| Race/ethnicity | ||||
| Non-Hispanic white | 2.36 | 2.35 to 2.38 | 2.49 | 2.49 to 2.49 |
| Black | 1.00 | 0.96 to 1.03 | 1.92 | 1.92 to 1.93 |
| Hispanic | 0.25 | 0.19 to 0.32 | 2.54 | 2.53 to 2.54 |
| Years since diagnosis† | ||||
| Never | 2.38 | 2.38 to 2.39 | ||
| ≤ 1 | −0.07 | −0.11 to −0.02 | ||
| 2-9 | 2.64 | 2.63 to 2.66 | ||
| ≥ 10 | 1.56 | 1.52 to 1.60 | ||
Annual percent changes were estimated on the basis of obesity rates by year, sex, and age group using Poisson regressions. P values for the interaction term between cancer status (yes/no) and time were all < .001.
Annual percent changes were compared with all cancer-free adults (the “Never” group).
Trends in Chronic Conditions
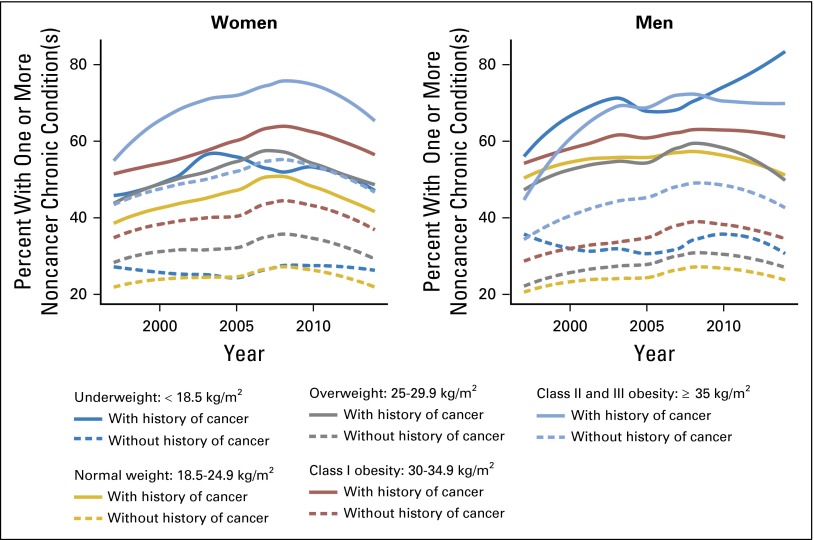
The percentages of adults with one or more noncancer chronic condition(s) increased over time independent of BMI and cancer history (Appendix Fig A1, online only). Within each BMI category, cancer survivors are systematically more likely to have one or more noncancer chronic condition(s) compared with adults without a history of cancer.
DISCUSSION
In a large, population-based, nationally representative sample, we observed a consistently increasing burden of obesity from 1997 to 2014 in US adults with and without a history of cancer. Across this time period, cancer survivors had a significantly faster increase in obesity prevalence compared with adults without a history of cancer. The faster increase in obesity prevalence was more pronounced in women, in breast and colorectal cancer survivors, and in non-Hispanic blacks. Our findings suggest that the trajectory of the US obesity burden will continue to increase, which will result in an increased burden of obesity in the general population and thus increase the number of obesity-related cancers, and also result in an increased burden of obesity among cancer survivors, including those cancers in which obesity is associated with poorer cancer outcomes.
On the basis of current trajectories, we identified population characteristics of cancer survivors at the highest risk of obesity, which are important patient populations in which oncology care providers should focus weight management efforts. In female colorectal cancer survivors, those who are young and non-Hispanic black and have been diagnosed within 2 to 9 years have the highest increasing rates of obesity. Similarly, among female breast cancer survivors, those who are young, were diagnosed within the past year, and are non-Hispanic white have the highest increasing obesity rate. Among male colorectal cancer survivors, the highest increases in obesity are among older men, non-Hispanic blacks, and those ≥ 10 years from diagnosis. In contrast, prostate cancer survivors with the highest increases in obesity burden are younger, non-Hispanic whites 2 to 9 years from diagnosis. Previous studies of weight change in prostate cancer showed similar patterns, which found younger age at diagnosis and lower baseline BMI to be predictors of weight gain after diagnosis.28,29 However, increased BMI was most likely to occur in the first year of therapy for prostate cancer survivors.30
Given that breast and colorectal cancer are the two cancers most closely linked to obesity, our findings can be partially explained by the growing population of patients with breast and colorectal cancer in whom obesity may have been in the causal pathway for disease.31-33 However, we have identified additional populations of cancer survivors at risk of obesity in whom the effects of obesity are not as well understood. In addition, it is important to consider obesity among cancer survivors not only in relation to cancer outcomes, but also in relation to other comorbid conditions. Although the association between increased BMI and recurrence and survival is not fully understood, it is well established that obesity can influence other medical conditions such as diabetes, heart disease, hypertension, and hypercholesterolemia, which may affect overall survival.1,2 In addition, specific chemotherapy agents have cardiac adverse effects that can be compounded by higher BMI.34 Among breast and prostate cancer survivors, the majority will die as a result of cardiovascular disease and not cancer.35,36 Studies of breast, colon, and prostate cancer showed that comorbid conditions increase 5-year all-cause and cancer-specific mortality.37,38 Therefore, it is important to consider obesity among cancer survivors not only in relation to cancer outcomes, but also in relation to other comorbid diseases.
A major strength of this study is that it was based on a large and nationally representative sample, making our results generalizable to the US adult population. Our estimates of prevalent cancer cases were similar to estimates of the National Cancer Institute’s SEER program. Using the NHIS data, we estimated that there were 14,250,194 cancer survivors in 2012 and 14,673,007 in 2013. In comparison, SEER reported that there were an estimated 13,776,251 cancer survivors in 2012,39 and the National Cancer Institute estimated that there were 14,483,830 cancer survivors in 2014.40 Our estimates were slightly higher than the SEER estimates of total prevalent cases, likely because our sample came from the noninstitutionalized population in the US, who may have been healthier than institutionalized individuals. Another strength of the study is that the ascertainment of cancer history was uniform and consistent across geographic regions and survey years, which helped reduce ascertainment bias. From this population-based sample of US adults, we were able to identify a large number of cancer survivors of different ages and race/ethnicities and at different stages of cancer survivorship, allowing for statistically robust comparisons in each subgroup of survivors. Nevertheless, our results do have limitations. First, the secular trends in obesity prevalence were observed using cross-sectional data over time; therefore, the observed faster increase in cancer survivors would be overestimated if leaner cancer survivors are at greater risk of mortality. Conversely, weight loss associated with heavy smoking41 and cachexia42 in cancer survivors may have biased our estimates toward the null. Second, the NHIS uses respondents' self-reported height and weight to calculate BMI, which may introduce information bias because obesity awareness has changed over time. It has been shown that self-reported weight and height data underestimate the prevalence of overweight and obesity.4,43 Our estimate of obesity prevalence in 2013 (29.6%) was comparable with findings from another self-reported national survey, the Behavioral Risk Factor Surveillance System, which reported the obesity prevalence for US adults 18 years of age and older as 28.9% in 2013.44 However, estimates of obesity prevalence from the National Health and Nutrition Examination Survey, which uses clinical measurements of height and weight, were slightly higher. The National Health and Nutrition Examination Survey results from 2011 to 2012 reported a 34.9% obesity prevalence for adults 20 years of age and older,45 as compared with the 29.6% estimate reported here. Third, because of the ecologic nature of this study, our findings may have limited inference on individual level obesity risk. Fourth, income may not be a good measure of socioeconomic status because it may reflect retirement income for older populations. Therefore, we examined additional socioeconomic factors, including education, employment, and health insurance coverage. Finally, we were unable to assess factors contributing to obesity because we lacked data on cancer stage, cancer treatment received, and detailed lifestyle behaviors, all of which can affect weight and cancer survival.
Our findings suggest that obesity is a growing public health burden for cancer survivors, which requires attention. Although it is well known that unfavorable lifestyle factors may contribute to the overall increase in obesity, future research should identify and act on modifiable risk factors that may be responsible for the steeper obesity trends in cancer survivors. Moreover, we have identified subgroups of cancer survivors who may be at the greatest risk of obesity and who may benefit from effective, targeted interventions by oncology care providers. These findings call for public health planning of effective and scalable weight management and control programs for cancer survivors, especially for breast and colorectal cancer survivors and for non-Hispanic blacks.
Appendix
Fig A1.
The percentages of adults with one or more noncancer chronic condition(s) increased over time independent of body mass index and cancer history.
Footnotes
Supported by National Cancer Institute Grant No. R21CA155973 (H.G.).
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Heather Greenlee, Zaixing Shi
Financial support: Heather Greenlee
Administrative support: Heather Greenlee
Collection and assembly of data: Heather Greenlee, Zaixing Shi
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Trends in Obesity Prevalence in Adults With a History of Cancer: Results From the US National Health Interview Survey, 1997 to 2014
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Heather Greenlee
Consulting or Advisory Role: EHE International
Zaixing Shi
No relationship to disclose
Christine L. Sardo Molmenti
No relationship to disclose
Andrew Rundle
Consulting or Advisory Role: EHE International, EHE International (Inst)
Wei Yann Tsai
No relationship to disclose
REFERENCES
- 1.Flegal KM, Kit BK, Orpana H, et al. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth GA, Nguyen G, Forouzanfar MH, et al. Estimates of global and regional premature cardiovascular mortality in 2025. Circulation. 2015;132:1270–1282. doi: 10.1161/CIRCULATIONAHA.115.016021. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 5.Wang YC, McPherson K, Marsh T, et al. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Panagiotou OA, Graubard BI. Estimating population attributable fractions to quantify the health burden of obesity. Ann Epidemiol. 2015;25:201–207. doi: 10.1016/j.annepidem.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institute of Medicine . The Role of Obesity in Cancer Survival and Recurrence: Workshop Summary. Washington, DC, The National Academies Press, 2012. [PubMed] [Google Scholar]
- 8.Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev. 2012;21:1244–1259. doi: 10.1158/1055-9965.EPI-12-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–3574. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kroenke CH, Chen WY, Rosner B, et al. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 11.Playdon MC, Bracken MB, Sanft TB, et al. Weight gain after breast cancer diagnosis and all-cause mortality: Systematic review and meta-analysis. J Natl Cancer Inst. 2015;107:djv275. doi: 10.1093/jnci/djv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cleveland RJ, Eng SM, Abrahamson PE, et al. Weight gain prior to diagnosis and survival from breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1803–1811. doi: 10.1158/1055-9965.EPI-06-0889. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin PJ, Ennis M, Pritchard KI, et al. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J Clin Oncol. 1999;17:120–129. doi: 10.1200/JCO.1999.17.1.120. [DOI] [PubMed] [Google Scholar]
- 14.Van Dongen-Melman JE, Hokken-Koelega AC, Hählen K, et al. Obesity after successful treatment of acute lymphoblastic leukemia in childhood. Pediatr Res. 1995;38:86–90. doi: 10.1203/00006450-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 16. American Institute for Cancer Research: AICR’S guidelines for cancer survivors. http://www.aicr.org/patients-survivors/aicrs-guidelines-for-cancer.html.
- 17.American Society of Clinical Oncology Obesity and cancer. https://www.asco.org/practice-guidelines/cancer-care-initiatives/prevention-survivorship/obesity-cancer.
- 18.Parsons VL, Moriarity C, Jonas K, et al. Design and estimation for the National Health Interview Survey, 2006-2015. Vital Health Stat 2. 2014:1–53. [PubMed] [Google Scholar]
- 19.NHLBI Obesity Education Initiative Expert Panel . Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report. Bethesda, MD: National Heart, Lung, and Blood Institute; 1998. [Google Scholar]
- 20.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 21.Dunn OJ. Multiple comparisons among means. J Am Stat Assoc. 1961;56:52–64. [Google Scholar]
- 22. Klein RJ, Schoenborn CA: Age adjustment using the 2000 projected U.S. population. Healthy People 2010 Stat Notes:1-10, 2001. [PubMed]
- 23.Cleveland WS, Devlin SJ. Locally weighted regression: An approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83:596–610. [Google Scholar]
- 24.Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9:1–19. [Google Scholar]
- 25. Reference deleted. [Google Scholar]
- 26.Haskell WL, Lee IM, Pate RR, et al. American College of Sports Medicine. American Heart Association Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 27.Falci L, Shi Z, Greenlee H. Multiple chronic conditions and use of complementary and alternative medicine among US adults: Results from the 2012 National Health Interview Survey. Prev Chronic Dis. 2016;13:E61. doi: 10.5888/pcd13.150501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seible DM, Gu X, Hyatt AS, et al. Weight gain on androgen deprivation therapy: Which patients are at highest risk? Urology. 2014;83:1316–1321. doi: 10.1016/j.urology.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Timilshina N, Breunis H, Alibhai SM. Impact of androgen deprivation therapy on weight gain differs by age in men with nonmetastatic prostate cancer. J Urol. 2012;188:2183–2188. doi: 10.1016/j.juro.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Kim HS, Moreira DM, Smith MR, et al. A natural history of weight change in men with prostate cancer on androgen-deprivation therapy (ADT): Results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. BJU Int. 2011;107:924–928. doi: 10.1111/j.1464-410X.2010.09679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: The Women’s Health Initiative (United States) Cancer Causes Control. 2002;13:741–751. doi: 10.1023/a:1020239211145. [DOI] [PubMed] [Google Scholar]
- 32.Giovannucci E, Ascherio A, Rimm EB, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 33.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: A meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev. 2007;16:2533–2547. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 34.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 35.Patnaik JL, Byers T, DiGuiseppi C, et al. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhatia N, Santos M, Jones LW, et al. Cardiovascular effects of androgen deprivation therapy for the treatment of prostate cancer: ABCDE steps to reduce cardiovascular disease in patients with prostate cancer. Circulation. 2016;133:537–541. doi: 10.1161/CIRCULATIONAHA.115.012519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jørgensen TL, Hallas J, Friis S, et al. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer. 2012;106:1353–1360. doi: 10.1038/bjc.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Søgaard M, Thomsen RW, Bossen KS, et al. doi: 10.2147/CLEP.S47150. The impact of comorbidity on cancer survival: A review. Clin Epidemiol 5:3-29, 2013 (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. National Cancer Institute: SEER Cancer Statistics Factsheets: All Cancer Sites. http://seer.cancer.gov/statfacts/html/all.html.
- 40.American Cancer Society . Cancer Treatment and Survivorship Facts & Figures 2014-2015. Atlanta, GA, American Cancer Society, 2014. [Google Scholar]
- 41.Winsløw UC, Rode L, Nordestgaard BG. High tobacco consumption lowers body weight: A Mendelian randomization study of the Copenhagen General Population Study. Int J Epidemiol. 2015;44:540–550. doi: 10.1093/ije/dyu276. [DOI] [PubMed] [Google Scholar]
- 42.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 43.Yun S, Zhu BP, Black W, et al. A comparison of national estimates of obesity prevalence from the behavioral risk factor surveillance system and the National Health and Nutrition Examination Survey. Int J Obes. 2006;30:164–170. doi: 10.1038/sj.ijo.0803125. [DOI] [PubMed] [Google Scholar]
- 44. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Health: DoP: BRFSS Prevalence & Trends Data. 2015. http://www.cdc.gov/brfss/brfssprevalence/
- 45.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]