Abstract
Purpose
Utility of combined-modality therapy for patients with limited-stage diffuse large B-cell lymphoma (DLBCL) was shown in the Southwest Oncology Group (SWOG) S8736 study, where three cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) plus radiotherapy (CHOP3RT) improved 5-year progression-free (PFS) and overall survival (OS) compared with eight cycles of CHOP (CHOP8). Subsequent analysis showed an unexpected overlap of the PFS curves. We aimed to confirm and investigate this observation by performing long-term analysis of SWOG S8736 and evaluating these data alongside data from similar patients receiving rituximab and CHOP3RT (SWOG S0014 study).
Patients and Methods
A subset of patients with limited-stage DLBCL randomly assigned to CHOP8 (n = 150) or CHOP3RT (n = 158) in S8736 was analyzed along with a 56-patient subset treated in S0014 for long-term PFS and OS.
Results
Median follow-up in S8736 was 17.7 years. In patients receiving CHOP8 and CHOP3RT, median PFS was 12.0 (95% CI, 8.8 to 14.3) and 11.1 years (95% CI, 8.9 to 14.4), respectively. There were no statistically significant differences in PFS between the groups (P = .73). Median OS was 13.0 (95% CI, 10.4 to 15.2) and 13.7 years (95% CI, 11.1 to 19.4) for patients treated with CHOP8 and CHOP3RT, respectively. Similarly, there were no statistically significant differences in OS between the groups (P = .38). With a median follow-up time 12 years in S0014, 5- and 10-year OS were 82% and 67%, respectively, with a persistent pattern of relapse despite the addition of rituximab.
Conclusion
Although 5-year PFS and OS were improved after early analysis in patients with limited-stage DLBCL receiving CHOP3RT versus CHOP8, extended survival data showed similar PFS and OS, with continuous treatment failure. The addition of rituximab (S0014) to combined-modality therapy did not mitigate the continued relapse risk, underscoring the value of prolonged clinical trial patient observation and possible unique biology of limited-stage DLBCL.
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of adult non-Hodgkin lymphoma (NHL). Anthracycline-based chemoimmunotherapy will cure approximately 50% to 60% of patients with advanced-stage disease, with a clear plateau in progression-free survival (PFS) and rare relapses beyond 5 years.1 In contrast, less is known about the curability of limited-stage DLBCL (Ann Arbor stage I to II,2 confined to a single irradiation field), which comprises approximately one third of patients with DLBCL.3 Standard treatment of limited-stage DLBCL includes extended chemoimmunotherapy (four to eight cycles4-7) versus abbreviated chemoimmunotherapy (three cycles) plus involved-field radiotherapy (IFRT).4,6-8 In Southwest Oncology Group (SWOG) S8736, a landmark study for this population, 401 patients with NHL were randomly assigned to receive eight cycles of chemotherapy with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone; CHOP8; n = 200) versus three cycles of chemotherapy with CHOP plus IFRT (CHOP3RT; n = 201).4 After a median follow-up of 4.4 years, initial observations included a statistically significant improvement in 5-year PFS (77% v 64%; P = .03) and overall survival (OS; 82% v 72%; P = .02) for the combined-modality arm compared with the chemotherapy-alone arm. Furthermore, the study report established the stage-modified International Prognostic Index (IPI) and led to widespread adoption of combined-modality therapy for patients with limited-stage DLBCL.4
Approximately 12 years ago, the addition of rituximab to chemotherapy in DLBCL changed the standard of care, with superior outcomes in chemoimmunotherapy-treated patients compared with chemotherapy alone. Multiple studies showed that the addition of rituximab to CHOP-based therapy (R-CHOP) resulted in an approximately 10% to 15% overall increase in survival for patients with advanced-stage DLBCL.6,9-12 To evaluate the impact of rituximab in patients with limited-stage disease, SWOG S0014 tested R-CHOP for three cycles followed by IFRT (R-CHOP3RT; 40 to 55 Gy) in high-risk patients with at least one adverse feature of the stage-modified IPI.8 With a median follow-up of 5.3 years, 4-year PFS and OS were 88% and 92%, respectively.8
Although both S8736 and S0014 were important contributions to limited-stage DLBCL management, follow-up was limited to 5 years at the times of the respective study publications. In a subsequent analysis of S8736 with 10-year follow-up published only in abstract form,13 the survival curves for CHOP8 and CHOP3RT, surprisingly, began to overlap. Additionally, for both S8736 and S0014, no plateau had been reached in the PFS curves. These data contrasted the expected cure rates for advanced-stage DLBCL, leading to the hypothesis that limited-stage DLBCL may have a unique biology from its advanced-stage counterpart. This final and long-term analysis of S8736 was pursued to describe clinical outcomes after an extended follow-up of almost two decades.
PATIENTS AND METHODS
Patients and Treatment
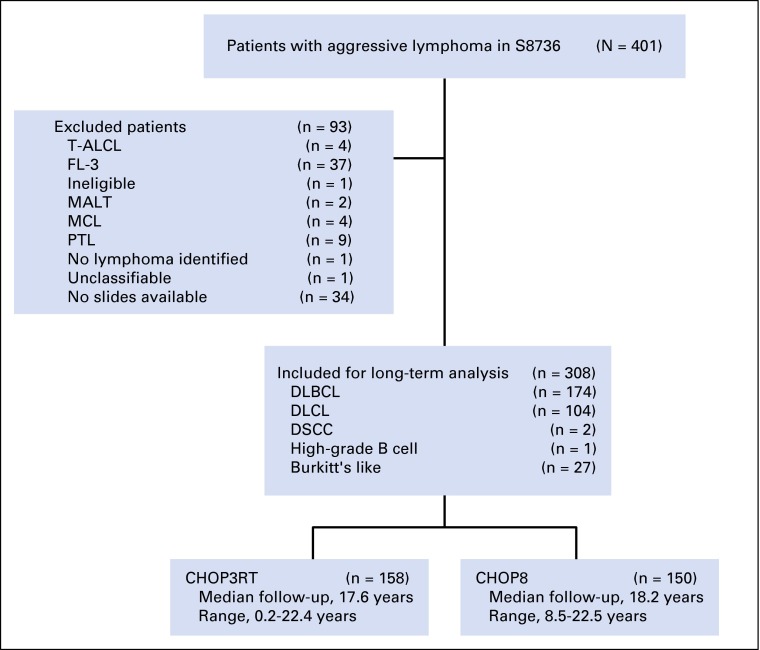
From 1988 to 1995, 401 patients with limited-stage NHL (including both B- and T-cell lymphomas) were randomly assigned to CHOP8 (n = 201) or CHOP3RT (n = 200; 40 to 55 Gy, started 3 weeks after last cycle of CHOP) in S8736, as described previously.4 For our long-term analysis, 93 patients were excluded for histologies inconsistent with DLBCL, leaving 308 patients with DLBCL-like histologies (Appendix Fig A1, online only). Of this subset, 150 and 158 received CHOP8 and CHOP3RT, respectively.
From 2000 to 2002, 60 patients with limited-stage DLBCL received R-CHOP3RT in S0014, as previously described.8 For our analysis, four patients were excluded for histologies inconsistent with DLBCL (Appendix Table A1, online only). For enrollment in S0014, all patients had a stage-modified IPI score of 1 or greater. In the stage-modified IPI, age older than 60 years, elevated lactate dehydrogenase, Ann Arbor stage II to IIE, and Eastern Cooperative Oncology Group (ECOG) performance status of 2 or greater are deemed adverse prognostic factors.4 Investigators at each institution obtained informed consent from each participant. The study was approved by local institutional review boards.
Statistical Analysis
The original design of S8736 required 200 eligible patients to be randomly assigned to each of the treatment arms to have power of 0.8 to detect a difference in long-term survival from 75% to 85% on the basis of a two-sided .05-level stratified log-rank test and assuming exponential distribution. PFS and OS were primary end points. In our analysis, PFS was defined as the time from random assignment to the first observation of progression or death resulting from any cause. Patients last known to be alive and progression free were censored at the date of last contact. OS and follow-up time were defined as the time from random assignment to date of death or last follow-up as recorded in the SWOG database. PFS and OS (with 95% CIs) were determined according to the Kaplan-Meier method. Treatment differences for PFS and OS were assessed by means of an unstratified log-rank test at two-sided α level of 0.05. Estimates of the cumulative incidence of progression and second primary malignancies were calculated using a nonparametric method.14,15 The Gray competing-risk test statistics were used for comparisons.16 For these analyses, death before disease progression or death before development of a secondary malignancy was considered a competing risk. Patient characteristics are described by treatment arm and compared combined S8736 and S0014 patients by χ2 or Fisher’s exact test at two-sided α level of 0.05. Cause of death was obtained via review of death certificates and medical records submitted to the SWOG database. Time to second malignancy, as recorded from medical records in the SWOG database, was calculated from date of treatment initiation.
RESULTS
Patient Characteristics
Baseline characteristics of the analyzed patient subset from S8736 and S0014 are listed in Table 1. Notably, the S0014 patients had higher-risk disease (by design), because patients with a stage-modified score of 0 were excluded; in contrast, approximately 30% of S8736 patients had a stage-modified score of 0. The baseline characteristics of the two arms of S8736 were similar, with a median age of 60 years (range, 17.9 to 85.1 years), 32% (98 of 308) of patients with Ann Arbor Stage II disease, and 4% (12 of 308) of patients with ECOG performance status of 2. Notably, the median age of S0014 patients was approximately 10 years older.
Table 1.
Baseline Patient (subset) Characteristics in Long-Term Follow-Up Studies S8736 and S0014
| Characteristic | ||||
|---|---|---|---|---|
| S8736 | S0014 | |||
| CHOP8 (n = 150) | CHOP3RT (n = 158) | R-CHOP3RT (n = 56) | P* | |
| Age, years | < .001† | |||
| Median | 59.8 | 59.4 | 69.3 | |
| Range | 18.8-83.3 | 17.9-85.1 | 26.3-85.1 | |
| Age > 60 years | 72 (48) | 78 (49) | 41 (73) | < .001 |
| Male sex | 95 (63) | 94 (59) | 41 (45) | .02 |
| Histology | < .001 | |||
| DLBCL | 83 (55) | 91 (58) | 56 (100) | |
| DLCL | 51 (34) | 53 (34) | 0 | |
| Other‡ | ||||
| Ann Arbor stage II | 47 (31) | 51 (32.2) | 24 (42.8) | .08 |
| Elevated LDH | 35 (23) | 36 (23) | 13 (23) | .97 |
| Missing | 1 (1) | 4 (3) | 0 | |
| ECOG PS 2 | 7 (5) | 5 (3) | 1 (2) | .70 |
| Stage-modified IPI | < .001§ | |||
| 0 | 45 (30) | 46 (29) | 0 | |
| 1 | 63 (42) | 63 (40) | 38 (68) | |
| 2 | 28 (18) | 31 (20) | 12 (21) | |
| 3 | 12 (8) | 13 (8) | 6 (11) | |
| 4 | 1 (1) | 1 (1) | 0 | |
| Unknown | 1 (1) | 4 (2) | 0 | |
NOTE. Data are presented as No. (%) unless otherwise indicated. Eligibility criteria for S8736 and S0014 were different, and S0014 intentionally enrolled higher-risk patients.
Abbreviations: CHOP3RT, three cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) plus radiotherapy; CHOP8, eight cycles of CHOP; DLBCL, diffuse large B-cell lymphoma; DLCL, diffuse large-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, International Prognostic Index; LDH, lactate dehydrogenase; R-CHOP3RT, rituximab plus CHOP3RT.
Compared combined S8736 with S0014 using χ2 or Fisher’s exact test (two-sided α = 0.05).
Wilcoxon rank sum test.
Other includes diffuse small cleaved-cell lymphoma (n = 2), high-grade B-cell lymphoma (n = 1), and Burkitt’s-like lymphoma (n = 27).
Stage-modified IPI (0 v 1 to 2 v 3 to 4).
Survival
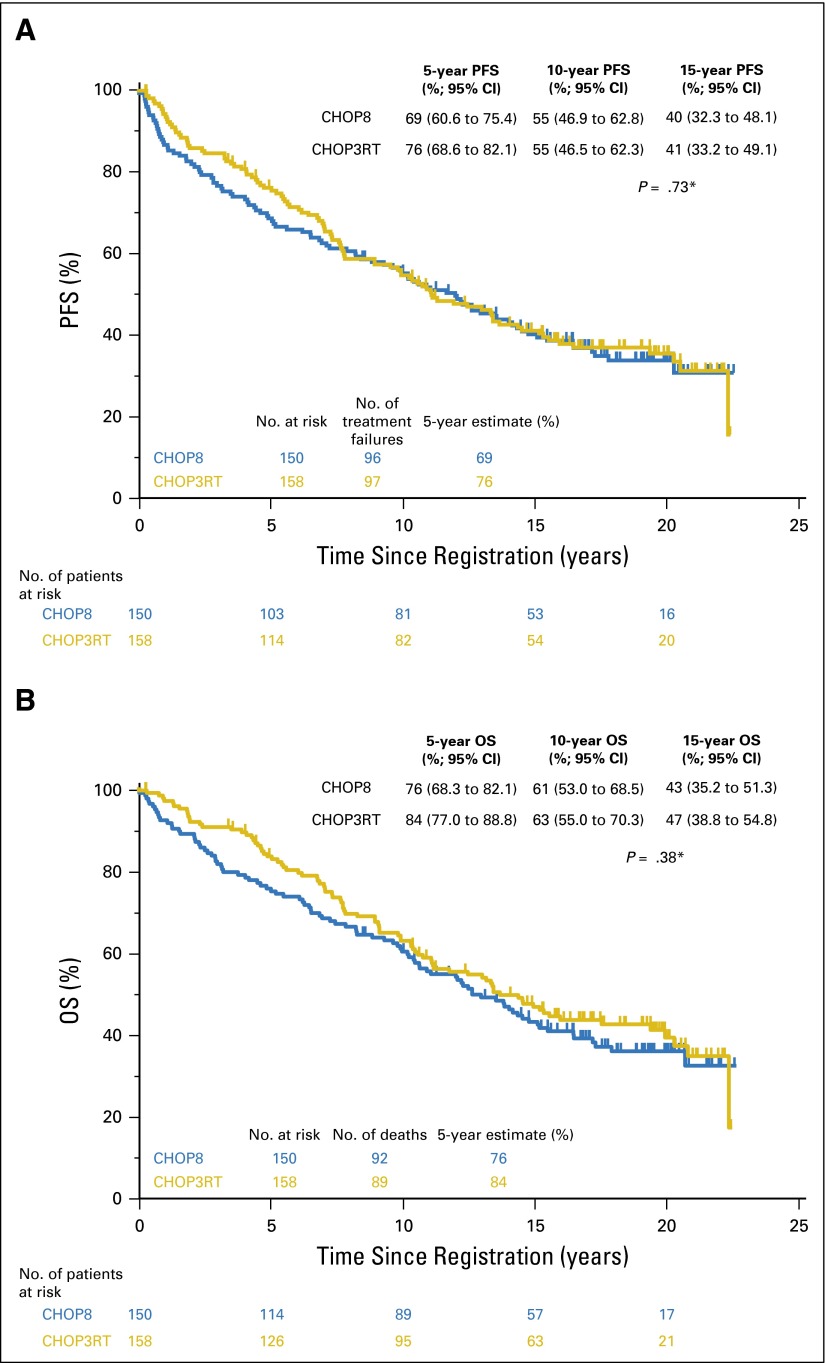
With a median follow-up of 18.2 years (range, 8.5 to 22.5 years) in patients randomly assigned to CHOP8 and 17.6 years (range, 0.2 to 22.4 years) in patients randomly assigned to CHOP3RT, median PFS was 12.0 (95% CI, 8.8 to 14.3) and 11.1 years (95% CI, 8.9 to 14.4), respectively. There were no statistically significant differences in PFS between the two groups, given the long-term follow-up (two-sided log-rank test P = .73; Fig 1A). The hazard ratio (HR) for PFS comparing the CHOP8 arm with the CHOP3RT arm was 1.05 (95% CI, 0.79 to 1.39).
Fig 1.
(A) Progression-free (PFS) and (B) overall survival (OS) of patients receiving CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) for eight cycles (CHOP8) or CHOP for three cycles plus radiotherapy (CHOP3RT) with prolonged follow-up. 5-, 10-, and 15-year estimates are included. (*) Two-sided log-rank test.
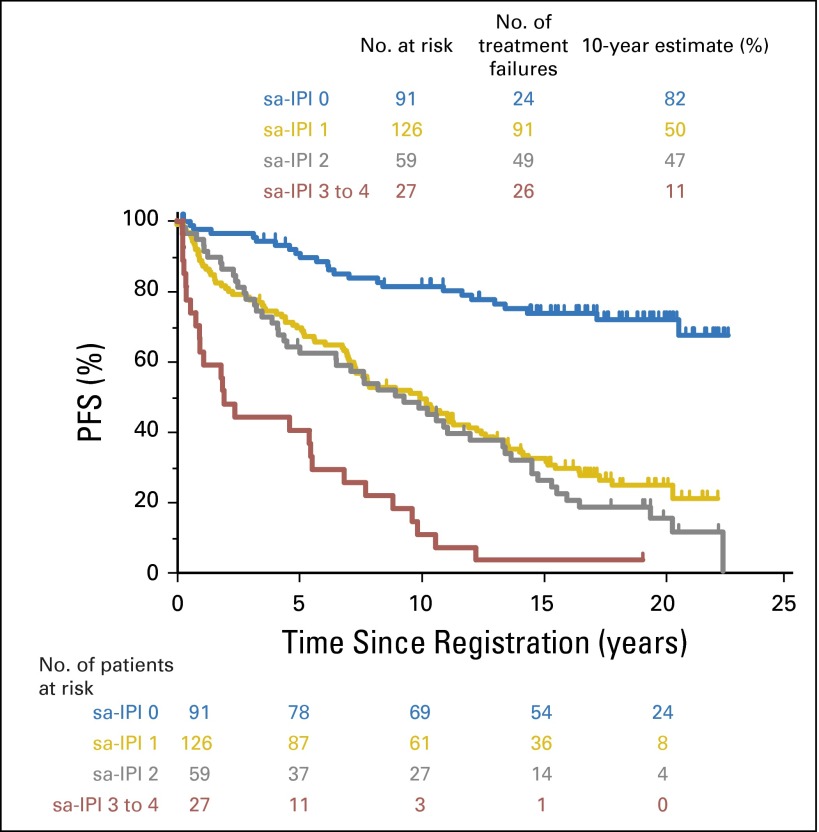
Median OS was 13.0 (95% CI, 10.4 to 15.2) and 13.7 years (95% CI, 11.1 to 19.4) for patients who received CHOP8 and CHOP3RT, respectively. Similarly, there were no statistically significant differences in OS between the groups (two-sided log-rank test P = .38; Fig 1B). The HR for OS comparing the CHOP8 arm with the CHOP3RT arm was 1.14 (95% CI, 0.85 to 1.53). PFS by stage-modified IPI is included in Appendix Figure A2 (online only).
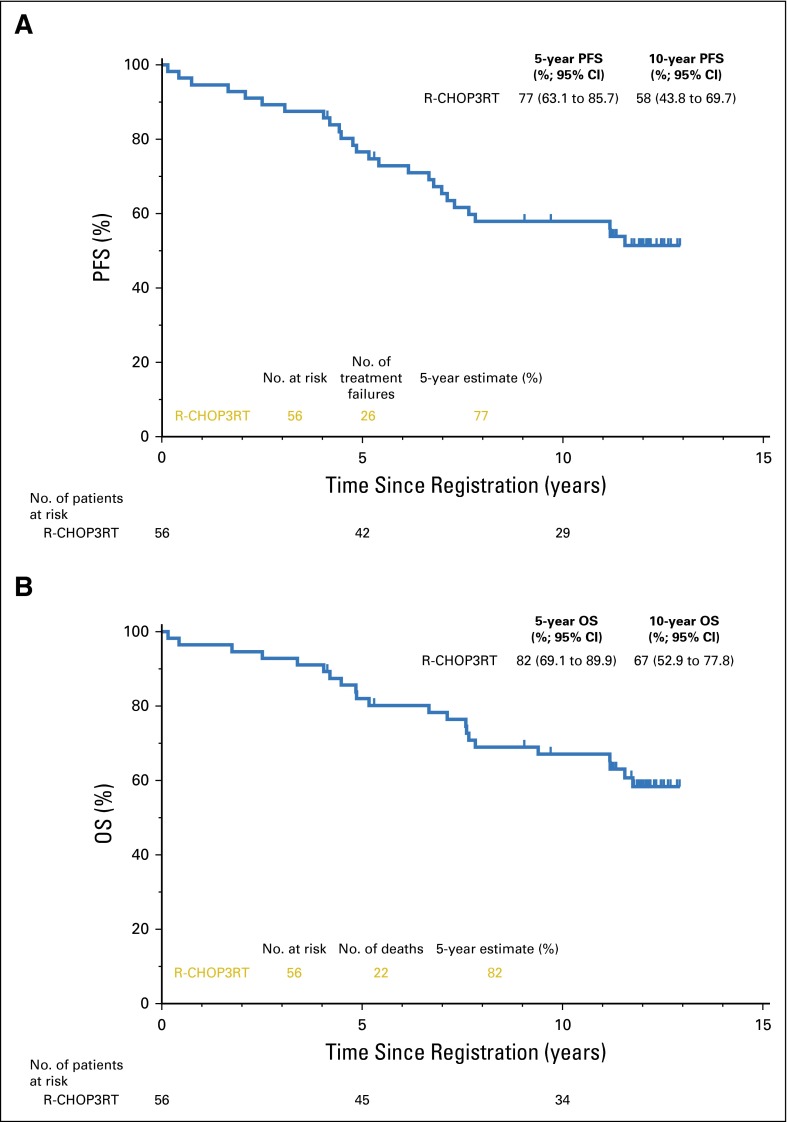
For S0014 patients receiving rituximab plus CHOP3RT, median follow-up was 12 years (range, 4.1 to 12.9 years). Median PFS and OS had not been reached (Figs 2A and 2B).
Fig 2.
(A) Progression-free (PFS) and (B) overall survival (OS) of patients receiving three cycles of rituximab plus CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) plus radiotherapy (R-CHOP3RT) with prolonged follow-up. 5- and 10-year estimates are included. Median follow-up was 12 years.
Cause of Death
Of 181 patient deaths in this S8736 subset, cause of death was known for 139 patients (CHOP8, n = 70; CHOP3RT, n = 69; Table 2). The most common cause of death was relapsed DLBCL, which occurred in 33 and 30 patients in the CHOP8 and CHOP3RT arms, respectively. The next most common causes of death were cardiovascular event, infection, and second malignancy. Notably, seven patients died as a result of congestive heart failure in the CHOP8 arm compared with one patient in the CHOP3RT arm. Additionally, 10 patients died as a result of second malignancy in the CHOP3RT arm, with four patients dying as a result of second malignancy in the CHOP8 arm.
Table 2.
Causes of Death for Patients in S8736
| Cause of Death | CHOP8 (n = 92) | CHOP3RT (n = 89) | Total Deaths (n = 181) |
|---|---|---|---|
| Relapsed DLBCL | 33 | 30 | 63 |
| Cardiovascular | 15 | 8 | 23 |
| Congestive heart failure | 7 | 1 | 8 |
| Myocardial infarction | 3 | 1 | 4 |
| Stroke | 4 | 3 | 7 |
| Cardiac arrhythmia | 1 | 1 | 2 |
| Abdominal aortic aneurysm rupture | 0 | 1 | 1 |
| Pulmonary embolism | 0 | 1 | 1 |
| Second malignancy | 4 | 10 | 14 |
| Lung | 1 | 5 | 6 |
| GI | 2 | 3 | 5 |
| Breast | 1 | 0 | 1 |
| Prostate | 0 | 1 | 1 |
| Melanoma | 0 | 1 | 1 |
| Infection | 8 | 7 | 15 |
| Miscellaneous* | 10 | 14 | 24 |
| Unknown | 22 | 20 | 42 |
Abbreviations: CHOP3RT, three cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) plus radiotherapy; CHOP8, eight cycles of CHOP; DLBCL, diffuse large B-cell lymphoma.
Amytrophic lateral sclerosis (n = 1), Alzheimer’s (n = 2), chronic obstructive pulmonary disease (n = 2), diabetes (n = 2), gastric outlet obstruction (n = 1), Lewy body dementia (n = 1), liver failure (n = 1), malnutrition (n = 2), Parkinson’s (n = 2), renal failure (n = 2), respiratory failure (n = 3), suicide (n = 1), surgical complication (n = 1), and trauma (n = 3).
Progressive Disease
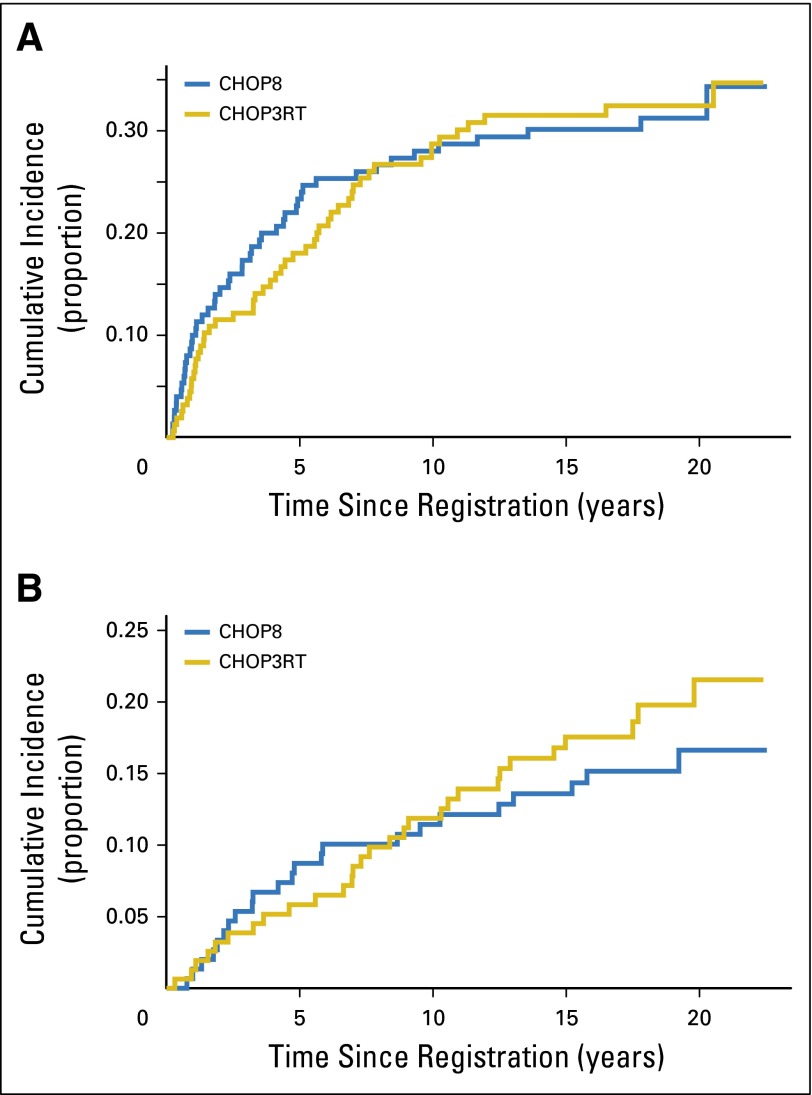
There were 193 patients who experienced DLBCL progression regardless of cause of death (CHOP8, n = 96; CHOP3RT, n = 97). In the CHOP8 cohort, 5- and 10-year cumulative incidence of progressive disease (PD) were 23% (95% CI, 16.9% to 30.4%) and 28% (95% CI, 21.1% to 35.4%), respectively. In the CHOP3RT cohort, 5- and 10-year cumulative incidence of PD were 18% (95% CI, 12.4% to 24.5%) and 29% (95% CI, 21.7% to 36.1%), respectively. Death before PD was considered a competing risk. There were no significant differences in cumulative incidence of PD between the groups (two-sided P = .91; Fig 3A).
Fig 3.
Cumulative incidence of (A) progression and (B) second malignancy for patients in S8736. Death before disease progression or death before development of a second malignancy was considered a competing risk. CHOP3RT, three cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) plus radiotherapy; CHOP8, eight cycles of CHOP.
Second Malignancies
Fifty-four S8736 patients developed second malignancies (Table 3). In the CHOP8 cohort, 24 patients developed a second malignancy at a median time of 4.8 years. In this group, 5- and 10-year cumulative incidence of a second cancer were 9% (95% CI, 4.9% to 14%) and 11% (95% CI, 6.9% to 17.2%), respectively. In the CHOP3RT cohort, 30 patients developed a second malignancy at a median time of 7.6 years. In this group, 5- and 10-year cumulative incidence a second cancer were 6% (95% CI, 2.9% to 10.3%) and 12% (95% CI, 7.3% to 17.6%), respectively. Death before development of a second malignancy was considered a competing risk. There were no significant differences in cumulative incidence of development of a second malignancy between the groups (two-sided P = .44; Fig 3B). The most common second cancer was nonmelanoma skin cancer (CHOP8, n = 11; CHOP3RT, n = 6). Six patients in the CHOP3RT group developed lung cancer in comparison with two patients in the CHOP8 group. Incidence of breast cancer was similar between the two cohorts (CHOP8, n = 4; CHOP3RT, n = 3). Two patients in the CHOP8 arm developed bladder cancer; there were no cases of bladder cancer in the CHOP3RT arm. There were no occurrences of myelodysplasia or acute leukemia in either cohort of this patient subset.
Table 3.
Second Malignancies Developed by Patients in S8736
| Second Malignancy Type | CHOP8 (n = 24) | CHOP3RT (n = 30) | Total (n = 54) |
|---|---|---|---|
| Nonmelanoma skin | 11 | 6 | 17 |
| Genitourinary | 4 | 5 | 9 |
| Prostate | 2 | 4 | 6 |
| Bladder | 2 | 0 | 2 |
| Cervical | 0 | 1 | 1 |
| Lung | 2 | 6 | 8 |
| Breast | 4 | 3 | 7 |
| GI | 1 | 4 | 5 |
| Colon | 0 | 2 | 2 |
| Rectum | 1 | 0 | 1 |
| Pancreas | 0 | 2 | 2 |
| Hematologic | 2 | 3 | 5 |
| Chronic myelomonocytic leukemia | 1 | 0 | 1 |
| Low-grade non-Hodgkin lymphoma | 0 | 2 | 2 |
| Multiple myeloma | 1 | 0 | 1 |
| Myelofibrosis | 0 | 1 | 1 |
| Melanoma | 0 | 3 | 3 |
Abbreviations: CHOP3RT, three cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) plus radiotherapy; CHOP8, eight cycles of CHOP.
DISCUSSION
DLBCL management is based on curative intent. For limited-stage DLBCL, accounting for nearly one third of DLBCLs, a variety of treatment approaches have been evaluated, including abbreviated chemotherapy with radiation therapy versus prolonged chemotherapy. The largest prospective phase III trial in this DLBCL subset, conducted in the prerituximab era, is S8736,4 in which patients were randomly assigned to receive either CHOP3RT or CHOP8. Although the initial study report4 showed an advantage for the combined-modality arm, a preliminary presentation after 10-year follow-up suggested increased late relapses and an overlap of the PFS curves after 7 years.13 Our analysis was conducted to evaluate this unexpected outcome and compare outcomes with those in a modern cohort of patients treated with abbreviated R-CHOP plus radiotherapy. With two decades of follow-up for S8736, we found no differences in PFS or OS between patients who received CHOP8 or CHOP3RT. Furthermore, despite the positive impact of rituximab on overall outcomes, there was still no suggestion of cure after abbreviated R-CHOP plus radiotherapy with 12 years of follow-up in S0014.
A key observation in the S8736 and S0014 studies, in contrast to mature data in advanced-stage DLBCL,1 was the pattern of continued late relapse in patients with limited-stage disease. For patients with advanced-stage DLBCL, relapses after 5 years from therapy have been reported, but they are rare. The GELA (Groupe d’Etude des Lymphomes de l’Adulte) LNH-98.5 study randomly assigned 399 elderly patients with advanced-stage DLBCL to either CHOP or R-CHOP and demonstrated a plateau of the PFS curves after 5 years, with only 7% of patients experiencing a late relapse.1 Similarly, a large analysis of almost 1,500 patients with advanced-stage DLBCL showed that relapse risk beyond 24 months matched that of age- and sex-matched controls.17 Two retrospective studies that identified patients with DLBCL who experienced relapse after 5 years from therapy reported that approximately 70% of this subgroup had limited-stage disease.18,19 The most common cause of death in S8736 was relapsed DLBCL, constituting 45% of the known causes of death; incidence was equally distributed between both arms at a constant rate of approximately 15% additional cases of PD in each five-year increment. These studies cumulatively suggest that late relapses occur predominantly in patients who initially present with limited-stage disease.
The cause of these distinct relapse patterns may support underlying biologic differences between limited- and advanced-stage disease, which is supported by early data from Roberts et al.20 This study showed that patients with limited-stage disease (n = 35) were more likely to have a cell-of-origin (COO) gene expression profile21 consistent with germinal center origin (GCB; 77%) compared with patients with advanced-stage DLBCL (49%; P = .01).20 A SWOG study of abbreviated chemotherapy plus radioimmunotherapy in patients with limited-stage disease similarly found twice as many cases of GCB using immunohistochemical algorithms for COO. Several early studies speculated that patients with GCB-subtype DLBCL gained relatively less clinical benefit with the addition of rituximab to chemotherapy compared with patients with non-GCB DLBCL.21,22 These speculations may explain why R-CHOP in S00148 did not improve survival compared with CHOP therapy in S8736.4 However, conclusions are limited, because the COO subtypes were not determined for S8736 patients; our study predated the routine determination of COO. Since publication of the SWOG 8736 and S0014 study reports,4,8 much effort has been made in the categorization of molecular and biologic DLBCL risk factors,23-25 but no specific data about the biologic underpinnings of limited- and advanced-stage disease have emerged.
The GELA LNH-936 and ECOG 14847 studies also evaluated chemotherapy (four to eight cycles of CHOP) and radiotherapy prospectively in similar populations of patients with limited-stage DLBCL. With respective 7- and 12-year median follow-up, the studies found no differences in OS between those who did or did not receive radiation therapy. Despite study differences (variations in treatment regimen and patient population) across these three trials, a pattern of no clear benefit of combined-modality therapy versus chemotherapy alone emerges.
Comparison of outcomes in S8736 with those in modern studies in limited-stage DLBCL is difficult, because S8736 was completed before the approval of rituximab, and there are few studies in the modern era specifically addressing limited-stage disease with prolonged follow-up time. S0014 focused on high-risk patients with limited-stage DLBCL, defined by having at least one risk factor in the stage-modified IPI. Despite being a decade older and having higher-risk disease, patients had outcomes roughly similar to those of patients in S8736. However, relapses continued to occur between years 5 and 10, with PFS dropping from 77% to 58% and OS similarly dropping from 82% to 67%. The MInT (MabThera International) trial was designed to test R-CHOP versus CHOP. A subset of patients in the MInT trial with stage II nonbulky disease with no age-adjusted IPI risk factors who received R-CHOP for six cycles demonstrated 6-year event-free and overall survival of 84% and 95%, respectively.11 These outcomes were better than those noted for patients in both S8736 and S0014; however, the MinT trial included a younger and lower-risk patient population, limiting direct comparison of the studies. Several large retrospective studies have also evaluated outcomes specifically for patients with limited-stage DLBCL treated with R-CHOP with or without radiotherapy.26-28 These studies were published after a short median follow-up of 4 to 5 years, but they still demonstrated no clear benefit of combined-modality regimens versus chemoimmunotherapy alone for patients with limited-stage DLBCL. Comparisons of these retrospective trials with our long-term follow-up of S8736 are somewhat limited by the retrospective nature of the trials, the addition of rituximab, and the short length of follow-up. In sum, these data raise the question of whether combined-modality therapy should be chosen over chemotherapy alone as the standard of care in patients with limited-stage DLBCL. One possible answer might be a risk-adapted approach and use of positron emission tomography scans to identify those with complete resolution of disease who could be spared additional chemotherapy or radiation therapy and attendant toxicities. This type of risk-adapted treatment approach for patients with limited-stage DLBCL is currently under investigation by SWOG in the S1001 study (Clinicaltrials.gov identifier NCT01359592).
Nonrelapse morbidity and mortality were primarily related to second malignancies and cardiovascular disease. In the S8736 subset, 17.9% (n = 55) of patients reported a second malignancy after treatment (median time to second malignancy for those receiving CHOP8, n = 4.8 years; CHOP3RT, n = 7.6 years), which is higher than reported in the RICOVER-60 (Rituximab With CHOP Over Age 60 Years) trial, where the incidence of second malignancy was approximately 5% and was not statistically different between patients assigned to R-CHOP with or without radiotherapy (P = .65)12; this is likely related to the short median follow-up of the study of only 2.9 years, in comparison with our long-term follow up data of S8736 of nearly 20 years. In S8736, approximately 10% of patients died as a result of second malignancies, which is comparable to what was observed in the GELA LNH-93 study (14%).6 Since S8736 enrollment completion in 1995, there have been significant advances in radiotherapy. Treatment fields are smaller and more focused, which could reduce toxicity, including second cancers. One retrospective study reviewed patients with limited-stage DLBCL who received three cycles of CHOP-like chemotherapy plus IFRT or involved-node radiotherapy with margins of 5 cm or smaller and found no difference in 10-year PFS or OS between the groups, indicating that reducing the field size did not affect outcome and may reduce toxiticy.29 Another common cause of nonrelapse mortality in S8736 was cardiovascular-related death. Patients in both groups experienced a similar incidence of cardiovascular events of 16.5%, with a higher rate of mortality in the chemotherapy-alone arm (CHOP8, 21.4%; CHOP3RT, 11.6%). In a SEER analysis of patients with limited-stage DLBCL (N = 15,454) who received chemotherapy or chemoradiotherapy, the actuarial incidence rates of cardiac-specific mortality at 15 years were higher in patients who were treated with chemotherapy alone versus chemoradiotherapy (16.1% v 13.8%; P < .001; HR, 1.35; 95% CI, 1.16 to 1.56).30 These data are comparable to those found in S8736; however, conclusions on the basis of our patient data are limited by the small number of events and the number of patients with missing causes of death (n = 42). Furthermore, six cycles of anthracycline-based chemotherapy have replaced eight cycles in modern DLBCL management, which diminished long-term cardiac consequences. In general, increased risk for second malignancy and cardiovascular disease should be considered when evaluating long-term survivors and choosing an initial treatment regimen.
In conclusion, although 5-year PFS and OS were initially improved in patients with limited-stage DLBCL receiving CHOP3RT versus CHOP8 in S8736, extended survival data with more than 17 years of follow-up showed similar outcomes, with continuous treatment failure and without a PFS plateau in either arm. The populations were not entirely identical; however, even the addition of rituximab as per S0014 to combined-modality therapy did not seem to mitigate the continued relapse risk, underscoring the value of prolonged observation of clinical trial patients and possible unique biology of limited-stage DLBCL.
Supplementary Material
Acknowledgment
We thank Thomas P. Miller, MD, for his many contributions and oversight of early-stage diffuse large B-cell lymphoma trials.
Appendix
Table A1.
Patients Excluded From Original S0014 Cohort
| Non-Hodgkin Lymphoma Subtype | No. of Patients |
|---|---|
| Anaplastic large-cell lymphoma | 1 |
| Burkitt’s lymphoma | 2 |
| T-cell–rich diffuse large B-cell lymphoma | 1 |
| Total excluded | 4 |
Fig A1.
CONSORT diagram for S8736. CHOP3RT, three cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) plus radiotherapy; DLBCL, diffuse large B-cell lymphoma; DLCL, diffuse large-cell lymphoma; DSCC, diffuse small cleaved-cell lymphoma; FL-3, grade 3 follicular lymphoma; MALT, mucosa-associated lymphoid tissue lymphoma; MCL, mantle cell lymphoma; PTL, primary thyroid lymphoma; T-ALCL, T-cell anaplastic large-cell lymphoma.
Fig A2.
Progression-free survival by stage-modified International Prognostic Index (IPI). sa, stage-adjusted.
Footnotes
Supported in part by National Institutes of Health (NIH)/National Cancer Institute (NCI)/National Clinical Trials Network Grants No. CA180888, CA180819, CA180801, CA 180834, CA 180846, CA180835, CA180818, CA180830, and CA180828 and by NIH/NCI Community Oncology Research Program Grants No. CA189953, CA189952, CA189954, CA189808, CA189830, CA189957, CA189822, CA189853, and CA189860.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00005089.
AUTHOR CONTRIBUTIONS
Conception and design: Deborah M. Stephens, Jonathan W. Friedberg, Sonali M. Smith
Collection and assembly of data: Deborah M. Stephens, Hongli Li, Soham D. Puvvada, Jonathan W. Friedberg, Sonali M. Smith
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Continued Risk of Relapse Independent of Treatment Modality in Limited-Stage Diffuse Large B-Cell Lymphoma: Final and Long-Term Analysis of Southwest Oncology Group Study S8736
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Deborah M. Stephens
No relationship to disclose
Hongli Li
No relationship to disclose
Michael L. LeBlanc
No relationship to disclose
Soham D. Puvvada
Consulting or Advisory Role: AbbVie/Genentech, Pharmacyclics
Speakers’ Bureau: Seattle Genetics
Research Funding: Spectrum Pharmaceuticals (Inst), Takeda Pharmaceuticals (Inst), Janssen Oncology (Inst), AbbVie/Genentech (Inst), Seattle Genetics (Inst)
Travel, Accommodations, Expenses: Genentech
Daniel Persky
Consulting or Advisory Role: Spectrum Pharmaceuticals, Cardinal Health, Genentech
Speakers’ Bureau: Gilead Sciences
Research Funding: Merck
Jonathan W. Friedberg
Consulting or Advisory Role: Bayer HealthCare Pharmaceuticals
Research Funding: Seattle Genentics (Inst)
Sonali M. Smith
Consulting or Advisory Role: Genentech, Celgene, Onyx Pharmaceuticals, Seattle Genetics, TG Therapeutics, Gilead Sciences, Immunogenix, Pharmacyclics
REFERENCES
- 1.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–1636. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Harris NL, et al. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissue. (ed 4) Lyon, France: International Agency on Research for Cancer; 2008. [Google Scholar]
- 4.Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1998;339:21–26. doi: 10.1056/NEJM199807023390104. [DOI] [PubMed] [Google Scholar]
- 5.Pfreundschuh M, Trümper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet C, Fillet G, Mounier N, et al. CHOP alone compared with CHOP plus radiotherapy for localized aggressive lymphoma in elderly patients: A study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2007;25:787–792. doi: 10.1200/JCO.2006.07.0722. [DOI] [PubMed] [Google Scholar]
- 7.Horning SJ, Weller E, Kim K, et al. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-Hodgkin’s lymphoma: Eastern Cooperative Oncology Group study 1484. J Clin Oncol. 2004;22:3032–3038. doi: 10.1200/JCO.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 8.Persky DO, Unger JM, Spier CM, et al. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol. 2008;26:2258–2263. doi: 10.1200/JCO.2007.13.6929. [DOI] [PubMed] [Google Scholar]
- 9.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 10.Pettengell R, Linch D, Haemato-Oncology Task Force of the British Committee for Standards in Haematology Position paper on the therapeutic use of rituximab in CD20-positive diffuse large B-cell non-Hodgkin’s lymphoma. Br J Haematol. 2003;121:44–48. doi: 10.1046/j.1365-2141.2003.04274.x. [DOI] [PubMed] [Google Scholar]
- 11.Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013–1022. doi: 10.1016/S1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- 12.Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: A randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9:105–116. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 13. Miller TP, LeBlanc M, Spier C: CHOP alone compared to CHOP plus radiotherapy for early stage aggressive non-Hodgkin's lymphomas: Update of the Southwest Oncology Group (SWOG) randomized trial. Blood 98:724a-725a, 2001 (abstr 3024) [Google Scholar]
- 14.Nelson W. Theory and applications of hazard plotting for censored failure data. Technometrics. 1972;14:945–966. [Google Scholar]
- 15.Aalen OO. Nonparametric inference for a family of counting processes. Ann Stat. 1978;6:701–726. [Google Scholar]
- 16.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 17.Maurer MJ, Ghesquières H, Jais JP, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32:1066–1073. doi: 10.1200/JCO.2013.51.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larouche JF, Berger F, Chassagne-Clément C, et al. Lymphoma recurrence 5 years or later following diffuse large B-cell lymphoma: Clinical characteristics and outcome. J Clin Oncol. 2010;28:2094–2100. doi: 10.1200/JCO.2009.24.5860. [DOI] [PubMed] [Google Scholar]
- 19.Vose JM, Weisenburger DD, Loberiza FR, et al. Late relapse in patients with diffuse large B-cell lymphoma. Br J Haematol. 2010;151:354–358. doi: 10.1111/j.1365-2141.2010.08330.x. [DOI] [PubMed] [Google Scholar]
- 20. Roberts RA, Rimsza LM, Staudt L, et al: Gene expression differences between low and high stage diffuse large B cell lymphoma (DLBCL). Blood 108, 2006 (abstr 809) [Google Scholar]
- 21.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 22.Winter JN, Weller EA, Horning SJ, et al. Prognostic significance of Bcl-6 protein expression in DLBCL treated with CHOP or R-CHOP: A prospective correlative study. Blood. 2006;107:4207–4213. doi: 10.1182/blood-2005-10-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salles G, de Jong D, Xie W, et al. Prognostic significance of immunohistochemical biomarkers in diffuse large B-cell lymphoma: A study from the Lunenburg Lymphoma Biomarker Consortium. Blood. 2011;117:7070–7078. doi: 10.1182/blood-2011-04-345256. [DOI] [PubMed] [Google Scholar]
- 24.Iqbal J, Shen Y, Huang X, et al. Global microRNA expression profiling uncovers molecular markers for classification and prognosis in aggressive B-cell lymphoma. Blood. 2015;125:1137–1145. doi: 10.1182/blood-2014-04-566778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Q, Tan C, Ni S, et al. Identification and validation of a two-gene expression index for subtype classification and prognosis in diffuse large B-cell lymphoma. Sci Rep. 2015;5:10006. doi: 10.1038/srep10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomita N, Takasaki H, Miyashita K, et al. R-CHOP therapy alone in limited stage diffuse large B-cell lymphoma. Br J Haematol. 2013;161:383–388. doi: 10.1111/bjh.12281. [DOI] [PubMed] [Google Scholar]
- 27.Dabaja BS, Vanderplas AM, Crosby-Thompson AL, et al. Radiation for diffuse large B-cell lymphoma in the rituximab era: Analysis of the National Comprehensive Cancer Network lymphoma outcomes project. Cancer. 2015;121:1032–1039. doi: 10.1002/cncr.29113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odejide OO, Cronin AM, Davidoff AJ, et al. Limited stage diffuse large B-cell lymphoma: Comparative effectiveness of treatment strategies in a large cohort of elderly patients. Leuk Lymphoma. 2015;56:716–724. doi: 10.3109/10428194.2014.930853. [DOI] [PubMed] [Google Scholar]
- 29.Campbell BA, Connors JM, Gascoyne RD, et al. Limited-stage diffuse large B-cell lymphoma treated with abbreviated systemic therapy and consolidation radiotherapy: Involved-field versus involved-node radiotherapy. Cancer. 2012;118:4156–4165. doi: 10.1002/cncr.26687. [DOI] [PubMed] [Google Scholar]
- 30.Pugh TJ, Ballonoff A, Rusthoven KE, et al. Cardiac mortality in patients with stage I and II diffuse large B-cell lymphoma treated with and without radiation: A Surveillance, Epidemiology, and End-Results analysis. Int J Radiat Oncol Biol Phys. 2010;76:845–849. doi: 10.1016/j.ijrobp.2009.02.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.