Abstract
Purpose
The use of radiographic response as the primary end point in phase II osteosarcoma trials may limit optimal detection of treatment response because of the calcified tumor matrix. We performed this study to determine if time to progression could be used as an end point for subsequent studies.
Patients and Methods
We performed a retrospective analysis of outcome for patients with recurrent/refractory osteosarcoma enrolled in one of seven phase II trials conducted by the Children’s Oncology Group and predecessor groups from 1997 to 2007. All trials used RECIST or WHO radiographic response criteria and the primary end point of response rate. The following potential prognostic factors—age, trial, number of prior chemotherapy regimens, sex, and race/ethnicity—were evaluated for their impact on event-free survival (EFS). We used data from a phase II study (AOST0221) of patients with osteosarcoma who were given inhaled granulocyte-macrophage colony-stimulating factor with first pulmonary recurrence who had an EFS as well as biologic end point to determine the historical disease control rate for patients with fully resected disease.
Results
In each included trial, the drugs tested were determined to be inactive on the basis of radiographic response rates. The EFS for 96 patients with osteosarcoma and measurable disease was 12% at 4 months (95% CI, 6% to 19%). There was no significant difference in EFS across trials according to number of prior treatment regimens or patient age, sex, and ethnicity. The 12-month EFS for the 42 evaluable patients enrolled in AOST0221 was 20% (95% CI, 10% to 34%).
Conclusion
The EFS was uniformly poor for children with recurrent/refractory osteosarcoma in these single-arm phase II trials. We have now constructed baseline EFS outcomes that can be used as a comparison for future phase II trials for recurrent osteosarcoma.
INTRODUCTION
Osteosarcoma is the most common primary malignant tumor of the bone and occurs primarily in children, adolescents, and young adults. The most recent major advance in the treatment of osteosarcoma occurred in the 1980s, when multiagent chemotherapy was demonstrated to improve overall survival compared with surgery alone.1 The combination of surgical resection and systemic chemotherapy with doxorubicin, cisplatin, high-dose methotrexate, and, in some regimens, ifosfamide, is considered standard treatment of osteosarcoma. With the exception of liposomal muramyl tripeptide phosphatidyl ethanolamine (L-MTP-PE), which is not approved for use in the United States,2,3 there have been no new chemotherapeutic, small-molecule–targeted, or immunotherapeutic agents found to be active in osteosarcoma. As a result, there has been little improvement in the survival of these patients in more than three decades.
This can be contrasted with the overall aggregate improvement in outcome for all other pediatric cancers combined in the same time period; specifically, the 5-year relative survival rate for children diagnosed from 1975 to 1977 versus 2002 to 2008 increased from 58% to 83%.4 An example of a similar malignancy for which there has been an improvement in patient outcome is nonmetastatic Ewing sarcoma.5,6
This begs the question: why have advances in osteosarcoma lagged behind? Radiographic response as the primary end point in osteosarcoma trials poses challenges for the identification of agents that are active in the treatment of osteosarcoma.
RECIST was developed in 20007 and has been used extensively in clinical trials. However, it has significant limitations.8-10 A particular problem with the evaluation of osteosarcoma response to treatment by radiographic imaging is the tendency for this tumor to stabilize or even increase in radiographically assessed size because of mineralization of the stromal tissue with tumor necrosis. Even if the tumor has few residual viable tumor cells after treatment, it will still occupy substantial volume because of the matrix produced by the malignant cells, which does not disappear when the cells die. Hence, objective radiographic responses are rare in osteosarcoma, even with proven complete necrosis in the tumor after neoadjuvant chemotherapy in patients with newly diagnosed disease. In addition, an increase in osteosarcoma tumor size often does not consistently correlate with disease progression.11 Consequently, the radiographic behavior of osteosarcoma may lead to the inability to detect clinical activity of novel therapies in clinical trials that use this as an end point.
Moreover, the standard clinical approach to recurrent osteosarcoma is to surgically resect disease whenever possible, because this is proven to result in long-term survival for a small subset of patients. Although rendering patients in surgical complete remission is the only proven therapeutic strategy that affects outcome in recurrent osteosarcoma,12-17 this approach makes patients ineligible for trials that require measurable disease for enrollment. This may represent a missed opportunity for the evaluation of activity of novel agents in the context of minimal residual disease.
The goal of this analysis was to use data from previous phase II trials from the Children’s Oncology Group (COG) and its predecessor groups (the Children’s Cancer Group and the Pediatric Oncology Group) to establish a baseline of expected time for disease progression in patients with relapsed osteosarcoma. We plan to use these data to facilitate alternate designs for future phase II trials, which we hope would avoid the aforementioned pitfalls, and more accurately identify active agents for osteosarcoma.
PATIENTS AND METHODS
Patients
Seven phase II trials for children with refractory/recurrent solid tumors with an osteosarcoma cohort conducted by the COG and its predecessor groups from 1997 to 2007 that had final study reports completed in July 2009, when this project was initiated, were included in this analysis. Protocols were reviewed by institutional review boards at participating institutions. Informed consent was obtained from all patients or guardians in accordance with institutional policies and as approved by the US Department of Health and Human Services.
The primary outcome measure for these trials was radiographic response (WHO or RECIST). Three trials—ADVL0122 (imatinib), ADVL0421 (oxaliplatin), and ADVL0524 (ixabepilone)—included time to disease progression as one of the study’s aims. For all trials included in this analysis, time to disease progression was collected prospectively. All patients enrolled were observed for status, including all occurrences of disease, as well as death, until loss to follow-up or a minimum of 5 years after enrollment (whichever occurred first). Table 1 lists the study drug and dose, study primary end point, number of osteosarcoma enrollees, and drug activity. Studies used either a two-stage or three-stage design with null (uninteresting) response rates of either 5% or 10% and alternative (interesting) response rates of 25% or 30%. The trials were designed so that the number of patients to be enrolled would maintain a type I error rate of no more than 10% and a power of at least 85%.
Table 1.
Summary of Responses of Patients With Osteosarcoma Enrolled in Seven Phase II Trials for Refractory/Recurrent Pediatric Solid Tumors and AOST0221 (phase II aerosolized GM-CSF)
| Study (years open) | Agent (dose) | End Point | No. of Enrolled Patients With Osteosarcoma/No. of Patients With Evaluable Response | No. of Patients Who Demonstrated Response According to Study Criteria | Activity According to Study End Point |
|---|---|---|---|---|---|
| CCG096218 (1997-2001) | Docetaxel 125 mg/m2 every 21 days | Radiographic (WHO) | 22/21 | 2 | No activity |
| A0971319 (1999-2003) | Topotecan 0.3 mg/m2 continuous 21-day infusion every 28-day cycle | Radiographic (WHO) | 11/11 | 0 | No activity |
| P976120 (1999-2005) | Irinotecan 50 mg/m2 for 5 days every 21 days | Radiographic (WHO) | 10/9 | 0 | No activity |
| P996321 (2000-2004) | Rebeccamycin 650 mg/m2 every 21 days | Radiographic (RECIST) | 17/16 | 0 | No activity |
| ADVL012222 (2002-2004) | Imatinib 440 mg/m2/day continuously | Radiographic (RECIST)* | 12/10 | 0 | No activity |
| ADVL042123 (2004-2006) | Oxaliplatin 130 mg/m2 every 21 days | Radiographic (RECIST)* | 13/10 | 0 | No activity |
| ADVL052424 (2006-2007) | Ixabepilone 8 mg/m2 once per day for 5 days every 21 days | Radiographic (RECIST)* | 11/10 | 0 | No activity |
| AOST022125 (2004-2008) | Aerosolized GM-CSF 250 µg-1750 µg twice per day on alternate weeks | Biologic (expression of Fas/Fas ligand and presence of dendritic cells) | 43/42 | 12-month EFS, 20% | No observed biologic activity, no improvement in outcome |
Abbreviation: EFS, event-free survival; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Studies that also evaluated time to progression.
AOST0221 was a phase II study specific to first pulmonary recurrence of osteosarcoma, which determined the effect of inhaled granulocyte-macrophage colony-stimulating factor (GM-CSF) on disease-free survival and assessed its immunomodulatory effect on pulmonary lesions post treatment (Table 1). Inclusion criteria included patients younger than 40 years old with suspected first isolated resectable (defined as able to be removed without pneumonectomy) pulmonary recurrence of osteosarcoma, no pleural effusion, at least one parenchymal nodule, only one other prior treatment regimen, ability to undergo complete surgical resection of pulmonary metastases, ability to perform inhalational therapy, and no evidence of pulmonary dysfunction at baseline.
After two cycles of treatment, patients underwent thoracotomy to have the tumor resected and to have pulmonary nodules analyzed for the expression of Fas/Fas ligand and the presence of dendritic cells by immunostains (CD1a, clusterin, and S100). Forty-two patients who were disease free after two cycles of GM-CSF were considered in this analysis. There was no detectable immunostimulatory effect in osteosarcoma pulmonary metastases.25
Statistical Methods
All patients enrolled in the studies noted in the Patients section, including those unevaluable for the primary study end point, were included in this retrospective analysis. The cutoff dates for data preparation for each of the trials are identified in the primary publication for each particular study.
Outcome definition.
Event-free survival (EFS)—defined as time from study enrollment until date of last contact, date of disease progression, or detection of disease at a previously uninvolved site, or date of death— was calculated for each patient. Patients who died or experienced disease progression were considered to have experienced an EFS event; otherwise, the patient was considered censored at the date of last follow-up. EFS was a function of time, because study enrollment was estimated according to the Kaplan-Meier method.26 Study records were reviewed to determine the reason patients terminated protocol therapy. Patients who stopped protocol therapy because of patient or family preference or because of toxicity and who subsequently died without reporting the date of disease recurrence were considered to have disease progression at the time of death.
For patients who were not enrolled in AOST0221, potential prognostic factors examined for their influences on risk of an EFS event included study of enrollment, age group at enrollment (coded as ≤ 9 years of age v 10 to 17 years of age v ≥ 18 years of age), number of chemotherapy regimens received prior to enrollment on the particular study (coded as 1 v 2 v ≥ 3), patient sex (coded as male v female), and patient race/ethnicity (coded as white v black v other).
Statistical comparisons.
The equality of risk for EFS event across groups defined by the categories for each of the factors noted in the Outcome definition section was assessed with the log-rank test.27 A two-sided P value of .05 or less was considered evidence of a significant difference in risk for EFS event across the categories considered.
RESULTS
Patients
Ninety-six patients from A09713 (topotecan), ADVL0122 (imatinib), ADVL0421 (oxaliplatin), ADVL0524 (ixabepilone), CCG-0962 (docetaxel), P9761 (irinotecan), and P9963 (rebeccamycin analog) were identified for inclusion in this data set. Patient characteristics, such as age, gender, race/ethnicity, and the number of prior chemotherapy regimens, are listed in Table 2. Only one patient was enrolled in more than one trial (enrolled first on CCG-0962 and then on A9713). Each enrollment was retained in the analysis and was considered an independent observation for the purposes of the analytic methodology.
Table 2.
EFS and Patient Demographic and Clinical Characteristics in the Phase II Study
| Characteristic | No. of Patients | Events | EFS at 4 Months (%) | ||
|---|---|---|---|---|---|
| None | Relapse | Death | |||
| All eligible patients | 96 | 2 | 84 | 10 | 12 |
| Study and drug | |||||
| A09713 (topotecan) | 11 | 0 | 11 | 0 | * |
| ADVL0122 (imatinib) | 12 | 0 | 8 | 4 | * |
| ADVL0421 (oxaliplatin) | 13 | 2 | 9 | 2 | 0.31 |
| ADVL0524 (ixabepilone) | 11 | 0 | 10 | 1 | 0.09 |
| CCG0962 (docetaxel) | 22 | 0 | 21 | 1 | 0.23 |
| P9963 (rebeccamycin) | 17 | 0 | 16 | 1 | 0.06 |
| P9761 (irinotecan) | 10 | 0 | 9 | 1 | * |
| No. of prior treatment regimens | |||||
| 1 | 51 | 1 | 43 | 7 | 0.12 |
| 2 | 34 | 1 | 31 | 2 | 0.12 |
| ≥ 3 | 10 | 0 | 9 | 1 | 0.10 |
| Age, years | |||||
| < 9 | 11 | 0 | 10 | 1 | 0.09 |
| 10-17 | 40 | 0 | 39 | 1 | 0.08 |
| > 18 | 45 | 2 | 35 | 8 | 0.16 |
| Sex | |||||
| Male | 62 | 2 | 4 | 6 | 0.13 |
| Female | 34 | 0 | 30 | 4 | 0.09 |
| Race | |||||
| White | 58 | 1 | 49 | 8 | 0.10 |
| Black | 16 | 1 | 14 | 1 | 0.19 |
| Other | 22 | 0 | 21 | 1 | 0.09 |
Abbreviation: EFS, event-free survival.
No patients were observed for follow-up at 4 months.
One patient was removed from protocol therapy on the day of enrollment and had no additional follow-up. Of the 96 patients included in the analysis, 83 experienced disease progression while receiving therapy on the particular protocol on which the patient was being followed. Of the remaining 13 patients, 10 stopped protocol therapy because of patient or family preference, or because of toxicity, and subsequently died (without reporting the date of disease recurrence) at a median of 37.5 days from the end of protocol therapy. These patients were considered to have an event for the purposes of this analysis on the day of death.
EFS in Patients With Measurable Disease Enrolled in Phase II Trials
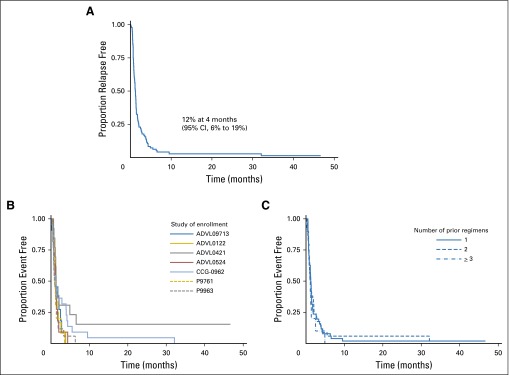
Of the 95 patients with some follow-up for EFS, 93 experienced an event. Two patients enrolled in ADVL0421 (oxaliplatin) were reported as alive and without an EFS event at 8 and 46 months after enrollment. The EFS was 12% at 4 months, and the 95% CI was 6% to 19% (Fig 1A).
Fig 1.
(A) Relapse-free survival of patients in the osteosarcoma cohort enrolled in seven phase II trials. (B) Event-free survival of patients in the osteosarcoma cohort by study. (C) Event-free survival of patients in the osteosarcoma cohort by number of prior treatments.
Impact of Covariates on EFS With Measurable Disease Enrolled in Phase II Trials
Patient characteristics such as age, sex, and ethnicity were not significantly related to the risk of disease progression (Table 2). There was no significant difference in the EFS across the different studies (Fig 1B) and number of prior treatments (Fig 1C).
EFS in Patients With Completely Resected Disease
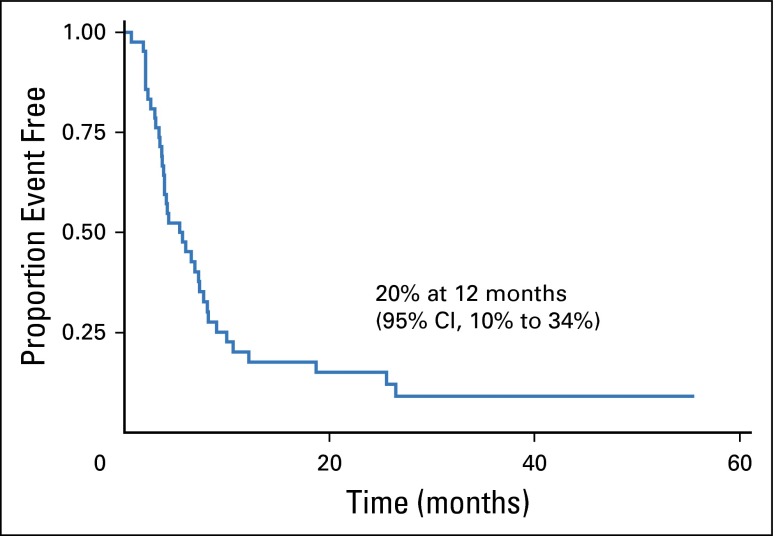
The intervention in AOST0221 (inhaled GM-CSF) was determined to lack activity on the basis of the primary outcome measure (biologic response). The 12-month EFS was 20%, with a 95% CI of 10% to 34% (Fig 2).
Fig 2.
Event-free survival of patients with osteosarcoma who received aerosolized granulocyte-macrophage colony-stimulating factor per Children’s Oncology Group study AOST0221.25
DISCUSSION
By characterizing EFS from prior studies in which agents were not considered efficacious according to conventional response criteria, this analysis allows for the introduction of benchmarks that can be used in the design of single-arm phase II trials that use EFS as an end point in osteosarcoma. Introduction of alternative end points, such as EFS or progression-free survival (PFS) in lieu of radiographic response, has previously been proposed for other diseases, such as metastatic melanoma.28 To date, however, osteosarcoma phase II trials have used objective response rate (ORR) primarily on the basis of RECIST criteria as the primary end point. The use of ORR as an end point may be particularly problematical in osteosarcoma because of several unique aspects of this disease: (1) radiographic response may not be the outcome that optimally reflects efficacy of an agent at the cellular level; (2) the standard approach to isolated pulmonary recurrence is surgical resection, which results in a significant number of patients with no radiographically measurable disease by the time of study entry, (ie, ineligible for enrollment); and (3) there is a realistic possibility of different drug activity in microscopic versus gross residual disease. Therefore, it is especially important in osteosarcoma that the COG and other clinical trials cooperative groups pursue phase II trials of new therapies in patients with recurrent and refractory osteosarcoma that have statistical design, eligibility criteria, and outcome measures that take into account these unique aspects of osteosarcoma.
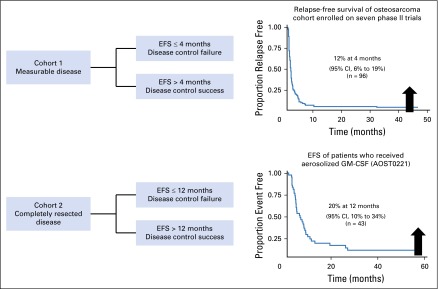
One possibility for future phase II trials in osteosarcoma is to conduct a single-arm phase II trial that uses EFS as the primary end point according to the historical benchmark derived from this analysis (Figure 3). We have used this approach in ongoing or recently completed COG trials (NCT02097238, NCT02470091, and NCT02484443). For example, for each patient with measurable, unresectable osteosarcoma, we would dichotomize EFS according to whether EFS is ≤ 4 months or > 4 months and define these as disease control failure (DCF) and disease control success (DCS), respectively. Because the statistical properties, including the type I and type II error rates, of two-stage phase II designs29 are well understood, the design shown in Table 3 can be used. If the DCS probability is 20%, which is at the upper 95% confidence bound for DCS probability for the historical population, the design identifies the agent as not of interest for additional development with a probability of 0.90. If the DCS probability is 40%, the design identifies the agent as of interest for additional development with a probability of 0.90.
Fig 3.
Future phase II osteosarcoma study design. EFS, event-free survival; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Table 3.
Single-Arm, Two-Stage, Phase II Trial Design for Patients With Measurable, Unresectable Osteosarcoma With an Event-Free Survival End Point According to the Historical Benchmark From This Analysis
| Stage | Cumulative No. Enrolled | Cumulative No. With Disease Control ≥ 4 Months | Decision |
|---|---|---|---|
| 1 | 19 | ≤ 3 | Terminate enrollment with the conclusion that the agent is not efficacious |
| 2 | 36 | ≥ 4 | Continue enrollment |
| ≤ 10 | Consider the agent ineffective | ||
| ≥ 11 | Consider the agent of sufficient efficacy for additional study |
We extended this approach to patients with completely resected disease by focusing on the 12-month time point. In this case, we dichotomized EFS according to whether EFS is ≤ 12 months or > 12 months and defined these as 12-month DCF (DCF12) and 12-month DCS (DCS12), respectively. A trial result with a DCS12 probability of 30% was considered insufficient for additional development, because 30% represents the largest plausible value for DCS according to the 95% CI from the historical benchmark derived from this analysis. The ongoing and recently completed COG phase II trials have used this definition of DCS, and have adjusted the definition of DCF and the trial design parameters to fit the novel agent that is studied. As an example, the ongoing COG trial of denosumab (NCT02470091) has a type I error rate of 9% if the DCS12 probability is 30% and has a power of 90% for a DCS12 of 50%. The number of patients needed for this study is 39.
COG has chosen to pursue single-arm phase II trials rather than randomized phase II trials, despite the limitations of single-arm phase II trials (discussed in the limitations paragraph here), primarily because osteosarcoma is a rare disease, so the number of patients available to enroll on clinical trials is limited. Nevertheless, this historical benchmark could also be used in the design of randomized phase II trials, in which patients are randomly assigned to two or more experimental agents, and EFS is compared between experimental arms and to a benchmark EFS derived from this analysis. A possible limitation of a single-arm phase II approach that uses a historical benchmark for EFS is that changes in patient management over time can shift the expected EFS above the historical benchmark. This is unlikely in osteosarcoma because the standard of care of treatment for newly diagnosed and recurrent disease has not changed in the past three decades. In addition, there have been no new active agents in osteosarcoma in the same era. Moreover, one of the most important prognostic factors in recurrent osteosarcoma is the ability to secure surgical remission.30,31 In this study, by specifically analyzing the EFS of patients with completely resected disease and of patients with gross disease separately, we have addressed this issue.
There are several limitations to this analysis. The schedule for routine evaluation of these trials varied across studies, although it was usually between 21 and 28 days. Carroll32 demonstrated that the schedule of patient evaluation can affect statistical estimation when a significant proportion of events are detected at routine screening.
Because of this, we elected to focus on the 4-month post-enrollment time point, by which time 90% of events had been identified. By focusing on the time point by which time 90% of events had been identified, we avoided a significant effect on the point estimate or its variance as a result of variations in follow-up schedules.
We determined that the phase II trials included in our analysis were of inactive agents on the basis of the primary end point for these trials which was, in all cases, ORR. This could have resulted in the inclusion of phase II trials of agents with some limited degree of activity in the analysis. Specifically, trials in the data set, such as a trial with docetaxel that had two long-term survivors, showed possible activity. Ten patients included in this analysis stopped protocol therapy because of patient or physician preference or because of toxicity, and the date of progression was not reported; therefore, the patient was considered to have disease progression at the date of death. Inclusion of trials of active drugs in our analysis and use of date of death for date of progression in these 10 patients would have the effect of increasing the proportion of patients who were event free at 4 months. Consequently, an agent demonstrated to be active in a trial that uses EFS as an end point compared with this historical benchmark might be even more likely to demonstrate activity in future clinical trials. Of note, the 12-month EFS for AOST0221 (inhaled GM-CSF) is relevant to patients with recurrent osteosarcoma who would have met the eligibility criteria for that trial. Given the restrictive nature of the AOST0221 eligibility criteria, the 12-month EFS derived from this trial represents the best-case scenario for completely resected recurrent osteosarcoma. If eligibility criteria for a single-arm phase II trial were broader than for AOST0221 and the trial used an EFS end point that compared with the AOST0221 historical benchmark, then an agent that resulted in a positive trial might be even more likely to demonstrate activity in future clinical trials.
In this paper, we summarize the poor outcome of patients enrolled in the osteosarcoma cohort of seven closed phase II studies for refractory/recurrent solid tumors from COG and its predecessor groups. This evaluation provides a baseline for disease progression in a population of children and young adults with recurrent/refractory osteosarcoma that can be used as comparison for the design of future phase II trials in osteosarcoma. We hope this method will permit rapid screening of drug activity in patients with recurrent osteosarcoma. Active agents will be tested in a randomized manner along with standard of care chemotherapy.
Footnotes
Supported by National Clinical Trials Network (NCTN) Operations Center Grant No. U10CA180886, NCTN Statistics and Data Center Grant No. U10CA180899, Chair Grant No. U10CA98543, and Statistics and Data Center Grant No. U10CA98413, all from the National Cancer Institute. Additional research support was provided by a grant from the QuadW Foundation to the Children's Oncology Group.
Presented at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 29-June 5, 2015.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Joanne P. Lagmay, Mark D. Krailo, Richard Gorlick, Katherine A. Janeway
Collection and assembly of data: All authors
Data analysis and interpretation: Joanne P. Lagmay, Mark D. Krailo, Ha Dang, Douglas S. Hawkins, Holcombe E. Grier, Susan M. Blaney, Richard Gorlick, Katherine A. Janeway
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Outcome of Patients With Recurrent Osteosarcoma Enrolled in Seven Phase II Trials Through Children's Cancer Group, Pediatric Oncology Group, and Children's Oncology Group: Learning From the Past to Move Forward
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Joanne P. Lagmay
No relationship to disclose
Mark D. Krailo
No relationship to disclose
Ha Dang
No relationship to disclose
AeRang Kim
No relationship to disclose
Douglas S. Hawkins
No relationship to disclose
Orren Beaty III
No relationship to disclose
Brigitte C. Widemann
No relationship to disclose
Theodore Zwerdling
Research Funding: Jazz Pharmaceuticals (Inst), Novartis (Inst), GlaxoSmithKline (Inst)
Lisa Bomgaars
Consulting or Advisory Role: Janssen Pharmaceuticals (Inst)
Research Funding: Boeringer Ingelheim (Inst), Baxter (Inst)
Anne-Marie Langevin
Research Funding: Genentech/Roche (Inst)
Holcombe E. Grier
No relationship to disclose
Brenda Weigel
Travel, Accommodations, Expenses: Eli Lilly, ImClone Systems, Genentech, Nektar
Susan M. Blaney
No relationship to disclose
Richard Gorlick
Stock or Other Ownership: Oncolytics Biotech
Consulting or Advisory Role: Oncolytics Biotech
Travel, Accommodations, Expenses: Bayer
Katherine A. Janeway
No relationship to disclose
REFERENCES
- 1.Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–1606. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 2.Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: A randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23:2004–2011. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 3.Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival—A report from the Children’s Oncology Group. J Clin Oncol. 2008;26:633–638. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 5. Nesbit ME, Gehan EA, Burgert EO, et al: Multimodal therapy for the management of primary, nonmetastatic Ewing sarcoma of bone: A long-term follow-up of the first Intergroup Study. J Clin Oncol 8:1664-1674, 1990. [DOI] [PubMed]
- 6.Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the Children’s Oncology Group. J Clin Oncol. 2012;30:4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gehan EA, Tefft MC. Will there be resistance to the RECIST (Response Evaluation Criteria in Solid Tumors)? J Natl Cancer Inst. 2000;92:179–181. doi: 10.1093/jnci/92.3.179. [DOI] [PubMed] [Google Scholar]
- 8.McHugh K, Kao S. Response evaluation criteria in solid tumours (RECIST): Problems and need for modifications in paediatric oncology? Br J Radiol. 2003;76:433–436. doi: 10.1259/bjr/15521966. [DOI] [PubMed] [Google Scholar]
- 9.van Klaveren RJ, Aerts JG, de Bruin H, et al. Inadequacy of the RECIST criteria for response evaluation in patients with malignant pleural mesothelioma. Lung Cancer. 2004;43:63–69. doi: 10.1016/s0169-5002(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 10.Trillet-Lenoir V, Freyer G, Kaemmerlen P, et al. Assessment of tumour response to chemotherapy for metastatic colorectal cancer: Accuracy of the RECIST criteria. Br J Radiol. 2002;75:903–908. doi: 10.1259/bjr.75.899.750903. [DOI] [PubMed] [Google Scholar]
- 11.Schuetze SM, Baker LH, Benjamin RS, et al. Selection of response criteria for clinical trials of sarcoma treatment. Oncologist. 2008;13:32–40. doi: 10.1634/theoncologist.13-S2-32. [DOI] [PubMed] [Google Scholar]
- 12.Goorin AM, Delorey MJ, Lack EE, et al. Prognostic significance of complete surgical resection of pulmonary metastases in patients with osteogenic sarcoma: Analysis of 32 patients. J Clin Oncol. 1984;2:425–431. doi: 10.1200/JCO.1984.2.5.425. [DOI] [PubMed] [Google Scholar]
- 13.Meyer WH, Schell MJ, Kumar AP, et al. Thoracotomy for pulmonary metastatic osteosarcoma: An analysis of prognostic indicators of survival. Cancer. 1987;59:374–379. doi: 10.1002/1097-0142(19870115)59:2<374::aid-cncr2820590235>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Saeter G, Høie J, Stenwig AE, et al. Systemic relapse of patients with osteogenic sarcoma: Prognostic factors for long-term survival. Cancer. 1995;75:1084–1093. doi: 10.1002/1097-0142(19950301)75:5<1084::aid-cncr2820750506>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari S, Briccoli A, Mercuri M, et al. Postrelapse survival in osteosarcoma of the extremities: Prognostic factors for long-term survival. J Clin Oncol. 2003;21:710–715. doi: 10.1200/JCO.2003.03.141. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins DS, Arndt CA. Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer. 2003;98:2447–2456. doi: 10.1002/cncr.11799. [DOI] [PubMed] [Google Scholar]
- 17.Duffaud F, Digue L, Mercier C, et al. Recurrences following primary osteosarcoma in adolescents and adults previously treated with chemotherapy. Eur J Cancer. 2003;39:2050–2057. doi: 10.1016/s0959-8049(03)00435-0. [DOI] [PubMed] [Google Scholar]
- 18.Zwerdling T, Krailo M, Monteleone P, et al. Phase II investigation of docetaxel in pediatric patients with recurrent solid tumors: A report from the Children’s Oncology Group. Cancer. 2006;106:1821–1828. doi: 10.1002/cncr.21779. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins DS, Bradfield S, Whitlock JA, et al. Topotecan by 21-day continuous infusion in children with relapsed or refractory solid tumors: A Children’s Oncology Group study. Pediatr Blood Cancer. 2006;47:790–794. doi: 10.1002/pbc.20739. [DOI] [PubMed] [Google Scholar]
- 20.Bomgaars LR, Bernstein M, Krailo M, et al. Phase II trial of irinotecan in children with refractory solid tumors: A Children’s Oncology Group Study. J Clin Oncol. 2007;25:4622–4627. doi: 10.1200/JCO.2007.11.6103. [DOI] [PubMed] [Google Scholar]
- 21.Langevin AM, Bernstein M, Kuhn JG, et al. A phase II trial of rebeccamycin analogue (NSC #655649) in children with solid tumors: A Children’s Oncology Group study. Pediatr Blood Cancer. 2008;50:577–580. doi: 10.1002/pbc.21274. [DOI] [PubMed] [Google Scholar]
- 22.Bond M, Bernstein ML, Pappo A, et al. A phase II study of imatinib mesylate in children with refractory or relapsed solid tumors: A Children’s Oncology Group study. Pediatr Blood Cancer. 2008;50:254–258. doi: 10.1002/pbc.21132. [DOI] [PubMed] [Google Scholar]
- 23.Beaty O, III, Berg S, Blaney S, et al. A phase II trial and pharmacokinetic study of oxaliplatin in children with refractory solid tumors: A Children’s Oncology Group study. Pediatr Blood Cancer. 2010;55:440–445. doi: 10.1002/pbc.22544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs S, Fox E, Krailo M, et al. Phase II trial of ixabepilone administered daily for five days in children and young adults with refractory solid tumors: A report from the children’s oncology group. Clin Cancer Res. 2010;16:750–754. doi: 10.1158/1078-0432.CCR-09-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arndt CA, Koshkina NV, Inwards CY, et al. Inhaled granulocyte-macrophage colony stimulating factor for first pulmonary recurrence of osteosarcoma: Effects on disease-free survival and immunomodulation. A report from the Children’s Oncology Group. Clin Cancer Res. 2010;16:4024–4030. doi: 10.1158/1078-0432.CCR-10-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27. Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY, John Wiley and Sons, 2002.
- 28.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 29.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 30.Kempf-Bielack B, Bielack SS, Jürgens H, et al. Osteosarcoma relapse after combined modality therapy: An analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23:559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 31. Bielack SS, Kempf-Bielack B, Branscheid D, et al: Second and subsequent recurrences of osteosarcoma: Presentation, treatment, and outcomes of 249 consecutive cooperative osteosarcoma study group patients. J Clin Oncol 27:557-565, 2009. [DOI] [PubMed]
- 32.Carroll KJ. Analysis of progression-free survival in oncology trials: Some common statistical issues. Pharm Stat. 2007;6:99–113. doi: 10.1002/pst.251. [DOI] [PubMed] [Google Scholar]