Abstract
Purpose
DNA mismatch repair deficiency (dMMR) hallmarks consensus molecular subtype 1 of colorectal cancer. It is being routinely tested, but little is known about dMMR rectal cancers. The efficacy of novel treatment strategies cannot be established without benchmarking the outcomes of dMMR rectal cancer with current therapy. We aimed to delineate the impact of dMMR on prognosis, the predicted response to fluoropyrimidine-based neoadjuvant therapy, and implications of germline alterations in the MMR genes in rectal cancer.
Methods
Between 1992 and 2012, 62 patients with dMMR rectal cancers underwent multimodality therapy. Oncologic treatment and outcomes as well as clinical genetics work-up were examined. Overall and rectal cancer–specific survival were calculated by the Kaplan-Meier method.
Results
The median age at diagnosis was 41 years. MMR deficiency was most commonly due to alterations in MSH2 (53%) or MSH6 (23%). After a median follow-up of 6.8 years, the 5-year rectal cancer–specific survival was 100% for stage I and II, 85.1% for stage III, and 60.0% for stage IV disease. Fluoropyrimidine-based neoadjuvant chemoradiation was associated with a complete pathologic response rate of 27.6%. The extent of surgical resection was influenced by synchronous colonic disease at presentation, tumor height, clinical stage, and pelvic radiation. An informed decision for a limited resection focusing on proctectomy did not compromise overall survival. Five of the 11 (45.5%) deaths during follow-up were due to extracolorectal malignancies.
Conclusion
dMMR rectal cancer had excellent prognosis and pathologic response with current multimodality therapy including an individualized surgical treatment plan. Identification of a dMMR rectal cancer should trigger germline testing, followed by lifelong surveillance for both colorectal and extracolorectal malignancies. We herein provide genotype-specific outcome benchmarks for comparison with novel interventions.
INTRODUCTION
DNA mismatch repair (MMR) status is one of the most well-established biomarkers in colorectal cancer (CRC). The DNA MMR system helps maintain genetic fidelity, and when defective, genetic errors accumulate, leading to microsatellite instability (MSI) and intestinal carcinogenesis.1,2 DNA MMR status is being increasingly tested universally for all CRCs.3-9 MMR deficiency (dMMR) has been associated with a favorable prognosis and a predicted poorer response to fluoropyrimidine-based adjuvant therapy in colon cancer.1,10 Emerging evidence suggests that dMMR can be predictive of significant response and survival gain from immune checkpoint (eg, programmed cell death protein 1) inhibitors.11 Finally, finding dMMR in CRC triggers the detection of a potential heritable germline deficiency in MMR. Patients with identifiable pathogenic mutations have Lynch syndrome (LS), whereas those without have been termed as having mutation-negative LS12,13 or Lynch-like syndrome.14,15
Novel treatment trials are being rapidly developed for dMMR CRCs, using immunotherapy alone or in combination with conventional therapy. However, the implications of dMMR status remain undefined in rectal cancer. First, the prognosis for dMMR rectal cancer treated with conventional therapy has not been benchmarked because of a paucity of long-term survival data for dMMR rectal cancers specifically,16 despite evidence that colon and rectal cancers may differ biologically.17 Second, the response rate of dMMR rectal cancers to fluoropyrimidine-based neoadjuvant therapy with radiation has not been established.18 Third, key issues in clinical genetics and optimal management of patients with rectal cancer with LS or mutation-negative LS remain controversial.
We therefore aimed to determine the impact of dMMR status on the long-term prognosis and the pathologic response rate to standard multimodality therapy and to establish cornerstones of clinical genetics care for patients with rectal cancers. Benchmarking outcomes of dMMR rectal cancers with current therapeutic and preventive strategies is a necessary first step to enable the development of novel biomarker-driven treatment trials in the future.
METHODS
Study Cohort
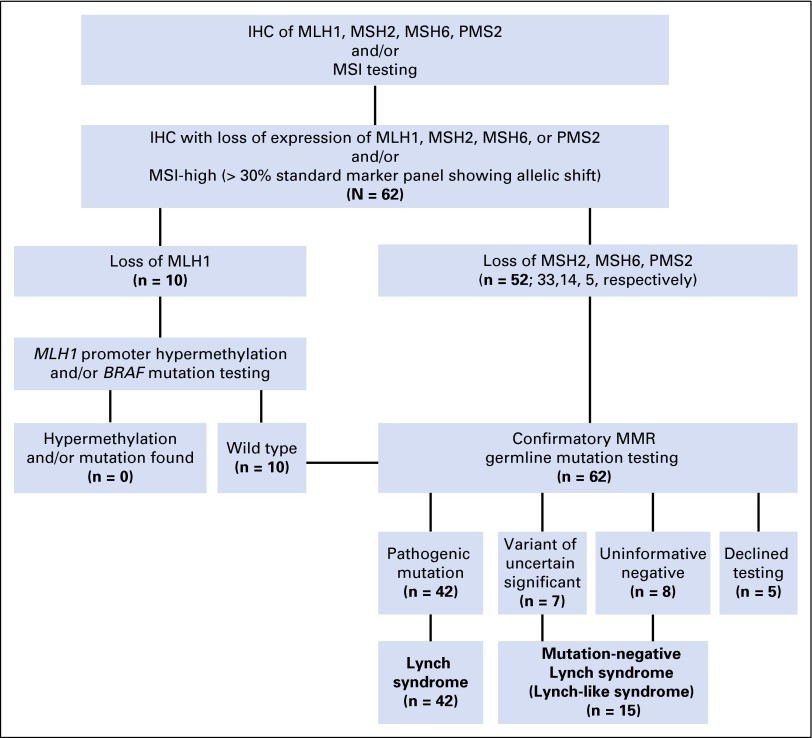
After approval from the University of Texas MD Anderson Cancer Center Institutional Review Board, the prospectively maintained Colorectal Surgery and Gastrointestinal Genetic Counseling databases were queried to identify patients (age > 18 years) with dMMR rectal adenocarcinoma diagnosed between 1992 and 2012. Tumor MMR testing was performed through a standardized institutional algorithm at the Clinical Laboratory Improvement Amendments–certified Molecular Diagnostics Laboratory (Fig 1).19 Immunohistochemistry for the MMR proteins MLH1, MSH2, MSH6, and PMS2 and polymerase chain reaction–based MSI testing were performed on tumor and adjacent normal tissue. Untreated tumor tissue was used whenever possible. dMMR tumor genotype was defined as either MSI-high (MSI-H; ie, > 30% of the standardized panel of markers showed allelic shift) and/or loss of expression of at least one MMR protein by immunohistochemistry.20
Fig 1.
A standardized algorithm for testing colorectal cancer for DNA mismatch repair (MMR) status at University of Texas MD Anderson Cancer Center was used in identifying our study cohort of 62 patients with MMR-deficiency rectal cancers. All clinical testing has been updated to the current standard of care. IHC, immunohistochemistry; MSI, microsatellite instability.
Clinicopathologic Data and Multimodality Treatments of Rectal Cancer
Records were reviewed for demographics, clinicopathologic characteristics, and treatments. Rectal cancer was considered the index CRC if it was the first cancer in the colon or rectum. Clinical staging was based on physical examination and computed tomography before 2004 and on endorectal ultrasound and/or pelvic magnetic resonance imaging more recently.
Surgical procedures were classified as segmental when the goal was proctectomy for rectal cancer and the abdominal colon was mostly left intact. Procedures were classified as extended when the abdominal colon was removed in addition to proctectomy for rectal cancer. Transanal local excision (LE) was classified separately (Table 1).
Table 1.
Patient and Tumor Characteristics Among 62 Patients With MMR-Deficient Rectal Cancer
| Characteristic | No. of Patients (%) |
|---|---|
| Age at rectal cancer diagnosis, median (IQR), years | 41 (35-54) |
| Sex | |
| Male | 32 (52) |
| Female | 30 (48) |
| Race/ethnicity | |
| White | 51 (82) |
| Black | 3 (5) |
| Hispanic | 4 (6.5) |
| Asian | 4 (6.5) |
| Rectal cancer presentation | |
| Rectal cancer as index cancer | 58 (93.5) |
| Synchronous high-risk colon lesion | 12 (19.4) |
| Prior colon cancer | 4 (6.4) |
| Tumor distance from anal verge, median (IQR), cm | 7.5 (4.6-12) |
| Tumor histologic grade | |
| Well differentiated | 2 (3.2) |
| Moderately differentiated | 53 (85.5) |
| Poorly differentiated | 7 (11.3) |
| Mucinous tumor | 38 (61.3) |
| Tumor-infiltrating lymphocytes | 3 (4.8) |
| Clinical stage of rectal cancer at diagnosis | |
| cT1-T2N0 | 17 (27.4) |
| cT3-4N0 | 14 (22.5) |
| cTanyN-positive | 26 (41.9) |
| cTanyNanyM-positive | 5 |
| Surgical procedure* | 59 |
| Segmental | 41 (69.5) |
| Low anterior resection | 25 |
| Coloanal anastomosis | 5 |
| Abdominal perineal resection/partial or total pelvic exenteration | 11 |
| Extended | 11 (18.6) |
| Near-TPC, ileo–low rectal anastomosis | 4 |
| TPC, IPAA | 2 |
| TPC, end ileostomy | 5 |
| Local excision | 7 (11.9) |
| Neoadjuvant therapy (fluorouracil and long-course radiation) | 30 (75% of 40 patients with cT3 to 4 or cN-positive) |
Abbreviations: IPAA, ileal pouch anal anastomosis; IQR, interquartile range; MMR, mismatch repair; TPC, total proctocolectomy.
Three patients declined surgical resection because of: complete clinical response to neoadjuvant therapy (one), metastatic progression (one), and concurrent lymphoma (one).
Pathologic assessment included the percentage of viable tumor remaining after neoadjuvant therapy. Pathologic complete response (pCR) was defined as no viable tumor cell in the surgical specimen (ie, ypT0N0), whereas downstaging was defined as a pathologic stage lower than the clinical stage.
Clinical Genetics Germline Mutation Testing and Genetic Risk Assessment
Patients with dMMR CRC and deficiency in MLH1 underwent secondary testing for MLH1 promoter hypermethylation and/or BRAF mutation (Fig 1).19 Patients with MLH1 deficiency but no evidence of promoter hypermethylation and/or BRAF mutation, as well as patients with deficiency in MSH2, MSH6, and PMS2, underwent genetic counseling for confirmatory germline mutation testing (Fig 1; Data Supplement, Method). Cases with identified pathogenic mutations were consistent with LS. Cases where germline test reported a variant of unknown significance (VUS) on the basis of functionality assessment21 using in silico tools22-26 or an uninformative negative result27 were consistent with mutation-negative LS12,13 (Fig 1; Data Supplement, Method). The three-generation pedigree collected by genetic counselors was categorized as meeting Amsterdam I or II and revised Bethesda criteria.28 We further quantified family history using the PREMM1,2,6 score, which was calculated by incorporating sex, diagnosis of CRC, and endometrial and extracolorectal cancers in the proband and first- and second-degree relatives.29
Oncologic Follow-Up for Rectal Cancer and Surveillance for Extracolorectal Malignancies
All (100%) patients were followed until their last contact or death (Appendix Figure A1, online only). Vital status and cause of death were obtained from medical records, tumor registry correspondence, or death certificates. Minimum surveillance included semiannual laboratory testing and annual thoracic, abdominal, and pelvic imaging. Lower endoscopy was performed every 12 to 24 months. Additional screening for gastric, genitourinary, skin, and endometrial cancers were based on individual patient’s personal and family cancer history (Data Supplement, Table).30
Statistical Analysis
Categorical data were described as number and percent and continuous data as median and interquartile range (IQR). The primary end points were overall survival (OS) and rectal cancer-specific survival (RC-SS). Rectal cancer recurrence was defined as either local only (if disease was present in the pelvis only) or distant (if disease was present in extrapelvic organs). The Kaplan-Meier method was used to estimate the survival outcomes. In addition, factors influencing the choice of surgical procedures were identified using analysis of variance for continuous and χ2 for categorical variables. The incidence of metachronous colonic adenoma with high-grade dysplasia (HGD) or carcinoma and the incidence of metachronous extracolorectal cancers were tabulated. All statistical analyses were generated using SPSS Statistics 22 (Chicago, IL).
RESULTS
Patient and Tumor Characteristics
Sixty-two patients met our inclusion criteria. The median age at diagnosis was 41 years (IQR, 35 to 54 years), with 48% being women and 82% being white (Table 1). Rectal cancer was the index CRC in nearly all patients (58; 93.5%); four patients had five prior colon cancers (Table 2). Twelve (19.4%) patients harbored a synchronous colonic adenoma with HGD or cancer, and 14 (22.6%) had 19 prior extracolorectal cancers (Table 2).
Table 2.
Personal History of Malignancies in Patients With MMR-Deficient Rectal Cancers
| Cancer Type | Before Rectal Cancer | Metachronous to Rectal Cancer |
|---|---|---|
| Colon | 4 (6.4) | 8 (16.7)* |
| Distal | 1 | 7 |
| Proximal | 4 | 1 |
| Extracolorectal | 14 (22.6) | 14 (22.6) |
| Endometrial | 6 | 4 |
| Urothelial/transitional | 2 | 2 |
| Small bowel | 1 | 2 |
| Gastric | 1 | |
| Pancreatic | 1 | |
| Sebaceous skin | 1 | 1 |
| Ependymal | 1 | |
| Sarcoma | 1 | 1 |
| Lymphoma/leukemia | 2 | 1 |
| Breast | 1 | |
| Squamous cell skin† | 2 | 5 |
| Cervix/vagina | 1 | 1 |
| Lung | 1 | 2 |
| Prostate | 1 |
NOTE. Data presented as No. of patients (%).
Abbreviation: MMR, mismatch repair.
Among 48 patients with remaining at-risk colon.
Eyelid, cheek, foot, thumb, scalp.
The majority of the rectal cancers were moderately differentiated (85.5%) and mucin producing (61.3%; Table 1). The median tumor distance from the anal verge was 7.5 cm (IQR, 4.6 to 12 cm). Forty patients (64.5%) presented with clinical stage II (n = 14) or III (n = 26) disease (Table 1). Five patients (8.1%) had stage IV disease involving the liver (three), liver and ovary (one), and soft tissue (one).
Molecular Testing and Clinical Genetic Risk Assessment
dMMR was most frequently due to defective MSH2 (33 patients, 53%), followed by MSH6 (14 patients, 23%), MLH1 (10 patients, 16%), and PMS2 (five patients, 8%). No patient had evidence of MLH1 promoter methylation or BRAF mutation (Fig 1). Confirmatory MMR germline testing was completed in 57 patients (92%) and declined by five patients. Forty-two patients (74%) had a pathogenic mutation. Seven patients (12%) had a VUS and eight (14%) had uninformative negative results (Fig 1). Table 3 illustrates the clinical characteristics of VUS cases.
Table 3.
Variants of Uncertain Significance Detected in Patients With MMR-Deficient Rectal Cancer: Clinical Characteristics and In Silico Analysis at RNA and Protein Levels
| Pt | Gene | DNA Change | Protein Change | Mut | In Silico RNA Analysis | In Silico Protein Analysis | Age at Dx (years) | Sex | Other CA | Stage at Dx | Ams | Beth | PREMM1,2,6 Score | VS | FU (years) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NNSplice22 | SplicePort24 | PP223 | FATH-MM25 | Mut Taster26 | ||||||||||||||
| 1 | MLH1 | c.110A>G | p.E37G | Ms | NE | NE | PrD | D | D | 65 | F | Endometrial | cT4N0M0 | Yes | Yes | 88.3 | A | 10.8 |
| 2 | MLH1 | c.1958T>G | p.L653R | Ms | NE | AbSS | PrD | D | D | 41 | M | Colon | cT3N0M0 | No | Yes | 74 | A | 5.1 |
| 3 | MSH2 | c.328A>C | p.K110Q | Ms | NE | NE | PrD | D | D | 36 | M | Colon | cT4N1M0 | No | Yes | 28.5 | A | 11.8 |
| 4 | MSH2 | c.2030C>G | p.T677R | Ms | NE | NE | PrD | D | D | 45 | F | None | cT3N1M0 | No | Yes | 3.8 | A | 9 |
| 5 | MSH2 | c.2234T>G | p.I745R | Ms | NE | NE | PrD | D | D | 46 | F | Endometrial | cT1N0M0 | No | Yes | 19.5 | A | 9.7 |
| 6 | MSH2 | c.2320A>G | p.I774V | Ms | AbSS | AbSS | PsD | D | D | 30 | M | Osteosarcoma | cT3N1M0 | No | Yes | 33.3 | A | 8.2 |
| 7 | MSH6 | c.2600T>G | p.V867G | Ms | NE | NE | Neu | D | D | 41 | M | None | cT4N2M0 | No | Yes | 8 | A | 3.3 |
NOTE. The variants may be classified as likely pathogenic if one or two programs at the RNA levels predicted aberrant splicing or two or more programs at the protein level predicted damaging (Data Supplement, Method).
Abbreviations: A, alive; AbSS, aberrant splicing site; Ams, Amsterdam I or II; Beth, Bethesda; CA, cancer; D, damaging; Dx, diagnosis; FU, follow-up; Ms, missense; Mut, mutation; NE, no effect; Neu, neutral; PP2, polyphen2 (HumVar); PrD, probably damaging; PsD, possibly damaging; Pt, patient; SS, splicing site; VS, vital status.
Nearly all patients (98%) met the revised Bethesda criteria, but only 15% met Amsterdam I or II criteria. The median PREMM 1,2,6 score was 25.6 (IQR, 12.1 to 51.0), with 58 patients (94%) having a PREMM1,2,6 score > 5%.
Response to Fluorouracil-Based Neoadjuvant Chemoradiation in Locally Advanced Rectal Cancer
Among 40 patients with clinical stage II or III disease, 30 (75%) received neoadjuvant fluoropyrimidine-based chemotherapy and long-course pelvic radiation. Twenty-nine underwent surgical resection (one patient with a complete clinical response declined surgery). Eight (27.6%) patients had a pCR. All remaining patients except one (n = 16; 55.5%) were downstaged. The median percent of viable tumor cells was 10% (IQR, 0 to 50).
Surgical Treatment and Factors Associated With Surgical Decisions
Overall, 59 (95.1%) patients underwent surgical resection of curative intent: segmental in 41 (69.5%), extended in 11 (18.6%), and LE in seven (11.9%; Table 1). LE was performed for clinical T1N0 rectal cancers (n = 6) and for complete clinical response after neoadjuvant therapy (n = 1). The decision to perform extended over segmental resections was associated with the presence of synchronous high-risk colonic lesions at presentation (P < .001), upper rectal cancer (median of 12 v 7 cm from the anal verge; P = .004), earlier clinical stage (P = .002), and no need for neoadjuvant radiation (P = .01; Table 4). Forty-one (66.1%) patients received adjuvant chemotherapy.
Table 4.
Association Between Preoperative Clinical Factors and the Extent of Surgical Resection in Patients With MMR-Deficient Rectal Cancer
| Factor | All Patients Undergoing Resection (N = 59) | Segmental Procedures (n = 41; 69.5%) | Extended Procedure (n = 11; 18.6%) | Local Excision (n = 7; 11.9%) | P |
|---|---|---|---|---|---|
| Age at rectal cancer diagnosis (median), years | 41 | 40 | 44 | 56 | .047 |
| Sex, female | 28 (47.5) | 20 (48.8) | 3 (27.3) | 5 (71.4) | .179 |
| Rectal cancer presentation | |||||
| Index and only cancer | 55 (93.2) | 40 (97.5) | 10 (90.9) | 5 (71.4) | .037 |
| Index, with synchronous colonic HGD or adenocarcinoma | 12 (20.3) | 4 (9.7) | 7 (63.6) | 1 (17.3) | < .001 |
| Metachronous to a prior colon cancer | 3 (5.1) | 3 (7.3) | 0 (0) | 0 (0) | .500 |
| Rectal cancer distance from anal verge (median), cm | 7 | 7 | 12 | 2 | .004 |
| Clinical stage of rectal cancer | .002 | ||||
| cT1-2N0 | 15 (25.4) | 5 (12.2) | 4 (36.4) | 6 (85.7) | |
| cT3-4N0 | 13 (22.0) | 10 (24.4) | 3 (27.3) | 0 (0) | |
| cTanyN-positive | 26 (44.1) | 22 (53.7) | 3 (27.3) | 1 (14.3) | |
| cTanyNanyM-positive | 4 (6.8) | 4 (9.7) | 0 (0) | 0 (0) | |
| Neoadjuvant chemoradiation | 33 (55.9) | 28 (68.3) | 4 (36.4) | 1 (14.3) | .010 |
| Multivisceral resection | 17 (28.8) | 13 (31.7) | 4 (36.4) | 0 (0) | .191 |
| Pathogenic germline MMR mutation identified | 39 (66.1) | 27 (65.9) | 8 (72.7) | 4 (57.1) | .792 |
NOTE. Data presented as No. (%).
Abbreviations: HGD, high-grade dysplasia; MMR, mismatch repair.
Long-Term Prognosis and Metachronous Cancers
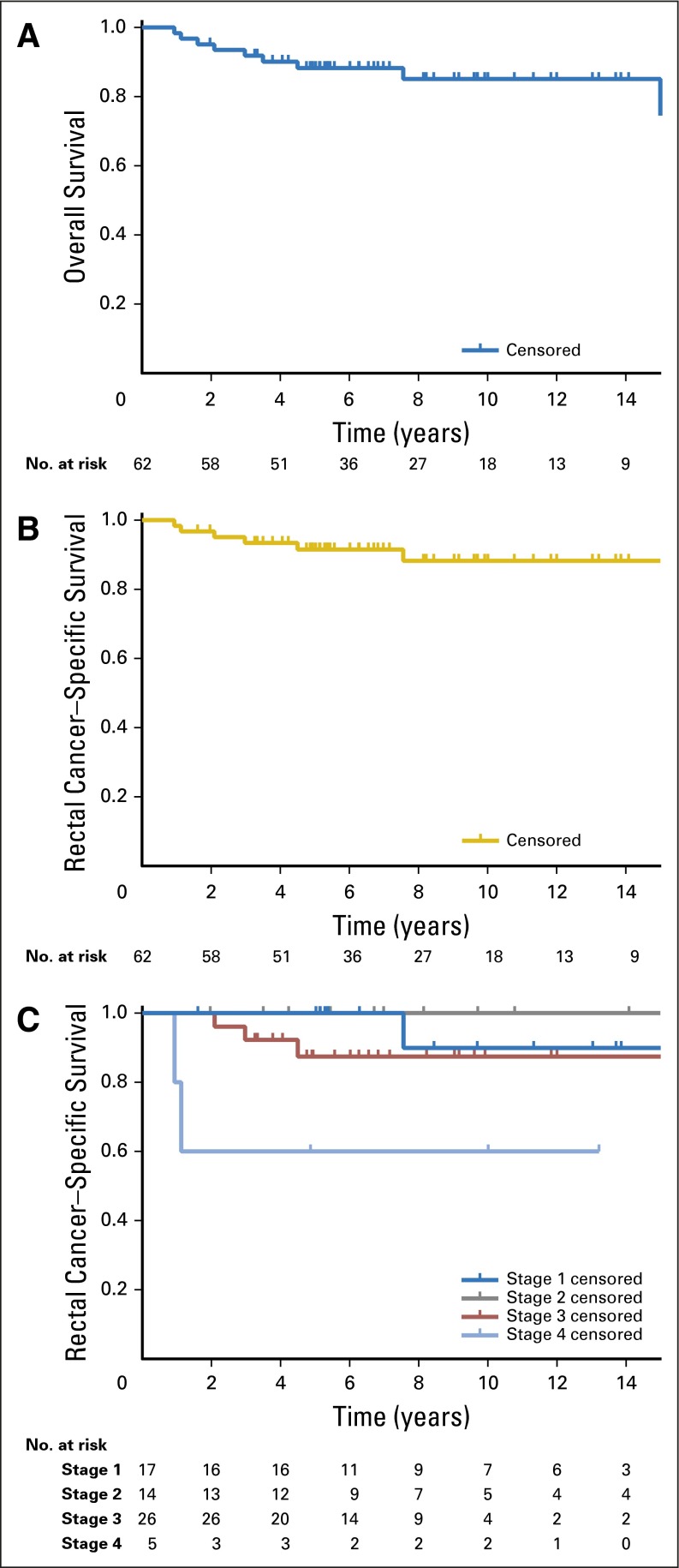
After a median follow-up of 6.8 years (IQR, 4.9 to 11.2), the overall 5-year OS was 87% and RC-SS was 90.6% (Figs 2A and 2B). The RC-SS differed by stage (P = .030; Fig 2C), with 5-year RC-SS of 100% for stage I and II, 85.1% for stage III, and 60.0% for stage IV. Disease recurred in 11 (18.6%) of the 59 patients who had curative-intent resection, eight locally and three distantly. Multimodal salvage therapy including repeat resection was feasible in nine patients.
Fig 2.
(A) Overall survival, (B) rectal cancer–specific survival, and (C) stage-stratified rectal cancer–specific survival.
At the last follow-up, 11 patients were deceased: six from rectal cancer (median, 2.5 years). Five (45.5%) died of extracolorectal cancers, including lymphoma (two), and urothelial cancer, transitional cell renal cancer, and duodenal cancer (one each) after 17.3 (median) years (Fig A1). Overall, 22 extracolorectal cancers developed in 14 (22.6%) patients (Table 2). Among the 48 patients who had segmental resection or LE for rectal cancer, 12 (25%) developed a metachronous colonic adenoma with HGD (four) or cancer (eight) at a median of 7.8 years (IQR, 2.2 to 9.2 years). Metachronous lesions were treated with: endoscopic mucosal resection (four), completion total proctocolectomy with end ileostomy (four), right hemicolectomy (three), and palliation only (one). There was no detectable difference in OS between the patients who underwent segmental versus extended procedures for their rectal cancer (5-year OS, 88.2 v 100%; P = .40).
DISCUSSION
Although universal testing of MMR for CRC has increased,8,9,11 the recent potential for novel immunotherapies to revolutionize the treatment of dMMR tumors has further sparked the need for MMR testing. In this largest clinical series of patients with dMMR rectal cancer reported to date to our knowledge, we established that current multimodality treatment provided a potential for cure in stage I and II disease and 5-year survival rates of 85.1% and 60% for stage III and IV disease, respectively. For locally advanced disease, fluoropyrimidine-based neoadjuvant chemoradiation was associated with a 27.6% pCR rate. Finally, all dMMR rectal cancers in our study were hereditary (related to either LS or mutation-negative LS). The extent of surgical resection was influenced by the location and clinical stage of the rectal cancer as well as age and synchronous colonic lesions at presentation, but choice of operation did not influence survival. Taken together, these data provide benchmarks for expected prognosis and neoadjuvant response rates with standard curative-intent therapy and highlight the key issues for clinical genetics care.
The long-term prognosis of dMMR rectal cancers with curative-intent therapy has not been established to date. Prior studies have been conflicting: one reported 5-year OS of 50% for 22 MSI-H rectal cancers,31 and another reported 3-year disease-free survival of 90% for 20 MSI-H rectal cancers.16 The Colon Cancer Family Registry reported 5-year OS of 83% for 37 MSI-H rectal cancers, but the prognosis was not stage stratified.32 Among our 62 patients, we observed an outstanding prognosis at all stages when compared with contemporaneous institutional data of MMR-proficient CRCs: 5-year recurrence-free survival of 70% for locally advanced rectal cancer33 and 5-year OS of 50% for metastatic CRC.34 The absence of concurrent BRAF mutation in our cohort likely contributed to the excellent outcomes.35 Thus, an aggressive approach in the event of disease recurrence and/or metachronous CRC as we have taken herein seems warranted. Importantly, dMMR CRCs induce formation of antigens, immune mediators, and cytotoxic lymphocytes, perhaps contributing to the favorable outcomes and highlighting the potential for treatment response to emerging immunotherapy strategies.11 The prognosis associated with standard therapy provides a benchmark for novel immune-based therapeutic strategies in these tumors.
Despite the general agreement that dMMR predicts poor response to adjuvant fluropyrimidine,10 its impact on response to fluoropyrimidine given with neoadjuvant radiation is controversial.18 Radiation response, available only in small series with little clinical detail, has been heterogeneously defined and has ranged between 0% and 60%.18,36-39 To our knowledge, our series of 29 patients with dMMR rectal cancer who underwent surgery after neoadjuvant chemoradiation constitutes the largest series to date. The observed pathologic response was excellent and compared favorably to a pCR rate of 18% and a downstaging rate of 47% among patients without LS treated with neoadjuvant chemoradiation.33 MMR system proteins play multiple roles in the cellular DNA damage response pathway and mediate the switch between cell-cycle progression and cell death, depending on DNA integrity.39 Thus, fluoropyrimidine as a radio-sensitizing agent for dMMR rectal cancer seems to be associated with favorable pathologic response. However, the potential for combination or sequential treatment approaches incorporating novel immunotherapy remains to be determined.
In our study, once dMMR is identified in a rectal cancer, the probability of identifying a pathogenic germline mutation was high at 74%, and the predicted probability on the basis of the PREMM model was 94%. Patients with dMMR rectal cancer exhibited deficiency in the MSH2/MSH6 complex most frequently. These MMR genes have been associated with higher incidences of extracolorectal cancers3: 22.6% of our cohort indeed developed and 45% of the mortalities arose from extracolorectal cancers. Thus, multiorgan cancer surveillance is critically important. Furthermore, a high incidence of family history of rectal cancer had been observed,16,40 and identifying a germline mutation in the proband enables predictive testing of all blood relatives. Thus, whenever a dMMR rectal cancer is identified, confirmatory germline testing should be pursued, either through direct testing of MMR genes6-8 or as part of a multiplex gene panel.9 Enrollment in specialized registries such as our Familial High-Risk Gastrointestinal Cancer Clinic can facilitate their long-term care (Data Supplement).3,41
The extent of surgical resection for dMMR rectal cancers associated with LS is controversial. Achieving best oncologic outcome is balanced by competing risks from metachronous colon and extracolorectal cancers and preservation of sphincter function and quality of life.42,43 Our surgical decision making considered disease presentation (synchronous colonic lesions), tumor height, clinical stage, and need for pelvic radiation. The high proportion of patients (69.5%) undergoing segmental resections likely reflected the many patients needing pelvic radiation and/or multivisceral resection, where an ileal pouch–anal anastomosis may be technically difficult and result in uncertain functional outcomes.42,44 More extended resections were more willingly performed for an upper rectal cancer where an ileo–low rectal anastomosis might be feasible. Other factors were synchronous colon lesions and early-stage rectal cancer in younger patients with long life expectancy. The risk of metachronous colon cancer has been reported40,45-47 as 19% at 10 years up to 69% at 30 years among 79 mutation carriers,47 with one report of a six-fold increase in mortality risk with developing a metachronous cancer.40 We observed eight metachronous colon cancers (16.7%) among 48 patients who had segmental resection after 7.8 years but detected no significant survival difference on the basis of the extent of resection. Thus, our data support an individualized approach to surgical management accounting for synchronous colonic lesions at rectal cancer diagnosis, tumor location and stage, and the need for neoadjuvant radiation. An informed decision to pursue segmental resection can lead to excellent long-term oncologic outcomes when patients are carefully followed.
Our study includes the largest molecularly characterized and clinically annotated cohort of patients with dMMR rectal cancer to our knowledge but it remains limited by tertiary-referral bias and its retrospective nature. Despite our median follow-up of 6.8 years (IQR, 4.9 to 11.2 years), late relapse or deaths could have remained uncaptured. Furthermore, five patients (8%) had declined germline testing, and surveillance of extracolorectal malignancies was not prospectively standardized. These variations may influence the prognostic outcomes reported herein. Finally, factors that likely contributed to the decision to perform segmental versus extended resections were only determined retrospectively, and we did not have direct data documenting patient preferences influencing the decision.
In conclusion, dMMR rectal cancer is a genetically defined subclass of CRC facing the potential for revolutionized care with novel immunotherapeutic approaches, but the prognostic and predictive implications of dMMR had not been specifically established to date. We herein benchmarked the expected stage-specific prognosis and response rates of dMMR rectal cancers after standard curative-intent multimodality therapy with neoadjuvant single-agent fluoropyrimidine. Key issues for clinical genetics care include confirmatory germline testing, individualized surgical decision making, and institution of a lifelong multiorgan surveillance program. By bridging the previous knowledge gaps, the efficacy of novel therapy and preventive efforts for patients with dMMR rectal cancer can be accurately assessed and improved.
Acknowledgment
We thank Sarah Bannon, CGC, and Maureen Mork, CGC, for their dedication to our patients and our Familial High-Risk Gastrointestinal Cancer Clinic. We also thank Amanda Cuddy, BS, for her oversight and management of the Hereditary Colorectal Cancer Syndrome Registry.
Appendix
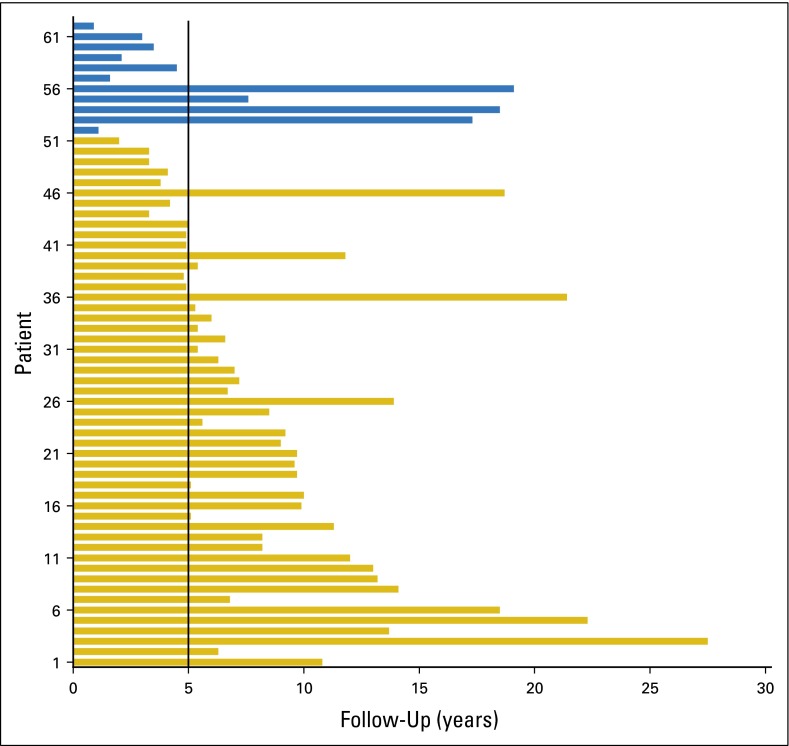
Fig A1.
The length of follow-up for 62 patients with mismatch repair–deficient rectal cancer. The follow-up was > 5 years in the majority of the patients, with a median follow-up of 7.0 years (interquartile ratio, 5.0 to 11.1 years) among 51 alive patients (gold bars), and 3.5 years (interquartile ratio, 1.9 to 12.4 years) among 11 deceased patients (blue bars).
Footnotes
Supported by the University of Texas MD Anderson Cancer Center G.S. Hogan Gastrointestinal Cancer Research Grant (Y.N.Y.); a gift from the Feinberg Family (E.V.); the University of Texas MD Anderson Cancer Center Janice Davis Gordon Memorial Postdoctoral Fellowship in Colorectal Cancer Prevention (E.B.); and U.S. National Institutes of Health/National Cancer Institute University of Texas MD Anderson Cancer Center Core Support Grant No. P30CA016672.
Presented in part at the American Society of Clinical Oncology Annual Meeting, June 4-8, 2010, Chicago, IL, and at the Society of Pelvic Surgeons Annual Meeting, July 6-12, 2013, Dusseldorf, Germany.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Nicole de Rosa, Miguel A. Rodriguez-Bigas, George J. Chang, Patrick M. Lynch, Eduardo Vilar, Y. Nancy You
Financial support: Eduardo Vilar
Provision of study materials or patients: Miguel A. Rodriguez-Bigas, George J. Chang, Ester Borras, John M. Skibber, Barry W. Feig, Patrick M. Lynch, Eduardo Vilar, Y. Nancy You
Collection and assembly of data: Nicole de Rosa, Jula Veerapong, Patrick M. Lynch, Eduardo Vilar, Y. Nancy You
Data analysis and interpretation: Nicole de Rosa, Miguel A. Rodriguez-Bigas, George J. Chang, Ester Borras, Sunil Krishnan, Brian Bednarski, Craig A. Messick, John M. Skibber, Barry W. Feig, Patrick M. Lynch, Eduardo Vilar, Y. Nancy You
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
DNA Mismatch Repair Deficiency in Rectal Cancer: Benchmarking Its Impact on Prognosis, Neoadjuvant Response Prediction, and Clinical Cancer Genetics
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Nicole de Rosa
No relationship to disclose
Miguel A. Rodriguez-Bigas
Patents, Royalties, Other Intellectual Property: UpToDate, Author
George J. Chang
Consulting or Advisory Role: Ethicon
Research Funding: Agendia NV
Travel, Accommodations, Expenses: Ethicon, Intuitive Surgical
Jula Veerapong
No relationship to disclose
Ester Borras
No relationship to disclose
Sunil Krishnan
Research Funding: Elekta, Shell Oil, Malaysian Palm Oil Board
Patents, Royalties, Other Intellectual Property: Royalties from Taylor and Francis: book sales (nanotechnology); Licensed IP back to self from MD Anderson (nanotechnology)
Brian Bednarski
Travel, Accommodations, Expenses: Intuitive Surgical
Craig A. Messick
No relationship to disclose
John M. Skibber
No relationship to disclose
Barry W. Feig
No relationship to disclose
Patrick M. Lynch
Consulting or Advisory Role: Thetis Pharmaceuticals
Speakers’ Bureau: Myriad Genetics
Patents, Royalties, Other Intellectual Property: Cancer Prevention Pharmaceuticals
Eduardo Vilar
No relationship to disclose
Y. Nancy You
No relationship to disclose
REFERENCES
- 1.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer: The stable evidence. Nat Rev Clin Oncol. 2010;7:153–162. doi: 10.1038/nrclinonc.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vilar E, Tabernero J. Molecular dissection of microsatellite instable colorectal cancer. Cancer Discov. 2013;3:502–511. doi: 10.1158/2159-8290.CD-12-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoffel EM, Mangu PB, Gruber SB, et al. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol. 2015;33:209–217. doi: 10.1200/JCO.2014.58.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weissman SM, Burt R, Church J, et al. Identification of individuals at risk for Lynch syndrome using targeted evaluations and genetic testing: National Society of Genetic Counselors and the Collaborative Group of the Americas on Inherited Colorectal Cancer joint practice guideline. J Genet Couns. 2012;21:484–493. doi: 10.1007/s10897-011-9465-7. [DOI] [PubMed] [Google Scholar]
- 5.Hampel H. Point: Justification for Lynch syndrome screening among all patients with newly diagnosed colorectal cancer. J Natl Compr Canc Netw. 2010;8:597–601. doi: 10.6004/jnccn.2010.0044. [DOI] [PubMed] [Google Scholar]
- 6.Dineen S, Lynch PM, Rodriguez-Bigas MA, et al. A prospective six sigma quality improvement trial to optimize universal screening for genetic syndrome among patients with young-onset colorectal cancer. J Natl Compr Canc Netw. 2015;13:865–872. doi: 10.6004/jnccn.2015.0103. [DOI] [PubMed] [Google Scholar]
- 7.Beamer LC, Grant ML, Espenschied CR, et al. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058–1063. doi: 10.1200/JCO.2011.38.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreira L, Balaguer F, Lindor N, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308:1555–1565. doi: 10.1001/jama.2012.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yurgelun MB. Next-generation strategies for hereditary colorectal cancer risk assessment. J Clin Oncol. 2015;33:388–393. doi: 10.1200/JCO.2014.58.9895. [DOI] [PubMed] [Google Scholar]
- 10.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.You YN, Vilar E. Classifying MMR variants: Time for revised nomenclature in Lynch syndrome. Clin Cancer Res. 2013;19:2280–2282. doi: 10.1158/1078-0432.CCR-13-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mork ME, You YN, Ying J, et al. High prevalence of hereditary cancer syndromes in adolescents and young adults with colorectal cancer. J Clin Oncol. 2015;33:3544–3549. doi: 10.1200/JCO.2015.61.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mas-Moya J, Dudley B, Brand RE, et al. Clinicopathological comparison of colorectal and endometrial carcinomas in patients with Lynch-like syndrome versus patients with Lynch syndrome. Hum Pathol. 2015;46:1616–1625. doi: 10.1016/j.humpath.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Carethers JM. Differentiating Lynch-like from Lynch syndrome. Gastroenterology. 2014;146:602–604. doi: 10.1053/j.gastro.2014.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong SP, Min BS, Kim TI, et al. The differential impact of microsatellite instability as a marker of prognosis and tumour response between colon cancer and rectal cancer. Eur J Cancer. 2012;48:1235–1243. doi: 10.1016/j.ejca.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Tamas K, Walenkamp AM, de Vries EG, et al. Rectal and colon cancer: Not just a different anatomic site. Cancer Treat Rev. 2015;41:671–679. doi: 10.1016/j.ctrv.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Lim SH, Chua W, Henderson C, et al. Predictive and prognostic biomarkers for neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Crit Rev Oncol Hematol. 2015;96:67–80. doi: 10.1016/j.critrevonc.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 19. MD Anderson Cancer Center: Molecular diagnostics laboratory. https://www.mdanderson.org/education-and-research/resources-for-professionals/scientific-resources/core-facilities-and-services/molecular-diagnostics-lab/index.html.
- 20.Rodriguez-Bigas MA, Boland CR, Hamilton SR, et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: Meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89:1758–1762. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 21.Thompson BA, Spurdle AB, Plazzer JP, et al. Application of a 5-tiered scheme for standardized classification of 2,360 unique mismatch repair gene variants in the InSiGHT locus-specific database. Nat Genet. 2014;46:107–115. doi: 10.1038/ng.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reese MG, Eeckman FH, Kulp D, et al. Improved splice site detection in Genie. J Comput Biol. 1997;4:311–323. doi: 10.1089/cmb.1997.4.311. [DOI] [PubMed] [Google Scholar]
- 23.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dogan RI, Getoor L, Wilbur WJ, et al. SplicePort--an interactive splice-site analysis tool. Nucleic Acids Res. 2007;35:W285-91. doi: 10.1093/nar/gkm407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shihab HA, Gough J, Cooper DN, et al. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum Mutat. 2013;34:57–65. doi: 10.1002/humu.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwarz JM, Cooper DN, Schuelke M, et al. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat Methods. 2014;11:361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 27. Myriad Pro: Genetic test results. https://www.myriadpro.com/for-your-practice/genetic-test-results/
- 28.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kastrinos F, Steyerberg EW, Mercado R, et al. The PREMM(1,2,6) model predicts risk of MLH1, MSH2, and MSH6 germline mutations based on cancer history. Gastroenterology. 2011;140:73–81. doi: 10.1053/j.gastro.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: A consensus statement by the US Multi-society Task Force on colorectal cancer. Am J Gastroenterol. 2014;109:1159–1179. doi: 10.1038/ajg.2014.186. [DOI] [PubMed] [Google Scholar]
- 31.Samowitz WS, Curtin K, Wolff RK, et al. Microsatellite instability and survival in rectal cancer. Cancer Causes Control. 2009;20:1763–1768. doi: 10.1007/s10552-009-9410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phipps AI, Lindor NM, Jenkins MA, et al. Colon and rectal cancer survival by tumor location and microsatellite instability: The Colon Cancer Family Registry. Dis Colon Rectum. 2013;56:937–944. doi: 10.1097/DCR.0b013e31828f9a57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770–1776. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brouquet A, Mortenson MM, Vauthey JN, et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: Classic, combined or reverse strategy? J Am Coll Surg. 2010;210:934–941. doi: 10.1016/j.jamcollsurg.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 35.Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst. 2013;105:1151–1156. doi: 10.1093/jnci/djt173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertolini F, Bengala C, Losi L, et al. Prognostic and predictive value of baseline and posttreatment molecular marker expression in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1455–1461. doi: 10.1016/j.ijrobp.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 37.Cecchin E, Agostini M, Pucciarelli S, et al. Tumor response is predicted by patient genetic profile in rectal cancer patients treated with neo-adjuvant chemo-radiotherapy. Pharmacogenomics J. 2011;11:214–226. doi: 10.1038/tpj.2010.25. [DOI] [PubMed] [Google Scholar]
- 38.Charara M, Edmonston TB, Burkholder S, et al. Microsatellite status and cell cycle associated markers in rectal cancer patients undergoing a combined regimen of 5-FU and CPT-11 chemotherapy and radiotherapy. Anticancer Res. 2004;24:3161–3167. [PubMed] [Google Scholar]
- 39.Shin JS, Tut TG, Yang T, et al. Radiotherapy response in microsatellite instability related rectal cancer. Korean J Pathol. 2013;47:1–8. doi: 10.4132/KoreanJPathol.2013.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cirillo L, Urso ED, Parrinello G, et al. High risk of rectal cancer and of metachronous colorectal cancer in probands of families fulfilling the Amsterdam criteria. Ann Surg. 2013;257:900–904. doi: 10.1097/SLA.0b013e31826bff79. [DOI] [PubMed] [Google Scholar]
- 41.Vasen HF, Blanco I, Aktan-Collan K, et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): Recommendations by a group of European experts. Gut. 2013;62:812–823. doi: 10.1136/gutjnl-2012-304356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wertzberger BE, Sherman SK, Byrn JC. Differences in short-term outcomes among patients undergoing IPAA with or without preoperative radiation: A National Surgical Quality Improvement Program analysis. Dis Colon Rectum. 2014;57:1188–1194. doi: 10.1097/DCR.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu XR, Kiran RP, Remzi FH, et al. Preoperative pelvic radiation increases the risk for ileal pouch failure in patients with colitis-associated colorectal cancer. J Crohn’s Colitis. 2013;7:e419–e426. doi: 10.1016/j.crohns.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 44.Coffey JC, Winter DC, Neary P, et al. Quality of life after ileal pouch-anal anastomosis: An evaluation of diet and other factors using the Cleveland Global Quality of Life instrument. Dis Colon Rectum. 2002;45:30–38. [PubMed] [Google Scholar]
- 45.Kalady MF, Lipman J, McGannon E, et al. Risk of colonic neoplasia after proctectomy for rectal cancer in hereditary nonpolyposis colorectal cancer. Ann Surg. 2012;255:1121–1125. doi: 10.1097/SLA.0b013e3182565c0b. [DOI] [PubMed] [Google Scholar]
- 46.Lee JS, Petrelli NJ, Rodriguez-Bigas MA. Rectal cancer in hereditary nonpolyposis colorectal cancer. Am J Surg. 2001;181:207–210. doi: 10.1016/s0002-9610(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 47.Win AK, Parry S, Parry B, et al. Risk of metachronous colon cancer following surgery for rectal cancer in mismatch repair gene mutation carriers. Ann Surg Oncol. 2013;20:1829–1836. doi: 10.1245/s10434-012-2858-5. [DOI] [PMC free article] [PubMed] [Google Scholar]