Abstract
Purpose
The clinicopathologic significance of mismatch repair (MMR) defects in endometrioid endometrial cancer (EEC) has not been definitively established. We undertook tumor typing to classify MMR defects to determine if MMR status is prognostic or predictive.
Methods
Primary EECs from NRG/GOG0210 patients were assessed for microsatellite instability (MSI), MLH1 methylation, and MMR protein expression. Each tumor was assigned to one of four MMR classes: normal, epigenetic defect, probable mutation (MMR defect not attributable to MLH1 methylation), or MSI-low. The relationships between MMR classes and clinicopathologic variables were assessed using contingency table tests and Cox proportional hazard models.
Results
A total of 1,024 tumors were assigned to MMR classes. Epigenetic and probable mutations in MMR were significantly associated with higher grade and more frequent lymphovascular space invasion. Epigenetic defects were more common in patients with higher International Federation of Gynecology and Obstetrics stage. Overall, there were no differences in outcomes. Progression-free survival was, however, worse for women whose tumors had epigenetic MMR defects compared with the MMR normal group (hazard ratio, 1.37; P < .05; 95% CI, 1.00 to 1.86). An exploratory analysis of interaction between MMR status and adjuvant therapy showed a trend toward improved progression-free survival for probable MMR mutation cases.
Conclusion
MMR defects in EECs are associated with a number of well-established poor prognostic indicators. Women with tumors that had MMR defects were likely to have higher-grade cancers and more frequent lymphovascular space invasion. Surprisingly, outcomes in these patients were similar to patients with MMR normal tumors, suggesting that MMR defects may counteract the effects of negative prognostic factors. Altered immune surveillance of MMR-deficient tumors, and other host/tumor interactions, is likely to determine outcomes for patients with MMR-deficient tumors.
INTRODUCTION
Uterine cancer is the most common gynecologic malignancy in the United States, with an estimated 60,050 new cases in 2016.1 Most uterine cancers are endometrial carcinomas (ECs). The histologic and biologic heterogeneity of EC has been recognized for more than two decades,2,3 and recent molecular characterization of ECs has emphasized the etiologic heterogenety.4 Endometrioid EC (EEC) is the most common subtype, making up approximately 80% of cases.5,6 Risk factors for EC include hyperestrinism, obesity, nulliparity, and inherited mutations in mismatch repair (MMR) genes resulting in Lynch syndrome.7,8
Most EECs present at early stage. For women with stage I or II disease, the overall 5-year survival approaches 90%.1,9,10 However, outcomes are poor for women with advanced-stage or recurrent disease. Although the absolute risk for recurrence for women with early-stage (I or II) EEC is low, the large number of patients means there is significant morbidity and mortality associated with early-stage EEC.
Loss of MMR is a frequent event in EEC, with reported rates ranging from approximately 20% to 40%.4,11-15 In fact, the rate of defective MMR in EC is nearly twice that in colorectal cancers.
Defective MMR results in greatly increased rate of strand-slippage mutations leading to microsatellite instability (MSI). Many tumors with defective MMR fail to express one or more MMR proteins. Although the vast majority of ECs with defective MMR are sporadic, 3% to 5% of cases develop disease because of inherited mutations in DNA MMR genes (Lynch syndrome). Tumor MSI and MMR immunohistochemistry (IHC) are used by many centers as part of screening for Lynch syndrome in patients with EC, and universal tumor screening has been recommended.16-18 The same approach to screening for Lynch syndrome in patients with colorectal cancer has been widely adopted.19 In addition to identifying potential germline mutation carriers, MMR analysis of colorectal tumors has use as both a prognostic and a predictive test.20-22 The relationship between MMR defects and outcomes in patients with EC has not been fully established. Some studies have suggested improved outcomes for women whose tumors have MMR defects, whereas others indicated worse or no difference in outcome.12,13,23-42 Differences in the methods used to assess MMR abnormalities and the types of cancers studied may account for the variable findings reported to date.
In the study reported here, we limited analysis to women with EEC enrolled in an NRG trial, GOG210. We hypothesized that comprehensive MMR typing in a large cohort would reveal associations between different MMR classes and clinicopathologic features. Understanding the relationship between tumor MMR status and outcomes, including response to adjuvant therapy, will be critical to the design and implementation of trials for treating advanced-stage and recurrent EEC, including biologic therapies such as immune checkpoint blockade.
METHODS
Patient Cohort and Clinical and Demographic Data
Subjects were investigated as part of NRG/Gynecologic Oncology Group’s GOG8020 protocol. They were recruited to the GOG0210 study between July 2003 and September 2007, during which time 2,471 eligible EEC cases were registered.43 An NRG/Gynecologic Oncology Group (GOG) Tissue Bank pathologist (N.R.) reviewed 1,673 cases for tumor cellularity and necrosis. Adequate high neoplastic cellularity tissues (estimated > 66% tumor cell content and < 25% necrosis) were available for 611 subjects. Formalin-fixed paraffin-embedded tissue sections were microdissected for an additional 432 subjects.14 Clinical reports and tumor slides for the 1,043 subjects were centrally reviewed by GOG/NRG pathologists. Analyses were limited to EEC, the histologic type in which MMR defects are most common.44 Molecular studies were approved by the Washington University Human Studies Committee (201102157).
Analysis of Tumors and Normal DNA
All tumors were assessed for MSI, expression of MMR proteins, and MLH1 methylation. DNA preparation and MSI and MLH1 methylation analyses were carried out as previously described.14,45,46 Briefly, MSI testing was performed using a five-plex assay for the National Cancer Institute consensus markers.47 When MSI was seen with a single marker, the finding was confirmed with repeat polymerase chain reaction and the tumor classified as MSI-low. Tumors with MSI at two or more markers were classified as MSI-high. MLH1 methylation was evaluated using pyrosequencing and/or combined bisulfite restriction analysis.14 IHC for MSH6, MSH2, and MLH146,48 and PMS2 for a subset of tumors has been described for this cohort.14
Statistical Analysis
The relationship between MMR status and clinical and demographic features was assessed using χ2 and analysis of deviance tests. Disease-specific survival (endometrial cancer–specific survival, ECS) was defined as the time (months) from date of surgery to death due to EC. Those subjects who did not die as a result of EC were censored at the date of last contact. Progression-free survival (PFS) was defined as the time from surgery to recurrence or progression. The Kaplan-Meier product limit method was used to estimate survival. The log-rank test was used to test for differences in survival (ECS and PFS) by MMR status. Cox proportional hazard regression was used to estimate the effect of MMR status on ECS and PFS adjusting for covariates.49 Clinically accepted prognostic factors significant on univariate analysis were included in the model, including age, stage, and tumor grade. All analyses were two-sided, and significance was set at a P value of .05. Statistical analyses were performed using R.50
RESULTS
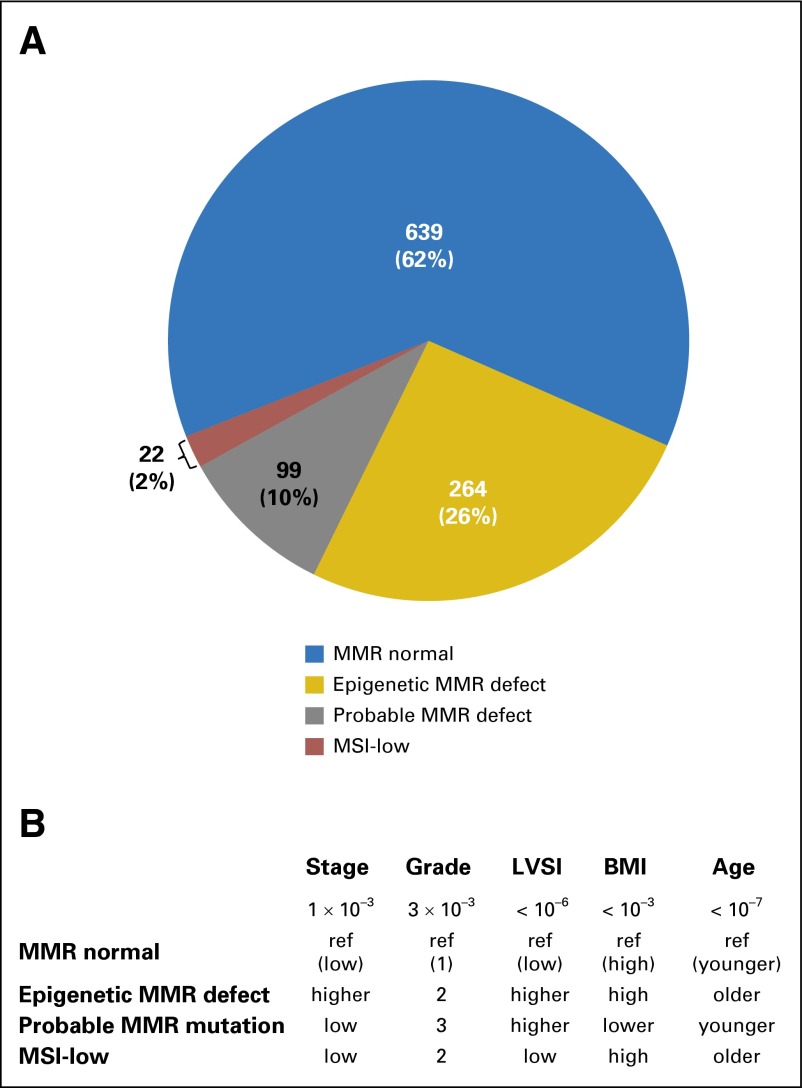
Combined MSI, MLH1 methylation, and IHC analyses were undertaken to assign 1,024 tumors to one of four molecular MMR classes. Nineteen additional tumors could not be classified because of failure of one or more tests. Six hundred thirty-nine tumors (62.40%) were classified as MMR normal (no MSI, no IHC defect), 264 (25.78%) as epigenetic MMR defective (MSI-positive with MLH1 methylation), and 99 (9.67%) as probable genetic MMR mutation (MSI-positive and/or IHC defect with absence of MLH1 methylation; Fig 1A). Only 22 tumors (2.15% of cohort) were classified as MSI-low.
Fig 1.
Mismatch repair (MMR) status and association with clinicopathologic and demographic variables for 1,024 endometrioid endometrial cancers. (A) Frequency distribution of four different MMR classes. (B) Patterns seen across the four MMR types for significantly associated variables. P values for Pearson’s χ2 tests. BMI, body mass index; LVSI, lymph-vascular space invasion; MSI, microsatellite instability.
MMR status was significantly associated with age at diagnosis and body mass index (BMI; Table 1). As has been previously reported, women whose tumors were classified as having epigenetic MMR defects were older than MMR normal or probable mutation cases.14,51,52 Sixty-nine percent of women with tumors classified as having an epigenetic defect were ≥ 60 years of age at diagnosis, in contrast to 36% of women with tumors classified as probable MMR mutation. Women whose tumors were classified as MSI-low were older as well (68.20% were ≥ 60 years). Probable MMR mutation cases had lower BMIs, with 26.26% being ≤ 25 kg/m2 (normal or underweight), and only 50.50% were obese (BMI ≥ 30 kg/m2), compared with approximately 69.70%, 67.68%, and 63.64% being obese in the MMR normal, epigenetic MMR defect, and MSI-low groups, respectively. MMR tumor class was not associated with race.
Table 1.
Association Between Tumor MMR Status and Clinicopathologic and Demographic Variables
| Clinicopathologic Factor | MMR Normal | Epigenetic MMR Defect | Probable MMR Mutation | MSI-Low | P* |
|---|---|---|---|---|---|
| Age, years | |||||
| < 60 | 311 (48.67) | 82 (31.06) | 63 (63.64) | 7 (31.82) | < .001 |
| ≥ 60 | 328 (51.33) | 182 (68.94) | 36 (36.36) | 15 (68.18) | |
| BMI | |||||
| < 25 (normal or underweight) | 72 (11.30) | 37 (14.07) | 26 (26.26) | 2 (9.09) | < .001 |
| ≥ 25-30 (overweight) | 121 (19.00) | 48 (18.25) | 23 (23.23) | 6 (27.27) | |
| ≥ 30-35 (obese class I) | 135 (21.19) | 78 (29.66) | 15 (15.15) | 5 (22.73) | |
| ≥ 35 (severe or super obese) | 309 (48.51) | 100 (38.02) | 35 (35.35) | 9 (40.91) | |
| Race | |||||
| White | 572 (89.51) | 243 (92.04) | 92 (92.93) | 19 (86.36) | NS |
| Black | 38 (5.95) | 14 (5.30) | 5 (5.05) | 1 (4.54) | |
| Other (Asian, Native American, unknown) | 29 (4.54) | 7 (2.65) | 2 (2.02) | 2 (9.09) | |
| Grade | |||||
| 1 | 295 (46.17) | 89 (33.71) | 38 (38.38) | 9 (40.91) | < .01 |
| 2 | 260 (40.69) | 132 (50.00) | 37 (37.37) | 11 (50.00) | |
| 3 | 84 (13.14) | 43 (16.29) | 24 (24.24) | 2 (9.09) | |
| Stage | |||||
| I | 491 (76.84) | 181 (68.56) | 81 (81.82) | 18 (81.82) | .001 |
| II | 63 (9.86) | 25 (9.47) | 5 (5.05) | 1 (4.54) | |
| III | 72 (11.27) | 57 (21.59) | 9 (9.09) | 3 (13.64) | |
| IV | 13 (2.03) | 1 (0.38) | 4 (4.04) | 0 (0) | |
| LVSI | |||||
| Present | 108 (17.25) | 87 (33.46) | 30 (30.93) | 3 (13.64) | < .001 |
| Absent | 518 (82.75) | 173 (66.54) | 67 (69.07) | 19 (86.36) | |
| Depth of invasion | |||||
| None | 119 (19.10) | 30 (11.67) | 19 (20.43) | 3 (13.64) | NS |
| Inner half | 344 (55.22) | 151 (58.75) | 50 (53.76) | 12 (54.54) | |
| Outer half or serosal | 160 (25.68) | 76 (29.57) | 24 (25.81) | 7 (31.82) | |
| Adjuvant therapy | |||||
| Any adjuvant therapy | 111 (17.42) | 63 (23.95) | 26 (26.26) | 5 (22.73) | .05 |
| No further treatment | 526 (82.57) | 200 (76.05) | 73 (73.74) | 17 (77.27) |
NOTE. Data presented as No. (%).
Abbreviations: LVSI, lymphovascular space invasion; MMR, mismatch repair; MSI, microsatellite instability; NS, not significant.
Pearson’s χ2 tests. Missing data: LVSI for 19 patients, depth of invasion for 29 patients, adjuvant therapy for three patients, and BMI for three patients.
Grade, stage, and lymphovascular space invasion (LVSI) were all significantly associated with MMR status (Table 1). Tumors with epigenetic MMR defects were more common in patients diagnosed with higher stage than the other three tumor classes (Fig 1B). Nearly 22% of the epigenetic MMR defect group had stage III or IV disease compared with 13% to 14% for the other three groups (Table 1). MMR defects in tumors (either epigenetic or probable mutation) were significantly associated with traditional prognostic features that portend poor outcomes: higher grade and LVSI (Fig 1B). MMR normal cases more frequently had grade 1 disease (46.17%) than the other three groups (combined, 35.49% grade 1). The majority of tumors with epigenetic MMR defects were grade 2 (50%). The most striking difference was with LVSI: the two groups with MMR defects (epigenetic and probable mutation combined) had a 32.77% rate of LVSI compared with 17.13% for MMR normal group (odds ratio, 2.36; 95% CI, 1.74 to 3.19; P < .001). Tumor MMR status did not vary by depth of invasion or use of adjuvant therapy (Table 1).
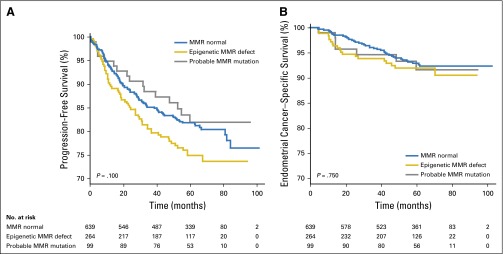
Outcome analyses were performed comparing the MMR normal, epigenetic MMR defects, and probable MMR mutation groups. The MSI-low group was not included because of the small number of cases. MMR status was not associated with PFS or ECS when the three classes were considered (Fig 2). Univariate analysis did, however, suggest worse PFS for women whose tumors had epigenetic defects (P = .100; analysis of deviance; Appendix Table A1, online only). In fact, Kaplan-Meier analysis gave a hazard ratio of 1.37 for cases with epigenetic defects (P ≤ .05; 95% CI, 1.00 to 1.86; Appendix Table A1). A trend toward better survival was observed for the MMR mutation group. Stage, grade, presence of LVSI, and myometrial invasion were all associated with reduced survival in univariate analyses (Appendix Table A1). Age was significantly associated with PFS but not ECS. When the five factors significant in univariate analysis were included in a multivariable analysis (along with adjuvant therapy), there was no evidence of association between MMR class and outcome (Appendix Table A2, online only).
Fig 2.
Tumor mismatch repair (MMR) status and outcomes. (A) Progression-free survival. (B) Endometrial cancer–specific survival. P values for likelihood ratio tests. Blue line, MMR normal; gold line, epigenetic defects; gray line, probable mutations.
Given the substantial body of literature indicating that MMR defects are predictive in colorectal cancer,53-55 we assessed MMR status and treatment interactions. The differences in efficacy of adjuvant therapy with respect to PFS across MMR classes were not statistically significant using Cox regression analysis (P = .08; Appendix Table A3, online only). Nonetheless, we explored what seemed to be trend for interaction by including the covariates stage, grade, presence of LVSI, myometrial invasion, and age in the analysis and examining the individual MMR classes. This further analysis suggested that trend observed in the Cox regression for PFS is driven by the probable mutation cases. The hazard ratio comparing adjuvant therapy with no therapy is greater for tumors with probable MMR mutation, compared with MMR normal tumors. The hazard ratio comparing therapy to untreated is .80 for MMR normal tumors and is 0.80 × 0.24 = 0.19 for tumors with probable mutation (Appendix Table A4, online only). The factor 0.24 (P = .07; 95% CI, 0.05 to 1.16), although not statistically significant, attributes a four-fold change in the advantage of adjuvant therapy for tumors with probable mutation, compared with MMR normal tumors. That is, adjuvant therapy reduces the hazard ratio from 1.0 to 0.8 in MMR normal tumors, but the reduction is from 1.0 to 0.19 in tumors with probable MMR mutation. An effect of similar magnitude was seen for the ECS, where the hazard ratio measuring the effect of adjuvant therapy was 0.87 for normal tumors and was reduced by a factor of 0.18 (P = .13; 95% CI, 0.02 to 1.70) to be 0.18 × 0.87 = 0.16 for probable mutation tumors in the multivariable analysis (Appendix Table A4).
DISCUSSION
In this study, we assessed the relationship between tumor MMR status and clinicopathologic features in what is the largest series of EECs investigated to date. We identified highly significant associations between MMR abnormalities and known negative prognostic factors. To our knowledge, this is the first large-scale study in which tumor MMR phenotyping has fully integrated MSI, IHC, and MLH1 methylation analyses. Our molecular classification of tumors allowed us to assign each case to one of four nonoverlapping MMR classes. Earlier studies that have tested for relationships between MMR defects and clinicopathologic variables have in general relied on either MSI or IHC findings. In some instances, subsets of tumors investigated were assessed using both MSI and IHC methods. MLH1 methylation has also been included in some studies, typically for a selected subset of tumors.
MMR defects (epigenetic or probable MMR mutation classes) were significantly associated with clinical features that portend poor outcomes (Fig 1B). Higher tumor grade and presence of LVSI were associated with both epigenetic MMR defects and probable MMR mutations. The association with grade was reported previously for a large EEC cohort45 in which tumors were classified based on MSI status, but that did not include MMR IHC or MLH1 methylation. The MSI-positive group would thus include all of the epigenetic and many of the probable MMR mutation cases. In a series of 473 ECs, 379 of which were EECs, reported by Black et al,33 the association with higher grade was not statistically significant. There was, however, a clear trend toward higher frequency of grade 2 tumors among the MSI-positive tumors, and it is possible that the difference in grade would be statistically significant when only EECs were considered. There have been multiple reports that higher grade is associated with MMR defects.23,27,39,56 On the other hand, many studies did not see an association with grade.12,24,31,32,36,41
The relationship with LVSI has not been explored extensively. In the cohort we investigated, the association between MMR defects and presence of LVSI was highly significant (P ≤ .001; Table 1), with an odds ratio of 2.34 (95% CI, 1.73 to 3.17) for MMR-deficient tumors having LVSI compared with MMR normal cases. An association between absent MLH1 and LVSI was reported previously in two smaller series. Cohn et al34 analyzed 336 tumors using IHC for MLH1, MSH2, MSH6, and PMS2. The increased frequency of LVSI and MMR defects was limited to tumors with absent MLH1. Bilbao et al35 investigated 93 tumors in which MMR status was determined by MSI analysis, and Shih et al reported increase frequency of LVSI in young patients with EC with MMR defects.56 No association was seen in two large series.31,33 In a recent study in which MMR status using IHC, MSI, and methylation analysis, association was seen only with some MMR classes.42 Again, the inclusion of non-EEC cases and differences in MMR typing may explain the difference between our study and what was reported previously.
The association with higher-stage disease was limited to women whose tumors had epigenetic MMR defects. Among the MMR normal cases, 13.3% had stage III or IV disease, whereas nearly 22% of patients with an epigenetic MMR defect cases were diagnosed with stage III or IV (Table 1). This finding is consistent with previous reports, noting many of these earlier studies included non-EEC cases.27,33,35,36,39
Given the increased rate of LVSI and advanced-stage and higher-grade tumors associated with MMR defects in the GOG210 cohort, we expected poorer outcomes for women with MMR-deficient tumors. There were, however, no significant differences in PFS or ECS when the three MMR groups (MMR normal, epigenetic MMR defect, and probable MMR mutation) were compared (Fig 2). The better-than-anticipated outcomes for women with tumors with defective MMR could reflect differences in T-cell or other immune responses to tumors with MMR defects that balance the effects of grade, stage, and LVSI.57-59 Trends in the survival curves indicated worse outcomes for those women with tumors with epigenetic MMR defects. In fact, the hazard ratio for reduced PFS was 1.37 (95% CI, 1.00 to 1.86; Appendix Table A1) for women with epigenetic MMR defects, demonstrating their outcomes were worse. As expected, the multivariable analyses showed no association between MMR status in PFS or ECS (Appendix Table A2). The expected associations with age, stage, grade, and LVSI, on the other hand, were evident (Appendix Table A1).
Our analysis also suggests that MMR status is associated with response to adjuvant therapy. Improved PFS and ECS were seen for those women who had adjuvant therapy and whose tumors were classified as having a probable MMR mutation who underwent additional treatment. In a recent report on 221 Japanese patients, a similar trend was noted.42 Cox regression analysis PFS assessing the MMR status by treatment interaction was suggestive (P = .09 for MMR status and P = .08 for MMR status and adjuvant status combined; Appendix Table A3). In multivariable analysis, this trend remained, with the survival advantage greatest for those women with probable MMR-mutant tumors receiving adjuvant therapies (Appendix Table A4). No such effect was seen in the epigenetic defect group, and the differences in outcomes for patients with the two different classes of MMR defect are highly suggestive. Despite the large size of our study, our power to detect differences in survival is limited by the modest number of cases whose tumors were classified as probable mutation (99, 10% of cohort) and further by the fact that only 26 subjects received adjuvant therapy.
We, and others, have previously demonstrated that the group classified as probable MMR mutation includes germline cases (women with Lynch syndrome) and cases with somatic mutations.14,52 The published literature on EC outcomes for Lynch syndrome is extremely limited.60 However, it is generally accepted that outcomes are better for patients with colon cancer with Lynch syndrome than for other patients with colon cancer.61-63 Possible explanations for why patients with colon cancer with Lynch syndrome have better survival include reduced viability of the tumor cells overall because of their high mutation burden/genetic instability,64-67 the fact that Lynch colon cancers are diploid,68,69 and because Lynch tumors have increased T-cell infiltration.70,71 These factors are unlikely to explain differences in response to adjuvant therapy for EEC for the epigenetic and probable mutation classes. Both tumor groups are deficient in MMR and in principle have the same tumor mutation burden.4,14 However, it is known that some somatic mutations distinguish epigenetic and MMR mutant tumors, the best example being BRAF mutations in colorectal cancers.72 The younger age of women with probable MMR defects compared with those with epigenetic defects could in part explain why the two MMR classes differ with respect to their outcomes. However, the same pattern as observed overall is reproduced within the subclasses of women younger than age 60 years and older than age 60 years. Immune surveillance of tumors in the two molecularly defined groups could, in part, explain differences in outcomes and, in particular, responses to adjuvant treatment.
From this analysis of a large, prospectively collected cohort of patients with EEC, we can conclude that MMR deficiency is associated with traditional prognostic factors, including stage, grade, and presence of LVSI. Although women with epigenetic defects had somewhat lower PFS, the overall effect was less than would be expected given the strong association with higher grade (66% grade 2 or 3), higher stage (22% stage III or IV), and frequent LVSI (33%). One possible explanation for the better-than-expected outcomes is that the MMR-deficient tumors are eliciting an antitumor immune response, as has been described for POLE ultramutated tumors.73 One of the most clinically relevant and intriguing findings for this study is that MMR status may be associated with response to adjuvant therapy. Given the fact that many centers routinely test for MMR defects and MLH1 methylation, it should be possible to rapidly undertake retrospective studies to validate the findings we report here.
Appendix
Table A1.
Univariate Outcome Analysis of All Subjects With EC (N = 1,024)
| Clinicopathologic Factor | PFS | ECS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P* | HR | 95% CI | P* | |
| Age (older v < 60 years) | 1.89 | 1.40 to 2.54 | < .001 | 1.21 | 0.75 to 1.94 | NS |
| Race (ref = white) | NS | NS | ||||
| Black | 1.37 | 0.81 to 2.32 | 1.33 | 0.54 to 3.32 | ||
| Other | 0.67 | 0.28 to 1.54 | 1.17 | 0.37 to 3.7 | ||
| Stage (ref = I) | < .001 | < .001 | ||||
| II | 1.14 | 0.69 to 1.86 | 1 | 0.36 to 2.83 | ||
| III | 1.93 | 1.37 to 2.74 | 5.13 | 3.10 to 8.49 | ||
| IV | 4.77 | 2.57 to 8.83 | 9.7 | 4.05 to 23.14 | ||
| BMI (categorical ref < 25) | NS | NS | ||||
| 25-30 | 1.2 | 0.73 to 1.96 | 0.61 | 0.27 to 1.39 | ||
| > 30-35 | 1.26 | 0.78 to 2.03 | 0.99 | 0.49 to 2.04 | ||
| > 35 | 0.97 | 0.62 to 1.52 | 0.72 | 0.36 to 1.41 | ||
| Grade (ref = 1) | < .001 | < .001 | ||||
| 2 | 1.41 | 1.02 to 1.97 | 1.62 | 0.83 to 3.14 | ||
| 3 | 3.14 | 2.19 to 4.51 | 7.56 | 4.06 to 14.09 | ||
| LVSI (present or absent) | 1.98 | 1.48 to 2.65 | < .001 | 4.15 | 2.60 to 6.61 | < .01 |
| Adjuvant therapy (yes or no) | 0.97 | 0.69 to 1.36 | NS | 0.6 | 0.36 to 1.00 | .06 |
| Myometrial invasion (ref = none) | < .001 | < .001 | ||||
| Inner half | 1.58 | 0.96 to 2.58 | 1.27 | 0.56 to 2.91 | ||
| Outer half or serosal | 2.99 | 1.82 to 4.94 | 3.28 | 1.45 to 7.41 | ||
| MMR status (ref = MMR normal) | .10 | NS | ||||
| Epigenetic MMR defect | 1.37 | 1.00 to 1.86 | 1.23 | 0.72 to 2.10 | ||
| Probable MMR mutation | 0.88 | 0.52 to 1.48 | 1.1 | 0.49 to 2.45 | ||
Abbreviations: BMI, body mass index; ECS, endometrial cancer–specific survival; HR, hazard ratio; LVSI, lymphovascular space invasion; MMR, mismatch repair; NS, not significant; PFS, progression-free survival.
Analysis of deviance
Table A2.
Multivariable Analysis for All 1,024 Subjects
| Clinicopathologic Factor | PFS | ECS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P* | HR | 95% CI | P* | |
| Age | ||||||
| ≥ 60 years | 1.78 | 1.30 to 2.44 | < .001 | 1.29 | 0.78 to 2.13 | NS |
| Stage | .01 | < .01 | ||||
| II | 1.02 | 0.60 to 1.73 | NS | 0.94 | 0.32 to 2.70 | NS |
| III | 1.55 | 1.02 to 2.37 | < .05 | 3.16 | 1.67 to 5.98 | < .001 |
| IV | 3.33 | 1.62 to 6.81 | .001 | 3.46 | 1.20 to 9.99 | .02 |
| Grade | < .001 | < .001 | ||||
| 2 | 1.19 | 0.84 to 1.71 | NS | 1.50 | 0.73 to 3.09 | NS |
| 3 | 2.35 | 1.54 to 3.59 | < .001 | 4.79 | 2.29 to 10.01 | < .001 |
| Myometrial invasion | .04 | NS | ||||
| Inner half | 1.33 | 0.79 to 2.24 | NS | 0.92 | 0.37 to 2.32 | NS |
| Outer half or serosal | 1.92 | 1.09 to 3.39 | .02 | 1.13 | 0.41 to 3.11 | NS |
| LVSI | ||||||
| Present | 1.23 | 0.85 to 1.78 | NS | 1.81 | 0.98 to 3.32 | .06 |
| MMR status | NS | NS | ||||
| Epigenetic MMR defect | 1.10 | 0.79 to 1.54 | NS | 0.78 | 0.43 to 1.41 | NS |
| Probable MMR mutation | 0.88 | 0.51 to 1.53 | NS | 0.91 | 0.40 to 2.07 | NS |
| Adjuvant therapy | ||||||
| Yes | 0.62 | 0.42 to 0.92 | .02 | 0.71 | 0.39 to 1.27 | NS |
Abbreviations: ECS, endometrial cancer–specific survival; HR, hazard ratio; LVSI, lymph-vascular space invasion; MMR, mismatch repair; NS, not significant; PFS, progression-free survival.
Likelihood ratio P values for full tests and Wald P values for individual tests.
Table A3.
Cox Regression for PFS Assessing MMR Status: Adjuvant Therapy Interaction
| Variable | Log Likelihood | χ2 | df | P* |
|---|---|---|---|---|
| Null | −1288.7 | |||
| MMR status | −1286.3 | 4.90 | 2 | .09 |
| Adjuvant therapy | −1286.3 | 0.02 | 1 | .90 |
| MMR status: adjuvant | −1283.8 | 5.01 | 2 | .08 |
Abbreviations: MMR, mismatch repair; PFS, progression-free survival.
Analysis of deviance.
Table A4.
Multivariable Analysis Including Treatment × MMR Status Interaction
| Clinicopathologic Factor | PFS | ECS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P* | HR | 95% CI | P* | |
| Age | ||||||
| ≥ 60 years | 1.75 | 1.27 to 2.39 | < .001 | 1.21 | 0.73 to 2.02 | NS |
| Stage | ||||||
| II | 1.00 | 0.59 to 1.71 | NS | 0.95 | 0.33 to 2.76 | NS |
| III | 1.54 | 1.00 to 2.36 | NS | 3.04 | 1.58 to 5.85 | < .001 |
| IV | 3.20 | 1.54 to 6.64 | < .01 | 3.44 | 1.15 to 10.24 | .03 |
| Grade | ||||||
| 2 | 1.19 | 0.83 to 1.70 | NS | 1.49 | 0.72 to 3.08 | NS |
| 3 | 2.36 | 1.54 to 3.60 | < .001 | 4.83 | 2.30 to 10.14 | < .001 |
| Myometrial invasion | ||||||
| Inner half | 1.32 | 0.79 to 2.22 | NS | 0.92 | 0.37 to 2.31 | NS |
| Outer half or serosal | 1.93 | 1.09 to 3.42 | .02 | 1.16 | 0.42 to 3.20 | NS |
| LVSI | ||||||
| Present | 1.22 | 0.84 to 1.76 | NS | 1.75 | 0.94 to 3.23 | .08 |
| MMR status | ||||||
| Epigenetic MMR defect | 1.20 | 0.83 to 1.75 | NS | 0.83 | 0.42 to 1.65 | NS |
| Probable MMR mutation | 1.20 | 0.67 to 2.17 | NS | 1.44 | 0.58 to 3.56 | NS |
| Adjuvant therapy | ||||||
| Yes | 0.80 | 0.49 to 1.30 | NS | 0.88 | 0.42 to 1.84 | NS |
| MMR status × treatment | ||||||
| Epigenetic MMR defect plus adjuvant | 0.67 | 0.30 to 1.50 | NS | 0.89 | 0.25 to 3.13 | NS |
| Probable MMR mutation plus adjuvant | 0.24 | 0.05 to 1.16 | .07 | 0.18 | 0.02 to 1.70 | NS |
Abbreviations: ECS, endometrial cancer–specific survival; HR, hazard ratio; LVSI, lymph-vascular space invasion; MMR, mismatch repair; NS, not significant; PFS, progression-free survival.
Wald test P values.
Footnotes
Listen to the podcast by Dr Yurgelun at www.jco.org/podcasts
Deceased.
Supported by SPORE in Endometrial Cancer Grant No. P50 CA134254 to Washington University School of Medicine, Barnes-Jewish Hospital and Siteman Cancer Center, The Ohio State University James Comprehensive Cancer Center, and National Cancer Institute Grants No. CA 27469 (Gynecologic Oncology Group [GOG] Administrative Office), CA 37517 (GOG Statistical Office), 1 U10 CA180822 (NRG Oncology Group), and U10 CA27469, U24 CA114793, and U10 CA180868 (GOG Tissue Bank).
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00340808.
AUTHOR CONTRIBUTIONS
Conception and design: D. Scott McMeekin, David G. Mutch, Richard Zaino, Feng Gao, Shamshad Ali, Kathleen M. Darcy, Paul J. Goodfellow
Provision of study materials or patients: D. Scott McMeekin, Matthew A. Powell, Lisa M. Landrum, Michael L. Pearl, Paul A. DiSilvestro
Collection and assembly of data: D. Scott McMeekin, Heather A. Lankes, Melissa A. Geller, Matthew A. Powell, Lisa M. Landrum, Richard Zaino, Russell D. Broaddus, Nilsa Ramirez, Shamshad Ali, Kathleen M. Darcy, Michael L. Pearl, Paul A. DiSilvestro, Paul J. Goodfellow
Data analysis and interpretation: David L. Tritchler, David E. Cohn, David G. Mutch, Floor J. Backes, Richard Zaino, Russell D. Broaddus, Michael L. Pearl, Shashikant B. Lele, Paul J. Goodfellow
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Clinicopathologic Significance of Mismatch Repair Defects in Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
D. Scott McMeekin
No relationship to disclose
David L. Tritchler
No relationship to disclose
David E. Cohn
Honoraria: Up To Date (Contributor), Elsevier Deputy Director, Gynecol Oncol
David G. Mutch
Honoraria: AstraZeneca
Heather A. Lankes
No relationship to disclose
Melissa A. Geller
Consulting or Advisory Role: OvaGene Oncology
Research Funding: Tesaro, Ovagene Oncology
Matthew A. Powell
Honoraria: Genentech, AstraZeneca
Consulting or Advisory Role: Genentech
Speakers’ Bureau: Genentech, AstraZeneca
Floor J. Backes
No relationship to disclose
Lisa M. Landrum
No relationship to disclose
Richard Zaino
Consulting or Advisory Role: Repros Therapeutics
Russell D. Broaddus
No relationship to disclose
Nilsa Ramirez
No relationship to disclose
Feng Gao
No relationship to disclose
Shamshad Ali
No relationship to disclose
Kathleen M. Darcy
No relationship to disclose
Michael L. Pearl
Honoraria: Ethicon
Consulting or Advisory Role: Ethicon
Patents, Royalties, Other Intellectual Property: Vitatex
Paul A. DiSilvestro
Research Funding: Genentech (Inst), Janssen Oncology (Inst), Tesaro (Inst), AstraZeneca (Inst)
Shashikant B. Lele
Consulting or Advisory Role: Genentech
Paul J. Goodfellow
No relationship to disclose
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Hendrickson M, Ross J, Eifel P, et al. Uterine papillary serous carcinoma: A highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6:93–108. doi: 10.1097/00000478-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stewart BW, Wild C (eds): World Cancer Report 2014. http://www.iarc.fr/en/publications/books/wcr/wcr-order.php.
- 6.Rutgers JK. Update on pathology, staging and molecular pathology of endometrial (uterine corpus) adenocarcinoma. Future Oncol. 2015;11:3207–3218. doi: 10.2217/fon.15.262. [DOI] [PubMed] [Google Scholar]
- 7.MacMahon B. Risk factors for endometrial cancer. Gynecol Oncol. 1974;2:122–129. doi: 10.1016/0090-8258(74)90003-1. [DOI] [PubMed] [Google Scholar]
- 8.Sorosky JI. Endometrial cancer. Obstet Gynecol. 2012;120:383–397. doi: 10.1097/AOG.0b013e3182605bf1. [DOI] [PubMed] [Google Scholar]
- 9.Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95:S105–S143. doi: 10.1016/S0020-7292(06)60031-3. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 10.Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 11.Risinger JI, Berchuck A, Kohler MF, et al. Genetic instability of microsatellites in endometrial carcinoma. Cancer Res. 1993;53:5100–5103. [PubMed] [Google Scholar]
- 12.MacDonald ND, Salvesen HB, Ryan A, et al. Frequency and prognostic impact of microsatellite instability in a large population-based study of endometrial carcinomas. Cancer Res. 2000;60:1750–1752. [PubMed] [Google Scholar]
- 13.Zighelboim I, Goodfellow PJ, Gao F, et al. Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol. 2007;25:2042–2048. doi: 10.1200/JCO.2006.08.2107. [DOI] [PubMed] [Google Scholar]
- 14.Goodfellow PJ, Billingsley CC, Lankes HA, et al. Combined microsatellite instability, MLH1 methylation analysis, and immunohistochemistry for Lynch syndrome screening in endometrial cancers from GOG210: An NRG Oncology and Gynecologic Oncology Group Study. J Clin Oncol. 2015;33:4301–4308. doi: 10.1200/JCO.2015.63.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Backes FJ, Leon ME, Ivanov I, et al. Prospective evaluation of DNA mismatch repair protein expression in primary endometrial cancer. Gynecol Oncol. 2009;114:486–490. doi: 10.1016/j.ygyno.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 16. Lancaster JM, Powell CB, Chen LM, et al: Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol 136:3-7, 2015 [Erratum: Gynecol Oncol 138:765, 2015. [DOI] [PubMed]
- 17.Lu KH, Ring KL. One size may not fit all: The debate of universal tumor testing for Lynch syndrome. Gynecol Oncol. 2015;137:2–3. doi: 10.1016/j.ygyno.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Frolova AI, Babb SA, Zantow E, et al. Impact of an immunohistochemistry-based universal screening protocol for Lynch syndrome in endometrial cancer on genetic counseling and testing. Gynecol Oncol. 2015;137:7–13. doi: 10.1016/j.ygyno.2015.01.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoffel EM, Mangu PB, Gruber SB, et al. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol. 2015;33:209–217. doi: 10.1200/JCO.2014.58.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim GP, Colangelo LH, Wieand HS, et al. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: A National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol. 2007;25:767–772. doi: 10.1200/JCO.2006.05.8172. [DOI] [PubMed] [Google Scholar]
- 22.Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caduff RF, Johnston CM, Svoboda-Newman SM, et al. Clinical and pathological significance of microsatellite instability in sporadic endometrial carcinoma. Am J Pathol. 1996;148:1671–1678. [PMC free article] [PubMed] [Google Scholar]
- 24.Wong YF, Ip TY, Chung TK, et al. Clinical and pathologic significance of microsatellite instability in endometrial cancer. Int J Gynecol Cancer. 1999;9:406–410. doi: 10.1046/j.1525-1438.1999.99054.x. [DOI] [PubMed] [Google Scholar]
- 25.Basil JB, Goodfellow PJ, Rader JS, et al. Clinical significance of microsatellite instability in endometrial carcinoma. Cancer. 2000;89:1758–1764. doi: 10.1002/1097-0142(20001015)89:8<1758::aid-cncr16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 26.Fiumicino S, Ercoli A, Ferrandina G, et al. Microsatellite instability is an independent indicator of recurrence in sporadic stage I-II endometrial adenocarcinoma. J Clin Oncol. 2001;19:1008–1014. doi: 10.1200/JCO.2001.19.4.1008. [DOI] [PubMed] [Google Scholar]
- 27.Baldinu P, Cossu A, Manca A, et al. Microsatellite instability and mutation analysis of candidate genes in unselected Sardinian patients with endometrial carcinoma. Cancer. 2002;94:3157–3168. doi: 10.1002/cncr.10606. [DOI] [PubMed] [Google Scholar]
- 28.Muresu R, Sini MC, Cossu A, et al. Chromosomal abnormalities and microsatellite instability in sporadic endometrial cancer. Eur J Cancer. 2002;38:1802–1809. doi: 10.1016/s0959-8049(02)00152-1. [DOI] [PubMed] [Google Scholar]
- 29.Koul A, Willén R, Bendahl PO, et al. Distinct sets of gene alterations in endometrial carcinoma implicate alternate modes of tumorigenesis. Cancer. 2002;94:2369–2379. doi: 10.1002/cncr.10498. [DOI] [PubMed] [Google Scholar]
- 30.Ørbo A, Eklo K, Kopp M. A semiautomated test for microsatellite instability and its significance for the prognosis of sporadic endometrial cancer in northern Norway. Int J Gynecol Pathol. 2002;21:27–33. doi: 10.1097/00004347-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Peiró G, Lohse P, Mayr D, et al. Insulin-like growth factor-I receptor and PTEN protein expression in endometrial carcinoma. Correlation with bax and bcl-2 expression, microsatellite instability status, and outcome. Am J Clin Pathol. 2003;120:78–85. doi: 10.1309/C1KA-H1PR-L1UB-W798. [DOI] [PubMed] [Google Scholar]
- 32.Pijnenborg JM, Dam-de Veen GC, de Haan J, et al. Defective mismatch repair and the development of recurrent endometrial carcinoma. Gynecol Oncol. 2004;94:550–559. doi: 10.1016/j.ygyno.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 33.Black D, Soslow RA, Levine DA, et al. Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J Clin Oncol. 2006;24:1745–1753. doi: 10.1200/JCO.2005.04.1574. [DOI] [PubMed] [Google Scholar]
- 34.Cohn DE, Frankel WL, Resnick KE, et al. Improved survival with an intact DNA mismatch repair system in endometrial cancer. Obstet Gynecol. 2006;108:1208–1215. doi: 10.1097/01.AOG.0000239097.42987.0c. [DOI] [PubMed] [Google Scholar]
- 35.Bilbao C, Lara PC, Ramírez R, et al. Microsatellite instability predicts clinical outcome in radiation-treated endometrioid endometrial cancer. Int J Radiat Oncol Biol Phys. 2010;76:9–13. doi: 10.1016/j.ijrobp.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackay HJ, Gallinger S, Tsao MS, et al. Prognostic value of microsatellite instability (MSI) and PTEN expression in women with endometrial cancer: Results from studies of the NCIC Clinical Trials Group (NCIC CTG) Eur J Cancer. 2010;46:1365–1373. doi: 10.1016/j.ejca.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 37.Steinbakk A, Malpica A, Slewa A, et al. Biomarkers and microsatellite instability analysis of curettings can predict the behavior of FIGO stage I endometrial endometrioid adenocarcinoma. Mod Pathol. 2011;24:1262–1271. doi: 10.1038/modpathol.2011.75. [DOI] [PubMed] [Google Scholar]
- 38.Diaz-Padilla I, Romero N, Amir E, et al. Mismatch repair status and clinical outcome in endometrial cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2013;88:154–167. doi: 10.1016/j.critrevonc.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Bilbao-Sieyro C, Ramírez R, Rodríguez-González G, et al. Microsatellite instability and ploidy status define three categories with distinctive prognostic impact in endometrioid endometrial cancer. Oncotarget. 2014;5:6206–6217. doi: 10.18632/oncotarget.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanopienė D, Smailytė G, Vidugirienė J, et al. Impact of microsatellite instability on survival of endometrial cancer patients. Medicina (Kaunas) 2014;50:216–221. doi: 10.1016/j.medici.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz I, Martín-Arruti M, Lopez-Lopez E, et al. Lack of association between deficient mismatch repair expression and outcome in endometrial carcinomas of the endometrioid type. Gynecol Oncol. 2014;134:20–23. doi: 10.1016/j.ygyno.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 42.Shikama A, Minaguchi T, Matsumoto K, et al. Clinicopathologic implications of DNA mismatch repair status in endometrial carcinomas. Gynecol Oncol. 2016;140:226–233. doi: 10.1016/j.ygyno.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 43.Brinton LA, Felix AS, McMeekin DS, et al. Etiologic heterogeneity in endometrial cancer: Evidence from a Gynecologic Oncology Group trial. Gynecol Oncol. 2013;129:277–284. doi: 10.1016/j.ygyno.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu KH, Daniels M. Endometrial and ovarian cancer in women with Lynch syndrome: Update in screening and prevention. Fam Cancer. 2013;12:273–277. doi: 10.1007/s10689-013-9664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zighelboim I, Schmidt AP, Gao F, et al. ATR mutation in endometrioid endometrial cancer is associated with poor clinical outcomes. J Clin Oncol. 2009;27:3091–3096. doi: 10.1200/JCO.2008.19.9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dewdney SB, Rimel BJ, Thaker PH, et al. Aberrant methylation of the X-linked ribosomal S6 kinase RPS6KA6 (RSK4) in endometrial cancers. Clin Cancer Res. 2011;17:2120–2129. doi: 10.1158/1078-0432.CCR-10-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 48.Djordjevic B, Barkoh BA, Luthra R, et al. Relationship between PTEN, DNA mismatch repair, and tumor histotype in endometrial carcinoma: Retained positive expression of PTEN preferentially identifies sporadic non-endometrioid carcinomas. Mod Pathol. 2013;26:1401–1412. doi: 10.1038/modpathol.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cox D. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 50. The R CoreTeam. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2013.
- 51.Whelan AJ, Babb S, Mutch DG, et al. MSI in endometrial carcinoma: Absence of MLH1 promoter methylation is associated with increased familial risk for cancers. Int J Cancer. 2002;99:697–704. doi: 10.1002/ijc.10429. [DOI] [PubMed] [Google Scholar]
- 52.Buchanan DD, Tan YY, Walsh MD, et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. J Clin Oncol. 2014;32:90–100. doi: 10.1200/JCO.2013.51.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinicrope FA. DNA mismatch repair and adjuvant chemotherapy in sporadic colon cancer. Nat Rev Clin Oncol. 2010;7:174–177. doi: 10.1038/nrclinonc.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawakami H, Zaanan A, Sinicrope FA. Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options Oncol. 2015;16:30. doi: 10.1007/s11864-015-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Webber EM, Kauffman TL, O’Connor E, et al. Systematic review of the predictive effect of MSI status in colorectal cancer patients undergoing 5FU-based chemotherapy. BMC Cancer. 2015;15:156. doi: 10.1186/s12885-015-1093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shih KK, Garg K, Levine DA, et al. Clinicopathologic significance of DNA mismatch repair protein defects and endometrial cancer in women 40years of age and younger. Gynecol Oncol. 2011;123:88–94. doi: 10.1016/j.ygyno.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Schwitalle Y, Linnebacher M, Ripberger E, et al. Immunogenic peptides generated by frameshift mutations in DNA mismatch repair-deficient cancer cells. Cancer Immun. 2004;4:14. [PubMed] [Google Scholar]
- 58.Suemori T, Susumu N, Iwata T, et al. Intratumoral CD8+ lymphocyte infiltration as a prognostic factor and its relationship with cyclooxygenase 2 expression and microsatellite instability in endometrial cancer. Int J Gynecol Cancer. 2015;25:1165–1172. doi: 10.1097/IGC.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 59.Bauer K, Nelius N, Reuschenbach M, et al. T cell responses against microsatellite instability-induced frameshift peptides and influence of regulatory T cells in colorectal cancer. Cancer Immunol Immunother. 2013;62:27–37. doi: 10.1007/s00262-012-1303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoo HJ, Lim MC, Son Y, et al. Survival outcome in endometrial cancer patients according to hereditary predisposition. Taiwan J Obstet Gynecol. 2015;54:24–28. doi: 10.1016/j.tjog.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 61.Watson P, Lin KM, Rodriguez-Bigas MA, et al. Colorectal carcinoma survival among hereditary nonpolyposis colorectal carcinoma family members. Cancer. 1998;83:259–266. [PubMed] [Google Scholar]
- 62.Stigliano V, Assisi D, Cosimelli M, et al. Survival of hereditary non-polyposis colorectal cancer patients compared with sporadic colorectal cancer patients. J Exp Clin Cancer Res. 2008;27:39. doi: 10.1186/1756-9966-27-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drescher KM, Sharma P, Lynch HT. Current hypotheses on how microsatellite instability leads to enhanced survival of Lynch Syndrome patients. Clin Dev Immunol. 2010;2010:170432. doi: 10.1155/2010/170432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sankila R, Aaltonen LA, Järvinen HJ, et al. Better survival rates in patients with MLH1-associated hereditary colorectal cancer. Gastroenterology. 1996;110:682–687. doi: 10.1053/gast.1996.v110.pm8608876. [DOI] [PubMed] [Google Scholar]
- 65.Rhyu MS. Molecular mechanisms underlying hereditary nonpolyposis colorectal carcinoma. J Natl Cancer Inst. 1996;88:240–251. doi: 10.1093/jnci/88.5.240. [DOI] [PubMed] [Google Scholar]
- 66.Muzny DM, Bainbridge MN, Chang K, et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim TM, Laird PW, Park PJ. The landscape of microsatellite instability in colorectal and endometrial cancer genomes. Cell. 2013;155:858–868. doi: 10.1016/j.cell.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kouri M, Laasonen A, Mecklin JP, et al. Diploid predominance in hereditary nonpolyposis colorectal carcinoma evaluated by flow cytometry. Cancer. 1990;65:1825–1829. doi: 10.1002/1097-0142(19900415)65:8<1825::aid-cncr2820650827>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 69.Frei JV. Hereditary nonpolyposis colorectal cancer (Lynch syndrome II). Diploid malignancies with prolonged survival. Cancer. 1992;69:1108–1111. doi: 10.1002/cncr.2820690507. [DOI] [PubMed] [Google Scholar]
- 70.Dolcetti R, Viel A, Doglioni C, et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999;154:1805–1813. doi: 10.1016/S0002-9440(10)65436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drescher KM, Sharma P, Watson P, et al. Lymphocyte recruitment into the tumor site is altered in patients with MSI-H colon cancer. Fam Cancer. 2009;8:231–239. doi: 10.1007/s10689-009-9233-0. [DOI] [PubMed] [Google Scholar]
- 72.Parsons MT, Buchanan DD, Thompson B, et al. Correlation of tumour BRAF mutations and MLH1 methylation with germline mismatch repair (MMR) gene mutation status: A literature review assessing utility of tumour features for MMR variant classification. J Med Genet. 2012;49:151–157. doi: 10.1136/jmedgenet-2011-100714. [DOI] [PubMed] [Google Scholar]
- 73.van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21:3347–3355. doi: 10.1158/1078-0432.CCR-15-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]