Abstract
Purpose:
To investigate the safety and potential efficacy of ranibizumab for prevention of radiation complications in patients treated with proton irradiation for choroidal melanoma
Methods:
Forty patients with tumors located within 2 disc diameters of the optic nerve and/or macula were enrolled in this open-label study. Participants received ranibizumab 0.5 mg or 1.0 mg at tumor localization and every 2 months thereafter for the study duration of 24 months. The incidence of adverse events, visual acuity, and other measures of ocular morbidity related to radiation complications were assessed. Historical controls with similar follow-up meeting the eligibility criteria for tumor size, location, and baseline visual acuity were assembled for comparison.
Results:
Fifteen patients with large tumors and 25 patients with small/medium tumors were enrolled. Thirty-two patients completed the month 24 visit. No serious ocular or systemic adverse events related to ranibizumab were observed. At 24 months, the proportion of patients with visual acuity ≥ 20/200 was 30/31 (97%) in the study group versus 92/205 (45%) in historical controls (P < .001). The proportion of patients with visual acuity ≥20/40 was 24/31 (77%) in the study group versus 46/205 (22%) in controls at 24 months (P<.001). Clinical evidence of radiation maculopathy at month 24 was seen in 8/24 (33%) patients with small/medium tumors versus 42/62 (68%) of controls (P = .004). Three patients with large tumors developed metastases.
Conclusions:
In this small pilot study, prophylactic ranibizumab appears generally safe in patients treated with proton irradiation for choroidal melanoma. High rates of visual acuity retention were observed through 2 years.
INTRODUCTION
Choroidal melanoma is the most common primary intraocular malignancy in adult patients and is one of the few potentially fatal ocular diseases. In recent decades, local irradiation has replaced enucleation as the preferred treatment for most patients with choroidal melanoma. Two major radiotherapy modalities are currently employed: external beam radiation therapy, most commonly with protons, and radioactive plaques, which are placed over the sclera in the area of the tumor. As a charged particle, protons can provide highly localized radiation dose distributions and by doing so may potentially reduce ocular morbidity.
Despite this advantage, radiation retinopathy remains a frequent complication for patients undergoing radiation for choroidal melanoma. It can be quite devastating and can lead to total vision loss or necessitate removal of the eye. In some cases, radiation retinopathy and its sequelae resolve without treatment. However, for many patients undergoing radiotherapy, radiation retinopathy remains a major source of ocular morbidity. There remains no standard of care for these patients, and available therapeutic options are limited with variable results.
PATHOPHYSIOLOGY OF RADIATION RETINOPATHY
Ionizing radiation can cause a variety of damaging effects on tissues in the body. Damage occurs directly through the blockage of cellular mitotic activity and indirectly through damage to the vasculature, which nourishes the tissue. Within the eye, the retina and optic nerve are relatively radioresistant tissues, but damage may develop secondary to disruptions in their vascular supply. Radiation retinopathy is a delayed-onset, slowly progressive vaso-occlusive complication of ionizing radiation exposure to the retina. Following radiation, the destruction of endothelial cells and capillary closure induces ischemic and proliferative changes similar to those found in other retinal vascular diseases such as diabetic retinopathy.1
Radiation-induced damage to the macula, “radiation maculopathy”, is characterized by edema, hemorrhage, telangiectasia, microaneurysm formation, nerve fiber layer infarcts, capillary nonperfusion, and atrophy of the retina pigment epithelium (RPE). Radiation-induced damage to the optic nerve, “radiation papillopathy”, includes edema, microvascular changes, and hemorrhage. Later manifestations include pallor and neovascularization of the disc. The severity of radiation complications has been shown to be dependent on the total dose of radiation, the fraction size, the fraction interval, and the volume of retina irradiated.1–4
THE ROLE OF VASCULAR ENDOTHELIAL GROWTH FACTOR IN RADIATION VASCULOPATHY
The pivotal role of vascular endothelial growth factor (VEGF) in ocular neovascular disease has been demonstrated in primate models. VEGF was demonstrated to be correlated with iris neovascularization in a monkey model of ischemic retinopathy and iris neovascularization.5 Injections of recombinant human VEGF in concentrations similar to those measured in eyes with neovascularization were carried out in normal monkey eyes, leading to iris neovascularization and neovascular glaucoma, as well as many of the changes of diabetic retinopathy, including vessel dilation, tortuosity, microaneurysm formation, hemorrhage, edema, capillary dropout and intraretinal neovascularization.6,7 These manifestations of diabetic retinopathy are similar to some of the characteristics observed in radiation vasculopathy.
The clinical experience confirming the role of VEGF in ischemic retinal diseases continues to grow. Measurement of VEGF levels in vitrectomy specimens showed increased VEGF levels in patients with proliferative disease from diabetes or retinal vein occlusion.8,9 Surgical specimens from eyes with proliferative diabetic retinopathy have also shown VEGF expression, and postmortem studies have demonstrated increased VEGF expression in eyes with retinal neovascularization.10,11 In addition to clinical trials demonstrating the efficacy of anti-VEGF therapy in choroidal neovascularization, there is also significant experience using this class of therapy for retinal proliferative disease such as proliferative diabetic retinopathy.12,13
After irradiation, the resulting environment of inflammation and ischemia may stimulate the production of any number of growth factors and cytokines in response to the injury, including VEGF.14,15 Radiation-induced changes in the retinal vasculature may parallel disruption of the blood-brain barrier seen in central nervous system radiation injury. In a rat model of radiation-induced spinal cord injury, increased VEGF expression was detected in astrocytes beginning at 16 weeks after radiation.16 Additionally, decreased functional VEGF expression appeared to be protective against the development of paralysis in a transgenic mouse model of radiation-induced spinal cord injury.17 Hypoxia resulting from initial radiation damage to the retinal vasculature could similarly stimulate increased VEGF expression in the retina leading to further changes in microvascular permeability and resultant macular edema. Widespread ischemia due to large treatment fields may result in higher VEGF levels and neovascular glaucoma. Therefore, inhibiting VEGF in the setting of ocular irradiation could potentially decrease vision-threatening complications.
RADIATION COMPLICATIONS AFTER PROTON IRRADIATION FOR CHOROIDAL MELANOMA
Based on treatment outcomes of over 3000 patients with choroidal melanoma treated at the Harvard Cyclotron Laboratory/Francis H. Burr Proton Therapy Center at Massachusetts General Hospital between 1975 and 2006, the incidence and risk factors for radiation complications are well characterized. The highest annual rates of radiation maculopathy are observed 2 (19%) to 3 years (11.5%) after treatment.18 This risk is significantly associated with the proximity of the tumor to the macula. Similarly, patients with tumors located near the optic nerve are at higher risk of developing papillopathy. In a study of patients with tumors within 4 disc diameters of the optic nerve or macula, annual rates of papillopathy peaked at 2 years (15%) after proton irradiation.4
In patients treated with proton beam irradiation, irrespective of tumor location, the probability of vision loss to worse than 20/200 increases from 52% at 5 years to 71% at 15 years. The development of radiation vasculopathy is associated with an even poorer visual prognosis; one year after diagnosis, visual acuity in these patients dropped below 20/200 in 60% and 77% of patients with maculopathy and papillopathy, respectively.18
Rates of eye loss range from 9% at 5 years to 16% at 15 years for all patients and increases to 27% at 5 years in high-risk patients, that is patients with large tumors or tumors located near the optic disc or macula. Neovascular glaucoma (NVG) is the primary reason for enucleation, and the majority of enucleations are performed during the first three years post irradiation.18
Iris neovascularization is significantly related to larger tumor volume and posterior tumor location. Rubeosis is a relatively early event. Few patients develop this complication more than three years post-irradiation. Approximately 40% of patients with high-risk characteristics (height >6 mm, >25% of lens exposed to >35 Gy (Gray; relative biological effectiveness (RBE)) develop NVG compared to less than 5% of patients in the low risk group (height <3 mm, <5% of lens exposed to > 35 Gy(RBE)) within 3 years of treatment.19
HISTORY OF TREATMENT OF RADIATION RETINOPATHY
A variety of treatment approaches for radiation retinopathy have been investigated. Some therapies have been supported only by anecdotal reports, such as hyperbaric oxygen20,21 and oral pentoxyfylline, a vasodilator that increases ocular blood flow.22,23 Treatments that have been investigated more extensively include laser photocoagulation and pharmacologic therapies such as corticosteroids and anti-VEGF agents.
Laser
Laser photocoagulation has been utilized in treating macular edema due to radiation vasculopathy, inducing regression of proliferative radiation retinopathy, and in prophylaxis of retinopathy. Kinyoun performed a combination of focal and grid (to areas of nonperfusion) laser in 19 eyes with macular edema with non-proliferative radiation retinopathy and demonstrated better final visual acuity compared to 23 eyes that did not receive laser treatment.24 A regression analysis in this study demonstrated a significant treatment benefit, but patients were noted to lose vision despite laser treatment. Hykin and colleagues similarly compared outcomes in 19 patients treated with focal laser for radiation maculopathy versus 23 matched controls.25 They found that while focal laser appeared beneficial in improving vision and reducing edema at 6 months after treatment, this benefit was not sustained at 12 and 24 months.
Panretinal photocoagulation has demonstrated efficacy in regressing neovascularization in proliferative radiation retinopathy. Kinyoun and colleagues observed complete resolution of radiation-induced neovascularization in 10 (91%) of 11 eyes following pan retinal photocoagulation.26 However, in this series, visual prognosis in eyes with proliferative radiation retinopathy was shown to be poor with 12 (86%) of 14 eyes having a final vision of 20/200 or worse after mean follow-up time of 75 months. Sectoral laser photocoagulation has also been shown to induce regression of non-proliferative retinopathy.27
Not surprisingly, verteporfin photodynamic therapy (PDT) has been successfully used to treat cases of choroidal neovascularization arising in the context of radiation maculopathy.28,29 However, there is also a report of the use of PDT for edema due to radiation maculopathy in the absence of choroidal neovascularization.30 In this small series, 3 such patients were noted to have improvement in edema and hard exudates as well as improvement in visual acuity.
Pharmacologic Therapies
Triamcinolone acetonide is a synthetic corticosteroid that may regulate capillary permeability as well as help restore a compromised inner blood-retinal barrier. It has been used to treat macular edema related to vascular diseases, including radiation maculopathy. Sutter and Gillies reported a case in which intravitreal triamcinolone significantly improved visual acuity, decreased retinal thickness and decreased vascular leakage in a patient with radiation retinopathy in whom focal laser failed to show benefit.31 In a series of 31 patients with radiation-induced macular edema, Shields and colleagues demonstrated that 4 mg intravitreal triamcinolone resulted in decreased central foveal thickness and stable or improved visual acuity in 14 of 31(45%) at 6 months.32 The same group has also suggested that intravitreal triamcinolone may also have some benefit in cases of acute radiation papillopathy.33
Recently, the sustained-release dexamethasone implant (Osurdex®) has emerged as a promising treatment for radiation-induced macular edema. Some case reports and small case series report reduction of macular edema and visual acuity improvement or stabilization.34–36 Several of these patients had failed prior therapy with bevacizumab.
Other therapies that have been described only anecdotally include hyperbaric oxygen and oral pentoxifylline. Gall and colleagues reported a case of a patient who developed radiation optic neuropathy and retinopathy after brachytherapy.21 After 20 sessions of hyperbaric oxygen treatment, they noted total resolution of retinal exudates and improvement of visual fields. Borruat and coauthors reported a similar case in which hyperbaric oxygen treatment was correlated with improved visual field defects in a patient with radiation-induced optic neuropathy.20 However, increased oxygenation of tissues during radiotherapy may potentiate radiation damage. Stanford reported a case in which simultaneous irradiation and hyperbaric oxygen resulted in a severe vaso-occlusive response.37 Pentoxifylline is commonly used to treat peripheral vascular disease, as it decreases the viscosity of blood and increases erythrocyte flexibility. It has a direct vasodilatory effect and has been shown to increase ocular blood flow.22 Gupta and colleagues described a patient who experienced visual acuity improvement following pentoxifylline treatment for radiation retinopathy related to whole brain radiotherapy for a temporal lobe medulloblastoma.23
Anti-VEGF Therapy
Radiation Maculopathy. Considerable experience with anti-VEGF agents for treatment of radiation maculopathy after plaque radiotherapy for choroidal melanoma has been reported. Finger and colleagues evaluated 99 patients treated with bevacizumab (n=92) or ranibizumab (n=7) for radiation maculopathy over a 10-year period from 2005–2015.38 The mean treatment period for these patients was 38 months (range 6–108 months) and the mean follow-up from time of initial tumor diagnosis was 81 months (range 11–130). Kaplan-Meier analysis of visual results in this study demonstrated a 69% probability of retaining visual acuity (VA) within 2 lines of pre-anti-VEGF treatment VA at 5 years and 38% at 8 years. Evaluation of central foveal thickness by spectral-domain OCT on 63 patients revealed an initial decrease, followed by stabilization, and perhaps a late increase over the course of anti-VEGF therapy. Dose escalation was performed for progressive macular edema during the last 4 years of the study period. At last follow-up, 49/99 (49%) of the patients had required dose escalation to 2.0 mg, 2.5 mg or 3.0 mg bevacizumab or 2.0 mg ranibizumab.
Shah and coauthors reported results of bevacizumab treatment for early signs of radiation maculopathy detected by SD-OCT in 159 patients.39 The mean time to development of radiation maculopathy in this study was 15.8 months. The authors observed that 77/159 (48%) patients had visual acuity of 20/40 or better and 81 (51%) had 20/50 or better with median follow-up of 36 months, which compared favorably to the results of the Collaborative Ocular Melanoma Study in which 31% of patients treated with plaque brachytherapy were noted to have 20/40 vision or better at 36 months. The patients with 20/50 or better at last follow-up had a mean of 5 injections over a mean period of 17.6 months. Of note, 16/81 (20%) of the patients retaining 20/50 or better had laser photocoagulation or pars plana vitrectomy in addition to bevacizumab, one patient had additional intravitreal triamcinolone, and 20/81 (25%) had cataract extraction.
A shorter term study from Wills Eye Institute evaluated the results of treating macular edema after plaque brachytherapy with 4 consecutive monthly injections of bevacizumab in 36 patients assessed at 4–6 months after the first injection.40 The median time to detection of macular edema was 14 months from plaque radiotherapy. In this series, 20/36 (56%) patients had decreased (>10% decrease vs. baseline) central macular thickness (CMT) on OCT and 15/36 (42%) had improved vision (>1 line improvement), while 16 (44%) had stable vision. Notably, the authors observed that there was an increase in CMT in all patients from the time of the fourth and final injection and the last follow-up, suggesting that long-term therapy is required.
Other small series indicate that the effects of bevacizumab are transient and variable. In a series of 10 patients with macular edema following plaque brachytherapy who were treated with a single injection of bevacizumab, Mason and colleagues observed decreased edema at 6 weeks after injection with mild gains in visual acuity. However, at 4 months, all patients had recurrent macular edema, and the mean visual acuity returned to pretreatment levels.41 Gupta and coauthors noted short-term efficacy in patients with new onset macular edema after 1 or 2 injections, but no effect in patients with chronic maculopathy of 3–5 years duration.42 Bakri and colleagues also noted inconsistent responses to bevacizumab in a series of 5 patients. Three of these patients showed complete resolution of macular edema after treatment with intravitreal triamcinolone acetonide, and the remaining 2 patients showed complete resolution after a combination of bevacizumab and triamcinolone acetonide.43 A case of response to ranibizumab after tachyphylaxis to bevacizumab has also been reported. Jutley and coauthors presented a patient with radiation maculopathy following stereotactic radiotherapy for optic nerve meningioma who was treated with 6 monthly injections of bevacizumab, but developed recurrence of macular edema and decreased vision after the third injection. He then received ranibizumab with resolution of macular edema and improved visual acuity.44
Neovascularization. Anti-VEGF therapy also plays a role in managing neovascular complications of radiation for choroidal melanoma. Case series reporting the use of bevacizumab after the development of neovascular glaucoma demonstrate that regression of iris neovascularization, reduction of intraocular pressure, and globe salvage may be achieved.45–47 However, there is little visual benefit in these advanced cases. Experience with ranibizumab appears to be similar.48,49
Optic Neuropathy. Description of the use of bevacizumab for radiation-induced optic neuropathy is more limited. Gondo and colleagues reported a single case in which one injection of 1.25mg intravitreal bevacizumab improved visual acuity to 20/20 and reduced radiation-induced optic disc swelling, hemorrhage, and exudates following external beam therapy for maxillary sinus cancer.50 The improvements were maintained through 12 months of follow-up. Finger and Chin reported a series of 14 patients treated with bevacizumab for radiation-induced optic neuropathy following plaque radiotherapy for choroidal melanoma.51 The median time to onset of optic neuropathy in these patients was 24.5 months after radiotherapy. They received a median of 13 injections over a median duration of 22.5 months. The authors reported stable or improved vision in 9/14 (64%) patients over a median follow-up period of 29 months (range 4–39). Of note, 5 (36%) of these 14 patients had visual acuity of 20/200 or worse at last follow-up.
Prevention of Radiation Complications after Radiotherapy for Uveal Melanoma. Recognizing the inconsistent results of treatment for established radiation vasculopathy, several groups have attempted prophylactic therapies in patients undergoing radiotherapy for uveal melanoma. Finger and Kurli described 16 patients with choroidal melanoma posterior to the equator treated with prophylactic scatter laser photocoagulation with mean follow-up of 23.2 months from plaque and 16.5 months from laser.27 They reported that 3 (19%) of these patients developed retinopathy, only one involving the macula, and all responding to additional laser.
Horgan and colleagues performed a randomized controlled trial evaluating the efficacy of 3 periocular triamcinolone injections given at 4 month intervals, starting at the time of plaque application, for preventing macular edema.52 They reported that macular edema developed in 32/55 (58%) patients in the control group, compared to 39/108 (36%) patients who were treated with periocular triamcinolone through 18 months of follow up. Triamcinolone treatment was significantly associated with lower risk of macular edema in multivariate analysis (hazard estimate, 0.45; 95% confidence interval, 0.19 – 0.70; P =.001). The triamcinolone group also demonstrated significantly lower rates of moderate and severe vision loss at 18 months.
Materin and coauthors investigated sector panretinal photocoagulation in combination with the same periocular triamcinolone regimen as studied by Horgan and colleagues in 29 patients. At the 12-month and 24-month follow-up, cystoid macular edema was observed in 5/29 (17%) and 6/25 (24%) patients, respectively.
The group at Wills Eye Institute recently published a series of patients undergoing prophylactic bevacizumab every 4 months for 2 years, starting at the time of plaque removal.53 This study included 292 patients in the bevacizumab group and 126 patients in a nonrandomized control group. The mean follow-up was 17 months for the bevacizumab patients and 21 months for the control group. Only 24 (9%) of the bevacizumab group received all 7 injections; the mean number of injections was 4. Despite the low frequency of injections, the authors reported significant benefits of bevacizumab therapy as assessed by overall incidence of macular edema on OCT (75/292 (26%) vs. 51/126 (40%), P=.004), clinically significant radiation maculopathy, moderate vision loss, and poor vision (VA worse than 5/200).
The surgical technique of pars plana vitrectomy and silicone oil injection at the time of plaque applications has also been evaluated as a means of reducing visual loss from radiation complications. Ahuja and colleagues demonstrated that silicone oil attenuates radiation dose in vitro and reported on 3 patients in which vitrectomy and silicone oil injection was performed with plaque radiotherapy.54 They noted that no patient developed radiation retinopathy over 10–24 months of follow-up. However, one patient did develop a rhegmatogenous retinal detachment. McCannel and McCannel reported results of this procedure in 20 patients compared to 20 control patients matched for tumor height, sex, and tumor location.55 The average follow-up time was 22.1 months in case patients and 19.4 months in control patients. They found that patients with vitrectomy and silicone oil placement experienced fewer clinically detectable macular abnormalities and less macular edema as measured by OCT. Observed complications included retinal tears, total serous retinal detachment, macular hole, and macular pucker.
The purpose of our study was to evaluate the role of prophylactic ranibizumab treatment in patients treated with proton beam irradiation for choroidal melanoma. We hypothesize that ranibizumab is safe when administered to these patients consistently for two years and may reduce vision loss and ocular morbidity from radiation complications
METHODS
This was a single center, phase I, prospective study conducted by the Ocular Oncology/Retina Service of the Massachusetts Eye and Ear. The Human Studies Committee of the Massachusetts Eye and Ear provided institutional review board approval. All subjects provided informed consent prior to participating in this study. This study conformed to the guidelines of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act (HIPAA). The trial was registered with ClinicalTrials.gov and assigned the identifier NCT00765921. This study was conducted under an investigator-initiated investigational new drug (IND) application (IKK; #101244).
The study was performed in 2 cohorts. The first 20 patients were enrolled in an open-label phase of the study to evaluate the safety of 0.5 mg ranibizumab given intravitreally every 2 months for 22 months (12 injections). After an acceptable safety profile was demonstrated, enrollment of a second cohort of 20 subjects in a randomized phase of the study was begun, with subjects assigned to receive 0.5 mg ranibizumab or 1.0 mg ranibizumab every 2 months for 22 months. A randomization was required by the FDA to study this second cohort of patients. However, the second cohort was not powered to detect a difference in efficacy between the two doses. A final assessment was performed at 24 months for both cohorts.
Patients with newly diagnosed choroidal melanoma meeting the inclusion/exclusion criteria were stratified into 2 groups: 1) Large tumors (N=15; >15 mm in diameter and/or >5 mm in height) and 2) Small and medium tumors at high risk for radiation retinopathy or papillopathy (N= 25; ≤ 15 mm in diameter and ≤5 mm in height, located ≤2 disc diameters (DD) from optic disc and/or macula). All patients in the large tumor group received ranibizumab 0.5 mg in the open-label phase. Five patients in the small/medium tumor group received ranibizumab 0.5 mg in the open-label phase. The remaining 20 patients in the small/medium tumor group were randomized 1:1 between standard dose (0.5 mg in 0.05 ml) and high dose ranibizumab (1.0 mg in 0.1 ml). During a short period of time when 2.0 mg in 0.05 ml was available from Genentech, 2 patients each received 2 injections with this preparation. However, Genentech discontinued this formulation during the course of the study and therefore the high dose group was transitioned to the 1.0 mg dose. Detailed inclusion criteria are provided in Table 1 and exclusion criteria in Table 2.
TABLE 1.
INCLUSION CRITERIA
| Ability to provide written informed consent and comply with study assessments for the full duration of the study |
| Age ≥ 18 years |
| Large Tumor Group |
| >15mm in largest diameter and/or >5 mm in height |
| Small/Medium Tumor Group |
| ≤ 15 mm in largest diameter and ≤ 5 mm in height |
| ≤ 2 DD from optic disc and/or macula |
| Baseline best-corrected visual acuity 20/100 or better in the study eye |
TABLE 2.
EXCLUSION CRITERIA
| History of prior treatment for choroidal melanoma |
| Presence of diabetic retinopathy |
| History of retinal vascular occlusion or other retinal vascular disease |
| Active ocular inflammation or history of uveitis in either eye |
| History of uncontrolled glaucoma (defined as intraocular pressure >30mmHg despite treatment with anti-glaucoma medication) or filtering surgery in the study eye |
| History of allergy to fluorescein |
| Inability to obtain fundus photographs of sufficient quality |
| Inability to comply with study or follow-up procedures |
| Previous intravitreal injections of bevacizumab in the study eye or in the non-study eye within 30 days. |
| Concurrent use of systemic anti-VEGF* therapy |
| Current treatment for active systemic infection |
| Pregnancy or lactation |
| History of other disease, metabolic dysfunction, physical examination finding or clinical laboratory finding giving reasonable suspicion of a disease or condition that contraindicates the use of an investigational drug or that might affect interpretation of the results of the study or render the subject at high risk for treatment complications. |
| Participation in another simultaneous medical investigation or trial |
| Any other condition that the investigator believes would pose a significant hazard to the subject if the investigational therapy were initiated |
The initial intravitreal injection of ranibizumab was given at the time of tumor localization surgery. Proton beam irradiation was given according to the standard clinical protocol in 5 fractions beginning approximately 2 weeks after surgery. Radiation treatments were performed at the Francis H. Burr Proton Therapy Center at Massachusetts General Hospital. The large tumor group received the standard dose of 70 Gy(RBE). Patients with small/medium sized tumors within 1 DD of the optic disc or fovea received 50 Gy(RBE), according to current clinical practice. This dose was demonstrated to adequately control tumors in a dose reduction trial of patients with similarly sized tumors.56 The remainder of the small/medium tumor group received 70 Gy(RBE).
Subjects had bi-monthly visits throughout the study for injections as well as evaluation of safety and efficacy. At each visit (every 60 ± 14 days), subjects had examination by the investigator prior to study treatment. Best corrected visual acuity (BCVA), color fundus photography, fluorescein angiography, ultrasonography, and optical coherence tomography (OCT) were performed at screening, month 6, 12, 18, and 24. For the first cohort of 20 patients, the Stratus time-domain OCT (Carl Zeiss Meditec, Inc.) was used. The Cirrus spectral-domain OCT (Carl Zeiss Meditec, Inc.) was used for the second cohort of 20 patients. A complete medical history and physical by an internist and liver function tests were performed at baseline, month 12, and month 24. Other staging or surveillance imaging was not required by the study protocol.
OUTCOME MEASURES
Since this was a phase I study, the primary outcome measures were the incidence and severity of ocular adverse events, as identified by eye examination, including rate of local tumor control, and the incidence and severity of other adverse events, as identified by physical examination and subject reporting, including metastasis and tumor-related death. Secondary outcome measures were exploratory measures to determine the potential efficacy of ranibizumab treatment in reducing ocular morbidity from proton beam irradiation in patients with choroidal melanoma by determining for all patients: 1) the degree of tumor regression as measured by ultrasonography and 2) the proportion of patients with final BCVA (measured with ETDRS chart at 4 meters) better than or equal to 20/200. For the large tumor group, the following additional measures were assessed: 1) the proportion of patients developing neovascular glaucoma (defined as presence of anterior segment neovascularization, i.e. iris or angle, with intraocular pressure >21 mmHg), 2) the proportion of patients with exudative retinal detachment, 3) the proportion of patients requiring enucleation. For the small/medium tumor group additional measures included: 1) the proportion of clinically evident radiation maculopathy, 2) the proportion of patients with radiation papillopathy, 3) the proportion of patients with final vision better than or equal to 20/40, and 4) quantitative and qualitative evaluation of macular findings on OCT.
HISTORICAL CONTROLS
Because this study did not include a control group, we assembled a group of historical controls meeting the eligibility criteria for tumor size, tumor location, and baseline visual acuity, with similar follow-up schedules and assessments. These controls are part of a uveal melanoma registry that includes data on patient demographics, tumor characteristics, treatment parameters, and both ocular and systemic outcomes after treatment (visual acuity, tumor regression, tumor recurrence, radiation complications, enucleation, metastasis and melanoma-related mortality). These outcomes are ascertained through active surveillance, and the use of databases such as the Social Security Death Index and National Death Index (metastasis and mortality data). For patients who receive their care at MEE, chart review to ascertain data is also completed. For patients enrolled in the large tumor group, historical controls were selected from patients treated during a 10-year period, 1987–1996. During this period, surveillance activities were completed on an annual basis to ascertain both ocular outcomes and survival, with follow-up through April 1998, ensuring mature data (>2 years) for most patients. We selected patients for the small/medium control group from registry patients who had participated in a dose reduction trial (standard dose of 70 Gy (RBE) vs. lower dose of 50 Gy (RBE)) between 1989 and 1994.56 This trial had similar eligibility criteria to the present trial in terms of tumor size and location, and provided a cohort of patients with available follow-up data who had received a dose of 50 Gy (RBE). We also selected control patients for the small/medium tumor group from patients in the registry who were treated from 2004 through 2013. The standard clinical treatment protocol was modified in 2004 to allow for the lower dose of 50 Gy (RBE) to be used for tumors within 1 DD of the fovea or optic nerve with tumor height ≤ 5 mm and largest diameter ≤ 15 mm.
Controls were selected for inclusion in the evaluation of rates of visual acuity retention and survival if they completed at least one follow-up visit after treatment before the endpoint was met, and up to 2 years of follow-up if the endpoint was not met. Observations were censored at 2 years for those patients with more than 2 years of follow-up. All patients identified from our uveal melanoma registry meeting the eligibility and follow-up criteria were included as historical controls.
STATISTICAL ANALYSIS
Cumulative rates were calculated using the Kaplan-Meier method. Differences in proportions of discrete outcomes (visual acuity of 20/40 or better, visual acuity of 20/200 or better, presence of complications (radiation maculopathy, radiation papillopathy) between patients treated with proton irradiation and ranibizumab and patients treated with proton irradiation alone were assessed at annual time points (12 months and 24 months after treatment) using Fisher’s exact test. For continuous variables, e.g., tumor regression, macular thickness, tumor dimensions, and age, the Mann-Whitney test was used to test for significant differences.
RESULTS
PATIENT CHARACTERISTICS
Forty patients were enrolled as planned in the study: 15 large tumor patients and 25 small/medium tumor patients. Eight patients discontinued the study: 1 patient (large tumor) requested enucleation during the study, 5 patients withdrew or were lost to follow-up (4 large tumor patients, 1 small/medium), 2 patients (large tumors) developed metastatic disease and were exited from the study. One patient in the large tumor group completed the month 24 visit, but underwent vitrectomy with silicone oil (outside Massachusetts Eye and Ear) about 2 weeks prior to this visit to repair an exudative retinal detachment. Therefore, the visual acuity at month 24 for this patient was not included in the analyses. Twenty-nine patients (73%) received all 12 possible injections. Three patients who completed the study received 10 (1 patient) or 11 injections (2 patients). The baseline characteristics of the study cohort as well as historical controls are presented in Table 3.
TABLE 3.
BASELINE CHARACTERISTICS
| SMALL/MEDIUM | LARGE | |||||
|---|---|---|---|---|---|---|
|
RBZ+PBI n=25 |
PBI n=100 |
P |
RBZ+PBI n=15 |
PBI n=275 |
P | |
| Age at Tx (mean, years) | 59.4 (40.9–84.5) | 57.9 (19.5–8.0) | .80* | 54.8 (28.3–86.2) | 57.3 (15.2–91.9) | .39* |
| Gender (% male) | 14 (56.0) | 51 (51) | .66^ | 8 (53.3) | 129 (46.9) | .79^ |
| LTD (Mean, mm) | 10.78 (6.5–15) | 10.55 (6–15) | .63* | 17.27 (11–24) | 15.78 (9–22) | .08* |
| Height (Mean, mm) | 2.76 (1.7–4.6) | 2.86 (1–5.5) | .54* | 7.32 (2.1–15.6) | 7.9 (1.8–17.1) | .32* |
| Distance to optic disc (mean, DD) | 1.68 (0–4) | 1.16 (0–4) | .05* | 2.47 (0–8) | 2.39 (0–8) | .81* |
| Distance to macula (mean, DD) | 1.08 (0–5) | 0.94 (0–5) | .69* | 2.27 (0–7) | 2.37 (0–8) | .70* |
| Radiation dose 70 Gy (%) | 6 (24.0) | 6 (6.0) | .01^ | 15 (100) | 275 (100) | --- |
| Baseline VA ≥ 20/40 (%) | 22 (88.0) | 68 (68.0) | .05^ | 8 (55.3) | 130 (47.3) | .79^ |
| Baseline VA ≥ 20/200 (%) | 25 (100) | 100 (100) | --- | 12 (80.0) | 225 (81.8) | .74^ |
Mann-Whitney test
Fisher’s exact test
RBZ: ranibizumab; PBI: proton beam irradiation; Tx: treatment; LTD: largest tumor diameter; DD: disc diameter; Gy: Gray; VA: visual acuity
SAFETY
Adverse Events
The most common ocular adverse events reported are listed in Table 4. These symptoms were attributed to the expected side effects of radiation treatment and the intravitreal injection procedure. The most common systemic adverse events reported are listed in Table 5. Eight serious adverse events occurred during the study, which are listed in Table 6. No ocular or systemic adverse events were determined to be related to the pharmacologic effects of ranibizumab.
TABLE 4.
MOST COMMON OCULAR ADVERSE EVENTS
| ADVERSE EVENT | NUMBER OF EVENTS |
|---|---|
| Eye pain | 45 |
| Vision Changes* | 17 |
| Floaters | 13 |
| Eye miscellaneous | 12 |
| Radiation lid burn | 9 |
| Flashes | 8 |
| Eye irritation* | 6 |
| Bilateral dryness, itching* | 5 |
| Tearing* | 5 |
| Cataract* | 4 |
| Increased intraocular pressure | 4 |
| Foreign body sensation | 4 |
| Light sensitivity | 4 |
| Eye discharge | 3 |
| Foreign body in eye | 2 |
| Eye burning | 2 |
| Scotoma | 2 |
| Hypotony | 2 |
At least 1 instance reported as severe
TABLE 5.
MOST COMMON SYSTEMIC ADVERSE EVENTS
| ADVERSE EVENT | NUMBER OF EVENTS |
|---|---|
| Headache* | 13 |
| Cold | 11 |
| Nausea* | 9 |
| Bronchitis* | 9 |
| Miscellaneous trauma* | 6 |
| Vertigo/dizziness | 5 |
| Benign tumor (any location)* | 5 |
| Back pain* | 5 |
| Cough | 5 |
| Colon polyps | 4 |
| Dental problems | 4 |
| Diarrhea/loose stools | 3 |
| Sinus/ear infection | 3 |
| Rash* | 3 |
| Influenza | 3 |
| Neck pain* | 3 |
| Pneumonia* | 3 |
| Vomiting* | 2 |
| Hypertension* | 2 |
| Transient ischemic attack* | 2 |
| Anemia | 2 |
| Shoulder pain* | 2 |
| Rash* | 2 |
| Spongiotic dermatitis | 2 |
| Skin infection | 2 |
At least 1 instance reported as severe
TABLE 6.
SERIOUS ADVERSE EVENTS
| DIAGNOSIS |
SAE CRITERIA MET |
TREATMENT | OUTCOME |
RELATEDNESS TO STUDY TREATMENT |
|---|---|---|---|---|
| Colloid Cyst of the Third Ventricle | Life-threatening Hospitalization | Surgical procedure | Stable | Not related (Pt. with history of hydrocephalus with shunt) |
| Enlargement Fibroid Tumor | Hospitalization | Procedure | Resolved | Not related |
| Gastroenteritis, UTI | Hospitalization | Medication | Resolved | Not related |
| Colitis | Hospitalization | Medication | Stable | Not related |
| Growth of Mesenteric Mass | Hospitalization | Surgical resection | Resolved | Not related (Mass present prior to study entry) |
| Carcinoid Tumor, Lung (left lower lobe) | Hospitalization | Procedure | Resolved | Not related (Mass present prior to study entry) |
| Pneumonia | Hospitalization | Medication | Resolved | Not related |
| Metastatic Adenocarcinoma | Life-threatening | Chemo-therapy | Ongoing | Not related (Pt.with history of colon and pancreatic cancer) |
SAE: Serious adverse event; UTI: Urinary tract infection
Tumor Control, Metastasis, and Mortality
One patient in the small/medium tumor group was noted to have a local recurrence at the month 24 visit. The recurrence appeared to be regrowth of a focal area of the previously treated tumor. This patient initially received a dose of 50 Gy (RBE), was retreated with proton beam irradiation at a dose of 70 Gy (RBE) in 5 fractions, and remains without evidence of metastatic disease. Comparison of recurrence rates in the study patients versus historical controls did not reveal a significant difference. The recurrence rate in the small/medium tumor group was 1/68 (1.5%) in historical controls vs. 1/24 (4.2%) in the ranibizumab-treated patients (P = .46, Fisher’s exact test). For the overall group, there were recurrences in 8/286 (2.8%) of historical controls versus 1/32 (3.1%) of ranibizumab-treated patients (P=.62, Fisher’s exact test).
Two patients in the large tumor group died of metastatic melanoma during the study. These two patients had very large tumors (largest basal diameter 21 mm for both, tumor height 11 mm and 9.3 mm). A third patient (largest basal diameter 15 mm, height 6.3 mm) in the large tumor group was diagnosed with metastatic melanoma 19 months after radiation treatment and died 1 year later (6 months after study completion). No patients in the small/medium tumor group developed metastases during the study period.
The observed melanoma-related mortality in the study patients was compared to mortality within the first 2 years after treatment for historical controls as shown in Table 7. The mortality rate in the large tumor patients treated with ranibizumab was higher than recorded in historical controls but did not quite reach statistical significance.
TABLE 7.
MELANOMA-RELATED MORTALITY
| SMALL/MEDIUM | LARGE | OVERALL | |
|---|---|---|---|
| RBZ + PBI (%) | 0/25 (0.0) | 2/15 (13.3) | 2/40 (5.0) |
| PBI (%) | 1/100 (1.0) | 6/275 (2.2) | 7/375 (1.9) |
| P* | .80 | .06 | .21 |
Fisher’s exact test
RBZ: ranibizumab; PBI: proton beam irradiation
SECONDARY OUTCOMES
Visual Acuity
Visual Acuity 20/200 or Better. The proportion of patients retaining visual acuity better than or equal to 20/200 at month 12 and 24 follow-up visits is shown in Table 8. A significantly higher proportion of patients treated with ranibizumab were observed to have acuity ≥ 20/200 at 24 months in both the small/medium tumor group and the large tumor group as compared to historical controls. All patients in the small/medium tumor group (24/24) retained this level of acuity 24 months after radiation treatment compared to 43/62 (69.3%) of historical controls (P=.001). Overall, 30/31 (96.8%) study patients versus 92/205 (44.9%) controls retained this level of visual acuity (P <.001).
TABLE 8.
PROPORTION OF PATIENTS WITH VISUAL ACUITY OF 20/200 OR BETTER
| SMALL/MEDIUM | LARGE | OVERALL | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Time post Tx |
RBZ + PBI |
PBI | P* |
RBZ+ PBI |
PBI | P* |
RBZ + PBI |
PBI | P* |
| 12 months (%) | 24/24 (100) | 68/79 (75.5) | .045 | 8/12 (66.7) | 104/217 (47.9) | .17 | 32/36 (88.9) | 172/296 (58.1) | <.001 |
| 24 months (%) | 24/24 (100) | 43/62 (69.3) | .001 | 6/7 (85.7) | 49/143 (34.3) | .01 | 30/31 (96.8) | 92/205 (44.9) | <.001 |
Fisher’s exact test
Tx:treatment; RBZ: ranibizumab; PBI: proton beam irradiation
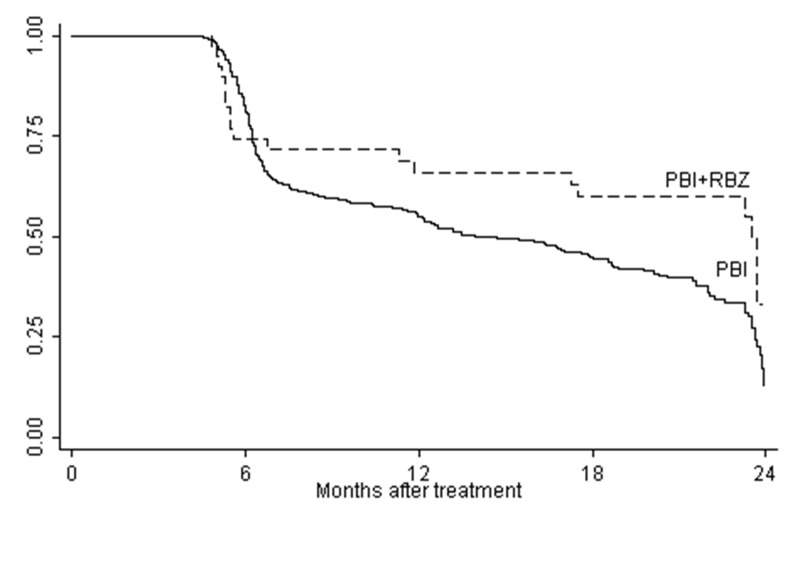
Cumulative rates of visual acuity retention of 20/200 or better were also evaluated. These data are shown in Figures 1–3. Rates of vision retention at this level were significantly higher in ranibizumab-treated patients for those with small/medium tumors as well as both groups combined.
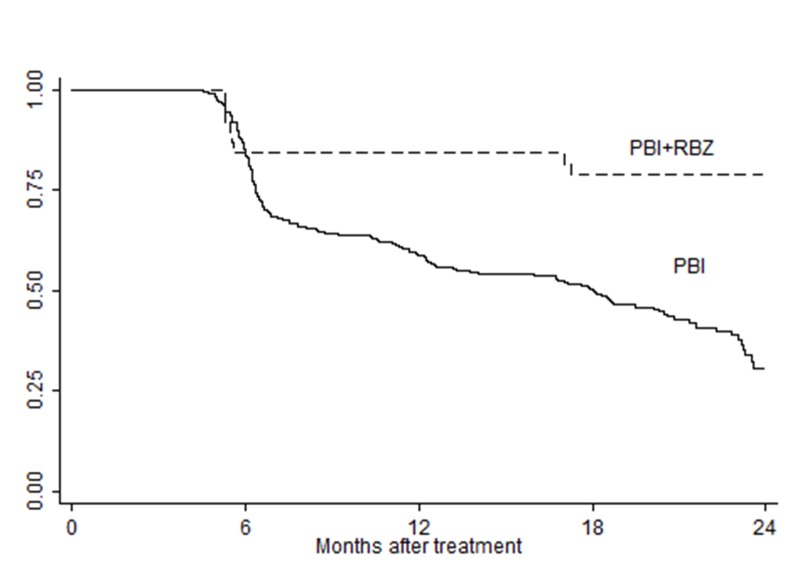
FIGURE 1.
Cumulative rates of visual acuity retention of ≥20/200 for overall study cohort. The difference between the curves is statistically significant, P<.001 (Log-rank test).
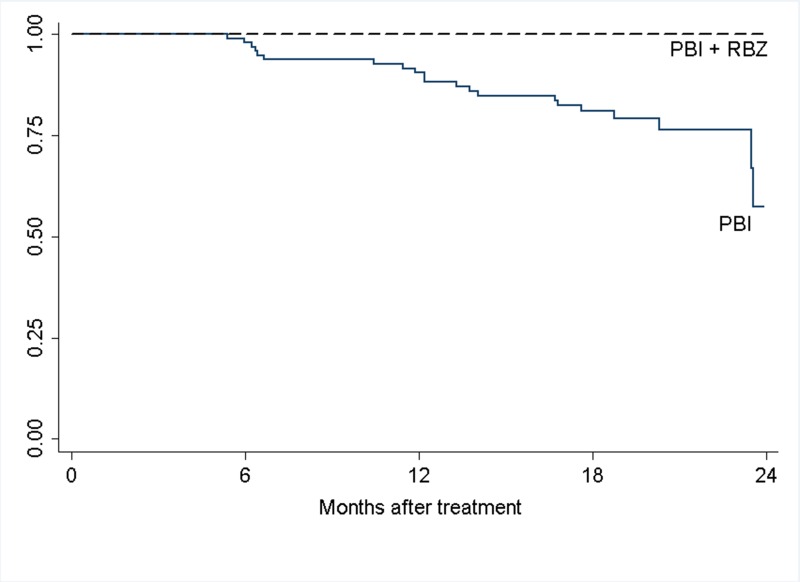
FIGURE 2.
Cumulative rates of visual acuity retention of ≥20/200 for large tumor group. The curves are not significantly different, P=.62 (Log-rank test).
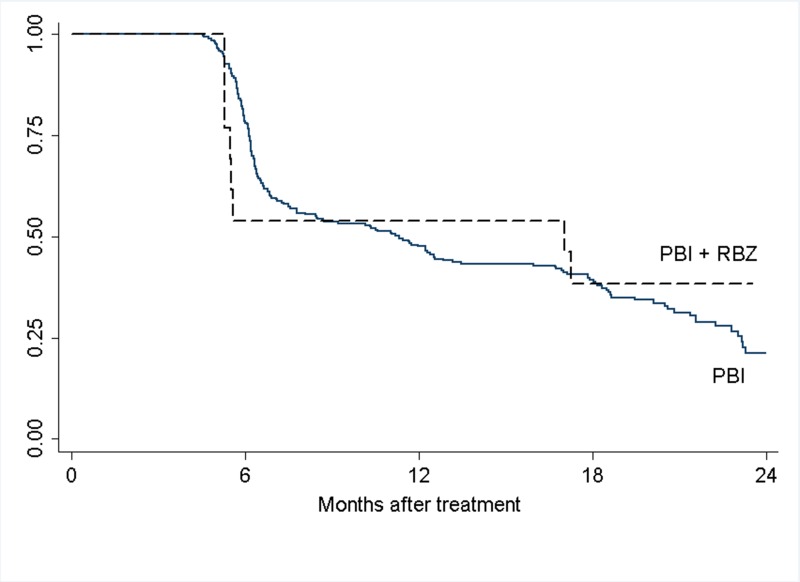
FIGURE 3.
Cumulative rates of visual acuity retention of ≥20/200 for small/medium tumor group. The difference between the curves is statistically significant, P=.006 (Log-rank test).
Visual Acuity 20/40 or Better. The proportion of patients with visual acuity better than or equal to 20/40 at the month 12 and 24 follow-up visits is shown in Table 9. For the small/medium tumor group, 21/24 (87.5%) retained this level of visual acuity at month 24 compared to 29/62 (46.8%) of historical controls (P<.001). Expectedly, the proportion of large tumor patients retaining this level of vision was much smaller (3/7). However, this was still higher than seen in historical controls (P=.05).
TABLE 9.
PROPORTION OF PATIENTS WITH VISUAL ACUITY OF 20/40 OR BETTER
| SMALL/MEDIUM TUMORS | LARGE TUMORS | OVERALL | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Time post Tx |
RBZ + PBI |
PBI | P* |
RBZ + PBI |
PBI | P* |
RBZ + PBI |
PBI | P* |
| 12 months (%) | 20/24 (83.3) | 52/79 (65.8) | .08 | 5/12 (41.7) | 47/217 (21.7) | .11 | 25/36 (69.4) | 99/296 (33.5) | <.001 |
| 24 months (%) | 21/24 (87.5) | 29/62 (46.8) | <.001 | 3/7 (42.9) | 17/143 (11.9) | .05 | 24/31 (77.4) | 46/205 (22.4) | <.001 |
Fisher’s exact test
Tx:treatment; RBZ: ranibizumab; PBI: proton beam irradiation
Since the control group of small/medium tumor patients had a lower proportion with baseline visual acuity of 20/40 or better, an analysis was performed stratifying the groups by baseline visual acuity (Table 10). A significantly greater percentage of those patients entering the study with acuity of 20/40 or better retained this level of vision compared to historical controls.
TABLE 10.
PROPORTION OF PATIENTS WITH VISUAL ACUITY OF 20/40 OR BETTER, BY BASELINE VISUAL ACUITY (SMALL/MEDIUM TUMORS)
| BLVA 20/40 OR BETTER | BLVA WORSE THAN 20/40 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Time post Tx |
RBZ+ PBI (%) |
PBI (%) |
P* |
RBZ+ PBI (%) |
PBI (%) |
P* |
| 12 months | 19/22 (86.4) | 44/53 (83.0) | .51 | 1/2 (50.0) | 3/18 (16.7) | .37 |
| 24 months | 20/22 (90.9) | 26/44 (59.1) | .007 | 1/2 (50.0) | 8/26 (30.8) | .55 |
Fisher’s exact test
BLVA: baseline visual acuity; Tx:treatment; RBZ: ranibizumab; PBI: proton beam irradiation
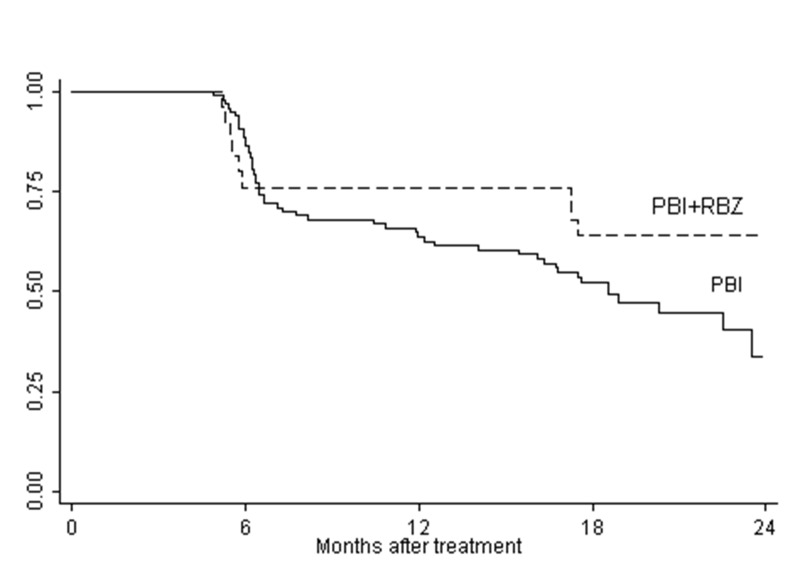
The cumulative rates of visual acuity retention of 20/40 or better for the small/medium tumor group are shown in Figure 4. While there does appear to be a difference in the curves in favor of the ranibizumab-treated group, this difference does not achieve statistical significance (P=.11)
FIGURE 4.
Cumulative rates of visual acuity retention of ≥20/40 for small/medium tumor group. The difference between the curves is not statistically significant, P=.11 (Log-rank test).
Moderate Vision Loss. The proportion of patients losing 3 or more lines of vision at months 12 and 24 based on Snellen visual acuity measurements was also evaluated. These data are shown in Table 11. The proportion of patients with moderate vision loss at 24 months after radiation treatment was significantly lower with ranibizumab treatment in the small/medium tumor group and the overall study cohort compared to historical controls. However, the difference in cumulative rates of vision retention (<3 line loss from baseline) only reached significance for the overall group (Figure 5).
TABLE 11.
PROPORTION OF PATIENTS WITH VISUAL ACUITY LOSS OF 3 OR MORE LINES (MODERATE VISION LOSS)
| SMALL/MEDIUM TUMORS | LARGE TUMORS | OVERALL | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Time post Tx |
RBZ + PBI (%) |
PBI (%) |
P* |
RBZ + PBI (%) |
PBI (%) |
P* |
RBZ + PBI (%) |
PBI (%) |
P* |
| 12 months | 2/24 (8.3) | 18/79 (22.8) | .10 | 6/12 (50.0) | 120/217 (55.3) | .47 | 8/36 (22.2) | 138/296 (46.6) | .004 |
| 24 months | 5/24 (20.8) | 28/62 (45.2) | .03 | 3/7 (42.9) | 102/143 (71.3) | .12 | 8/31 (25.8) | 130/205 (63.4) | <.001 |
Fisher’s exact test
Tx:treatment; RBZ: ranibizumab; PBI: proton beam irradiation
FIGURE 5.
Cumulative rates of vision retention within 3 lines from baseline (<3 line loss) for overall study cohort. The difference in curves is of borderline significance, P =.04 (Log-rank test)
Exploratory Subgroup Analyses. Since the second cohort of 20 small/medium tumor patients was randomized between ranibizumab 0.5 mg and 1.0 mg, an exploratory analysis for possible differences in visual outcome based on dose was performed. The 5 patients with small/medium tumors given ranibizumab 0.5 mg in the first cohort were also included in this analysis. The proportion of patients with visual acuity of 20/40 or better was slightly higher in the 0.5 mg dose group compared to the 1.0 mg dose group at 12 (13/15 vs. 7/9) and 24 months (14/15 vs. 7/9) . However, this difference was not statistically significant (P=.31 at month 24, Fisher’s exact test).
Additionally, visual acuity retention of 20/200 and 20/40 or better based on relative tumor proximity to the fovea and/or optic disc was examined for the small/medium tumor patients. These results appear in Tables 12 and 13. There is the suggestion that patients at highest risk for both radiation maculopathy and papillopathy based on tumor location within 2 DD of both the disc and fovea might benefit the most from ranibizumab treatment.
TABLE 12.
PROPORTION OF PATIENTS WITH VISUAL ACUITY 20/200 OR BETTER, BY TUMOR LOCATION (SMALL/MEDIUM TUMORS)
| TIME POST TX | 12 MONTHS | 24 MONTHS | ||||
|---|---|---|---|---|---|---|
| Tumor Location |
RBZ + PBI (%) |
PBI (%) |
P^ |
RBZ + PBI(%) |
PBI (%) |
P^ |
| ≤ 2DD from both optic disc and fovea | 11/11 (100) | 39/48 (81.3) | .13 | 11/11 (100) | 22/37 (59.5) | .009 |
| ≤ 2DD from fovea and > 2DD from optic disc | 8/8 (100) | 15/16 (93.8) | .67 | 8/8 (100) | 13/14 (92.9) | .64 |
| ≤ 2DD from optic disc and > 2DD from fovea | 5/5 (100) | 14/15 (93.3) | .75 | 5/5 (100) | 8/11 (72.7) | .29 |
Fisher’s exact test
Tx:treatment; RBZ: ranibizumab; PBI: proton beam irradiation
TABLE 13.
PROPORTION OF PATIENTS WITH VISUAL ACUITY 20/40 OR BETTER, BY TUMOR LOCATION (SMALL/MEDIUM TUMORS)
| TIME POST TX | 12 MONTHS | 24 MONTHS | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Tumor Location |
RBZ + PBI (%) |
PBI (%) |
P^ |
RBZ + PBI (%) |
PBI (%) |
P^ |
| ≤ 2DD from both optic disc and fovea | 9/11 (81.8) | 27/48 (56.2) | .11 | 9/11 (81.8) | 13/37 (35.1) | .008 |
| ≤ 2DD from fovea and > 2DD from optic disc | 6/8 (75.0) | 13/16 (81.3) | .56 | 7/8 (87.5) | 9/14 (64.3) | .26 |
| ≤ 2DD from optic disc and > 2DD from fovea | 5/5 (100) | 12/15 (80) | .40 | 5/5 (100) | 7/11 (63.6) | .18 |
Fisher’s exact test
Tx:treatment; RBZ: ranibizumab; PBI: proton beam irradiation
Anatomic outcomes
Tumor regression. Tumor regression in the ranibizumab-treated patients at months 12 and 24 was compared to that seen in historical controls as shown in Table 14. At month 24, there appeared to be slightly greater reduction in apical height of the small/medium tumors compared to historical controls. There was no significant difference for the large tumors and overall group.
TABLE 14.
TUMOR HEIGHT REGRESSION AT ANNUAL TIME POINTS
| SMALL/MEDIUM | LARGE | OVERALL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Time post Tx |
RBZ + PBI |
PBI | P |
RBZ + PBI |
PBI | P |
RBZ + PBI |
PBI | P | |
| 12 mo | N (%)regressed | 22/24 (91.7) | 48/70 (68.6) | .02* | 11/12 (91.7) | 148/173 (85.6) | .48 | 33/36 (91.7) | 196/243 (80.7) | .08* |
| Median regression (mm) (range) | −.6 (−1.4, −.1) | −.7 (−2.7, −.1) | .50† | −1.8 (−7.2, −.1) | −2.25 (−11.5, −.1) | .71† | −.8 (−7.2, −.1) | −1.7 (−11.5, −.1) | .0008† | |
| 24 mo | N (%)regressed | 22/24 (91.6) | 46/55 (83.6) | .28* | 8/8# (100) | 101/111 (91.0) | .48 | 30/32 (93.8.) | 147/166 (88.6) | .30* |
| Median regression (mm) (range) | −1.15 (−4.1, −4) | −.7 (−2.7, −.1) | .02† | −3.05# (−7.2, −1) | −3.3 (−9.5, −.1) | .80† | −1.25 (−7.2, −.4) | −2.3 (−9.5, −.1) | .08† | |
Fisher’s exact test
Mann-Whitney
Includes one measurement taken at month 22 (month 24 value unable to be obtained due to silicone oil tamponade)
Tx:treatment; RBZ: ranibizumab; PBI: proton beam irradiation
Neovascular Glaucoma and Retinal Detachment. For the patients with large tumors, rates of neovascular glaucoma (NVG) and exudative retinal detachment were explored. No cases of NVG were noted in the 8 large tumor patients for whom 24-month follow-up was available. For comparison, 38/146 (26.1%) patients in the historical control group of patients with large tumors were noted to have NVG at 24 months after radiation. However, the difference between these two groups did not reach significance (P=.10, Fisher’s exact test). Eleven of 15 (73%) patients with large tumors were noted to have exudative retinal detachment at baseline. In 6/11 patients, the detachment had resolved by month 24. Data regarding exudative retinal detachment was not consistently available for the historical control group.
Radiation Maculopathy and Papillopathy. Rates of radiation maculopathy and papillopathy were evaluated for the small/medium tumor group as shown in Table 15. At month 24, rates of clinically evident maculopathy and papillopathy were significantly lower in the ranibizumab-treated patients compared to historical controls. Additionally, the proportion of patients with signs of maculopathy but with visual acuity ≥ 20/40 or ≥20/200 were significantly higher in ranibizumab-treated patients. Only 2 patients developed papillopathy in the ranibizumab-treated group. Both retained visual acuity of 20/200 or better at month 24, and one retained vision of 20/40 or better.
TABLE 15.
PROPORTION OF PATIENTS WITH RADIATION COMPLICATIONS AT MONTH 24 FOR SMALL/MEDIUM TUMORS
|
RBZ + PBI (%) |
PBI | P^ | |
|---|---|---|---|
| Maculopathy | 8/24 (33.3) | 42/62 (67.7) | .004 |
| Maculopathy with VA>20/40 | 6/8 (75.0) | 15/42 (35.7) | .048 |
| Maculopathy with VA>20/200 | 8/8 (100.0) | 25/42 (59.5) | .03 |
| Papillopathy | 2/24 (8.3) | 20/62 (32.3) | .02 |
| Papillopathy with VA>20/40 | 1/2 (50.0) | 4/20 (20.0) | .41 |
| Papillopathy with VA>20/200 | 2/2 (100.0) | 10/20 (50.0) | .29 |
Fisher’s exact test
VA: Visual Acuity
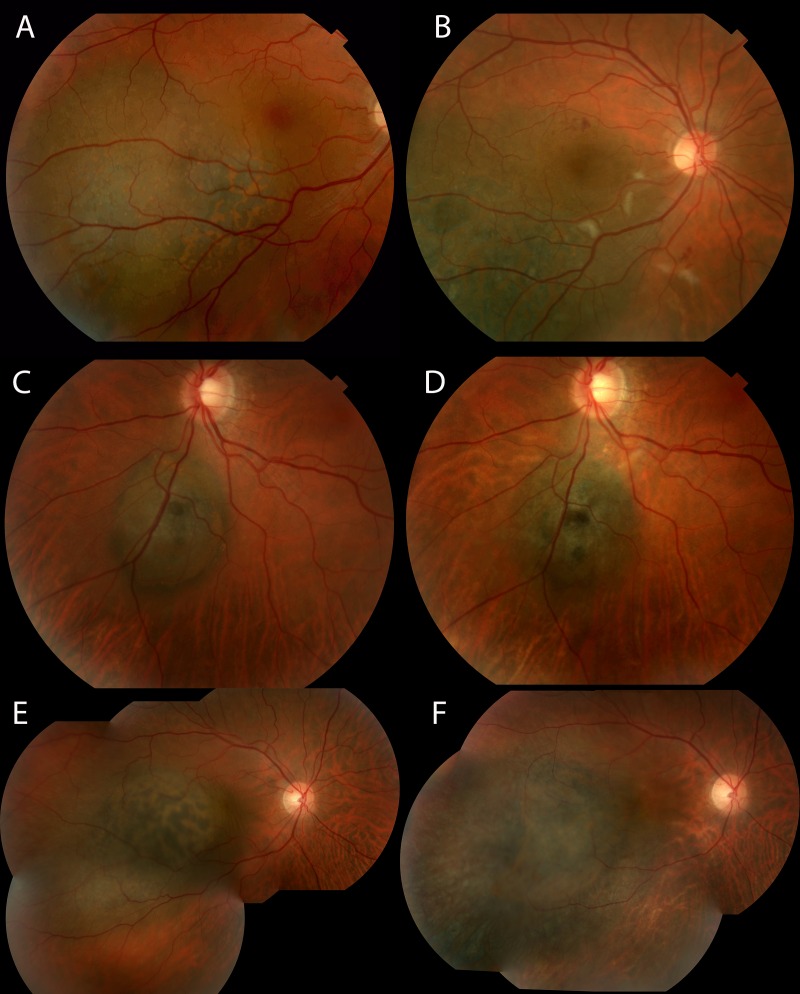
A few representative cases from the small/medium tumor group are shown in Figure 6. The first patient retained 20/16 vision through month 24 despite clinical evidence of mild radiation maculopathy. The second patient retained 20/20 vision through month 24 with no papillopathy despite tumor location adjacent to the optic disc. The final patient’s vision declined from 20/25 at baseline to 20/63 at month 24.
FIGURE 6.
Representative examples of patients from small/medium tumor group. A. Color fundus photograph of a patient at baseline with visual acuity 20/16. B. Same patient at month 24 with some clinical signs of radiation maculopathy. Visual acuity remains 20/16. C. Color fundus photograph of a patient at baseline with visual acuity 20/20. D. Same patient at month 24 with visual acuity 20/20. E. Color fundus photograph of patient at baseline with visual acuity 20/25. F. Same patient at month 24 with no signs of radiation maculopathy but decrease in visual acuity to 20/63.
Optical Coherence Tomography. The mean central subfield thickness (CST) for patients with small/medium tumors at baseline and months 12 and 24 is shown in Table 16. The mean change in central retinal thickness at the annual time points is shown in Table 17. While there appeared to be a slightly greater reduction in CST in patients treated with the higher dose of ranibizumab, this difference did not reach significance. At month 24, 10/24 (42%) patients had cystoid changes on OCT. Two of these 10 had a central retinal thickness >300 microns, one of whom had an epiretinal membrane (ERM), and another 5 out of the 10 had central retinal thickness >250 microns, one with ERM.
TABLE 16.
MEAN OCT CENTRAL SUBFIELD THICKNESS (SMALL/MEDIUM TUMORS)
| RBZ DOSE |
BASELINE (MICRONS) |
12 MONTHS (MICRONS) |
24 MONTHS (MICRONS) |
BASELINE VS. MONTH 24^ |
|---|---|---|---|---|
| Overall | 307 (105–658) n=25 |
252 (179–339) n=24 |
254 (173–402) n=24 |
P =.04 |
| Low dose (0.5mg) | 296 (197–515) n=15 |
254 (179–339) n=15 |
259 (173–402) n=15 |
P = .36 |
| High dose (1.0 mg) | 323 (105–658) n=10 |
248 (193–300) n=9 |
245 (191–292) n=9 |
P =.05 |
| Low v. High^ | P =.72 | P = .70 | P = .83 |
Mann-Whitney
OCT: Optical Coherence Tomography; RBZ: Ranibizumab
TABLE 17.
MEAN CHANGE IN OCT CENTRAL SUBFIELD THICKNESS (SMALL/MEDIUM TUMORS)
| RBZ DOSE |
12 MONTHS (MICRONS) |
24 MONTHS (MICRONS) |
|---|---|---|
| Overall | −54 (−456 – 150) n=24 |
−52 (−463 – 141) n=24 |
| Low dose (0.5mg) | −42 (−252 – 51) n=15 |
−37 (−281 – 46) n=15 |
| High dose (1.0 mg) | −74 (−456 – 150) n=9 |
−77 (−463 – 141) n=9 |
| Low v. High^ | P = .88 | P = .79 |
Mann-Whitney
OCT: Optical Coherence Tomography; RBZ: Ranibizumab
DISCUSSION
This pilot study of intravitreal ranibizumab given every 2 months for 24 months in patients undergoing proton beam irradiation for choroidal melanoma suggests an acceptable safety profile and provides preliminary evidence of visual benefit. The primary goal of this phase I study was to demonstrate that the use of ranibizumab in this setting would not compromise local tumor control or increase ocular adverse events. The lack of efficacy of anti-VEGF therapy (bevacizumab) as monotherapy to induce regression of choroidal melanoma has been reported.57 Additionally, some have advised caution regarding the potential for anti-VEGF induced ischemia in tumors leading to an increased resistance to radiotherapy.58 On the contrary, there is also literature suggesting that anti-VEGF therapy may have beneficial effects when combined with radiotherapy stemming from vascular normalization effects in tumors.59 In contrast to prior studies, the initial injection of ranibizumab in this protocol was given approximately 2 weeks prior to the first session of proton irradiation. We did observe one case of local recurrence in a patient with a small/medium tumor at the final study visit (month 24). However, the overall recurrence rate in the study patients (3.1%) is not significantly different from the historical controls and is similar to previously published rates for proton irradiation of choroidal melanoma.56,60 Furthermore, comparison of tumor regression rates between study patients and historical controls do not demonstrate any significant differences. While limited, these data suggest that the regimen of ranibizumab combined with proton irradiation used in this study is neither antagonistic nor synergistic with respect to tumor regression. Therefore, regarding the timing of initial anti-VEGF therapy, while it is possible that there may be a window of vascular normalization decreasing hypoxia within the tumor and therefore potentiating the effects of radiation, finding this window after intravitreal injection will require much further study.
The two cases of death from metastatic melanoma observed in this series should be considered carefully. This rate was higher than seen in our cohort of historical controls of patients with large tumors, but this difference did not reach statistical significance. A report of a series of patients with extra large melanomas (mean thickness 8.6 mm, mean largest basal diameter 18.7 mm) treated with proton irradiation demonstrated a 10% probability of metastases at 24 months after treatment.61 Out of the 21 patients in that series, 3 developed metastatic disease. One died at 15 months after treatment and the other at 28 months after treatment. In another series of 354 patients with large tumors (mean thickness 9 mm, mean base 14 mm), the Kaplan-Meier estimate of metastatic disease was 10% at 2 years and 30% at 5 years.62 The patients in our study that developed metastases within the study period had very large tumors in both thickness and basal diameter. Additionally, baseline abdominal imaging was not performed prior to radiation and study enrollment. Given these factors and the small number of patients in our cohort, it is not possible to determine if treatment with ranibizumab contributed to melanoma-related mortality in the patients with large tumors. Further study of the use of ranibizumab in eyes with very large choroidal melanomas will be needed to determine safety.
For patients with small or medium tumors close to the optic nerve or fovea, the visual acuity results observed in this study appear promising. These observations are consistent with a prior study of bevacizumab given prophylactically in patients undergoing plaque radiotherapy although the measured outcomes were somewhat different.53 Shah and colleagues treated patients with bevacizumab every 4 months for 2 years starting at the time of plaque removal and compared outcomes to a control group of patients who declined treatment with bevacizumab. Only 24 of 292 (9%) patients in the bevacizumab group received all 7 possible injections. The mean number of injections was 4. They reported that the proportion of patients with moderate vision loss (3 or more lines) “overall” in the study was 96/292 (33%) in the bevacizumab group compared to 72/126 (57%) in the control group (P < .001). The proportion of patients with moderate vision loss at 24 months was 73/292 (25%) in the bevacizumab group vs. 40/126 (32%) in the control group, but no P value was provided for this comparison, and it is not clear how many patients had follow-up through 24 months. In our study, the overall proportion of moderate vision loss at 24 months was 8/31 (26%) in the ranibizumab group vs. 130/205 (63%) in historical controls (P < .001). Examining cumulative rates of vision loss with Kaplan-Meier analysis, Shah and coauthors reported 44.7% moderate vision loss at 24 months in the bevacizumab group vs. 40.0% in the controls, which was not statistically significant. Our Kaplan-Meier analysis examined vision retention, but translates roughly to 67% moderate vision loss at 24 months in the ranibizumab group vs. 87% in the control group. This difference reached statistical significance (P = .04).
One significant difference between the study by Shah and colleagues and ours is that their study population was not limited to those cases at highest risk of radiation complications based on tumor size or location, whereas our study included only high-risk patients. The mean distance to the fovea and optic nerve in their patients was about 4 mm with mean tumor thickness of about 5 mm.
In our study, the proportion of patients with small/medium tumors with visual acuity of 20/40 or better was significantly higher at 24 months than in the historical controls. Of the patients treated with ranibizumab, 21/24 (87.5%) retained visual acuity of 20/40 or better at 24 months. These visual results are quite encouraging given that these patients had tumors within 2 disc diameters of the fovea or optic nerve. The analysis of cumulative rates of visual acuity retention reached significance in favor of ranibizumab treatment for visual acuity of 20/200 or better but not for 20/40 or better. One drawback of the Kaplan-Meier analysis in this setting is that it does not consider those whose vision improves after dropping below the level specified. For example, if a patient is recorded as having visual acuity of 20/60 at month 12, but then improves to 20/40 at subsequent visits, this patient will be counted as having lost vision at month 12 in the analysis of cumulative rates and could not be counted at later time points as retaining 20/40 vision.
The number of large tumor patients with 24-month follow-up is limited, but the results do suggest that there may be a visual acuity benefit in this group as well. There was a greater proportion of patients retaining visual acuity of 20/200 or better (P =.01) as well as 20/40 or better, although borderline in significance (P = .05). The observation that there were no cases of neovascular glaucoma amongst those with 24-month follow-up is notable as these patients would be considered at high risk for this complication. Given the small numbers, this finding could not be shown to be significantly different compared to historical controls. However, a study by Mantel and colleagues supports the prophylactic use of anti-VEGF agents to prevent iris neovascularization.63 They reported the results of 24 patients with large melanomas (mean largest tumor diameter 19.3 mm and mean thickness 8.7 mm) and associated ischemic retinal detachment treated with proton irradiation who received prophylactic bevacizumab at the time of tumor localization and then every 2 months for 6 months, followed by every 3 months until resolution of the detachment. Once the detachment resolved, scatter laser was applied to the ischemic retina. A retrospective control group of 44 eyes was also evaluated. This group did not receive bevacizumab, but also received scatter laser after retinal reattachment. Over a mean censored follow-up time of 23.9 months, the rate of rubeosis in the bevacizumab group was 4% (1 eye) versus 36% (16 eyes) in the control group (P = .02, Log-rank test). There was also the suggestion that bevacizumab treatment might hasten the resolution of retinal detachment.
There are limitations to our study. The small numbers and lack of a randomized control group preclude any definitive conclusions. Nearly half of the large tumor patients did not complete the study, and there were some imbalances between the study patients and the control group. Specifically, for patients with small/medium tumors, the proportion of patients receiving a radiation dose of 70 Gy(RBE) in the study cohort was higher than in the historical controls (24% (6/25) vs. 6% (6/100)). Given that fewer control patients received the higher dose of radiation, this might have led to better visual outcomes in the control group. However, despite this imbalance, the data suggest better visual outcomes in the ranibizumab-treated patients. Additionally, there was a greater proportion of small/medium tumor patients in the study cohort with baseline visual acuity of 20/40 or better. Nevertheless, when considering only those patients with baseline vision of 20/40 or better in both the study and control groups, a significantly higher proportion of ranibizumab-treated patients retained this level of vision through 24 months.
Given the known latency between radiation exposure and clinical manifestations of radiation maculopathy or papillopathy, the 2-year follow-up period in this study is relatively short.1,18 Patients certainly remain at high risk for vision loss through at least 3 years after proton beam irradiation. Continued follow-up of this study cohort will provide additional information regarding the value of anti-VEGF therapy in this setting. However, since this study only evaluated the use of ranibizumab, it is not known if other anti-VEGF agents would produce similar outcomes. Additionally, the optimal frequency and duration of injections remains to be determined. The bi-monthly schedule in this study was selected as a compromise between the recommended monthly dosing schedule for ranibizumab based on the known ocular pharmacokinetics and the need to limit the number of office visits for the patients, many of whom live quite far from Boston. The prior study by Shah and coauthors demonstrated some benefit with bevacizumab, even with injections given every 4 months.53
Despite the limitations, this is one of the only prospective studies investigating an anti-VEGF agent for the prevention of vision loss resulting from the sequelae of radiation in patients with choroidal melanoma. The enrollment of a well-defined population at high risk for radiation complications on the basis of tumor size and location along with the use of a historical control group closely matched for those same factors are strengths of this study. The significant visual benefits seen in this pilot trial provide some rationale for further study of the role of prophylactic anti-VEGF therapy in patients with uveal melanoma at high risk for vision loss due to tumor location near the fovea and/or optic nerve. Literature on blood-brain barrier (BBB) damage due to radiation suggests that initial injury to vascular endothelial cells leads to BBB disturbance causing edema and perfusion abnormalities and subsequent hypoxia.17,64 The hypoxia leads to VEGF upregulation, which causes further BBB disruption through permeability effects, exacerbating neuronal damage. An analogous scenario may occur in the retina with radiation disrupting the blood-retinal barrier. Inhibiting VEGF in this setting may limit this damage and therefore limit subsequent neuronal cell death in the retina.
Acknowledgments
A. Funding/Support: Grant from Genentech (FVF4384s; IKK); Grimshaw-Gudewicz Foundation (ESG).
B. Financial Disclosures: Dr. Kim has served as a consultant for Genentech, Inc., Iconic Therapeutics, and Allergan. She has received clinical trial support from Acucela and Ophthotech. Dr. Gragoudas has served as a consultant for Iconic Therapeutics, Aura Pharmaceuticals, Ocata Therapeutics, and MPM Capital.
C. Contributions of Authors: Design and conduct of the study (IKK, ESG, AML, PJ); Collection, management, analysis, and interpretation of the data (IKK, ESG, AML, PJ); Preparation, review, or approval of the manuscript (IKK, AML, ESG, CA, PJ).
D. Other Acknowledgments: Helen A. Shih, MD, Yen-Lin Chen, MD, Shannon M. MacDonald, MD, John Munzenrider, MD, J. Michael Collier, PhD, and Alexi Trofimov, PhD of the Department of Radiation Oncology, Massachusetts General Hospital, Boston, MA for their collaboration in planning and supervising the proton therapy of the patients in this study. Joyce Galonsky, RN, Jacqueline Honerlaw RN, MPH, Joshua Yocum, Elizabeth Reader, and Edward Miretsky for assisting as study coordinators for portions of this trial during their time at Massachusetts Eye and Ear. Ashley Flibotte, the current study coordinator. Andrea Gibson for assistance in designing and obtaining funding for this study during her tenure as a Medical Science Liaison, Genentech. Kathleen Tarnowski, Medical Science Liaison, Genentech.
REFERENCES
- 1.Archer DB. Doyne Lecture. Responses of retinal and choroidal vessels to ionising radiation. Eye. 1993;7((Pt 1)(1)):1–13. doi: 10.1038/eye.1993.3. [DOI] [PubMed] [Google Scholar]
- 2.Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR. Radiation retinopathy after external-beam irradiation: analysis of time-dose factors. Radiation Oncology Biology. 1994;30(4):765–773. doi: 10.1016/0360-3016(94)90347-6. [DOI] [PubMed] [Google Scholar]
- 3.Danesh-Meyer HV. Radiation-induced optic neuropathy. Journal of Clinical Neuroscience. 2008;15(2):95–100. doi: 10.1016/j.jocn.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Gragoudas ES, Li W, Lane AM, Munzenrider J, Egan KM. Risk factors for radiation maculopathy and papillopathy after intraocular irradiation. Ophthalmology. 1999;106(8):1571–1577. doi: 10.1016/S0161-6420(99)90455-4. [DOI] [PubMed] [Google Scholar]
- 5.Miller JW, Adamis AP, Shima DT, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1994;145(3):574–584. [PMC free article] [PubMed] [Google Scholar]
- 6.Tolentino MJ, Miller JW, Gragoudas ES, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996;103(11):1820–1828. doi: 10.1016/s0161-6420(96)30420-x. [DOI] [PubMed] [Google Scholar]
- 7.Tolentino MJ, Miller JW, Gragoudas ES, Chatzistefanou K, Ferrara N, Adamis AP. Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch Ophthalmol. 1996;114(8):964–970. doi: 10.1001/archopht.1996.01100140172010. [DOI] [PubMed] [Google Scholar]
- 8.Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118(4):445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 9.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 10.Lutty GA, McLeod DS, Merges C, Diggs A, Plouët J. Localization of vascular endothelial growth factor in human retina and choroid. Arch Ophthalmol. 1996;114(8):971–977. doi: 10.1001/archopht.1996.01100140179011. [DOI] [PubMed] [Google Scholar]
- 11.Pe’er J, Shweiki D, Itin A, Hemo I, Gnessin H, Keshet E. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest. 1995;72(6):638–645. [PubMed] [Google Scholar]
- 12.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J of Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 13.Simunovic MP, Maberley D. Anti-vascular endothelial growth factor therapy for proliferative diabetic retinopathy: A Systematic Review and Meta-Analysis. Retina. 2015;35(10):1931–42. doi: 10.1097/IAE.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 14.Ramakrishnan S, Anand V, Roy S. Vascular Endothelial Growth Factor Signaling in Hypoxia and Inflammation. J Neuroimmune Pharmacol. 2014;9(2):142–160. doi: 10.1007/s11481-014-9531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crafts TD, Jensen AR, Blocher-Smith EC, Markel TA. Vascular endothelial growth factor: Therapeutic possibilities and challenges for the treatment of ischemia. Cytokine. 2015;71(2):385–393. doi: 10.1016/j.cyto.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Tsao MN, Li YQ, Lu G, Xu Y, Wong CS. Upregulation of vascular endothelial growth factor is associated with radiation-induced blood-spinal cord barrier breakdown. J Neuropathol Exp Neurol. 1999;58(10):1051–1060. doi: 10.1097/00005072-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Nordal RA, Nagy A, Pintilie M, Wong CS. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res. 2004;10(10):3342–3353. doi: 10.1158/1078-0432.CCR-03-0426. [DOI] [PubMed] [Google Scholar]
- 18.Gragoudas E, Lane AM. Uveal Melanoma: Proton Beam Irradiation. Ophthalmology Clinics of North America. 2005;18(1):111–118. doi: 10.1016/j.ohc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Gragoudas ES, Munzenrider JE, Lane AM, Collier JM. Eye. In: Delaney TF, Kooy HM, editors. Proton and Charged Particle Radiotherapy. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 151–161. [Google Scholar]
- 20.Borruat FX, Schatz NJ, Glaser JS, Feun LG, Matos L. Visual recovery from radiation-induced optic neuropathy. The role of hyperbaric oxygen therapy. J Clin Neuroophthalmol. 1993;13(2):98–101. [PubMed] [Google Scholar]
- 21.Gall N, Leiba H, Handzel R, Pe’er J. Severe radiation retinopathy and optic neuropathy after brachytherapy for choroidal melanoma, treated by hyperbaric oxygen. Eye. 2007;21(7):1010–1012. doi: 10.1038/sj.eye.6702820. [DOI] [PubMed] [Google Scholar]
- 22.Schmetterer L, Kemmler D, Breiteneder H, et al. A randomized, placebo-controlled, double-blind crossover study of the effect of pentoxifylline on ocular fundus pulsations. Am J Ophthalmol. 1996;121(2):169–176. doi: 10.1016/s0002-9394(14)70581-1. [DOI] [PubMed] [Google Scholar]
- 23.Gupta P, Meisenberg B, Amin P, Pomeranz HD. Radiation retinopathy: the role of pentoxifylline. Retina. 2001;21(5):545–547. doi: 10.1097/00006982-200110000-00026. [DOI] [PubMed] [Google Scholar]
- 24.Kinyoun JL. Long-term visual acuity results of treated and untreated radiation retinopathy (an AOS thesis) Trans Am Ophthalmol Soc. 2008;106:325–335. [PMC free article] [PubMed] [Google Scholar]
- 25.Hykin PG, Shields CL, Shields JA, Arevalo JF. The efficacy of focal laser therapy in radiation-induced macular edema. Ophthalmology. 1998;105(8):1425–1429. doi: 10.1016/S0161-6420(98)98023-X. [DOI] [PubMed] [Google Scholar]
- 26.Kinyoun JL, Lawrence BS, Barlow WE. Proliferative radiation retinopathy. Arch Ophthalmol. 1996;114(9):1097–1100. doi: 10.1001/archopht.1996.01100140299007. [DOI] [PubMed] [Google Scholar]
- 27.Finger PT. Laser photocoagulation for radiation retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol. 2005;89(6):730–738. doi: 10.1136/bjo.2004.052159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakri SJ, Beer PM. Photodynamic therapy with verteporfin for classic choroidal neovascularization secondary to focal laser photocoagulation for radiation retinopathy. Ophthalmic Surg Lasers Imaging. 2002;34(6):475–7. [PubMed] [Google Scholar]
- 29.Lee SC, Song JH, Chung EJ, Kwon OW. Photodynamic therapy of subretinal neovascularization in radiation retinopathy. Eye. 2004;18(7):745–746. doi: 10.1038/sj.eye.6700736. [DOI] [PubMed] [Google Scholar]
- 30.Bakri SJ, Beer PM. Photodynamic therapy for maculopathy due to radiation retinopathy. Eye. 2004;19(7):795–799. doi: 10.1038/sj.eye.6701637. [DOI] [PubMed] [Google Scholar]
- 31.Sutter FKP, Gillies MC. Intravitreal triamcinolone for radiation-induced macular edema. Arch Ophthalmol. 2003;121(10):1491–1493. doi: 10.1001/archopht.121.10.1491. [DOI] [PubMed] [Google Scholar]
- 32.Shields CL, Demirci H, Dai V, et al. Intravitreal triamcinolone acetonide for radiation maculopathy after plaque radiotherapy for choroidal melanoma. Retina. 2005;25(7):868–874. doi: 10.1097/00006982-200510000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Shields CL, Demirci H, Marr BP, et al. Intravitreal triamcinolone acetonide for acute radiation papillopathy. Retina. 2006;26(5):537–544. doi: 10.1097/00006982-200605000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Russo A, Avitabile T, Uva M, et al. Radiation macular edema after Ru-106 plaque brachytherapy for choroidal melanoma resolved by an intravitreal dexamethasone 0.7-mg implant. Case Rep Ophthalmol. 2012;3(1):71–76. doi: 10.1159/000337144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baillif S, Maschi C, Gastaud P, Caujolle JP. Intravitreal dexamethasone 0.7-mg implant for radiation macular edema after proton beam therapy for choroidal melanoma. Retina. 2013;33(9):1784–1790. doi: 10.1097/IAE.0b013e31829234fa. [DOI] [PubMed] [Google Scholar]
- 36.Hellman JB, Garcia-Gonzalez JM. Dexamethasone 0.7 mg implant for the treatment of recalcitrant radiation maculopathy after proton radiotherapy for carcinoma of the maxillary sinus. Journal of Ocular Diseases and Therapeutics. 2014;2(1):30–35. [Google Scholar]
- 37.Stanford MR. Retinopathy after irradiation and hyperbaric oxygen. J R Soc Med. 1984;77(12):1041–1043. doi: 10.1177/014107688407701211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finger PT, Chin KJ, Semenova EA. Intravitreal anti-VEGF therapy for macular radiation retinopathy: a 10-year study. Eur J Ophthalmol. 2015;26(1):60–66. doi: 10.5301/ejo.5000670. [DOI] [PubMed] [Google Scholar]
- 39.Shah NV, Houston SK, Markoe AM, Feuer W, Murray TG. Early SD-OCT diagnosis followed by prompt treatment of radiation maculopathy using intravitreal bevacizumab maintains functional visual acuity. Clin Ophthalmol. 2012;6:1739–1748. doi: 10.2147/OPTH.S34949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mashayekhi A, Rojanaporn D, Al-Dahmash S, Shields CL, Shields JA. Monthly intravitreal bevacizumab for macular edema after iodine-125 plaque radiotherapy of uveal melanoma. Eur J Ophthalmol. 2013;24(2):228–234. doi: 10.5301/ejo.5000352. [DOI] [PubMed] [Google Scholar]
- 41.Mason JO, Albert MA, Persaud TO, Vail RS. Intravitreal bevacizumab treatment for radiation macular edema after plaque radiotherapy for choroidal melanoma. Retina. 2007;27(7):903–907. doi: 10.1097/IAE.0b013e31806e6042. [DOI] [PubMed] [Google Scholar]
- 42.Gupta A, Muecke JS. Treatment of radiation maculopathy with intravitreal injection of bevacizumab (Avastin) Retina. 2008;28(7):964–968. doi: 10.1097/IAE.0b013e3181706302. [DOI] [PubMed] [Google Scholar]
- 43.Bakri SJ, Larson TA. The variable efficacy of intravitreal bevacizumab and triamcinolone acetonide for cystoid macular edema due to radiation retinopathy. Semin Ophthalmol. 2015;30(4):276–280. doi: 10.3109/08820538.2013.847110. [DOI] [PubMed] [Google Scholar]
- 44.Jutley G, Shona OA, Leen RC, Lee N, Olver JM, George SM. Response to ranibizumab following tachyphylaxis to bevacizumab in a patient with radiation maculopathy following stereotactic fractionated radiotherapy for optic nerve meningioma. Arch Ophthalmol. 2012;130(11):1466–1470. doi: 10.1001/archophthalmol.2012.1542. [DOI] [PubMed] [Google Scholar]
- 45.Vásquez LM, Somani S, Altomare F, Simpson ER. Intracameral bevacizumab in the treatment of neovascular glaucoma and exudative retinal detachment after brachytherapy in choroidal melanoma. Can J Ophthalmol. 2009;44(1):106–107. doi: 10.3129/i08-171. [DOI] [PubMed] [Google Scholar]
- 46.Yeung SN, Paton KE, Waite C, Maberley DA. Intravitreal bevacizumab for iris neovascularization following proton beam irradiation for choroidal melanoma. Can J Ophthalmol. 2010;45(3):269–273. doi: 10.3129/i09-259. [DOI] [PubMed] [Google Scholar]
- 47.Nagendran ST, Finger PT. Anti-VEGF intravitreal bevacizumab for radiation-associated neovascular glaucoma. Ophthalmic Surg Lasers Imaging Retina. 2015;46(2):201–207. doi: 10.3928/23258160-20150213-08. [DOI] [PubMed] [Google Scholar]
- 48.Caujolle JP, Maschi C, Freton A, Pages G, Gastaud P. Treatment of neovascular glaucoma after proton therapy for uveal melanomas with ranibizumab injection: preliminary results. Ophthalmic Res. 2012;47(2):57–60. doi: 10.1159/000328633. [DOI] [PubMed] [Google Scholar]
- 49.Dunavoelgyi R, Zehetmayer M, Simader C, Schmidt-Erfurth U. Rapid improvement of radiation-induced neovascular glaucoma and exudative retinal detachment after a single intravitreal ranibizumab injection. Clin Experiment Ophthalmol. 2007;35(9):878–880. doi: 10.1111/j.1442-9071.2007.01632.x. [DOI] [PubMed] [Google Scholar]
- 50.Gondo M, Sakai T, Tsuneoka H, Kanehira C. Intravitreal bevacizumab for delayed radiation maculopathy and papillopathy after irradiation for maxillary sinus cancer. Clin Ophthalmol. 2011:1217–1219. doi: 10.2147/OPTH.S23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finger PT, Chin KJ. Antivascular endothelial growth factor bevacizumab for radiation optic neuropathy: secondary to plaque radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(2):789–798. doi: 10.1016/j.ijrobp.2010.11.075. [DOI] [PubMed] [Google Scholar]
- 52.Horgan N, Shields CL, Mashayekhi A, et al. Periocular triamcinolone for prevention of macular edema after plaque radiotherapy of uveal melanoma:. A randomized controlled trial. Ophthalmology. 2009;116(7):1383–1390. doi: 10.1016/j.ophtha.2009.01.051. [DOI] [PubMed] [Google Scholar]
- 53.Shah SU, Shields CL, Bianciotto CG, et al. Intravitreal bevacizumab at 4-month intervals for prevention of macular edema after plaque radiotherapy of uveal melanoma. Ophthalmology. 2014;121(1):269–275. doi: 10.1016/j.ophtha.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 54.Ahuja Y, Kapoor KG, Thomson RM, et al. The effects of intraocular silicone oil placement prior to iodine 125 brachytherapy for uveal melanoma: a clinical case series. Eye. 2012;26(11):1487–1489. doi: 10.1038/eye.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCannel TA, McCannel CA. Iodine 125 brachytherapy with vitrectomy and silicone oil in the treatment of uveal melanoma: 1-to-1 matched case-control series. Int J Radiat Oncol Biol Phys. 2014;89(2):347–352. doi: 10.1016/j.ijrobp.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 56.Gragoudas ES, Lane AM, Regan S, et al. A randomized controlled trial of varying radiation doses in the treatment of choroidal melanoma. Arch Ophthalmol. 2000;118(6):773–778. doi: 10.1001/archopht.118.6.773. [DOI] [PubMed] [Google Scholar]
- 57.Lima BR, Schoenfield LR, Singh AD. The impact of intravitreal bevacizumab therapy on choroidal melanoma. Am J Ophthalmol. 2011;151(2):323–328.e2. doi: 10.1016/j.ajo.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 58.Filali el M, van der Velden PA, Luyten GPM, Jager MJ. Anti-angiogenic therapy in uveal melanoma. Dev Ophthalmol. 2012;49:117–136. doi: 10.1159/000329591. [DOI] [PubMed] [Google Scholar]
- 59.Jain RK. Normalizing Tumor Microenvironment to Treat Cancer: Bench to Bedside to Biomarkers. J Clin Oncol. 2013;31(17):2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dendale R, Lumbroso-Le Rouic L, Noel G, et al. Proton beam radiotherapy for uveal melanoma: Results of Curie Institut–Orsay Proton Therapy Center (ICPO) Int J Radiat Oncol Biol Phys. 2006;65(3):780–787. doi: 10.1016/j.ijrobp.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 61.Conway RM, Poothullil AM, Daftari IK, Weinberg V, Chung JE, O’Brien JM. Estimates of ocular and visual retention following treatment of extra-large uveal melanomas by proton beam radiotherapy. Arch Ophthalmol. 2006;124(6):838–843. doi: 10.1001/archopht.124.6.838. [DOI] [PubMed] [Google Scholar]
- 62.Shields CL, Naseripour M, Cater J, et al. Plaque radiotherapy for large posterior uveal melanomas (> or =8-mm thick) in 354 consecutive patients. Ophthalmology. 2002;109(10):1838–1849. doi: 10.1016/s0161-6420(02)01181-8. [DOI] [PubMed] [Google Scholar]
- 63.Mantel I, Schalenbourg A, Bergin C, Petrovic A, Weber DC, Zografos L. Prophylactic use of bevacizumab to avoid anterior segment neovascularization following proton therapy for uveal melanoma. Am J Ophthalmol. 2014;158(4):693–701. doi: 10.1016/j.ajo.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Li YQ, Ballinger JR, Nordal RA, Su ZF, Wong CS. Hypoxia in radiation-induced blood-spinal cord barrier breakdown. Cancer Res. 2001;61(8):3348–3354. [PubMed] [Google Scholar]