Abstract
Liriopogons (Liriope and Opiopogon) species are used as a main medicinal ingredient in several Asian countries. The Liriopes Radix (tuber, root of Liriope platyphylla) has to be a promising candidate due to their source of phytochemicals. Steroidal saponins and their glycosides, phenolic compounds, secondary metabolites are considered of active constituents in Liriopes Radix. Spicatoside A, a steroidal saponin, could be more efficacious drug candidate in future. In this review, we summarized the available knowledge on phytochemical and pharmacological activities for spicatoside A. It significantly suppressed the level of NF-κB, NO, iNOS, Cox-2, IL-1β, IL-6 and MAPKs in LPS-stimulated inflammation. The production of MUC5AC mucin was increased. MMP-13 expression was down-regulated in IL-1β-treated cells and reduced glycosaminoglycan release from IL-1α-treated cells. The neurite outgrowth activity, PI3K, Akt, ERK1/2, TrkA and CREB phosphorylation and neurotropic factors such as NGF and BDNF were upregulated with increased latency time. It also showed cell growth inhibitory activity on various carcinoma cells. From this, spicatoside A exerts anti-inflammation, anti-asthma, anti-osteoclastogenesis, neurite outgrowth, memory consolidation and anticancer activities. Further studies are needed on spicatoside A in order to understand mechanisms of action to treat various human diseases.
Keywords: Liriopes, Saponins, L. platypylla, Neurite outgrowth, Memory consolidation, Anti-inflammatory
INTRODUCTION
The search for new bioactive natural products from naturally occurring drugs that may be effective for the prevention and treatment of various human diseases is currently a worldwide pursuit. In the last years, extensive research and development have generated over 25% of the approved clinical drugs that are derived or tailored from natural products (Khaled et al., 2013). In Korea, many Korean medicinal herbs and Korean traditional prescriptions are expected to be safe medicinal agents without major adverse effects (Kang et al., 1998). The uses of natural agents derived in traditional oriental medicine are attractive sources for developing novel therapeutics or prophylactics because of their safety, affordability, long-term use and ability to target multiple pathways. In recent decades, the huge number of researches related to plant drugs and its compounds is provided as a source to produce medicines, nutraceuticals and to develop functional foods.
LIRIOPES RADIX
Liriopes Radix (LR) commonly known as Liriope or Liriopis or Liriopsis Tuber, the tuber of the Liriopsis plant, is a medicinal ingredient traditionally used in several Asian countries, especially China, Korea and Japan for hundreds of years to treat dry hacking cough, dry tongue and mouth, insomnia, constipation (Wang et al., 2013), asthma, bronchial and lung inflammation and sputum (Kim et al., 2012). LR is the swelling part of the roots from Liriope platyphylla Wang et Tang, Ophiopogon japonicus Ker-Gawl., O. stolonifer Levl. et Vant., Mondo japonicum (L. f.) Farwell and L. spicata (Thunb.) Lour., used for medicinal purposes (Kim and Lee, 2008). Botanically, liriopogons may be either Liriope or Ophiopogon are native to East Asia and belonging to the Liliaceae family, is abundantly distributed in subtropical and temperate regions globally (Li et al., 2011). Liriopogons have become a popular and economically important groundcover has been found to be marked under incorrect names. Considering that LR has many synonyms, the vernacular names are maegmundong (맥문동) in Korean, maimendong (麥 門 冬) in Mandarin and ryunohige (リュウノヒゲ) or janohige (ジャノヒゲ) in Japanese.
Quality evaluation of traditional medicinal products is very important for guaranteeing safety, efficacy, and stability. Almost all traditional medicines contain multiple known or unknown constituents that vary greatly in content, chemical, and physical properties. Recently, many studies have revealed steroidal saponins and their glycosides, phenolic compounds, secondary metabolites from L. platyphylla and L. spicata whereas steroidal glycosides and homoisoflavones from O. japonicus are considered of active constituents in Liriopes Radix.
SAPONINS
The medicinal activities of plants are generally due to their secondary metabolites. Plant synthesize diverse classes of secondary metabolites mainly to defend themselves (Sahu et al., 2008). Saponins are a group of widely naturally occurring plant glycosides, structurally contain a steroidal or triterpenoid aglycone with one or more sugar chains. Saponin-contained plants have been known for their beneficial effects on health long before saponins were isolated as the effective compounds. Saponins have been reported to possess a wide range of biological activities (Sun et al., 2015).
STEROIDAL SAPONINS
Steroidal saponins have drawn much attention in the last few decades, not only as economically important, but also as biologically active compounds and as ingredients for cosmetics. Steroidal saponins are usually higher polar compounds occurring as complex mixtures with highly branched oligosaccharide moieties and present a formidable task for their purification and structure elucidation (Sahu et al., 2008). Steroidal saponins are generally consisting of two to five kinds of oligosaccharide sugar units such as d-glucose, d-galactose, d-xylose, l-arabinose and l-fucose as their carbohydrate moieties. These sugar moieties are linked to the aglycone through hydroxyl groups either in a linear or branched fashion (Backer et al., 1972). Steroidal saponins are present in different types of plants and expresses a wide range of pharmacological applications indicates that each steroidal saponin has distinct biological effects (Escobar-Sánchez et al., 2015). The tubers of liriopogons have steroidal saponin named spicatoside A. In this review, we summarize the effects of spicatoside A and the relevant mechanisms reported in recent years in order to facilitate future research and support the utilization of it as a novel drug.
SPICATOSIDE A
The pursuit of structurally novel steroidal saponins with more effective, less toxic, and less resistance remains to be a highly challenging task and has great interest in discovery and development of new functional drugs. Although there are many phytochemicals present in each plant, a single phytochemical that could be used in the treatment of diseases would be a necessity. We are hopeful that spicatoside A from liriopogons could provide the impetus to investigate novel and more efficacious drug in the future that will open up new opportunities for researchers interested in steroidal saponins based medicinal chemistry and drug designing.
Separation
Saponins are one of the main effective ingredients of various medicinal plants. However, it is normally a time consuming and laborious process to extract saponins using traditional techniques (Chen et al., 2009). It is difficult to determine saponins by a UV detector, for they mostly subject to no chromophores in their structures and the UV absorption is weak or only has end absorption (Bai et al., 2008). An adequate quadratic polynomial model for predicting the values of spicatoside A yield from L. platyphylla was determined according to the optimization designs (Kim et al., 2010). A pressurized liquid extraction technique by high performance liquid chromatography coupled with charged aerosol detector (HPLC-CAD) was investigated for the extraction of spicatoside A from L. platyphylla. Response surface analysis indicated that the highest predicted content of spicatoside A might be 0.0161% of the optimized extract at extraction time 20 min, extraction temperature 130°C and ethanol concentration 86% which agrees with the one predicted by analysis of variance. The validation of this method showed good results of linearity, precision and accuracy (Kim et al., 2010).
Structure
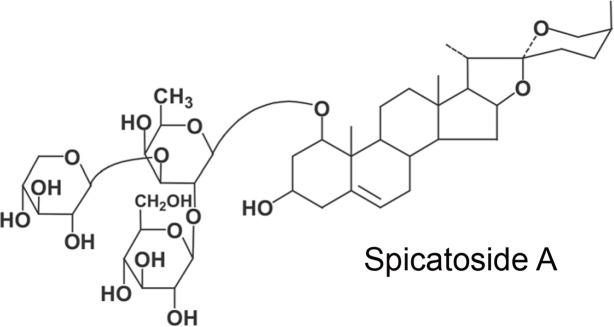
Spicatoside A, m.p. 243–5°C, showed a strong absorption band of hydroxyl groups and characteristic absorption bands of a 25(S)-spiroketal moiety in the IR spectrum (Wall et al., 1952). On acidic hydrolysis of Spicatoside A with 2N HCl-dioxane, it gave glucose, xylose, fucose and an aglycone. These results suggested that spicatoside A was considered to be a triglycoside of 25(S)-ruscogenin possessing one mole each of glucose, xylose and fucose. Based on the above results, the structure of spicatoside A was established to be 25(S)-ruscogenin 1-O-β-d-glucopyranosyl (l→2)-[β-d-xylopyranosyl (1→3)]-β-d-fucopyranoside (Lee et al., 1989). The structure was diagrammed in Fig. 1.
Fig. 1.
The structure of spicatoside A.
PHARMACOLOGICAL ACTIVITIES
Anti-inflammatory activities
Inflammation is one of the natural defensive mechanisms managed primarily by phagocytic cells. Macrophages play a central role in various inflammatory responses through the release of inflammatory mediators, such as nitric oxide (NO), generated by activating inducible NO synthase (iNOS), and cyclooxygenase-2 (Cox-2), and proinflammatory cytokines, such as interleukin-1β (IL-1β) and IL-6. Excessive inflammation mediated by inflammatory mediators has been closely linked to systemic inflammatory responses in many inflammatory diseases with severe tissue damage. Treatment with prosapogenin III of spicatoside A at concentrations of 10, 50 and 100 μM did not result in a decrease in cell viability. Lipopolysaccharide (LPS)-mediated inflammatory response resulted in higher production of NO, however, treatment of prosapogenin III of spicatoside A significantly decreased NO production in RAW264.7 cells. It also resulted in suppression of the nuclear translocation of NF-κB through suppression of NO, iNOS, Cox-2, IL-1β, and IL-6. Treatment with prosapogenin III of spicatoside A resulted in the potent inhibition of phosphorylation of three mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase (ERK1/2), p38, and c-Jun N-terminal kinase (JNK) in LPS-stimulated RAW264.7 macrophages (Han et al., 2013).
Anti-asthma activities
Asthma is defined as a chronic inflammatory disease of the airways accompanying the overproduction of mucus, airway wall remodeling, bronchial hyperreactivity and airway obstruction. The potential of L. platyphylla radix extract (LPP) inhalation as a simultaneous bronchodilator and anti-inflammatory agent was reported on the asthmatic mouse model. LPP inhalation reduced inflammatory cytokine levels in bronchoalveolar lavage fluid (BALF) and improved airway hyperresponsiveness (AHR). Ophiopogonin D, spicatoside A and spicatoside B were the chemical constituents of LPP through SIM chromatography (Kim et al., 2015) related to the treatment of respiratory disease and appeared to show anti-inflammatory activity.
Mucus in the pulmonary system is pivotal in defensive action against invading pathogenic microorganisms, chemicals and particles. Mucins are high molecular weight glycoproteins present in the airway mucus and produced by goblet cells in the surface epithelium as well as mucous cells in the submucosal gland. Among the twenty-one MUC genes coding human mucins reported, MUC5AC was mainly expressed in goblet cells in the airway surface epithelium (Rogers and Barnes, 2006; Voynow and Rubin, 2009). Phorbol 12-myristate 13-acetate (PMA) reported to stimulate the endogenous activator of protein kinase C (PKC) also can induce the MUC5AC gene expression and production in NCI-H292 cells (Park et al., 2014). Spicatoside A increased basal production of MUC5AC mucin from NCI-H292 cells. The amounts of mucin in the cells of spicatoside A-treated cultures were reported as 100, 119, 152 and 195% for control, spicatoside A 10−6M, spicatoside A 10−5M and spicatoside A 10−4M, respectively. Spicatoside A did not inhibit but increased PMA-induced MUC5AC production from NCI-H292 airway epithelial cells (Park et al., 2014).
Anti-osteoclastogenesis activities
Excess osteoclastogenesis or activation of mature osteoclasts causing the bone destruction is observed in advanced cases of rheumatoid arthritis and the neoplastic diseases, osteoporosis, periodontitis, multiple myeloma and bone metastasis. The balance between bone formation and resorption is tightly regulated by osteoblast and osteoclast to main the homeostasis of the skeleton (Youn et al., 2008). The spicatoside A down-regulated matrix metalloproteinase (MMP)-13 expression in IL-1β-treated human chondrocyte cell line, SW1353, at pharmacologically relevant concentrations (Lim et al., 2015). In the cartilage, residing chondrocytes synthesize and release MMP to the extracellular matrix (ECM) (Hadler-Olsen et al., 2011). Of these, MMP-13 collagenase that degrade collagen matrix of the cartilage. It participates in the normal turnover of the cartilage materials, but in some situations such as arthritic conditions or aging process, they are highly induced and degrade the ECM rapidly, resulting in pathological conditions like osteoarthritis (OA) (Mitchell et al., 1996; Takaishi et al., 2008). Spicatoside A concentration-dependently inhibited MMP-13 expression at 0.5 to 5 μM, moreover, spicatoside A at 5 μM reduced MMP-13 expression in IL-1β-treated SW1353 cells without any cytotoxic effect (Lim et al., 2015). Spicatoside A reduced glycosaminoglycan (GAG) release from IL-1α-treated rabbit joint cartilage culture. It can be suggested that spicatoside A may be beneficial for protecting against cartilage degradation in certain conditions such as arthritis (Lim et al., 2015).
Neurite outgrowth activities
Nerve growth factor (NGF) is one of several neurotrophic factors that play a crucial role in the neuronal development and maintenance within the central nervous system (CNS) and the peripheral nervous system (PNS). The binding of NGF to its receptor causes dimerization and leads to activation of the intracellular kinase domain of Trk via conformational changes that enable the phosphorylation of tyrosine residues in its auto-regulatory loop and other pathways. These signaling cascades lead to an array of protein expression, modification, and translocation events that facilitate the neuronal survival and neurite outgrowth response of characteristic neurotrophins. Spicatoside A (1, 5 and 10 μg/ml) exhibited neurite outgrowth in undifferentiated PC12 cells, which were similar to that of NGF at 50 ng/ml, indicating that spicatoside A may exhibit neuritogenic activity, which induces the neuronal differentiation of PC12 cells (Hur et al., 2009).
Activation of the Ras-MAPK and PI3K pathways is responsible for the differentiation and survival of PC12 cells. Therefore, an investigation was launched into whether or not the neuritogenic effects of spicatoside A are linked to MAPK-mediated intracellular signaling events. The results also demonstrate that PD98059, an MEK1/2 inhibitor, reversed neurite outgrowth induced by spicatoside A. Furthermore, spicatoside A (10 μg/ml) stimulated ERK1/2 phosphorylation suggesting that spicatoside A activation of ERK1/2 mediates the neurite outgrowth of PC12 cells. Spicatoside A increased PI3K activity as well as Akt phosphorylation levels. Furthermore, the phosphorylation of the tyrosine residue 490 in TrkA was detected in the spicatoside A-treated PC12 cells. This suggests that spicatoside A stimulated TrkA tyrosine phosphorylation and subsequently activated ERK1/2 and PI3K (Hur et al., 2009).
The cAMP-responsive element-binding protein (CREB) is an essential molecule that promotes NGF-dependent cell survival and differentiation. NGF and spicatoside A-stimulated the phosphorylation of CREB by ERK1/2 and PI3K may contribute to neurite outgrowth in spicatoside A-treated PC12 cells. When TrkA siRNA was transfected into PC12 cells, the neurite outgrowth effect of NGF and spicatoside A disappeared (Hur et al., 2009).
Astrocytes play an essential role in maintaining the physiological function of neurons. Neurotropic factors produced and released by astrocytes contribute to the differentiation and survival of neuronal cells (Barres and Barde, 2000). Spicatoside A and the butanol fraction of L. platyphylla were shown to directly or indirectly function as NGF mimetics or NGF inducers. Spicatoside A induce neuronal cell differentiation and upregulate neurotrophic factors such as NGF and BDNF. Spicatoside A also promotes the secretion of neurotrophic factors in C6 glioma and primary astrocyte cells to enhance long-term potentiation (LTP) (Hur et al., 2004; Venkatesan et al., 2015).
Memory consolidation activities
Memory consolidation is a process that converts acquired memory into a solid thing and makes it strengthened. Brain-derived neurotrophic factor (BDNF) has been regarded as one of the key neurotrophic factors in the memory consolidation. It modulates activity-dependent LTP, a form of synaptic plasticity underlying long term memory (LTM). The deprivation of BDNF by genetic modification or infusion of anti-sense oligonucleotides impairs learning and memory in mice (Bartoletti et al., 2002). The administration of a GABAA receptor antagonist enhances memory consolidation, which results from the enhancement of mature BDNF levels.
When, spicatoside A (2.5, 5, 10 and 20 mg/kg) was administrated orally to mice after an acquisition trial for memory consolidation in the passive avoidance task, spicatoside A (20 mg/ kg) showed the longer latency time in the retention trial compared to the vehicle-treated control group. The groups treated with spicatoside A (20 mg/kg) immediately (0 h) or 1 h after the acquisition trial showed a significant enhancement of latency in the retention trial, but not observed in the 3 h group (Kwon et al., 2014).
Mature BDNF levels in the hippocampus gradually increased, peaking at 6 h after the treatment, and then returned to baseline levels by 24 h was reported by western blot analysis. They also observed that spicatoside A increases pERK levels in the hippocampal tissue in vivo. Therefore, it is reported that spicatoside A increases mature BDNF levels through the ERK pathway in the hippocampal tissue. They suggest the positive possibility of spicatoside A to be a therapeutic agent in patients suffering from cognitive dysfunctions (Kwon et al., 2014).
Anti-cancer activities
Aside from tumor cell proliferation, expansion and metastasis, angiogenesis is one of the essential steps in tumor progression. Spicatoside A (5, 10, 50 and 100 μg/ml) isolated from the tuber of L. platyphylla was tested for cancer cell viability against A549, SK-OC-3, SK-Mel-2, XF-498 and HCT-15 cancer cell lines. Spicatoside A at 50 and 100 μg/ml concentrations showed very promising cell growth inhibitory activity on carcinoma cells, and the IC50 values against A549, SK-OC-3, SK-Mel-2, XF-498 and HCT-15 cancer cells were determined to be 17.3, 21.7, 14.9, 18.8 and 15.6 μg/ml, respectively. Moreover, the tuber of L. platyphylla (80% aqueous MeOH and solvent-fractionated with EtOAc, n-BuOH and H2O) indicated that the n-BuOH fraction to be the highest in the activity of growth inhibitory activity on various carcinoma cells, such as A549, SK-OC-3, SK-Mel-2, XF-498 and HCT-15 (Baek et al., 1998).
CONCLUSIONS AND FUTURE PROSPECTS
Herbal medicine provides a foundation for a large number of popular remedies in common use of various traditional medicine systems. Because of the increasing demand for high quality plant drugs, extensive studies on the chemical composition and their biological effects of crude drugs are required. Liriopes Radix (LR) are a potential source of natural compounds mainly steroidal saponins and their glycosides, phenolic compounds, homoisoflavones and secondary metabolites. Although, there are many phytochemicals present in each plant, the isolation of purified compounds and deeper research studies in order to understand their mechanisms of action is still a necessity in the treatment of various diseases. One such compound, spicatoside A, is a bioactive steroidal saponin, contained in the radix of liriopogons showing diverse biological effects. In this review, we have summarized the existing uses of the phytochemical properties and pharmacological activities of spicatoside A for treatment of various diseases. Spicatoside A exerts biological activities including anti-inflammation, anti-asthma, anti-osteoclastogenesis, neurite outgrowth, memory consolidation and anti-cancer activities. This is the first time that spicatoside A reported publications were summarized. The structural composition of individual aglycone moiety and the number of structures of monosaccharide units of spicatoside A are important for its biological activity. The different molecular mechanisms modified by spicatosie A is of great interest. We believe in the enhanced future research with in vitro and in vivo studies will be needed to screen the medicinal efficiency of spicatoside A and support the utilization of it as a novel drug for various human diseases such as osteoporosis, neurodegenerative diseases and cancer.
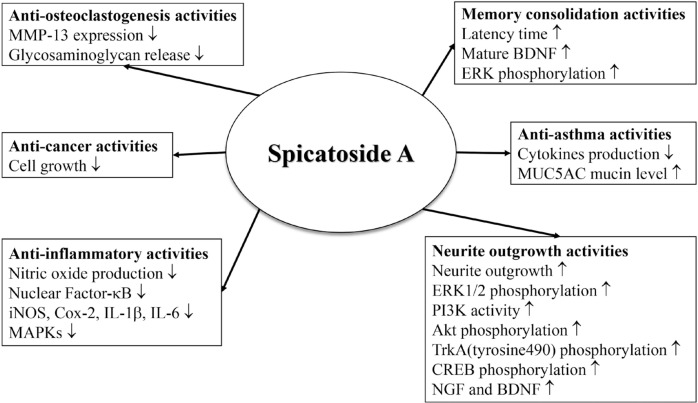
Fig. 2.
Schematic diagram for functions of spicatoside A.
Table 1.
Major pharmacological activities reported for spicatoside A
| Pharmacological activities | Active dose | References |
|---|---|---|
| Anti-inflammatory activities in RAW264.7 macrophage cells | 10, 50 and 100 µM | Han et al., 2013 |
| Anti-asthma activities in NCI-H292 airway epithelial cells | 100, 10 and 1 nM | Park et al., 2014 |
| Anti-osteoclastogenesis activities in SW1353 human chondrocyte cells | 0.5 to 5 µM | Lim et al., 2015 |
| Neurite outgrowth activities in undifferentiated PC12 cells | 1, 5 and 10 µM | Hur et al., 2009 |
| Memory consolidation activities in mice | 2.5, 5, 10 and 20 mg/kg | Kwon et al., 2014 |
| Anti-cancer activities in A549, SK-OC-3, SK-Mel-2, XF-498 and HCT-15 cancer cells | 5, 10, 50 and 100 µg/ml | Baek et al., 1998 |
Acknowledgments
This work was supported by grants from Kyung Hee University (KHU-20150703) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korean Ministry of Education, Science and Technology (2013R1A1A2006613).
REFERENCES
- Backer RC, Bianchi E, Cole JR. A phytochemical investigation of Yucca schottii (Liliaceae) J Pharm Sci. 1972;61:1665–1666. doi: 10.1002/jps.2600611034. [DOI] [PubMed] [Google Scholar]
- Baek N-I, Cho S-J, Bang M-H, Lee I, Park C, Kim M, Kim K, Sung J. Cytotoxicity of steroid-saponins from the tuber of Liriope platyphylla W. T. Agric Chem Biotechnol. 1998;41:390–394. [Google Scholar]
- Bai C-C, Han S-Y, Chai X-Y, Jiang Y, Li P, Tu P-F. Sensitive determination of saponins in radix et rhizoma notoginseng by charged aerosol detector coupled with HPLC. J Liq Chromatogr Relat Technol. 2008;32:242–260. doi: 10.1080/10826070802603187. [DOI] [Google Scholar]
- Barres BA, Barde Y. Neuronal and glial cell biology. Curr Opin Neurobiol. 2000;10:642–648. doi: 10.1016/S0959-4388(00)00134-3. [DOI] [PubMed] [Google Scholar]
- Bartoletti A, Cancedda L, Reid SW, Tessarollo L, Porciatti V, Pizzorusso T, Maffei L. Heterozygous knock-out mice for brain-derived neurotrophic factor show a pathway-specific impairment of long-term potentiation but normal critical period for monocular deprivation. J Neurosci. 2002;22:10072–10077. doi: 10.1523/JNEUROSCI.22-23-10072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Meng F, Zhang S, Liu Z. Effects of ultrahigh pressure extraction conditions on yields and antioxidant activity of ginsenoside from ginseng. Sep Purif Technol. 2009;66:340–346. doi: 10.1016/j.seppur.2008.12.026. [DOI] [Google Scholar]
- Escobar-Sánchez ML, Sánchez-Sánchez L, Sandoval-Ramírez J. Steroidal saponins and cell death in cancer. In: Ntuli T, editor. In Cell Death - Autophagy, Apoptosis and Necrosis. InTech; 2015. pp. 331–352. [Google Scholar]
- Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg JO. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 2011;278:28–45. doi: 10.1111/j.1742-4658.2010.07920.x. [DOI] [PubMed] [Google Scholar]
- Han Y, Jung HW, Lee DH, Kwon SY, Son KH, Park YK. Anti-inflammatory effects of prosapogenin III from the dried roots of Liriope platyphylla in LPS-stimulated RAW264.7 cells. J Asian Nat Prod Res. 2013;15:1038–1049. doi: 10.1080/10286020.2013.825253. [DOI] [PubMed] [Google Scholar]
- Hur J, Lee P, Kim J, Kim AJ, Kim H, Kim SY. Induction of nerve growth factor by butanol fraction of Liriope platyphylla in C6 and primary astrocyte cells. Biol Pharm Bull. 2004;27:1257–1260. doi: 10.1248/bpb.27.1257. [DOI] [PubMed] [Google Scholar]
- Hur J, Lee P, Moon E, Kang I, Kim SH, Oh MS, Kim SY. Neurite outgrowth induced by spicatoside A, a steroidal saponin, via the tyrosine kinase A receptor pathway. Eur J Pharmacol. 2009;620:9–15. doi: 10.1016/j.ejphar.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Kang B-J, Lee H-H, Kim N-J, Hong W-S, Park K-J. Activities of Korean medicinal herbs and traditional prescriptions Against Herpes simplex virus type-1. Pharm Biol. 1998;36:287–294. doi: 10.1076/phbi.36.4.287.4582. [DOI] [Google Scholar]
- Khaled M, Jiang ZZ, Zhang LY. Deoxypodophyllotoxin: a promising therapeutic agent from herbal medicine. J Ethnopharmacol. 2013;149:24–34. doi: 10.1016/j.jep.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Kim JE, Hwang IS, Choi SI, Lee HR, Lee YJ, Goo JS, Lee HS, Son HJ, Jang MJ, Lee SH, Kang BC, Hwang DY. Aqueous extract of Liriope platyphylla, a traditional Chinese medicine, significantly inhibits abdominal fat accumulation and improves glucose regulation in OLETF type II diabetes model rats. Lab Anim Res. 2012;28:181–191. doi: 10.5625/lar.2012.28.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Cho DH, Yang HJ, Choi EK, Shin MH, Kim KH, Ahn KS, Ha IJ, Na YC, Um JY, Chung WS, Jung HJ, Jung SK, Jang HJ. Effects of the inhaled treatment of liriope radix on an asthmatic mouse model. Am J Chin Med. 2015;43:425–441. doi: 10.1142/S0192415X15500275. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kim HK, Yang ES, Lee KY, Kim SD, Kim YC, Sung SH. Optimization of pressurized liquid extraction for spicatoside A in Liriope platyphylla. Sep Purif Technol. 2010;71:168–172. doi: 10.1016/j.seppur.2009.11.016. [DOI] [Google Scholar]
- Kim SJ, Lee K. Extracts of Liriopsis tuber protect AMPA induced brain damage and improve memory with the activation of insulin receptor and ERK I/II. Phytother Res. 2008;22:1450–1457. doi: 10.1002/ptr.2475. [DOI] [PubMed] [Google Scholar]
- Kwon G, Lee HE, Lee DH, Woo H, Park SJ, Gao Q, Ahn YJ, Son KH, Ryu JH. Spicatoside A enhances memory consolidation through the brain-derived neurotrophic factor in mice. Neurosci Lett. 2014;572:58–62. doi: 10.1016/j.neulet.2014.04.034. [DOI] [PubMed] [Google Scholar]
- Lee D-Y, Son K-H, Do J-C, Kang SS. Two new steroidal saponins from the tubers of Liriope spicata. Arch Pharm Res. 1989;12:295–299. doi: 10.1007/BF02911063. [DOI] [Google Scholar]
- Li G, Ra W-H, Park J-W, Kwon S-W, Lee J-H, Park C-B, Park Y-J. Developing EST-SSR markers to study molecular diversity in Liriope and Ophiopogon. Biochem Syst Ecol. 2011;39:241–252. doi: 10.1016/j.bse.2011.08.012. [DOI] [Google Scholar]
- Lim H, Min DS, Kang Y, Kim HW, Son KH, Kim HP. Inhibition of matrix metalloproteinase-13 expression in IL-1β-treated articular chondrocytes by a steroidal saponin, spicatoside A, and its cellular mechanisms of action. Arch Pharm Res. 2015;38:1108–1116. doi: 10.1007/s12272-015-0581-z. [DOI] [PubMed] [Google Scholar]
- Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Lee HJ, Ryu J, Son KH, Kwon SY, Lee SK, Kim YS, Hong JH, Seok JH, Lee CJ. Effects of ophiopogonin D and spicatoside A derived from Liriope Tuber on secretion and production of mucin from airway epithelial cells. Phytomedicine. 2014;21:172–176. doi: 10.1016/j.phymed.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med. 2006;38:116–125. doi: 10.1080/07853890600585795. [DOI] [PubMed] [Google Scholar]
- Sahu NP, Banerjee S, Mondal NB, Mandal D. Steroidal saponins. Fortschr Chem Org Naturst. 2008;89:45–141. doi: 10.1007/978-3-211-74019-4_2. [DOI] [PubMed] [Google Scholar]
- Sun A, Xu X, Lin J, Cui X, Xu R. Neuroprotection by saponins. Phytother Res. 2015;29:187–200. doi: 10.1002/ptr.5246. [DOI] [PubMed] [Google Scholar]
- Takaishi H, Kimura T, Dalal S, Okada Y, D’Armiento J. Joint diseases and matrix metalloproteinases: a role for MMP-13. Curr Pharm Biotechnol. 2008;9:47–54. doi: 10.2174/138920108783497659. [DOI] [PubMed] [Google Scholar]
- Venkatesan R, Ji E, Kim SY. Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: a comprehensive review. BioMed Res Int. 2015;2015:814068. doi: 10.1155/2015/814068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest. 2009;135:505–512. doi: 10.1378/chest.08-0412. [DOI] [PubMed] [Google Scholar]
- Wall ME, Eddy CR, McClennan ML, Klumpp ME. Detection and estimation of steroidal sapogenins in plant tissue. Anal Chem. 1952;24:1337–1341. doi: 10.1021/ac60068a018. [DOI] [Google Scholar]
- Wang HC, Wu CC, Cheng TS, Kuo CY, Tsai YC, Chiang SY, Wong TS, Wu YC, Chang FR. Active constituents from Liriope platyphylla root against cancer growth in vitro. Evid Based Complement Alternat Med. 2013;2013:857929. doi: 10.1155/2013/857929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn YN, Lim E, Lee N, Kim YS, Koo MS, Choi SY. Screening of Korean medicinal plants for possible osteoclastogenesis effects in vitro. Genes Nutr. 2008;2:375–380. doi: 10.1007/s12263-007-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]