Abstract
PICK1, a PDZ domain-containing protein, is known to increase the reuptake activities of dopamine transporters by increasing their expressions on the cell surface. Here, we report a direct and functional interaction between PICK1 and dopamine D3 receptors (D3R), which act as autoreceptors to negatively regulate dopaminergic neurons. PICK1 colocalized with both dopamine D2 receptor (D2R) and D3R in clusters but exerted different functional influences on them. The cell surface expression, agonist affinity, endocytosis, and signaling of D2R were unaffected by the coexpression of PICK1. On the other hand, the surface expression and tolerance of D3R were inhibited by the coexpression of PICK1. These findings show that PICK1 exerts multiple effects on D3R functions.
Keywords: Dopamine D2 receptor, Dopamine D3 receptor, Dopamine transporter, PICK1, Cell surface expression, Tolerance
INTRODUCTION
Dopamine D2 and D3 receptors (D2R, D3R) are expressed in dopaminergic neurons together with dopamine transporters (DAT) (Gurevich and Joyce, 1999; Diaz et al., 2000). Functional interactions between D2Rs and DATs have been characterized. For example, the stimulation of D2R activates the up-take activity of DAT (Mayfield and Zahniser, 2001), and the cell surface localization of DAT is facilitated through an interaction between the amino-terminus of DAT and the third intracellular loop of D2R (Lee et al., 2007).
Among the dopamine receptor subtypes characterized, D2R and D3R are important targets for the treatment of various diseases in motor, emotional, and endocrine functions, such as Parkinson’s disease, schizophrenia, and pituitary tumors (for review, see ref. Cho et al., 2010b). D2R and D3R show different spatial expression patterns within the brain. D2R is heavily expressed in the regions responsible for motor and endocrine functions (for example, striatum and pituitary glands) whereas D3R is more predominant in emotional and mental functions areas (for example, nucleus accumbens, olfactory tubercle, and islands of calleja) (Bunzow et al., 1988; Sokoloff et al., 1990; Gurevich and Joyce, 1999; Cho et al., 2010b). Since current antipsychotics show low selectivity toward the two receptor types, the treatment of schizophrenia with these agents result in the disturbances of motor and endocrine functions, which are presumably caused by the inhibition of D2R. Hence, cellular components or cellular events involved in the selective manipulation of D2R and D3R could be used as potential targets to separate the desired therapeutic effects from the unwanted side effects of antipsychotics.
PICK1 (protein interacting with C kinase 1) was originally identified by its interaction with PKCα in a yeast two hybrid-screen (Staudinger et al., 1995). A previous study has shown that PICK1 and DAT coimmunoprecipitate from mice striatal membranes and colocalize in cultured dopaminergic neurons (Torres et al., 2001). The uptake activity of DAT was enhanced via an increase in surface expression when PICK1 was coexpressed (Torres et al., 2001). Since D2R and D3R are expressed in dopaminergic neurons and functionally related to DAT, it is expected that PICK1 has functional interactions with D2R and D3R.
In this study, we tested whether PICK1 exerts any regulatory effects on the D2R and D3R.
MATERIALS AND METHODS
Materials
Human embryonic kidney cells (HEK-293) were obtained from the American Type Culture Collection (Rockville, MD, USA). Cell culture media and fetal bovine serum were obtained from Life Technologies, Inc. (Carlsbad, CA, USA). Dopamine (DA), (-) quinpirole, haloperidol, PMA, anti-Flag M2 antibodies, anti-Flag-conjugated agarose beads, antibodies for green fluorescence protein (GFP), horseradish peroxidase (HRP)-labeled secondary antibodies, and glutathione beads were purchased from Sigma/Aldrich Chemical (St Louis, MO, USA). [3H]-Sulpride (87 Ci/mmol) and [3H]-spiperone (85.7 Ci/mmol) were purchased from PerkinElmer Life Sciences (Boston, MA, USA).
Plasmid constructs
The mammalian expression constructs for human dopamine D2 receptor (D2R) and D3 receptor (D3R) have been described previously (Kim et al., 2001; Beom et al., 2004). The PICK1 construct was provided by Dr. Hanley (University of Bristol, Bristol, UK). The GST fusion protein constructs of the 3rd intracellular loops of D2R and D3R were described in a previous study (Zheng et al., 2011).
Determination of receptor expression levels
To determine receptor expression levels, cells were incubated with 3 nM [3H]-spiperone with 100 μg of membranes for 1 h at room temperature. Nonspecific binding was determined in the presence of 10 μM haloperidol. Reactions were terminated by rapid filtration over GF/B filters, followed by three washes with ice-cold buffer (100 mM NaC1, 50 mM Tris, pH 7.2).
Endocytosis assay
Endocytosis of D2R and D3R were measured based on the hydrophilic properties of [3H]-sulpiride as previously described (Cho et al., 2010a). Briefly, HEK-293 cells expressing D2R or D3R were seeded 1 day after transfection at a density of 1.5×105 cells/well in 24-well plates. The cells were stimulated with 10 μM DA, 0.1 μM PMA, or 1.0 μM PMA for 0–60 min as indicated. The cells were then incubated with 250 μl of [3H]-sulpiride (final concentration; 2.2 nM for D2R, 7.2 nM for D3R) at 4°C for 150 min in the absence and presence of unlabeled competitive inhibitor (10 μM haloperidol). Subsequently, the cells were washed three times with ice-cold serum-free media and 1% SDS was added. The samples were mixed with 2 ml Lefkofluor scintillation fluid and counted on a liquid scintillation counter.
Reporter gene assay
Cellular cAMP was measured by an indirect method which had been used for the determination of D2R and D3R signaling as described previously (Cho et al., 2007; Cho et al., 2010a). A reporter plasmid containing the firefly luciferase gene under the control of multiple cAMP responsive elements (CRE) was used with a pRL-TK control vector (Promega, Madison, WI, USA).
Immunoprecipitation
Cells were lysed in RIPA buffer (150 mM NaCl, 50 mM Tris, pH 8.0, 1% NP-40, 0.5% deoxycholate, 0.1% SDS) on a rotation wheel for 1 h at 4°C. The supernatants were mixed with 35 μl of a 50% slurry of anti-Flag-agarose beads for 2–3 h on the rotation wheel. The beads were washed with a buffer (50 mM Tris, pH 7.4, 137 mM NaCl, 10% glycerol, 1% NP-40) three times for 10 min each. The immunoprecipitates were analyzed by immunoblotting.
Immunocytochemistry
The HEK-293 cells were plated on cover slips and fixed with 4% paraformaldehyde in PBS for 10 min at room temperature. The cells were permeabilized with 0.1% Triton X-100 in PBS at room temperature. Next, the cells were incubated with PBS containing 3% fetal bovine serum and 1% bovine serum albumin for 1 h and incubated with anti-Flag antibodies at 1:1000 dilutions for 1 h at room temperature. After three washes, cells were incubated with Alexa 555-conjugated anti-mouse antibodies (Invitrogen, Carlsbad, CA, USA) at 1:500 dilutions. After another three washes with the washing buffer, the cells were mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA) and visualized with a laser scanning confocal microscope (TCS SP5/AOBS/Tandem, Wetzlar, Germany).
Pharmacological sequestration assay
The pharmacological sequestration of D3R was conducted as previously described (Min et al., 2013). Briefly, HEK-293 cells expressing the corresponding receptors were stimulated with a vehicle or 1 μM DA for 5 min and washed three times with a low pH buffer (150 mM NaCl, 50 mM acetic acid, pH 2.0), followed by two washes with HEPES buffer (20 mM, pH 7.4) on ice. Subsequently, the cells were incubated with 250 μl of [3H]-sulpiride (7.2 nM) at 4°C for 150 min in the absence or presence of 10 μM haloperidol. The cells were then washed and lysed with 1% SDS, and the remaining radioactivity was counted using a liquid scintillation counter.
Data analyses
The values are expressed as mean ± SEM for the number of independent experiments indicated in the figure legends. Student’s t-test was used to compare the results. A p-value <0.05 was considered significant. Comparisons among experimental groups were performed using ANOVA and Tukey’s simultaneous test.
RESULTS
PICK1 forms clusters with dopamine D2 and D3 receptors
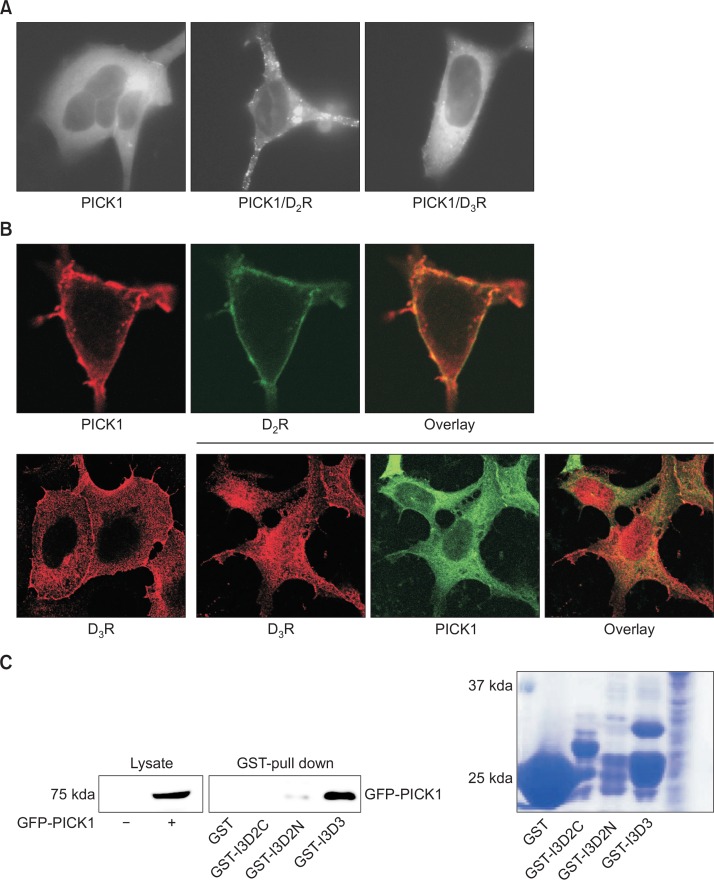
PICK1 is expressed in dopaminergic neurons and has been shown to colocalize with DATs to increase their uptake activities (Torres et al., 2001). Since dopamine D2 receptor (D2R) and D3 receptor (D3R) act as autoreceptors in dopaminergic neurons, the functional interactions between PICK1 and D2R/D3R were tested. As shown in the left panel of Fig. 1A, PICK1 was distributed throughout the cytosol and near the plasma membrane but not in the nucleus. When D2R was coexpressed, PICK1 formed extensive clusters on the plasma membrane (Fig. 1A, middle panel). When D3R was coexpressed, PICK1 also formed some clusters on the plasma membrane, but the majority of the clusters stayed in the cytosol (Fig. 1A, right panel). Consistent with these results, PICK1 and D2R showed colocalizations on the plasma membrane (Fig. 1B, upper panel), whereas PICK1 and D3R colocalized mainly in the cytosol (Fig. 1, lower panel). When the interactions were tested by GST pull-down assays, the 3rd intracellular loop of D3R showed more abundant interactions with PICK1 than those of D2R.
Fig. 1.
Interaction between PICK1 and D2R/D3R. (A) The effects of D2R or D3R on the subcellular distribution of PICK1. HEK-293 cells were transfected with Flag-tagged PICK1 (left panel) or with D2R (middle panel) or D3R (right panel) (2.7 and 2.1 pmol/mg protein, respectively). Cells were labeled with anti-Flag antibodies. (B) Colocalization between PICK1 and D2R or D3R. (Upper panel) HEK-293 cells were transfected with Flag-PICK1 and D2R-GFP. Cells were labeled with anti-Flag antibodies. (Lower panel) HEK-293 cells were transfected with Flag-D3R with or without GFP-PICK1. Cells were labeled with anti-Flag antibodies. (C) GST pull-down assay for the interaction between PICK1 and the intracellular loops of D2R and D3R. Constructs for the GST fusion protein with the 3rd intracellular loop of D2R (I3D2) and D3R (I3D3) and the functionalities of these constructs were described in a previous study (Zheng et al., 2011). I3D2C and I3D2N represent carboxyl region and amino region of I3D2. Bacterial lysates containing GST-binding agarose beads along with GST-I3D2 or GST-I3D3 were incubated with the lysates of HEK-293 cells expressing GFP-PICK1. After washing three times, bound proteins were eluted with SDS sample buffer. Eluents were analyzed on the SDS-PAGE gel and blotted with antibodies against GFP. On the right is an SDS-PAGE analysis of the after-wash of bacterial cell lysates.
Effects of PICK1 on the signaling activities of D2R and D3R
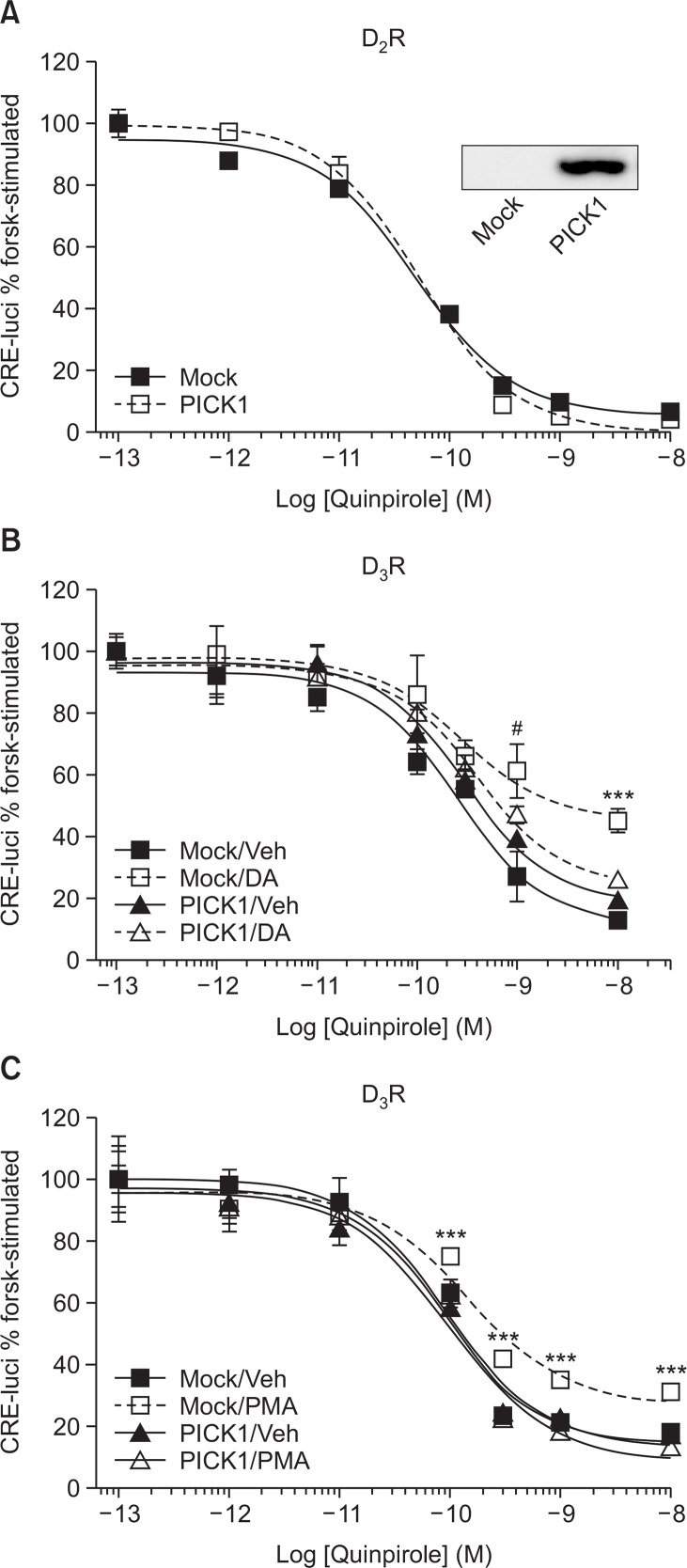
Next, we examined the effect of PICK1 coexpression on the signaling activities of D2R and D3R. The coexpression of PICK1 did not affect the signaling of D2R (Fig. 2A) or D3R (Fig. 2B, compare Mock/Veh vs. PICK1/Veh).
Fig. 2.
Effects of PICK1 on the signaling via D2R and D3R. (A) HEK-293 cells stably expressing D2R (1.9 pmol/mg of protein) were transfected with Mock vector or Flag-PICK1. Reporter gene assay was conducted with increasing concentrations of quinpirole. Cell lysates were blotted with anti-Flag antibodies. (B) HEK-293 cells stably expressing D3R (2.1 pmol/mg of protein) were transfected with either Mock vector or PICK1. Cells were pretreated with either vehicle or 10 μM DA for 5 min, washed 5 times with warm serum-free media and dose-responses were obtained with increasing concentrations of quinpirole. ***p<0.001 compared with other groups. #p<0.05 when Mock/DA group was compared with PICK1/ Veh or PICK1/DA group. (C) HEK-293 cells stably expressing D3R (2.1 pmol/mg of protein) were transfected with either Mock vector or PICK1. Cells were pretreated with either vehicle or 100 nM PMA for 5 min, washed 3 times with warm serum-free media and dose-responses were obtained with increasing concentrations of quinpirole. ***p<0.001 compared with other groups.
It was previously reported that D3R but not D2R undergoes tolerance in response to pretreatment with agonists (Min et al., 2013) (Fig. 2B, compare Mock/Veh vs. Mock/DA group). The coexpression of PICK1 significantly inhibited the tolerance of D3R (Fig. 2B, compare Veh vs. DA group in Mock- and PICK1-transfected cells). D3R also undergoes PKC-mediated desensitization (Cho et al., 2007) (Fig. 2C, compare Mock/Veh vs. Mock/PMA), which was blocked by the coexpression of PICK1 (Fig. 2C, compare PICK1/Veh vs. PICK1/PMA).
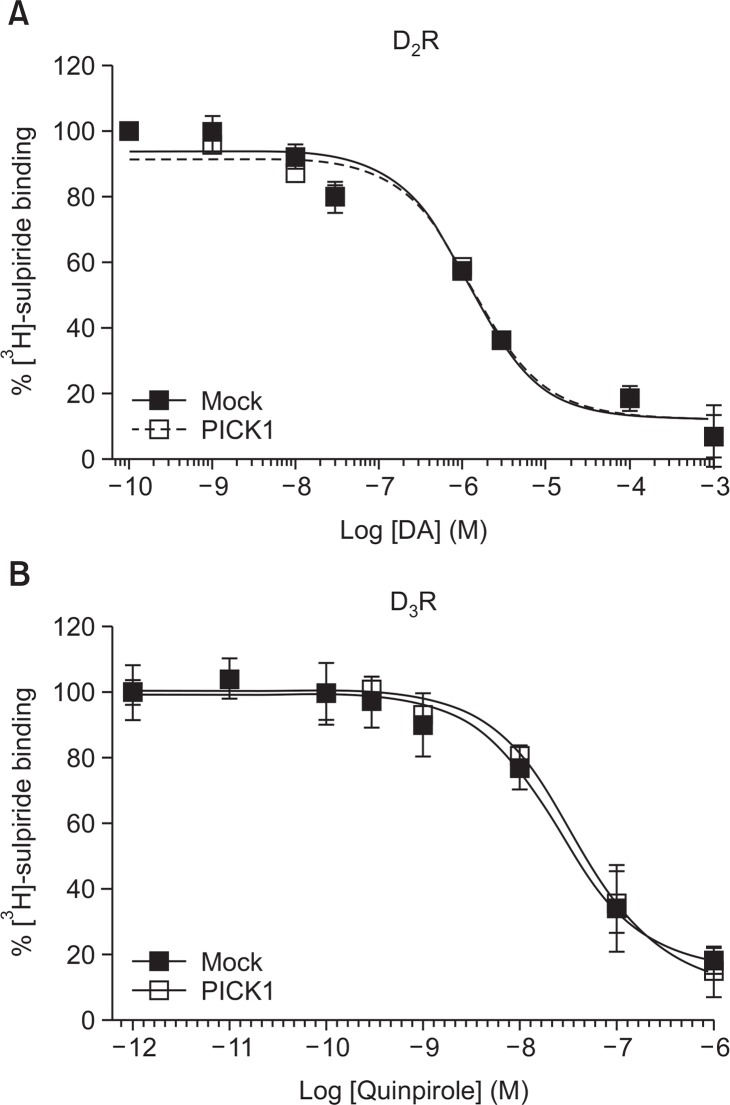
Effects of PICK1 on the agonist affinity of D2R and D3R
Since D2R and D3R formed clusters with PICK1, we examined the effects of PICK1 on agonist affinities. Cells were labelled with [3H]-sulpiride and challenged with increasing concentrations of DA or quinpirole. As shown in Fig. 3A, the Ki of D2R for DA was about 0.98 μM and this was not affected by the coexpression of PICK1. Similarly, the Ki of D3R for quinpirole was about 12.7 nM and this was not affected by the coexpression of PICK1 (Fig. 3B).
Fig. 3.
The effects of PICK1 on the agonist affinity of D2R and D3R. (A) HEK-293 cells expressing D2R (1.9 pmol/mg protein) were labeled with 2.2 nM [3H]-sulpiride with increasing concentrations of DA for 1 h. Cells were washed 3 times with ice-cold serum-free media for 5 min per each wash. Cells were incubated with 1% SDS overnight and radioactivity was measured with liquid scintillation counter. (B) HEK-293 cells expressing D3R (2.1 pmol/mg protein) were labeled with 7.2 nM [3H]-sulpiride with increasing concentrations of quinpirole for 1 h. Ki value was calculated according to the Cheng-Prusoff equation (Cheng and Prusoff, 1973).
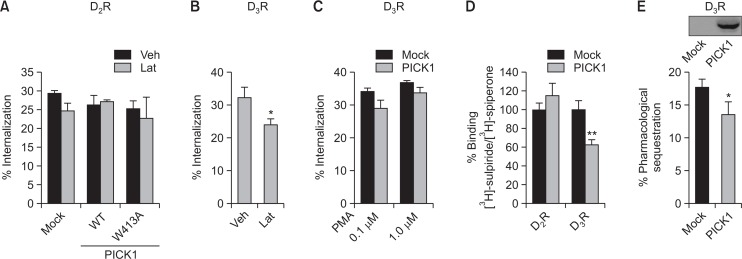
Effects of PICK1 on the endocytosis and cell surface expression of D2R and D3R
Receptor endocytosis is closely related to the regulation of signaling. PICK1 is known to interact with glutamate receptors such as metabotropic glutamate receptors 7 or 3 (Dev et al., 2000; Hirbec et al., 2002) and AMPA receptors (Xia et al., 1999). According to studies on the GluR2 subunit of the AMPA receptor, PICK1 interacts with Arp2/3 complex to increase the internalization of AMPA receptors by inducing actin depolymerization (Rocca et al., 2008). However, this does not seem to be the case with D2R because latrunculin A (LatA), an inhibitor of actin assembly, did not have any effect. Furthermore, W413A-PICK1, a PICK1 mutant which inhibits Arp2/3 complex-mediated actin polymerization (Rocca et al., 2008), had no effect on the endocytosis of D2R (Fig. 4A).
Fig. 4.
The effects of PICK1 on the endocytosis and expression of D2R and D3R at the cell surface. (A) HEK-293 cells expressing D2R were transfected with Mock vector, Flag-PICK1, or Flag-W413A-PICK1 plasmid. Cells were treated with either a vehicle or 1 μM latrunculin A (LatA) for 30 min, followed by 10 μM DA for 1 h. (B) HEK-293 cells expressing D3R were treated with either a vehicle or 1 μM LatA for 30 min, followed by 100 nM PMA for 30 min. *p<0.05 compared with Veh group. (C) HEK-293 cells expressing D3R were transfected with Mock vector or Flag-PICK1 plasmid. Cells were treated with 0.1 and 1 μM PMA for 30 min. (D) HEK293 cells expressing D2R or D3R were transfected with either Mock vector or Flag-PICK1 plasmid. Cells were labelled with 2.2 or 7.2 nM [3H]-sulpiride for D2R and D3R, respectively. Both cell groups were labeled with 2 nM [3H]-spiperone. **p<0.01 compared with D3R/Mock group. (E) HEK-293 cells expressing D3R were transfected with Mock vector or Flag-PICK1 plasmid. Cells were treated with 1 μM DA for 5 min. *p<0.05 compared with Mock group. Cell lysates were blotted with anti-Flag antibodies.
In contrast to D2R which mainly undergoes GRK2-mediated endocytosis, D3R mainly undergoes PKC-mediated endocytosis (Cho et al., 2007). When cells were treated with LatA, PMA-induced internalization of D3R was inhibited (Fig. 4B), suggesting that polymerized actin was required for the PKC-mediated internalization of D3R, which is the opposite to the regulation of glutamate receptors. As expected, PICK1 did not affect the internalization of D3R (Fig. 4C).
Next, roles of PICK1 were tested for the subcellular distribution of D2R and D3R by utilizing the hydrophilicity and hydrophobicity of [3H]-sulpiride and [3H]-speperone, respectively. As expected from Fig. 1B, coexpression of PICK1 did not affect the subcellular distribution of D2R but decreased the expression of D3R on cell surface (Fig. 4D). These results indicate that the alterations of the number of functional receptors at the cell membrane might not be enough to affect the basal signaling of D3R.
Pharmacological sequestration is defined as a shift of receptors to a more hydrophobic fraction within the plasma membrane without the actual translocation of receptors to cytosolic regions (Mostafapour et al., 1996). A subsequent study has shown that pharmacological sequestration can be used to predict the short-term tolerance of GPCRs, including D3R (Min et al., 2013). As shown in Fig. 4E, PICK1 moderately inhibited the pharmacological sequestration of D3R, suggesting that PICK1-mediated attenuation of pharmacological sequestration could be one of the factors which are involved in the PICK1-mediated inhibition of D3R tolerance.
DISCUSSION
At the cell membrane, the function of GPCRs and transporters can be regulated by multiple protein kinases and arresting proteins. The regulation of G protein-coupled receptors (GPCRs) occurs through two different pathways: homologous and heterologous. The homologous pathway involves GRK-mediated receptor phosphorylation followed by an association with arrestin. Arrestins also function as adaptors for the endocytosis of GPCRs at a later stage (Sibley et al., 1987). In comparison, the heterologous desensitization pathway involves the phosphorylation of the receptor proteins by protein kinase A (PKA) or protein kinase C (PKC), which may be activated by other cellular pathways. Thus, in addition to the regulation of receptor functions through receptor phosphorylation and binding with arrestin proteins, this study showed that the functions of D3R can be regulated through interactions with PICK1.
From Fig. 1A, PICK1 seemed to interact more robustly with D2R than D3R. In addition, D2R and PICK1 formed clusters and colocalized on the plasma membrane (Fig. 1B). On the other hand, the GST pull-down assay showed that the 3rd cytoplasmic loop of D3R interacted more strongly with PICK1 than those of D2R. D2R signaling, endocytosis, agonist affinity, and subcellular localization were not altered by the coexpression of PICK1. On the contrary, some of functional aspects of D3R were altered by the coexpression of PICK1. The functional significance of the interaction between D2R and PICK1 in clusters on the plasma membrane is not clear at this time point. More in depth and diverse examinations of receptor functions are needed to understand their interactions.
Fig. 4D shows that the coexpression of PICK1 caused a 40% decrease in D3R expression on the cell surface without altering the signaling of D3R (Fig. 2B). Assuming that the signaling of GPCRs via G proteins occurs on the plasma membrane, these results could be explained by spare receptors. It is possible that an excess number of D3R are functional on the plasma membrane despite the decreased receptor expression with the coexpression of PICK1.
Desensitization or tolerance of GPCRs represents a decrease in receptor functions through prolonged or repeated exposure to an agonist (Lohse et al., 1989; Hausdorff et al., 1990). According to the current paradigm of GPCR desensitization based on studies of the β2 adrenergic receptor (β2AR), receptor phosphorylation and β-arrestin translocation are key cellular events that explain GPCR desensitization. However, this paradigm does not seem to explain the desensitization of certain GPCRs. For example, receptor phosphorylation (Pao and Benovic, 2002; Ferguson, 2007) or GRK2 (Violin et al., 2008) was not required for the desensitization of certain GPCRs. A more recent study suggested that pharmacological sequestration explains the desensitization of D3R (Min et al., 2013). In agreement with the previous study, we found that the pharmacological sequestration of D3R was inhibited by the coexpression of PICK1 (Fig. 4E). Interestingly, PKC-mediated desensitization of D3R was also inhibited by the coexpression of PICK1. Further studies are required to clarify whether certain cellular events, which are commonly involved in homologous and heterologous desensitization, are regulated by PICK1. In addition, the molecular targets that PICK1 act on to block the receptor tolerance need to be identified.
It was suggested that class I PDZ domains bind to a carboxyl terminal consensus sequence S/T-X-V/I (where X is any amino acid); class II PDZ domains preferentially bind to a carboxyl terminal sequence ϕ-X-ϕ (where ϕ represents any hydrophobic residue) (Songyang et al., 1997). D2R contains a consensus sequence within the 3rd intracellular loop, 267SPI269, which is supposed to bind with class I PDZ domain. There are multiple ϕ-X-ϕ motifs within the 3rd intracellular loop of D2R and D3R. However, these sequence are not located at the carboxyl terminus, and further studies are needed to confirm whether these sites are responsible for the interaction with PICK1.
Our results showed that PICK1 regulated the surface expression and tolerance of D3R. Thus, these findings demonstrate that in addition to the regulation of receptor functions through receptor phosphorylation and binding with arresting proteins, the signaling of D2R and D3R can be differentially regulated depending on the expression levels of PICK1.
Acknowledgments
This work was funded by KRF- 2014R1A2A2A01002547. We thank the Korea Basic Science Institute for technical support.
REFERENCES
- Beom S, Cheong D, Torres G, Caron MG, Kim KM. Comparative studies of molecular mechanisms of dopamine D2 and D3 receptors for the activation of extracellular signal-regulated kinase. J Biol Chem. 2004;279:28304–28314. doi: 10.1074/jbc.M403899200. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Van Tol HH, Grandy DK, Albert P, Salon J, Christie M, Machida CA, Neve KA, Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988;336:783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cho D, Zheng M, Min C, Ma L, Kurose H, Park JH, Kim KM. Agonist-induced endocytosis and receptor phosphorylation mediate resensitization of dopamine D2 receptors. Mol Endocrinol. 2010a;24:574–586. doi: 10.1210/me.2009-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DI, Zheng M, Kim KM. Current perspectives on the selective regulation of dopamine D2 and D3 receptors. Arch Pharm Res. 2010b;33:1521–1538. doi: 10.1007/s12272-010-1005-8. [DOI] [PubMed] [Google Scholar]
- Cho EY, Cho DI, Park JH, Kurose H, Caron MG, Kim KM. Roles of protein kinase C and actin-binding protein 280 in the regulation of intracellular trafficking of dopamine D3 receptor. Mol Endocrinol. 2007;21:2242–2254. doi: 10.1210/me.2007-0202. [DOI] [PubMed] [Google Scholar]
- Dev KK, Nakajima Y, Kitano J, Braithwaite SP, Henley JM, Nakanishi S. PICK1 interacts with and regulates PKC phosphorylation of mGLUR7. J Neurosci. 2000;20:7252–7257. doi: 10.1523/JNEUROSCI.20-19-07252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, Sokoloff P. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci. 2000;20:8677–8684. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS. Phosphorylation-independent attenuation of GPCR signalling. Trends Pharmacol Sci. 2007;28:173–179. doi: 10.1016/j.tips.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Joyce JN. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology. 1999;20:60–80. doi: 10.1016/S0893-133X(98)00066-9. [DOI] [PubMed] [Google Scholar]
- Hausdorff WP, Caron MG, Lefkowitz RJ. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- Hirbec H, Perestenko O, Nishimune A, Meyer G, Nakanishi S, Henley JM, Dev KK. The PDZ proteins PICK1, GRIP, and syntenin bind multiple glutamate receptor subtypes. Analysis of PDZ binding motifs. J Biol Chem. 2002;277:15221–15224. doi: 10.1074/jbc.C200112200. [DOI] [PubMed] [Google Scholar]
- Kim KM, Valenzano KJ, Robinson SR, Yao WD, Barak LS, Caron MG. Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and beta-arrestins. J Biol Chem. 2001;276:37409–37414. doi: 10.1074/jbc.M106728200. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Pei L, Moszczynska A, Vukusic B, Fletcher PJ, Liu F. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 2007;26:2127–2136. doi: 10.1038/sj.emboj.7601656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MJ, Lefkowitz RJ, Caron MG, Benovic JL. Inhibition of beta-adrenergic receptor kinase prevents rapid homologous desensitization of beta 2-adrenergic receptors. Proc Natl Acad Sci USA. 1989;86:3011–3015. doi: 10.1073/pnas.86.9.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield RD, Zahniser NR. Dopamine D2 receptor regulation of the dopamine transporter expressed in Xenopus laevis oocytes is voltage-independent. Mol Pharmacol. 2001;59:113–121. doi: 10.1124/mol.59.1.113. [DOI] [PubMed] [Google Scholar]
- Min C, Zheng M, Zhang X, Caron MG, Kim KM. Novel roles for beta-arrestins in the regulation of pharmacological sequestration to predict agonist-induced desensitization of dopamine D3 receptors. Br J Pharmacol. 2013;170:1112–1129. doi: 10.1111/bph.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafapour S, Kobilka BK, Von Zastrow M. Pharmacological sequestration of a chimeric beta 3/beta 2 adrenergic receptor occurs without a corresponding amount of receptor internalization. Recept Signal Transduct. 1996;6:151–163. [PubMed] [Google Scholar]
- Pao CS, Benovic JL. Phosphorylation-independent desensitization of G protein-coupled receptors? Sci STKE. 2002;2002:PE42. doi: 10.1126/stke.2002.153.pe42. [DOI] [PubMed] [Google Scholar]
- Rocca DL, Martin S, Jenkins EL, Hanley JG. Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis. Nat Cell Biol. 2008;10:259–271. doi: 10.1038/ncb1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley DR, Benovic JL, Caron MG, Lefkowitz RJ. Molecular mechanisms of beta-adrenergic receptor desensitization. Adv Exp Med Biol. 1987;221:253–273. doi: 10.1007/978-1-4684-7618-7_19. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- Staudinger J, Zhou J, Burgess R, Elledge SJ, Olson EN. PICK1: a perinuclear binding protein and substrate for protein kinase C isolated by the yeast two-hybrid system. J Cell Biol. 1995;128:263–271. doi: 10.1083/jcb.128.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres GE, Yao WD, Mohn AR, Quan H, Kim KM, Levey AI, Staudinger J, Caron MG. Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1. Neuron. 2001;30:121–134. doi: 10.1016/S0896-6273(01)00267-7. [DOI] [PubMed] [Google Scholar]
- Violin JD, Dipilato LM, Yildirim N, Elston TC, Zhang J, Lefkowitz RJ. beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem. 2008;283:2949–2961. doi: 10.1074/jbc.M707009200. [DOI] [PubMed] [Google Scholar]
- Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/S0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Zheng M, Cheong SY, Min C, Jin M, Cho DI, Kim KM. beta-arrestin2 plays permissive roles in the inhibitory activities of RGS9-2 on G protein-coupled receptors by maintaining RGS9-2 in the open conformation. Mol Cell Biol. 2011;31:4887–4901. doi: 10.1128/MCB.05690-11. [DOI] [PMC free article] [PubMed] [Google Scholar]