Abstract
Neuropathic pain (NPP) is the main culprit among chronic pains affecting the normal life of patients. Procaine is a frequently-used local anesthesia with multiple efficacies in various diseases. However, its role in modulating NPP has not been reported yet. This study aims at uncovering the role of procaine in NPP. Rats were pretreated with procaine by intrathecal injection. Then NPP rat model was induced by sciatic nerve chronic compression injury (CCI) and behavior tests were performed to analyze the pain behaviors upon mechanical, thermal and cold stimulations. Spinal expression of Janus kinase 2 (JAK2) and signal transducer and activator of transcription 3 (STAT3) was detected by qRT-PCR and western blot. JAK2 was also overexpressed in procaine treated model rats for behavior tests. Results showed that procaine pretreatment improved the pain behaviors of model rats upon mechanical, thermal and cold stimulations, with the best effect occurring on the 15th day post model construction (p<0.05). Procaine also inhibited JAK2 and STAT3 expression in both mRNA (p<0.05) and protein levels. Overexpression of JAK2 increased STAT3 level and reversed the improvement effects of procaine in pain behaviors (p<0.01). These findings indicate that procaine is capable of attenuating NPP, suggesting procaine is a potential therapeutic strategy for treating NPP. Its role may be associated with the inhibition on JAK2/STAT3 signaling.
Keywords: Neuropathic pain, Procaine, JAK2/STAT3 signaling, CCI rat model
INTRODUCTION
Neuropathic pain (NPP) is caused by lesion- or disease-affected somatosensory system, which is usually accompanied with altered functions of peripheral and central nervous system (Treede et al., 2008). Nowadays, NPP is becoming a global burden with a high prevalence among middle-aged people, as well as cancer patients (García de Paredes et al., 2011). NPP includes both negative and positive sensory symptoms. The former are deficits of different somatosensory like hypoesthesia, anesthesia and loss of vibratory sensation; the latter includes hyperalgesia, allodynia, paroxysmal pain and ongoing superficial pain (Nickel et al., 2012). NPP is a consequence of multiple altered mechanisms and processes such as sensitization of nociceptors, aberrant ectopic excitability of affected neurons, pro-nociceptive facilitation at the spinal dorsal horn, disinhibition of the spinal inhibitory network, sympathetically maintained pain, and central nervous system reorganization processes (Campbell and Meyer, 2006; Zhuo, 2007; Nickel et al., 2012). For its long course and poor therapeutic effects, NPP is a main culprit among chronic pains that affects the life quality of patients. Molecular therapeutic strategy is under investigation (Backonja and Woolf, 2010), which is of great significance for controlling and treating NPP.
Procaine is a widely-used local anesthesia, though it is gradually replaced by more effective anesthetics such as lidocaine (Hodgson et al., 2000). Furthermore, its effect in treating diseases has been revealed in recent years. For example, injection of 4 mL 1% procaine HCl in median nerves improves the clinical and electrophysiological evaluations of carpal tunnel syndrome patients (Karadaş et al., 2012a, 2012b). Procaine is a potential agent for treating bladder cancer treatment for its regulation of different cell apoptotic pathways (Sun et al., 2012) and is a suppressor in human nasopharyngeal carcinoma cell line CNE-2Z (Zhou et al., 2007). It also facilitates the treatment of skin eruption (Kim et al., 2011). Its new functions are attracting attentions and a growing number of researches are emerging.
In this study, we aimed to investigate the role of procaine in modulating NPP. Rats with procaine injected intrathecally received sciatic nerve chronic compression injury (CCI) surgery were used as the model for NPP study. Then behavior tests were performed to analyze the pain behaviors from three aspects–mechanical, thermal and cold stimulation. The role of procaine in NPP was connected with the Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling by detecting expression of JAK2 and STAT3 and overexpressing JAK2. This study would provide a general understanding for procaine in modulating NPP and offer fundamental information for further application of procaine in alleviating NPP.
MATERIALS AND METHODS
Animal
Sprague-Dawley female rats (SPF grade, 180–210 g) purchased from Vital River Laboratories (Beijing, China) were raised in controlled temperature (22°C) and relative humidity (about 40%) with adequate supply of food and water. They were raised for over five days for acclimation. The animal experiments were approved by a local ethics committee and were performed according to the instructions of our institute.
Drug treatment
Pentobarbital sodium (40 mg/kg) was intraperitoneally injected to the rats for anesthesia, and a vertical incision was made on the spine to expose L5 and L6 spinous processes. The ligamentum flavum was found and impaled by a blunt-tipped needle, and a sudden twitch of the rat was observed. Then a PE-10 catheter was inserted in the subarachnoid space for about 3 cm, and the backflow of cerebrospinal fluid indicated clearness of the catheter, after which the catheter was guided through a subcutaneous tunnel and fixed on the skin. The wound was cleaned by saline and closed. The rats were observed for 2 d after the operation, and those with infection, movement disorder, or unsuccessful intrathecal catheter insertion were excluded.
Procaine treatment was performed at three time points (3 d before model construction, 1 d before model construction and just before model construction) by intrathecally injecting of 2% procaine HCl in DMSO (10 μL/kg, Selleck Chemicals, Houston, TX, USA).
Model construction
The NPP rat model in this study was induced by sciatic nerve CCI. The rats was randomly divided into three groups, namely, sham surgery group, CCI group and CCI+procaine group, with 6 individuals in each group. The rats in the CCI groups all received the whole procedure of sciatic nerve CCI surgery, while the sham surgery groups underwent the surgery without ligation of sciatic nerve. Briefly, the rat was anesthetized by intraperitoneally injecting pentobarbital sodium (40 mg/kg). At 2 mm above the start of sciatic nerve, four ligations spanning about 4 mm on the nerve were made to slightly compress the nerve without interruption of blood flow. The same procedures were performed on the nerves on both sides. After the surgery, the rats were kept in warm and quiet raising environment.
Behavior test
Changes in rat behavior were detected from three aspects, namely, mechanical withdrawal threshold (MWT), paw withdrawal thermal latency (PWTL) and number of withdrawal upon cold stimulation, reflecting the resistance against mechanical, thermal and cold stimulations, respectively. The behavior tests were performed at night on 0, 5, 10, 15 and 20 d post CCI surgery.
MWT was detected by a monitor Electronic von Frey Plenthysmometer (IITC Life Science, Woodland Hills, CA, USA) based on the method proposed by Vivancos G et al. (Vivancos et al., 2004). The rat was placed in a transparent glass box to adapt to the environment. The back heels of both sides were stimulated by a metal probe vertically, with gradually increasing stimulus intensity. The rat withdrew or touched its feet quickly, and the minimal stimulus intensity that could lead to withdrawals was recorded. Detection on the same rat was repeated for 3 times, with 1 min of interval between two detections. MWT was calculated as the mean of 3 repeated results.
PWTL was measured by a monitor Plantar Test Apparatus (IITC Life Science) (Hargreaves et al., 1988). The rat back feet were exposed to the light of the equipment. When the rat withdrew or touched its feet, the light exposure duration was recorded. Detection on the same rat was repeated for 3 times, with 3 min of interval between two detections. PWTL was calculated as the mean of 3 repeated results.
The number of withdrawal upon cold stimulation was measured based on the method proposed by Datta et al. (2010). Briefly, 0.1 mL of acetone was dropped on the rat back foot, and the rat withdrew or touched its foot upon the acetone-induced cold stimulation, which was considered to be positive reaction. Detection on the same rat was repeated for 3 times, with 3 min of interval between two detections. The number of withdrawal per minute was recorded.
JAK2 overexpression
Another 24 rat individuals were used for procaine and JAK2 treatment. They were randomly divided into four groups (6 individuals in each group): the procaine-treated groups were injected with 2% procaine HCl in DMSO (10 μL/kg) and the control group with the same volume of DMSO. To overexpressing JAK2 in rats, we injected 100 μL of JAK2 overexpression vector (300 ng/mL) through the intrathecal catheter to rats on 1 d before model construction. The JAK2 overexpression vector was constructed via ligating the coding sequence of rat Jak2 (GenBank No. NM_031514) into pcDNA3.1 (Thermo Scientific, Carlsbad, CA, USA). The same amount of blank vector was injected as a control.
Tissue sampling
After behavior tests at 20 or 15 d post surgery, the rats were anesthetized by intraperitoneally injected with pentobarbital sodium (40 mg/kg) and then sacrificed for tissue sampling. The L4-L6 spinal dorsal horn was sampled and quickly frozen in liquid nitrogen for further RNA and protein extraction.
qRT-PCR
Total RNAs were extracted from tissues by TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. The RNAs were purified with DNase I (Invitrogen) to remove any DNA contamination. Reverse transcription was performed using PrimeScript 1st Strand cDNA Synthesis Kit (TaKaRa, Dalian, China). qRT-PCR was conducted on Quant-Studio 6 Flex Realtime PCR System (Applied Biosystems, Carlsbad, CA, USA) with each reacting system containing 20 ng of cDNAs and the specific primers for rat Jak2 (Fw: 5′-GGT TCA TTC AGC AGT TCA GTC A-3′ and Rv: 5′-GCA GGG TCT CCA GGT TTA TG-3′) and Stat3 (Fw: 5′-TAC CAC AAA AGT CAG GTT GCT G-3′ and Rv: 5′-ACA TCC CCA GAG TCC TTA TCA A-3′). Data were normalized by Gapdh (Fw: 5′-CGC ATT GCC AGA CAT ATC AGC-3′ and Rv: 5′-AGG TGA AGC AGG CTC AAT CAA-3′) and analyzed with 2−ΔΔCt method.
Western blot
Protein samples were extracted from tissues by RIPA lysis buffer (Sigma-Aldrich, Shanghai, China) according to the manufacturer’s instructions. The same amount of protein (20 μg) from each sample were separated on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then blotted to a polyvinylidene fluoride membrane. The membrane was blocked in 5% skim milk for 2 h at room temperature and incubated in specific antibodies for JAK2 (ab108596), STAT3 (ab68153) and GAPDH (ab8245, abcam, Cambridge, UK) overnight at 4°C. GAPDH was used as an internal reference. Then the membrane was washed twice in phosphate buffered saline and incubated in horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. ECL Plus Western Blotting Substrate was used to develop the signals, and the density of bands was analyzed with ImageJ 1.49 (National Institutes of Health, Public Domain, USA).
Statistical analysis
All the experiments were repeated for three times. Results were represented as the mean ± standard deviation. Data were analyzed by F test for homogeneity test of variance and then t test for statistical significance. p<0.05 was considered significant difference between groups.
RESULTS
Procaine attenuates NPP of model rats
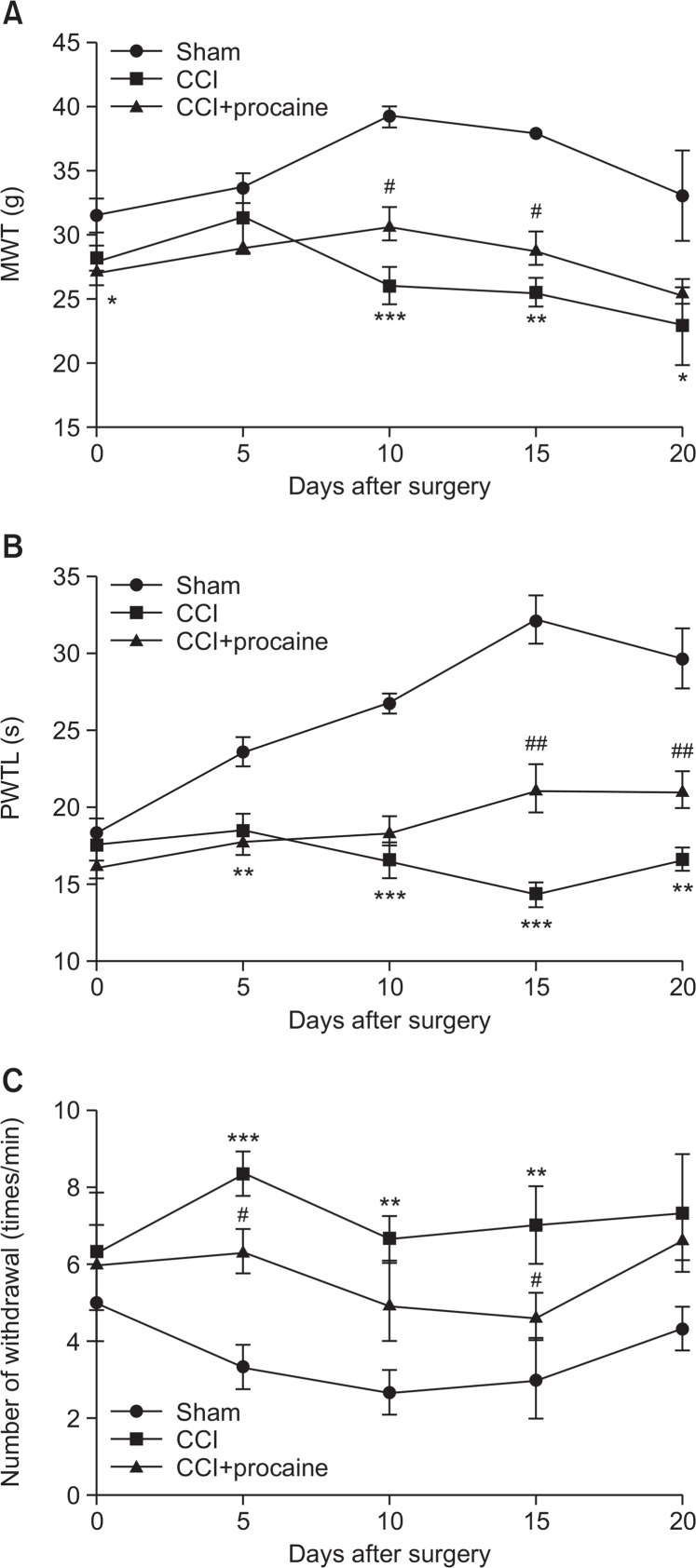
In the CCI-induced NPP model rats with or without procaine pretreatment, behavior tests were performed to analyze the effect of procaine on NPP control. A higher MWT, a longer PWTL and a smaller number of withdrawal upon cold stimulation indicated the higher pain tolerance. Results showed that the at 10, 15 and 20 d post surgery, significant differences were found in MWT between CCI group and sham or CCI+ procaine group (Fig. 1A). CCI significantly decreased MWT compared to sham group (*p<0.05), and procaine significantly increased MWT compared to CCI group (#p<0.05). CCI could decrease PWTL when detected at 5 d post surgery (**p<0.01, Fig. 1B) compared to sham group, and the obvious effect of procaine in recovering PWTL was detected at 15 and 20 d post surgery (##p<0.01). As to cold stimulation, the number of withdrawal was increased significantly by CCI when detected at 5 d post surgery (***p<0.001, Fig. 1C), and was significantly decreased by procaine pretreatment at 5 and 15 d post surgery (#p<0.05). Taken together, the pain behaviors of CCI-induced rats were improved by procaine treatment, with the most obvious effect observed at the 15th day post surgery.
Fig. 1.
Behavior tests in NPP model rats upon mechanical, thermal and cold stimulations. (A) Mechanical withdrawal threshold (MWT) reflecting the reaction upon mechanical stimulation. (B) Paw withdrawal thermal latency (PWTL) reflecting the reaction upon thermal stimulation. (C) Number of withdrawal reflecting reaction upon cold stimulation. sham, rats undergone sham surgery. CCI, rats undergone sciatic nerve chronic compression injury (CCI) as the neuropathic pain (NPP) model. CCI+procaine, NPP model rats pretreated with procaine. Behavior tests are performed on 0, 5, 10, 15 and 20 d post surgery (n=3). *p<0.05, **p<0.01 and ***p<0.001 compared to sham group. #p<0.05 and ##p<0.01 compared to CCI group.
Procaine inhibits JAK2 and STAT3 expression
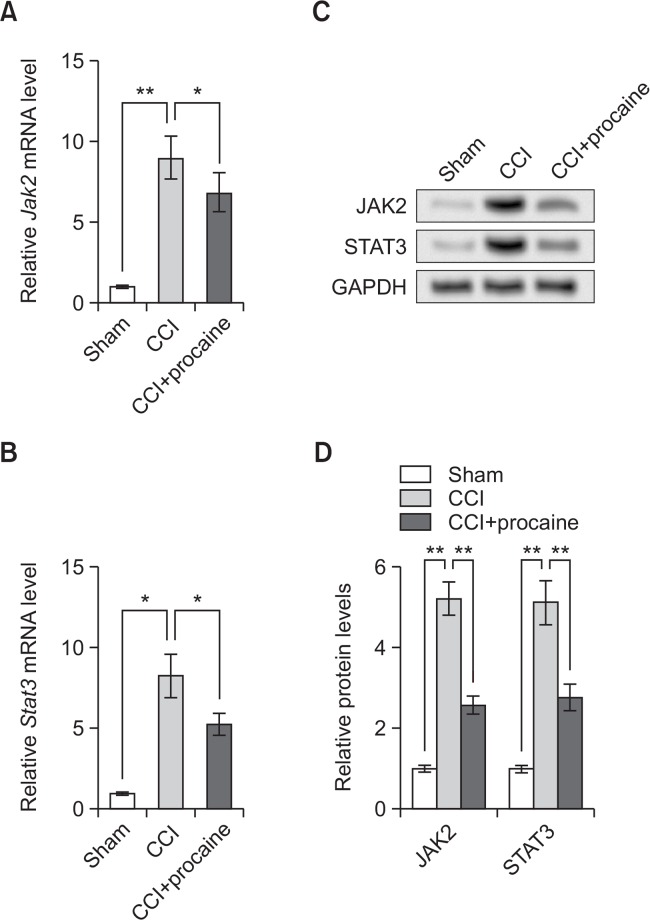
Next we tried to investigate the mechanism involved in procaine functions. It is reported that the JAK2/STAT3 signaling is activated upon NPP (Yamauchi et al., 2006), so we detected expression levels of JAK2 and STAT3 by qRT-PCR and western blot on the 20th day post surgery. qRT-PCR showed that both Jak2 and Stat3 mRNA levels were elevated by CCI surgery (**p<0.01 or *p<0.05, Fig. 2A, 2B), which might support the previously reported JAK2/STAT3 activation during NPP. However, the NPP model rats with procaine pretreatment possessed obviously lower Jak2 and Stat3 levels (*p<0.05), suggesting procaine treatment might inhibit the expression of JAK2 and STAT3. Western blot results showed the similar changing patterns of JAK2 and STAT3 proteins (Fig. 2C), and significant differences were detected between groups (**p<0.01, Fig. 2D). These results suggested that procaine inhibited JAK2 and STAT3 expression in NPP model rats.
Fig. 2.
Procaine pretreatment inhibits JAK2 and STAT3 expression. (A) Relative Jak2 mRNA level detected by qRT-PCR. (B) Relative Stat3 mRNA level detected by qRT-PCR. (C) JAK2 and STAT3 protein expression detected by western blot. (D) Relative protein levels of JAK2 and STAT3 based on Western blot results. sham, rats undergone sham surgery. CCI, rats undergone sciatic nerve chronic compression injury (CCI) as the neuropathic pain (NPP) model. CCI+procaine, NPP model rats pretreated with procaine. The detection is performed on the 20th day post surgery (n=3). GAPDH is used as an internal reference. *p<0.05, **p<0.01. JAK2, Janus kinase 2. STAT3, signal transducer and activator of transcription 3.
JAK2 overexpression abrogates the effects of procaine
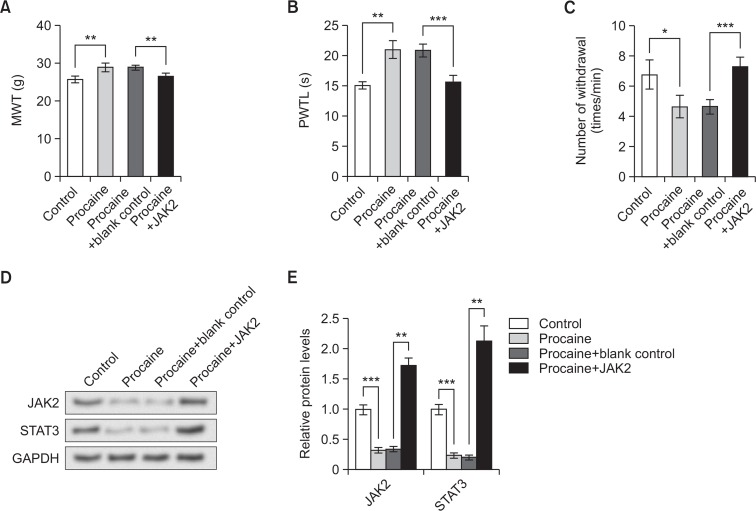
In order to test the involvement of JAK2/STAT3 signaling in the functional mechanism of procaine, JAK2 was overexpressed by its overexpression vector before CCI surgery, and then the rats were raised until the 15th day post surgery, when behavior tests were performed. This time point was selected because procaine pretreatment was most effective according to the above results (Fig. 1).
Behavior test results showed that procaine pretreatment in NPP model rats increased MWT and PWTL, and decreased the number of withdrawal upon cold stimulation compared to control group (*p<0.05, Fig. 3A–3C). JAK2 overexpression in model rats with procaine pretreatment significantly reduced MWT (**p<0.01) and PWTL (***p<0.001), and significantly increased the number of withdrawal upon cold treatment (***p<0.001), suggesting that JAK2 overexpression aggravated pain behaviors of NPP model rats and reversed the effect of procaine.
Fig. 3.
JAK2 overexpression abrogates the effect of procaine pretreatment in NPP model rats. (A) Mechanical withdrawal threshold (MWT) reflecting the reaction upon mechanical stimulation. (B) Paw withdrawal thermal latency (PWTL) reflecting the reaction upon thermal stimulation. (C) Number of withdrawal reflecting reaction upon cold stimulation. (D) Western blot showing the expression change of JAK2 and STAT3 proteins. (E) Relative protein levels of JAK2 and STAT3 based on Western blot results. Control, neuropathic pain (NPP) model rats with no pretreatment. Procaine, NPP model rats pretreated with procaine. Procaine+blank control, NPP model rats pretreated with procaine and injected with blank vectors as the control for JAK2 overexpression. Procaine+JAK2, NPP model rats pretreated with procaine and injected with JAK2 overexpression vector. Behavior test and western blot are performed on the 15th day post surgery. GAPDH is used as an internal control in western blot. *p<0.05, **p<0.01, ***p<0.001. JAK2, Janus kinase 2. STAT3, signal transducer and activator of transcription 3.
The expression level of JAK2 and STAT3 was further detected by western blot and results showed that both JAK2 and STAT3 protein levels were reduced by procaine pretreatment in model rats, but were elevated by JAK2 overexpression (Fig. 3D), with significant differences between control and procaine groups (***p<0.001), as well as procaine+blank control and procaine+JAK2 groups (**p<0.01, Fig. 3E). This result verified the successful overexpression of JAK2 and the consequent up-regualted STAT3, which abrogated the effect of procaine in inhibiting STAT3.
DISCUSSION
In this study, we pretreat CCI-induced NPP rat model with procaine and uncover the protective role of procaine against NPP. The rats treated with procaine were less sensitive to mechanical, thermal and cold stimulation, and possessed lower JAK2 and STAT3 levels compared to control group. Overexpression of JAK2 could abrogate the effect of procaine, making the model rats generating more severe pain behaviors.
The JAK/STAT signaling pathways and MAPK cascades are vital signal transduction pathways for neuropoietic cytokines such as interleukin-6 (Heinrich et al., 2003; Dubový et al., 2010; Vallejo et al., 2010), among which the JAK2/STAT3 signaling can be activated by almost all the cytokines acting on JAKs (Imada and Leonard, 2000). Peripheral nerve injury evokes the activation of JAK2/STAT3 with increased phosphorylation of STAT3, contributing to NPP (Dominguez et al., 2008, 2010). In a study of rats with spinal nerve injury, JAK/ STAT3 inhibitor induces less dorsal horn astrocyte proliferation and promotes tactile allodynia recovery (Tsuda et al., 2011). Besides, spinal IL-33/ST2 signaling aggravates NPP via activating the JAK2/STAT3 signaling (Liu et al., 2015). WP1066, an inhibitor of STAT3 signaling, relieves the pain behavior of CCI rats, thus being a promising agent for treating NPP (Xue et al., 2014). Based on these references, the JAK2/STAT3 signaling is activated in both peripheral and spinal nerve injury, while its inhibition alleviates NPP. Similarly in this study, over-expression of JAK2 via intrathecal injection of vector induced the up-regulation of both JAK2 and STAT3 protein, which suggested the activated JAK2/STAT3 signaling. Meanwhile, the activated signaling was accompanied with increased sensitivity to mechanical, thermal and cold stimulations, indicating the aggravated pain behavior, which also supports the former finding that the JAK2/STAT3 signaling is associated with NPP regulation.
Procaine acts as a sodium channel blocker for its application in anesthesia. For example, it inhibits the opening of Ca2+ channels, which is a factor contributing to local anesthesia (Ahn and Karaki, 1988; Ribeiro and Costa, 2003). Its role in relieving pains inspires us to investigate its effect on NPP of this study. Results showed that procaine pretreatment in CCI-induced NPP model rats could alleviate pain behaviors upon mechanical, thermal and cold stimulations. CCI surgery induces the peripheral nerve injury, and its affections were detected in spinal nerves, as was indicated by JAK2 and STAT3 changes in spinal tissues. On this basis, intrathecal injection of procaine could alleviate pain behaviors and inhibit the JAK2/ STAT3 signaling, suggesting procaine treatment is effective in controlling NPP.
We also found that JAK2 overexpression in procaine-treated model rats was capable of reversing the effects of procaine, including the procaine-relieved pain behaviors, and procaine-inhibited JAK2 and STAT3 expression, which implies the potential association of procaine and the JAK2/STAT3 signaling during regulation of NPP. Previous studies have confirmed the role of some drugs in controlling NPP is via inhibiting the JAK2/STAT3 signaling. For example, triptolide attenuates spinal nerve ligation-induced NPP via inhibiting spinal astrocyte activation and JAK2/STAT3 activation (Tang et al., 2012). Aspirin-triggered lipoxin A4 suppresses mechanical allodynia in NPP rats through controlling spinal JAK2/STAT3 (Wang et al., 2014). So given the results that JAK2/STAT3 activation could reverse procaine effects, it is likely that the role of procaine in attenuating NPP is via suppressing the JAK2/STAT3 signaling. But there is possibility that procaine may alternatively affect the MAPK signaling to suppress NPP, because MAPK is another functioning mechanism of triptolide in NPP (Wang et al., 2012). Still, more and stronger evidence are to be found in further studies.
To sum up, this study reveals a new role of procaine in attenuating NPP in CCI-induced model rats, indicating the potential application of procaine in treating NPP. This neo-function of procaine is associated with inhibited JAK2/STAT3 signaling, but the detailed and explicit mechanism remains to be investigated.
Footnotes
CONFLICT OF INTERESTS
The authors report no declarations of interest.
REFERENCES
- Ahn HY, Karaki H. Inhibitory effects of procaine on contraction and calcium movement in vascular and intestinal smooth muscles. Br J Pharmacol. 1988;94:789–796. doi: 10.1111/j.1476-5381.1988.tb11590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backonja M, Woolf CJ. Future directions in neuropathic pain therapy: closing the translational loop. Oncologist. 2010;15(Suppl 2):24–29. doi: 10.1634/theoncologist.2009-S502. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Chatterjee K, Kline RH, 4th, Wiley RG. Behavioral and anatomical characterization of the bilateral sciatic nerve chronic constriction (bCCI) injury: correlation of anatomic changes and responses to cold stimuli. Mol. Pain. 2010;6:7. doi: 10.1186/1744-8069-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez E, Mauborgne A, Mallet J, Desclaux M, Pohl M. SOCS3-mediated blockade of JAK/STAT3 signaling pathway reveals its major contribution to spinal cord neuroinflammation and mechanical allodynia after peripheral nerve injury. J Neurosci. 2010;30:5754–5766. doi: 10.1523/JNEUROSCI.5007-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez E, Rivat C, Pommier B, Mauborgne A, Pohl M. JAK/STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J Neurochem. 2008;107:50–60. doi: 10.1111/j.1471-4159.2008.05566.x. [DOI] [PubMed] [Google Scholar]
- Dubový P, Klusáková I, Svízenská I, Brázda V. Satellite glial cells express IL-6 and corresponding signal-transducing receptors in the dorsal root ganglia of rat neuropathic pain model. Neuron Glia Biol. 2010;6:73–83. doi: 10.1017/S1740925X10000074. [DOI] [PubMed] [Google Scholar]
- García de Paredes ML, del Moral González F, Martínez del Prado P, Martí Ciriquián JL, Enrech Francés S, Cobo Dols M, Esteban González E, Ortega Granados AL, Majem Tarruella M, Cumplido Burón JD, Gascó Hernández A, López Miranda E, Ciria Santos JP, de Castro Carpeño FJ. First evidence of oncologic neuropathic pain prevalence after screening 8615 cancer patients. Results of the On study. Ann Oncol. 2011;22:924–930. doi: 10.1093/annonc/mdq449. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/bj20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson PS, Liu SS, Batra MS, Gras TW, Pollock JE, Neal JM. Procaine compared with lidocaine for incidence of transient neurologic symptoms. Reg Anesth Pain Med. 2000;25:218–222. doi: 10.1016/s1098-7339(00)90001-4. [DOI] [PubMed] [Google Scholar]
- Imada K, Leonard WT. The Jak-STAT pathway. Mol Immunol. 2000;37:1–11. doi: 10.1016/S0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- Karadaş Ö, Omac OK, Tok F, Ozgul A, Odabasi Z. Effects of steroid with repetitive procaine HCl injection in the management of carpal tunnel syndrome: an ultrasonographic study. J Neurol Sci. 2012a;316:76–78. doi: 10.1016/j.jns.2012.01.023. [DOI] [PubMed] [Google Scholar]
- Karadaş Ö, Tok F, Akarsu S, Tekin L, Balaban B. Triamcinolone acetonide vs procaine hydrochloride injection in the management of carpal tunnel syndrome: randomized placebo-controlled study. J Rehabil Med. 2012b;44:601–604. doi: 10.2340/16501977-0990. [DOI] [PubMed] [Google Scholar]
- Kim HY, Kim BJ, Kim JH, Yoo BH. Early congenital syphilis presenting with skin eruption alone: a case report. Korean J Pediatr. 2011;54:512–514. doi: 10.3345/kjp.2011.54.12.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Mi WL, Li Q, Zhang MT, Han P, Hu S, Mao-Ying QL, Wang YQ. Spinal IL-33/ST2 Signaling Contributes to Neuropathic Pain via Neuronal CaMKII-CREB and Astroglial JAK2-STAT3 Cascades in Mice. Anesthesiology. 2015;123:1154–1169. doi: 10.1097/ALN.0000000000000850. [DOI] [PubMed] [Google Scholar]
- Nickel FT, Seifert F, Lanz S, Maihofner C. Mechanisms of neuropathic pain. Eur Neuropsychopharmacol. 2012;22:81–91. doi: 10.1016/j.euroneuro.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Ribeiro MA, Costa PF. The sensitivity of sodium channels in immature and mature rat CA1 neurones to the local anaesthetics procaine and lidocaine. Brain Res Dev Brain Res. 2003;146:59–70. doi: 10.1016/j.devbrainres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Sun R, Chang L, Wang K, Sun H, Cong X. Procaine Inhibiting Human Bladder Cancer Cell Proliferation by Inducing Apoptosis and Demethylating APAF1 CpG Island Hypermethylated. Chem Res Chin Univ. 2012;28:1017–1021. [Google Scholar]
- Tang J, Li ZH, Ge SN, Wang W, Mei XP, Wang W, Zhang T, Xu LX, Li JL. The Inhibition of Spinal Astrocytic JAK2-STAT3 Pathway Activation Correlates with the Analgesic Effects of Triptolide in the Rat Neuropathic Pain Model. Evid Based Complement Alternat Med. 2012;1851;2012:67. doi: 10.1155/2012/185167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Kohro Y, Yano T, Tsujikawa T, Kitano J, Tozaki-Saitoh H, Koyanagi S, Ohdo S, Ji RR, Salter MW, Inoue K. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain. 2011;134:1127–1139. doi: 10.1093/brain/awr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10:167–184. doi: 10.1111/j.1533-2500.2010.00367.x. [DOI] [PubMed] [Google Scholar]
- Vivancos GG, Verri WA, Jr, Cunha TM, Schivo IR, Parada CA, Cunha FQ, Ferreira SH. An electronic pressure-meter nociception paw test for rats. Braz J Med Biol Res. 2004;37:391–399. doi: 10.1590/S0100-879X2004000300017. [DOI] [PubMed] [Google Scholar]
- Wang W, Mei XP, Chen L, Tang J, Li JL, Wu SX, Xu LX, Li YQ. Triptolide prevents and attenuates neuropathic pain via inhibiting central immune response. Pain Physician. 2012;15:E995–E1006. [PubMed] [Google Scholar]
- Wang ZF, Li Q, Liu SB, Mi WL, Hu S, Zhao J, Tian Y, Mao-Ying QL, Jiang JW, Ma HJ, Wang YQ, Wu GC. Aspirin-triggered Lipoxin A4 attenuates mechanical allodynia in association with inhibiting spinal JAK2/STAT3 signaling in neuropathic pain in rats. Neuroscience. 2014;273:65–78. doi: 10.1016/j.neuroscience.2014.04.052. [DOI] [PubMed] [Google Scholar]
- Xue ZJ, Shen L, Wang ZY, Hui SY, Huang YG, Ma C. STAT3 inhibitor WP1066 as a novel therapeutic agent for bCCI neuropathic pain rats. Brain Res. 2014;1583:79–88. doi: 10.1016/j.brainres.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Osuka K, Takayasu M, Usuda N, Nakazawa A, Nakahara N, Yoshida M, Aoshima C, Hara M, Yoshida J. Activation of JAK/STAT signalling in neurons following spinal cord injury in mice. J Neurochem. 2006;96:1060–1070. doi: 10.1111/j.1471-4159.2005.03559.x. [DOI] [PubMed] [Google Scholar]
- Zhou H, Xu MF, Luo GQ, Zhang YF. Effects of procaine on human nasopharyngeal carcinoma cell strain CNE-2Z. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;21:1118–1121. [PubMed] [Google Scholar]
- Zhuo M. Neuronal mechanism for neuropathic pain. Mol. Pain. 2007;3:14. doi: 10.1186/1744-8069-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.