Abstract
β-Arrestins are one of the protein families that interact with G protein-coupled receptors (GPCRs). The roles of β-arrestins are multifaceted, as they mediate different processes including receptor desensitization, endocytosis, and G protein-independent signaling. Thus, determining the GPCR regions involved in the interactions with β-arrestins would be a preliminary step in understanding the molecular mechanisms involved in the selective direction of each function. In the current study, we determined the roles of the N-terminus, intracellular loops, and C-terminal tail of a representative GPCR in the interaction with β-arrestin2. For this, we employed dopamine D2 and D3 receptors (D2R and D3R, respectively), since they display distinct agonist-induced interactions with β-arrestins. Our results showed that the second and third intracellular loops of D2R are involved in the agonist-induced translocation of β-arrestins toward plasma membranes. In contrast, the N- and C-termini of D2R exerted negative effects on the basal interaction with β-arrestins.
Keywords: β-Arrestin, G protein-coupled receptors, Dopamine D2 receptor, Dopamine D3 receptor
INTRODUCTION
G protein-coupled receptors (GPCRs) are classified into rhodopsin-like, secretin-like, and metabotropic glutamate-like families based on the size and shape of their N-terminal region (Bockaert and Pin, 1999). Rhodopsin-like GPCRs are further classified into subfamilies, which bind to different ligands including catecholamines, peptides, and glycoprotein hormones. Dopamine D2 and D3 receptors (D2R and D3R, respectively) belong to the catecholamine subfamily and are important targets for the treatment of various diseases associated with motor, emotional, and endocrine dysfunction (Thomas et al., 2008; Cho et al., 2010b).
GPCRs including D2R and D3R contain relatively well-conserved transmembrane regions, but the sizes and amino acid sequences of the N-termini and intracellular loops are variable (Cho et al., 2010b). According to the crystal structure of D3R (Chien et al., 2010), the three-dimensional structure around the TM domains was clearly defined, and the ionic interactions among the well-conserved amino acid residues of the TM regions and adjacent submolecular domains were well predicted. However, the structural features of the other re gions, especially those of the N-terminal region could not be determined.
Upon stimulation by agonists, GPCRs activate G proteins and are phosphorylated by G protein-coupled receptor kinases (GRKs). In response to agonistic stimulation, some GPCRs undergo GRK2/3-mediated phosphorylation and interact with β-arrestins, which results in desensitization of the GPCRs, and this was exemplified by β2 adrenergic receptor (β2AR) (Lohse et al., 1990; Lohse et al., 1992). When exposed to agonists for extended periods, GPCRs are endocytosed in a complex with β-arrestin (Ferguson et al., 1996; Goodman et al., 1996), and may or may not dissociate from the co-internalized β-arrestins after entering the cells. Based on the postendocytic behaviors of GPCRs with β-arrestins, GPCRs are classified as either class A or class B (Oakley et al., 2000).
Recent studies have suggested that β-arrestins mediate G protein-independent atypical signaling, and evidence for this was mainly demonstrated by ERK activation (Ahn et al., 2003; Ahn et al., 2004). It remains unclear how β-arrestins mediate various cellular processes such as desensitization, endocytosis, and atypical signaling. As an initial step toward understanding the multifaceted roles of β-arrestins in these processes, we determined the roles of distinct GPCR subregions in the interactions with β-arrestins.
MATERIALS AND METHODS
Materials
Human embryonic kidney 293 (HEK-293) cells were purchased from American Type Culture Collection (Manassas, VA, USA). HEK-293 cells were cultured in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, MA USA), 100 units/ml penicillin and 100 μg/ml streptomycin in a humidified atmosphere containing 5% CO2. The cells were transfected using polyethyleneimine (PEI) (Warrington, PA, USA). [3H]-spiperone was purchased from PerkinElmer Life Sciences (Boston, MA, USA). Dopamine (DA), antibodies to Flag, and anti-M2 Flag antibody-conjugated agarose beads were obtained from Sigma/Aldrich Chemical Co. (St. Louis, MO, USA). Antibodies to β-arrestin were kindly provided by Dr. Lefkowitz (Duke University, NC, USA).
Plasmid constructs
The human genes encoding for D2R and D3R in the pCMV5 vector, either untagged or tagged with Flag epitopes at the N-termini, were described elsewhere (Kim et al., 2001; Cho et al., 2007). D2R and D3R chimeric receptors, in which the second and third intracellular loops or N/C-termini were exchanged, were described previously (Robinson and Caron, 1997; Zheng et al., 2011), or prepared by site-directed mutagenesis. Specifically, in D2R-(IL2/3-D3R), the second and third intracellular loops of D2R were exchanged with those of D3R. In D3R-(IL2/3-D2R), the second and third intracellular loops of D3R were exchanged with those of D2R. D2R mutants lacking either the N-terminus or C-terminus were prepared by site-directed mutagenesis.
Immunoprecipitation
Cells were lysed in RIPA buffer (150 mM NaCl, 50 mM Tris, pH 8.0, 1% NP-40, 0.5% deoxycholate, 0.1% SDS) on a rotation wheel for 1 h at 4°C. The supernatant was mixed with 35 μL of 50% slurry of anti-Flag antibody-conjugated agarose beads for 2–3 h on the rotation wheel. The beads were washed with washing buffer (50 mM Tris, pH 7.4, 137 mM NaCl, 10% glycerol, 1% NP-40) three times for 10 min each.
Confocal microscopy
For β-arrestin translocation assays, HEK-293 cells were transfected with β-arrestin2-GFP and corresponding GPCRs (Kim et al., 2001). One day after transfection, cells were seeded onto 35-mm dishes containing a centered, 1-cm well and allowed to recover for 1 day. Cells were then incubated with 2 mL MEM containing 20 mM HEPES (pH 7.4) and viewed on a Zeiss laser scanning confocal microscope.
RESULTS
D2R but not D3R mediates agonist-induced β-arrestin translocation
Upon binding to agonists, GPCRs undergo conformational changes resulting in signaling and a series of regulatory processes. For example, Gβγ helps recruit GPCR kinase2/3 (GRK2/3) from the cytosol to the plasma membrane to phosphorylate activated receptors (Pitcher et al., 1992). Phosphorylated receptors exhibit a higher affinity to β-arrestin, causing translocation of β-arrestins from the cytosol to the extracellular membrane (Barak et al., 1997). Translocated β-arrestins bind to agonist-occupied GPCRs to which they exert multiple effects such as desensitization and receptor endocytosis. Thus, agonist-induced translocation of β-arrestins is a biomarker to predict the uncoupling of the receptors from G proteins and GPCR endocytosis.
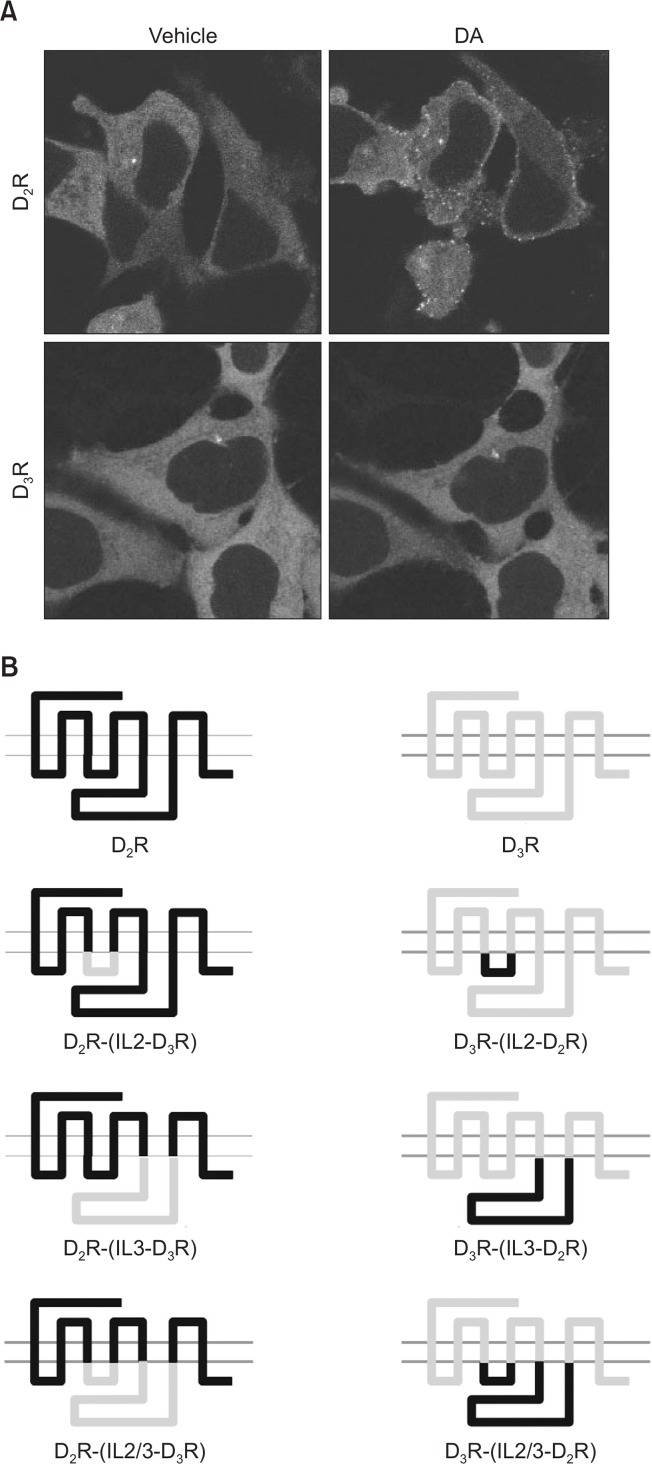
As shown in Fig. 1A, agonist stimulation of D2R but not D3R resulted in β-arrestin2 translocation, suggesting that these two receptors could be used to determine the particular regions involved in β-arrestin2 translocation. The second and third intracellular loops are the main regions involved in GPCR interactions with various proteins including G proteins (Robinson and Caron, 1996). To test whether the second and third loops are involved in the agonist-induced β-arrestin2 translocation, six different D2R and D3R chimeric receptors were prepared (Fig. 1B). For each chimeric receptor, either the second or third intracellular loop or both loops were replaced with those of the other receptor.
Fig. 1.
Comparison between D2R and D3R in agonist-induced β-arrestin translocation. (A) Visualization of β-arrestin2-GFP translo-cation in response to agonistic stimulation of D2R or D3R in HEK-293 cells. Cells stably expressing D2R or D3R (2.7 and 3.5 pmol/mg protein, respectively) were transfected with 2 μg β-arrestin2-GFP per 100 mm culture dish. Cells were stimulated with 10 μM DA for 5 min. Receptor expression levels were determined by [3H]-spiperone binding at saturating concentrations (3 nM). (B) Schematic diagram of D2R and D3R chimeric receptors. Detailed information about these constructs is described in previous studies (Robinson and Caron, 1996; Robinson and Caron, 1997).
The second and third intracellular loops are both involved in agonist-induced translocation of β-arrestin2
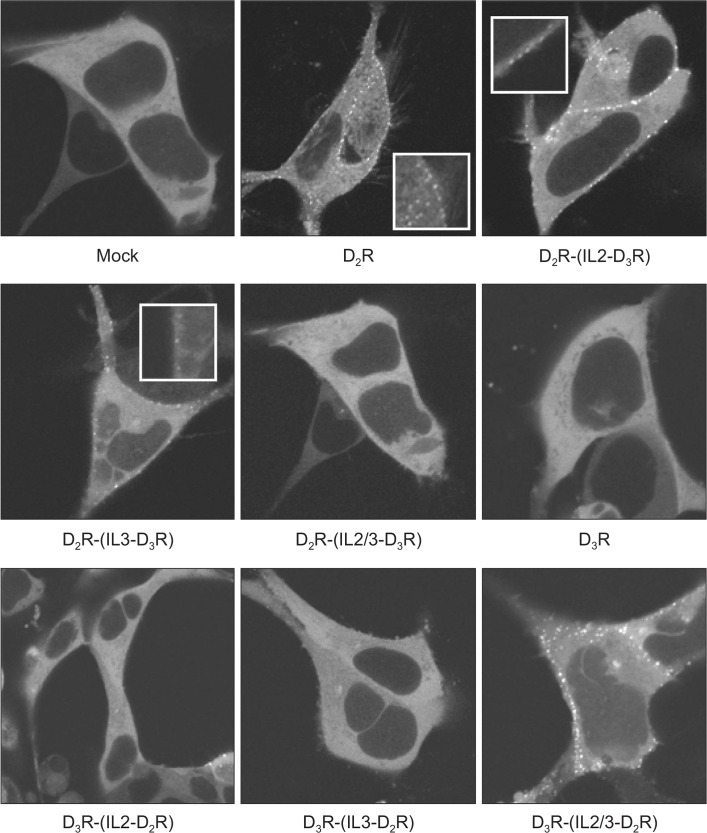
To understand the roles of the intracellular loops of D2R in β-arrestin2 translocation, chimeras of D2R and D3R were utilized. The first loop was excluded because point mutations in serine and threonine residues located within the first loop did not affect D2R endocytosis, which is a direct downstream event of β-arrestin translocation (Cho et al., 2010a). As shown in Fig. 2, D2R clearly mediated the translocation of β-arrestin2, whereas D3R failed to accomplish this (second and sixth images). As the second or third loop of D2R was replaced with that of D3R, D2R showed a decreased ability to recruit β-arrestin2 (third and fourth images) and failed to recruit β-arrestin2 when both loops were replaced (fifth image). In the reverse situation, D3R gained the ability to translocate β-arrestin2, as the second or third intracellular loop was replaced with that of D2R (seventh and eighth images), and the functional phenotype of D3R was changed to that of D2R when both loops were exchanged (ninth image). However, it should be noted that D2R clearly retained the ability to translocate β-arrestin2 when the second or third intracellular loop was replaced with that of D3R, while D3R could not, as clearly, gain the ability to translocate β-arrestin2 when both loops were replaced with those of D2R. Thus, it could be concluded that the second and third intracellular loops of D2R play central roles in the translocation of β-arrestin2, though other subregions also possess certain roles.
Fig. 2.
Evaluation of β-arrestin2-GFP translocation after agonist activation of D2R, D3R, or D2R and D3R chimeric receptors. HEK-293 cells expressing D2R (2.3 pmol/mg of protein), D3R (3.7 pmol/mg of protein), or D2R and D3R chimeric receptors (between 2.0 and 3.5 pmol/mg protein) were transfected with 1 μg of β-arrestin2-GFP. Cells were stimulated with 10 μM DA for 5 min and viewed on a Zeiss laser scanning confocal microscope.
Constitutive interactions are controlled by the N- or C-terminus
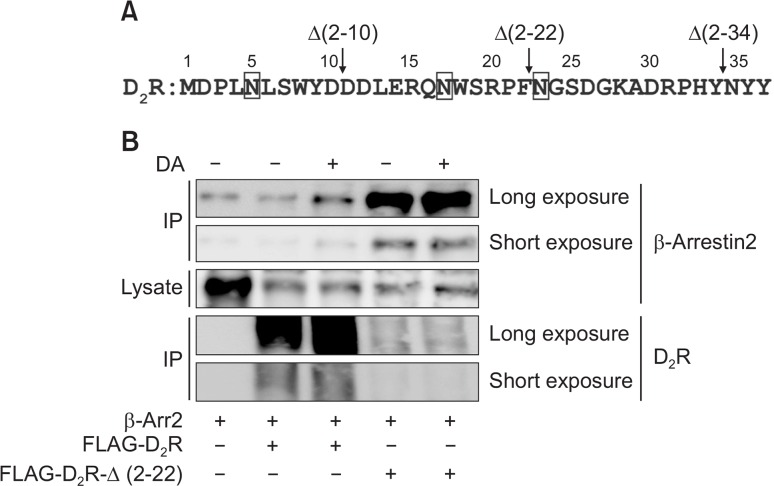
The roles of the N-terminus of D2R or D3R in the interaction with β-arrestins have not been reported. The N-terminus of D2R consists of 37 amino acids and contains three N-linked glycosylation sites (N-X-S/T) (Fig. 3A). Since N-linked glycosylation is important in the surface expression of certain GP-CRs, we prepared three constructs in which approximately 10 consecutive amino acids were deleted. For example, Δ(2–10) represents a deletion mutant of D2R in which the amino acids 2 through 10 were deleted. Surface expression of Δ(2–34) was too seriously impaired (data not shown), and thus could not be used for functional studies. As shown in Fig. 3B, agonist stimulation of D2R resulted in an increase in the interaction between D2R and β-arrestin2. However, deletion of 21 amino acid residues located within the N-terminus of D2R, Δ(2–22), resulted in a marked increase in the basal interaction with β-arrestin2.
Fig. 3.
Role of the N-terminal region of D2R in the interaction with β-arrestin2. (A) Alignment of the amino acid sequences within the N-terminal regions of D2R. The numbers represent the position of the amino acid residues starting from Met1. Vertical arrows show the positions at which deletions were made. Squares represent consensus N-linked glycosylation sites. (B) Effects of shortening the N-terminal region on the interaction between D2R and β-arrestin2. HEK-293 cells were transfected with 0.8 μg of Flag-tagged D2R and 8 μg Flag-D2R-Δ(2–22) in pCMV5 per 100 mm culture dishes. Cells were treated with 10 μM DA for 5 min. Cell lysates were immunoprecipitated with Flag beads, and analyzed by an immunoblot assay with antibodies against β-arrestin2 or Flag. Cell lysates were immunoblotted with antibodies against β-arrestin2. Data represent results from three independent experiments with similar outcomes.
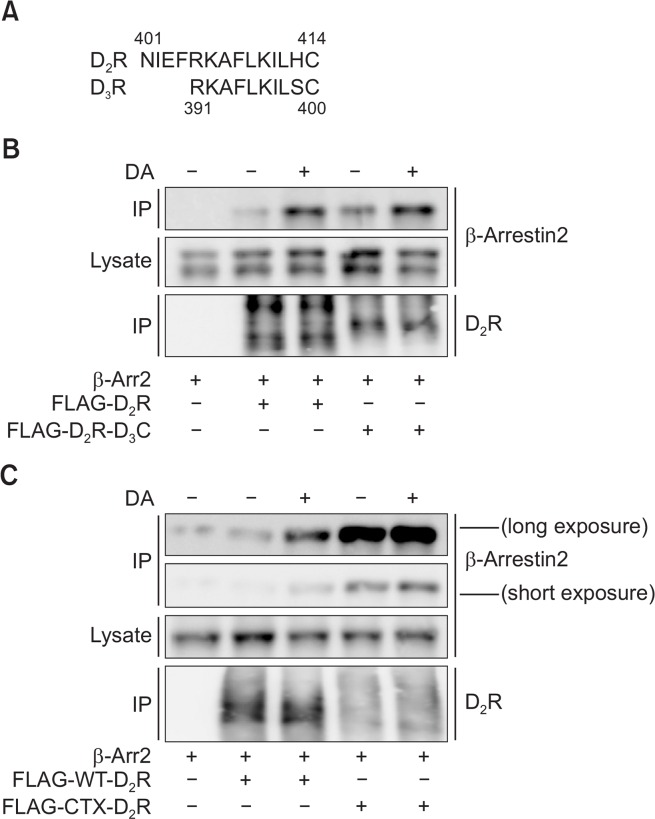
When the carboxyl tails of D2R and D3R are compared, it becomes apparent that D2R contains four extra residues near the seventh transmembrane domain. Other than this difference, the only difference found between the two C-termini was one amino acid residue that precedes the cysteine residue, which is the last amino acid residue of each receptor (Fig. 4A). Replacement of the C-terminus of D2R with that of D3R (D2R-D3C) did not affect the pattern of the interaction with β-arrestin2, that is, the interaction was increased in response to treatment with the agonist (Fig. 4B). Conversely, deletion of the C-terminus of D2R (CTX-D2R) inhibited the receptor expression levels, but also markedly increased the basal interaction with β-arrestin2 (Fig. 4C).
Fig. 4.
Role of the C-terminal tail of D2R in the interaction with β-arrestin2. (A) Alignment of the amino acid sequences within the C-terminal tail of D2R and D3R. The numbers represent the position of the amino acid residues starting from Met1. (B) Effects of replacing the C-terminus of D2R with that of D3R on the interaction with β-arrestin2. HEK-293 cells were transfected with Flag-D2R or Flag-D2R-D3C in pCMV5 together with β-arrestin2 in pCMV5. Cells were treated with 10 μM DA for 5 min. Immunoprecipitates were analyzed by an immunoblot assay with antibodies against β-arrestin2 or Flag. Cell lysates were immunoblotted with antibodies against β-arrestin2. Data represent results from three independent experiments with similar outcomes. (C) Effects of deletion of the C-terminus of D2R on the interaction with β-arrestin2. HEK-293 cells were transfected with Flag-D2R or Flag-CTX-D2R in pCMV5 together with β-arrestin2 in pCMV5. Cells were treated with 10 mM DA for 5 min. Immunoprecipitates were analyzed by an immunoblot assay with antibodies against β-arrestin2 or Flag. Cell lysates were immunoblotted with antibodies against β-arrestin2. Data represent results from three independent experiments with similar outcomes.
DISCUSSION
Like other GPCRs, D2R and D3R contain the characteristic topology of heptahelical receptors: N-terminus, seven trans-membrane (TM) domains, three extracellular and intracellular loops, and C-terminal tail. Although the TM regions are relatively well-conserved among GPCRs, most GPCRs including D2R and D3R, differ in their amino acid sequences and in the lengths of their N-terminal region, intracellular loops, and C-terminal tail (Cho et al., 2010b).
The roles of the intracellular loops and C-terminal tails of some GPCRs have been extensively characterized in terms of G-protein coupling and intracellular trafficking (Ostrowski et al., 1992; Cho et al., 2010b). However, both D2R and D3R have relatively short C-terminal tails, consisting of fourteen and ten amino acid residues, respectively; thus, it was difficult to study functional roles of the C-termini of D2R and D3R. It is unlikely that the C-terminal tail of D2R plays a major role in determining the interaction with β-arrestin2, as the results presented herein show that the replacement of the C-terminus of D2R with that of D3R did not affect the interaction with β-arrestin2.
The roles of the N-terminal regions of rhodopsin family GP-CRs have been difficult to study owing to technical limitations such as challenges in getting three-dimensional information from their crystal structures (Chien et al., 2010). Additionally, it is unclear how the N-terminus of D2R, which is located extracellularly, affects the interaction between D2R and β-arrestin2, which presumably occurs through intracellular regions of D2R. It could be speculated that shortening the N-terminus alters the overall conformation of D2R, such that potential strains that prevent the constitutive interaction between D2R and β-arrestin2 are relieved.
In a previous study, it was reported that the second and third intracellular loops of GPCRs are involved in the translocation of β-arrestins (Kim et al., 2001). In this previous study, a β-arrestin translocation assay was conducted in the presence of exogenous GRK2, which potentiates the translocation of β-arrestins. Since over-expression of GRK2 potentiates the translocation of β-arrestins, there is possibility that roles of each loop in recruiting cytosolic β-arrestin2 toward plasma membrane could be over-estimated. In the current study, experiments were conducted under endogenous conditions, and we could determine the roles of each loop in agonist-induced β-arrestin2 translocation more precisely.
In conclusion, we showed that both the N-terminus and the C-terminal tail exert negative effects on the interaction between D2R and β-arrestin2. Though further studies are needed to understand completely the molecular details involved this process, the results presented herein may provide clues for elucidating the functional roles of the N- and C-termini of D2R in the interaction with β-arrestin2.
Acknowledgments
This work was funded by KRF-2014R1A2A2A01002547. We thank the Korea Basic Science Institute for technical support.
REFERENCES
- Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc Natl Acad Sci USA. 2003;100:1740–1744. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- Barak LS, Ferguson SS, Zhang J, Caron MG. A beta-arrestin/green fluorescent protein biosensor for detecting G protein-coupled receptor activation. J Biol Chem. 1997;272:27497–27500. doi: 10.1074/jbc.272.44.27497. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Pin JP. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien EY, Liu W, Zhao Q, Katritch V, Han GW, Hanson MA, Shi L, Newman AH, Javitch JA, Cherezov V, Stevens RC. Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science. 2010;330:1091–1095. doi: 10.1126/science.1197410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D, Zheng M, Min C, Ma L, Kurose H, Park JH, Kim KM. Agonist-induced endocytosis and receptor phosphorylation mediate resensitization of dopamine D(2) receptors. Mol Endocrinol. 2010a;24:574–586. doi: 10.1210/me.2009-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DI, Zheng M, Kim KM. Current perspectives on the selective regulation of dopamine D2 and D3 receptors. Arch Pharm Res. 2010b;33:1521–1538. doi: 10.1007/s12272-010-1005-8. [DOI] [PubMed] [Google Scholar]
- Cho EY, Cho DI, Park JH, Kurose H, Caron MG, Kim KM. Roles of protein kinase C and actin-binding protein 280 in the regulation of intracellular trafficking of dopamine D3 receptor. Mol Endocrinol. 2007;21:2242–2254. doi: 10.1210/me.2007-0202. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Downey WE, 3rd, Colapietro AM, Barak LS, Ménard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Kim KM, Valenzano KJ, Robinson SR, Yao WD, Barak LS, Caron MG. Differential Regulation of the Dopamine D2 and D3 Receptors by G Protein-coupled Receptor Kinases and beta -Arrestins. J Biol Chem. 2001;276:37409–37414. doi: 10.1074/jbc.M106728200. [DOI] [PubMed] [Google Scholar]
- Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- Lohse MJ, Andexinger S, Pitcher J, Trukawinski S, Codina J, Faure JP, Caron MG, Lefkowitz RJ. Receptor-specific desensitization with purified proteins. Kinase dependence and receptor specificity of beta-arrestin and arrestin in the beta 2-adrenergic receptor and rhodopsin systems. J Biol Chem. 1992;267:8558–8564. [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- Ostrowski J, Kjelsberg MA, Caron MG, Lefkowitz RJ. Mutagenesis of the beta 2-adrenergic receptor: how structure elucidates function. Annu Rev Pharmacol Toxicol. 1992;32:167–183. doi: 10.1146/annurev.pa.32.040192.001123. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PJ, Kim C, Benovic JL, Kwatra MM, Caron MG, Lefkowitz RJ. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- Robinson SW, Caron MG. Chimeric D2/D3 dopamine receptors efficiently inhibit adenylyl cyclase in HEK 293 cells. J Neurochem. 1996;67:212–219. doi: 10.1046/j.1471-4159.1996.67010212.x. [DOI] [PubMed] [Google Scholar]
- Robinson SW, Caron MG. Selective inhibition of adenylyl cyclase type V by the dopamine D3 receptor. Mol Pharmacol. 1997;52:508–514. doi: 10.1124/mol.52.3.508. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Cheong SY, Min C, Jin M, Cho DI, Kim KM. β-arrestin2 plays permissive roles in the inhibitory activities of RGS9-2 on G protein-coupled receptors by maintaining RGS9-2 in the open conformation. Mol Cell Biol. 2011;31:4887–4901. doi: 10.1128/MCB.05690-11. [DOI] [PMC free article] [PubMed] [Google Scholar]