Abstract
Severe complications associated with EV71 infections are a common cause of neonatal death. Lack of effective therapeutic agents for these infections underlines the importance of research for the development of new antiviral compounds. In the present study, the anti-EV71 activity of norwogonin, oroxylin A, and mosloflavone from Scutellaria baicalensis Georgi was evaluated using a cytopathic effect (CPE) reduction method, which demonstrated that all three compounds possessed strong anti-EV71 activity and decreased the formation of visible CPEs. Norwogonin, oroxylin A, and mosloflavone also inhibited virus replication during the initial stage of virus infection, and they inhibited viral VP2 protein expression, thereby inhibiting viral capsid protein synthesis. However, ribavirin has a relatively weaker efficacy compared to the other drugs. Therefore, these findings provide important information that will aid in the utilization of norwogonin, oroxylin A, and mosloflavone for EV71 treatment.
Keywords: Enterovirus 71, Antiviral activity, Norwogonin, Oroxylin A, Mosloflavone
INTRODUCTION
Enterovirus 71 (EV71) is the causative agent of hand, foot, and mouth disease (HFMD) and herpangina and can also cause severe neurological diseases such as brainstem encephalitis and poliomyelitis-like paralysis (Wang et al., 2003; Chang et al., 2007). EV71-infected children can develop severe neurological complications, which can lead to rapid clinical deterioration and even death (Lum et al., 1998; Wong et al., 2000; Mizuta et al., 2005; Chang et al., 2007). According to data from the national surveillance system of HFMD in mainland China in 2008–2012, the fatality rate of HFMD was 0.03%, and the case-severity rate was 1.1% (Xing et al., 2014).
EV71 is a positive-stranded RNA virus that belongs to the enterovirus genus of the Picornaviridae family (McMinn, 2002). The genes encoding the capsid proteins (VP proteins: VP1, VP2, VP3, and VP4) of EV71 contain various important neutralization epitopes and have been extensively used for molecular typing (Oberste et al., 1999). Monitoring the genetic variations of the circulating EV71 strains and the emergence of new types or recombinants of EV71 in the epidemic regions are important for vaccine development and drug discovery.
In the current study, we investigated the possibility of using norwogonin, oroxylin A, and mosloflavone from Scutellaria baicalensis Georgi, which possesses antiviral activity against EV71. Furthermore, to elucidate the action of norwogonin, oroxylin A, and mosloflavone on EV71 multiplication, we investigated the effects of the three compounds on the infection cycle of EV71 through a time-of-addition study and western blot analysis. Consequently, we found that norwogonin, oroxylin A, and mosloflavone inhibited EV71 replication in Vero cells and prevented the cytotoxicity induced by EV71 infection.
MATERIALS AND METHODS
Virus, cells, and reagents
The EV71 virus was purchased from the Division of Vaccine Research of the Korea Center Disease Control and Prevention (KCDC, Cheongwon, Korea) and then propagated at 37°C in Vero cells (ATCC, Manassas, VA, USA), which is a kidney epithelial cell line that originated from an African green monkey. Vero cells were maintained in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) and 0.01% antibiotic-antimycotic. Antibiotic-antimycotic, trypsin-EDTA, FBS, and MEM were supplied by Gibco BRL (Grand Island, NY, USA). Tissue culture plates were purchased from Falcon (BD Biosciences, Franklin Lakes, NJ, USA). Ribavirin and sulforhodamine B were purchased from Sigma-Aldrich (St Louis, MO, USA).
Isolation of active compounds from the aerial parts of S. baicalensis Georgi
The dried aerial parts of S. baicalensis Georgi (1.2 kg) were cut into pieces and extracted with methanol (3×2 L) in an ultrasonic apparatus at room temperature after evaporation of the solvent. The methanol extract was suspended in H2O and successively partitioned into chloroform, ethyl acetate, n-butanol, and water fractions after removal of the solvents under vacuum. These fractions were then subjected to a sulforhodamine B (SRB)-based antiviral activity assay, and the chloroform fraction was found to have antiviral activity against EV71. Next, the chloroform fraction was subjected to C18 column silica gel column adsorption chromatography (40–63 μm, 300 g) (Merck and Co., Kenilworth, NJ, USA) and eluted with a gradient consisting of methanol:water (3:7, 4:6, 6:4, 8:2, and 10:0; 2×500 mL). The fraction was separated on a Sephadex LH-20 column (Sigma-Aldrich, St. Louis, MO, USA) using 100% methanol, and norwogonin, oroxylin A, and mosloflavone were obtained. The chemical structures of norwogonin, oroxylin A, and mosloflavone were identified using electrospray ionization mass spectrometry, 1H-nuclear magnetic resonance (NMR), and 13C-NMR (Kim et al., 2014).
Assays of antiviral activity
Assays of antiviral activity were performed with the SRB method that assesses cytopathic effect (CPE) reduction, which was previously reported by Choi et al. (2009a). The effect of norwogonin, oroxylin A, and mosloflavone on EV71-induced CPE was observed. Briefly, Vero cells were seeded onto a 96-well culture plate at a concentration of 2×104 cells per well. Next day, the medium was removed and washed with PBS, and 0.09 mL of diluted virus suspension of EV71 containing TCID50 (50% tissue culture infective dose) of the virus stock to produce an appropriate cytopathic effects within 2 days after infection and 0.01 mL of norwogonin, oroxylin A, mosloflavone, and ribavirin (0.4, 2, 10, and 50 μg/mL, respectively) were added. After incubation at 37°C in 5% CO2 for 2 days, the morphology of the cells was observed under a microscope at 32×10 magnifications (AXIOVERT10, ZEISS, Oberkochen, Germany), and the images were recorded.
Time-of-addition assays
The time-of-addition effects of norwogonin, oroxylin A, and mosloflavone were examined in accordance with previously described procedures (Chiang et al., 2003) with minor modifications. Vero cells were seeded onto 96-well culture plates at a density of 2×104 cells per well and incubated for 1 day. After washing with phosphate-buffered saline (PBS), 50 μg/mL of norwogonin, oroxylin A, and mosloflavone were then added to the cells either before (−1 h), during (0 h), or after (1, 2, 4, 6, and 8 h) EV71 infection. After 2 days, the antiviral activity was tested using the SRB assay, and ribavirin was used as the positive control.
Real-time PCR analysis
Vero cells were seeded onto a 96-well culture plate at a concentration of 2×104 cells per well. The next day, medium was removed and the cells were washed with 1×PBS. Then, 90 μL of diluted virus suspension and 10 μL of medium supplemented with 1% FBS containing norwogonin, oroxylin A, and mosloflavone of 50 μg/mL were added. After incubation at 37°C in 5% CO2 for 48 h, the next step was performed. Total RNA was extracted from Vero cells using a QIAamp® viral RNA mini kit (Qiagen, Limburg, Holland, Germany). Reverse transcription was performed using SuperScriptTM II reverse transcriptase (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s instructions. For real-time PCR analysis, the cDNA was serially diluted 10-fold and amplified using a 7500 real time PCR system (Applied Biosystems, Foster City, CA, USA) with Power SYBR® Green PCR master mix (Applied Biosystems). We used the following primers: β-actin (sense 5′-CCA TCA TGA AGT GTG ACG TGG-3′, antisense 5′-GTC CGC CTA GAA GCA TTT GCG-3′) and EV-NCR (sense 5′-CCG GCC CCT GAA TGC GG-3′, antisense 5′-ATT CTT TAA TTG TCA CCA TAA GCA GCC A-3′).
Western blot analysis
Western blot analysis was evaluated using a previously reported method (Song et al., 2014). Vero cells were plated onto 6-well culture plates at a density of 5×105 cells/well 24 h before infection with EV71. EV71-infected cells were treated with norwogonin, oroxylin A, mosloflavone, and ribavirin at a concentration of 50 mg/mL for 48 h for detection of viral VP2 protein. Mock-infected cells treated with 0.1% DMSO and EV71-infected cells treated with 0.1% DMSO were used as controls. The cells were lysed in ice-cold RIPA lysis buffer containing 50 mM Tris-HCl pH7.4, 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 1% SDS, 5 mg/mL aprotinin, 5 mg/mL leupeptin, 1 mM PMSF, 5 mM sodium fluoride, and 5 mM sodium orthovanadate. The preparation of sample protein (30 mg) was boiled for 10 min at 100°C and separated in 12% acrylamide gels run at 100 V for 1 h (for detection of VP2). The SeeBlue®Plus2 prestained protein ladder (Invitrogen, Carlsbad, CA) was used as a molecular weight standard. The gels were transferred to a nitrocellulose membrane using the Invitrogen iBlot®Gel Transfer Device (Invitrogen) at 20 V for 7 min.
For detection of VP2, the membranes were blocked with 5% skim milk (Difco) dissolved in phosphate-buffered saline-Tween 20 (PBST) overnight at 4°C on a shaker. The blots were washed three times with PBST before being incubated with primary mouse anti-EV71 monoclonal antibody (Millipore, Darmstadt, Germany) dissolved in 5% skim milk at a dilution of 1:1,000. For the loading control, separate blots containing the same samples were incubated with primary α-tubulin mouse monoclonal IgG1 (Santacruz Biotechnology, Texas, USA) dissolved in 5% skim milk at a dilution of 1:1,000. The blots were incubated with primary antibodies at room temperature on a shaker. The blots were then washed three times with PBST (10 min each time). This was followed by incubation with secondary polyclonal goat anti-mouse IgG (H+L)-HRP (DenDE-POT, Texas, USA) for 1 h at room temperature on shaker. Dilution of secondary antibody was done in 5% skim milk at a ratio of 1:5,000. Membranes were then rinsed three times with PBST (10 min each time). Membranes were developed by the enhanced chemiluminescence (ECL) method using West-Q chemiluminescent substrate (GenDEPOT).
Statistical analysis
To compare multiple groups, we performed one-way ANOVA followed by the Tukey post hoc test using GraphPad Prism version 5 software (Graphpad, San Diego, CA, USA). Values of p<0.05 were considered significant at a 95% confidence interval.
RESULTS
Antiviral activity of norwogonin, oroxylin A, and mosloflavone against EV71
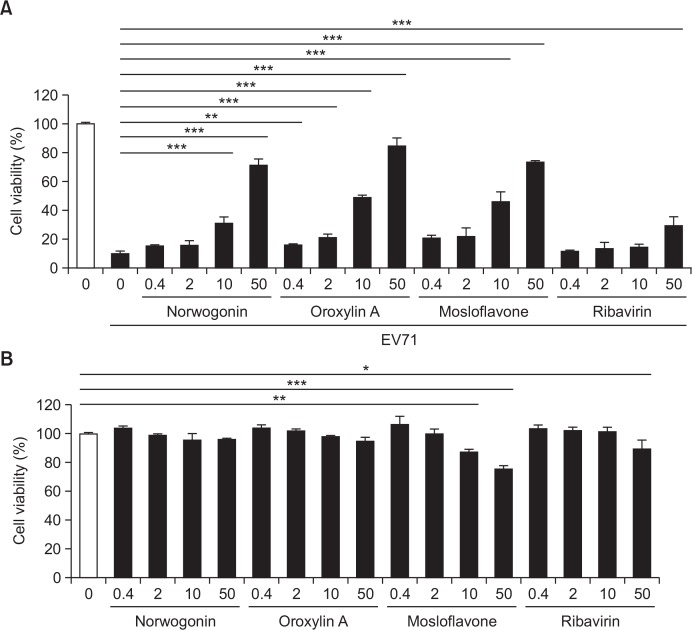
Norwogonin, oroxylin A, and mosloflavone were investigated for their antiviral activity against EV71. The antiviral activity was evaluated by measuring the enhanced cell viability of EV71-infected Vero cells by treatment with norwogonin, oroxylin A, and mosloflavone. Norwogonin, oroxylin A, and mosloflavone exhibited strong antiviral activity against EV71 at a concentration of 50 μg/mL, and they showed antiviral activity against EV71 in a dose-dependent manner (Fig. 1). Also, EC50 values of norwogonin, oroxylin A, and mosloflavone revealed 31.83, 14.91, 37.72 μg/ml (Table 1). However, ribavirin demonstrated weak antiviral activity (30%) against EV71 at a concentration of 50 μg/mL (Fig. 1).
Fig. 1.
Antiviral activity of norwogonin, oroxylin A, mosloflavone, and ribavirin against Enterovirus 71 (EV71). (A) Antiviral activity and (B) cytotoxicity of norwogonin, oroxylin A, mosloflavone and ribavirin were measured in Vero cells. Vero cells were infected with EV71 and then treated with the indicated concentrations (0.4, 2, 10, and 50 μg/mL) of norwogonin, oroxylin A, mosloflavone for 48 h. The antiviral activity was investigated using a CPE reduction assay. Data are presented as mean ± SD. from three independent experiments, each carried out in triplicate. *p<0.01; **p<0.001; ***p<0.0001. one-way analysis of variance (ANOVA) with Tukey’s post hoc test.
Table 1.
The antiviral activity of norwogonin, oroxylin A, mosloflavone and ribavirin against EV71
| Enterovirus 71 | |||
|---|---|---|---|
|
| |||
| CC50a | IC50b | TIc | |
| Norwogonin | > 50 | 31.83 ± 6.21 | > 1.57 |
| Oroxylin A | > 50 | 14.19 ± 4.22 | > 3.52 |
| Mosloflavone | > 50 | 37.72 ± 0.78 | > 1.33 |
| Ribavirin | > 50 | NDd | - |
Results are presented as the mean IC50 values ± standard deviation (SD) obtained from 3 independent experiments each carried out in triplicate.
Concentration required to reduce cell growth by 50% (μg/mL).
Concentration required to inhibit virus-induced CPE by 50% (μg/mL).
Therapeutic index = CC50/IC50.
Not determined.
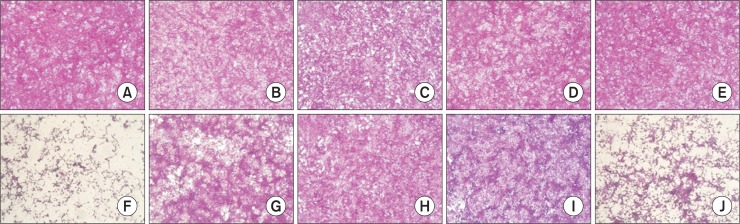
The effect of norwogonin, oroxylin A, and mosloflavone on EV71-induced CPE
Without EV71 infection, mock cells (Fig. 2A) or cells treated with 50 μg/mL norwogonin, oroxylin A, and mosloflavone, respectively (Fig. 2B–2D), or ribavirin (Fig. 2E) showed typical spread-out shapes and normal morphology. At this concentration, no signs of cytotoxicity of norwogonin, oroxylin A, and mosloflavone were observed. Infection with EV71 in the absence of norwogonin, oroxylin A, and mosloflavone resulted in a severe CPE (Fig. 2F). Addition of norwogonin, oroxylin A, and mosloflavone to infected Vero cells inhibited the formation of a visible CPE (Fig. 2G–2I). However, the addition of ribavirin in EV71-infected Vero cells weakly prevented the CPE (Fig. 2J). Thus, the CPE of the viral infection is prevented by the presence of norwogonin, oroxylin A, and mosloflavone.
Fig. 2.
The effect of norwogonin, oroxylin A, mosloflavone, and ribavirin on EV71-induced cytopathic effect. The virus-infected cells were treated with 50 μg/mL norwogonin, oroxylin A, mosloflavone, and ribavirin. After incubation at 37°C in 5% CO2 for 48 h, the cell viability was evaluated using the sulforhodamine B (SRB) assay, and the morphology of cells was determined using a microscope. (A) Non-infected cells; (B) non-infected cells treated with norwogonin; (C) non-infected cells treated with oroxylin A; (D) non-infected cells treated with mosloflavone; (E) non-infected cells treated with ribavirin; (F) EV71-infected cells; (G) EV71-infected cells treated with norwogonin; (H) EV71-infected cells treated with oroxylin A; (I) EV71-infected cells treated with mosloflavone; (J) EV71-infected cells treated with ribavirin.
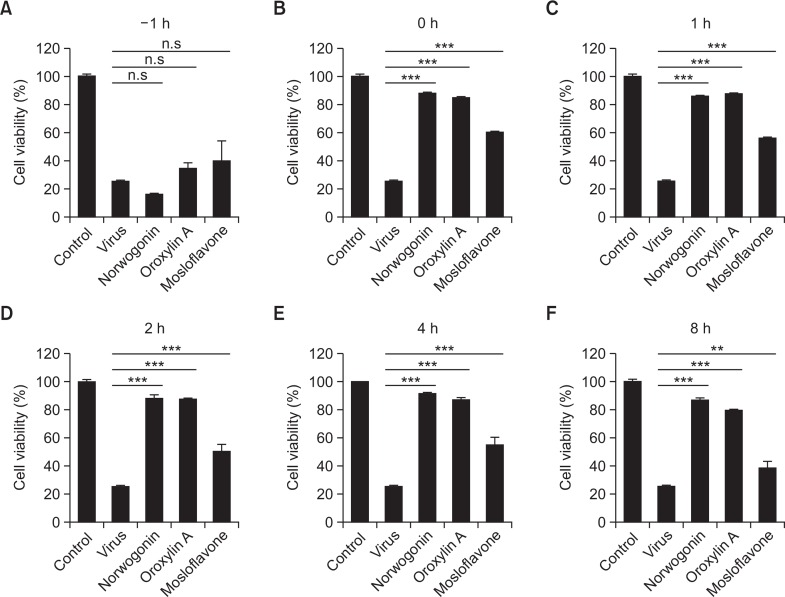
Time-course of compound addition
Norwogonin, oroxylin A, and mosloflavone were added at different time points (before, during, and after) of EV71 infection. The results showed that norwogonin, oroxylin A, and mosloflavone suppressed EV71 infection when added just after virus inoculation (0 h) and during the early stages after virus inoculation (1, 2, and 4 h), and the effect decreased when they were added just after virus inoculation (8 h). The inhibitory levels of norwogonin, oroxylin A, and mosloflavone were higher than 80% (Fig. 3). However, the inhibitory levels of the four compounds declined to ≤20% when added prior (−1 h) to infection. This result indicated that norwogonin, oroxylin A, and mosloflavone affects the initial stage of EV71 infection, but does not block viral attachment and entry.
Fig. 3.
Time-of-addition effect of norwogonin, oroxylin A, and mosloflavone on EV71 replication in Vero cells. Each compound (50 μg/mL) was added either before (−1 h), during (0 h), or after (1, 2, 4, 6, and 8 h) virus infection. After 2 days, the inhibition was evaluated by SRB method and expressed as the inhibition rate. Each value is the result of mean ± SD. of three independent experiments. **p<0.001; ***p<0.0001 using one-way ANOVA with Tukey’s post hoc test.
Norwogonin, oroxylin A, and mosloflavone inhibit viral replication
To further confirm the inhibitory effects of norwogonin, oroxylin A, and mosloflavone on viral replication of EV71 in Vero cells, we performed real-time PCR analysis for EV71 NCR gene. The RNA extraction was performed at 48 h after EV71 infection. Norwogonin and oroxylin A (50 μg/mL) strongly decreased the expression of EV71 NCR gene at 48 h after infection, but mosloflavone (50 μg/mL) showed marginal effect on expression of the EV71 NCR gene (Fig. 4A).
Fig. 4.
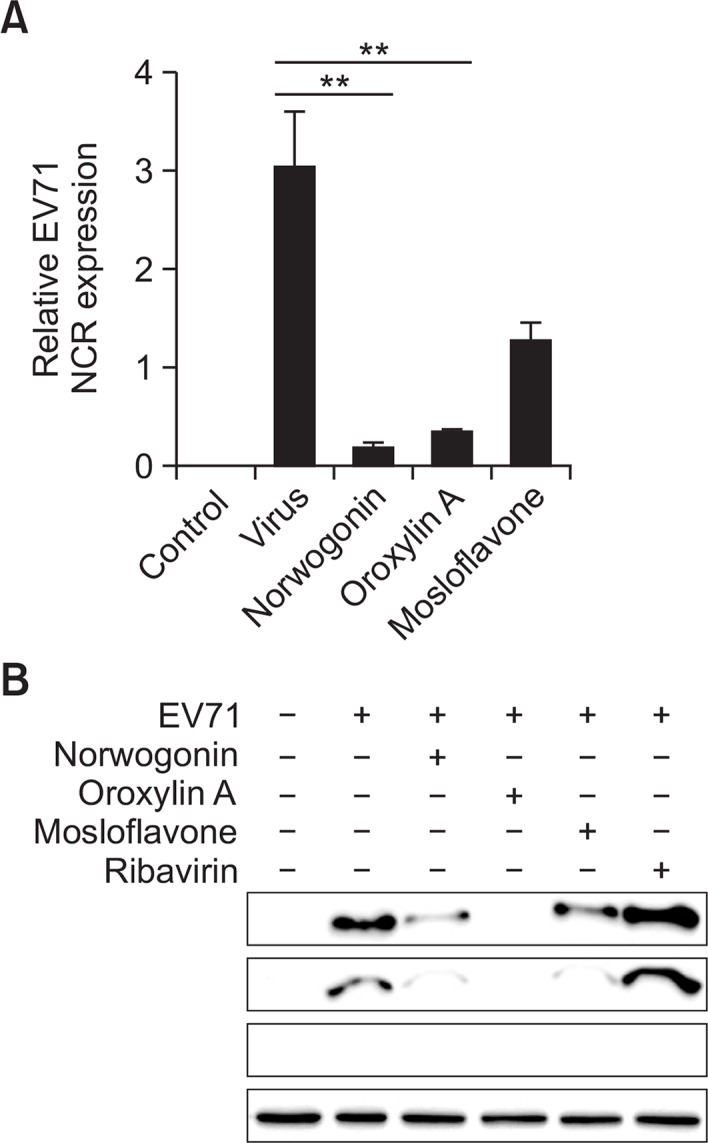
The effect of norwogonin, oroxylin and mosloflavone on EV71 replication. (A) Real-time PCR analyses were performed to determine the effect of norwogonin, oroxylin A and mosloflavone on EV71 NCR gene expression levels. Replication of EV71 from Vero cells at 48 h after infection by EV71 in the presence of 50 μg/mL norwogonin, oroxylin A and mosloflavone was determined by real-time PCR. Vehicle (0.1% DMSO)-treated cells without EV71 infection was used as control. (B) Western blot analyses were performed to determine the effect of norwogonin, oroxylin A, mosloflavone, and ribavirin on the production of EV71 VP2 proteins. The reduction in protein expression of EV71 VP2 was identified after treatment with 50 μg/mL norwogonin, oroxylin A, mosloflavone, and ribavirin for 48 h. α-tubulin was used as a loading control for each set of samples. **p<0.001 using one-way ANOVA with Tukey’s post hoc test.
Effect of norwogonin, oroxylin A, and mosloflavone on viral VP2 protein synthesis
Viral VP2 proteins synthesis was compared between drug-treated and untreated infected cells. As shown in Fig. 4, when cells were infected with virus and cultured in the absence of drugs until western blot analysis, the viral VP2 protein could be detected in the untreated cells. The size of the EV71 VP2 protein was determined to be 34 kDa, and α-tubulin was used as the loading control in the experiment as well as to ensure that norwogonin, oroxylin A, and mosloflavone used in this study did not affect the synthesis and expression of host cellular proteins. The western blot analysis also showed that viral VP2 protein expression was decreased dramatically by norwogonin, oroxylin A, and mosloflavone (50 μg/mL) at 48 h after infection with EV71 (Fig. 4B). Out of the compounds tested, oroxylin A significantly decreased viral VP2 protein expression at same time. However, ribavirin used as the positive control did not show cytotoxicity as well as antiviral activity in vitro and during western blot analysis.
DISCUSSION
In 1969, EV71 was first isolated in California, United States and was first isolated as the cause of epidemics of hand-foot-and-mouth disease (HFMD) in Japan in 1973 (Schmidt et al., 1974; Hagiwara et al., 1978). Since 2008, EV71 outbreak associated with HFMD has been an area of concern in mainland China (Tan et al., 2011; Xing et al., 2014). In addition, in the past ten years, EV71 was isolated in numerous European and American countries, such as France (Schuffenecker et al., 2011; Kassab et al., 2013), Denmark (Badran et al., 2011), Spain (Cabrerizo et al., 2014), Portugal (Venancio et al., 2013), Brazil (Lamarao et al., 2003), Canada (Merovitz et al., 2000), and the United States. Most commonly, EV71 causes HFMD in children, which is considered to be a mild syndrome (Kuo and Shih, 2013). However, some young children infected by the virus have developed severe neurological syndromes, such as aseptic meningitis, encephalitis, poliomyelitis-like paralysis, and even death (Chang et al., 1999).
During the last few years, efforts have been made to discover or develop substances with antiviral activity and the substances discovered belong to classes of nucleoside analogues, such as acyclovir and ribavirin (De Clercq, 2004). However, the therapeutic potency of most antiviral agents developed so far is counteracted by their severe side effects in humans (Choi et al., 2009b). Hence, new approaches for the control of EV71 infections must be explored.
In the current study, the EV71 activity of norwogonin, oroxylin A, and mosloflavone were evaluated in vitro. Norwogonin, oroxylin A, and mosloflavone were shown to exhibit anti-EV71 activity by reducing the formation of a visible CPE in the CPE reduction assay against EV71. Similarly, our recent study showed antiviral activity of hederasaponin B obtained from Hedera helix against EV71 (Song et al., 2014).
The effect of norwogonin, oroxylin A, and mosloflavone on each stage of the EV71 infection cycle and the treatment period was assessed to observe the antiviral activity. From the results, pre-incubation of Vero cells with norwogonin, oroxylin A, and mosloflavone did not protect the cells from EV71 infection (Fig. 3). Furthermore, norwogonin, oroxylin A, and mosloflavone only inhibited EV71 virus infection when added at 1, 2, and 4 h after virus inoculation (Fig. 3). These results are similar to the effects of quercetin 7-rhamnoside on porcine epidemic diarrhea virus (Choi et al., 2009a). Collectively, we found that norwogonin, oroxylin A, and mosloflavone are early-stage inhibitor for EV71 replication or translation, but did not inhibit the entry step of EV71 on target cells.
In addition, the inhibitory effects of norwogonin, oroxylin A, mosloflavone, and ribavirin against EV71 were analyzed by western blot assay. The expression of EV71 VP proteins was inhibited in the presence of 50 μg/mL norwogonin, oroxylin A, and mosloflavone (Fig. 4). However, ribavirin did not show any inhibitory effects against EV71 infection, which is consistent with previous reports by Song et al. (Song et al., 2014).
Collectively, these results suggest that norwogonin, oroxylin A, and mosloflavone possessed antiviral activity against EV71 by inhibition of viral protein expression, and thus, could be considered as antiviral drug candidates for the treatment of EV71.
Further studies will be required to explore the detailed antiviral mechanism of action of norwogonin, oroxylin A, and mosloflavone known as flavonoid. We will carry out research focusing on suppression of enterovirus replication by norwogonin, oroxylin A, and mosloflavone because baicalin, a flavonoid derived from Scutellaria baicalensis, has been demonstrated that it is inhibited viral nucleotide synthesis against dengue virus (Moghaddam et al., 2014).
Acknowledgments
This work was supported by a grant from the R&D project (2015-NG48001-00) at the Korea National Institute of Health. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2014R1A2A2A01002576, NRF-2014R1A1A2003820).
Footnotes
CONFLICT OF INTEREST
The authors have declared that they have no conflicts of interest.
REFERENCES
- Badran SA, Midgley S, Andersen P, Bottiger B. Clinical and virological features of enterovirus 71 infections in Denmark, 2005 to 2008. Scand J Infect Dis. 2011;43:642–648. doi: 10.3109/00365548.2011.577094. [DOI] [PubMed] [Google Scholar]
- Cabrerizo M, Tarrago D, Munoz-Almagro C, Del Amo E, Dominguez-Gil M, Eiros JM, Lopez-Miragaya I, Perez C, Reina J, Otero A, Gonzalez I, Echevarria JE, Trallero G. Molecular epidemiology of enterovirus 71, coxsackievirus A16 and A6 associated with hand, foot and mouth disease in Spain. Clin Microbiol Infect. 2014;20:O150–O156. doi: 10.1111/1469-0691.12361. [DOI] [PubMed] [Google Scholar]
- Chang LY, Lee CY, Kao CL, Fang TY, Lu CY, Lee PI, Huang LM. Hand, foot and mouth disease complicated with central nervous system involvement in Taiwan in 1980–1981. J Formos Med Assoc. 2007;106:173–176. doi: 10.1016/S0929-6646(09)60236-9. [DOI] [PubMed] [Google Scholar]
- Chang LY, Lin TY, Hsu KH, Huang YC, Lin KL, Hsueh C, Shih SR, Ning HC, Hwang MS, Wang HS, Lee CY. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet. 1999;354:1682–1686. doi: 10.1016/S0140-6736(99)04434-7. [DOI] [PubMed] [Google Scholar]
- Chiang LC, Chang JS, Chen CC, Ng LT, Lin CC. Anti-Herpes simplex virus activity of Bidens pilosa and Houttuynia cordata. Am J Chin Med. 2003;31:355–362. doi: 10.1142/S0192415X03001090. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Kim JH, Lee CH, Ahn YJ, Song JH, Baek SH, Kwon DH. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res. 2009a;81:77–81. doi: 10.1016/j.antiviral.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Lim CH, Song JH, Baek SH, Kwon DH. Antiviral activity of raoulic acid from Raoulia australis against Picornaviruses. Phytomedicine. 2009b;16:35–39. doi: 10.1016/j.phymed.2008.10.012. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Antiviral drugs in current clinical use. J Clin Virol. 2004;30:115–133. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Hagiwara A, Tagaya I, Yoneyama T. Epidemic of hand, foot and mouth disease associated with enterovirus 71 infection. Intervirology. 1978;9:60–63. doi: 10.1159/000148922. [DOI] [PubMed] [Google Scholar]
- Kassab S, Saghi T, Boyer A, Lafon ME, Gruson D, Lina B, Fleury H, Schuffenecker I. Fatal case of enterovirus 71 infection and rituximab therapy, france, 2012. Emerging Infect Dis. 2013;19:1345–1347. doi: 10.3201/eid1908.130202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Kim JG, Yang C, Ki H, Jo J, Ihee H. Pump-Probe X-ray Solution Scattering Reveals Accelerated Folding of Cytochrome Upon Suppression of Misligation. Bull Korean Chem Soc. 2014;35:695–696. doi: 10.5012/bkcs.2014.35.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo RL, Shih SR. Strategies to develop antivirals against enterovirus 71. Virol J. 2013;10:28. doi: 10.1186/1743-422X-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarao LM, Maciel AM, Gomes Mde L. First isolation of enterovirus 71 (EV-71) from Northern Brazil. Braz J Infect Dis. 2003;7:278–281. doi: 10.1590/S1413-86702003000400009. [DOI] [PubMed] [Google Scholar]
- Lum LC, Wong KT, Lam SK, Chua KB, Goh AY, Lim WL, Ong BB, Paul G, AbuBakar S, Lambert M. Fatal enterovirus 71 encephalomyelitis. J Pediatr. 1998;133:795–798. doi: 10.1016/S0022-3476(98)70155-6. [DOI] [PubMed] [Google Scholar]
- McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev. 2002;26:91–107. doi: 10.1111/j.1574-6976.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Merovitz L, Demers AM, Newby D, McDonald J. Enterovirus 71 infections at a Canadian center. Pediatr Infect Dis J. 2000;19:755–757. doi: 10.1097/00006454-200008000-00017. [DOI] [PubMed] [Google Scholar]
- Mizuta K, Abiko C, Murata T, Matsuzaki Y, Itagaki T, Sanjoh K, Sakamoto M, Hongo S, Murayama S, Hayasaka K. Frequent importation of enterovirus 71 from surrounding countries into the local community of Yamagata, Japan, between 1998 and 2003. J Clin Microbiol. 2005;43:6171–6175. doi: 10.1128/JCM.43.12.6171-6175.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam E, Teoh BT, Sam SS, Lani R, Hassandarvish P, Chik Z, Yueh A, Abubakar S, Zandi K. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci Rep. 2014;4:5452. doi: 10.1038/srep05452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste MS, Maher K, Kilpatrick DR, Flemister MR, Brown BA, Pallansch MA. Typing of human enteroviruses by partial sequencing of VP1. J Clin Microbiol. 1999;37:1288–1293. doi: 10.1128/jcm.37.5.1288-1293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt NJ, Lennette EH, Ho HH. An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis. 1974;129:304–309. doi: 10.1093/infdis/129.3.304. [DOI] [PubMed] [Google Scholar]
- Schuffenecker I, Mirand A, Antona D, Henquell C, Chomel JJ, Archimbaud C, Billaud G, Peigue-Lafeuille H, Lina B, Bailly JL. Epidemiology of human enterovirus 71 infections in France, 2000–2009. J Clin Virol. 2011;50:50–56. doi: 10.1016/j.jcv.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Song J, Yeo SG, Hong EH, Lee BR, Kim JW, Kim J, Jeong H, Kwon Y, Kim H, Lee S, Park JH, Ko HJ. Antiviral Activity of Hederasaponin B from Hedera helix against Enterovirus 71 Subgenotypes C3 and C4a. Biomol. Ther. (Seoul) 2014;22:41–46. doi: 10.4062/biomolther.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Huang X, Zhu S, Chen H, Yu Q, Wang H, Huo X, Zhou J, Wu Y, Yan D, Zhang Y, Wang D, Cui A, An H, Xu W. The persistent circulation of enterovirus 71 in People’s Republic of China: causing emerging nationwide epidemics since 2008. PLoS ONE. 2011;6:e25662. doi: 10.1371/journal.pone.0025662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venancio P, Oliveira M, Silva R, Conceicao C, Brito MJ. First case of severe enterovirus 71 infection in Portugal. Pediatr Infect Dis J. 2013;32:581–582. doi: 10.1097/INF.0b013e31828689ab. [DOI] [PubMed] [Google Scholar]
- Wang SM, Lei HY, Huang KJ, Wu JM, Wang JR, Yu CK, Su IJ, Liu CC. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: roles of cytokines and cellular immune activation in patients with pulmonary edema. J Infect Dis. 2003;188:564–570. doi: 10.1086/376998. [DOI] [PubMed] [Google Scholar]
- Wong KT, Lum LC, Lam SK. Enterovirus 71 infection and neurologic complications. N Engl J Med. 2000;342:356–358. doi: 10.1056/NEJM200002033420514. [DOI] [PubMed] [Google Scholar]
- Xing W, Liao Q, Viboud C, Zhang J, Sun J, Wu JT, Chang Z, Liu F, Fang VJ, Zheng Y, Cowling BJ, Varma JK, Farrar JJ, Leung GM, Yu H. Hand, foot, and mouth disease in China, 2008–12: an epidemiological study. Lancet Infect Dis. 2014;14:308–318. doi: 10.1016/S1473-3099(13)70342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]