Abstract
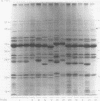
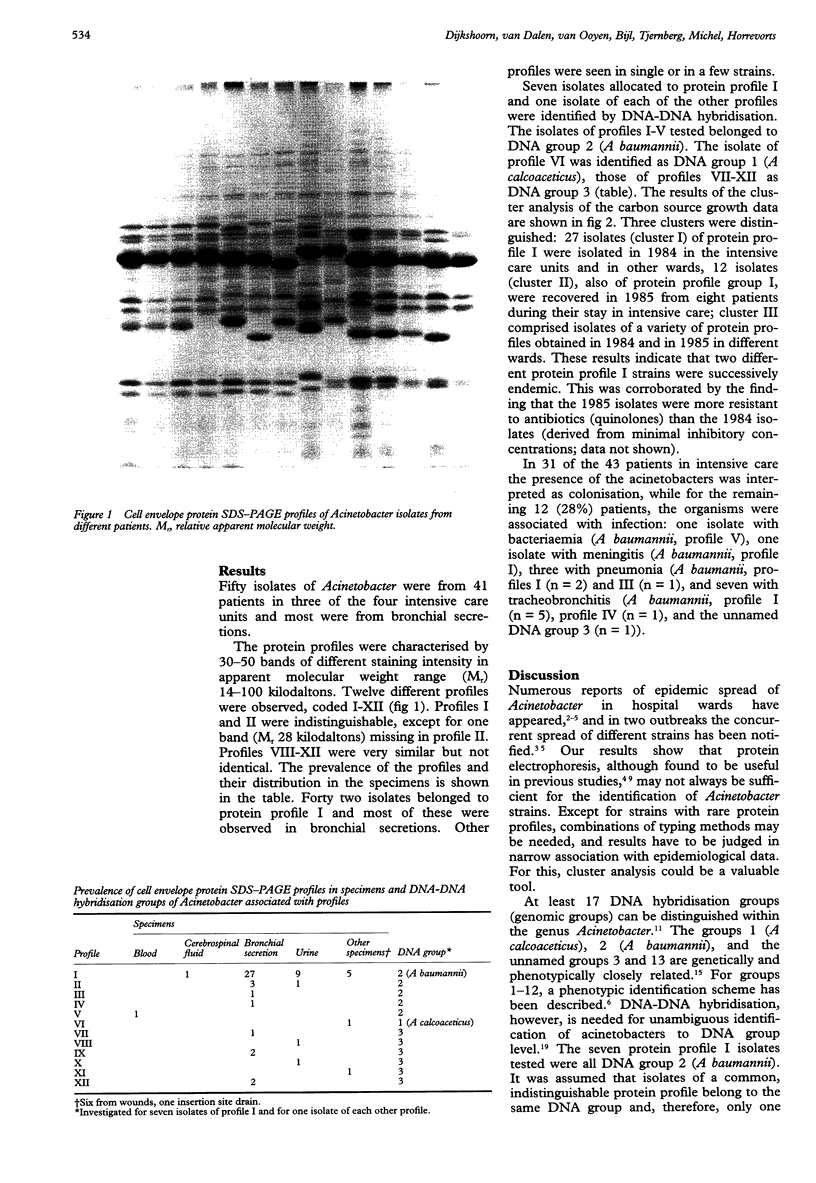
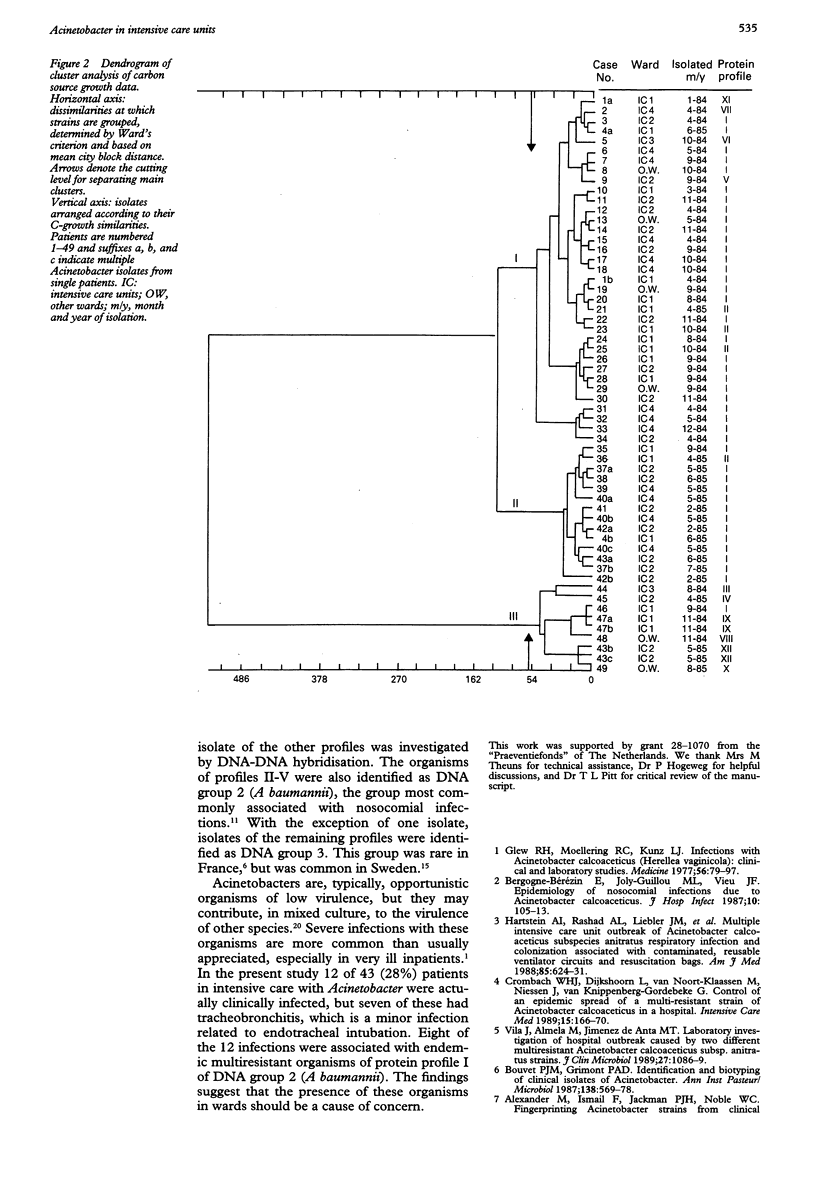
AIMS--To assess whether Acinetobacter isolates obtained over 20 months in a tertiary care hospital were epidemiologically related; to establish the clinical importance of the organisms; and to identify the isolates according to the recent taxonomy. METHODS--Fifty eight Acinetobacter isolates from 49 patients collected during 1984 and 1985 were investigated. Most isolates were from respiratory tract specimens from intensive care patients. The organisms were typed by cell envelope protein electrophoresis and by a quantitative carbon source growth assay; patients' charts were reviewed to differentiate between colonisation and infection; representative isolates were identified to species level by DNA-DNA hybridisation. RESULTS--Twelve protein profiles were distinguished in the isolates. Forty two isolates were of the same protein profile (profile I); other profiles were observed in a few or single isolates. Cluster analysis of carbon source growth divided profile I isolates into two groups--one of isolates from 1984 and one from 1985. They were identified as A baumannii and associated with infections in eight patients. Four other infections were caused by acinetobacters with other protein profiles (three of A baumannii; one of the unnamed DNA group 3). CONCLUSIONS--Apart from sporadic strains, two strains of the same protein profile, but distinguishable by carbon source growth, were successively endemic. Cluster analysis was a valuable tool in the interpretation of typing and epidemiological data. The 12 (28%) infections of Acinetobacter in 43 patients in intensive care suggest that the presence of these organisms in wards of severely ill patients should be a cause of concern.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M., Ismail F., Jackman P. J., Noble W. C. Fingerprinting Acinetobacter strains from clinical sources by numerical analysis of electrophoretic protein patterns. J Med Microbiol. 1984 Aug;18(1):55–64. doi: 10.1099/00222615-18-1-55. [DOI] [PubMed] [Google Scholar]
- Bergogne-Bérézin E., Joly-Guillou M. L., Vieu J. F. Epidemiology of nosocomial infections due to Acinetobacter calcoaceticus. J Hosp Infect. 1987 Sep;10(2):105–113. doi: 10.1016/0195-6701(87)90135-6. [DOI] [PubMed] [Google Scholar]
- Bouvet P. J., Grimont P. A. Identification and biotyping of clinical isolates of Acinetobacter. Ann Inst Pasteur Microbiol. 1987 Sep-Oct;138(5):569–578. doi: 10.1016/0769-2609(87)90042-1. [DOI] [PubMed] [Google Scholar]
- Bouvet P. J., Jeanjean S., Vieu J. F., Dijkshoorn L. Species, biotype, and bacteriophage type determinations compared with cell envelope protein profiles for typing Acinetobacter strains. J Clin Microbiol. 1990 Feb;28(2):170–176. doi: 10.1128/jcm.28.2.170-176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombach W. H., Dijkshoorn L., van Noort-Klaassen M., Niessen J., van Knippenberg-Gordebeke G. Control of an epidemic spread of a multi-resistant strain of Acinetobacter calcoaceticus in a hospital. Intensive Care Med. 1989;15(3):166–170. doi: 10.1007/BF01058568. [DOI] [PubMed] [Google Scholar]
- Dijkshoorn L., Michel M. F., Degener J. E. Cell envelope protein profiles of Acinetobacter calcoaceticus strains isolated in hospitals. J Med Microbiol. 1987 Jun;23(4):313–319. doi: 10.1099/00222615-23-4-313. [DOI] [PubMed] [Google Scholar]
- Dijkshoorn L., Van Ooyen A., Hop W. C., Theuns M., Michel M. F. Comparison of clinical Acinetobacter strains using a carbon source growth assay. Epidemiol Infect. 1990 Jun;104(3):443–453. doi: 10.1017/s0950268800047452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner J. S., Jarvis W. R., Emori T. G., Horan T. C., Hughes J. M. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988 Jun;16(3):128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- Gerner-Smidt P., Tjernberg I., Ursing J. Reliability of phenotypic tests for identification of Acinetobacter species. J Clin Microbiol. 1991 Feb;29(2):277–282. doi: 10.1128/jcm.29.2.277-282.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glew R. H., Moellering R. C., Jr, Kunz L. J. Infections with Acinetobacter calcoaceticus (Herellea vaginicola): clinical and laboratory studies. Medicine (Baltimore) 1977 Mar;56(2):79–97. doi: 10.1097/00005792-197703000-00001. [DOI] [PubMed] [Google Scholar]
- Hartstein A. I., Rashad A. L., Liebler J. M., Actis L. A., Freeman J., Rourke J. W., Jr, Stibolt T. B., Tolmasky M. E., Ellis G. R., Crosa J. H. Multiple intensive care unit outbreak of Acinetobacter calcoaceticus subspecies anitratus respiratory infection and colonization associated with contaminated, reusable ventilator circuits and resuscitation bags. Am J Med. 1988 Nov;85(5):624–631. doi: 10.1016/s0002-9343(88)80233-x. [DOI] [PubMed] [Google Scholar]
- Obana Y. Pathogenic significance of Acinetobacter calcoaceticus: analysis of experimental infection in mice. Microbiol Immunol. 1986;30(7):645–657. doi: 10.1111/j.1348-0421.1986.tb02991.x. [DOI] [PubMed] [Google Scholar]
- Tjernberg I., Ursing J. Clinical strains of Acinetobacter classified by DNA-DNA hybridization. APMIS. 1989 Jul;97(7):595–605. doi: 10.1111/j.1699-0463.1989.tb00449.x. [DOI] [PubMed] [Google Scholar]
- Vila J., Almela M., Jimenez de Anta M. T. Laboratory investigation of hospital outbreak caused by two different multiresistant Acinetobacter calcoaceticus subsp. anitratus strains. J Clin Microbiol. 1989 May;27(5):1086–1089. doi: 10.1128/jcm.27.5.1086-1089.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]