Abstract
Background
Atherosclerosis, a response to injury, may be thought of as scarring in the artery wall. TGF-β and associated signaling molecules have been implicated in the pathophysiology of keloid scarring, Dupuytren’s Contracture and atherosclerotic plaques in independent studies.
Purpose
To test the hypothesis that excess cutaneous scarring and Dupuytren’s contractures predispose independently to carotid atherosclerosis.
Methods
Among 1,747 patients with plaque measurements and complete data for multivariable regression analysis, 57 Caucasian patients had Dupuytren’s contractures and 12 had keloid scars. Carotid total plaque area (TPA) was measured by 2-Dimensional ultrasound.
Results
In linear multivariable regression analysis with coronary risk factors, keloid scars were associated with TPA (P= 0.018), but Dupuytren’s contractures were not. Patients with keloid scarring were younger (P<0.0001), and more likely to be diabetic (P<0.0001)
Conclusions
Keloid scarring is a clinical clue to excess atherosclerosis not explained by traditional risk factors. Such patients may benefit from therapy directed at targets related to signalling molecules common to both the process of keloid scarring and atherosclerosis. These findings suggest previously unexplored possibilities for the prevention and treatment of atherosclerosis. The differences between Dupuytren’s and keloid scars that may identify such targets are discussed.
Traditional risk factors for atherosclerosis include age, sex, blood pressure (BP), cholesterol levels, smoking history, and diabetes. These risk factors are essentially the same in different levels of the arterial tree.1–4 It has become increasingly clear, however, that these risk factors do not completely account for the degree of atherosclerosis in many patients.5,6 It is thus important to identify previously unrecognized risk factors for atherosclerosis. Doing so provides insights into the pathophysiology of the disease as well as the potential for new therapeutic approaches.
This study began with the presentation in 1994 of a young man with unusually severe atherosclerosis for his age. At age 31 he had required bilateral femoropopliteal bypass, although he did not have unusually high levels of traditional risk factors. However, he had been treated on seven occasions by plastic surgeons to remove keloid scar tissue. What made this remarkable was that he was Caucasian. As atherosclerosis shares some components with scar tissue – a reaction in the artery wall to injury7,8 – JDS wondered if this association may be more than a coincidence. As the result of this experience, excess cutaneous scarring became a routine part of the physical examination and functional inquiry for subsequent patients referred to our vascular prevention clinic. In this paper we present the results of our observations since this young man first presented.
The purpose of this study was to test the hypothesis that keloid scarring is a marker for “unexplained atherosclerosis” (UE); that is, atherosclerosis not explained by traditional coronary risk factors. Specifically, our aim was to determine whether keloid formation and Dupuytren’s contractures are independently associated with carotid plaque burden, after adjusting in multiple regression for traditional risk factors
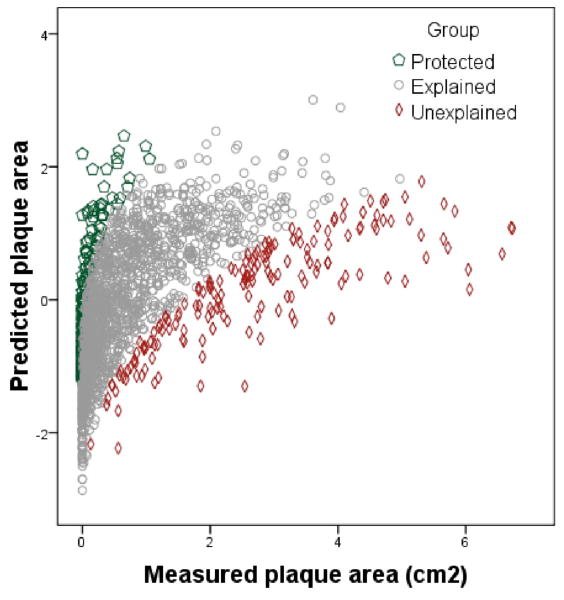
We began measuring carotid total plaque area (TPA) as a measure of atheroma burden in 19909 and, since 1995, have routinely used this measurement in the management of patients referred for treatment of atherosclerosis.10 Validation work from our laboratory has shown that both baseline TPA and progression of TPA identify patients at higher risk of cardiovascular events: after adjustment for age, sex, cholesterol, systolic blood pressure, pack-years of smoking, total homocysteine, diabetes and treatment of blood pressure and lipids, patients in the top quartile of plaque area had 3.4 times higher risk of stroke, death or myocardial infarction over 5 years and, those with progression, had twice the risk of those with stable plaque or regression.11 Our findings have been validated in a population-based study of over 6,000 participants in the Tromsø Study.12 In our earlier studies, we used multivariable linear regression analysis to identify determinants of carotid plaque area (TPA).13 We found that traditional risk factors were significant determinants of variation in TPA, accounting for 52% of variance in TPA.5 Patients with high residual scores in the regression model (i.e. those far to the right of the regression line) were recognized as having more carotid atherosclerosis than would be predicted by traditional risk factors. Thus, the term “unexplained atherosclerosis” (UE) was introduced to refer to a marked difference between observed and expected TPA5. This concept is illustrated in Figure 1.
FIGURE 1.
Unexplained atherosclerosis. Predicted plaque area (transformed) on the vertical axis, vs measured plaque area on the horizontal axis. The red diamonds represent patients with residual scores in the top 10% (>= 1.281); i.e. those with the most atherosclerosis not explained by traditional risk factors (“Unexplained atherosclerosis”); the green pentagons represent those with residual scores in the lowest 10% (<- 1.25); i.e. those with protection from atherosclerosis; the grey circles represent the 80% of patients with residual scores nearest the regression line (“Explained atherosclerosis”).
Methods
Study population and data collection
We analyzed a cohort of 1,747 patients from the Stroke Prevention and Atherosclerosis Research Centre at the Robarts Research Institute, London, Canada. Included in the analysis were all those with complete data for all variables used in the regression model. For these patients, the following information was collected from the initial visit: age, sex, systolic blood pressure (SBP), total cholesterol, pack-years of smoking, presence of diabetes mellitus, whether the patients were on antihypertensive therapy or lipid-lowering agents, and the baseline TPA.
Baseline TPA was measured as previously described.11 Briefly, total plaque area was defined as the sum of the cross-sectional areas of all plaques seen in the left and right internal, external, and common carotid arteries. Plaques were defined as localized increases in thickness of the intima-media complex >1 mm in thickness. Each plaque was measured in the longitudinal plane in which it was biggest, by tracing with a track-ball cursor its cross-sectional area. Total plaque area is the sum of these areas for all plaques seen in the left and right common, internal and external carotid.
As we are reporting on the results of clinical practice from our own patients, such reports were exempted from a requirement for explicit signed consent by the UWO ethics review board in 1977.
Statistical analysis
Multivariable linear regression, using a model that we have previously described7, was used to assess whether the different forms of cutaneous scarring were independent predictors of TPA. For this model, the distribution of TPA was normalized by a cube-root transformation. The following variables maximally explain the variance in TPA: age, sex, SBP, total cholesterol, smoking history in pack-years, a history of treatment for hypertension and a history of treatment of for hyperlipidemia (each P < 0.05). To this model were added either keloid scarring or Dupuytren’s contractures.
Chi-square was used to compare non-parametric variables, and ANOVA to compare continuous variables across the three groups of patients: no scarring, Dupuytren’s contractures or keloid scarring.
All statistical analyses were performed using SPSS for Windows (Version 16). A two-tailed P value of 0.05 was deemed to be statistically significant.
Results
Among the patients with complete data for all the variables used in the multivariable regression model, 57 had Dupuytren’s contractures, and 12 had keloid scars.
The total cholesterol was not available for one patient, who was not on a lipid-lowering agent. For this patient, an imputed baseline total cholesterol value of 5.59 mmol/L was used from the mean level for patients in the database who were not on a lipid-lowering agent at the time of the first visit, but who subsequently received such treatment.
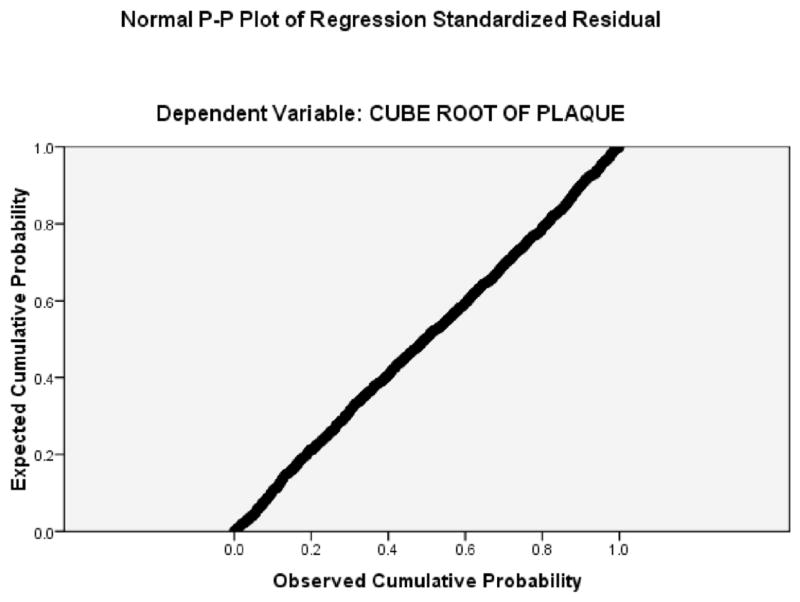
The adjusted R2 value for the multivariable regression model was 0.51, - 51% of the variance in the transformed TPA variable was explained by age, sex, SBP, total cholesterol, pack-years of smoking and treatment of lipids or hypertension. (The R2 for untransformed TPA was 0.36.) Figure 2, a cumulative probability plot of residual scores, shows that the regression model is very robust.
FIGURE 2.
Cumulative probability plot of residual scores in the regression model for keloid scarring (Table 1).
Tables 1 and 2 show the results of adding to the regression model keloid scarring or Dupuytren’s as independent predictors of TPA. The presence of Dupuytren’s contracture was not significant as an independent predictor of TPA (p = 0.653), but keloid scarring was significantly associated with TPA (p= 0.018).
TABLE 1.
Multivariable linear regression for carotid total plaque area with Keloid scarring added to the model
| Coefficientsa | ||||||
|---|---|---|---|---|---|---|
| Model | Unstandardized Coefficients | Standardized Coefficients | t | P | ||
| B | Std. Error | Beta | ||||
| 1 | (Constant) | −0.405 | 0.050 | −8.166 | 0.000 | |
| Age (yr) | 0.017 | 0.001 | 0.572 | 29.798 | 0.000 | |
| Sex (female =1, male=0) | −0.121 | 0.015 | −0.140 | −8.198 | 0.000 | |
| Cholesterol (mmol/L) | 0.024 | 0.007 | 0.062 | 3.583 | 0.000 | |
| On hypertensive meds | 0.054 | 0.016 | 0.063 | 3.480 | 0.001 | |
| On lipid lowering meds | 0.107 | 0.017 | 0.124 | 6.496 | 0.000 | |
| Smoking (pack-years) | 0.004 | 0.000 | 0.187 | 10.919 | 0.000 | |
| Keloid scarring | 0.209 | 0.088 | 0.040 | 2.377 | 0.018 | |
Dependent Variable: Cube root transformation of total plaque area
R2= 0.510; n=1747
TABLE 2.
Multivariable linear regression for carotid total plaque area with Dupuytren’s contractures added to the model
| Coefficientsa | ||||||
|---|---|---|---|---|---|---|
| Model | Unstandardized Coefficients | Standardized Coefficients | t | P | ||
| B | Std. Error | Beta | ||||
| 1 | (Constant) | −0.399 | 0.050 | −8.028 | 0.000 | |
| Age (years) | 0.017 | 0.001 | 0.571 | 29.409 | 0.000 | |
| Sex (female=1, male=0) | −0.122 | 0.015 | −0.141 | −8.202 | 0.000 | |
| Cholesterol (mmol/L) | 0.024 | 0.007 | 0.061 | 3.515 | 0.000 | |
| On hypertensive meds | 0.055 | 0.016 | 0.063 | 3.474 | 0.001 | |
| On lipid lowering meds | 0.109 | 0.017 | 0.126 | 6.568 | 0.000 | |
| Smoking (pack-years) | 0.004 | 0.000 | 0.186 | 10.841 | 0.000 | |
| Dupuytren’s | −0.018 | 0.040 | −0.008 | −0.450 | 0.653 | |
Dependent Variable: Cube root transformation of total plaque area
Adusted R2= 0.509 n=1747
Baseline values for the variables used in the multivariable regression model are shown in Table 3. The three groups of scarring were different in age, with the keloid scarring patients being younger, and were all more likely to be male; those with Dupuytren’s contractures were predominantly male. Patients with keloid scarring were much more likely to be diabetic.
TABLE 3.
Baseline values of the variables used in the regression model, for the three groups of patients
| No scarring | Dupuytren’s | Keloid | P | |
|---|---|---|---|---|
| Number | 1679 | 57 | 12 | |
| Age (yr) | 56.94 (1.04) | 66.11 (9.85) | 50.00 (14.81) | <0.0001 |
| Male % | 53% | 80.7% | 58.3% | <0.0001 |
| Diabetic % | 8.9% | 5.3% | 16.7% | 0.0001 |
| Smoking Pack-yr | 12.51 (18.08) | 16.92 (20.03) | 5.58 (7.39) | 0.08 |
| Total cholesterol (mmol/L) | 5.16 (1.10) | 4.99 (1.16) | 4.82 (0.87) | 0.31 |
| Systolic blood pressure (mmHg) | 139.74 (21.47) | 139.30 (21.47) | 149.08 (19.29) | 0.32 |
| Antihypertensive therapy % | 56.8% | 42.1% | 66.7% | 0.069 |
| Lipid-lowering therapy | 47.65 | 42.1% | 66.7% | 0.30 |
| Carotid plaque area (cm2) | 0.85 (1.04) | 1.35 (1.51) | 1.03 (1.03) | 0.002 |
Chi-square for percentages, ANOVA for continuous variables
Discussion
Our results suggest that keloid scarring is a physical finding that may identify patients with excess atherosclerosis not explained by traditional risk factors. To our knowledge, this study represents the first association between these two processes. Patients with this form of scarring were shown to have increased TPA when compared to controls with similar traditional risk factors. Furthermore, keloid scarring was an independent predictor of TPA in multivariable regression. The finding of an independent association between keloid scarring and excess TPA despite the small number of patients with this phenomenon suggests a powerful biological link. Plausible links may be found in inflammation and scarring.
A weakness of our study is the small number of patients with keloid scarring. Strengths are the measurement of carotid plaque, as opposed to intima-media thickness, and the adjustment for traditional coronary risk factors in multivariable regression. Carotid total plaque area is biologically and genetically distinct from IMT9,14,15, and total plaque area is a stronger predictor of coronary events than are IMT measurements that do not include plaque thickness.16 Recently we showed that lipoprotein (a) is a strong predictor of carotid stenosis and occlusion, but not plaque area17, highlighting the importance of distinguishing between carotid ultrasound phenotypes. Table 3 shows that patients with Dupuytren’s had more plaque, but they were older, smoked more, and were predominantly male. Adjustment in multivariable regression makes it clear that the Dupuytren’s was not an independent predictor of plaque.
Atherosclerosis may be thought of as an inflammatory process.18–20 The molecular mechanisms of this inflammatory process are complex and involve cytokines with pro-inflammatory and anti-inflammatory activity.20,21 Of the many cytokines involved, recent studies suggest that transforming growth factor-beta (TGF-beta) and vascular endothelial growth factor (VEGF) may play key roles in atherosclerosis, including the formation of carotid atherosclerotic plaques.22–27 It is interesting that these same cytokines have been implicated in the pathogenesis of abnormal cutaneous scarring28–34 and suggested as components of Dupuytren’s contracture.35,36
Atherosclerosis, a response to injury7,8, includes a major component of scarring in the artery wall. Dustan37 hypothesized that keloid formation may be associated with excess risk of hypertension and vascular disease among blacks, and Dupuytren’s contracture has been associated with diabetes mellitus and its microvascular complications.38,39
Mechanisms involved in such associations include effects of transforming growth factor beta (TGFβ). Wang et al. demonstrated that cells derived from abnormal cutaneous scar tissue produce more TGFβ than normal skin cells.28 Similarly, exogenous TGFβ-1 causes a greater increase in collagen synthesis in keloid-derived fibroblasts compared to fibroblasts derived from normal dermis.30 Both Wu et al. 31 and Gira et al. 32 have shown increased levels of VEGF in keloids. Dupuytren’s Contracture cord and wound granulation tissue have similar histological features, leading to the view that DD may be a dysregulated, fibrotic wound healing response.40 TGFβ-1 has not only been implicated in promoting myofibroblast development in Dupuytren’s Contracture33.41, but primary cells derived from Dupuytren’s Contracture cord are reported to have an enhanced sensitivity to its effects.29
TGFβ signalling triggers long-term responses through a variety of signalling intermediates42 and some of these molecules have been demonstrated to be components of atherosclerosis, keloid scarring and DD. One of these is β-catenin, a signalling molecule regulated by a variety of growth factors including TGFβ: β-catenin accumulation has been implicated in atherosclerosis43, abnormal cutaneous scarring and wound healing 44,45 and Dupuytren’s Contracture 46 in independent studies. Thus, it is quite possible that the changes in gene expression initiated by β-catenin accumulation and transcription factor transactivation may underlie some of the molecular mechanisms leading to the formation of keloid scar tissue, Dupuytren’s contracture cords and atherosclerosis. Other TGFβ signalling intermediates, however, may not be common components of these conditions. Connective Tissue Growth Factor (CTGF) is a TGFβ-inducible signalling component of keloid scar formation47 and atherosclerotic plaques that is not evident in microarray analyses of gene expression in DD tissues or primary DD cells.48 Thus, there is molecular evidence that atherosclerosis, keloid scarring and DD share some, but not all, of the TGFβ-mediated intermediates associated with the wound healing response.
Accumulation of β-catenin and CTGF up-regulation are both associated with the differentiation of fibroblasts into myofibroblasts.49 One intriguing possibility is that myofibroblasts in atherosclerotic plaques and keloid scars may be derived from similar cellular precursors while the myofibroblasts in DD cord may be derived from different precursor cells via overlapping, but different signalling pathways. This idea is suggested by a recent report suggesting that, while myofibroblasts in lung fibrosis are largely derived from resident fibroblasts, the phenotypically identical cell type is contributed by hepatic stellate cells in liver fibrosis and media smooth muscle cells in atheromatous plaques.50 Consistent with this speculation, therapies that inhibit the mammalian target of rapamycin (mTOR) have been shown to prevent both atherosclerotic plaque formation in an animal model51 and myofibroblast development by keloid fibroblasts52 in independent studies. Research focused on identifying the molecular pathways that promote myofibroblast differentiation in patients prone to keloid scar development and atherosclerotic plaque formation may therefore reveal additional potential targets for therapeutic interventions.
Conclusion
Keloid scarring, but not Dupuytren’s contractures, was associated with excess atherosclerosis not accounted for by traditional risk factors. This discrepancy suggests that factors differentiating these two types of excess scarring may point to factors linking scarring to atherosclerosis, and thus to new potential therapeutic targets for atherosclerosis. Keloid scarring may be a clinical clue to excessive atherosclerosis not explained by traditional coronary risk factors.
Acknowledgments
Funded by the Heart & Stroke Foundation of Ontario, including grants grant numbers T2956, T5017, NA4990, T5704 and NA5912. Also supported by donations to the Stroke Prevention & Atherosclerosis Research Centre.
References
- 1.van dMI, Iglesias del SA, Hak AE, Bots ML, Hofman A, Witteman JC. Risk factors for progression of atherosclerosis measured at multiple sites in the arterial tree: the Rotterdam Study. Stroke. 2003;34:2374–9. doi: 10.1161/01.STR.0000088643.07108.19. [DOI] [PubMed] [Google Scholar]
- 2.Espeland MA, Tang R, Terry JG, Davis DH, Mercuri M, Crouse JR., III Associations of risk factors with segment-specific intimal-medial thickness of the extracranial carotid artery. Stroke. 1999;30:1047–55. doi: 10.1161/01.str.30.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB. Hazards, risks, and threats of heart disease from the early stages to symptomatic coronary heart disease and cardiac failure. Cardiovasc Drugs Ther. 1997;11(Suppl 1):199–212. doi: 10.1023/a:1007792820944. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulatio. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 5.Spence JD, Barnett PA, Bulman DE, Hegele RA. An approach to ascertain probands with a non traditional risk factor for carotid atherosclerosis. Atherosclerosis. 1999;144:429–34. doi: 10.1016/s0021-9150(99)00003-9. [DOI] [PubMed] [Google Scholar]
- 6.Spence JD. Technology Insight: ultrasound measurement of carotid plaque--patient management, genetic research, and therapy evaluation. Nat Clin Pract Neurol. 2006;2:611–9. doi: 10.1038/ncpneuro0324. [DOI] [PubMed] [Google Scholar]
- 7.Haust MD. Reaction patterns of intimal mesenchyme to injury, and repair in atherosclerosis. Adv Exp Med Biol. 1974;43:35–57. doi: 10.1007/978-1-4684-3243-5_3. [DOI] [PubMed] [Google Scholar]
- 8.Ross R, Glomset J, Harker L. Response to injury and atherogenesis. Am J Pathol. 1977;86:675–84. [PMC free article] [PubMed] [Google Scholar]
- 9.Spence JD. Ultrasound measurement of carotid plaque: Uses in patient management, genetic research and evaluation of new therapies. Nat Clin Prac Neurol. 2006;2:611–9. doi: 10.1038/ncpneuro0324. [DOI] [PubMed] [Google Scholar]
- 10.Spence JD. Point: uses of carotid plaque measurement as a predictor of cardiovascular events. Prev Cardiol. 2005;8:118–21. doi: 10.1111/j.1520-037x.2005.03908.x. [DOI] [PubMed] [Google Scholar]
- 11.Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. Carotid Plaque Area: A Tool for Targeting and Evaluating Vascular Preventive Therapy. Stroke. 2002;33:2916–22. doi: 10.1161/01.str.0000042207.16156.b9. [DOI] [PubMed] [Google Scholar]
- 12.Johnsen SH, Mathiesen EB, Joakimsen O, et al. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow-up study of 6226 persons: the Tromso Study. Stroke. 2007;38:2873–80. doi: 10.1161/STROKEAHA.107.487264. [DOI] [PubMed] [Google Scholar]
- 13.Barnett PA, Spence JD, Manuck SB, Jennings JR. Psychological stress and the progression of carotid atherosclerosis. J Hypertension. 1997;15:49–55. doi: 10.1097/00004872-199715010-00004. [DOI] [PubMed] [Google Scholar]
- 14.Spence JD, Hegele RA. Noninvasive phenotypes of atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:e188–e189. doi: 10.1161/01.ATV.0000146160.22637.33. [DOI] [PubMed] [Google Scholar]
- 15.Pollex RL, Hegele RA. Genetic determinants of carotid ultrasound traits. Current Atherosclerosis Reports. 2006;8:206–15. doi: 10.1007/s11883-006-0075-z. [DOI] [PubMed] [Google Scholar]
- 16.Johnsen SH, Mathiesen EB, Joakimsen O, et al. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow-up study of 6226 persons: the Tromso Study. Stroke. 2007;38:2873–2880. doi: 10.1161/STROKEAHA.107.487264. [DOI] [PubMed] [Google Scholar]
- 17.Klein JH, Hegele RA, Hackam DG, Koschinsky ML, Huff MW, Spence JD. Lipoprotein(a) is associated differentially with carotid stenosis, occlusion, and total plaque area. Arterioscler Thromb Vasc Biol. 2008;28:1851–6. doi: 10.1161/ATVBAHA.108.169292. [DOI] [PubMed] [Google Scholar]
- 18.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 19.Schillinger M, Exner M, Mlekusch W, et al. Inflammation and Carotid Artery--Risk for Atherosclerosis Study (ICARAS) Circulation. 2005;111:2203–9. doi: 10.1161/01.CIR.0000163569.97918.C0. [DOI] [PubMed] [Google Scholar]
- 20.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–81. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 21.Hansson GK. Inflammation, Atherosclerosis, and Coronary Artery Disease. N Engl J Med. 2005;252:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 22.Bobik A, Agrotis A, Kanellakis P, et al. Distinct patterns of transforming growth factor-beta isoform and receptor expression in human atherosclerotic lesions. Colocalization implicates TGF-beta in fibrofatty lesion development. Circulation. 1999;99:2883–91. doi: 10.1161/01.cir.99.22.2883. [DOI] [PubMed] [Google Scholar]
- 23.Panutsopulos D, Papalambros E, Sigala F, Zafiropoulos A, Arvanitis DL, Spandidos DA. Protein and mRNA expression levels of VEGF-A and TGF-beta1 in different types of human coronary atherosclerotic lesions. Int J Mol Med. 2005;15:603–10. [PubMed] [Google Scholar]
- 24.Morsi WG, Shaker OG, Ismail EF, et al. HO-1 and VGEF gene expression in human arteries with advanced atherosclerosis. Clin Biochem. 2006 doi: 10.1016/j.clinbiochem.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Inoue M, Itoh H, Ueda M, et al. Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: possible pathophysiological significance of VEGF in progression of atherosclerosis. Circulation. 1998;98:2108–16. doi: 10.1161/01.cir.98.20.2108. [DOI] [PubMed] [Google Scholar]
- 26.Papalambros E, Sigala F, Georgopoulos S, et al. Vascular endothelial growth factor and matrix metalloproteinase 9 expression in human carotid atherosclerotic plaques: relationship with plaque destabilization via neovascularization. Cerebrovasc Dis. 2004;18:160–5. doi: 10.1159/000079736. [DOI] [PubMed] [Google Scholar]
- 27.Lee SH, Jeong MH, Bae HR, et al. Circulating levels of interleukin-8 and vascular endothelial growth factor in patients with carotid stenosis. J Korean Med Sci. 2001;16:198–203. doi: 10.3346/jkms.2001.16.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang R, Ghahary A, Shen Q, Scott PG, Roy K, Tredget EE. Hypertrophic scar tissues and fibroblasts produce more transforming growth factor-beta1 mRNA and protein than normal skin and cells. Wound Repair Regen. 2000;8:128–37. doi: 10.1046/j.1524-475x.2000.00128.x. [DOI] [PubMed] [Google Scholar]
- 29.Bisson MA, McGrouther DA, Mudera V, Grobbelaar AO. The different characteristics of Dupuytren’s disease fibroblasts derived from either nodule or cord: expression of alpha-smooth muscle actin and the response to stimulation by TGF-beta1. J Hand Surg [Br] 2003;28:351–6. doi: 10.1016/s0266-7681(03)00135-9. [DOI] [PubMed] [Google Scholar]
- 30.Bettinger DA, Yager DR, Diegelmann RF, Cohen IK. The effect of TGF-beta on keloid fibroblast proliferation and collagen synthesis. Plast Reconstr Surg. 1996;98:827–33. doi: 10.1097/00006534-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Zhang Q, Ann DK, et al. Increased vascular endothelial growth factor may account for elevated level of plasminogen activator inhibitor-1 via activating ERK1/2 in keloid fibroblasts. Am J Physiol Cell Physiol. 2004;286:C905–C912. doi: 10.1152/ajpcell.00200.2003. [DOI] [PubMed] [Google Scholar]
- 32.Gira AK, Brown LF, Washington CV, Cohen C, Arbiser JL. Keloids demonstrate high-level epidermal expression of vascular endothelial growth factor. J Am Acad Dermatol. 2004;50:850–3. doi: 10.1016/j.jaad.2003.11.061. [DOI] [PubMed] [Google Scholar]
- 33.Badalamente MA, Sampson SP, Hurst LC, Dowd A, Miyasaka K. The role of transforming growth factor beta in Dupuytren’s disease. J Hand Surg [Am] 1996;21:210–5. doi: 10.1016/S0363-5023(96)80102-X. [DOI] [PubMed] [Google Scholar]
- 34.Lee TY, Chin GS, Kim WJ, Chau D, Gittes GK, Longaker MT. Expression of transforming growth factor beta 1, 2, and 3 proteins in keloids. Ann Plast Surg. 1999;43:179–84. [PubMed] [Google Scholar]
- 35.Bains W. Vasoprotective VEGF as a candidate for prevention of recurrence of fibrotic diseases such as Dupuytren’s contracture. Med Hypotheses. 2003;60:793–6. doi: 10.1016/s0306-9877(02)00397-3. [DOI] [PubMed] [Google Scholar]
- 36.Bayat A, Stanley JK, Watson JS, Ferguson MW, Ollier WE. Genetic susceptibility to Dupuytren’s disease: transforming growth factor beta receptor (TGFbetaR) gene polymorphisms and Dupuytren’s disease. Br J Plast Surg. 2003;56:328–33. doi: 10.1016/s0007-1226(03)00176-0. [DOI] [PubMed] [Google Scholar]
- 37.Dustan HP. Does keloid pathogenesis hold the key to understanding black/white differences in hypertension severity? Hypertension. 1995;26:858–62. doi: 10.1161/01.hyp.26.6.858. [DOI] [PubMed] [Google Scholar]
- 38.Stradner F, Ulreich A, Pfeiffer KP. Dupuytren’s contracture as a concomitant disease in diabetes mellitus. Wien Med Wochenschr. 1987;137:89–92. [PubMed] [Google Scholar]
- 39.Arkkila PE, Kantola IM, Viikari JS. Dupuytren’s disease: association with chronic diabetic complications. J Rheumatol. 1997;24:153–9. [PubMed] [Google Scholar]
- 40.Fitzgerald AM, Kirkpatrick JJ, Naylor IL. Dupuytren’s disease. The way forward? J Hand Surg [Br] 1999;24:395–9. doi: 10.1054/jhsb.1999.0207. [DOI] [PubMed] [Google Scholar]
- 41.Berndt A, Kosmehl H, Mandel U, et al. TGF beta and bFGF synthesis and localization in Dupuytren’s disease (nodular palmar fibromatosis) relative to cellular activity, myofibroblast phenotype and oncofetal variants of fibronectin. Histochem J. 1995;27:1014–20. [PubMed] [Google Scholar]
- 42.Grotendorst GR, Rahmanie H, Duncan MR. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J. 2004;18:469–79. doi: 10.1096/fj.03-0699com. [DOI] [PubMed] [Google Scholar]
- 43.Barbieri SS, Weksler BB. Tobacco smoke cooperates with interleukin-1beta to alter beta-catenin trafficking in vascular endothelium resulting in increased permeability and induction of cyclooxygenase-2 expression in vitro and in vivo. FASEB J. 2007;21:1831–43. doi: 10.1096/fj.06-7557com. [DOI] [PubMed] [Google Scholar]
- 44.Cheon SS, Wei Q, Gurung A, et al. Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. FASEB J. 2006;20:692–701. doi: 10.1096/fj.05-4759com. [DOI] [PubMed] [Google Scholar]
- 45.Cheon SS, Cheah AY, Turley S, et al. beta-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci SA. 2002;99:6973–8. doi: 10.1073/pnas.102657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varallo VM, Gan BS, Seney S, et al. Beta-catenin expression in Dupuytren’s disease: potential role for cell-matrix interactions in modulating beta-catenin levels in vivo and in vitro. Oncogene. 2003;22:3680–4. doi: 10.1038/sj.onc.1206415. [DOI] [PubMed] [Google Scholar]
- 47.Khoo YT, Ong CT, Mukhopadhyay A, et al. Upregulation of secretory connective tissue growth factor (CTGF) in keratinocyte-fibroblast coculture contributes to keloid pathogenesis. J Cell Physiol. 2006;208:336–43. doi: 10.1002/jcp.20668. [DOI] [PubMed] [Google Scholar]
- 48.Cicha I, Yilmaz A, Klein M, et al. Connective tissue growth factor is overexpressed in complicated atherosclerotic plaques and induces mononuclear cell chemotaxis in vitro. Arterioscler Thromb Vasc Biol. 2005;25:1008–13. doi: 10.1161/01.ATV.0000162173.27682.7b. [DOI] [PubMed] [Google Scholar]
- 49.Caraci F, Gili E, Calafiore M, et al. TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts The myofibroblast: one function, multiple origins. Pharmacol Res. 2008 doi: 10.1016/j.phrs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Hinz B, Phan SH, Tannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mueller MA, Beutner F, Teupser D, Ceglarek U, Thiery J. Prevention of atherosclerosis by the mTOR inhibitor everolimus in LDLR−/− mice despite severe hypercholesterolemia. Atherosclerosis. 2008;198:39–48. doi: 10.1016/j.atherosclerosis.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 52.Ong CT, Khoo YT, Mukhopadhyay A, et al. mTOR as a potential therapeutic target for treatment of keloids and excessive scars. Exp Dermatol. 2007;16:394–404. doi: 10.1111/j.1600-0625.2007.00550.x. [DOI] [PubMed] [Google Scholar]