Abstract
Microglia mediate neuroinflammation and regulate brain development and homeostasis. Microglial abnormalities are implicated in a range of neuropsychiatric pathology, including Tourette syndrome (TS) and autism. Histamine (HA) is both a neurotransmitter and an immune modulator. HA deficiency has been implicated as a rare cause of TS and may contribute to other neuropsychiatric conditions. In vitro studies suggest that HA can regulate microglia, but this has never been explored in vivo. We used immunohistochemistry to examine the effects of HA deficiency in histidine decarboxylase (Hdc) knockout mice and of HA receptor stimulation in wild-type animals. We find HA to regulate microglia in vivo, via the H4 receptor. Chronic HA deficiency in Hdc knockout mice reduces ramifications of microglia in the striatum and (at trend level) in the hypothalamus, but not elsewhere in the brain. Depletion of histaminergic neurons in the hypothalamus has a similar effect. Microglia expressing IGF-1 are particularly reduced, However, the microglial response to challenge with lipopolysacchariade (LPS) is potentiated in Hdc knockout mice. Genetic abnormalities in histaminergic signaling may produce a vulnerability to inflammatory challenge, setting the state for pathogenically dysregulated neuroimmune responses.
Keywords: basal ganglia, H4R receptor, IGF-1, lipopolysaccharide
INTRODUCTION
Histamine (HA) is produced both by immune cells and by a group of neurons in the posterior tuberomamillary nucleus of the hypothalamus; in the central nervous system it functions as a neurotransmitter, with diverse functions in different circuitries (1). Histamine receptors are prominent in many brain regions, especially in the cortico-basal ganglia circuitry (1). Dysregulation of brain histamine is increasingly appreciated as a potential contributor to neuropsychiatric disease (2). For example, disruption of histamine biosynthesis has been implicated as a rare genetic cause of Tourette syndrome (TS) (3-6). Abnormalities in histaminergic signaling have also been hypothesized to contribute to Parkinson’s disease (7), Huntington’s disease (8, 9), Alzheimer disease’s (10, 11), narcolepsy (12, 13), and drug addiction (1, 2).
Peripheral HA is an important modulator of immune and allergic responses (e.g. 14, 15). Dysregulated neuroinflammation has been proposed to contribute to TS and a variety of other neuropsychiatric conditions (16-18), although these connections remain unproven in most cases. The possible role of HA as a regulator of neuroinflammatory processes in the brain has received scant attention.
Microglia are the primary inflammatory cells in the brain; inflammatory challenge, such as administration of lipopolysaccharide (LPS), induces them to produce inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). More recently it has been appreciated that microglia are required for normal brain development, homeostasis, synaptic function, and adult neurogenesis, even in the absence of inflammation (19-21). Consistent with this variety of functional roles, microglia can take on a range of phenotypes. Insulin-like growth factor 1 (IGF-1) is an important mediator of homeostatic microglial function. IGF-1 is upregulated in neuroprotective microglia induced by TH2 cytokines such as IL-4 (22). Support by IGF-1 expressing neurotrophic microglia is required for survival of cortical neurons during postnatal development (22), for neurogenesis induced by environmental enrichment and exercise in the adult (23, 24), and for neuroprotection after ischemic injury (25, 26). IGF-1-positive microglia also serve a neuroprotective role in animal models of neurodegenerative diseases such as amyotrophic lateral sclerosis (27) and retinitis pigmentosum (28).
Microglial dysregulation is seen in a variety of neuropsychiatric conditions, including TS, although whether such dysregulation is central to pathophysiology remains to be established (18, 29-31). Inflammation-related genes have been found to be upregulated in postmortem brain from individuals with TS (31-33), and microglia in postmortem tissue have morphological hallmarks of activation, though they are normal in number (31). A recent positron emission tomography study similarly suggested increased microglial activation in patients (30). In mice, knockout of the HoxB8 gene, which is expressed in a subset of microglia, leads to microglial deficits and a marked increase in grooming behavior; similar grooming phenotypes have been described in other animal models of TS (34), obsessive compulsive disorder (OCD) (35-37), and autism (21, 38). Strikingly, these behavioral manifestations of the HoxB8 knockout have been reported to be rescued by repopulation of the brain with wild-type microglia (39).
These observations led us to investigate the role of histamine in the regulation of microglia. A few in vitro studies have attempted to address this question, but much is unclear. In N9 cells, a microglia-like cell line, HA can induce migration, through activation of the H4 histamine receptor (but not the H1 receptor) (40). N9 cells, like brain microglia, produce pro-inflammatory cytokines such as IL-1β and TNF-α when stimulated with LPS. When they are stimulated by HA, however, N9 cells do not produce these pro-inflammatory cytokines – in fact, HA blocks LPS-induced N9 cell motility and IL-1β secretion. This leads to the hypothesis that HA might oppose the pro-inflammatory activation of microglia – a role that has also been suggested for other aminergic neurotransmitters (41). Similar findings have recently been reported in microglial cultures (42).
In contrast, other reports using primary microglial cultures from neonatal rat brain suggest that HA can have a pro-inflammatory effect. In one recent study, Dong et al (43) found that HA activated microglia in vitro in a dose- and time-dependent manner. Activation was mediated by both the H1 and the H4 receptors and led to the production of the inflammatory cytokines interleukin-6 (IL-6) and TNF-α. Zhu and colleagues reported similar results, also in primary microglial cultures (44). HA has been reported to induce nitric oxide synthase (iNOS), another marker of an inflammatory phenotype, in cultured microglia (45).
These divergent results may derive from the fact that all of these studies have been performed in vitro. In vitro studies have examined microglia isolated from neonates; even if they accurately recapitulate events in vivo in pups, this may not extrapolate to adult animals. Microglia in the adult have a less inflammatory expression profile than those seen in neonates or aging animals (46). In addition, such preparations eliminate the multitudinous cell-cell interactions that, it is increasingly recognized, fine-tune microglial phenotype and function in vivo. It remains unclear, therefore, how histamine modulates microglial activation in vivo, and whether this varies depending on brain region or animal state. We therefore used genetic and pharmacological tools to examine the regulation of microglia by HA in the intact brain.
MATERIALS AND METHODS
Mice
All experiments were conducted under the auspices of the Yale Institutional Animal Care and Use Committee, in accordance with NIH guidelines. Adult male mice aged 2-4 months were used for all experiments. C57Bl/6 wild-types were purchased from Jackson Laboratories (www.jax.org). Hdc-KO mice have been previously described (3, 47); the mice used in these experiments were backcrossed onto C57Bl/6 for ≥ 9 generations. Knockout mice were generated by intercrossing heterozygotes in our vivarium; WT littermates were used as controls. Targeted depletion of histaminergic neurons was performed in Hdc-cre transgenic mice (48), which were obtained from Jackson Laboratories (jaxmice.jax.org/strain/021198) and backcrossed with C57Bl/6J mice in our vivarium. HDC-Cre negative littermates were used as negative controls in these experiments.
Drugs
Histamine dihydrochloride and VUF8430 dihydrobromide were purchased from Sigma Aldrich. 4-methylhistamine dihydrochloride, 2-pyridylethylamine dihydrochloride and JNJ 10191584 maleate were purchased from Tocris Biosciences. Lipopolysaccharide from Escherichia coli (055:B5) was purchased from Santa Cruz Biotechnology. Except for JNJ 10191584, which was dissolved in 1% carboxymethylcellulose (Calbiochem), all the drugs were dissolved in sterile 0.9% sodium chloride.
Surgery
Intracerebroventricular (ICV) drug infusion was performed under anesthesia using standard sterile stereotaxic technique. Animals were anesthetized with ketamine:xylazine 100:10 mg/kg. An infusion syringe was inserted into the lateral ventricle at coordinates −0.70 mm anteroposterior, 2 mm dorsoventral, and 1.4 mm mediolateral (relative to bregma, using the atlas of Paxinos (49)). Drugs were infused in a total volume of 1 μl using an UltraMicroPump III (World Precision Instruments Inc) at a rate of 100 nl/min.
For specific depletion of HDC cells in the tuberomammillary nucleus (TMN), we used an AAV5-flex-taCasp3-TEVp virus (50) (1×1012 viral particles, University of North Carolina Vector Core). Viral infusion injections were performed bilaterally in the TMN, at coordinates −2 mm anteroposterior, −5.2 mm dorsoventral, +/−0.6 mm mediolateral. A volume of 0.5 ul was injected in each hemisphere (1 ul total) at a flow rate of 100 nl/min (51).
Perfusion and tissue preparation
For histology, animals were deeply anesthetized using ketamine/xylazine and transcardially perfused with 0.5-1 vol/weight PBS followed by 1-2 vol/weight of 4% paraformaldehyde (PFA) in PBS. Brains were removed and postfixed in 4% PFA overnight, then transferred to sucrose 15% and 30% successively. Brains were sliced using a cryostat; 30 μm sections were collected and stored floating in cryoprotectant solution (30% glycerin + 30% ethylene glycol in PBS) at 4°C until use.
Immunohistochemistry and bright field microscopy
Brain slices were washed thoroughly with PBS. Endogenous peroxidases were inactivated with Bloxall blocking solution (Vector Labs). Nonspecific staining was reduced by incubation of samples with 3% normal goat serum in PBS + 0.1% Triton X100. Sections were then incubated overnight at 4°C with anti-Iba1 primary antibody (Wako, Japan) diluted 1:500 with 3% normal goat serum in PBS + 0.1% Triton X100. Samples were washed and then incubated with biotinylated goat anti-rabbit antibody (Vector Labs) in PBS + 0.1% Triton X100 with 2% normal goat serum for 1 hour at room temperature. Brain slices were washed again and developed using the Vectastain Elite ABC kit and diaminobenzidine (DAB; Vector Labs). Slices were mounted on charged slides, allowed to dry completely, counterstained with Nuclear Fast Red (Vector Labs), dehydrated through graded alcohol washes followed by CitriSolv (CitraSolv), and then mounted and coverslipped using D.P.X. (Sigma Aldrich). All sections compared within each experiment were processed and stained in parallel to ensure comparability.
Mounted sections were visualized using a Leica DM1000 microscope. Images in the middorsal striatum were collected at 200×. Several images were collected for each mouse, but only one field was collected for each section, to prevent overlap. For microglial cell counts, cells were manually counted with the Photoshop counter tool in each image by an investigator blind to experimental condition, and averaged across all sections from that mouse. Total optical density analysis was performed using the same images, using an automated procedure in ImageJ software, as illustrated in Supplemental Figure 1.
Immunofluorescence and confocal microscopy
Free-floating sections were washed with PBS, treated with Bloxall Solution, washed again, incubated in 5% normal donkey serum solution in PBS 0.3% Triton X100 for 1 hour, and then incubated overnight with goat polyclonal anti-IGF-1 1:50 (R&D Systems) and rabbit anti-Iba1 1:500 (Wako, Japan). After 3 washes, slices were incubated with Cy3-conjugated donkey anti-goat antibody and Alexa 488-conjugated donkey anti-rabbit antibody (Jackson Immunoresearch), both at 1:400, for an hour at room temperature. Samples were washed again and mounted in Vectashield HardSet Mounting Medium (Vector Labs) and analyzed by confocal microscopy.
Confocal imaging was performed by sequential scanning of slices using an Olympus Fluoview FV-1000 confocal microscope, using a Kalman filter with an acquisition speed of 4 pixel/sec. 15 μm z-stacks were generated by using a fixed step size of 0.5 μm.
Quantification of double-positive cells was performed blind to genotype by counting fluorescent cells above background in the striatum bilaterally in multiple views from multiple slices for each animal; all counted slices were averaged for each animal for analysis.
For counting of Hdc-postiive cells in TMN-depleted animals, coronal slices samples were washed and incubated for 1 hour in 5% normal donkey serum (Jackson ImmunoResearch), and then incubated ON at 4°C with rabbit anti-HDC (1:500, Acris). For secondary detection an Alexafluor555 donkey anti rabbit antibody (1:400, Life sciences) was used. Confocal imaging was performed as described above. Quantification was performed by counting single positive cells above background in the TMN bilaterally without overlap. Four sections per mouse were used per experiment.
In situ hybridization
In situ hybridization was performed as described previously (52). Briefly, probes were generated by in vitro transcription from templates amplified by PCR from mouse cDNA. See Supplementary Table 1 for primer sequences. PCR products were verified by sequencing. Radiolabeled probes were generated using the MAXIscript kit (Ambion) with T7 polymerase and [35S]rCTP, purified through a mini Quick Spin RNA Columns (Roche) and hybridized [hybridization buffer: 50% (v/v) formamide, 3×SSC, 50 mM NaPO4,10 mM dithiothreitol, 1×Denhart’s, 0.25 g/l tRNA, 10% dextran SO4] to post-fixed (4% PFA) coronal sections mounted on glass slides. Following overnight hybridization at 55°C, slides were washed several times with increasingly dilute SSC buffer and then rinsed in dH2O, dehydrated in 100% ethanol, air dried for several hours and exposed to autoradiographic film (Amersham Hyperfilm MP, GE Healthcare Life Sciences). Images were captured using a computer-controlled digital camera (Cohu Inc.) and imported into ImageJ for densitometric analysis of the signal of antisense probes. No signal was detected with sense sequence controls in the regions of interest for H1 or H4 genes.
Real Time rt-PCR
Unfixed whole striatum samples were quickly dissected immediately after euthanasia and frozen in liquid nitrogen. For quantification of cytokines after LPS challenge, mice were injected approximately 4 hours before dissection with 1 mg/kg LPS (53). Tissue was homogenized in 1 ml of Trizol reagent (Invitrogen), and RNA was isolated following the manufacturer’s instructions. Poly(A)+ mRNA was purified from total RNA using the PolyATract mRNA Isolation System III (Promega) following the manufacturer’s instructions. These samples were subjected to retrotranscription using the SuperScript III First-Strand Synthesis SuperMix (Invitrogen). Real Time PCR was carried out in a 7500 Fast Real-Time PCR System (Applied Biosystems) using SybrGreen PCR Core Reagent (Applied Biosystems). See Supplementary Table 1 for primer sequences. Absolute amounts of cDNA present on each sample were determined with respective standard curves, and genes of interest were normalized to actin.
Statistical analysis
Most data were analyzed using t-tests or ANOVAs Prism 6.0d (GraphPad), as detailed in the Results section and figure legends for each analysis. A mixed model analysis in SPSS 19.0 (IBM) was used to analyze LPS response data to allow for differing number of samples across groups (Figure 5). Alpha was set at 0.05 for all analyses. 2-tailed tests were used in most cases; 1-tailed tests were used as appropriate for confirmatory experiments, where detailed in the text. In immunohistochemical experiments in which multiple slices were examined from each animal, data from all slices was averaged before analysis – that is, the unit of analysis was the animal, not the slice.
RESULTS
Modulation of microglia by histamine in vivo
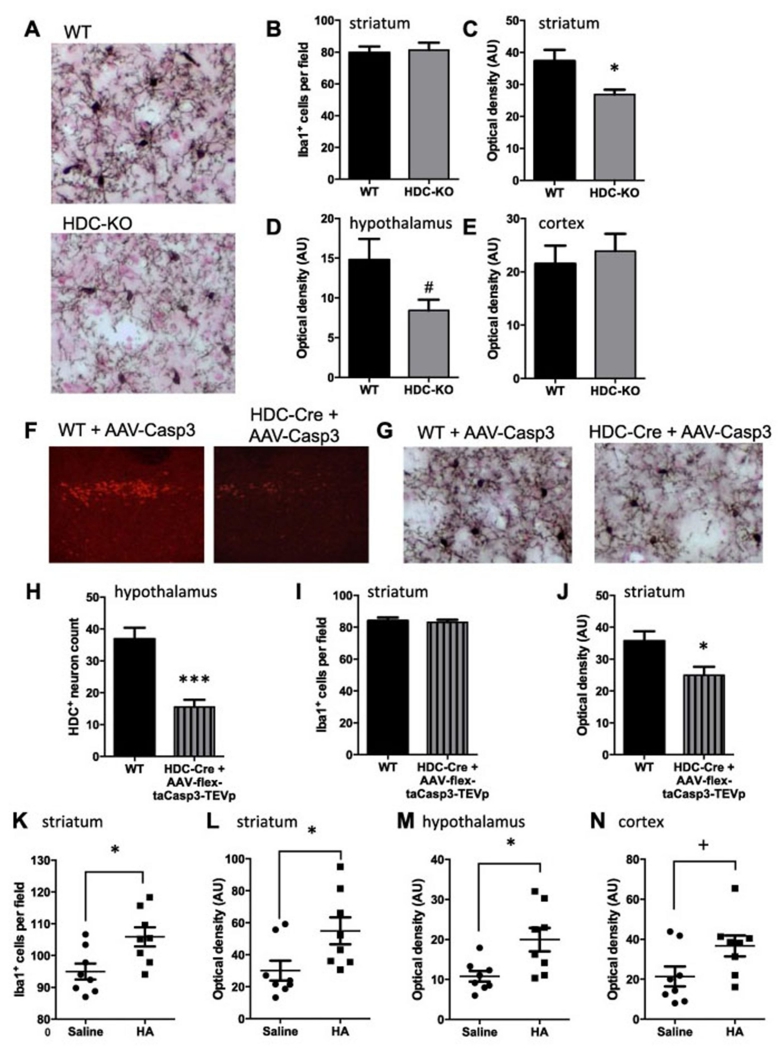
We evaluated the effect of histamine deficiency on microglial number and activation by examining microglia in the dorsal striatum of Hdc knockout mice, which have undetectable levels of histamine in the striatum (3, 47), using anti-Iba1 immunohistochemistry. We focused on the striatum because of our interest in TS, in which striatal abnormalities are well established (54, 55), and because we have previously documented neuronal abnormalities in this structure in these mice (3, 56). Stained microglial processes were less prominent in Hdc-KO striatum than in controls (Figure 1A). To quantify this we computed the % area of tissue covered by Iba1-positive cells and processes, using ImageJ (Supplementary Figure 1). This revealed a reduced area covered by Iba1 immunoreactivity in knockout animals. The total number of microglia, on the other hand, was not altered (Figure 1B, C). As the number of microglia cells is not reduced in the Hdc-KO mice, and the soma size is qualitatively normal, the decrease of optical density could be attributed to decreased ramifications (Figure 1A). Alterations in Iba1 immuonstaining density were not uniform throughout the brain: a trend towards reduced Iba1 immunostaining denisty was seen in hypothalamus, where histaminergic neuronal cell bodies are located (p = 0.054, Figure 1D), but there was no alteration in other regions examined, including motor cortex (Figure 1E) hippocampus, substantia nigra, or globus pallidus (Supplemental Figure 2). This pattern confirms the importance of studying microglial regulation in vivo; regional variation in microglial activation would not be apparent in in vitro studies.
Figure 1. Microglial modulation by histamine deficiency or stimulation.
A. Representative Iba-1 stained striatal fields from WT and Hdc-KO mice, with nuclear fast red counterstaining. B. Microglia number was quantified as Iba1-positive cells per high-power field in the dorsal striatum, blind to genotype. (See also Supplementary Figure 2.) There was no difference in total microglia in Hdc-KO mice (N = 7-9 mice per group; two-tailed t-test: t[14] = 0.26, p = 0.8). C. Optical density of Iba1 immunostaining in dorsal striatum (see Supplementary Figure 1) was reduced in KO mice (t[10.7] = 2.75 [Welch’s correction], p = 0.019). D. Similar reduction in Iba1 staining was observed in the hypothalamus: two-tailed t-test: t[11.7] = 2.1 [Welch’s correction], p = 0.054). E. There was no alteration in Iba1 staining in motor cortex in Hdc-KO mice (two-tailed t-test: t[14] = 0.45, p = 0.6; see also Supplementary Figure 2). F. HDC-expressing neurons in the TMN were selectively ablated using an AAV5-flex-taCasp3-TEVp virus in HDC-Cre mice (50); controls were wild-type mice identically infused with virus. G. Depletion of HDC neurons in the TMN produced abnormalities in striatal microglia similar to those seen in Hdc-KO mice. H. HDC neurons were depleted ~ 50% (N = 5-6 mice per group; two-tailed t-test: t[9] = 5.3, p = 0.0005). I. Striatal microglia cell number was not modified by this treatment (N = 5-6 mice per group; two-tailed t-test: t[9] = 0.31, p = 0.76). J. Optical density of Iba1 was reduced in the striatum after HDC neuronal depletion, similarly to what was observed in Hdc-KO mice (N = 5-6 mice per group; two-tailed t-test: t[9] = 2.633, p = 0.027). K. Histamine dihydrochloride (20 μg) was infused into the lateral cerebral ventricle of C57Bl/6 mice (as in ref. 3); microglial activation was examined by Iba1 immunostaining 7 days later (see Supplementary Figure 3 for other time points). Iba1-positive cells were slightly but significantly increased following HA infusion (N = 8 mice per group; two-tailed t-test: t[14] = 2.8, p = 0.014). L. Optical density for Iba1 staining was more markedly increased after HA (two-tailed t-test: t[14] = 2.4, p = 0.031). M. Iba1 optical density was also increased in the hypothalamus after HA infusion (N = 8,8; two-tailed t-test:[g23] t[14] = 2.847, p = 0.013). N. Total Iba1 staining was also increased in the motor cortex of wildtype mice compared to vehicle-treated mice (N = 8,8; two-tailed t-test: t[14] = 2.116, p = 0.053). All values are shown as mean ± SEM. *p<0.05. #p = 0.054. +p = 0.053.
Much HA in the brain is produced by Hdc-expressing histaminergic neurons in the tuberomamillary nucleus (TMN) in the posterior hypothalamus (1). To further confirm the effect of HA deficiency on microglia in the striatum, we selectively depleted histaminergic neurons in the TMN through targeted ablation. A virus that expresses constitutively active Caspase 3 when activated by cre recombinase (50) was stereotaxically introduced, bilaterally, into the TMN of Hdc-Cre transgenic mice. This produced a ~50% reduction in the number of Hdc-expressing cells, relative to wild-type mice injected with the same virus (Figure 1 F, H) (51). Iba1 staining in the striatum showed a normal number of microglia cells but reduced area covered by microglia processes in Hdc-ablated mice, compared to controls (Figure 1 G, I, J), paralleling what is seen in Hdc knockout mice. This indicates that neurally derived HA, specifically, can regulate microglia.
To test whether increased histamine is able to regulate microglia acutely in vivo, we infused HA into wild-type mice, as in a previous study (3). Wild-type adult male C57Bl/6 mice were infused ICV with 20 μg of histamine dihydrochloride in 1 μl sterile saline, or saline alone, under ketamine/xylazine anesthesia. In a pilot experiment, mice were sacrificed 1-14 days later and tissue was stained for Iba1. Histamine increased both microglia number and density of ramifications in the striatum, with an increase apparent as early as 3 days after HA infusion, prominent at 7 days, and persisting at least 14 days (Supplementary Figure 3). We replicated this effect at 7 days and documented a robust increase in both microglial number and Iba1 immunostaining density (Figure 1K, L). The microglial response to exogenous histamine was apparent more broadly than the abnormalities in Hdc-KO mice but still showed some regional specificity: increased Iba1 staining was also observed in the hypothalamus, the motor cortex and globus pallidus after HA infusion, whereas substantia nigra and hippocampus showed no response to exogenous HA (Figure 1M, N, and Supplementary Figure 3).
Microglial regulation by the histamine H4 receptor in vivo
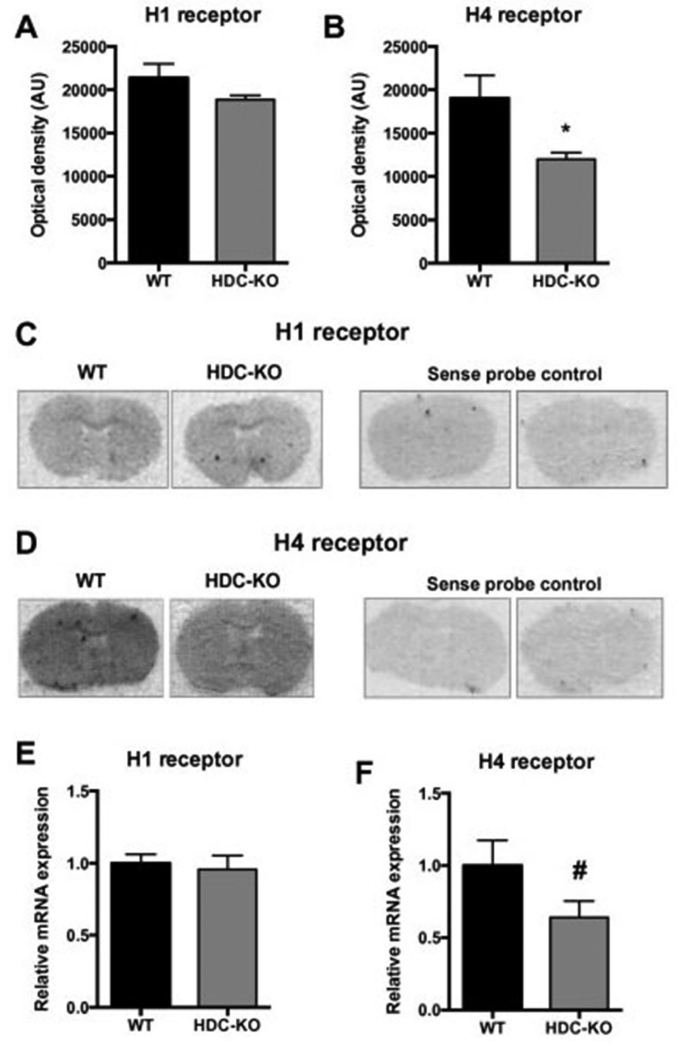
HA acts on four receptors (H1-H4), all of which are present in the central nervous system (1). H1 and H4 are expressed on microglia and regulate them in primary culture, but only H4 regulates N9 cells (43, 44). H1 is also present on neurons; H4 is generally believed not to be (1). We quantified the expression of H1 and H4 mRNA in the striatum of Hdc knockout mice by in situ hybridization. H1 mRNA was not significantly altered in KO mice (Figure 2A, C). H4 mRNA, on the other hand, was significantly decreased in KO mice (Figure 2B, D). These results were confirmed using real time rt-PCR measurement of mRNA in striatal homogenate: H4 mRNA levels were reduced in the Hdc-KO mice, but no changes were seen for H1 mRNA (Figure 2E, F).
Figure 2. Differential expression of histamine receptors in HDC-KO mice.
A. H1 receptor, visualized using in situ hybridization with a specific riboprobe, was unaltered in Hdc-KO mice (N = 3-4 mice per group; two-tailed t-test: t[5] = 1.3, p = 0.26). Quantification was performed relative to a sense control probe, which showed minimal binding. B. H4 receptor expression was decreased in Hdc-KO mice (two-tailed t-test: t[6] = 2.6, p = 0.043). C, D. Representative images of H1 and H4 receptor autoradiographies respectively; sense control probes showed minimal binding. E, F. H1 and H4 expression was confirmed using rtPCR from dissected striatal tissue from Hdc-KO and control mice; again, H1 mRNA did not differ between genotypes (N = 10-11 mice per group; two-tailed t-test: t[19] = 0.37, p = 0.7), but H4 expression was reduced, confirming in situ hybridization results (N = 16 mice per group; one-tailed t-test: t[30]=1.733, p = 0.0467). All values are shown as mean ± SEM. *p<0.05 by 2-tailed t-test; # p<0.05 by 1-tailed t-test.
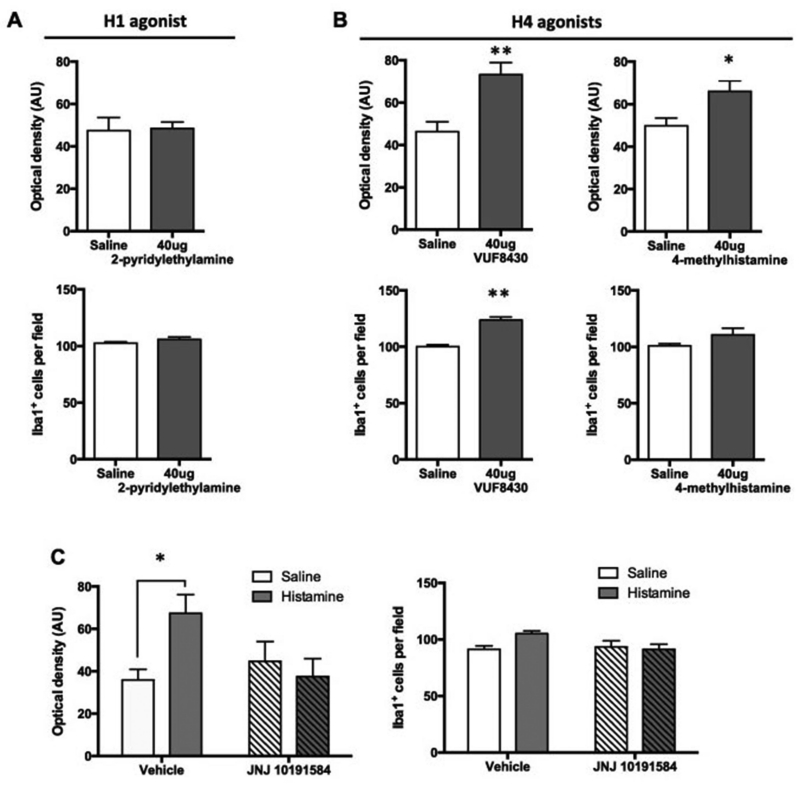
We next tested the ability of H1 and H4 agonists to recapitulate the regulation of microglia by HA in vivo in wild-type mice. H1 and H4 agonists were infused ICV over a range of concentrations in a dose-finding study (Supplementary Figure 4), and then the highest concentration of each was then infused in a larger number of animals in a follow-up experiment. Mice were euthanized 3 days after drug infusion (corresponding to the first marked activation by HA under similar conditions; see Supplementary Figure 3).
The H1 agonist 2-pyridylethylamine has been reported to activate microglia in primary cultures (43) but not in an immortalized microglia-like cell line (40). It produced no effect on either Iba1 staining density or microglial number even at a high dose of 40 ug (Supplementary Figure 4 and Figure 3A).
Figure 3. Microglial activation in vivo is mediated by the H4 receptor.
A. The H1 agonist 2-pyridylethylamine, administered ICV, produced no detectable alteration in dorsal striatal microglia 3 days later (n = 8,8, two-tailed t-test for total density: t[14] = 0.15, p = 0.89; Iba1+ cells: t[14] = 1.5, p = 0.15). B. The H4 receptor agonist VUF8430, administered ICV, produced dose-dependent changes in microglial number and activation 3 days later. (n = 8-9 mice per group, two-tailed t-test for total density: t[15] = 3.66, p = 0.0023; Iba1+ cells: t[15] = 8.02, p < 0.001). The H4 agonist 4-methylhistamine showed similar effects on striatal microglia (n = 8-9 mice per group, two-tailed t-test for total density: t[15] = 2.702, p = 0.016; two-tailed t-test with Welch’s correction for Iba1+ cells: t[8.408] = 1.6, p = 0.15). C. H4 antagonism mitigated the HA-induced increase in Iba1 immunostaining [g523]N = 6-7 mice per group; 2-way ANOVA: main effect of histamine, F[1,22] = 2.3, p = 0.14; main effect of JNJ 10191584, F[1,22] = 1.7, p = 0.20; interaction: F[1,22] = 5.8, p = 0.025). H4 antagonism appeared to also mitigate the small HA-induced increase in microglial number, though this interactive effect did not quite reach statistical significance (2-way ANOVA: main effect of histamine, F[1,22] = 2.2, p = 0.15; main effect of JNJ 10191584, F[1,22] = 2.26, p = 0.15; interaction: F[1,22] = 4.0, p = 0.056). * p < 0.05; ** p < 0.01, main effect of dose.
In contrast, ICV infusion of 40 ug of the H4 agonist VUF 8430 produced an increase in the total Iba1-immunopositive area and in microglia cell number, similarly to HA infusion (Figure 3B; Supplementary Figure 4). Similar results were obtained with another H4 agonist, 4-methylhistamine (4-MH), which produced an increase in total Iba1 staining area (Figure 3B, Supplementary Figure 4). There was a slight increase of Iba1+ cells after 4-MH, but it did not reach statistical significance (Figure 3B).
We next asked whether H4 antagonism could attenuate the changes produced by HA in microglial cells. Mice were treated systemically with the H4 antagonist JNJ 10191584 (10 mg/kg intraperitoneal) prior to 20 μg ICV HA (as in Figure 1; JNJ 10191584 was given 15 minutes before the induction of surgical anesthesia, approximately 45 minutes before the initiation of the HA infusion). H4 antagonism blocked the effects of HA on microglial cells, as measured by Iba1 staining density (Figure 3C); effects of HA on microglial number were not significant in this experiment.
HDC-KO mice have reduced IGF1-positive microglia
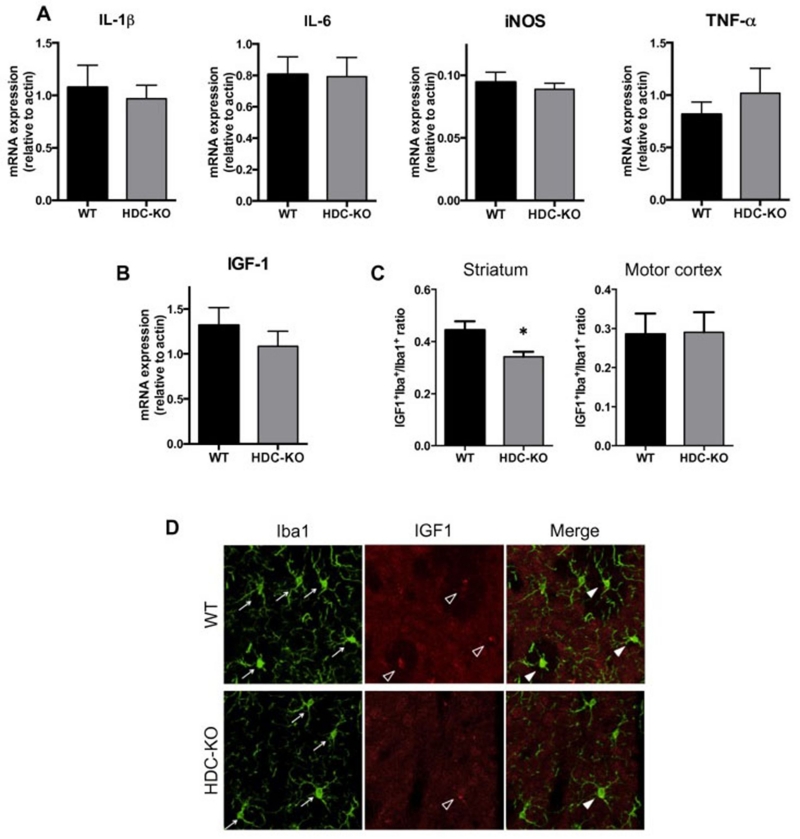
Microglia can take on a range of phenotypes in vivo and can be proinflammatory or neuroprotective. We used rtPCR on acutely dissected striatal homogenate to examine expression of cytokines associated with inflammatory and neuroprotective profiles (Figure 4). IL-1β, TNF-α, IL-6 and iNOS have all been reported to be regulated by HA in vitro, though published data have been inconsistent. In vivo, between-genotype comparisons did not reveal significant differences in any of these comparisons (Figure 4A). There was a nominal difference suggesting a decrease in the neurotrophin IGF-1, though it did not reach statistical significance (Figure 4B). IGF-1 is an important factor in microglial support for neuronal function and a mediator of microglial neuroprotection in a variety of contexts (22, 23, 27, 57).
Figure 4. Regulation of IGF-1, a putative marker of a neuroprotective microglial phenotype, by HA.
A-B. mRNA of inflammatory markers in the striatum of Hdc-KO mice, measured from whole tissue by rtPCR. There were no statistically significant differences between groups, but a trend in IGF-1 motivated further examination. n = 7,7. 2-tailed t-test: IL-1beta, t[12] = 0.44, p = 0.67; IL-6, t[12] = 0.09, p = 0.93; TNF-alpha, t[12] = 0.76, p = 0.46; iNOS, t[11] = 0.63, p = 0.54; IGF-1, t[12] = 0.9, p = 0.38. C-D. IGF-1 expression in microglial cells was detected by double immunofluorescence for Iba1 and IGF-1, followed by confocal microscopy and quantification of IGF-1 expressing microglia as a subset of total Iba1 positive cells. IGF-1+Iba1+ cells, as a proportion of total Iba1+ cells, were decreased in the dorsal striatum of Hdc-KO mice relative to WT sibling controls (N = 5 WT, 4 KO; t[7] = 2.5, p = 0.043). No difference was found in the motor cortex (t[7] = 0.064, p = 0.95). All values shown as mean ± SEM. *p<0.05.
IGF-1 is significantly retained in cells and therefore can be examined with cell-type specificity in vivo using co-immunofluorescence (Figure 4D). We found the number of IGF1+Iba1+ double-positive cells, as a fraction of all Iba1+ cells, to be significantly reduced in the Hdc-KO mice (Figure 4C, D). This reduction is region-specific: no differences between Hdc-KO and WT mice were seen in the motor cortex (Figure 4C).
Deficiency of histamine results in increased susceptibility to inflammatory challenge
IGF-1 expressing microglia have been proposed to be neuroprotective and to limit inflammatory responses (25, 26). We speculated that the reduction in IGF-1-expressing microglia in Hdc-KO mice would lead to a dysregulated response to pro-inflammatory stimulation. Such an effect would be consistent with the inhibitory effect that HA has on the response of N9 cells (40) and neonatal microglial cultures (42) to lipopolysaccharide (LPS) in some studies in vitro (though not in all).
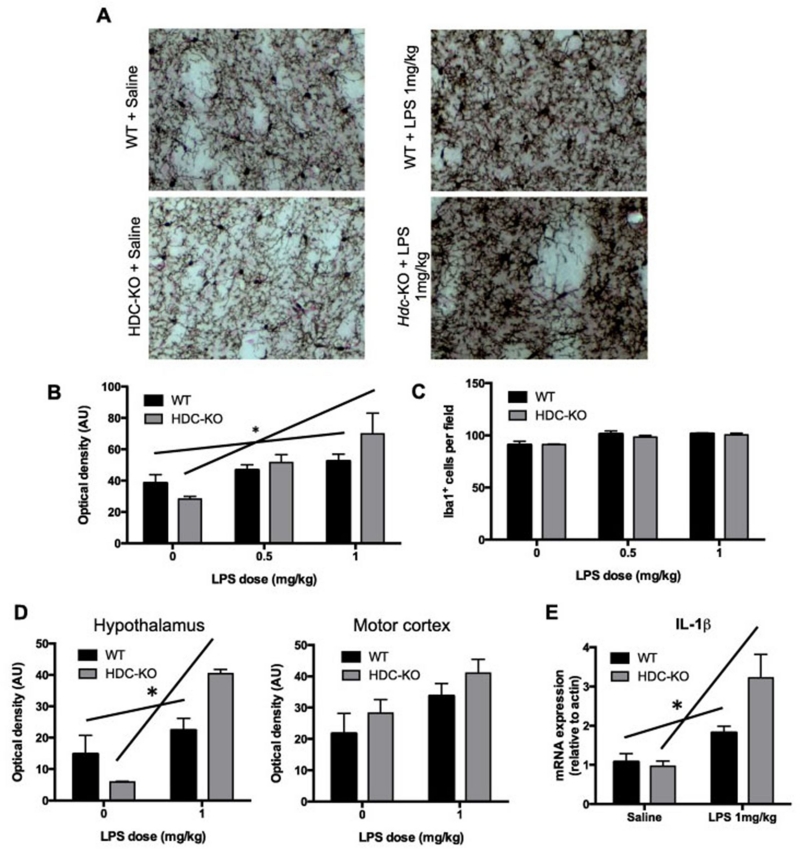
We treated HDC-KO and WT mice with LPS at 0.5 and 1.0 mg/kg and evaluated microglial number and activation in the striatum by immunohistochemistry, 7 days later (the peak of HA effects on microglia; see Supplemental Figure 3). Saline-injected KO mice had a reduced density of Iba1, as before (Figure 5A, B). However, microglia in Hdc-KO mice responded significantly more robustly to LPS, with a particularly marked effect after 1 mg/kg LPS (Figure 5A, B). This effect was not seen in the effect of LPS on total Iba1-positive cell count (Figure 5C). Overall, these dose-response curves indicate an overexuberant response to inflammatory challenge in mice that lack HA. This differential response of microglia in Hdc-KO mice is brain-region specific: the response of microglia to LPS was also exacerbated in the hypothalamus of Hdc-KO mice, but not in the motor cortex (Figure 5D).
Figure 5. Increased susceptibility of Hdc-KO mice to LPS challenge.
A. Hdc-KO mice and wild-type littermate controls were injected IP with saline or various doses of LPS (0.5 to 1 mg/kg); microglial activation in the dorsal striatum was examined by Iba1 immunostaining 7 days later. B. The density of Iba1 immunostaining was disproportionally increased by LPS in a dose-dependent fashion in Hdc-KO mice (N = 3-4 mice per group; mixed model: main effect of LPS, F[1,18] = 18.9, p < 0.0001; main effect of genotype, F[1,18] = 1.4, p = 0.25; genotype × LPS interaction, F[1,18] = 4.7, p = 0.043). The relationship between LPS dose and Iba1 staining density was significant for WT mice (r = 0.612, p = 0.045) but much more significant for KO animals (r = 0.776, p = 0.005). C. The density of microglial cells was increased in a dose-dependent fashion, with no difference between genotypes (mixed model: main effect of LPS: F[1,18] = 22.972, p < 0.001; main effect of genotype: F[1,18] = 0.037, p = 0.85; genotype × LPS interaction: F[1,18] = 0.09, p = 0.76). D. Microglial response to LPS was also enhanced in the hypothalamus of Hdc-KO mice (N = 4,4; 2-way ANOVA: main effect of LPS, F[1,12] = 35.27, p < 0.0001; main effect of genotype, F[1,12] = 1.615, p = 0.2278; genotype X LPS interaction: F[1,12] = 14.38, p = 0.0026), but not in the motor cortex (N = 4,4; 2-way ANOVA: main effect of LPS, F[1,12] = 6.519, p = 0.025; main effect of genotype, F[1,12] = 2.03, p = 0.18; genotype × LPS interaction: F[1,12] = 0.007, p > 0.9). E. IL-1β mRNA showed a greater response in Hdc-KO mice after 1 mg/kg LPS challenge, corroborating the differential microglial response (n = 7,7; 2-way ANOVA: main effect of LPS, F[1,24] = 20.20, p = 0.0002; main effect of genotype, F[1,24] = 3.717, p = 0.066; genotype × LPS interaction: F[1,24] = 5.080, p = 0.034). See also Supplementary Figure 5. * p<0.05 genotype × dose interaction.
To corroborate the differential effect of LPS challenge in Hdc-KO mice, cytokine mRNA was quantified 4 hr after LPS challenge (1 μg/ml). Hdc-KO mice showed an exaggerated production of IL-1β in response to LPS compared to WT controls (Figure 5E). A similar trend was seen for TNF-α (Supplementary Figure 5). In contrast, arginase-1, a neuroprotective factor, showed a trend toward reduction after LPS in Hdc-KO mice, but not in controls (Supplementary Figure 5).
DISCUSSION
Microglia, the brain’s resident inflammatory cells, have critical roles both in the response to injury or infection and in normal brain development and homeostasis. They are dysregulated in a variety of neuropsychiatric disease states (18), although the causal role of this dysregulation remains to be established in most cases. They are modulated by innumerable neurotransmitters, cytokines, and other factors.
We have examined the modulation of microglia by histamine (HA) in vivo, using exogenous HA infusion, chronic histamine deficiency in the Hdc knockout mouse, selective removal of the primary source of histamine in the brain (histaminergic neurons in the TMN), and a range of pharmacological manipulations. The potential importance of HA regulation of microglia (45) is highlighted by the fact that both HA and microglial dysregulation are implicated in neuropsychiatric disease states such as Tourette syndrome (TS) and a range of neurodegenerative syndromes. Our finding of microglial abnormalities in the Hdc knockout mouse, a validated model of the pathophysiology of TS (Figure 1) (3), further emphasizes the importance of understanding HA’s role in regulating microglial function. Previous examination of HA regulation of microglia has been limited to in vitro studies of immortalized N9 cells (40) or primary microglial cultures from neonates (42-44). These studies have found HA to regulate microglia but have produced conflicting data as to the nature of that regulation – specifically, whether HA-stimulated microglia have a pro-inflammatory or neuroprotective phenotype (57) (40). Our study is the first examination of these questions in vivo.
Several findings stand out. We confirm that HA can regulate microglia in vivo, as manifested by increased total area of Iba1 staining after ICV HA infusion, and decreased Iba1-immunoreactive density in Hdc-KO mice. In neonatal cultures, the H1 and H4 receptors have been implicated in microglial activation; the H4 receptor plays a particularly prominent role in immortalized cells (40). Our data indicate that, under these conditions, the H4 receptor is the primary mediator of microglial modulation by histamine: ICV treatment with an H1 agonist, at comparable doses (43), has no significant effect on microglia, and an H4 antagonist blocks the effect produced by ICV histamine (Figure 3).
These in vivo effects may result from direct HA action on microglia; alternatively, they may be an indirect result of HA effects on other cell types. The H4 receptor has not been reported to be expressed in striatal neurons or glia, apart from microglia. Most studies have described its expression in the brain as being largely restricted to hematopoetic cells (1); if this is the case, then the effects of H4 agonists (Figure 2) suggest a direct effect on microglia. H4 receptor RNA and protein have been identified in many regions of the brain (58, 59), but cell-type specificity has not been characterized in detail in vivo. One study has reported electrophysiological effects of H4 agonists on neurons in somatosensory cortex, suggesting the presence of functional receptors in these cells (58); however, it remains possible that this effect is indirect, via H4 receptors on microglia or other local cell types. There is, to our knowledge, no evidence suggesting the presence of the H4 receptors on astrocytes. H4 expression has, on the other hand, been reported in isolated microglia and in in vitro studies of immortalized microglia-like cells (40, 44).
Chronic HA deficiency in the Hdc knockout mouse (3) leads to effects opposite to those of exogenous HA administration, across multiple experiments. Hdc knockout mice have a normal number of microglia but a reduced density of microglial ramifications, as measured by the total area occupied by Iba1 expression (Figure 1). Interestingly, this effect is also observed in the hypothalamus, but not elsewhere in the brain (Supplementary Figure 2). This pattern correlates with levels of HDC protein, which we have found to be high in hypothalamus (where the histaminergic cell bodies are located) and in striatum, relative to other forebrain structures (60). H4 receptor expression is reduced in Hdc-KO mice (Figure 2), suggesting that chronic HA deficiency may also lead to a reduction in the mechanisms of microglial HA responsivity. Selective depletion of Hdc-expressing neurons in the TMN induces similar abnormalities, suggesting that neurotransmitter histamine is a key modulator of striatal microglia (as opposed to perivascular mast cells, or autocrine signal from microglia cells themselves).
Our data suggest that HA deficiency makes microglial cells more vulnerable to an inflammatory challenge. HA deficiency in the Hdc-KO mouse leads to a reduction in IGF-1 positive microglia (Figure 4). IGF-1-expressing microglia are also induced by TH2 cytokines such as IL-4. They have been shown to protect against neural cell death in organotypic hippocampal cultures (61), to support cortical neurons during development (22), and to promote neurogenesis and preserve hippocampal function in adulthood (23, 24), suggesting a neuroprotective function (57). In pathological contexts, IGF-1 positive microglia protect the brain after ischemic injury and in neurodegenerative conditions (25-28). Their reduction in Hdc-KO mice may produce a state of vulnerability.
Consistent with this, the Hdc-KO mouse shows an exaggerated microglial response to a pro-inflammatory challenge with LPS (Figure 5). These data suggest an overly exuberant inflammatory response to an immune challenge. Such a model is consistent with in vitro findings in an immortalized microglia-like cell line, in which HA was shown to activate N9 cells (by a chemotaxis assay) but to antagonize the activation produced by LPS stimulation (40); similar findings have recently been reported in primary microglial cultures (42). Our in vivo findings contrast, however, with other studies in acutely isolated microglia from neonates, which suggest that HA induces a pro-inflammatory phenotype, characterized by production of such cytokines as IL-6, IL-1β, and TNF-α, and oxidative enzymes such as iNOS (43-45).
Microglial heterogeneity
It is increasingly appreciated that microglia in the brain exhibit remarkable phenotypic heterogeneity (57) and are responsive to a wide range of factors in the milieu (62). Different protocols for microglial isolation, culture, and study may produce markedly different cellular phenotypes and thus divergent molecular and functional results (62); this may explain the divergent reports of the effects of HA on microglia studied in vitro (42-44). This reinforces the importance of studying them in vivo.
Several other aspects of our findings further emphasize the importance of in vivo studies. We find abnormalities in microglia in Hdc-KO animals in the striatum and in the hypothalamus, but not elsewhere; such regional variation would be lost in studies of isolated or cultured microglia. Our observation that neuron-derived HA is a key regulator of microglia would, additionally, not be possible in vitro. In vivo examinations such as we present here are technically challenging, but they are essential to characterize the full panoply of functions of these enigmatic cells in the brain.
We have focused largely on microglia in the adult mouse striatum. HA’s regulatory effects may differ in other contexts; the fact that microglial abnormalities are not uniform throughout the brain of Hdc-KO mice (Figure 1 and Supplementary Figure 2) supports this. Development may also modulate microglial responses to HA. The ability of HA to produce a pro-inflammatory phenotype in previous studies (43, 44) may be an artifact of in vitro analysis in primary cultures, but it might equally well be a result of the fact that those studies were performed on microglia from neonates, not adults. In vivo examination of the variation in the ability of HA to regulate microglia across development is an important goal for future work.
One distinction that has garnered substantial recent interest is the distinction between brain-resident microglia, which populate the central nervous system early in development, and macrophage-lineage cells that enter the brain at a later point in ontogeny. We have not examined this distinction in the current work. The possibility that HA differentially regulates these two populations merits future study.
A gene-environment interaction in the regulation of CNS inflammation
Many aspects of brain function, and most forms of psychopathology, are thought to result from gene-environment interactions, rather than from purely genetic or purely environmental causes. Our finding of differential inflammatory response in Hdc-KO mice exemplifies such an interaction. In unchallenged Hdc-KO mice, raised with minimal immunological challenge in a virus antigen-free vivarium environment, microglial Iba1 immunoreactivity density is lower than that in controls. However, after immunological challenge, the Hdc-KO mice mount an exaggerated response, such that after moderate LPS challenge the Iba1 pattern is reversed, with KO mice showing greater Iba1 expression extent than controls (Figure 5A, B). This is accompanied by increased production of IL-1β, confirming an increased inflammatory response.
This observation is particularly intriguing in the context of the fact that HA deficiency, in the Hdc-KO mice and in humans carrying mutations in this and other components of histamine signaling pathways, has been proposed as a potential cause of Tourette syndrome (3-6, 63). TS is well established to be substantially genetic, with recent estimates of heritability centering at around 50% (64). There is also substantial evidence that immunological processes contribute to TS, at least in some cases. Elevated inflammatory microglial markers in the striatum, including Iba1, have been documented in TS, post-mortem (31-33) and in a recent PET imaging study (30). Alterations in peripheral immune markers (65-68) and the response to inflammatory challenge (69, 70) have also been described.
These observations suggest a ‘two-hit’ model of inflammatory dysregulation. A genetic abnormality such as Hdc deficiency may produce only subtle deficits in isolation (3) but may create vulnerability for an enhanced or abnormal response to immunologic challenge. A subsequent infectious challenge – rare in mice raised in a virus antigen free vivarium environment but universal in the real world – may lead in such a genetic context to an overly exuberant inflammatory response, such as we see here after LPS challenge (Figure 5). This exaggerated inflammatory response, which resembles that seen in the basal ganglia in TS post-mortem (31) and in vivo (30), may be an important pathophysiological contributor to disease.
Supplementary Material
HIGHLIGHTS.
Histamine regulates microglia in vivo in the striatum
The H4 receptor activates microglia
Histamine deficiency leads to reduce neuroprotective microglia
Histamine deficiency leads to a potentiated response to inflammatory challenge.
ACKNOWLEDGEMENTS
We gratefully acknowledge Stacey Wilber and Jessica André for assistance with mouse care and genotyping and Eric Wohleb and Ania Majewska for constructive discussions and comments on the manuscript. This work was supported by the Allison Family Foundation (CP), the Tourette Syndrome Association (LF), the Massachusetts General Hospital Research Fund (CP), and the State of Connecticut through its support of the Ribicoff Research Facilities at the Connecticut Mental Health Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have no conflict of interest of relevance to this work to disclose.
References
- 1.Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88(3):1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 2.Panula P, Nuutinen S. The histaminergic network in the brain: basic organization and role in disease. Nature reviews. Neuroscience. 2013;14(7):472–487. doi: 10.1038/nrn3526. [DOI] [PubMed] [Google Scholar]
- 3.Castellan Baldan L, et al. Histidine decarboxylase deficiency causes tourette syndrome: parallel findings in humans and mice. Neuron. 2014;81(1):77–90. doi: 10.1016/j.neuron.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ercan-Sencicek AG, et al. L-histidine decarboxylase and Tourette’s syndrome. N Engl J Med. 2010;362(20):1901–1908. doi: 10.1056/NEJMoa0907006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez TV, et al. Rare copy number variants in tourette syndrome disrupt genes in histaminergic pathways and overlap with autism. Biological psychiatry. 2012;71(5):392–402. doi: 10.1016/j.biopsych.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karagiannidis I, et al. Support of the histaminergic hypothesis in Tourette syndrome: association of the histamine decarboxylase gene in a large sample of families. Journal of medical genetics. 2013;50(11):760–764. doi: 10.1136/jmedgenet-2013-101637. [DOI] [PubMed] [Google Scholar]
- 7.Shan L, Bossers K, Unmehopa U, Bao AM, Swaab DF. Alterations in the histaminergic system in Alzheimer’s disease: a postmortem study. Neurobiology of aging. 2012;33(11):2585–2598. doi: 10.1016/j.neurobiolaging.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 8.van Wamelen DJ, et al. Functional increase of brain histaminergic signaling in Huntington’s disease. Brain pathology. 2011;21(4):419–427. doi: 10.1111/j.1750-3639.2010.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodchild RE, et al. Distribution of histamine H3-receptor binding in the normal human basal ganglia: comparison with Huntington’s and Parkinson’s disease cases. The European journal of neuroscience. 1999;11(2):449–456. doi: 10.1046/j.1460-9568.1999.00453.x. [DOI] [PubMed] [Google Scholar]
- 10.Shan L, et al. Alterations in the histaminergic system in the substantia nigra and striatum of Parkinson’s patients: a postmortem study. Neurobiology of aging. 2012;33(7):1488, e1481–1413. doi: 10.1016/j.neurobiolaging.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Naddafi F, Mirshafiey A. The neglected role of histamine in Alzheimer’s disease. American journal of Alzheimer’s disease and other dementias. 2013;28(4):327–336. doi: 10.1177/1533317513488925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.John J, et al. Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Annals of neurology. 2013;74(6):786–793. doi: 10.1002/ana.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valko PO, et al. Increase of histaminergic tuberomammillary neurons in narcolepsy. Annals of neurology. 2013;74(6):794–804. doi: 10.1002/ana.24019. [DOI] [PubMed] [Google Scholar]
- 14.Passani MB, Ballerini C. Histamine and neuroinflammation: insights from murine experimental autoimmune encephalomyelitis. Frontiers in systems neuroscience. 2012;6:32. doi: 10.3389/fnsys.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nature reviews. Immunology. 2014;14(7):478–494. doi: 10.1038/nri3690. [DOI] [PubMed] [Google Scholar]
- 16.Hornig M, Lipkin WI. Immune-mediated animal models of Tourette syndrome. Neuroscience and biobehavioral reviews. 2013;37(6):1120–1138. doi: 10.1016/j.neubiorev.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell RH, Goldstein BI. Inflammation in children and adolescents with neuropsychiatric disorders: a systematic review. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(3):274–296. doi: 10.1016/j.jaac.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Frick LR, Williams K, Pittenger C. Microglial dysregulation in psychiatric disease. Clinical & developmental immunology. 2013;2013:608654. doi: 10.1155/2013/608654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji K, Miyauchi J, Tsirka SE. Microglia: an active player in the regulation of synaptic activity. Neural plasticity. 2013;2013:627325. doi: 10.1155/2013/627325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schafer DP, Lehrman EK, Stevens B. The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61(1):24–36. doi: 10.1002/glia.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhan Y, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nature neuroscience. 2014;17(3):400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- 22.Ueno M, et al. Layer V cortical neurons require microglial support for survival during postnatal development. Nature neuroscience. 2013;16(5):543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- 23.Kohman RA, DeYoung EK, Bhattacharya TK, Peterson LN, Rhodes JS. Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain, behavior, and immunity. 2012;26(5):803–810. doi: 10.1016/j.bbi.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziv Y, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nature neuroscience. 2006;9(2):268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 25.Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(10):2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DH, et al. Early immature neuronal death initiates cerebral ischemia-induced neurogenesis in the dentate gyrus. Neuroscience. 2014;284C:42–54. doi: 10.1016/j.neuroscience.2014.09.074. [DOI] [PubMed] [Google Scholar]
- 27.Chiu IM, et al. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(46):17913–17918. doi: 10.1073/pnas.0804610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arroba AI, Alvarez-Lindo N, van Rooijen N, de la Rosa EJ. Microglia-mediated IGF-I neuroprotection in the rd10 mouse model of retinitis pigmentosa. Investigative ophthalmology & visual science. 2011;52(12):9124–9130. doi: 10.1167/iovs.11-7736. [DOI] [PubMed] [Google Scholar]
- 29.Siskova Z, Tremblay ME. Microglia and synapse: interactions in health and neurodegeneration. Neural plasticity. 2013;2013:425845. doi: 10.1155/2013/425845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar A, Williams MT, Chugani HT. Evaluation of Basal Ganglia and Thalamic Inflammation in Children With Pediatric Autoimmune Neuropsychiatric Disorders Associated With Streptococcal Infection and Tourette Syndrome: A Positron Emission Tomographic (PET) Study Using 11C-[R]-PK11195. Journal of child neurology. 2014 doi: 10.1177/0883073814543303. [DOI] [PubMed] [Google Scholar]
- 31.Lennington JB, et al. Transcriptome Analysis of the Human Striatum in Tourette Syndrome. Biological psychiatry. 2014 doi: 10.1016/j.biopsych.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong JJ, et al. Microarray analysis in Tourette syndrome postmortem putamen. Journal of the neurological sciences. 2004;225(1-2):57–64. doi: 10.1016/j.jns.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Morer A, et al. Elevated expression of MCP-1, IL-2 and PTPR-N in basal ganglia of Tourette syndrome cases. Brain, behavior, and immunity. 2010;24(7):1069–1073. doi: 10.1016/j.bbi.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Xu M, et al. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2015 doi: 10.1073/pnas.1419533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmari SE, et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340(6137):1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shmelkov SV, et al. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nature medicine. 2010;16(5):598–602. doi: 10.1038/nm.2125. 591p following 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welch JM, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448(7156):894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peca J, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472(7344):437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen SK, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141(5):775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira R, et al. Histamine modulates microglia function. Journal of neuroinflammation. 2012;9:90. doi: 10.1186/1742-2094-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off signals control microglia. Trends in neurosciences. 2007;30(11):596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Iida T, et al. Histamine H3 receptor in primary mouse microglia inhibits chemotaxis, phagocytosis, and cytokine secretion. Glia. 2015;63(7):1213–1225. doi: 10.1002/glia.22812. [DOI] [PubMed] [Google Scholar]
- 43.Dong H, et al. Histamine induces upregulated expression of histamine receptors and increases release of inflammatory mediators from microglia. Molecular neurobiology. 2014;49(3):1487–1500. doi: 10.1007/s12035-014-8697-6. [DOI] [PubMed] [Google Scholar]
- 44.Zhu J, Qu C, Lu X, Zhang S. Activation of microglia by histamine and substance p. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2014;34(3):768–780. doi: 10.1159/000363041. [DOI] [PubMed] [Google Scholar]
- 45.Rocha SM, Pires J, Esteves M, Graca B, Bernardino L. Histamine: a new immunomodulatory player in the neuron-glia crosstalk. Frontiers in cellular neuroscience. 2014;8:120. doi: 10.3389/fncel.2014.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crain JM, Nikodemova M, Watters JJ. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. Journal of neuroscience research. 2013;91(9):1143–1151. doi: 10.1002/jnr.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohtsu H, et al. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS letters. 2001;502(1-2):53–56. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- 48.Zecharia AY, et al. GABAergic inhibition of histaminergic neurons regulates active waking but not the sleep-wake switch or propofol-induced loss of consciousness. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(38):13062–13075. doi: 10.1523/JNEUROSCI.2931-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Compact 2nd Ed. Elsevier Academic Press; Amsterdam ; Boston: 2004. [Google Scholar]
- 50.Yang CF, et al. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 2013;153(4):896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rapanelli M, Frick LR, Pittenger C. Disruption of histaminergic neurotransmission in the striatum produces repetitive grooming. under review. [Google Scholar]
- 52.Duric V, et al. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16(1):69–82. doi: 10.1017/S1461145712000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-lbeta and antiinflammatory IL-10 cytokines. Brain, behavior, and immunity. 2009;23(3):309–3l7. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams K, Bloch MH, State MW, Pittenger C. Tourette syndrome. In: C DS, Buxbaum JD, Sklar P, Nestler EJ, editors. Neurobiology of Mental Illness. 4th edition. 4th Ed. Oxford University Press; New York: 2013. [Google Scholar]
- 55.Leckman JF, Bloch MH, Smith ME, Larabi D, Hampson M. Neurobiological substrates of Tourette’s disorder. Journal of child and adolescent psychopharmacology. 2010;20(4):237–247. doi: 10.1089/cap.2009.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rapanelli M, et al. Dysregulated intracellular signaling in the striatum in a pathophysiologically grounded model of Tourette syndrome. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2014;24(12):1896–1906. doi: 10.1016/j.euroneuro.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olah M, Biber K, Vinet J, Boddeke HW. Microglia phenotype diversity. CNS & neurological disorders drug targets. 2011;10(1):108–118. doi: 10.2174/187152711794488575. [DOI] [PubMed] [Google Scholar]
- 58.Connelly WM, et al. The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS. British journal of pharmacology. 2009;157(1):55–63. doi: 10.1111/j.1476-5381.2009.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strakhova MI, et al. Localization of histamine H4 receptors in the central nervous system of human and rat. Brain research. 2009;1250:41–48. doi: 10.1016/j.brainres.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 60.Krusong K, et al. High levels of histidine decarboxylase in the striatum of mice and rats. Neurosci Lett. 2011;495(2):110–114. doi: 10.1016/j.neulet.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butovsky O, Talpalar AE, Ben-Yaakov K, Schwartz M. Activation of microglia by aggregated beta-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-gamma and IL-4 render them protective. Molecular and cellular neurosciences. 2005;29(3):381–393. doi: 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Pannell M, Szulzewsky F, Matyash V, Wolf SA, Kettenmann H. The subpopulation of microglia sensitive to neurotransmitters/neurohormones is modulated by stimulation with LPS, interferon-gamma, and IL-4. Glia. 2014;62(5):667–679. doi: 10.1002/glia.22633. [DOI] [PubMed] [Google Scholar]
- 63.Bloch M, State M, Pittenger C. Recent advances in Tourette syndrome. Curr Opin Neurol. 2011;24(2):119–125. doi: 10.1097/WCO.0b013e328344648c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davis LK, et al. Partitioning the heritability of Tourette syndrome and obsessive compulsive disorder reveals differences in genetic architecture. PLoS genetics. 2013;9(10):e1003864. doi: 10.1371/journal.pgen.1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kawikova I, et al. Children with Tourette’s syndrome may suffer immunoglobulin A dysgammaglobulinemia: preliminary report. Biological psychiatry. 2010;67(7):679–683. doi: 10.1016/j.biopsych.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawikova I, et al. Decreased numbers of regulatory T cells suggest impaired immune tolerance in children with tourette syndrome: a preliminary study. Biological psychiatry. 2007;61(3):273–278. doi: 10.1016/j.biopsych.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 67.Leckman JF, et al. Increased serum levels of interleukin-12 and tumor necrosis factor-alpha in Tourette’s syndrome. Biological psychiatry. 2005;57(6):667–673. doi: 10.1016/j.biopsych.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 68.Matz J, et al. Altered monocyte activation markers in Tourette’s syndrome: a case-control study. BMC psychiatry. 2012;12:29. doi: 10.1186/1471-244X-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Servello D, Sassi M, Gaeta M, Ricci C, Porta M. Tourette syndrome (TS) bears a higher rate of inflammatory complications at the implanted hardware in deep brain stimulation (DBS) Acta neurochirurgica. 2011;153(3):629–632. doi: 10.1007/s00701-010-0851-y. [DOI] [PubMed] [Google Scholar]
- 70.Weidinger E, et al. Impaired activation of the innate immune response to bacterial challenge in Tourette syndrome. The world journal of biological psychiatry: the official journal of the World Federation of Societies of Biological Psychiatry. 2014;15(6):453–458. doi: 10.3109/15622975.2014.907503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.