Abstract
Catheter-associated urinary tract infections (CAUTI) are the most common type of hospital-acquired infection, with more than 30 million catheters placed annually in the US and a 10–30% incidence of infection. Candida albicans forms fungal biofilms on the surfaces of urinary catheters and is the leading cause of fungal urinary tract infections. As a step toward new strategies that could prevent or reduce the occurrence of C. albicans-based CAUTI, we investigated the ability of antifungal β-peptide-based mimetics of antimicrobial peptides (AMPs) to kill C. albicans and prevent biofilm formation in synthetic urine. Many α-peptide-based AMPs exhibit antifungal activities, but are unstable in high ionic strength media and are easily degraded by proteases—features that limit their use in urinary catheter applications. Here, we demonstrate that β-peptides designed to mimic the amphiphilic helical structures of AMPs retain 100% of their structural stability and exhibit antifungal and anti-biofilm activity against C. albicans in a synthetic medium that mimics the composition of urine. We demonstrate further that these agents can be loaded into and released from polymer-based multilayer coatings applied to polyurethane, polyethylene, and silicone tubing commonly used as urinary catheters. Our results reveal catheters coated with β-peptide-loaded multilayers to kill planktonic fungal cells for up to 21 days of intermittent challenges with C. albicans and prevent biofilm formation on catheter walls for at least 48 hours. These new materials and approaches could lead to advances that reduce the occurrence of fungal CAUTI.
Keywords: Antifungal, Biofilms, β-Peptides, Coatings, Multilayers, Urinary Catheters
Graphical abstract
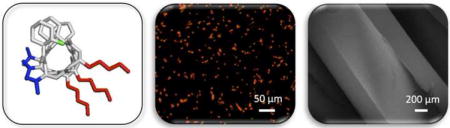
1. Introduction
Urinary tract infections are the most common type of hospital acquired infection and, in most cases, are associated with the presence of a urinary catheter [1, 2]. An estimated 30 million urinary catheters are placed annually in the US, with catheter-associated urinary tract infection (CAUTI) accounting for up to 40% of all nosocomial infections [2–4]. Candida species are the third most common cause of infection and Candida albicans is the most prevalent cause of fungal urinary infections [4–7]. Planktonic (free-floating) C. albicans is capable of colonizing the surfaces of urinary catheters and forming biofilms—a step that is a precursor to the development and progression of CAUTI [4, 8, 9]. Fungal infections continue to pose serious problems, despite the implementation of clinical best-practices such as improved hygiene, the use of closed catheter drainage systems, and daily cleansing with antiseptics and antimicrobials [10]. Many antifungal drugs such as amphotericin B and fluconazole currently used to treat fungal infections cause significant side effects and their widespread use contributes to the development of drug resistant strains [6, 11–13]. In the specific context of CAUTI, these drugs also exhibit reduced and variable activity in urine and generally exhibit reduced effectiveness against biofilms in urinary environments [6, 8]. Many different surface-based strategies have also been developed to prevent or reduce CAUTI, including the impregnation of catheters with silver, antiseptics, and antibiotics, however many of these methods of prophylaxis also elicit mammalian cell toxicity and/or kill fungal cells through mechanisms that can also contribute to the development of resistance [1, 14–19].
The work reported here was motivated by past studies demonstrating that several types of antimicrobial peptides (AMPs), components of the innate immune system in many species, can kill fungal cells and prevent biofilm formation [20, 21]. AMPs represent promising alternatives to other types of drugs because they typically kill fungal cells through membrane disruption mechanisms that have been suggested to be less likely to lead to the development of resistance [22]. Furthermore, AMPs can be designed to result in low toxicity to mammalian cells. Unfortunately, these natural α-peptide-based agents generally exhibit substantial reductions in antimicrobial activity at physiological conditions and they are susceptible to proteolytic degradation in vivo [23–27], which has thus far limited their therapeutic potential—particularly in the context of CAUTI, in which fungal cells grow and colonize the surfaces of catheters bathed in high osmolar and denaturing urine environments. We sought to develop new approaches that exploit the specific advantages of AMPs using β-peptide-based mimics of AMPs designed to fold into helical structures that are more stable in complex and higher ionic strength media [28–30]. Past studies have shown that β-peptide mimics of anti-bacterial AMPs can be designed to exhibit potent and specific antifungal activities, and that they fold into helical structures that are not disrupted at physiological ionic strength (150 mM) [31–34]. This combination of features and properties renders β-peptide AMP mimics promising as candidates for preventing fungal infections in urinary catheters.
Here, we demonstrate that an antifungal β-peptide that exhibits high antifungal activity and high specificity for C. albicans in conventional physiological culture media remains structurally stable in synthetic urine (SU), and that this β-peptide agent retains activity against planktonic C. albicans and against biofilms in catheters challenged with SU containing C. albicans. We further demonstrate (i) that antifungal β-peptides can be loaded into and released from polymer multilayer coatings fabricated in three commonly used catheter materials (polyurethane, polyethylene, and silicone tubes), and (ii) that coated catheters loaded with β-peptide are effective in killing planktonic C. albicans cells and preventing C. albicans biofilm formation in vitro over short (hours) and long (weeks) periods in SU media. Overall, our results demonstrate that amphiphilic, helical β-peptides can exhibit antifungal activity in complex urinary environments and that the fabrication of urinary catheters designed to locally release these agents offers a new and promising approach to prevent fungal biofilm formation on urinary catheters, with the potential to reduce the incidence and severity of CAUTI.
2. Materials and Methods
2.1 Materials
Silicone tubing (inner diameter = 0.04 inches) and polyurethane tubing (inner diameter = 0.025 inches) were purchased from Instech Laboratories (Plymouth Meeting, PA) polyethylene tubing (PE-100, inner diameter = 0.034 inches) from Intramedic (Franklin Lakes, NJ). Poly-L-lysine hydrobromide (PLL, MW = 15,000–30,000), chitosan (CH, medium molecular weight), poly-L-glutamic acid sodium salt (PGA, MW = 50,000–100,000), and branched polyethyleneimine (BPEI, MW = 25,000) were purchased from Sigma-Aldrich (Milwaukee, WI). Sodium hyaluronate (HA, MW = 1,500,000 – 2,200,000), Tween-20, MgCl2, and urea were obtained from Acros Organic (Pittsburgh, PA). RPMI 1640 powder (with L-glutamine and phenol red, without HEPES and sodium bicarbonate), propidium iodide (PI), 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT), 3-(N-morpholino) propanesulfonic acid (MOPS), Tris-base, KCl, CaCl2, KH2PO4, glucose, phosphate-buffered saline (PBS) liquid concentrate (10×), and NaCl were purchased from Fisher Scientific (Pittsburgh, PA). Menadione, glutaraldehyde, Na2-oxalate, Na2SO4, NH4Cl, creatinine, yeast nitrogen base medium, and formaldehyde were purchased from Sigma-Aldrich (Milwaukee, WI). Magainin-2 was purchased from American Peptide Company (Sunnyvale, California). Na3-Citrate was purchased from Mallinckrodt (St. Louis, MO). Osmium tetroxide (4%) was purchased from Electron Microscopy Sciences (Hatfield, PA).
2.2 General Considerations
Synthetic urine (SU) media was prepared as described elsewhere [35] and consisted of CaCl2 (0.65 g/liter), MgCl2 (0.65 g/liter), NaCl (4.6 g/liter), Na2SO4 (2.3 g/liter), Na3-citrate (0.65 g/liter), Na2-oxalate (0.02 g/liter), KH2PO4 (2.8 g/liter), KCl (1.6 g/liter), NH4Cl (1.0 g/liter), urea (25.0 g/liter), creatinine (1.1 g/liter), 5% yeast nitrogen base (YNB) medium (vol/vol), and 2% glucose (wt/vol). The pH was adjusted to 6.0 and the media was filter sterilized by passing it through a 0.22 μm pore filter. For circular dichroism experiments, a minimal SU media was prepared consisting of only the three major non-chiral constituents of this SU medium [urea (25.0 g/liter), NaCl (4.6 g/liter), and KH2PO4 (2.8 g/liter), with pH adjusted to 6.0 and filter sterilization as described above]. Coumarin-linker-(ACHC-β3hVal-β3hLys)3 (referred to from hereon as β-peptide 1) was synthesized by solid phase synthesis using a CEM Discover microwave reactor and methods reported previously [31, 36]. Fluorescence micrographs were obtained using an Olympus IX71 microscope with the MetaMorph Advanced version 7.8.1.0 software package. Images were processed using NIH Image J software and fluorescence images of labeled β-peptide were false colored yellow. Experiments to characterize the release of β-peptide 1 from polymer coatings were performed by measuring the β-peptide fluorescence in solution using a NanoDrop3300 (Thermo Scientific). Catheter tube-based experiments were either performed by leaving the ends of the tubes open in a humid environment or by reversibly plugging the ends of the tubes with custom made metal stoppers. C. albicans colonies on agar plates were counted manually for viability experiments. Scanning electron microscopy (SEM) and critical point drying of SEM samples were performed using a LEO SEM microscope and a Critical Point Dryer (Tousimis Samdri-815), respectively. Absorbance measurements for XTT assays were acquired at 490 nm using a plate reader (EL800 Universal Microplate Reader, Bio-Tek Instruments, Inc.) and the sonication of tubes for biofilm inhibition assays was performed using a sonication bath (Branson 2510R-MT). Circular dichroism of β-peptide 1 was measured using a circular dichroism spectrometer (Aviv, model 420). All chemicals were used as received without any further purification unless otherwise noted.
2.3 Characterization of Circular Dichroism of β-Peptide Solutions
Circular dichroism was measured at 0.1 mM of β-peptide 1 in PBS, methanol, or a minimal SU medium (prepared without the addition of interfering salts and chiral molecules such as glucose and YNB; see General Considerations). Briefly, β-peptide 1 was dissolved at 0.2 mg/mL in deionized H2O and then divided into desired amounts using a gas tight syringe (Hamilton). After lyophilization, peptides were dissolved in either PBS, methanol, or minimal SU medium. Circular dichroism was measured at 20 °C with a 1 mm path length cell and 5 second averaging times.
2.4 Planktonic Antifungal Susceptibility Testing
The antifungal activities of the β-peptides and antimicrobial α-peptide magainin-2 controls against C. albicans cells were characterized in 96-well plates according to the planktonic susceptibility testing guidelines provided by the Clinical and Laboratory Standards Institute (formally, National Committee for Clinical Laboratory Standards) broth microdilution assay, modified to include a quantitative XTT assessment of cell metabolic activity [31]. These assays were performed separately in both RPMI and SU media. Two-fold serial dilutions (100 µL) of β-peptides in RPMI (pH adjusted to 7.2 with MOPS) or SU were mixed with 100 µL of a C. albicans strain SC5314 cell suspension (grown for 24 hours at 35 °C and concentration adjusted to 1–5 × 103 cells/mL based on absorbance at 600 nm) and the plates were incubated at 35 °C for 48 hours. Wells lacking β-peptide (cell controls) and wells lacking both peptide and cells (medium sterility controls) were included in every plate that was assayed. After 48 hours, the MICs were recorded visually and by using an XTT metabolic assay. 100 μL of XTT solution (0.5 g/L in PBS, pH 7.4, containing 3 μM menadione in acetone) was added to all wells and plates were incubated at 37 °C in the dark for 1.5 and 2.5 hours for RPMI and SU based experiments, respectively. The supernatants (75 μL) from all wells were transferred to a fresh plate and absorbance measurements at 490 nm were recorded. The metabolic activity was then plotted as a function of β-peptide concentration. The metabolic activity was calculated as:
where A490, , and are the average absorbance values at 490 nm of the supernatant from wells containing a specific concentration of β-peptide, cell control wells lacking β-peptide, and medium sterility control wells, respectively. Experiments were performed in triplicate and repeated on at least two different days. The lowest assayed concentration of β-peptide that resulted in less than 10% average metabolic activity was taken as the minimum inhibitory concentration (MIC) of the peptide under that condition.
2.5 Fluorescence Imaging of Planktonic C. albicans Cells
To image the interaction of β-peptide 1 with C. albicans cells in SU media, a cell suspension of 1×105 cells/mL was prepared. Two-fold serial dilutions (from 4–64 µg/mL) of β-peptide 1, a positive kill control with methanol, and β-peptide-free control were prepared, and 50 µL of each solution was added to an equal volume of cell suspension. Cells were incubated with the solutions for 3.5 hours at 37 °C, and PI was then added at a final concentration of 1 µg/mL. Plates were incubated at 37 °C for an additional 45 minutes, after which cells were imaged using a fluorescence microscope with a red filter for PI and a blue filter for coumarin-labeled β-peptide 1.
2.6 Fungal Biofilm Formation in the Presence of β-Peptide
C. albicans biofilm susceptibility to β-peptide 1 in SU media and RPMI was performed as described previously [31, 33]. Briefly, overnight cultures of C. albicans were adjusted to 106 cells/mL in SU media or RPMI media. Two-fold serial dilutions of β-peptide in respective media were added to equal volumes of cells. Plates were incubated at 37 °C for 48 hours and XTT was used to evaluate biofilm MICs as described previously. Biofilm MICs in RPMI or SU media were defined as the lowest assayed concentration of β-peptide that resulted in less than a 10% average metabolic activity.
2.7 Fabrication of Polyelectrolyte Multilayer Coatings in Urinary Catheters
Solutions of BPEI, PLL, PGA (1 mg/mL), HA (2 mg/mL), and CH (in 0.1M acetic acid; 2 mg/mL) were prepared using a NaCl solution (0.15 M) in deionized water. A single layer of BPEI was first deposited on the inner surfaces of catheter tube segments by infusing a solution of BPEI into the tube and allowing it to incubate in the tube for 30 min. The tubes were then rinsed twice using a solution of NaCl (0.15 M) in water for 1 min each. PEM films were then deposited using a previously developed ‘fill-and-purge’ approach [37, 38]. Briefly, (i) a solution of negatively charged polymer (PGA or HA) was infused into the tube and allowed to incubate (for 3 min or 5 min, respectively), (ii) tubes were rinsed twice by infusing a rinse solution of NaCl solution in water (0.15 M) for 1 min each, (iii) tubes were infused with a solution of positively charged polymer (PLL or CH for 3 min or 5 min, respectively), and (iv) tubes were rinsed again in the manner described in step (ii). This was repeated until 19.5 PGA/PLL or HA/CH bilayers had been deposited (such that all films were terminated by the deposition of a final layer of the cationic polymer, as reported previously [37, 38]).
2.8 Loading of β-Peptide into Film-Coated Catheters
A 10 mg/mL solution of β-peptide 1 in 0.15 M NaCl was infused into tubes coated with HA/CH or PGA/PLL multilayers. The peptide solution was allowed to remain in the tubes at room temperature for 24 hours. The peptide solution was then removed from the tubes and the tubes were rinsed consecutively using a solution of PBS for 5 min, a solution of Tris-buffered saline Tween (TBST; 10 mM Tris–HCl, 100 mM NaCl, 0.1% Tween–20) for 1 hour, and a solution of PBS for 5 min. Tubes fabricated using this procedure were either used immediately or stored in a dried state at room temperature until use. Control uncoated tubes (no multilayer coating) were also treated with peptide in the manner described above. Film-coated control tubes (no peptide) were fabricated as described above but were not loaded with β-peptide.
2.9 Characterization of Intraluminal Release Profiles
Characterization of β-peptide release profiles was performed either in PBS or modified SU media (prepared without glucose, with 1% (v/v) Penicillin Streptomycin antibiotic to reduce the likelihood of contamination). Solutions were infused to completely fill a 2 cm segment of β-peptide-treated tubes and the filled tubes were incubated at 37 °C. The tubes were removed from the incubator at predetermined intervals and solution fluorescence was measured (at an excitation of 336 nm and an emission of 402 nm, corresponding to the excitation and emission maxima of the β-peptide). After each measurement, the tubes were infused with fresh solution and returned to the incubator. Fluorescence measurements were converted to β-peptide concentrations using a calibration curve created with known concentrations of β-peptide. Release profiles were constructed by cumulatively summing the concentration of β-peptide released into solution at individual time points.
2.10 Characterization of the Antifungal Activity of Catheter Tubes
Overnight cultures of C. albicans SC5314 cells grown at 30 °C in liquid yeast extract-peptone-dextrose (YPD) medium were washed with PBS and re-suspended in SU medium. For evaluation of short-term antifungal activity, 15 μL of C. albicans cell suspensions (adjusted to 106 cfu/mL with SU) were infused into 3 cm segments of the polyethylene tubes to completely fill the tubes (both catheters coated with β-peptide-loaded films, and control (no-peptide and no-film/no-peptide) catheter tubes), and the samples were incubated at 37 °C for six hours. Post-incubation, the C. albicans inoculum was removed from the tubes, a 100-fold dilution of the inoculum was plated on YPD plates, and the plates were incubated at 37 °C for ~24 hours. The number of colonies formed on agar plates was then counted. To evaluate the antifungal activity of the tubes at later times, catheter segments were pre-incubated with PBS at 37 °C for 0, 3, 7, 14 or 21 days with buffer replaced every 3–4 days. The antifungal activities of the tubes were then evaluated by performing experiments as described above. At the end of the six-hour C. albicans inoculum incubation, solutions were placed in individual wells of a 96-well plate and, after 24 hours, an XTT assay was performed to quantify metabolic activity in cells in the inoculum from the tubes. Background absorbance from wells containing media and XTT was subtracted from every reading and the data was plotted relative to the absorbance value from wells containing samples of solution from no-film/no-peptide control tubes.
2.11 Biofilm Inhibition Assays
For in vitro biofilm assays, C. albicans SC5314 cells grown overnight at 30 °C in liquid YPD medium were washed with PBS and re-suspended in SU medium. C. albicans cell suspensions (adjusted to 106 cfu/mL with SU) were then completely infused into 3 cm segments of catheters coated with β-peptide-loaded films and control (no-peptide and no-film/no-peptide) catheters. The tubes were placed in separate wells of a 6-well plate containing 2 mL of SU medium per well and the plate was then incubated at 37 °C for 48 hours. At the end of 48 hours, the extent of biofilm formation in the tubes was characterized using either (i) a metabolic XTT assay or (ii) by visualizing the biofilm formed by SEM. For XTT assays, each tube was placed in an individual microcentrifuge tube and sonicated for 15 min to dislodge any strongly adherent biofilm. The inoculum was then removed from inside the tubes and placed in separate wells of a 96-well plate. The tubes were washed twice with PBS (40 μL per wash) and the wash solutions were added to respective wells of the 96-well plate. The microcentrifuge tubes were centrifuged and the recovered solution was also added to respective wells of the 96-well plate. XTT solution (40 μL; 0.5g/L in PBS, pH 7.4, containing 3 μM menadione in acetone) was added to each well containing biofilm solution and to wells containing only SU medium to serve as negative controls. After incubating the solution at 37 °C for 2–2.5 h, 75 μL of the supernatant was transferred to a 96-well plate and the absorbance of the solution at 490 nm was measured to determine the relative metabolic activity of biofilms formed in the different tubes. Background absorbance from wells containing media and XTT was subtracted from all readings and data was plotted relative to the absorbance value from wells containing samples of solution from no-film/no-peptide control tubes. For characterization of biofilms by SEM, catheter tube segments were placed in fixative (1% glutaraldehyde (v/v) and 4% formaldehyde (v/v)) overnight at 4 °C. The samples were rinsed in PBS (0.1 M) for 10 min and then placed in osmium tetroxide (1%) for 30 min, followed by 10 min in phosphate buffer (0.1 M). The samples were then dehydrated by a series of ethanol washes (30%, 50%, 70%, 85%, 95%, and 100%) for 15 min each. Final desiccation was performed by critical-point drying. Samples were mounted on aluminum stubs and sliced open to reveal the inside of the catheter tubes, and then sputter coated with gold-palladium and characterized by SEM in high-vacuum mode at 5 kV.
2.12 Statistical Analysis
All experiments were conducted at least in triplicate and data are presented as means with standard deviation. Statistical analysis was performed using Microsoft Excel and comparisons between the groups were performed using a two-tailed Student’s t-test. Results were considered statistically significant at p < 0.05.
3. Results and Discussion
3.1 Structural Stability, Antifungal Activity, and Anti-Biofilm Activity of β-Peptides in SU Media
One challenge confronting the treatment of CAUTI using antifungal drugs, relative to the treatment of other fungal infections, is that urine has a higher osmolality and varies in composition compared to blood and other physiological environments [39, 40]. These differences can lead to reductions in the activities of conventional antifungal drugs such as amphotericin B, and are even more problematic for antimicrobial helical α-peptides that are structurally unstable in high ionic strength media [12, 25, 35]. Although past studies have demonstrated that certain 14-helical β-peptide mimics of antibacterial AMPs can retain their designed helical conformations at physiological ionic strengths and in the presence of denaturants [29, 41], it was not clear at the outset of these studies how the specific composition and higher osmolality of urine would impact the structures or the antifungal activities of helical β-peptides. To investigate the potential influence of salts, denaturants, and other small molecules commonly found in urine on the stability and antifungal activity of β-peptides, we characterized β-peptide 1 dissolved in synthetic urine (SU) medium. β-Peptide 1 contains a fluorescent coumarin label at the N-terminus to facilitate measurement of concentration and localization based on fluorescence. The unlabeled analogue of β-peptide 1 has been demonstrated to be antifungal against C. albicans in standard susceptibility testing medium (e.g., in RPMI, MIC = 8 μg/mL) and to have relatively low mammalian toxicity (e.g., on the basis of red blood cell hemolysis assays) [31].
We performed a series of experiments to characterize the structure (e.g., helicity) and activity (e.g., MIC against C. albicans planktonic cells and biofilm formation) of β-peptide 1 in SU medium using circular dichroism (CD) and bioassays, respectively (CD is a spectroscopic method that measures the differential adsorption of right and left-handed circularly polarized light by optically active chiral molecules and is often used as a measure of the secondary structure of peptides). For these experiments, we used a minimal SU medium prepared without the addition of interfering salts and chiral molecules (see Materials and Methods for additional details) because we were unable to acquire complete CD spectra in the complete SU medium used in other biological experiments below. As shown in Figure 1A (dotted line), solutions of β-peptide exhibited a CD minimum at ~207 nm in minimal SU medium. The wavelength and the intensity of that minimum were similar to those observed for β-peptide 1 in PBS (Figure 1A; solid line). These results demonstrate that this β-peptide retained its higher order structure in this minimal SU medium. We note that the minimum observed at ~207 nm is shifted from the wavelength of 214 nm that is characteristic of the secondary structure of many 14-helical β-peptides, including the unlabeled analog of β-peptide 1 in Tris-buffered saline [29] and in PBS. Past studies have reported shifts from 214 nm to 205 nm to correlate to the aggregation or ‘bundling’ of certain hydrophobic 14-helical β-peptides [42]. It is possible that the addition of the fluorescent coumarin label to the N-terminus of β-peptide 1 could also affect changes in aggregation state (additional support for this proposition was provided by the results of experiments conducted using solutions of this β-peptide in methanol (Figure 1A, dashed line), which revealed a minimum at 214 nm consistent with past reports that methanol can disrupt such aggregated states without disrupting 14-helicity [42]). Regardless, the results shown in Figure 1A demonstrate that this minimal SU medium does not perturb the native helical structure exhibited by this fluorescently end-labeled β-peptide in PBS. Further, as shown in Figure 1B, β-peptide 1 retained substantial antifungal activity in SU medium and exhibited a MIC of 16 µg/mL (open symbols). We note that the MIC of the β-peptide measured in SU medium is only two-fold higher than its MIC under standard planktonic susceptibility testing conditions in RPMI (Table 1 and Figure S1). This modest reduction in potency in SU medium contrasts significantly to the lack of antifungal activity observed for magainin-2 in SU medium (Figure 1B, closed symbols, MIC > 128 µg/mL; magainin-2 is an α-peptide AMP shown to be antifungal in certain low ionic-strength media, e.g., IC50= 0.3 μM in isotonic glucose phosphate buffer [43]).
Figure 1.
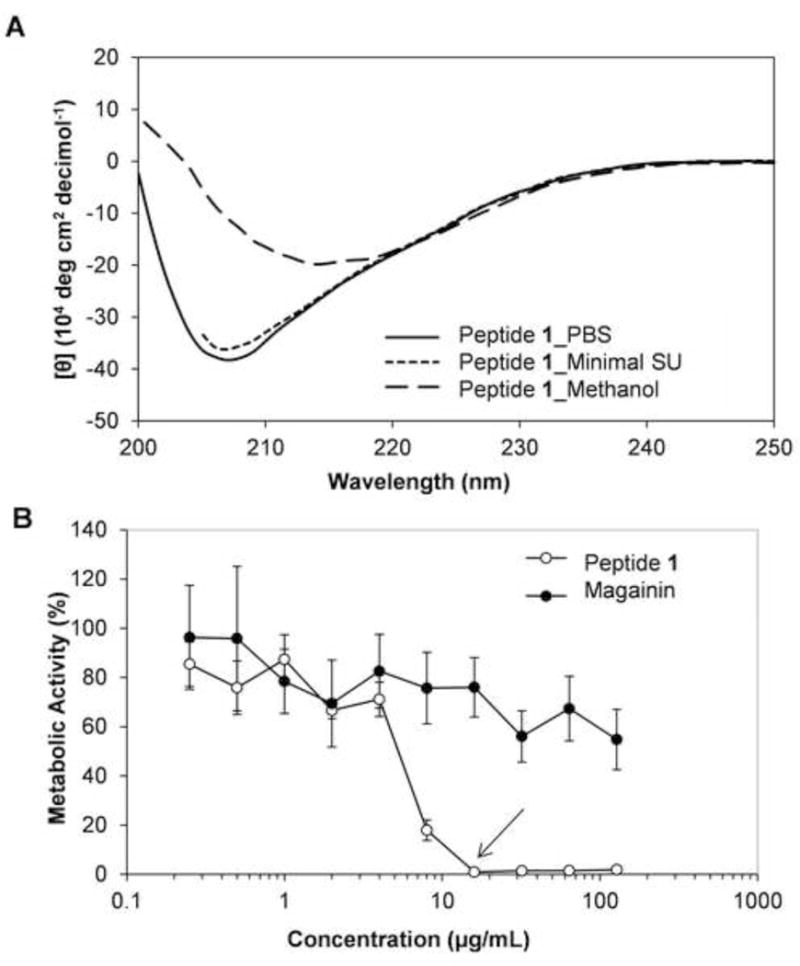
(A) Circular dichroism spectra of β-peptide 1 in PBS (solid line), in minimal SU medium (dotted line), and in methanol (dashed line). (B) Planktonic antifungal activity of β-peptide 1 (solid circles) and antimicrobial α-peptide magainin-2 (open circles) in SU media. C. albicans cells (103 cells/mL) were incubated in the presence of 2-fold dilutions of peptide for 48 hours and XTT was used to assess metabolic activity. Data points are averages of at least two independent experiments with triplicates in each and error bars denote standard deviation. Arrow indicates MIC of the peptide in SU medium.
Table 1.
Minimum Inhibitory Concentration (MIC) of β-peptide 1 against C. albicans planktonic cells and biofilms in RPMI and SU media.
| Media | Growth Condition | MICa(μg/mL) |
|---|---|---|
| SU | Planktonic | 16 |
| SU | Biofilm | 32 |
| RPMI | Planktonic | 8 |
| RPMI | Biofilm | 32 |
MICs were determined by average of three experiments of three replicate each by incubating C. albicans cells (103 cells/mL for planktonic and 106 cells/mL for biofilm) in RPMI or SU with β-peptides for 48 hours and β-peptide susceptibility was assessed using an XTT reduction assay to compare the absorbance at 490 nm for β-peptide-treated samples and untreated samples.
In addition to evaluating the MIC of β-peptide 1 against C. albicans in SU medium, we assessed the concentration-dependent killing of yeast in SU media by visualizing the extent of co-localization of β-peptide-associated fluorescence and a red fluorescent dye (PI) used to stain dead yeast cells (Figure 2). At concentrations of β-peptide as low as 8 µg/mL, we observed some cells stained positive for both the β-peptide and PI (Figure 2C and S2); at 16 µg/mL and at higher peptide concentrations (Figure 2D–F), we observed complete cell death, indicated by PI staining, and β-peptide present in all treated cells. Finally, to evaluate the ability of β-peptide 1 to prevent biofilm formation, we performed a series of experiments in which 2-fold dilutions of β-peptide were added to C. albicans cells for 48 hours in SU medium. As shown in Table 1 and Figure S1, the amount of β-peptide required to prevent biofilm formation was 32 µg/mL in both SU and RPMI media, indicating that the β-peptide retained the ability to prevent biofilm formation in SU medium without a reduction in antifungal activity. Overall, our results demonstrate that β-peptide 1 remains helical in SU medium and exhibits substantial antifungal activity and the ability to prevent fungal biofilm formation in complex SU medium containing salts, denaturants, and other small molecules.
Figure 2.
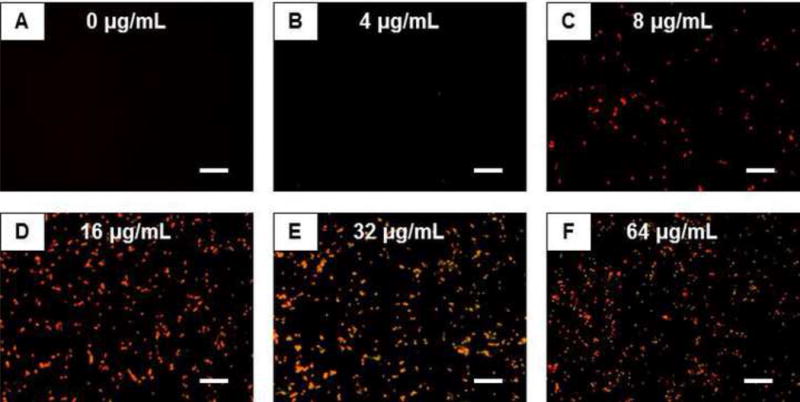
Fluorescence micrographs of C. albicans treated with β-peptide 1. Cells (105 cells/mL) were treated with the labeled β-peptide (yellow) at 2-fold varying concentrations from 0 to 64 µg/mL for 3.5 hours. Cells were stained with PI (red) to identify dead cells. Scale bars = 50 μm.
3.2 Loading and Release of β-Peptide from Polymer-Coated Catheters
We used two model biocompatible polyelectrolyte multilayer coating systems—a polypeptide-based system consisting of poly-L-lysine (PLL) and poly-L-glutamic acid (PGA) [37, 44–46], and a polysaccharide-based system consisting of hyaluronic acid (HA) and chitosan (CH) [38, 47–50] — to fabricate polymeric thin film coatings on the intraluminal walls of urinary catheters. Using a previously developed fill-and-purge method [37, 38], we fabricated PGA/PLL and HA/CH multilayers inside polyurethane, polyethylene, and silicone tubes commonly used to manufacture urinary catheters. β-Peptide was loaded into the coated catheter tubes by infusing β-peptide 1 into the tubes and allowing it to infiltrate the coatings by diffusion for 24 hours (see Materials and Methods for additional details of β-peptide loading). The extent of loading was assessed by fluorescence microscopy (Figure 3). Polyethylene and silicone tubes coated with PGA/PLL films and loaded with β-peptide exhibited similar levels of fluorescence (Figure 3A,C). In contrast, coated polyurethane tubes exhibited markedly lower fluorescence (Figure 3B), suggesting that lower levels of β-peptide were loaded into films fabricated in polyurethane tubes (we return to this observation again in the discussion below). However, all three catheter tubes coated with HA/CH films and loaded with β-peptide showed levels of fluorescence that were qualitatively similar to each other and greater than those observed in catheter tubes coated with β-peptide-loaded PGA/PLL films (Figure 3D–F). The higher apparent loading in the catheters coated with HA/CH films is consistent with our previous observations demonstrating that HA/CH films fabricated in polyethylene catheter tubes were thicker than those fabricated using PGA/PLL (e.g., 1340 ± 240 nm vs. 710 ± 50 nm) and released more β-peptide (e.g., ~ 4.3 µg vs. ~2.7 µg) under otherwise identical conditions [38]. In all cases, uncoated control tubes infused with β-peptide exhibited negligible fluorescence (Figure 3G–I).
Figure 3.
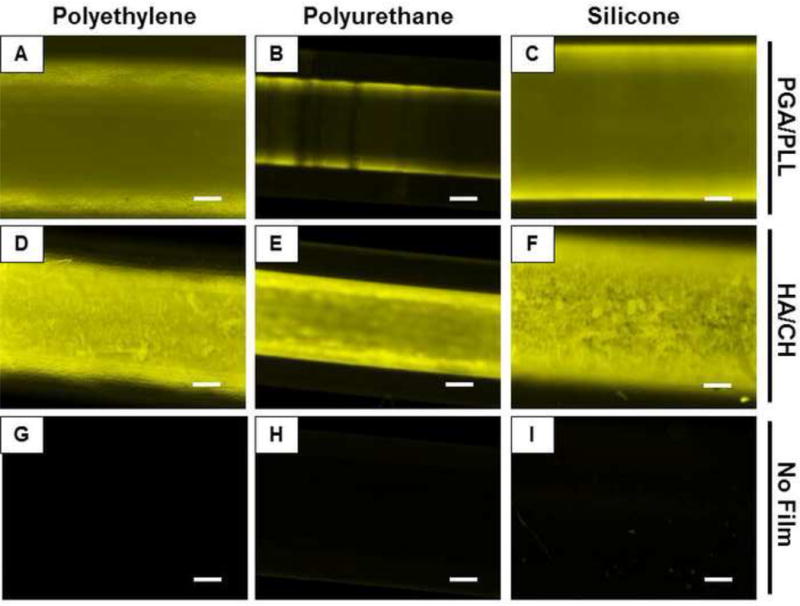
Fluorescence micrographs of catheter tube segments of polyethylene (A,D,G), polyurethane (B,E,H), and silicone (C,F,I) coated with multilayer films composed of PGA/PLL (A–C) or HA/CH (D–F) and incubated with β-peptide 1 for 24 hours. (G–I) Controls consisting of tube segments not coated with any multilayer film and incubated with β-peptide 1. Scale bars = 250 μm.
To characterize potential differences in the β-peptide release profiles from films fabricated in polyurethane, polyethylene, and silicone tubes, we incubated tubes prepared and loaded as described above in buffer solution for predetermined amounts of time and measured the amount of β-peptide released into the intraluminal space. For these long-term release experiments, we used PBS (instead of complex rich media with nutrient sources) to minimize chances of microbial contamination in the lumen of the tubes (which we occasionally observed when SU medium was used). Figure 4 shows the release of β-peptide from catheters coated with PGA/PLL multilayers (Figure 4A), HA/CH multilayers (Figure 4B), and uncoated control tubes (Figure 4C). To facilitate comparisons among tubes with different diameters (see Materials and Methods), Figure 4 shows the release profiles in terms of the concentration of β-peptide released normalized per unit area of the inner tubing wall. After 70 days of incubation, more than 70% of the β-peptide was released from the PGA/PLL-coated tubes (Figure 4A) and more than 80% of the β-peptide was released from the HA/CH-coated tubes (Figure 4B) for all catheter tube materials. During this time period, PGA/PLL coatings loaded with β-peptide in both polyethylene and silicone tubes released ~230 µg/mL/mm2 of β-peptide. In contrast, PGA/PLL in polyurethane tubes released only ~150 µg/mL/mm2 of β-peptide (Figure 4A). The HA/CH coatings released higher amounts of β-peptide (~445, ~360, and ~520 µg/mL/mm2 from coatings on polyethylene, silicone, and polyurethane, respectively). Subsequent short-term (e.g., 30-day) release experiments using β-peptide-loaded catheters infused with SU medium revealed that the initial release rates of peptide into SU were similar to those observed in PBS (Figure S3).
Figure 4.
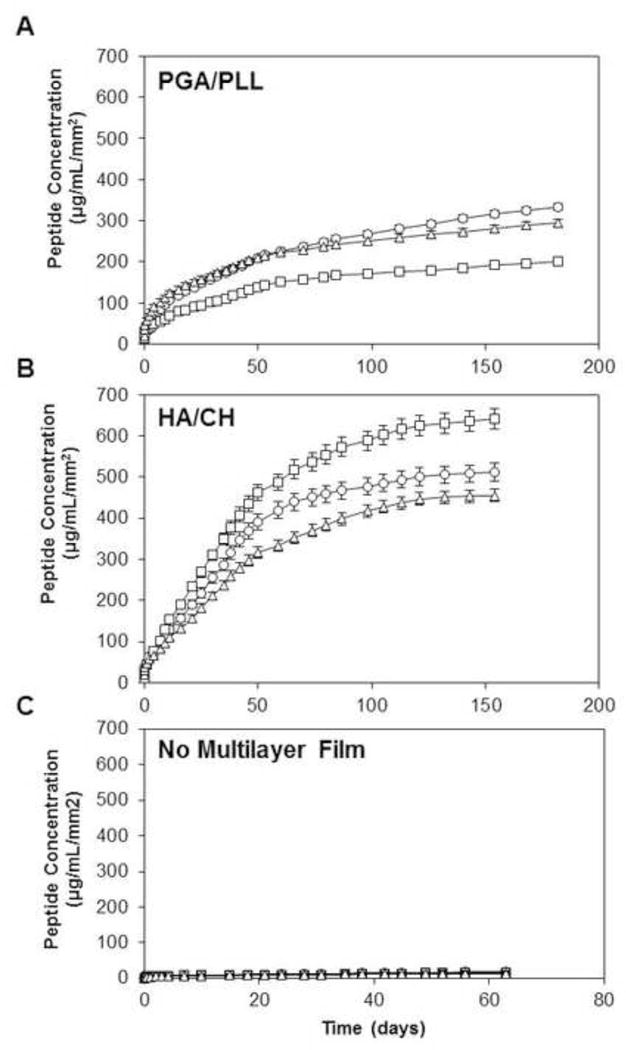
Plots of the release of β-peptide 1 into intraluminal buffer solution in polyethylene (circles), polyurethane (squares), and silicone (triangles) catheter tubes coated with β-peptide-loaded (A) PGA/PLL multilayers, (B) HA/CH multilayers, and (C) no multilayer film. Data points are averages of three technical replicates and error bars denote standard deviations.
Coated catheter tubes were longitudinally sliced and imaged using SEM to characterize film morphology and uniformity (Figure 5). We observed the presence of continuous and uniform coatings on the luminal surfaces of all catheters, with the exception of polyurethane tubes coated with PGA/PLL films (Figure 5B,E). Images of intentionally scratched films revealed PGA/PLL films in polyurethane tubes to be discontinuous and patchy (Figure 5B,E). Although we do not currently understand the reasons for this different film morphology, these results are consistent with the lower loadings and different release profiles observed for these PGA/PLL polyurethane catheter tubes observed in the studies described above (Figures 3 and 4).
Figure 5.
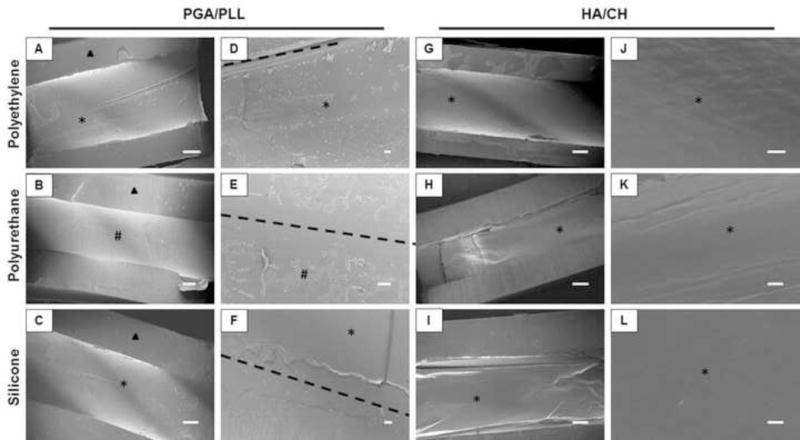
Scanning electron micrographs of longitudinal sections of polyethylene (A,D,G,J), polyurethane (B,E,H,K), and silicone (C,F,I,L) catheter tube segments coated with (A–F) PGA/PLL and (G–L) HA/CH multilayers. The dotted lines indicate where PGA/PLL coated catheters were intentionally scratched to more clearly evaluate the presence of the coatings. Triangles indicate catheter walls, asterisks (*) indicate uniform multilayer coatings on the inner surface of the tube, and pound symbols (#) indicate areas of patchy multilayer coatings. Scale bars = 200 μm (A–C,G–I), 20 μm (D–F,J–L).
3.3 Antifungal Activities of β-Peptide-Loaded Catheters in SU Media
We assessed the short-term antifungal activity of PE catheter tubes coated with β-peptide-loaded PGA/PLL and HA/CH multilayers in SU medium by incubating C. albicans (106 cells/mL in SU) in the catheters for 6 hours at 37 °C, plating the inoculum on agar, and counting colonies. For both β-peptide-loaded PGA/PLL-coated and HA/CH-coated catheters, there was a substantial and nearly complete reduction of viable C. albicans compared to control untreated catheter tubes (Figure 6A,B; p < 0.05 by two-tailed t-test). This observation is consistent with the release curves shown in Figure 4, which demonstrate that PE tubes coated with PGA/PLL or HA/CH released 19 ± 5 µg/mL and 19 ± 3 µg/mL of β-peptide over this time period. These values are above the MIC for β-peptide 1 in SU medium (it is important to note however that the release profiles shown in Figure 4 were obtained in the absence of C. albicans, and that the release rates in the presence of inoculum could vary). Tubes coated with PGA/PLL or HA/CH films alone (no β-peptide) did not result in a decrease in antifungal activity in SU medium (tube/film, Figure 6A,B). This result contrasts to the results of our past studies, which demonstrated that HA/CH coatings exhibit some degree of inherent antifungal activity (in the absence of β-peptide 1) when challenged with planktonic C. albicans in conventional physiological growth medium (e.g., RPMI) [38]. This result suggests that CH, which has been reported to have inherent antifungal activity in certain contexts [51, 52] and is believed to confer antifungal activity to these HA/CH films when inoculated with yeast in conventional media, may not exhibit inherent antifungal activity in SU medium.
Figure 6.
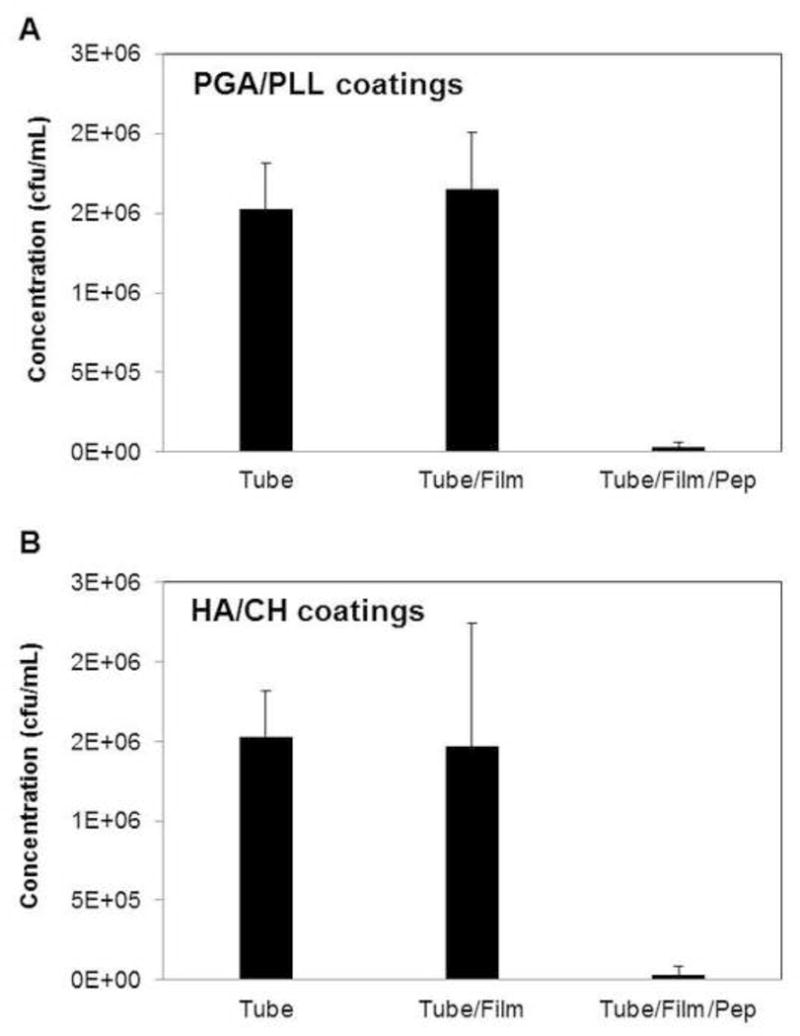
Antifungal activity of polyethylene catheter tubes coated with (A) PGA/PLL or (B) HA/CH multilayers loaded with antifungal β-peptide 1 (tube/film/pep) against C. albicans (106 cells/mL) in SU media. Antifungal activities of control untreated tube segments (tube) and tubes coated with multilayers but not loaded with β-peptide (tube/film) are also shown. Data points are averages of three independent experiments consisting of three technical repeats each and error bars denote standard deviation. Colony counts of solutions from tubes coated with β-peptide-loaded films (tube/film/pep) were statistically different (p < 0.05 by two-tailed t-test) from colony counts of solutions from untreated controls (tube).
We next conducted a series of experiments to evaluate the antifungal activities of these β-peptide-loaded catheter tubes at longer time points by pre-incubating polyethylene catheters with buffer solution for varying lengths of time and then subsequently quantifying the antifungal activity of the catheters in SU medium (these experiments were not performed using pre-incubation in SU medium to prevent microbial contamination during the pre-incubation period). Catheter segments were pre-incubated with buffer solution for 0, 3, 7, 14, or 21 days. At the end of the pre-incubation period, buffer solutions were removed and replaced with C. albicans in SU medium for 6 hours, as described above, and an XTT assay was used to quantify reductions in metabolic activity. Catheters coated with β-peptide-loaded PGA/PLL and HA/CH films exhibited strong and statistically significant antifungal activities for up to 21 days in these experiments (Figure 7A,B; white bars; p < 0.05 by two-tailed t-test). As expected, film-coated control tubes that were not loaded with β-peptide did not exhibit any significant antifungal activity (Figure 7A,B; grey bars).
Figure 7.
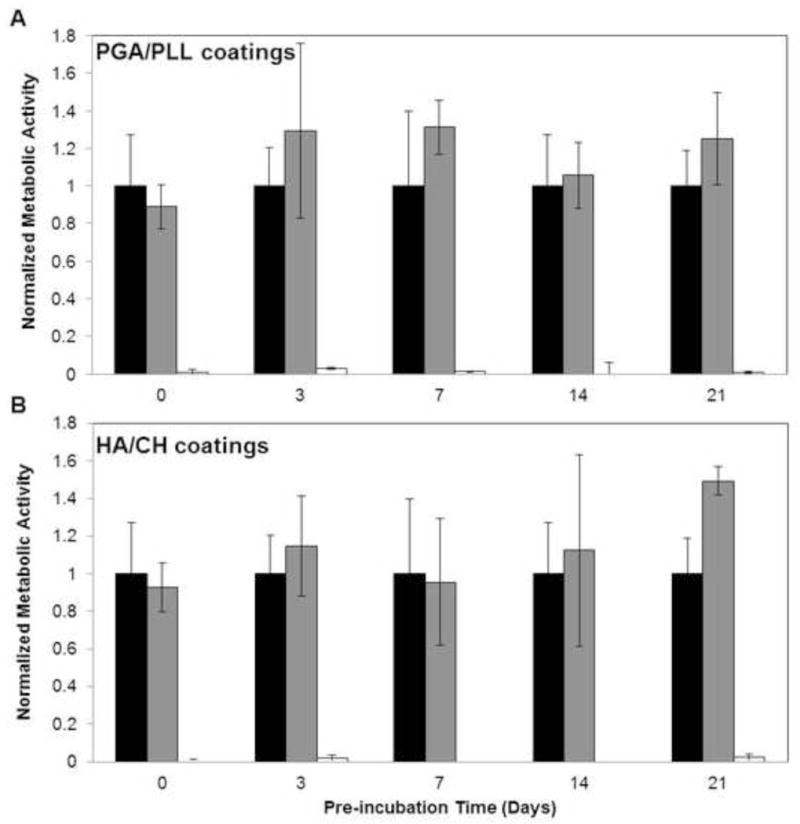
Antifungal activity of polyethylene catheter tubes coated with (A) PGA/PLL and (B) HA/CH in SU media after pre-incubation with PBS for extended times. Catheter tubes coated with multilayer film and loaded with β-peptide (black bars), tubes coated with multilayer films (no peptide; grey bars), and untreated bare control tubes were filled with PBS for pre-determined times, as indicated on the x-axis, after which the buffer was removed and antifungal activity was evaluated by adding C. albicans inoculum and using XTT to evaluate metabolic activity. Data points shown are average of three replicates and all values are normalized to the metabolic activity of the untreated catheter tubes at the same time point. For all pre-incubation times, reductions in metabolic activity for β-peptide-loaded films (tube/film/pep) were statistically different (p < 0.05 by two-tailed t-test) from untreated controls (tube) under the same conditions.
3.4 β-Peptide-Loaded Catheters Inhibit C. albicans Biofilm Formation in SU Medium
Finally, we characterized the ability of PGA/PLL and HA/CH film-coated catheter tubes loaded with β-peptide 1 to prevent C. albicans biofilm formation in SU medium by inoculating 106 cells/mL of C. albicans in SU medium in the catheters for 48 hours at 37 °C (see Methods for additional details) and evaluated the extents and metabolic activities of biofilms formed on intraluminal catheter surfaces by SEM and our XTT assay. For biofilm characterization using SEM, polyethylene catheter tubes were fixed, dried, longitudinally sliced, and the exposed intraluminal surfaces of the tubes were imaged. We observed robust biofilms on untreated control tubes (Figure 8A,F) and catheter tubes coated with both PGA/PLL (Figure 8B,G) and HA/CH multilayers (Figure 8C,H). However, we observed little to no biofilm on the surfaces of catheter tubes coated with β-peptide-loaded PGA/PLL (Figure 8D,I) or HA/CH (Figure 8 E,J) films. We also observed a significant reduction in the metabolic activities of biofilms formed on β-peptide-loaded PGA/PLL and HA/CH coated films (tube/film/pep; Figure 9A and B, respectively) in comparison to untreated control polyethylene (black), polyurethane (grey), and silicone (white) tubes (p < 0.05 by two-tailed t-test). Tubes coated with multilayers but not loaded with β-peptide did not demonstrate any decrease in biofilm formation (tube/film; Figure 9).
Figure 8.
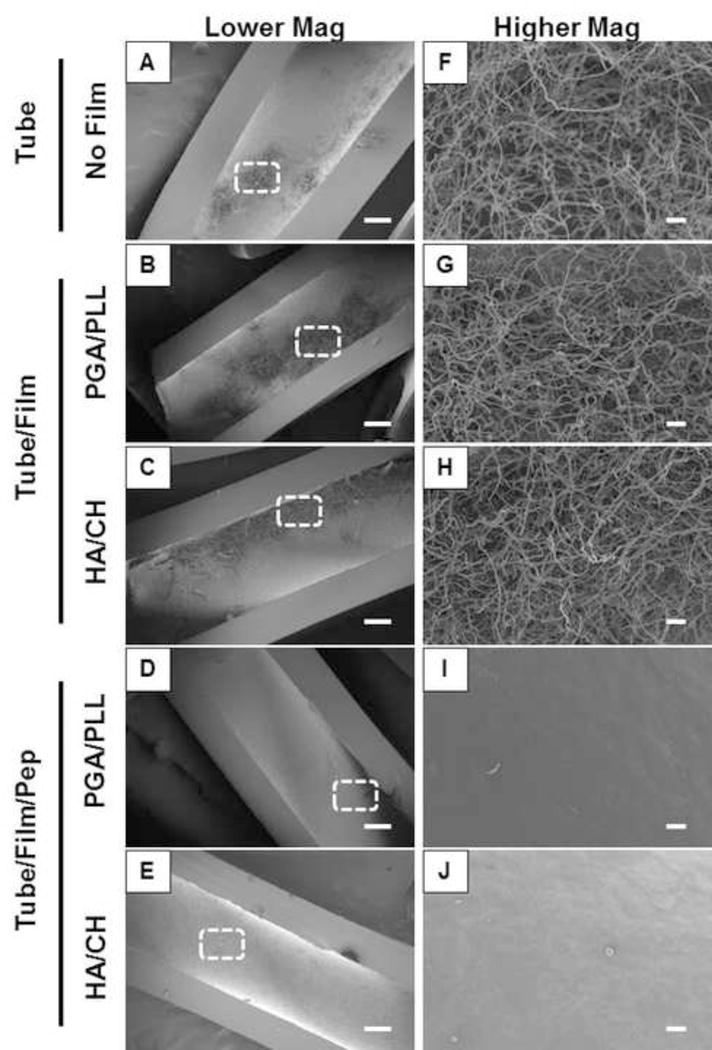
Lower (A–E) and higher (F–J) magnification scanning electron micrographs of C. albicans biofilms in SU media on (A,F) untreated polyethylene catheter tubes, (B,G) tubes coated with PGA/PLL multilayers, or (C,H) HA/CH multilayers, and (D,I) PGA/PLL films and, (E,J) HA/CH films loaded with β-peptide. The dotted boxes in A–E indicate the approximate region of the catheter from which the higher magnification images in F–J were taken. Biofilms were grown on the inner surfaces of polyethylene catheter tube segments in SU media for 48 hours and tubes were longitudinally sliced open and prepared for imaging. Scale bars = 200 μm (A–E), 20 μm (F–J).
Figure 9.
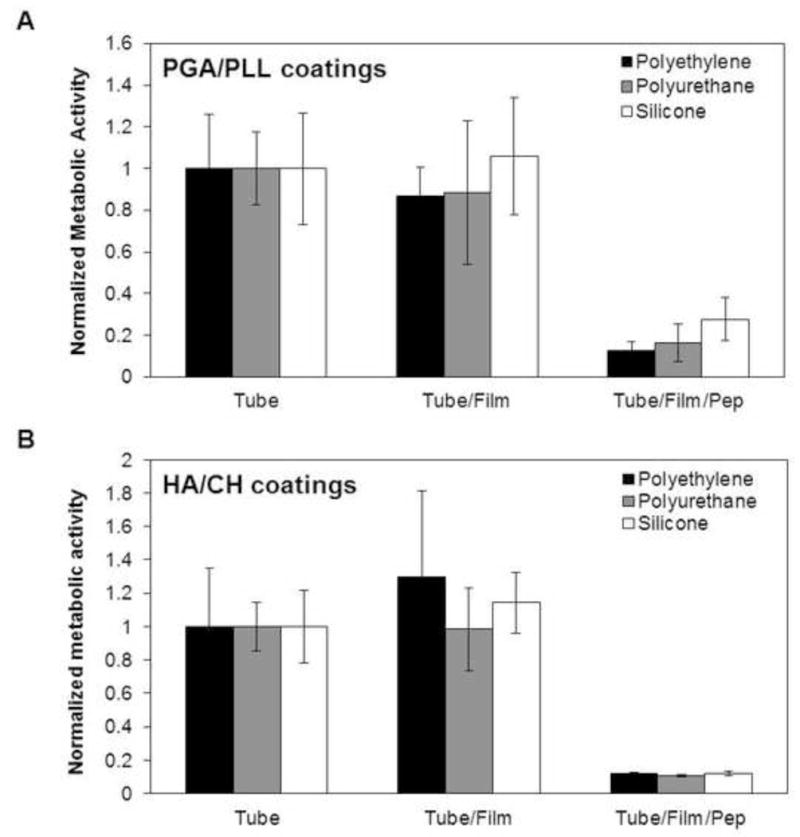
Quantitative characterization of biofilm formed in polyethylene (PE, black), polyurethane (PU, grey), and silicone (white) catheter tubes coated with (A) PGA/PLL and (B) HA/CH multilayer films loaded with β-peptide (tube/film/pep). Controls consisting of catheter tubes coated with PGA/PLL or HA/CH multilayer films (no peptide; tube/film) and untreated bare catheter tubes (tube) are also indicated. C. albicans biofilms were grown in the tubes for 48 hours in SU medium, after which an XTT assay was performed to quantify the metabolic activity of the biofilms. Data points represent the average of three replicates from three independent experiments each and error bars denote standard deviation. For all catheter tube material, metabolic activity observed from β-peptide-loaded films (tube/film/pep) were statistically different (p < 0.05 by two-tailed t-test) from untreated controls (tube) under the same conditions.
Catheters impregnated with antiseptics and antibiotics have been utilized in the past to design antimicrobial surfaces [14–16] but these approaches are likely to be ineffective against antibiotic-resistant pathogens [18, 19]. Li et al. recently demonstrated that salt-resistant mimics of AMPs can be immobilized onto silicone catheters covalently via a polymer brush interlayer and that this approach can reduce biofilm formation [53]. That study reported a method for designing antimicrobial surfaces that could prove useful in preventing infection from antibiotic-resistant pathogens; however the results of that study demonstrated only a ~40 % reduction in C. albicans biofilm formation compared to control silicone catheters, possibly because of reduced antifungal effects of the peptide after immobilization to the catheter surface [53]. In contrast, in the approach reported here, we utilize multilayer coatings as depots to store and gradually release antimicrobial β-peptide-based AMP mimics that are structurally stable and our results demonstrate that these β-peptide-eluting coatings kill planktonic cells and prevent biofilm formation with high efficiency. As indicated in Figure 9, all β-peptide-containing catheters demonstrated significant (>75%, and up to 90% in most cases) reductions in intraluminal biofilm formation in the tubes when incubated with C. albicans for at least 48 hours. When combined, these results demonstrate that β-peptides are stable and remain active in SU medium and that the loading of β-peptide into multilayer film-coated catheter tubes is an effective approach to prevent biofilm formation in urinary catheter materials.
4. Conclusions
Our results demonstrate that a β-peptide-based AMP mimic previously reported to be antifungal in physiological media retains its helical structure and antifungal activity and prevents biofilm formation in a more complex medium containing high ionic strength salts, denaturants, and other small molecules typical of urine. Polyethylene, polyurethane, and silicone catheters coated with HA/CH- and PGA/PLL-based polymer multilayers and loaded with β-peptide released β-peptide into intraluminal spaces gradually over a period of ~100 days. β-Peptide-loaded catheter tubes demonstrated excellent antifungal activities for short (6 hour) and longer durations (up to 21 days of intermittent challenges) and substantially reduced biofilm formation (by 75–90%) on the luminal surfaces of catheters in synthetic urine medium. The modular approach reported here permits the design of multifunctional antimicrobial surfaces by exploiting the versatility of layer-by-layer assembly and the physicochemical properties of polyelectrolyte-based multilayer coatings and provides future opportunities to improve the antifungal potencies of these surfaces through the design and incorporation of other synthetic β-peptide mimics of AMPs with enhanced potency. With further development, the strategies reported here could thus lead to new materials-based approaches that can reduce the extent of fungal colonization in urinary catheters and thereby reduce the incidence of CAUTI.
Supplementary Material
For Statement of Significance Only.
Catheter-associated urinary tract infections are the most common type of hospital-acquired infection. The human pathogen Candida albicans is the leading cause of fungal urinary tract infections, and forms difficult to remove ‘biofilms’ on the surfaces of urinary catheters. We investigated synthetic β-peptide mimics of natural antimicrobial peptides as an approach to kill C. albicans and prevent biofilm formation in media that mimics the composition of urine. Our results reveal these mimics to retain structural stability and activity against C. albicans in synthetic urine. We also report polymer-based approaches to the local release of these agents within urinary catheter tubes. With further development, these materials-based approaches could lead to advances that reduce the occurrence of fungal urinary tract infections.
Acknowledgments
Financial support for this work was provided by the National Institutes of Health (R01 AI092225). The authors acknowledge use of instrumentation supported by the National Science Foundation through grants provided to the UW-Madison Materials Research Science and Engineering Center (MRSEC; DMR-1121288).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information. Supporting information including planktonic and biofilm MIC plots, unmerged fluorescence micrographs of β-peptide interacting with cells, and comparisons of β-peptide release profiles into PBS and SU media can be found in the online version at doi:
References
- 1.Siddiq DM, Darouiche RO. New strategies to prevent catheter-associated urinary tract infections. Nat Rev Urology. 2012;9(6):305–14. doi: 10.1038/nrurol.2012.68. [DOI] [PubMed] [Google Scholar]
- 2.Tambyah PA. Catheter-associated urinary tract infections: diagnosis and prophylaxis. Int J Antimicrob Ag. 2004;24:S44–S48. doi: 10.1016/j.ijantimicag.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Cardo D, Horan T, Andrus M, Dembinski M, Edwards J, Peavy G, Tolson J, Wagner D, Syst N. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32(8):470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 4.Ramage G, Martinez JP, Lopez-Ribot JL. Candida biofilms on implanted biomaterials: a clinically significant problem. Fems Yeast Res. 2006;6(7):979–986. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 5.Tambyah PA, Knasinski V, Maki DG. The direct costs of nosocomial catheter-associated urinary tract infection in the era of managed care. Infect Cont Hosp Ep. 2002;23(1):27–31. doi: 10.1086/501964. [DOI] [PubMed] [Google Scholar]
- 6.Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23(2):253–273. doi: 10.1128/CMR.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher JF, Kavanagh K, Sobel JD, Kauffman CA, Newman CA. Candida urinary tract infection: pathogenesis. Clin Infect Dis. 2011;52:S437–S451. doi: 10.1093/cid/cir110. [DOI] [PubMed] [Google Scholar]
- 8.Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17(2):255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukherjee PK, Zhou GY, Munyon R, Ghannoum MA. Candida biofilm: a well-designed protected environment. Med Mycol. 2005;43(3):191–208. doi: 10.1080/13693780500107554. [DOI] [PubMed] [Google Scholar]
- 10.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambayh PA, Tenke P, Nicolle LE. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 11.Fisher JF. Candida urinary tract infections-epidemiology, pathogenesis, diagnosis, and treatment: executive summary. Clin Infect Dis. 2011;52:S429–S432. doi: 10.1093/cid/cir108. [DOI] [PubMed] [Google Scholar]
- 12.Fisher JF, Sobel JD, Kauffman CA, Newman CA. Candida urinary tract infections-treatment. Clin Infect Dis. 2011;52:S457–S466. doi: 10.1093/cid/cir112. [DOI] [PubMed] [Google Scholar]
- 13.Sanguinetti M, Posteraro B, Lass-Florl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses. 2015;58:2–13. doi: 10.1111/myc.12330. [DOI] [PubMed] [Google Scholar]
- 14.Gaonkar TA, Sampath LA, Modak SM. Evaluation of the antimicrobial efficacy of urinary catheters impregnated with antiseptics in an in vitro urinary tract model. Infect Cont Hosp Ep. 2003;24(7):506–513. doi: 10.1086/502241. [DOI] [PubMed] [Google Scholar]
- 15.Darouiche RO, Safar H, Raad II. In vitro efficacy of antimicrobial-coated bladder catheters in inhibiting bacterial migration along catheter surface. J Infect Dis. 1997;176(4):1109–1112. doi: 10.1086/516523. [DOI] [PubMed] [Google Scholar]
- 16.Hachem R, Reitzel R, Borne A, Jiang Y, Tinkey P, Uthamanthil R, Chandra J, Ghannoum M, Raad I. Novel antiseptic urinary catheters for prevention of urinary tract infections: correlation of in vivo and in vitro test results. 2009;cc 53(12):5145–5149. doi: 10.1128/AAC.00718-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher LE, Hook AL, Ashraf W, Yousef A, Barrett DA, Scurr DJ, Chen XY, Smith EF, Fay M, Parmenter CDJ, Parkinson R, Bayston R. Biomaterial modification of urinary catheters with antimicrobials to give long-term broadspectrum antibiofilm activity. J Control Release. 2015;202:57–64. doi: 10.1016/j.jconrel.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 18.Tambe SM, Sampath L, Modak SM. In vitro evaluation of the risk of developing bacterial resistance to antiseptics and antibiotics used in medical devices. J Antimicrob Chemoth. 2001;47(5):589–598. doi: 10.1093/jac/47.5.589. [DOI] [PubMed] [Google Scholar]
- 19.Sampath LA, Tambe SM, Modak SM. In vitro and in vivo efficacy of catheters impregnated with antiseptics or antibiotics: evaluation of the risk of bacterial resistance to the antimicrobials in the catheters. Infect Cont Hosp Ep. 2001;22(10):640–6. doi: 10.1086/501836. [DOI] [PubMed] [Google Scholar]
- 20.Diamond G, Beckloff N, Weinberg A, Kisich KO. The roles of antimicrobial peptides in innate host defense. Curr Pharm Design. 2009;15(21):2377–2392. doi: 10.2174/138161209788682325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 22.Shai Y, Makovitzky A, Avrahami D. Host defense peptides and lipopeptides: modes of action and potential candidates for the treatment of bacterial and fungal infections. Curr Protein Pept Sc. 2006;7(6):479–486. doi: 10.2174/138920306779025620. [DOI] [PubMed] [Google Scholar]
- 23.Hancock REW, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24(12):1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 24.van’t Hof W, Veerman ECI, Helmerhorst EJ, Amerongen AVN. Antimicrobial peptides: properties and applicability. Biol Chem. 2001;382(4):597–619. doi: 10.1515/BC.2001.072. [DOI] [PubMed] [Google Scholar]
- 25.Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zasloff M, Wilson JM. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88(4):553–60. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 26.Schmidtchen A, Frick IM, Andersson E, Tapper H, Bjorck L. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol Microbiol. 2002;46(1):157–68. doi: 10.1046/j.1365-2958.2002.03146.x. [DOI] [PubMed] [Google Scholar]
- 27.Chu HL, Yu HY, Yip BS, Chih YH, Liang CW, Cheng HT, Cheng JW. Boosting salt resistance of short antimicrobial peptides. 2013;cc 57(8):4050–4052. doi: 10.1128/AAC.00252-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porter EA, Wang XF, Lee HS, Weisblum B, Gellman SH. Antibiotics – non-haemolytic beta-amino-acid oligomers. Nature. 2000;404(6778):565–565. doi: 10.1038/35007145. [DOI] [PubMed] [Google Scholar]
- 29.Raguse TL, Porter EA, Weisblum B, Gellman SH. Structure-activity studies of 14-helical antimicrobial beta-peptides: probing the relationship between conformational stability and antimicrobial potency. J Am Chem Soc. 2002;124(43):12774–12785. doi: 10.1021/ja0270423. [DOI] [PubMed] [Google Scholar]
- 30.Porter EA, Weisblum B, Gellman SH. Mimicry of host-defense peptides by unnatural oligomers: antimicrobial beta-peptides. J Am Chem Soc. 2002;124(25):7324–7330. doi: 10.1021/ja0260871. [DOI] [PubMed] [Google Scholar]
- 31.Karlsson AJ, Pomerantz WC, Neilsen KJ, Gellman SH, Palecek SP. Effect of sequence and structural properties on 14-helical beta-peptide activity against Candida albicans planktonic cells and biofilms. ACS Chem Biol. 2009;4(7):567–79. doi: 10.1021/cb900093r. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson AJ, Pomerantz WC, Weisblum B, Gellman SH, Palecek SP. Antifungal activity from 14-helical beta-peptides. J Am Chem Soc. 2006;128(39):12630–12631. doi: 10.1021/ja064630y. [DOI] [PubMed] [Google Scholar]
- 33.Raman N, Lee MR, Lynn DM, Palecek SP. Antifungal activity of 14-helical beta-peptides against planktonic cells and biofilms of Candida species. Pharmaceuticals. 2015;8(3):483–503. doi: 10.3390/ph8030483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MR, Raman N, Gellman SH, Lynn DM, Palecek SP. Hydrophobicity and helicity regulate the antifungal activity of 14-helical beta-peptides. ACS Chem Biol. 2014;9(7):1613–1621. doi: 10.1021/cb500203e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uppuluri P, Dinakaran H, Thomas DP, Chaturvedi AK, Lopez-Ribot JL. Characteristics of Candida albicans biofilms grown in a synthetic urine medium. J Clin Microbiol. 2009;47(12):4078–4083. doi: 10.1128/JCM.01377-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karlsson AJ, Flessner RM, Gellman SH, Lynn DM, Palecek SP. Polyelectrolyte multilayers fabricated from antifungal beta-peptides: design of surfaces that exhibit antifungal activity against Candida albicans. Biomacromolecules. 2010;11(9):2321–2328. doi: 10.1021/bm100424s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raman N, Lee MR, Palecek SP, Lynn DM. Polymer multilayers loaded with antifungal beta-peptides kill planktonic Candida albicans and reduce formation of fungal biofilms on the surfaces of flexible catheter tubes. J Control Release. 2014;191:54–62. doi: 10.1016/j.jconrel.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raman N, Marchillo K, Lee MR, Rodriguez Lopez AL, Andes DR, Palecek SP, Lynn DM. Intraluminal release of an antifungal beta-peptide enhances the antifungal and anti-biofilm activities of multilayer-coated catheters in a rat model of venous catheter infection. ACS Biomater Sci Eng. 2016;2(1):112–121. doi: 10.1021/acsbiomaterials.5b00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rose C, Parker A, Jefferson B, Cartmell E. The characterization of feces and urine: a review of the literature to inform advanced treatment technology. Crit Rev Env Sci Tec. 2015;45(17):1827–1879. doi: 10.1080/10643389.2014.1000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook JD, Caplan YH, LoDico CP, Bush DM. The characterization of human urine for specimen validity determination in workplace drug testing: a review. J Anal Toxicol. 2000;24(7):579–588. doi: 10.1093/jat/24.7.579. [DOI] [PubMed] [Google Scholar]
- 41.Lee MR, Raguse TL, Schinnerl M, Pomerantz WC, Wang XD, Wipf P, Gellman SH. Origins of the high 14-helix propensity of cyclohexyl-rigidified residues in beta-peptides. Org Lett. 2007;9(9):1801–1804. doi: 10.1021/ol070511r. [DOI] [PubMed] [Google Scholar]
- 42.Pomerantz WC, Grygiel TLR, Lai JR, Gellman SH. Distinctive circular dichroism signature for 14-helix-bundle formation by beta-peptides. Org Lett. 2008;10(9):1799–1802. doi: 10.1021/ol800622e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helmerhorst EJ, Reijnders IM, van’t Hof W, Veerman ECI, Amerongen AVN. A critical comparison of the hemolytic and fungicidal activities of cationic antimicrobial peptides. Febs Lett. 1999;449(2–3):105–110. doi: 10.1016/s0014-5793(99)00411-1. [DOI] [PubMed] [Google Scholar]
- 44.Schultz P, Vautier D, Richert L, Jessel N, Haikel Y, Schaaf P, Voegel JC, Ogier J, Debry C. Polyelectrolyte multilayers functionalized by a synthetic analogue of an anti-inflammatory peptide, alpha-MSH, for coating a tracheal prosthesis. Biomaterials. 2005;26(15):2621–2630. doi: 10.1016/j.biomaterials.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 45.Etienne O, Gasnier C, Taddei C, Voegel JC, Aunis D, Schaaf P, Metz-Boutigue MH, Bolcato-Bellemin AL, Egles C. Antifungal coating by biofunctionalized polyelectrolyte multilayered films. Biomaterials. 2005;26(33):6704–6712. doi: 10.1016/j.biomaterials.2005.04.068. [DOI] [PubMed] [Google Scholar]
- 46.Etienne O, Picart C, Taddei C, Haikel Y, Dimarcq JL, Schaaf P, Voegel JC, Ogier JA, Egles C. Multilayer polyelectrolyte films functionalized by insertion of defensin: a new approach to protection of implants from bacterial colonization. 2004;cc 48(10):3662–3669. doi: 10.1128/AAC.48.10.3662-3669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cado G, Aslam R, Seon L, Garnier T, Fabre R, Parat A, Chassepot A, Voegel JC, Senger B, Schneider F, Frere Y, Jierry L, Schaaf P, Kerdjoudj H, Metz-Boutigue MH, Boulmedais F. Self-defensive biomaterial coating against bacteria and yeasts: polysaccharide multilayer film with embedded antimicrobial peptide. Adv Funct Mater. 2013;23(38):4801–4809. [Google Scholar]
- 48.Richert L, Lavalle P, Payan E, Shu XZ, Prestwich GD, Stoltz JF, Schaaf P, Voegel JC, Picart C. Layer by layer buildup of polysaccharide films: physical chemistry and cellular adhesion aspects. Langmuir. 2004;20(2):448–458. doi: 10.1021/la035415n. [DOI] [PubMed] [Google Scholar]
- 49.Thierry B, Winnik FM, Merhi Y, Tabrizian M. Nanocoatings onto arteries via layer-by-layer deposition: toward the in vivo repair of damaged blood vessels. J Am Chem Soc. 2003;125(25):7494–7495. doi: 10.1021/ja034321x. [DOI] [PubMed] [Google Scholar]
- 50.Chua PH, Neoh KG, Shi Z, Kang ET. Structural stability and bioapplicability assessment of hyaluronic acid-chitosan polyelectrolyte multilayers on titanium substrates. J Biomed Mater Res A. 2008;87A(4):1061–1074. doi: 10.1002/jbm.a.31854. [DOI] [PubMed] [Google Scholar]
- 51.Seyfarth F, Schliernann S, Elsner P, Hipler UC. Antifungal effect of high- and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-D-glucosamine against Candida albicans, Candida krusei and Candida glabrata. Int J Pharm. 2008;353(1–2):139–148. doi: 10.1016/j.ijpharm.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 52.Martinez LR, Mihu MR, Tar M, Cordero RJB, Han G, Friedman AJ, Friedman JM, Nosanchuk JD. Demonstration of antibiofilm and antifungal efficacy of chitosan against Candidal biofilms, using an in vivo central venous catheter model. J Infect Dis. 2010;201(9):1436–1440. doi: 10.1086/651558. [DOI] [PubMed] [Google Scholar]
- 53.Li X, Li P, Saravanan R, Basu A, Mishra B, Lim SH, Su X, Tambyah PA, Leong SS. Antimicrobial functionalization of silicone surfaces with engineered short peptides having broad spectrum antimicrobial and salt-resistant properties. Acta Biomater. 2014;10(1):258–66. doi: 10.1016/j.actbio.2013.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


