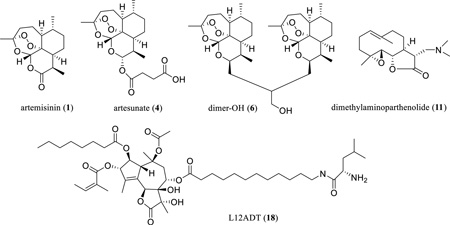
Abstract
Sesquiterpene lactones are of considerable interest due to their potent bioactivities, including cancer cell cytotoxicity and antineoplastic efficacy in in vivo studies. Among these compounds, artesunate, dimethylaminoparthenolide, and L12ADT peptide prodrug, a derivative of thapsigargin, are being evaluated in current cancer clinical or preclinical trials. Based on the structures of several antitumor sesquiterpene lactones, a number of analogues showing greater potency have been either isolated as natural products or partially synthesized, and some potential anticancer agents that have emerged from this group of lead compounds have been investigated extensively. The present review focuses on artemisinin, parthenolide, thapsigargin, and their naturally occurring or synthetic analogues showing potential anticancer activity. This provides an overview of the advances in the development of these types of sesquiterpene lactones as potential anticancer agents, including their structural characterization, synthesis and synthetic modification, and antitumor potential, with the mechanism of action and structure-activity relationships also discussed. It is hoped that this will be helpful in stimulating the further interest in developing sesquiterpene lactones and their derivatives as new anticancer agents.
Keywords: Artemisinin, Dimers of artemisinin, Dimethylaminoparthenolide, Parthenolide, Potential anticancer agents, Sesquiterpene lactones, Thapsigargin
Graphical abstract
The antimalarial artemisinin (1) shows potential anticancer activity, and a dimeric derivative, dimer-OH (6), exhibits an improved antitumor efficacy. Artesunate (4), dimethylaminoparthenolide (11), and a L12ADT (18) peptide prodrug of thapsigargin are being evaluated in current cancer clinical or preclinical trials.
1. INTRODUCTION
Sesquiterpene lactones (SQLs) are representative of a large number of natural products (NPs), containing 15 carbons with a common incorporation of three isoprenyl groups arranged in several characteristic ring systems, including one or more lactone rings. There are more than 5000 SQLs characterized as secondary metabolites in species of the plant kingdom, in particular in the family Asteraceae [1]. This type of NP has received considerable attention because of their diverse structure skeletons and chemotaxonomic significance [2, 3], as well as their potent bioactivities, including antibiotic, antitumor, antiulcer, insect-feeding deterrent, phytotoxic, and schistosomicidal effects [4, 5].
Many SQLs have been reported to exhibit cancer cell cytotoxicity and antitumor efficacy [1, 4, 6–10], and several of these compounds have been evaluated currently in cancer clinical trials [8], even though they have not always been regarded favorably as potential anticancer agents due to their toxicity and non-selectivity [11–13], as well as a tendency to promote allergic contact dermatitis [14, 15]. Among these compounds, artemisinin (ART, 1, a SQL endoperoxide) and its naturally occurring or synthetic analogues that show low side effects and high antitumor potency, are emerging as promising anticancer leads or prodrugs [8, 16–18].
Cancer is a major threat to human health, but the currently available cancer chemotherapy regimens may result in several critical problems, including insufficient effectiveness, serious adverse effects, and the development of multidrug resistance (MDR). Thus, more effective treatments that result from the discovery of new anticancer agents to resolve these problems would be highly desirable. NPs and their semi-synthetic derivatives have been used as anticancer agents widely and effectively over several decades, and plants historically have played a critical role in NP-related anticancer drug discovery [19, 20]. Plant-derived agents, including the vinca bisindole alkaloids, the epipodophyllotoxin analogues, the taxanes, and the camptothecins, have been among the most widely used cancer chemotherapeutics available, and interest in these agents and other NPs to combat cancer is increasing [19]. Recently, ART (1) and its synthetic analogues have been well documented for their promising anticancer activity with lower side effects and higher effectiveness [18]. In the present review, three SQL-type anticancer leads, ART (1), PTL (10), and TPG (17), and their natural or synthetic analogues are reviewed for their potential as anticancer agents, with the considerable interest in these SQL leads for their further development as oncological agents indicated.
2. CHARACTERIZATION OF ARTEMISININ: FROM AN ANTIMALARIAL DRUG TO AN ANTICANCER LEAD
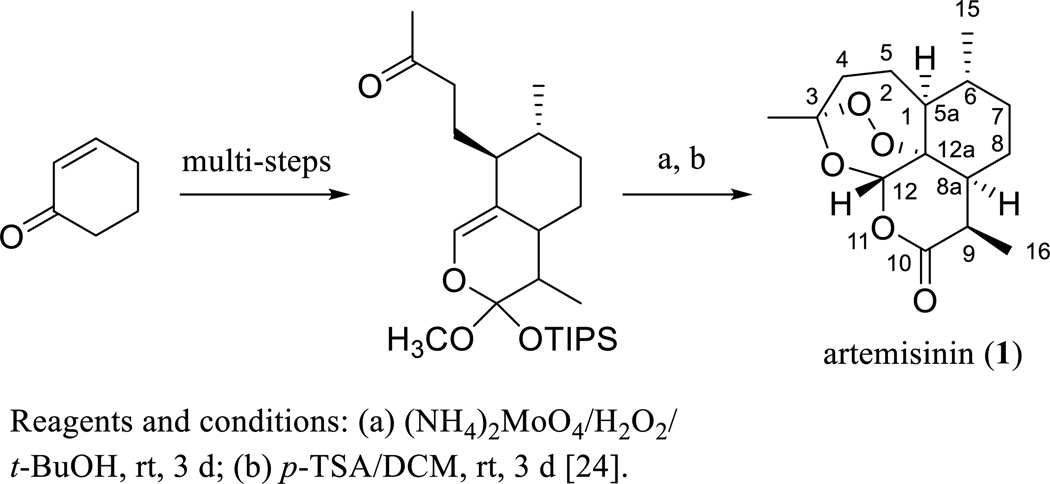
Artemisinin (ART, 1) was isolated and characterized originally from a Chinese herb, Artemisia annua L. (Asteraceae) [21], and subsequently from the same plant collected in the United States by Dr. Daniel L. Klayman’s group at Walter Reed Army Medical Center, Washington, DC, USA [22]. The structure of ART was established by analysis of its single-crystal X-ray diffraction data [23] and confirmed by total synthesis of the molecule (Scheme 1) [24]. This agent has been used both in an unmodified structural form and when derivatized for the treatment of malaria for several decades. The 2015 Nobel Prize in Physiology or Medicine was shared by Professor Youyou Tu for her discoveries concerning the novel ART-related therapy against Malaria (http://www.nobelprize.org/nobel_prizes/medicine/laureates/2015/press.html).
Scheme. (1).
Synthesis of artemisinin.
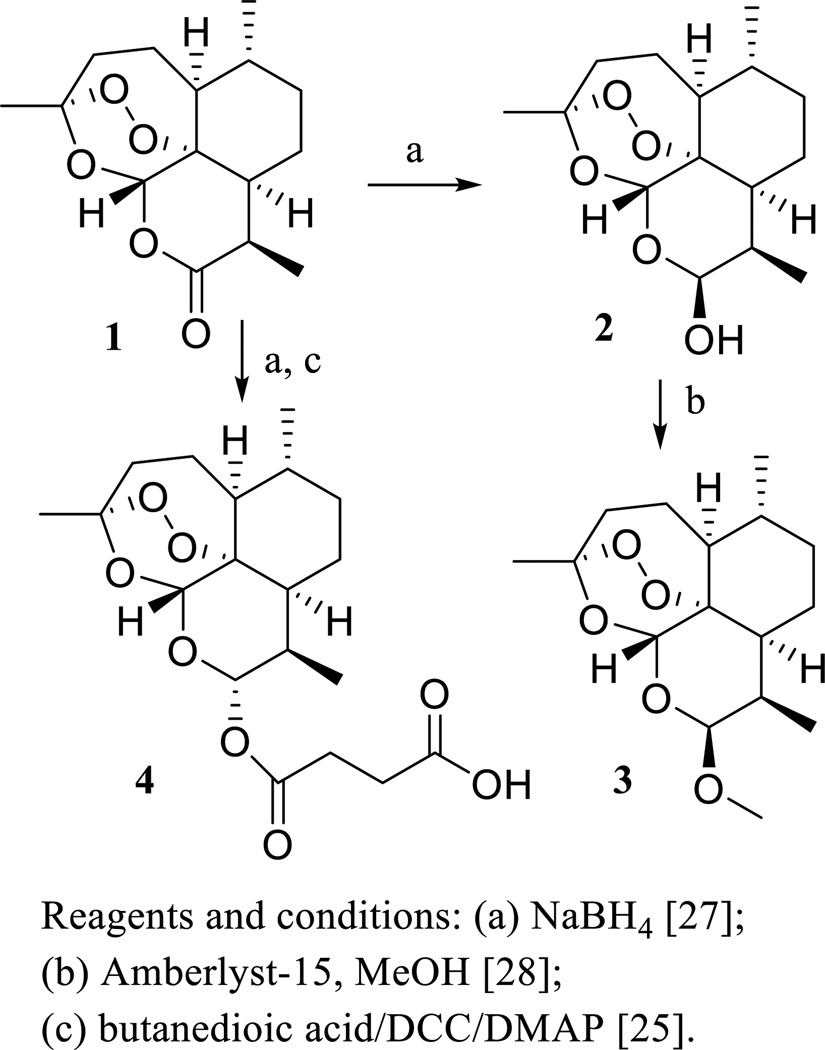
However, ART is not recommended currently as a stand-alone antimalarial agent due to the development of resistance by the malaria parasite [16]. To improve the antimalarial activity, a series of ART derivatives showing higher potency or enhanced stability or solubility have been prepared [25, 26], including dihydroartemisinin (DHA, 2) [27], artemether (ARM, 3) [28], and sodium artesunate (ARS, 4) [26] (Scheme 2). Consistent with its in vitro potency, DHA (2) was found to show more potent antimalarial activity than ART (1) in vivo, when Swiss mice bearing Plasmodium berghei parasites were injected intramuscularly (i.m.) or intraperitoneally (i.p.) with ART (1), DHA (2), and ARS (4) [25].
Scheme. (2).
Synthesis of artemisinin derivatives.
Interestingly, ART and its derivatives have also been found to show antifungal, antiinflammatory, antineoplastic, antiparastic, and antiviral activities, and their anticancer potential is attracting considerable interest [8, 16, 18, 29]. Detailed information on the cytotoxicity and antitumor efficacy of ART and its derivatives has been reviewed recently [18]. In the following paragraphs, the cancer cell line selectivity, MDR, and clinical trial studies of ART and its derivatives are discussed.
ART (1) exhibited selective cytotoxicity toward the human hepatoma HepG2 (p53 wild-type), Huh-7 (p53 mutant), BEL-7404 (p53mutant), and Hep3B (p53 null) cell lines, when compared with the 7702 non-neoplastic human liver cell line [30]. This agent suppressed DU 145 and PC-3 human prostate carcinoma cell growth by apoptosis induction through the mitochondrial pathway [31], with intracellular heme (Fe2+ protoporphyrin IX) serving as the molecular target [32]. When four- to six-week-old female athymic nude mice bearing HepG2 and Hep3B xenografts were treated with ART (1) and DHA (2) [both suspended in 5% sesame oil and plus 95% saline, 50 or 100 mg/kg, per os (p.o.), daily, 5 times a week for four weeks], tumor growth was inhibited significantly by both compounds, and no apparently toxic effects were observed [30]. The mechanism of anticancer activity of ART has remained controversial, but it is well documented that the essential endoperoxide bridge reacts with ferrous iron to generate free radicals leading to macromolecular damage and cancer cell death, and translationally controlled tumor protein (TCTP) was proposed as a potential target [16–18]. Also, ART activates the constitutive androstane receptor (CAR) to induce cytochrome P450 (CYP) enzymes to affect the pharmacokinetics of co-administered drugs and inhibits sarcoplasmic/ER Ca2+ ATPase (SERCA) to suppress cancer cell growth by altering calcium homeostasis [17].
To improve on the cytotoxicity of ART (1), several new analogues exhibiting greater potency, including DHA (2), ARM (3), and ARS (4), have been prepared through reduction of the C-10 carbonyl group followed by further modifications of the parent compound [26–28]. Among these derivatives, DHA (2) and ARS (4) were more potently cytotoxic than ART toward drug-sensitive CCRF-CEM and MDR CEM/ADR5000 leukemia cells [33], as well as toward human papillomavirus (HPV)-immortalized and transformed cervical cells [34]. DHA (2) also showed selective cytotoxicity toward human MOLT4-lymphoblastoid cells (compared with normal human lymphocytes) [35], HTB 27 breast cancer cells (compared with normal HTB 125 human breast cells) [36], and hepatoma HepG2 (p53 wild-type), Huh-7 (p53 mutant), BEL-7404 (p53mutant), and Hep3B (p53 null) hepatoma cell lines (compared with the 7702 normal human liver cell line) [30].
In an in vivo study, tumor growth was retarded significantly when female Fisher 344 rats bearing fibrosarcoma cells were treated with ferrous sulfate [dissolved in water (2 mg/mL), given daily by intubation at 20 mg/kg for 6 h] followed by treatment with 2 [dissolved in peanut oil, given daily by intubation at 2 mg/kg for 3 days followed by 5 mg/kg for seven days], and no significant adverse effects were observed [37]. When six- to ten-week-old female beagle dogs, with both sides of the oral mucosa abraded and infected with canine oral papilloma virus, were treated topically with 2 [100 µL of 78 mM DMSO solution, daily, 5 times a week for six weeks], tumor formation was not observed, while tumors were formed in the oral mucosa of the dogs treated by the vehicle control [34]. Mechanistically, DHA-induced cell death is involved in activation of the mitochondrial caspase pathway with resultant apoptosis induction [34], and its selective cytotoxicity was proposed to be related to the reaction of the endoperoxide bridge with ferrous iron due to a higher expression of transferrin receptors occurring in cancer cells than in normal cells [35].
ARM (3) was found to exhibit both antitumor and immunomodulatory properties in vivo, when BALB/c mice bearing spontaneous mouse mammary tumors (SMMT) were treated with ARM [dissolved in ethanol/PBS (1:2), 10 mg/kg, i.p., daily for three consecutive days followed by three more administrations on days 5, 7, and 9] [38]. A case study showed that when a 75-year-old male patient with pituitary macroadenoma was treated orally with ARM [40 mg (approximately 0.5 mg/kg), daily for 29 days, and every other day for a duration of 30 days, and then twice a week for 10 months] and vitamins C (500 mg) and E (200 units) (daily for the entire treatment), the tumor density was reduced, and the life quality of the patient was improved by relieving the cancer-related symptoms, including vision, hearing, and locomotion-related problems [39].
When five classes of established antimalarial drugs were screened against a panel of 91 human cancer cell lines, DHA (2) and ARS (4) emerged as potently cytotoxic agents [40]. Further studies showed that ARS (4) reversed MDR by reducing ATP-binding cassette sub-family G member 2 (ABCG2, a multidrug transporter) expression in Eca109/ABCG2 esophageal cancer cells [41]. It exhibited antineuroblastoma activity toward a panel of chemosensitive and chemoresistant neuroblastoma cell lines [42]. Recently, the cytotoxicity of 4 toward a small panel of human cancer cell lines was found to be enhanced using a nanoparticle technique. When 4 was loaded in poly(lactic-co-glycolic) acid (PLGA) nanoparticles using an oil/water emulsion evaporation method, the ARS-PLGA formulation reduced significantly human cancer cell viability when compared with free 4 [43]. Interestingly, 4 was found to activate programmed cell death (PCD) in cancer cells in a manner dependent on the presence of iron and the generation of reactive oxygen species (ROS) [44]. It triggered PCD via engagement of distinct interconnected PCD pathways, with hierarchical signaling from lysosomes to mitochondria [44]. Also, it induced DNA damage, and this was proposed to contribute to its therapeutic effect against cancer cells [45, 46].
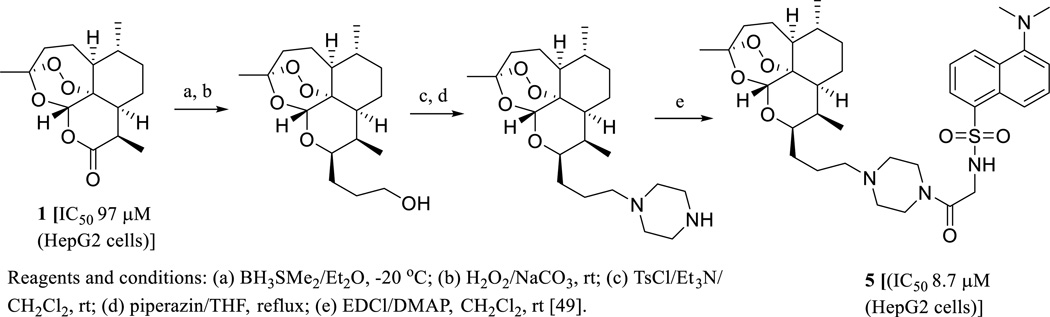
A safety and efficacy field study for ARS conducted in dogs bearing non-resectable tumors showed that oral administration of ARS for 7–385 days at a dosage of 651–1178 mg/m2 led to gastrointestinal and hematological toxicity and fever, but neither neurological nor cardiac toxicity was observed [47]. A clinical safety and tumor marker effect investigation conducted in non-pregnant female patients (between 39 and 70 years old) with advanced cervix carcinoma showed that oral administration of 4 for 28 days with a dose starting at 100 mg/day during the first week and then 200 mg/day for the subsequent three weeks induced clinical remission with seven days as a median time for the disappearance of the symptoms, while no adverse events of grades greater than three occurred [48]. Currently, ARS (4) is being evaluated in phase II cancer clinical trials for the treatment of non-small cell lung cancer, metastatic uveal melanoma, and laryngeal squamous cell carcinoma [8]. Interestingly, a fluorescent artemisinin derivative (5), which showed around ten-fold more potent cytotoxicity toward the human hepatoma HepG2 cells than ART (1), was synthesized (Scheme 3). The subcellular localization of 5 in the human hepatoma Hep3B cells was investigated, which indicated that endoplasmic reticulum (ER) rather than mitochondria is the major locating site of 5 (Scheme 3) [49]. This provides an important clue for the future characterization of the molecular target of ART and its analogues for their antitumor activity.
Scheme. (3).
Synthesis of a fluorescent artemisinin derivative.
A preliminary structure-activity relationship (SAR) study indicated that the endoperoxide group of ART is essential for its cytotoxicity, and that 9R-hydroxyartemisinin exhibited a higher effect than its 9S epimer [50, 51]. A C-16 exocyclic methylene increased the activity. Reduction of the C-10 ketone group to a C-9/C-10 carbon double bond retained activity, but opening of the lactone ring reduced the cytotoxicity [50]. Interestingly, a simple ether dimer of ART was found to be more potently cytotoxic than the parent compound [50, 51], indicating that dimeric ART may be more promising than ART itself for the development of new anticancer agents.
3. ARTEMISININ DIMERS SHOWING IMPROVED ANTITUMER POTENTIAL
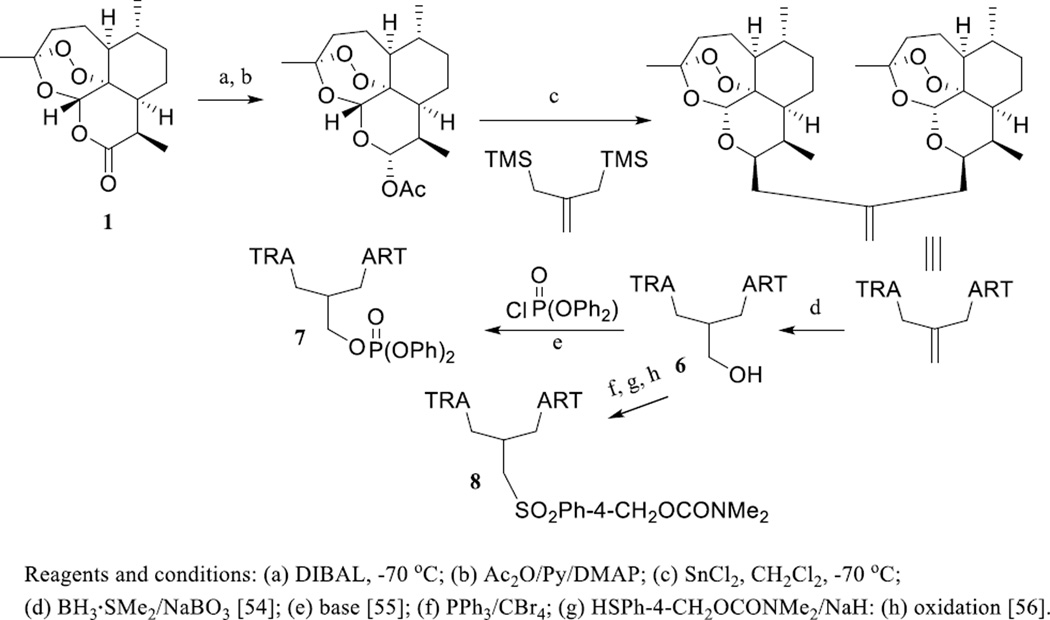
Dimeric SQLs are rare plant NPs discovered mainly from the family Asteraceae, and some of these dimers exhibit more potent bioactivities than their monomers, including cancer-related effects [12, 52]. An initial cytotoxicity screening against Ehrlich ascites tumor (EAT) cells for ART and its analogues showed that a simple ether dimeric ART was the most active compound [50]. Following this evidence, several dimeric ARTs have been prepared and evaluated, and the majority of these dimers were found to be potently cytotoxic toward a small panel of human cancer cell lines [53].
Continuous investigations on these dimers resulted in the discovery of a three-carbon atom linked trioxane dimer alcohol, dimer-OH (6) [54], which showed more potent in vitro cytotoxicity than the anticancer drug, doxorubicin, toward the aggressive tumorigenic and metastatic transgenic adenocarcinoma of mouse prostate (TRAMP) C2G and C2H cell lines [54]. Further work on this dimer with introduction of a phosphate ester or a sulfonyl functional group yielded two new dimers, 838 (7) and 832-4 (8) (Scheme 4) [55–59]. Dimer 838 (7) was highly cytotoxic toward TRAMP C2G and C2H and Jurkat T-acute lymphoblastic leukemia (ALL) cells, and it was more active than doxorubicin in the inhibition of human prostate cancer LNCaP cell growth [55, 59]. Dimer 832-4 (8) was cytotoxic toward human HL-60 promyelocytic leukemia, U-937 lymphoma, SK-MEL-5, UACC-62 melanoma, and HeLa cervical cancer cells, and of comparable potency to doxorubicin. Moreover, this dimer did not significantly affect WT-MEF and Hs888Lu non-cancerous immortalized fibroblast cells [56]. Furthermore, dimer 838 (7) and dimer 832-4 (8) exhibited cytotoxicity toward human HeLa cervical adenocarcinoma, HCT116 colorectal carcinoma, and 1205Lu melanoma cell lines accompanied with a lack of cytotoxicity toward non-cancer primary human foreskin fibroblast (HFF) cells [57], indicating that both dimers are selectively cytotoxic toward cancer cells.
Scheme. (4).
Synthesis of three-carbon atom linked artemisinin dimers.
Dimer-OH (6) was considerably more potent than the monomer, DHA (2), in the inhibition of rat mammary adenocarcinoma MTLn3 cell growth in vitro [58]. When female Fisher-344 rats implanted with mammary adenocarcinoma MTLn3 cells were treated daily by intubation of dimer-OH (6) (dissolved in olive oil) for five days at a dosage of 20 mg/kg, tumor growth was suppressed considerably in both cases [58]. A mechanistic study showed that dimer 838 (7) induced G0/G1 cell cycle arrest in human LNCaP prostate cancer cells and decreased cells in the S phase. These effects correlated with the modulation of G1 phase cell cycle proteins, including decreased levels of cyclin D1, cyclin E, and cdk2, and increased levels of p21waf1 and p27Kip1 [55].
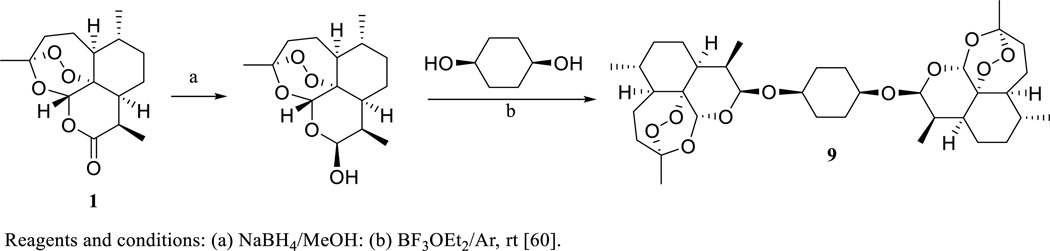
When a cyclohexane residue was used to link two DHA (2) molecules, the dimeric compound 9 was produced (Scheme 5). This dimer was found to be the most active of several artemisinin acetal dimers synthesized and evaluated for cytotoxicity in the NCI in vitro human tumor 60 cell line assay [60]. It showed activity comparable with paclitaxel in the NCI in vivo hollow fiber assay against a standard panel of 12 human cancer cell lines, when mice were treated (i.p. daily) with dimer 9 for four days. It was active on further evaluation in a NCI subcutaneous (s.c.) xenograft model of HL-60 human leukemia cells, when female athymic nude mice were treated (s.c., 25 mg/kg, daily) with this compound for 10 days [60].
Scheme. (5).
Synthesis of β,β-symmetrical cis-1,4-cyclohexanediol dihydroartemisinin acetal dimer.
Further modification of DHA dimers resulted in the production of a novel artemisinin–guanidine hybrid dimer. This hybrid dimer showed up to 600-fold more potent cytotoxicity than DHA (2) toward the human A549 non-small-cell lung adenocarcinoma, HT-29 colon cancer, and MDA-MB-231 breast cancer cell lines [61]. Recently, three artesunic acid homodimers have been synthesized and evaluated for their activity against human CCRF-CEM and MDR P-glycoprotein-overexpressing CEM/ADR 5000 leukemia cells, and the MDR cells used were found not to be cross-resistant to these new dimers [62].
4. PARTHENOLIDE, AN EPOXYLATED GERMACRANOLIDE EXHIBITING PROMISING ANTICANCER ACTIVITY
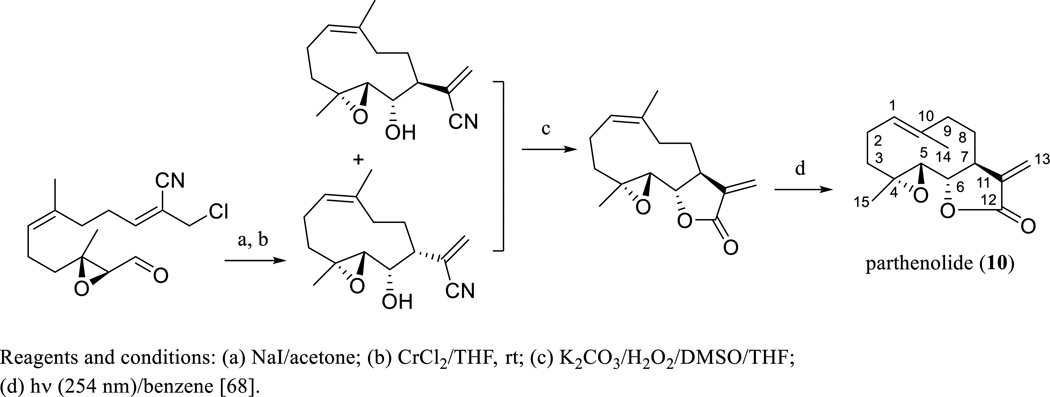
Parthenolide (PTL, 10) is an epoxylated germacranolide isolated from Chrysanthemum parthenium L. or Tanacetum parthenium L. (Asteraceae) (Scheme 6) [63], the feverfew plant, which is well known to contain SQLs and is used widely as a phytomedicine or botanical dietary supplement in Western countries (NIH National Library of Medicine's MedlinePlus Feverfew Listing: www.nlm.nih.gov/medlineplus/druginfo/natural/933.html). The isolation and characterization of PTL was reported briefly in 1959 and then in a more detailed manner in 1961, with a double bond determined at the C-2 and C-3 positions [63, 64]. Soon afterwards, the structure of PTL was revised through several degradation experiments and interpretation of NMR data, with the C-2/C-3 double bond assigned at the C-1 and C-10 positions, when it was isolated from the trunk bark of Michelia champaca L. (Magnoliaceae) [65]. The stereochemistry of the lactone ring of PTL was proposed by comparison of the analytical data of this epoxide with that of dihydrocostunolide [66], and supported later by analysis of its single-crystal X-ray diffraction data (Scheme 6) [67]. This was also confirmed by the total synthesis of PTL, which has been completed through a macrocyclic stereocontrolled Barbier reaction followed by a photoinduced Z/E isomerization (Scheme 6) [68].
Scheme. (6).
Synthesis of parthenolide.
As discussed in a recent review article, PTL shows cytotoxicity against various human cancer cells and has promising antitumor efficacy [69]. It induces a rapid apoptotic pre-B ALL cell death [70], inhibits tubulin carboxypeptidase activity in Hela cells [71], suppresses osteosarcoma cell proliferation [72], and prevents DNA methyltransferase 1 [73]. When six-week-old male nude mice bearing OUR-10 human early stage renal cell carcinoma (RCC) xenografts were treated with PTL (dissolved in ethanol and diluted with saline, either by s.c. injection, 3 µg/mouse, daily, 3 times/week for six weeks, or by p.o., 10 mg/kg, daily for six weeks), the tumor growth was significantly suppressed with both treatments [74]. When nude BALB/cByJ-Hfh11nu KRIBB mice bearing human cholangiocarcinoma Choi-CK and SCK xenografts were treated (i.p.) by PTL (2.5 mg/kg), Ro317549 (a protein kinase C-α inhibitor, 2.5 mg/kg), or PTL plus Ro317549, daily for four weeks, the tumor growth was significantly inhibited by all treatments, and the combination of PTL and Ro317549 efficiently improved tumor growth inhibition [75]. In addition, when six- to eight-week-old athymic nude mice inoculated with A549 human non-small cell lung cancer cells were treated with PTL (dissolved in PBS, 5 mg/kg, i.p., three times/week for four weeks), PTL (dissolved in PBS, 5 mg/kg, i.p., three times weekly for four weeks) plus paclitaxel (dissolved PBS, 5 mg/kg, i.p., weekly for four weeks), or paclitaxel (dissolved PBS, 5 mg/kg, i.p., weekly for four weeks), the efficacy of paclitaxel for the treatment of non-small cell lung tumor was potentiated significantly by PTL [76]. After MDA-MB-231 human breast cancer cells were injected into the mammary fat pad of six- to seven-week-old female nude mice for six weeks, the tumors were removed, and mice were treated with PTL (a slurry in 10% ethanol, 40 mg/kg, oral gavage, daily for 45 days), docetaxel (dissolved in 13% ethanol, 5 mg/kg, i.p., weekly for 45 days), or PTL (a slurry in 10% ethanol, 40 mg/kg, oral gavage, daily) plus docetaxel (in 13% ethanol, 5 mg/kg, i.p., weekly) for 45 days. PTL reduced metastasis and improved the survival of mice bearing a xenograft model of breast cancer when used as a combination with docetaxel [77]. Mechanistically, the antitumor efficacy of PTL is involved in inhibiting nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and STATs, arresting cell cycle, and interfering with microtubule formation [78].
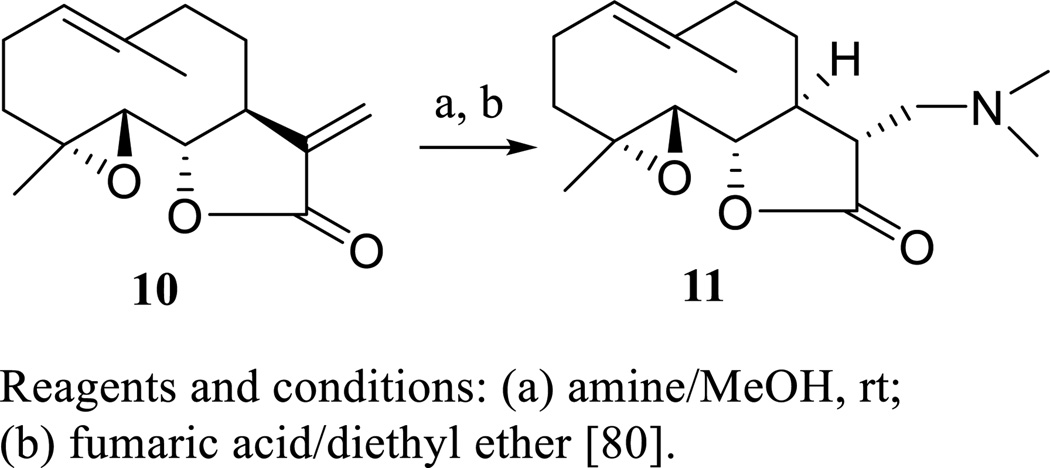
When a Phase I dose escalation trial was conducted for PTL given as a component of feverfew (T. parthenium) in cancer patients (12 adult patients with a male:female ratio of 11:1 and median age of 73 years), the low doses of this agent administered were unable to be detected, which was caused probably by its poor bioavailability [79]. To overcome this problem, several water-soluble aminoparthenolides have been partially synthesized (Scheme 7), and their cytotoxicity toward primary acute myeloid leukemia (AML) cells was evaluated [80]. Among these semisynthetic analogues, dimethylaminoparthenolide (DMAPT or LC-1, 11) was found to be potently cytotoxic against AML, CWR22Rv1 human androgen-independent prostate, and PC-3 human prostate cancer cells [80, 81].
Scheme. (7).
Synthesis of dimethylaminoparthenolide.
5. DIMETHYLAMINOPARTHENOLIDE (DMAPT), AN ANTICANCER PRO-DRUG DERIVED FROM PARTHENOLIDE
Dimethylaminoparthenolide (DMAPT, 11) was derived from PTL and showed improved cytotoxicity toward several human cancer cell lines and water solubility [80, 81]. When six- to eight-week-old female nude athymic mice bearing CWR22Rv1 or PC-3 tumors were treated with DMAPT (dissolved in sterile water, 100 mg/kg, oral gavage, daily for 61 days), docetaxel (diluted in 100% alcohol, 5 mg/kg, i.p., weekly for 61 days), or bicalutamide (dissolved in sterile water, 50 mg/kg, oral gavage, daily for 61 days), DMAPT was found to inhibit tumor growth substantially, which was more effective than bicalutamide alone in the CWR22Rv1 model and more effective than docetaxel alone in a PC-3 model [81]. Also, DMAPT enhanced X-ray-induced tumor growth delay in female athymic nude mice bearing A549 tumor xenografts, when mice were treated by DMAPT (in 2.5% mannitol, 100 mg/kg, oral gavage, daily for 7 days) [82].
Treatment with DMAPT (dissolved in sterile water, 50 or 100 mg/kg, oral gavage, daily for 48 days) was found to suppress tumor growth in athymic male nude mice bearing A549 human lung cancer and UMUC-3 human bladder transitional cell carcinoma xenografts [83]. When seven-month-old LSL-KrasG12D/+;Pdx-1-Cre mice were treated with DMAPT (dissolved in hydroxylpropyl methylcellulose with 0.2% Tween, 40 mg/kg, oral lavage, daily for 3 months) or DMAPT in combination with sulindac (dissolved in hydroxylpropyl methylcellulose with 0.2% Tween, 20 mg/kg, oral lavage, daily for 3 months) and/or gemcitabine (dissolved in PBS, 50 mg/kg, i.p., 2 times/week for 3 months), the percentage of normal pancreatic ducts was increased significantly by the combinations of DMAPT/sulindac, DMAPT/gemcitabine, sulindac/gemcitabine, and DMAPT/sulindac/gemcitabine, when compared to placebo treatment. In addition, the percentage of mouse pancreatic intraepithelial neoplasia-2 lesions was decreased significantly by a DMAPT/gemcitabine combination treatment [84]. These in vivo effects were consistent with the enhancement of the antiproliferative effects of gemcitabine by DMAPT through inhibition of NF-κB observed in vitro [85]. Also, DMAPT induced the rapid death of primary human leukemia stem cells (LSCs) from both myeloid and lymphoid leukemia and demonstrated approximately 70% oral bioavailability [86]. When NOD/SCID mice bearing AML tumor xenografts were treated orally with DMAPT using a single dose of 100 mg/kg, the cell response to oxidative stress was activated, and NF-κB was inhibited [86]. Mechanistically, DMAPT may exhibit antitumor efficacy through inhibition of NF-κB DNA binding and activation of the p53 tumor protein [86]. It has been evaluated in a phase I clinical trial for the treatment of ALL, AML, and other hematological tumors [8].
6. DE-EPOXYLATED ANALOGUES OF PARTHENOLIDE WITH POTENTIAL ANTITUMER EFFICACY
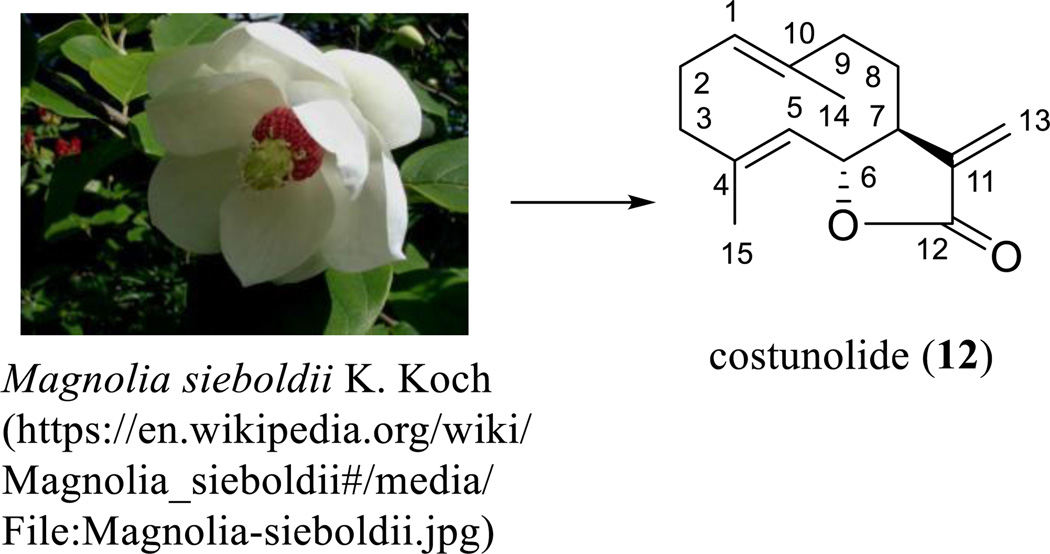
PTL (10) and its synthetic analogue, DMAPT (11), have been well documented for their potential antitumor efficacy. Interestingly, some de-epoxy analogues of PTL were also found to show this same activity. Costunolide (12) isolated from Magnolia sieboldii K. Koch (Magnoliaceae) (Scheme 8) was found to show cytotoxicity toward a panel of human cancer cell lines, including hormone-dependent (LNCaP) and independent (PC-3 and DU-145) prostate cancer cells [87–89]. When male inbred CDF1 mice bearing 3LL Lewis lung carcinoma tumors were treated (i.p.) with costunolide (12) using doses of 7.5, 15, or 30 mg/kg/day for 7 days, tumor growth was significantly suppressed and mouse survival was increased with all doses used [87].
Scheme. (8).
Costunolide derived from Magnolia sieboldii K. Koch.
After BALB/c athymic nude mice inoculated subcutaneously with SKOV3PT platinum-resistant human ovarian cancer cells were treated with costunolide (12, i.p., 5 and 10 mg/kg, daily for 10 days), tumor growth was retarded significantly, which was associated with activation of caspases-3, -8, and -9 and ROS generation [90]. Mechanistically, the cytotoxicity of costunolide toward PC-3 cells was proposed as being due to induction of a rapid overload of nuclear Ca2+, DNA damage response, and ataxia-telangiectasia, mutated and Rad3-related (ATR) phosphorylation [89]. Costunolide (12) induced human leukemia cell apoptosis via ROS and Bcl-2-dependent mitochondrial permeability transition and activation of JNK [87]. Also, it induced G2/M phase arrest and mitochondrial-mediated apoptosis in SGC-7901 human gastric adenocarcinoma cells [91]. In estrogen receptor-negative MDA-MB-231 breast cancer cells, costunolide (12) induced Fas-mediated extrinsic apoptosis, p21WAF1-related G2/M cell cycle arrest, and ROS generation [92].
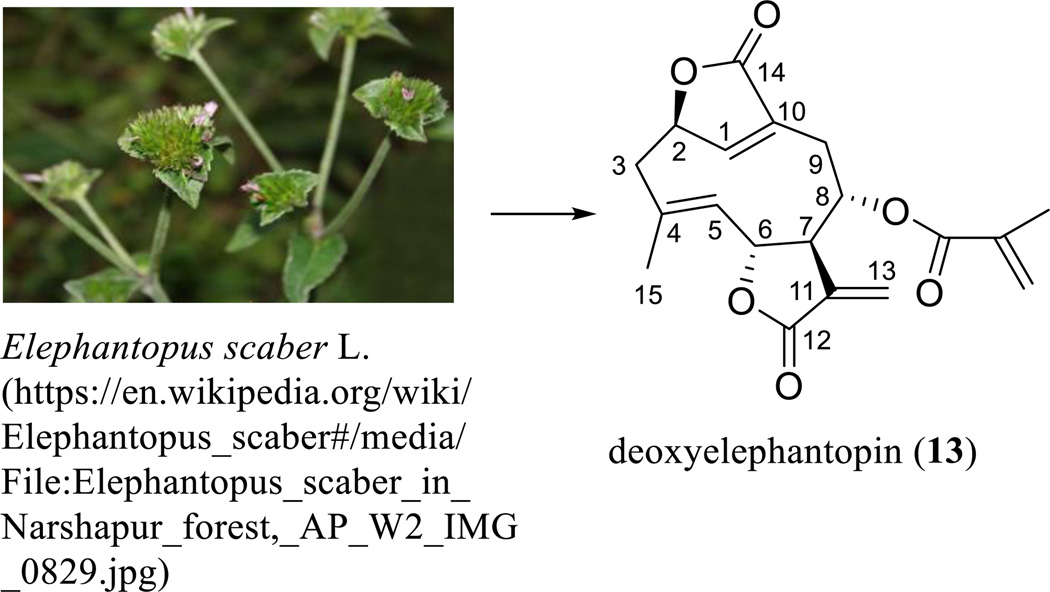
An interesting SQL with potential anticancer activity is deoxyelephantopin (13), a germacranolide containing a C-8 methacrylate group and additional lactone rings located at the C-2 and C-10 positions. This compound was isolated from Elephantopus scaber L. (Asteraceae) (Scheme 9) [93], and its structure was determined by comparison of its spectroscopic data with those of isodeoxyelephantopin dimethylamine adduct, which was investigated earlier by single-crystal X-ray diffraction measured on an Enraf-Nonius CAD-4 diffractometer using Cu-Ka radiation [94]. This original determination was confirmed by analysis of the single-crystal X-ray data of a close analogue, scabertopin, which were measured on a Rigaka RAXIS IIc image plate detector with MoKα radiation [95].
Scheme. (9).
Deoxyelephantopin derived from Elephantopus scaber L.
Several in vitro studies have shown that deoxyelephantopin (13) exhibits cytotoxicity toward human cancer KB cells [94]. It inhibited tumor necrosis factor-α (TNF-α) induced NF-κB activation in KBM-5 human chronic myeloid leukemia cells [96] and activated the nuclear hormone receptor and peroxisome proliferator-activated receptor-γ (PPAR-γ) in HeLa cells [97]. This compound inhibited significantly the colony formation, cell proliferation, migration, and invasion of murine mammary adenocarcinoma TS/A cells [98]. It induced G2/M arrest and apoptosis by up-regulation of JNK-mediated p21Waf1/Cip1 expression, caspase activation, and tumor necrosis factor (TNF)-α-induced matrix metalloproteinase-9 (MM-9) activity and NF-κB activation inhibition [98]. In in vivo testing, it inhibited tumor growth, reduced tumor blood vessel density, and doubled the survival time of female BALB/c mice bearing TS/A metastasizing murine mammary tumors, and these antitumor efficacies were found to be superior to paclitaxel, when mice were treated with this anticancer drug (5 mg/kg, i.p., daily for 28 days) or deoxyelephantopin (1 or 10 mg/kg, i.p., daily for 28 days) [98].
To investigate the molecular mechanisms underlying the differential efficacy between deoxyelephantopin (13) and paclitaxel, the protein profiles expressed in the nucleus and cytoplasm of TS/A cells treated by both agents were measured. This showed that endoplasmic reticulum (ER) stress was associated with apoptosis induced by both these test compounds, but only 13 inhibited proteasomal proteolysis [99]. Another mechanistic study showed that 13 inhibited CNE human nasopharyngeal cancer cell proliferation, causing cell cycle arrest in both the S and G2/M phases, and the apoptosis triggered was associated with dysfunction in the mitochondria [100]. A SAR study showed that the stereochemistry at the C-2 position affected the cytotoxicity of 13 toward SMMC7721 human hepatocarcinoma, human cervical carcinoma HeLa, and Caco-2 human colon carcinoma cells [101].
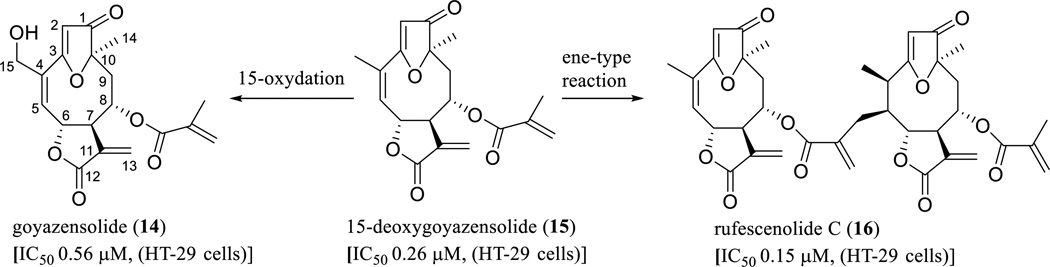
In a search for anticancer agents from higher plants by our group [19], several parthenolide analogues containing a furan ring between the C-3 and C-10 positions, a ketone group at the C-1 position, and an ester group at the C-8 position, including goyazensolide (14), 15-deoxygoyazensolide (15), and rufescenolide C (16), were characterized as active leads from Piptocoma rufescens Cass. (Asteraceae) (Scheme 10) [52, 102–104].
Scheme. (10).
Proposed biogenesis of rufescenolide C.
Goyazensolide (14) was initially isolated as a potential schistosomicidal constituent from Eremanthus goyazensis Sch.-Bip. [105], with the structure determined by single-crystal X-ray diffraction analysis [106]. The novel dimeric compound, rufescenolide C (16), was proposed as being formed through an ene-type reaction from 15, from which 14 was probably produced by C-15 oxidation (Scheme 10) [52]. All these compounds showed potent cytotoxicity toward HT-29 human colon cancer cells, and compound 14 was also found to inhibit the Sch10545 and Ben-Men-cell growth [52, 102–104]. This agent inhibited the proliferation of Sch10545 and Ben-Men cells by reducing the levels of cyclins A and E, phospho-AKT, and NF-κB, and was proposed as a potential agent for the treatment of neurofibromatosis type 2 (NF2)-associated schwannomas and meningiomas [103]. Also, it showed antitumor efficacy when tested in an in vivo hollow fiber assay in immunodeficient NCr nu/nu mice implanted with HT-29 cells with a dose of 12.5 mg/kg (i.p.) [104]. Mechanistically, it down-regulated IKK and IKKβ, up-regulated ROS levels and caspase-3 expression in HT-29 cells, and induced cellular apoptosis by a G1 block in the cell cycle progression [104].
It has been reported that the α-methylene-γ-lactone ring of SQLs plays a critical role in mediating cancer cell cytotoxicity, and introduction of an additional enone O=C-C=CH2 system into the molecule can increase this activity [107]. A quantitative structure–activity relationship (QSAR) study showed that a double bond between the C-1 and C-10 positions, a hydroxy group at the C-5 position, and an angeloyloxy group at the C-8 position are all important for SQLs to mediate their cytotoxicity [108]. In a study on the development of a structural model of SQLs for their NF-κB inhibitory activity, several germacranolides were found to possess this effect [109]. Consistent with these previous observations, rufescenolide C (16, a SQL dimer isolated originally from Piptocoma rufescens), contains four enone groups, and was found to be more cytotoxic toward the HT-29 cells than its monomer, 15-deoxygoyazensolide (15, Scheme 10) [52], which was reported to inhibit NF-κB activation in vitro [102].
7. 8-O-(12-{l-LEUCINOYLAMINO}DODECANOYL)-8-O-DEBUTANOYLTHAPSIGARGIN (L12ADT), AN ANTICANCER PRODRUG DERIVED FROM THAPSIGARGIN
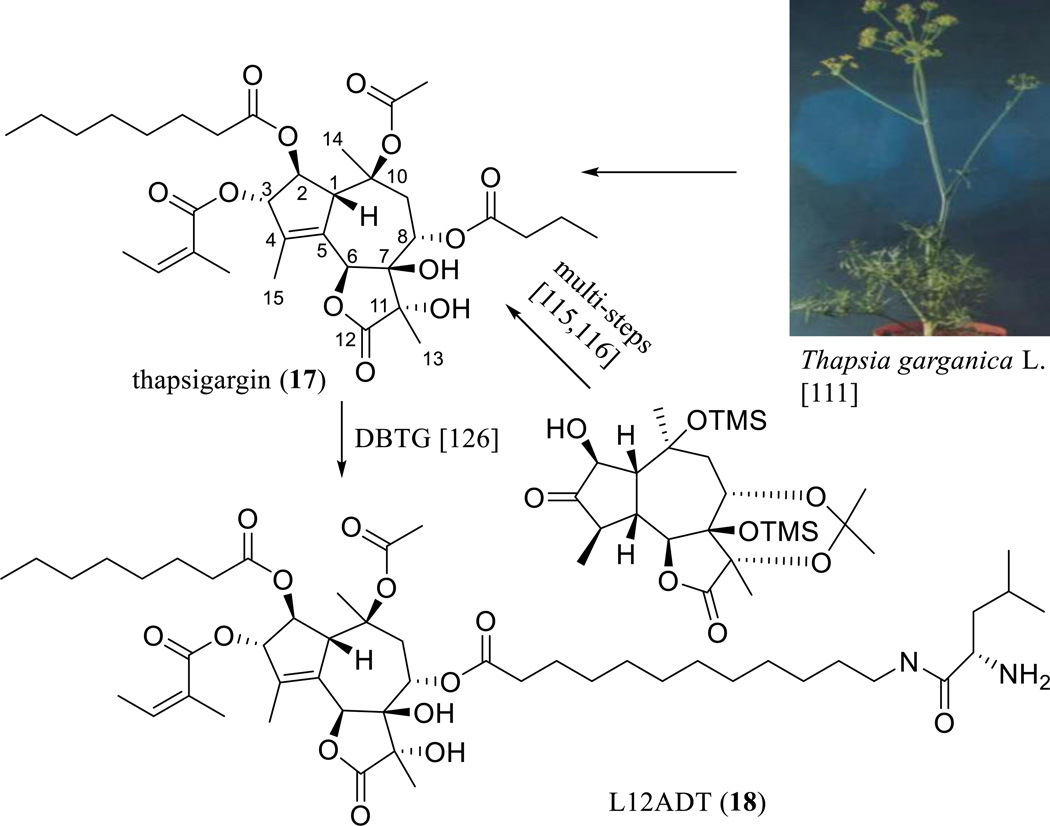
A major achievement in the search for anticancer guaianolides was the discovery of thapsigargin (TPG, 17) (Scheme 11) [110, 111]. This compound was originally isolated from the roots of Thapsia garganica L. (Apiaceae) by the group of Prof. Søren Brøgger Christensen (Danish University of Pharmaceutical Sciences, Copenhagen, Denmark) [112], with the structure and relative configuration determined by chemica1 and spectroscopic investigations, together with X-ray analysis of its 7,11-epoxide analogue [113]. The absolute configuration of TPG was established using CD spectroscopy with the application of Horeau’s method [114], and the enantioselective total synthesis of TPG has been completed through a total of 42 steps of reactions involving regioselective introduction of the internal olefin at C-4–C-5, judicious protecting group choice to allow chelation-controlled reduction at C-3, and selective esterification (Scheme 11) [115, 116].
Scheme. (11).
Synthesis of L12ADT.
TPG (17) was found to inhibit intracellular sarcoplasmic/ER Ca2+ transport ATPase (SERCA) [117], and exhibited cytotoxicity toward a panel of human cancer cell lines [118]. It synergized with the cytotoxicity of imatinib toward GIST-T1 gastrointestinal stromal tumor cells as a result of the expression of glucose-regulated protein 78 (GRP78) and activation of caspase-3 [119]. It induced Caki human renal cancer cell apoptosis, and such an effect was increased by co-treatment of melatonin through the up-regulation of cytidine-cytidine-adenosine-adenosine-thymidine (CCAAT)-enhancer-binding protein homologous protein (CHOP) [120]. Also, TPG (17) induced HCT116 human colon cancer cell apoptosis through a Bax-dependent signaling pathway [121] and sensitized the stromal-like tumor cells of giant cell tumor of bone (GCT) to TNF-related apoptosis-inducing ligand (TRAIL)-induced cell death, which is associated partially with up-regulation of the death receptor DR5 and down-regulation of the decoy receptor DcR1 [122].
However, a Lucena I MDR tumor cell line derived from human leukemic K562 cells was found to be highly resistant to TPG [123]. To improve the cytotoxicity of TPG, a number of analogues bearing an aromatic amine residue rather than an 8-O-butanoyl group have been synthesized and evaluated using a SERCA inhibition assay. An analogue containing a 3-(4-aminophenyl)propanoyl group was found to inhibit SERCA and to potently induce TSU-Pr1 human prostate cancer cell apoptosis [124]. A binding study on 8-O-N-tert-butoxycarbonyl-12-aminododecanoyl derivatives and a SERCA pump showed the importance of the length and flexibility of the side chain at the C-8 position of thapsigargin, and a series of 2-unsubstituted analogues showing equipotent SERCA inhibition have been constructed [125]. Among these products, only a 12-Boc-aminododecanoyl derivative possessing a conjugation site for a peptide moiety was apoptotic [125]. Furthermore, a 12-aminododecanoyl side-chain analogue of TPG (12ADT) was coupled successfully to leucine to produce a cytotoxic compound, 8-O-(12-[l-leucinoylamino]dodecanoyl)-8-O-debutanoylthapsigargin, namely, L12ADT (18) [126]. L12ADT was then coupled to a peptide carrier, a substrate for the prostate-specific antigen (PSA) protease to produce an aqueous soluble, cell-impermeable latent prodrug, which was specifically activated extracellularly within metastatic prostate cancer sites by PSA [127].
A kinetic study on L12ADT peptide prodrug showed that it is stable in human plasma and hydrolyzed efficiently by PSA [127]. It was selectively toxic to PSA-producing prostate cancer cells [127]. The L12ADT peptide prodrug inhibited tumor growth without substantial host toxicity observed in vivo, when nude BALB/c mice bearing LNCaP human prostate tumors were treated with this agent (dissolved in 2% DMSO in water, injected intravenously, 7 mg/kg, daily for three cycles of 5 days each) [127]. The mechanism of action of the peptide prodrug L12ADT was proposed as being due to depletion of androgen receptor (AR) protein via protein synthesis inhibition [128]. Currently, this agent is being developed for testing in clinical trials to treat metastatic prostate cancer [127, 128], and a new alternative production platform to produce TPG (17) from the moss Physcomitrella through biosynthesis is being investigated to meet the requirement of large compound quantities for the further development of this parent compound [129].
8. ANALOGUES OF THAPSIGARGIN WITHOUT ESTER GROUPS SHOWING POTENTIAL ANTITUMOR ACTIVITY
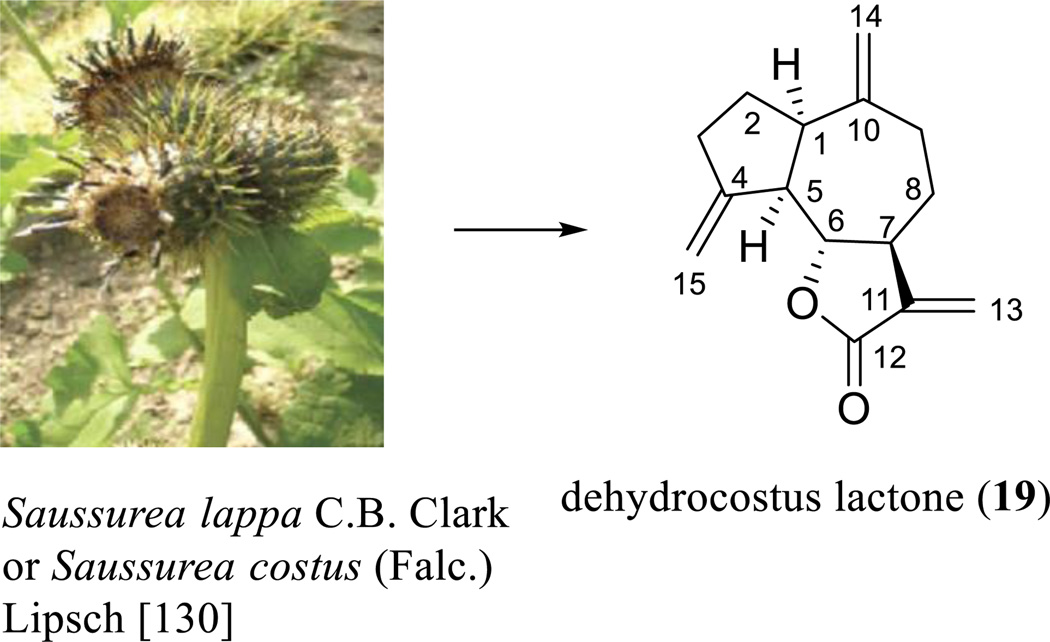
It has been evidenced that many biologically active SQLs possess either the guaianolide (with the C-15 methyl group attached at the C-4 position) or the pseudoguaianolide (with the C-15 methyl group attached at the C-5 position) skeleton [108, 109]. As presented in several of the paragraphs above, TPG (17), a guaianolide containing four ester groups, was found to show potent antitumor activity. Interestingly, a very simple analogue of TPG, dehydrocostus lactone (DHL, 19) and its analogues, were also found to show this same effect.
DHL (19) was extracted from Saussurea lappa C.B. Clark or Saussurea costus (Falc.) Lipsch (Asteraceae) (Scheme 12) [130] and has been found to show cytotoxicity against the HepG2 and PLC/PRF/5 human liver cancer cell lines [131] and to inhibit the growth of the DU145 human prostate cancer cell line [132]. The molecular structure of this agent was determined by analysis of its single-crystal X-ray diffraction parameters (Scheme 12) [133], and (±)-DHL was synthesized by formation of a five-membered ring through a 1,8-addition with a Grignard reagent, completion of a regio- and stereoselectively-butyrolactone via epoxide opening with dilithioacetate, and introduction of three exocyclic methylene groups [134]. It was found to inhibit the proliferation of the HepG2 and PLC/PRF/5 human liver cancer cell lines by inducing apoptosis through up-regulation of Bax and Bak, down-regulation of Bcl-2 and Bcl-XL, nuclear relocation of the mitochondrial apoptosis-inducing factor (AIF), and effects on endonuclease G (Endo G) [131]. It triggered ER stress in a p38 and extracellular signal-regulated kinase 1/2-dependent manner and subsequently caused JNK activation [131]. DHL (19) inhibited DU145 cell growth and induced cell apoptosis by activation of caspases 8, 9, 7, and 3 [132]. It protected osteoblastic MC3T3-E1 cells from antimycin A-induced cell damage [135] and enhanced TNF-α-induced apoptosis of the HL-60 human leukemia cell line [136]. Also, this compound enhanced poly(ADP-ribose) polymerase (PARP) cleavage, decreased Bcl-xL expression, and increased levels of Bax, Bak, Bok, Bik, Bmf, and t-Bid [132].
Scheme. (12).
Dehydrocostus lactone derived from Saussurea lappa C.B. Clark or Saussurea costus (Falc.) Lipsch.
Consistent with its in vitro cytotoxicity toward cancer cells, DHL (19) showed antitumor efficacy in vivo. When BALB/cA-nu mice bearing PLC/PRF/5 human hepatocellular carcinoma and MDA-MB-231 human breast tumor were treated by DHL (dissolved in a solution containing 25% polyethylene glycol, 10 mg/kg, i.p., daily for 45 day), tumor growth was significantly suppressed [131, 137]. Also, neo-vascularization was found to be significantly inhibited, when C57BL/6 mice were injected subcutaneously with matrigel mixed with or without DHL (1 mM and 10 mM), and the matrigels were dissected and photographed [138].
Mechanistically, DHL (19) prevented mitochondrial membrane potential dissipation, complex IV inactivation, ATP loss, cytochrome c release, intracellular calcium elevation, potassium loss, and ROS production induced by antimycin A in osteoblastic MC3T3-E1 cells [135]. It inhibited TNF-α-induced NF-κB activation in human leukemia HL-60 cells to induce cell apoptosis [136] and inhibited MCF-7 human breast cancer cell proliferation by inducing apoptosis through cell cycle blockade [137]. It increased suppressors of cytokine signaling (SOCS)-1 and SOCS-3 expression and inhibited the JAK/STAT3 pathway [137]. Treatment of human umbilical vein endothelial cells (HUVECs) with DHL resulted in cell cycle arrested at the G0/G1 phase to inhibit angiogenesis in vitro, which was proposed to target the Akt/GSK-3b and mTOR pathway [138].
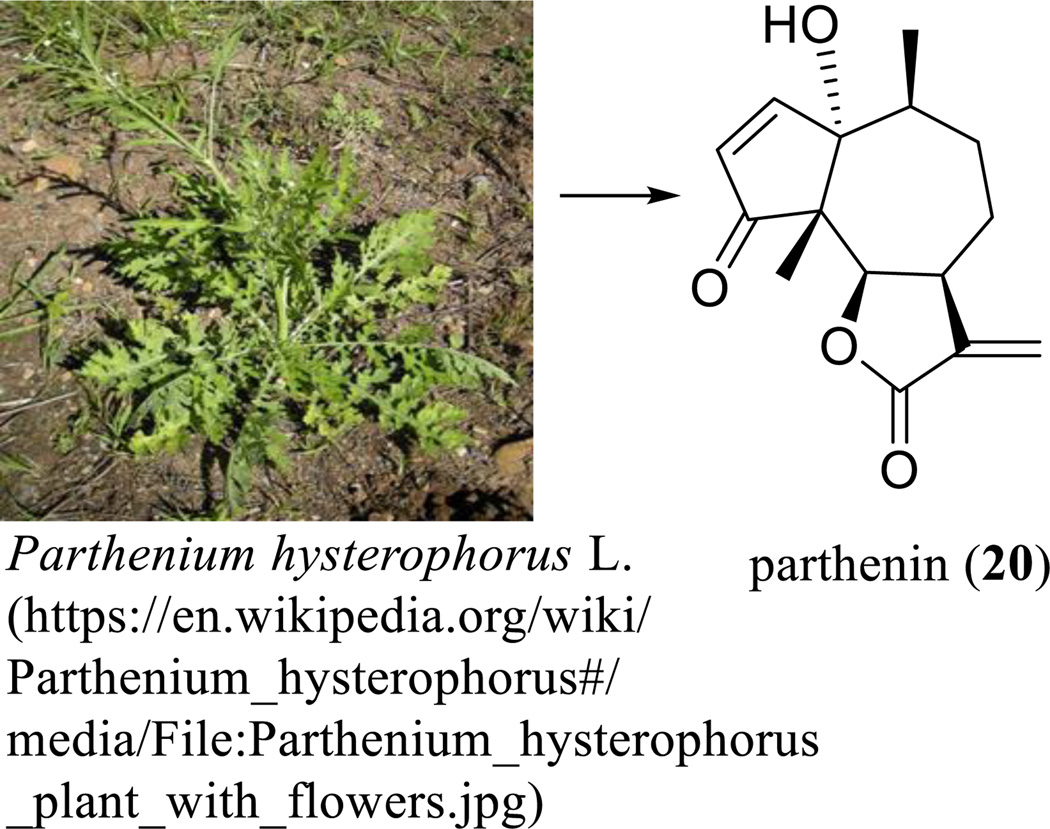
Parthenin (20) was isolated from Parthenium hysterophorus L. (Asteraceae) with the structure proposed from chemical transformations and the 10S absolute configuration established on the degradation of parthenin to S-(+)-α-methylglutaric acid [139]. This structure elucidation has been completed by analysis of its single-crystal X-ray diffraction [140], as supported later by total synthesis of the compound (Scheme 13) [141, 142].
Scheme. (13).
Parthenin derived from Parthenium hysterophorus L.
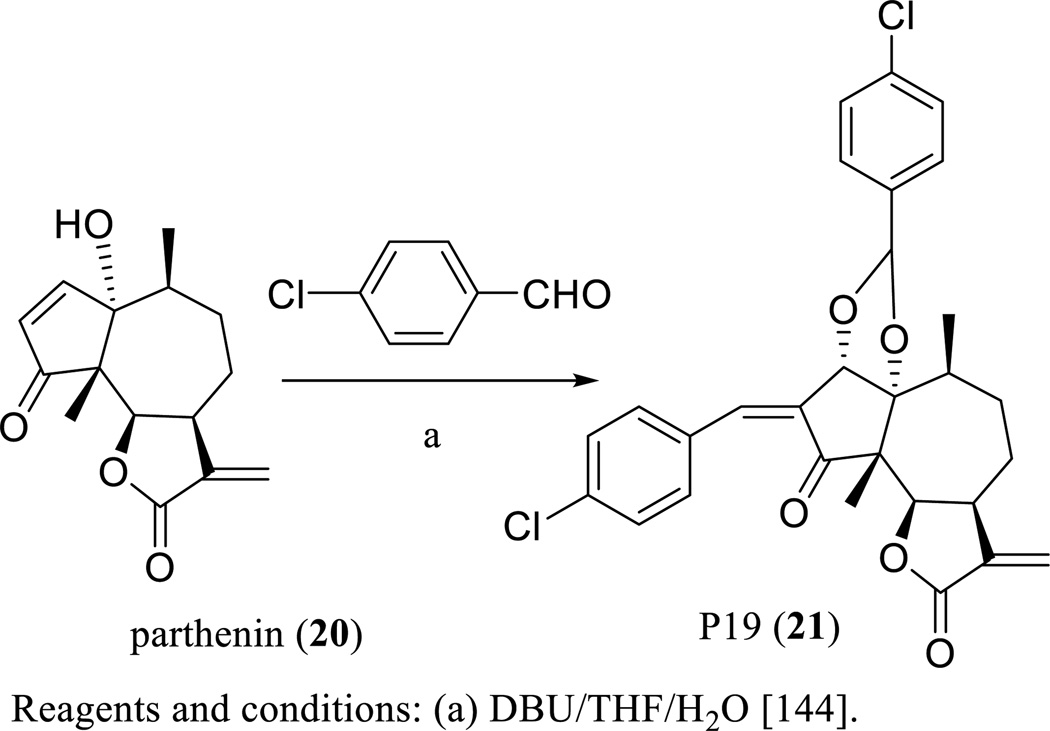
Parthenin (20) showed cytotoxicity against several human cancer cell lines, but the further development as a useful anticancer agent was hindered by its genotoxicity observed in an in vivo study [13]. To overcome this problem, several spiro derivatives of parthenin have been synthesized and evaluated, of which some compounds exhibited improved cytotoxicity toward a small panel of human cancer cells [143]. In an in vivo study on non-inbred Swiss mice bearing EAT performed on parthenin (20) and its spiro derivative, 2t, (both suspended in 1% gum acacia, 10, 25, 50, 100 or 200 mg/kg, i.p., daily for nine days), significant tumor growth inhibition was observed for compound 2t at a dose of 100 mg/kg, whereas a 200 mg/kg dose of this substance induced mortality of all mice by the fourth day of the treatment. However, a high level of toxicity without significant antitumor activity was recorded with the parthenin (20) treatments, which caused mortality of all mice by the second and third day of treatment at 25 and 50 mg/kg doses, respectively, suggesting that compound 2t showed more potent antitumor activity and lower toxicity in mammalians than the parent compound [143]. Following this evidence, further modification of parthenin (20) produced compound P19 (21) (Scheme 14) [144], which showed cytotoxicity toward the HeLa human cervical carcinoma cell line and the HL-60 human myeloid leukemia cell line [145].
Scheme. (14).
Synthesis of P19.
P19 (21) extended the median life span of CDF1 male mice bearing L1210 lymphoid leukemia, when mice were treated with this agent (dissolved in 1% gum acacia in normal saline, 10 mg/kg, i.p., for nine consecutive days) [146]. This compound suppressed EAT growth in Swiss albino mice, when mice were treated i.p. for nine consecutive days at doses of 15 and 25 mg/kg of P19 [146]. Also, P19 impeded Sarcoma-180 solid tumor growth in Balb/c mice treated with this compound for nine consecutive days at the i.p. doses of 15 mg/kg, but no toxicity or mortality occurred in animals during the treatment period [146]. Mechanistically, P19 produced selectively NO by countering ROS generation, disrupted mitochondrial integrity leading to cytochrome c release, activated caspases 3, 8, and 9, and inhibited NF-κB and the pro-survival proteins, pSTAT3 and survivin [146].
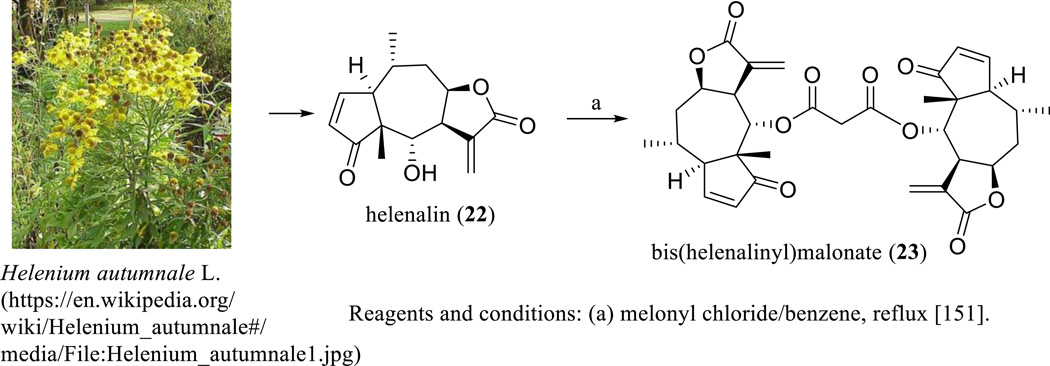
Helenalin (22) is a pseudoguaianolide with a five-membered lactone ring linked at the C-7 and C-8 positions. Helenalin was discovered as a major constituent of Helenium autumnale L. (Asteraceae) (Scheme 15), with its structure confirmed by analysis of the single-crystal X-ray diffraction of its brominated derivative, bromohelenalin [147].
Scheme. (15).
Synthesis of bis(helenalinyl)malonate.
Treatment of BDFl male mice with helenalin using a single dose of 25 mg/kg (i.p.) decreased significantly hepatic microsomal cytochrome P450 levels in mice [148]. A mechanistic study showed that helenalin (22) induced HL-60 cell differentiation via the protein kinase C (PKC)/extracellular signal-regulated kinase (ERK) signaling pathway and inhibition of NF-κB activity [149]. It was found to reverse Bcl-2-mediated chemoresistance in tumor cells by inhibition of Bcl-2-induced upregulation of NF-κB activity [150]. Also, this compound induced cell death and abrogated clonal survival in the highly apoptosis-resistant Bcl-2-overexpressing Jurkat cell line and other two Bcl-2-overexpressing solid MCF-7 mammary and L6.3pl pancreatic tumor cell lines [150]. It was proposed to target inhibition of augmented NF-κB activity in Bcl-2-overexpressing tumor cells and promotion of the production of ROS leading to necrosis, but not directly to target Bcl-2-induced mitochondrial resistance [150].
To improve antitumor efficacy, several dimeric analogues of helenalin were prepared (Scheme 15) [151], of which bis(helenalinyl)malonate (23) was found to be more cytotoxic toward P-388 human lymphocytic leukemia and human KB oral cancer cells than helenalin [151, 152]. Also, this dimer showed a greater potency and much lower toxicity to animals than helenalin (22) to suppress tumor growth, when BDF1 male mice bearing P-388 leukemia were treated with both agents (8 mg/kg, i.p., daily for 14 days) [151].
9. DISCUSSIONS AND PERSPECTIVES
Three SQLs showing promising anticancer activity in current cancer clinical trials, namely, ARS (4), DMAPT (11), and L12ADT (18), which were prepared from the plant-derived ART (1), PTL (10), and TPG (17), respectively, have been discussed in the present review. In addition, several close analogues of compounds 4, 11, and 18 that have also shown antitumor efficacy have been reviewed. It is worthy of note that all the naturally occurring antitumor SQLs discussed, except for TPG (17), including ART (1) [21], PTL (10) [63], costunolidde (12) [130], deoxyelephantopin (13) [93], goyazensolide and its analogues (14–16) [52, 102], DHL (19) [130], parthenin (20) [139], and helenalin (22) [147], were isolated from a large and widespread plant family, the Asteraceae (Sunflower family), from which lettuce (Lactuca sativa L.) and chicory (Chicorium intybus L.) are dietary sources of SQLs that has been consumed globally as common vegetables for many years [1]. This indicates that some species in the Asteraceae family may produce the specific enzymes that are probably responsible for generation of SQLs with potent cytotoxicity toward human cancer cells that are non-toxic at low concentrations.
It has been well documented that an α-methylene-γ-lactone ring, which causes the steric or chemical changes of the molecular target to affect their functions through alkylation of the enzymes, is essential for biological effects mediated by SQLs [1]. Consistent with this evidence, deoxyelephantopin (13), which contains two lactone rings, showed promising in vivo antitumor activity that was superior to paclitaxel in the animal model used [98]. Dimeric SQLs, such as rufescenolide C (16) and bis(helenalinyl)malonate (23), have been found to be more potently cytotoxic than their monomers toward human cancer cells [52, 151, 152], indicating that the antitumor potential of SQLs could be improved by the presence of additional α-methylene-γ-lactone rings.
Synthetic modifications on NPs in enhancing their therapeutic value through optimization of pharmacokinetics, stability, potency, and/or selectivity have yielded several successful drugs [28]. As discussed in the present review, the antitumor potency of the natural SQLs, including ART, PTL, and TPG, has been improved greatly by synthetic molecular modification, and dimerization has been found to be a promising method in such an effort. Several dimeric ARTs have been prepared and found to be more potently and selectively cytotoxic toward a small panel of human cancer cell lines than their monomers [55–60], and the MDR P-glycoprotein-overexpressing CEM/ADR 5000 leukemia cell line was determined as not being cross-resistant to several ART dimers [62]. Also, some ART dimers showed improved antitumor efficacy in vivo [58, 60], of which dimer 9 exhibited effects comparable with paclitaxel in the NCI in vivo hollow fiber assay [60]. The high effectiveness, low side effects, and reversal of MDR observed from ART dimers make these agents especially promising for further development as potential anticancer agents.
The endoperoxide bridge of ART reacts with ferrous iron to generate free radicals leading to cancer cell death [32]. Most cancer cells contain more intracellular free iron than normal cells, thus, ART and its analogs caused selective cancer cell apoptosis [18, 30, 36]. Inhibition of NF-κB can prohibit tumor cell resistance to the chemotherapeutic agents to sensitize cancer treatment [153]. Both PTL and DMAPT were found to potentiate the activity of other anticancer drugs, which was probably due to their inhibition of NF-κB [76–78, 86]. Thus, these SQLs provide important information for the design and discovery of new anticancer drugs by showing more selective and greater synergistic effects for the better treatment of cancer.
Acknowledgments
Some compounds mentioned in this review article that were isolated in our laboratory, were obtained as a result of grant P01 CA125066, funded by the National Cancer Institute, NIH, Bethesda, MD, USA. We thank Dr. Judith C. Gallucci, Department of Chemistry and Biochemistry, The Ohio State University, Columbus, Ohio, 43210, United States, for her comments on single-crystal X-ray analysis of the compounds covered by this manuscript. Also, we are grateful to Mr. Xiaoyu Lu for his initial work on reference searching and structural editing.
ABBREVIATIONS
- 12ADT
12-Aminododecanoyl side-chain analogue of thapsigargin
- ALL
Acute lymphoblastic leukemia
- AML
Acute myeloid leukemia
- AR
Androgen receptor
- ARM
Artemether
- ARS
Artesunate
- ART
Artemisinin
- CAR
Constitutive androstane receptor
- CCAAT
Cytidine-cytidine-adenosine-adenosine-thymidine
- CHOP
CCAAT-enhancer-binding protein homologous protein
- CYP
Cytochrome P450
- DHA
Dihydroartemisinin
- DHL
Dehydrocostus lactone
- DMAPT
Dimethylaminoparthenolide
- EAT
Ehrlich ascites tumor
- ER
Endoplasmic reticulum
- GCT
Giant cell tumor of bone
- HPV
Human papillomavirus
- HUVEC
Human umbilical vein endothelial cell
- i.p.
Intraperitoneally
- L12ADT
8-O-(12-[l-Leucinoylamino]dodecanoyl)-8-O-debutanoylthapsigargin
- LSC
Leukemia stem cell
- MDR
Multidrug resistance
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NP
Natural product
- p.o.
Per os (by mouth)
- PCD
Programmed cell death
- PPAR-γ
Peroxisome proliferator-activated receptor-γ
- PSA
Prostate-specifically antigen
- PTL
Parthenolide
- QSAR
Quantitative structure–activity-relationship
- ROS
Reactive oxygen species
- s.c.
Subcutaneous
- SAR
Structure-activity relationship
- SERCA
Sarcoplasmic/ER Ca2+ transport ATPase
- SQL
Sesquiterpene lactone
- TCTP
Translationally controlled tumor protein
- TNF-α
Tumor necrosis factor-α
- TPG
Thapsigargin
- TRAIL
TNF-related apoptosis-inducing ligand
- TRAMP
Transgenic adenocarcinoma of mouse prostate
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Chadwick M, Trewin H, Gawthrop F, Wagstaff C. Sesquiterpenoid lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013;14:12780–12805. doi: 10.3390/ijms140612780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staneva JD, Todorova MN, Evstatieva LN. Sesquiterpene lactones as chemotaxonomic markers in genus. Anthemis. Phytochemistry. 2008;69:607–618. doi: 10.1016/j.phytochem.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Zidorn C. Sesquiterpene lactones and their precursors as chemosystematic markers in the tribe Cichorieae of the Asteraceae. Phytochemistry. 2008;69:2270–2296. doi: 10.1016/j.phytochem.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez E, Towers GHN, Mitchell JC. Biological activities of sesquiterpene lactones. Phytochemistry. 1976;15:1573–1580. [Google Scholar]
- 5.Repetto MG, Boveris A. Bioactivity of sesquiterpenes: compounds that protect from alcohol-induced gastric mucosal lesions and oxidative damage. Mini-Rev. Med. Chem. 2010;10:615–623. doi: 10.2174/138955710791383992. [DOI] [PubMed] [Google Scholar]
- 6.Lee K-H, Huang E-S, Piantadosi C, Pagano JS, Geissman TA. Cytotoxicity of sesquiterpene lactones. Cancer Res. 1971;31:1649–1654. [PubMed] [Google Scholar]
- 7.Zhang S, Won Y-K, Ong C-N, Shen H-M. Anti-cancer potential of sesquiterpene lactones: Bioactivity and molecular mechanisms. Curr. Med. Chem. - Anti-Cancer Agents. 2005;5:239–249. doi: 10.2174/1568011053765976. [DOI] [PubMed] [Google Scholar]
- 8.Ghantous A, Gali-Muhtasib H, Vuorela H, Saliba NA, Darwiche N. What made sesquiterpene lactones reach cancer clinical trials? Drug Disc. Today. 2010;15:668–678. doi: 10.1016/j.drudis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Merfort I. Perspectives on sesquiterpene lactones in inflammation and cancer. Curr. Drug Targets. 2011;12:1560–1573. doi: 10.2174/138945011798109437. [DOI] [PubMed] [Google Scholar]
- 10.Orofino Kreuger MR, Grootjans S, Biavatti MW, Vandenabeele P, D'Herde K. Sesquiterpene lactones as drugs with multiple targets in cancer treatment: focus on parthenolide. Anti-cancer Drugs. 2012;23:883–896. doi: 10.1097/CAD.0b013e328356cad9. [DOI] [PubMed] [Google Scholar]
- 11.Suffness M, Douros J. Current status of the NCI plant and animal product program. J. Nat. Prod. 1982;45:1–14. doi: 10.1021/np50019a001. [DOI] [PubMed] [Google Scholar]
- 12.Hall IH, Grippo AA, Holbrook DJ, Chang J-J, Yang L-M, Chaney SG, Lee K-H. Renal, hepatic, cardiac and thymic acute toxicity afforded by bis(helenalinyl)malonate in BDF1 mice. Toxicology. 1990;64:205–216. doi: 10.1016/0300-483x(90)90136-5. [DOI] [PubMed] [Google Scholar]
- 13.Ramos A, Rivero R, Visozo A, Piloto J, Garcia A. Parthenin, a sesquiterpene lactone of Parthenium hysterophorus L. is a high toxicity clastogen. Mutat. Res. 2002;514:19–27. doi: 10.1016/s1383-5718(01)00321-7. [DOI] [PubMed] [Google Scholar]
- 14.Salapovic H, Geier J, Reznicek G. Quantification of sesquiterpene lactones in Asteraceae plant extracts: Evaluation of their allergenic potential. Sci. Pharm. 2013;81:807–818. doi: 10.3797/scipharm.1306-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amorim MHR, Gil da Costa RM, Lopes C, Bastos MMSM. Sesquiterpene lactones: Adverse health effects and toxicity mechanisms. Crit. Rev. Toxicol. 2013;43:559–579. doi: 10.3109/10408444.2013.813905. [DOI] [PubMed] [Google Scholar]
- 16.Chaturvedi D, Goswami A, Pratim Saikia P, Barua NC, Rao PG. Artemisinin and its derivatives: a novel class of anti-malarial and anti-cancer agents. Chem. Soc. Rev. 2010;39:435–454. doi: 10.1039/b816679j. [DOI] [PubMed] [Google Scholar]
- 17.Crespo-Ortiz MP, Wei MQ. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. J. Biomed. Biotechnol. 2012:1–18. doi: 10.1155/2012/247597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai HC, Singh NP, Sasaki T. Development of artemisinin compounds for cancer treatment. Invest. New Drugs. 2013;31:230–246. doi: 10.1007/s10637-012-9873-z. [DOI] [PubMed] [Google Scholar]
- 19.Kinghorn AD, Carcache-Blanco EJ, Chai H-B, Orjala J, Farnsworth NR, Soejarto DD, Oberlies NH, Wani MC, Kroll DJ, Pearce CJ, Swanson SM, Kramer RA, Rose WC, Fairchild CR, Vite GD, Emanuel S, Jarjoura D, Cope FO. Discovery of anticancer agents of diverse natural origin. Pure Appl. Chem. 2009;81:1051–1063. doi: 10.1351/PAC-CON-08-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cragg GM, Grothaus PG, Newman DJ. New horizons for old drugs and drug leads. J. Nat. Prod. 2014;77:703–723. doi: 10.1021/np5000796. [DOI] [PubMed] [Google Scholar]
- 21.Coordinating Group for Research on the Structure of Qing Hau Sau. A new type of sesquiterpene lactone – Qing Hau Sau. Kexue Tongbao. 1977;22:142. [Google Scholar]
- 22.Klayman DL, Lin AJ, Acton N, Scovill JP, Hoch JM, Milhous WK, Theoharides AD, Dobek AS. Isolation of artemisinin (qinghaosu) from Artemisia annua growing in the United States. J. Nat. Prod. 1984;47:715–717. doi: 10.1021/np50034a027. [DOI] [PubMed] [Google Scholar]
- 23.Lisgarten JN, Potter BS, Bantuzeko C, Palmer RA. Structure, absolute configuration, and conformation of the antimalarial compound, artemisinin. J. Chem. Crystallogr. 1998;28:539–543. [Google Scholar]
- 24.Zhu C, Cook SP. A concise synthesis of (+)-artemisinin. J. Am. Chem. Soc. 2012;134:13577–13579. doi: 10.1021/ja3061479. [DOI] [PubMed] [Google Scholar]
- 25.Janse CJ, Waters AP, Kos J, Lugt CB. Comparison of in vivo and in vitro antimalarial activity of artemisinin, dihydroartemisinin and sodium artesunate in the Plasmodium berghei-rodent model. Int. J. Parasitol. 1994;24:589–594. doi: 10.1016/0020-7519(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 26.Jung M, Lee K, Kim H, Park M. Recent advances in artemisinin and its derivatives as antimalarial and antitumor agents. Curr. Med. Chem. 2004;11:1265–1284. doi: 10.2174/0929867043365233. [DOI] [PubMed] [Google Scholar]
- 27.Brossi A, Venugopalan B, Dominguez Gerpe L, Yeh HJC, Flippen-Anderson JL, Buchs P, Luo XD, Milhous W, Peters W. Arteether, a new antimalarial drug: Synthesis and antimalarial properties. J. Med. Chem. 1988;31:645–650. doi: 10.1021/jm00398a026. [DOI] [PubMed] [Google Scholar]
- 28.Szychowski J, Truchon J-F, Bennani YL. Natural products in medicine: Transformational outcome of synthetic chemistry. J. Med. Chem. 2014;57:9292–9308. doi: 10.1021/jm500941m. [DOI] [PubMed] [Google Scholar]
- 29.Ho WE, Peh HY, Chan TK, Wong WSF. Artemisinins: Pharmacological actions beyond anti-malarial. Pharmacol. Ther. 2014;142:126–139. doi: 10.1016/j.pharmthera.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Hou J, Wang D, Zhang R, Wang H. Experimental therapy of hepatoma with artemisinin and its derivatives: In vitro and in vivo activity, chemosensitization and mechanisms of action. Clin. Cancer. Res. 2008;14:5519–5530. doi: 10.1158/1078-0432.CCR-08-0197. [DOI] [PubMed] [Google Scholar]
- 31.Nakase I, Gallis B, Takatani-Nakase T, Oh S, Lacoste E, Singh NP, Goodlett DR, Tanaka S, Futaki S, Lai H, Sasaki T. Transferrin receptor-dependent cytotoxicity of artemisinin–transferrin conjugates on prostate cancer cells and induction of apoptosis. Cancer Lett. 2009;274:290–298. doi: 10.1016/j.canlet.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, Gerhard GS. Heme mediates cytotoxicity from artemisinin and serves as a general anti-proliferation target. PLoS ONE. 2009;4:e7472. doi: 10.1371/journal.pone.0007472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soomro S, Langenberg T, Mahringer A, Konkimalla VB, Horwedel C, Holenya P, Brand A, Cetin C, Fricker G, Dewerchin M, Carmeliet P, Conway EM, Jansen H, Efferth T. Design of novel artemisinin-like derivatives with cytotoxic and anti-angiogenic properties. J. Cell. Mol. Med. 2011;15:1122–1135. doi: 10.1111/j.1582-4934.2010.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Disbrow GL, Baege AC, Kierpiec KA, Yuan H, Centeno JA, Thibodeaux CA, Hartmann D, Schlegel R. Dihydroartemisinin is cytotoxic to papillomavirus-expressing epithelial cells in vitro and in vivo. Cancer Res. 2005;65:10854–10861. doi: 10.1158/0008-5472.CAN-05-1216. [DOI] [PubMed] [Google Scholar]
- 35.Lai H, Singh NP. Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer Lett. 1995;91:41–46. doi: 10.1016/0304-3835(94)03716-v. [DOI] [PubMed] [Google Scholar]
- 36.Singh NP, Lai H. Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci. 2001;70:49–56. doi: 10.1016/s0024-3205(01)01372-8. [DOI] [PubMed] [Google Scholar]
- 37.Moore JC, Lai H, Li J-R, Ren R-L, McDougall JA, Singh NP, Chou C-K. Oral administration of dihydroartemisinin and ferrous sulfate retarded implanted fibrosarcoma growth in the rat. Cancer Lett. 1995;98:83–87. [PubMed] [Google Scholar]
- 38.Farsam V, Hassan ZM, Zavaran-Hosseini A, Noori S, Mahdavi M, Ranjbar M. Antitumor and immunomodulatory properties of artemether and its ability to reduce CD4+ CD25+ FoxP3+ T reg cells in vivo. Int. Immunopharmacol. 2011;11:1802–1808. doi: 10.1016/j.intimp.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Singh NP, Panwar VK. Case report of a pituitary macroadenoma treated with artemether Integr. Cancer Ther. 2006;5:391–394. doi: 10.1177/1534735406295311. [DOI] [PubMed] [Google Scholar]
- 40.Hooft van Huijsduijnen R, Guy RK, Chibale K, Haynes RK, Peitz I, Kelter G, Phillips MA, Vennerstrom JL, Yuthavong Y, Wells TNC. Anticancer properties of distinct antimalarial drug classes. PLoS One. 2013;8:e82962. doi: 10.1371/journal.pone.0082962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L, Zuo LF, Guo JW. Reversal of multidrug resistance by the anti-malaria drug artesunate in the esophageal cancer Eca109/ABCG2 cell line. Oncology Lett. 2013;6:1475–1481. doi: 10.3892/ol.2013.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michaelis M, Kleinschmidt MC, Barth S, Rothweiler F, Geiler J, Breitling R, Mayer B, Deubzer H, Witt O, Kreuter J, Doerr HW, Cinatl J, Cinatl J. Anti-cancer effects of artesunate in a panel of chemoresistant neuroblastoma cell lines. Biochem. Pharmacol. 2010;79:130–136. doi: 10.1016/j.bcp.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen HT, Tran TH, Kim JO, Yong CS, Nguyen CN. Enhancing the in vitro anti-cancer efficacy of artesunate by loading into poly-D,L-lactide-co-glycolide (PLGA) nanoparticles. Arch. Pharm. Res. 2015;38:716–724. doi: 10.1007/s12272-014-0424-3. [DOI] [PubMed] [Google Scholar]
- 44.Hamacher-Brady A, Stein HA, Turschner S, Toegel I, Mora R, Jennewein N, Efferth T, Eils R, Brady NR. Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J. Biol. Chem. 2011;286:6587–6601. doi: 10.1074/jbc.M110.210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li PCH, Lam E, Roos WP, Zdzienicka MZ, Kaina B, Efferth T. Artesunate derived from traditional Chinese medicine induces DNA damage and repair. Cancer Res. 2008;68:4347–4351. doi: 10.1158/0008-5472.CAN-07-2970. [DOI] [PubMed] [Google Scholar]
- 46.Berdelle N, Nikolova T, Quiros S, Efferth T, Kaina B. Artesunate induces oxidative DNA damage, sustained DNA double-strand breaks, and the ATM/ATR damage response in cancer cells. Mol. Cancer Ther. 2011;10:2224–2233. doi: 10.1158/1535-7163.MCT-11-0534. [DOI] [PubMed] [Google Scholar]
- 47.Rutteman GR, Erich SA, Mol JA, Spee B, Grinwis GCM, Fleckenstein L, London CA, Efferth T. Safety and efficacy field study of artesunate for dogs with non-resectable tumours. Anticancer Res. 2013;33:1819–1827. [PubMed] [Google Scholar]
- 48.Jansen FH, Aboubi I, Kouassi Comoe JC, De Cnodder T, Jansen N, Tschulakow A, Efferth T. First study of oral artenimol-R in advanced cervical cancer: clinical benefit, tolerability and tumor markers. Anticancer Res. 2011;31:4417–4422. [PubMed] [Google Scholar]
- 49.Liu Y, Lok C-N, Ko BC-B, Shum TY-T, Wong M-K, Che C-M. Subcellular localization of a fluorescent artemisinin derivative to endoplasmic reticulum. Org. Lett. 2010;12:1420–1423. doi: 10.1021/ol902890j. [DOI] [PubMed] [Google Scholar]
- 50.Woerdenbag HJ, Moskal TA, Pras N, Malingré TM, El-Feraly FS, Kampinga HH, Konings AWT. Cytotoxicity of artemisinin-related endoperoxides to Ehrlich ascites tumor cells. J. Nat. Prod. 1993;56:849–856. doi: 10.1021/np50096a007. [DOI] [PubMed] [Google Scholar]
- 51.Beekman AC, Barentsen ARW, Woerdenbag HJ, Van Uden W, Pras N, Konings AWT, El-Feraly FS, Galal AM, Wikström HV. Stereochemistry-dependent cytotoxicity of some artemisinin derivatives. J. Nat. Prod. 1997;60:325–330. doi: 10.1021/np9605495. [DOI] [PubMed] [Google Scholar]
- 52.Ren Y, Jiménez F, García R, Mejía M, Chai H, Farnsworth NR, Soejarto DD, Kinghorn AD. A cytotoxic dimeric furanoheliangolide from Piptocoma rufescens. Tetrahedron Lett. 2013;54:5457–5460. doi: 10.1016/j.tetlet.2013.07.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Posner GH, Ploypradith P, Hapangama W, Wang D, Cumming JN, Dolan P, Kensler TW, Klinedinst D, Shapiro TA, Zheng QY, Murray CK, Pilkington LG, Jayasinghe LR, Bray JF, Daughenbaugh R. Trioxane dimers have potent antimalarial, antiproliferative and antitumor activities in vitro. Bioorg. Med. Chem. 1997;5:1257–1265. doi: 10.1016/s0968-0896(97)00079-5. [DOI] [PubMed] [Google Scholar]
- 54.Posner GH, McRiner AJ, Paik I-H, Sur S, Borstnik K, Xie S, Shapiro TA, Alagbala A, Foster B. Anticancer and antimalarial efficacy and safety of artemisinin-derived trioxane dimers in rodents. J. Med. Chem. 2004;47:1299–1301. doi: 10.1021/jm0303711. [DOI] [PubMed] [Google Scholar]
- 55.Alagbala AA, McRiner AJ, Borstnik K, Labonte T, Chang W, D'Angelo JG, Posner GH, Foster BA. Biological mechanisms of action of novel C-10 non-acetal trioxane dimers in prostate cancer cell lines. J. Med. Chem. 2006;49:7836–7842. doi: 10.1021/jm060803i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenthal AS, Chen X, Liu JO, West DC, Hergenrother PJ, Shapiro TA, Posner GH. Malaria-infected mice are cured by a single oral dose of new dimeric trioxane sulfones which are also selectively and powerfully cytotoxic to cancer cells. J. Med. Chem. 2009;52:1198–1203. doi: 10.1021/jm801484v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He R, Mott BT, Rosenthal AS, Genna DT, Posner GH, Arav-Boger R. An artemisinin-derived dimer has highly potent anti-cytomegalovirus (CMV) and anti-cancer activities. PLoS ONE. 2011;6:e24334. doi: 10.1371/journal.pone.0024334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh NP, Lai HC, Park JS, Gerhardt TE, Kim BJ, Wang S, Sasaki T. Effects of artemisinin dimers on rat breast cancer cells in vitro and in vivo. Anticancer Res. 2011;31:4111–4114. [PubMed] [Google Scholar]
- 59.Mott BT, He R, Chen X, Fox JM, Civin CI, Arav-Boger R, Posner GH. Artemisinin-derived dimer phosphate esters as potent anti-cytomegalovirus (anti-CMV) and anti-cancer agents: A structure-activity study. Bioorg. Med. Chem. 2013;21:3702–3707. doi: 10.1016/j.bmc.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galal AM, Gul W, Slade D, Ross SA, Feng S, Hollingshead MG, Alley MC, Kaur G, ElSohly MA. Synthesis and evaluation of dihydroartemisinin and dihydroartemisitene acetal dimers showing anticancer and antiprotozoal activity. Bioorg. Med. Chem. 2009;17:741–751. doi: 10.1016/j.bmc.2008.11.050. [DOI] [PubMed] [Google Scholar]
- 61.Xie L, Zhao Y, Zhai X, Li P, Liu C, Li Y, Gong P. The application of tandem aza-Wittig reaction to synthesize artemisinin-guanidine hybrids and their anti-tumor activity. Arch. Pharm. Chem. Life Sci. 2011;344:631–638. doi: 10.1002/ardp.201000363. [DOI] [PubMed] [Google Scholar]
- 62.Reiter C, Herrmann A, Capci A, Efferth T, Tsogoeva SB. New artesunic acid homodimers: Potent reversal agents of multidrug resistance in leukemia cells. Bioorg. Med. Chem. 2012;20:5637–5641. doi: 10.1016/j.bmc.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 63.Herout V, Souček M, Šorm F. Parthenolide, another sesquiterpenic lactone with a ten-membered ring. Chem. Ind. 1959:1069–1070. [Google Scholar]
- 64.Souček M, Herout V, Šorm F. On terpenes. CXVIII. Constitution of parthenolide. Coll. Czech. Chem. Commun. 1961;26:803–810. [Google Scholar]
- 65.Govindachari TR, Joshi BS, Kamat VN. Structure of parthenolide. Tetrahedron. 1965;21:1509–1519. [Google Scholar]
- 66.Bawdekar AS, Kelkar GR, Bhattacharyya SC. Terpenoids. LXXXIX. Absolute configuration of parthenolide. Tetrahedron Lett. 1966:1225–1227. [Google Scholar]
- 67.Quick A, Rogers D. Crystal and molecular structure of parthenolide [4,5-epoxygermacra-1 (10), 11(13)-dien-12,6-olactone] J. Chem. Soc., Perkin Trans. II. 1976:465–469. [Google Scholar]
- 68.Long J, Zhang S-F, Wang P-P, Zhang X-M, Yang Z-J, Zhang Q, Chen Y. Total syntheses of parthenolide and its analogues with macrocyclic stereocontrol. J. Med. Chem. 2014;57:7098–7112. doi: 10.1021/jm5009456. [DOI] [PubMed] [Google Scholar]
- 69.Ghantous A, Sinjab A, Herceg Z, Darwiche N. Parthenolide: from plant shoots to cancer roots. Drug Discov. Today. 2013;18:894–905. doi: 10.1016/j.drudis.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 70.Zunino SJ, Ducore JM, Storms DH. Parthenolide induces significant apoptosis and production of reactive oxygen species in high-risk pre-B leukemia cells. Cancer Lett. 2007;254:119–127. doi: 10.1016/j.canlet.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 71.Fonrose X, Ausseil F, Soleilhac E, Masson V, David B, Pouny I, Cintrat J-C, Rousseau B, Barette C, Massiot G, Lafanechère L. Parthenolide inhibits tubulin carboxypeptidase activity. Cancer Res. 2007;67:3371–3378. doi: 10.1158/0008-5472.CAN-06-3732. [DOI] [PubMed] [Google Scholar]
- 72.Kishida Y, Yoshikawa H, Myoui A. Parthenolide, a natural inhibitor of NF-κB, inhibits lung colonization of murine osteosarcoma cells. Clin. Cancer Res. 2007;13:59–67. doi: 10.1158/1078-0432.CCR-06-1559. [DOI] [PubMed] [Google Scholar]
- 73.Liu Z, Liu S, Xie Z, Pavlovicz RE, Wu J, Chen P, Aimiuwu J, Pang J, Bhasin D, Neviani P, Fuchs JR, Plass C, Li P-K, Li C, Huang TH-M, Wu L-C, Rush L, Wang H, Perrotti D, Marcucci G, Chan KK. Modulation of DNA methylation by a sesquiterpene lactone parthenolide. J. Pharmacol. Exp. Ther. 2009;329:505–514. doi: 10.1124/jpet.108.147934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oka D, Nishimura K, Shiba M, Nakai Y, Arai Y, Nakayama M, Takayama H, Inoue H, Okuyama A, Nonomura N. Sesquiterpene lactone parthenolide suppresses tumor growth in a xenograft model of renal cell carcinoma by inhibiting the activation of NF-κB. Int. J. Cancer. 2007;120:2576–2581. doi: 10.1002/ijc.22570. [DOI] [PubMed] [Google Scholar]
- 75.Yun B-R, Lee M-J, Kim J-H, Kim I-H, Yu G-R, Kim D-G. Enhancement of parthenolide-induced apoptosis by a PKC-alpha inhibition through heme oxygenase-1 blockage in cholangiocarcinoma cells. Exp. Mol. Med. 2010;42:787–797. doi: 10.3858/emm.2010.42.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang D, Qiu L, Jin X, Guo Z, Guo C. Nuclear factor-κB inhibition by parthenolide potentiates the efficacy of taxol in non-small cell lung cancer in vitro and in vivo. Mol. Cancer Res. 2009;7:1139–1149. doi: 10.1158/1541-7786.MCR-08-0410. [DOI] [PubMed] [Google Scholar]
- 77.Sweeney CJ, Mehrotra S, Sadaria MR, Kumar S, Shortle NH, Roman Y, Sheridan C, Campbell RA, Murry DJ, Badve S, Nakshatri H. The sesquiterpene lactone parthenolide in combination with docetaxel reduces metastasis and improves survival in a xenograft model of breast cancer. Mol. Cancer Ther. 2005;4:1004–1012. doi: 10.1158/1535-7163.MCT-05-0030. [DOI] [PubMed] [Google Scholar]
- 78.Mathema VB, Koh Y-S, Thakuri BC, Sillanpää M. Parthenolide, a sesquiterpene lactone, expresses multiple anti-cancer and anti-inflammatory activities. Inflammation. 2012;35:560–565. doi: 10.1007/s10753-011-9346-0. [DOI] [PubMed] [Google Scholar]
- 79.Curry EA, III, Murry DJ, Yoder C, Fife K, Armstrong V, Nakshatri H, O'Connell M, Sweeney CJ. Phase I dose escalation trial of feverfew with standardized doses of parthenolide in patients with cancer. Invest. New Drugs. 2004;22:299–305. doi: 10.1023/B:DRUG.0000026256.38560.be. [DOI] [PubMed] [Google Scholar]
- 80.Neelakantan S, Nasim S, Guzman ML, Jordan CT, Crooks PA. Aminoparthenolides as novel anti-leukemic agents: discovery of the NF-κB inhibitor, DMAPT (LC-1) Bioorg. Med. Chem. Lett. 2009;19:4346–4349. doi: 10.1016/j.bmcl.2009.05.092. [DOI] [PubMed] [Google Scholar]
- 81.Shanmugam R, Kusumanchi P, Cheng L, Crooks P, Neelakantan S, Matthews W, Nakshatri H, Sweeney CJ. A water-soluble parthenolide analogue suppresses in vivo prostate cancer growth by targeting NFκB and generating reactive oxygen species. Prostate. 2010;70:1074–1086. doi: 10.1002/pros.21141. [DOI] [PubMed] [Google Scholar]
- 82.Estabrook NC, Chin-Sinex H, Borgmann AJ, Dhaemers RM, Shapiro RH, Gilley D, Huda N, Crooks P, Sweeney C, Mendonca MS. Inhibition of NF-κB and DNA double-strand break repair by DMAPT sensitizes non-small-cell lung cancers to X-rays. Free. Rad. Biol. Med. 2011;51:2249–2258. doi: 10.1016/j.freeradbiomed.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 83.Shanmugam R, Kusumanchi P, Appaiah H, Cheng L, Crooks P, Neelakantan S, Peat T, Klaunig J, Matthews W, Nakshatri H, Sweeney CJ. A water soluble parthenolide analog suppresses in vivo tumor growth of two tobacco-associated cancers, lung and bladder cancer, by targeting NF-κB and generating reactive oxygen species. Int. J. Cancer. 2011;128:2481–2494. doi: 10.1002/ijc.25587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yip-Schneider MT, Wu H, Hruban RH, Lowy AM, Crooks PA, Schmidt CM. Efficacy of dimethylaminoparthenolide and sulindac in combination with gemcitabine in a genetically engineered mouse model of pancreatic cancer. Pancreas. 2013;42:160–167. doi: 10.1097/MPA.0b013e318254f455. [DOI] [PubMed] [Google Scholar]
- 85.Holcomb BK, Yip-Schneider MT, Waters JA, Beane JD, Crooks PA, Schmidt CM. Dimethylamino parthenolide enhances the inhibitory effects of gemcitabine in human pancreatic cancer cells. J. Gastrointest Surg. 2012;16:1333–1340. doi: 10.1007/s11605-012-1913-7. [DOI] [PubMed] [Google Scholar]
- 86.Guzman ML, Rossi RM, Neelakantan S, Li X, Corbett CA, Hassane DC, Becker MW, Bennett JM, Sullivan E, Lachowicz JL, Vaughan A, Sweeney CJ, Matthews W, Carroll M, Liesveld JL, Crooks PA, Jordan CT. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110:4427–4435. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choi J-H, Lee K-T. Costunolide-induced apoptosis in human leukemia cells: Involvement of c-Jun N-terminal kinase activation. Biol. Pharm. Bull. 2009;32:1803–1808. doi: 10.1248/bpb.32.1803. [DOI] [PubMed] [Google Scholar]
- 88.Park HW, Lee JH, Choi S-U, Baek N-I, Kim S-H, Yang JH, Kim DK. Cytotoxic germacranolide sesquiterpenes from the bark of Magnolia kobus. Arch. Pharm. Res. 2010;33:71–74. doi: 10.1007/s12272-010-2227-5. [DOI] [PubMed] [Google Scholar]
- 89.Hsu J-L, Pan S-L, Ho Y-F, Hwang T-L, Kung F-L, Guh J-H. Costunolide induces apoptosis through nuclear calcium2+ overload and DNA damage response in human prostate cancer. J. Urol. 2011;185:1967–1974. doi: 10.1016/j.juro.2010.12.091. [DOI] [PubMed] [Google Scholar]
- 90.Yang Y-I, Kim J-H, Lee K-T, Choi J-H. Costunolide induces apoptosis in platinum-resistant human ovarian cancer cells by generating reactive oxygen species. Gynecol. Oncol. 2011;123:588–596. doi: 10.1016/j.ygyno.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 91.Rasul A, Yu B, Yang L-F, Arshad M, Khan M, Ma T, Yang H. Costunolide, a sesquiterpene lactone induces G2/M phase arrest and mitochondria-mediated apoptosis in human gastric adenocarcinoma SGC-7901 cells. J. Med. Plants Res. 2012;6:1191–1200. [Google Scholar]
- 92.Choi YK, Seo HS, Choi HS, Choi HS, Kim SR, Shin YC, Ko S-G. Induction of Fas-mediated extrinsic apoptosis, p21WAF1-related G2/M cell cycle arrest and ROS generation by costunolide in estrogen receptor-negative breast cancer cells, MDA-MB-231. Mol. Cell. Biochem. 2012;363:119–128. doi: 10.1007/s11010-011-1164-z. [DOI] [PubMed] [Google Scholar]
- 93.Kurokawa T, Nakanishi K, Wu W, Hsu HY, Maruyama M, Kupchan SM. Deoxyelephantopin and its interrelation with elephantopin. Tetrahedron Lett. 1970:2863–2866. [Google Scholar]
- 94.Zhang D, Haruna M, McPhail AT, Lee K-H. Cytotoxic germacranolides of Elephantopus carolinianus and the structure and stereochemistry of isodeoxyelephantopin. Phytochemistry. 1986;25:899–904. [Google Scholar]
- 95.But PP-H, Hon P-M, Cao H, Chan TWD, Wu B-M, Mak TCW, Che C-T. Sesquiterpene lactones from Elephantopus scaber. Phytochemistry. 1997;44:113–116. [Google Scholar]
- 96.Ichikawa H, Nair MS, Takada Y, Sheeja DBA, Kumar MAS, Oommen OV, Aggarwal BB. Isodeoxyelephantopin, a novel sesquiterpene lactone, potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis through suppression of nuclear factor-κB (NF-κB) activation and NF-κB-regulated gene expression. Clin. Cancer Res. 2006;12:5910–5918. doi: 10.1158/1078-0432.CCR-06-0916. [DOI] [PubMed] [Google Scholar]
- 97.Zou G, Gao Z, Wang J, Zhang Y, Ding H, Huang J, Chen L, Guo Y, Jiang H, Shen X. Deoxyelephantopin inhibits cancer cell proliferation and functions as a selective partial agonist against PPARγ. Biochem. Pharmacol. 2008;75:1381–1392. doi: 10.1016/j.bcp.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 98.Huang C-C, Lo C-P, Chiu C-Y, Shyur L-F. Deoxyelephantopin, a novel multifunctional agent, suppresses mammary tumour growth and lung metastasis and doubles survival time in mice. Br. J. Pharmacol. 2010;159:856–871. doi: 10.1111/j.1476-5381.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee W-L, Wen T-N, Shiau J-Y, Shyur L-F. Differential proteomic profiling identifies novel molecular targets of paclitaxel and phytoagent deoxyelephantopin against mammary adenocarcinoma cells. J. Proteome Res. 2010;9:237–253. doi: 10.1021/pr900543e. [DOI] [PubMed] [Google Scholar]
- 100.Su M, Chung HY, Li Y. Deoxyelephantopin from Elephantopus scaber L. induces cell-cycle arrest and apoptosis in the human nasopharyngeal cancer CNE cells. Biochem. Biophys. Res. Commun. 2011;411:342–347. doi: 10.1016/j.bbrc.2011.06.144. [DOI] [PubMed] [Google Scholar]
- 101.Xu G, Liang Q, Gong Z, Yu W, He S, Xi L. Antitumor activities of the four sesquiterpene lactones from Elephantopus scaber L. Exp. Oncol. 2006;28:106–109. [PubMed] [Google Scholar]
- 102.Ren Y, Muñoz Acuña U, Jiménez F, García R, Mejía M, Chai H, Gallucci JC, Farnsworth NR, Soejarto DD, Carcache de Blanco EJ, Kinghorn AD. Cytotoxic and NF-κB inhibitory sesquiterpene lactones from Piptocoma rufescens. Tetrahedron. 2012;68:2671–2678. doi: 10.1016/j.tet.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spear SA, Burns SS, Oblinger JL, Ren Y, Pan L, Kinghorn AD, Welling DB, Chang L-S. Natural compounds as potential treatments of NF2-deficient schwannoma and meningioma: cucurbitacin D and goyazensolide. Otol. Neurotol. 2013;34:1519–1527. doi: 10.1097/MAO.0b013e3182956169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Muñoz Acuña U, Shen Q, Ren Y, Lantvit DD, Wittwer JA, Kinghorn AD, Swanson SM, Carcache de Blanco EJ. Goyazensolide induces apoptosis in cancer cells in vitro and in vivo. Int. J. Cancer Res. 2013;9:36–53. doi: 10.3923/ijcr.2013.36.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vichnewski W, Sarti SJ, Gilbert B, Herz W. Goyazensolide, a schistosomicidal heliangolide from Eremanthus goyazensis. Phytochemistry. 1976;15:191–193. [Google Scholar]
- 106.Herz W, Goedken VL. Structure of goyazensolide and its congeners. J. Org. Chem. 1982;47:2798–2800. [Google Scholar]
- 107.Kupchan SM, Eakin MA, Thomas AM. Tumor inhibitors. 69. Structure-cytotoxicity relations among the sesquiterpene lactones. J. Med. Chem. 1971;14:1147–1152. doi: 10.1021/jm00294a001. [DOI] [PubMed] [Google Scholar]
- 108.Scotti MT, Fernandes MB, Ferreira MJP, Emerenciano VP. Quantitative structure-activity relationship of sesquiterpene lactones with cytotoxic activity. Bioorg. Med. Chem. 2007;15:2927–2934. doi: 10.1016/j.bmc.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 109.Wagner S, Hofmann A, Siedle B, Terfloth L, Merfort I, Gasteiger J. Development of a structural model for NF-κB inhibition of sesquiterpene lactones using self-organizing neural networks. J. Med. Chem. 2006;49:2241–2252. doi: 10.1021/jm051125n. [DOI] [PubMed] [Google Scholar]
- 110.Doan NTQ, Paulsen ES, Sehgal P, Møller JV, Nissen P, Denmeade SR, Isaacs JT, Dionne CA, Christensen SB. Targeting thapsigargin towards tumors. Steroids. 2015;97:2–7. doi: 10.1016/j.steroids.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Makunga NP, Jäger AK, van Staden J. Micropropagation of Thapsia garganica–a medicinal plant. Plant Cell Rep. 2003;21:967–973. doi: 10.1007/s00299-003-0623-8. [DOI] [PubMed] [Google Scholar]
- 112.Christensen SB, Rasmussen U, Christophersen C. Thapsigargin, constitution of a sesquiterpene lactone histamine liberator from Thapsia garganica. Tetrahedron Lett. 1980;21:3829–3830. [Google Scholar]
- 113.Christensen SB, Larsen IK, Rasmussen U, Christophersen C. Thapsigargin and thapsigargicin, two histamine liberating sesquiterpene lactones from Thapsia garganica. X-ray analysis of the 7,11-epoxide of thapsigargin. J. Org. Chem. 1982;47:649–652. [Google Scholar]
- 114.Christensen SB, Norup E. Absolute configurations of the histamine liberating sesquiterpene lactones thapsigargin and trilobolide. Tetrahedron Lett. 1985;26:107–110. [Google Scholar]
- 115.Ley SV, Antonello A, Balskus EP, Booth DT, Christensen SB, Cleator E, Gold H, Höegenauer K, Hüenger U, Myers RM, Oliver SF, Simic O, Smith MD, Søhoel H, Woolford AJA. Synthesis of the thapsigargins. Proc. Natl. Acad. Sci. 2004;101:12073–12078. doi: 10.1073/pnas.0403300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ball M, Andrews SP, Wierschem F, Cleator E, Smith MD, Ley SV. Total Synthesis of thapsigargin, a potent SERCA pump inhibitor. Org. Lett. 2007;9:663–666. doi: 10.1021/ol062947x. [DOI] [PubMed] [Google Scholar]
- 117.Inesi G, Sagara Y. Thapsigargin, a high affinity and global inhibitor of intracellular Ca2+ transport ATPases. Arch. Biochem. Biophys. 1992;298:313–317. doi: 10.1016/0003-9861(92)90416-t. [DOI] [PubMed] [Google Scholar]
- 118.Liu H, Jensen KG, Tran LM, Chen M, Zhai L, Olsen CE, Søhoel H, Denmeade SR, Isaacs JT, Christensen SB. Cytotoxic phenylpropanoids and an additional thapsigargin analogue isolated from Thapsia garganica. Phytochemistry. 2006;67:2651–2658. doi: 10.1016/j.phytochem.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 119.Jin T, Nakatani H, Taguchi T, Sonobe H, Morimoto N, Sugimoto T, Akimori T, Nakano T, Namikawa T, Okabayashi T, Kobayashi M, Araki K. Thapsigargin enhances cell death in the gastrointestinal stromal tumor cell line, GIST-T1, by treatment with imatinib (Glivec) J. Health Sci. 2006;52:110–117. [Google Scholar]
- 120.Min K-J, Kim HS, Park EJ, Kwon TK. Melatonin enhances thapsigargin-induced apoptosis through reactive oxygen species-mediated upregulation of CCAAT-enhancer-binding protein homologous protein in human renal cancer cells. J. Pineal Res. 2012;53:99–106. doi: 10.1111/j.1600-079X.2012.00975.x. [DOI] [PubMed] [Google Scholar]
- 121.Yamaguchi H, Bhalla K, Wang H-G. Bax plays a pivotal role in thapsigargin-induced apoptosis of human colon cancer HCT116 cells by controlling Smac/Diablo and Omi/HtrA2 release from mitochondria. Cancer Res. 2003;63:1483–1489. [PubMed] [Google Scholar]
- 122.Huang L, Xu J, Li K, Zheng MH, Kumta S-M. Thapsigargin potentiates TRAIL-induced apoptosis in giant cell tumor of bone. Bone. 2004;34:971–981. doi: 10.1016/j.bone.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 123.Wagner-Souza K, Echevarria-Lima J, Rodrigues LAP, Reis M, Rumjanek VM. Resistance to thapsigargin-induced intracellular calcium mobilization in a multidrug resistant tumour cell line. Mol. Cell. Biochem. 2003;252:109–116. doi: 10.1023/a:1025586225941. [DOI] [PubMed] [Google Scholar]
- 124.Christensen SB, Andersen A, Kromann H, Treiman M, Tombal B, Denmeade S, Isaacs JT. Thapsigargin analogs for targeting programmed death of androgen-independent prostate cancer cells. Bioorg. Med. Chem. 1999;7:1273–1280. doi: 10.1016/s0968-0896(99)00074-7. [DOI] [PubMed] [Google Scholar]
- 125.Søhoel H, Lund Jensen A-M, Møller JV, Nissen P, Denmeade SR, Isaacs JT, Olsen CE, Christensen SB. Natural products as starting materials for development of second-generation SERCA inhibitors targeted towards prostate cancer cells. Bioorg. Med. Chem. 2006;14:2810–2815. doi: 10.1016/j.bmc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 126.Jakobsen CM, Denmeade SR, Isaacs JT, Gady A, Olsen CE, Christensen SB. Design, synthesis, and pharmacological evaluation of thapsigargin analogues for targeting apoptosis to prostatic cancer cells. J. Med. Chem. 2001;44:4696–4703. doi: 10.1021/jm010985a. [DOI] [PubMed] [Google Scholar]
- 127.Denmeade SR, Jakobsen CM, Janssen S, Khan SR, Garrett ES, Lilja H, Christensen SB, Isaacs JT. Prostate-specific antigen-activated thapsigargin prodrug as targeted therapy for prostate cancer. J. Natl. Cancer Inst. 2003;95:990–1000. doi: 10.1093/jnci/95.13.990. [DOI] [PubMed] [Google Scholar]
- 128.Vander Griend DJ, Antony L, Dalrymple SL, Xu Y, Christensen SB, Denmeade SR, Isaacs JT. Amino acid containing thapsigargin analogues deplete androgen receptor protein via synthesis inhibition and induce the death of prostate cancer cells. Mol. Cancer Ther. 2009;8:1340–1349. doi: 10.1158/1535-7163.MCT-08-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Simonsen HT, Drew DP, Lunde C. Perspectives on using Physcomitrella patens as an alternative production platform for thapsigargin and other terpenoid drug candidates. Perspect. Med. Chem. 2009;3:1–6. doi: 10.4137/pmc.s2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zahara K, Tabassum S, Sabir S, Arshad M, Qureshi R, Amjad MS, Chaudhari SK. A review of therapeutic potential of Saussurea lappa–An endangered plant from Himalaya. Asian Pac. J. Trop. Med. 2014;7S1:S60–S69. doi: 10.1016/S1995-7645(14)60204-2. [DOI] [PubMed] [Google Scholar]
- 131.Hsu Y-L, Wu L-Y, Kuo P-L. Dehydrocostuslactone, a medicinal plant-derived sesquiterpene lactone, induces apoptosis coupled to endoplasmic reticulum stress in liver cancer cells. J. Pharmacol. Exp. Ther. 2009;329:808–819. doi: 10.1124/jpet.108.148395. [DOI] [PubMed] [Google Scholar]
- 132.Kim EJ, Lim SS, Park SY, Shin H-K, Kim J-S, Park JHY. Apoptosis of DU145 human prostate cancer cells induced by dehydrocostus lactone isolated from the root of Saussurea lappa. Food Chem. Toxicol. 2008;46:3651–3658. doi: 10.1016/j.fct.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 133.Fronczek FR, Vargas D, Parodi FJ, Fischer NH. Structure of the guaianolide dehydrocostus lactone. Acta Crystallogr. C. 1989;45:1829–1831. [Google Scholar]
- 134.Rigby JH, Wilson JZ. Total synthesis of guaianolides: (±)-dehydrocostus lactone and (±)-estafiatin. J. Am. Chem. Soc. 1984;106:8217–8224. [Google Scholar]
- 135.Choi EM. Dehydrocostus lactone prevents mitochondrial dysfunction in osteoblastic MC3T3-E1 cells. Eur. J. Pharmacol. 2011;664:1–7. doi: 10.1016/j.ejphar.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 136.Oh GS, Pae HO, Chung HT, Kwon JW, Lee JH, Kwon TO, Kwon SY, Chon BH, Yun YG. Dehydrocostus lactone enhances tumor necrosis factor-α-induced apoptosis of human leukemia HL-60 cells. Immunopharmacol. Immunotoxicol. 2004;26:163–175. doi: 10.1081/iph-120037712. [DOI] [PubMed] [Google Scholar]
- 137.Kuo P-L, Ni W-C, Tsai E-M, Hsu Y-L. Dehydrocostuslactone disrupts signal transducers and activators of transcription 3 through up-regulation of suppressor of cytokine signaling in breast cancer cells. Mol. Cancer Ther. 2009;8:1328–1339. doi: 10.1158/1535-7163.MCT-08-0914. [DOI] [PubMed] [Google Scholar]
- 138.Wang C-Y, Tsai A-C, Peng C-Y, Chang Y-L, Lee K-H, Teng C-M, Pan S-L. Dehydrocostuslactone suppresses angiogenesis in vitro and in vivo through inhibition of Akt/GSK-3β and mTOR signaling pathways. PLoS One. 2012;7:e31195. doi: 10.1371/journal.pone.0031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Herz W, Watanabe H, Miyazaki M, Kishida Y. Structures of parthenin and ambrosin. J. Am. Chem. Soc. 1962;84:2601–2610. [Google Scholar]
- 140.Gupta VK, Goswami KN, Bhutani KK. X-ray crystal structure analysis of parthenin–a sesquiterpene lactone. Cryst. Res. Technol. 1994;29:373–378. [Google Scholar]
- 141.Heathcock CH, Tice CM, Germroth TC. Synthesis of sesquiterpene antitumor lactones. 10. Total synthesis of (±)-parthenin. J. Am. Chem. Soc. 1982;104:6081–6091. [Google Scholar]
- 142.Shimoma F, Kusaka H, Azami H, Wada K, Suzuki T, Hagiwara H, Ando M. Total syntheses of (±)-hymenolin and (±)-parthenin. J. Org. Chem. 1998;63:3758–3763. [Google Scholar]
- 143.Reddy DM, Qazi NA, Sawant SD, Bandey AH, Srinivas J, Shankar M, Singh SK, Verma M, Chashoo G, Saxena A, Mondhe D, Saxena AK, Sethi VK, Taneja SC, Qazi GN, Kumar HMS. Design and synthesis of spiro derivatives of parthenin as novel anti-cancer agents. Eur. J. Med. Chem. 2011;46:3210–3217. doi: 10.1016/j.ejmech.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 144.Shah BA, Taneja SC, Sethi VK, Gupta P, Andotra SS, Chimni SS, Qazi GN. The formation of novel 1,3-dioxolanes: atypical Baylis-Hillman reaction of a sesquiterpene lactone parthenin. Tetrahedron Lett. 2007;48:955–960. [Google Scholar]
- 145.Shah BA, Kaur R, Gupta P, Kumar A, Sethi VK, Andotra SS, Singh J, Saxena AK, Taneja SC. Structure-activity relationship (SAR) of parthenin analogues with pro-apoptotic activity: Development of novel anti-cancer leads. Bioorg. Med. Chem. Lett. 2009;19:4394–4398. doi: 10.1016/j.bmcl.2009.05.089. [DOI] [PubMed] [Google Scholar]
- 146.Kumar A, Malik F, Bhushan S, Shah BA, Taneja SC, Pal HC, Wani ZA, Mondhe DM, Kaur J, Singh J. A novel parthenin analog exhibits anti-cancer activity: Activation of apoptotic signaling events through robust NO formation in human leukemia HL-60 cells. Chem. Biol. Interact. 2011;193:204–215. doi: 10.1016/j.cbi.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 147.Emerson MT, Caughlan CN, Herz W. The crystal and molecular structure of bromohelenalin. Tetrahedron Lett. 1964;12:621–625. [Google Scholar]
- 148.Chapman DE, Holbrook DJ, Chaney SG, Hall IH, Lee K-H. In vivo and in vitro effects of helenalin on mouse hepatic microsomal cytochrome P450. Biochem. Pharmacol. 1991;41:229–235. doi: 10.1016/0006-2952(91)90481-j. [DOI] [PubMed] [Google Scholar]
- 149.Kim SH, Oh SM, Kim TS. Induction of human leukemia HL-60 cell differentiation via a PKC/ERK pathway by helenalin, a pseudoguaianolide sesquiterpene lactone. Eur. J. Phamacol. 2005;511:89–97. doi: 10.1016/j.ejphar.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 150.Hoffmann R, von Schwarzenberg K, López-Anón N, Rudy A, Wanner G, Dirsch VM, Vollmar AM. Helenalin bypasses Bcl-2-mediated cell death resistance by inhibiting NF-κB and promoting reactive oxygen species generation. Biochem. Pharmacol. 2011;82:453–463. doi: 10.1016/j.bcp.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 151.Lee K-H, Ibuka T, Sims D, Muraoka O, Kiyokawa H, Hall IH, Kim HL. Antitumor agents. 44. Bis(helenalinyl) esters and related derivatives as novel potent antileukemic agents. J. Med. Chem. 1981;24:924–927. doi: 10.1021/jm00140a003. [DOI] [PubMed] [Google Scholar]
- 152.Chen C-H, Yang L-M, Lee TT-Y, Shen Y-C, Zhang D-C, Pan D-J, McPhail AT, McPhail DR, Liu S-Y, Li D-H, Cheng Y-C, Lee K-H. Antitumor agents–CLI. Bis(helenalinyl)glutarate and bis(isoalantodiol-B)glutarate, potent inhibitors of human DNA topoisomerase II. Bioorg. Med. Chem. 1994;2:137–145. doi: 10.1016/s0968-0896(00)82008-8. [DOI] [PubMed] [Google Scholar]
- 153.Nakanishi C, Toi M. Nuclear factor-κB inhibitors as sensitizers to anticancer drugs. Nat. Rev. Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]