Abstract
Background
Chronic rhinosinusitis (CRS) is a heterogeneous inflammatory condition of the sinonasal mucosa consisting of poorly defined subtypes and characterized by variable clinical manifestations, responses to therapy, and underlying pathophysiologies. In the related disorder of asthma, progress has been made in defining disease subtypes on both clinical and pathophysiologic levels, facilitating the development of targeted biologic pharmacotherapy. The potential role of these drugs for the management of CRS will be reviewed.
Objective
To highlight the emerging therapeutic targets in CRS in light of evolving treatment options for asthma and enhanced understandings of the clinical manifestations and pathophysiology of CRS.
Methods
The article is a review of recent studies regarding current and future advances in biomarker-directed therapies in the medical treatment of CRS.
Results
Various biologic therapies used in the management of asthma have demonstrated clinical promise for CRS, particularly within the CRS with nasal polyposis (CRSwNP) phenotype. Several randomized, double-blind, placebo-controlled studies increasingly support the targeting of immunoglobulin E and interleukin (IL)-5 pathways to improve outcome measures in CRSwNP patients. The IL-4/IL-13 pathway and other type 2 inflammatory pathways have also shown potential as targets for CRSwNP, but all pathways require further investigation.
Conclusion
Recalcitrant CRS in the United States and Europe is most commonly associated with nasal polyposis and a type 2 cytokine skewing in the tissue, resulting in tissue infiltration of eosinophils, mast cells, and basophils. Targeting biomarkers of the associated type 2 pathways may be a practical treatment option for recalcitrant CRSwNP in the future.
Keywords: chronic rhinosinusitis, nasal polyps, asthma, biologic therapy, monoclonal antibodies, molecular biomarkers, eosinophils
Introduction
Chronic rhinosinusitis (CRS) is a heterogeneous group of inflammatory disorders that involve the mucosa of the nasal passageways and paranasal sinuses. Currently, a diverse armamentarium exists for the medical management of CRS. The therapeutic mainstays for CRS consist of intranasal corticosteroids, short-term oral corticosteroids, and nasal saline irrigations, which are supported by high-level evidence.(1, 2) Antibiotics in both oral and topical preparations, leukotriene receptor antagonists, and topical nasal decongestants are also commonly included in the medical regimen, but limited evidence is available to support their recommended uses.(2–5) The estimated success rate of medical therapy in controlling both subjective and objective outcome parameters, however, ranges from 38% to 51%.(3, 6) For CRS patients who are refractory to medical management, surgery serves as a viable therapeutic option, but medical therapy still plays an important adjunctive role following surgery.(7, 8)
The widespread use of nonspecific therapies for CRS, as predominantly prescribed in the United States today, generates substantial residual morbidity. For example, the overuse of antibiotic therapy for CRS treatment is likely associated with the development of resistant bacteria.(9) Furthermore, antibiotic use for CRS oftentimes neglects the fact that CRS is primarily an inflammatory disorder, as opposed to an infectious condition. Hence, there is a compelling need for new treatment strategies. Recently, biologic therapies have become increasingly effective and attractive options for asthmatic patients based on their ability to target key asthma inflammatory profiles.(10, 11) Due to similarities in the underlying role of inflammation in asthma and CRS, interest has emerged on the application of biologic therapies to provide potential treatment options for CRS.(12, 13) This article serves as an overview of the current and future developments of biologic therapy for CRS.
Advances in understandings of CRS phenotypes and endotypes
Current research highlights the diverse and multifactorial nature of CRS pathogenesis.(14–16) Specifically, a dysfunctional interplay between different host susceptibilities and environmental modifiers is speculated to instigate and perpetuate the inflammatory response underlying the clinical syndrome. Environmental factors include infectious pathogens, pollutants, and inhaled allergens, while host contributions involve both congenital and acquired variables, such as anatomical obstruction of the osteomeatal complex, impaired mucociliary clearance, a defective epithelial barrier function, and an aberrant host immune response.(17) Despite the variety of hypotheses proposed to explain CRS, none has singly proven valid for the whole CRS spectrum.
To highlight the different etiologic factors involved with CRS pathogenesis, recent research has emphasized characterizing CRS as a heterogeneous spectrum of disease variants defined by clinically observable features, responses to therapeutic interventions, and presumably distinct, but overlapping, pathophysiologic pathways.(18–20) The attempt to elucidate the heterogeneity of CRS in terms of clinical manifestations and pathogenesis is rooted in prior studies on asthma, a similar inflammatory disorder that consists of multiple disease variants.(10, 21) Today, asthma phenotypes refer to common variants recognized by clinically observable properties, including severity of disease, age of disease onset, and association with atopy. Disease endotypes, on the other hand, are defined by the specific biological pathways driving the observable phenotypic characteristics. Identification of asthma endotypes according to the distinct pathophysiologic mechanisms of inflammation has increasingly given a role for biologic pharmacotherapy through the targeting of biomolecules specific to the endotypic variants. Most importantly, certain asthmatic endotypes have correlated with response to certain medications such as inhaled corticosteroid.(22)
The comorbid association between CRS and asthma is clearly established in epidemiologic studies, highlighting similar immunologic patterns of inflammation occurring at the epithelial cell layers of the upper and lower airways.(23–25) The subset of patients with the most severe forms of CRS and consequently those most likely to lack success with the traditional treatment regimens are commonly patients with comorbid asthma.(1, 26) Given the parallels in pathophysiology between asthma and CRS, various immunobiologic factors that are suspected to drive the inflammatory processes of asthma, such as immunoglobulin (Ig) E and interleukin (IL)-5, have by translation become likely targets for biologic therapy in CRS. Despite the potential utility of these therapies in CRS, the successful application of such agents requires the proper identification of patients who demonstrate the appropriate endotypic profiles.
As in asthma, phenotypes in CRS are based upon clinically observable characteristics, while CRS endotyping is based on different molecular pathways important in disease pathogenesis. For CRS, the absence or presence of nasal polyposis on endoscopic examination serves as the most frequent method of distinguishing two basic phenotypes: CRS with nasal polyposis (CRSwNP) and CRS without nasal polyposis (CRSsNP).(1, 27, 28) Over recent years, this distinction has proven to be overly broad and simplistic, as multiple studies have found significant clinical variations in disease course, responsiveness to therapeutic interventions, and risk of recidivism even within the CRSwNP and CRSsNP subgroups.(29, 30) Increasing emphasis has therefore been placed on the endotypic stratification of CRSwNP and CRSsNP subgroups according to the distinctive pathophysiologic mechanisms, which most likely account for the variations in clinical manifestations and outcomes. Definitions for CRS phenotypes and endotypes, however, remain less developed than those for asthma, which by comparison are based on stronger and more extensive data.
One current attempt to endotype CRS has focused on the inflammatory milieu composed of different cytokine profiles, which are elevated in both CRSwNP and CRSsNP. The most common scheme to subclassify CRSwNP and CRSsNP has been based upon the balance of type 1 and type 2 inflammatory patterns. Type 1 inflammation is characterized by the presence of neutrophils; elevated type 1 cytokines, such as interferon (IFN)-γ; and T-helper 1 (Th1) cells. On the other hand, type 2 inflammation is characterized by the high presence of eosinophils, mast cells, and basophils; elevated type 2 cytokines, including IL-4, IL-5, and IL-13; and T-helper 2 (Th2) cells. CRSwNP has been associated with skewed type 2 inflammation, in which IL-4 plays an important unifying role in supporting the induction of IgE-mediated immune responses and the upregulation of basophils, mast cells, and eosinophils. CRSsNP, in contrast, has been characterized by a relative skewing away from type 2 inflammation and toward more type 1 inflammation, with Th1 and T-helper 17 (Th17) cytokine patterns.(29, 31) CRSsNP is also more closely associated with neutrophilic tissue infiltration, although eosinophils are also commonly present in CRSsNP, albeit at significantly lower levels than in CRSwNP.(1) The general use of inflammatory profiles to characterize patterns of immunologic expression in patients with CRSwNP and CRSsNP, however, is not absolute and requires continued investigation.(1, 30–34)
Present research on the endotypic variants of CRS highlight the potential applications of certain biologic therapy that target specific biomarkers driving the inflammatory response of CRS. Multiple agents targeting type 2 cytokine inflammatory byproducts have been developed and more thoroughly studied for disease control in eosinophilic and allergic asthma and are thus potential therapeutic candidates for use in CRS.(21, 35) For CRS, significant attention regarding biologic therapies is also generally directed to CRSwNP in Caucasian populations, in whom the inflammatory response is induced by Th2 cells, eosinophils, and associated type 2 pro-inflammatory cytokines. However, the clinical efficacy and utility of these agents in CRS remain in the early and experimental phases of study. Table 1 highlights the currently available randomized controlled studies of biologic therapies specifically for CRS.
Table 1.
Published Randomized Controlled TrialsEvaluating Biologic Therapies for Chronic Rhinosinusitis in Human Subjects
| Study | Target pathway |
Drug name | Drug type and mechanism of action |
Study population |
Efficacy of therapeutic intervention |
|---|---|---|---|---|---|
| Gevaert et al, 2013 (36) |
IgE | Omalizumab | Monoclonal antibody binding to IgE |
CRSwNP with comorbid asthma (n = 24) |
Reduced total nasal endoscopic polyp scores and Lund-MacKay scores on radiologic imaging; improved disease-specific quality-of-life questionnaire scores |
| Pinto et al, 2010 (37) |
IgE | Omalizumab | Monoclonal antibody binding to IgE |
CRS, nonspecific for CRSwNP or CRSsNP (n = 14) |
Did not show significant clinical impact |
| Gevaert et al, 2011 (43) |
IL-5 | Mepolizumab | Monoclonal antibody binding to IL-5 |
CRSwNP refractory to corticosteroid therapy (n = 30) |
Reduced endoscopic nasal polyp score and computed tomography scan scores |
| Gevaert et al, 2006 (42) |
IL-5 | Reslizumab | Monoclonal antibody binding to IL-5 |
CRSwNP (n = 24) |
Reduced polyp size and blood eosinophil levels and improved patient-reported symptom scores |
| Bachert et al, 2016 (57) |
IL-4/IL-13 | Dupilumab | Monoclonal antibody binding to IL- 4Rα |
CRSwNP refractory to intranasal corticosteroid therapy (n = 60) |
Reduced nasal polyp burden and Lund-Mackay scores; improved both olfactory and disease-specific quality-of-life questionnaire scores |
Targeting the IgE pathway
The most promising biologic therapy for CRS to date has been omalizumab, which is an approved treatment option in the United States and Europe for patients with severe allergic asthma. Omalizumab has also demonstrated positive results for CRSwNP patients in a recent randomized, double-blind, placebo-controlled study.(36) The mechanism of action of omalizumab, a recombinant humanized monoclonal antibody, involves its selective binding to free circulating IgE, which decreases the expression of IgE receptors on mast cells, basophils, and dendritic cells and thereby interferes with activation of these effector cells. In the study by Gevaert et al, the subcutaneous administration of four to eight doses of omalizumab was found to result in significant reduction of polyp size on endoscopic examination, Lund-MacKay scores on radiologic imaging, and a variety of disease-specific quality-of-life questionnaire scores when compared to placebo. Interestingly, the improvements in upper and lower airway diseases following use of anti-IgE therapy were seen independent of serum IgE levels. These findings support the involvement of local mucosal IgE production in the upper airways in the pathogenesis of nasal polyps and suggest that omalizumab may offer therapeutic improvements for both allergic and nonallergic variants of CRSwNP. Due to the small study population in the existing study, however, further studies with larger population sizes are needed.
The results from the study by Gevaert et al are nonetheless encouraging, given that an earlier randomized, double-blinded, placebo-controlled trial with omalizumab had previously demonstrated a lack of significant difference between anti-IgE therapy and placebo in patients with severe CRS refractory to standard treatments, including sinus surgery.(37) In the earlier trial by Pinto et al, the treatment group was given subcutaneous doses of omalizumab at 4-week intervals over a course of 6 months. Outcome measurements included Lund-MacKay scores from sinus imaging and various qualitative gauges on symptomatic and quality-of-life improvements. The study, however, was underpowered due to recruitment problems after warnings were raised about anaphylactic events following administration of omalizumab. Moreover, this study did not provide details about distinguishing between CRSwNP and CRSsNP in the recruitment of study subjects for both treatment and control groups.
While the available data may suggest that more widespread application of omalizumab for CRSwNP is an increasingly viable option, regardless of asthma status or systemic atopy, the cost and toxicity of omalizumab remain prohibitive factors to the biologic agent’s broad application for CRSwNP. Chronic maintenance therapy may or may not be necessary to control polyp growth. Although the side effects of anti-IgE therapy in the two prior randomized, double-blinded, placebo-controlled trials have generally been negligible, issues have been raised in regard to three main areas: malignancy, cardiovascular disease, and anaphylaxis. The incidence of anaphylaxis is approximately 0.2%, thus mandating administration in a health care setting.(38) Recent data evaluating the longer-term risk of malignancy, however, have indicated no difference in the rate of malignancy in omalizumab-treated versus placebo-treated patients.(39) The rationale for the use of omalizumab in severe asthma, which has an uncommon but not insignificant mortality, is more easily supported when compared to use in CRS.
Targeting the IL-5 pathway
As a target for biologic therapy in asthma, IL-5 is a type 2 cytokine that serves as an essential role in the terminal differentiation of bone marrow eosinophilic progenitors to develop into mature eosinophils.(40, 41) The prevalence of IL-5 as an inflammatory mediator in CRSwNP has allowed for the targeting of IL-5 in CRS pharmacotherapy with the use of reslizumab and mepolizumab, both anti-IL-5 monoclonal antibodies. Efficacy of reslizumab was first suggested in a randomized, double-blind, placebo-controlled trial, in which CRSwNP patients were treated with single doses of reslizumab or placebo.(42) At 12 weeks following administration of reslizumab, the majority of study participants who received the agent at the 1 mg/kg dosage demonstrated smaller polyp sizes, improved patient-reported symptom scores, and reduced blood eosinophil levels when compared with their baseline values. The study patients who responded to reslizumab were found to have elevated nasal IL-5 levels at baseline, compared to the nonresponders. Interestingly, blood eosinophil counts initially dropped significantly in patients who received reslizumab, but after discontinuation of reslizumab, showed an increase above baseline values, suggesting a rebound effect with resultant hypereosinophilia.
A more recent phase II study with anti-IL-5 antibodies for CRSwNP by the same group of investigators further indicated the potential utility of IL-5 inhibition as a therapeutic approach in patients with severe eosinophilic CRSwNP.(43) In this randomized, placebo-controlled trial, CRSwNP patients were randomized to receive either two injections of mepolizumab given 28 days apart, or placebo. While the nasal polyp score improved in the treatment group 8 weeks following the first injection, it remained unchanged in the placebo group. In addition, significantly less opacification on CT imaging was observed in the treatment group. The subgroup of patients who responded to anti-IL-5 therapy with polyp shrinkage also demonstrated a decrease in some indicators of type 2 inflammation in nasal secretions. Although the group’s initial pilot trial with reslizumab suggested that nasal IL-5 levels could predict responders, this follow-up study showed that the effects of mepolizumab was independent of nasal IL-5 levels. Therefore, despite the encouraging results, no clinical or laboratory parameter, other than perhaps nasal polyp eosinophilia has been identified as a predictor of therapeutic response, has been identified as a predictor of therapeutic response that can enhance the success rate.
Benralizumab has been another recent biologic agent that imparts its inhibitory effect on the IL-5 pathway and the resulting inflammatory cascade related to the development of asthma and possibly CRS. Instead of directly neutralizing free IL-5 levels in serum, benralizumab serves as a humanized afucosylated monoclonal antibody that targets the IL-5 receptor (IL-5R) located on eosinophils, basophils, and their corresponding progenitors. The subsequent binding of benralizumab to IL-5R on effector cells is suspected to result in a competitive inhibition of IL-5 and also induction of apoptosis of eosinophils and basophils.(44–46) Laviolette et al recently conducted a randomized, placebo-controlled phase I trial with benralizumab in patients with eosinophilic asthma.(44) This clinical study found that patients who received either a single intravenous dose of benralizumab or three monthly subcutaneous doses of benralizumab demonstrated reduced eosinophil counts in the airway mucosa, sputum, bone marrow, and peripheral blood, when compared to the control groups receiving a placebo. Additionally, blood basophil levels were also lowered following administration of benralizumab. Compared to reslizumab and mepolizumab, benralizumab provides the unique targeting of not only eosinophils, but also basophils, which have been found in elevated levels in CRSwNP patients.(47) Continued studies on the effects of benralizumab on outcome measurements in asthma and especially CRSwNP patients are necessary.
Targeting the IL-4/IL-13 pathway
The IL-4/IL-13 signaling pathway serves as a prime example of how the immunohistopathologic similarities of the respiratory epithelium in the lower and upper airways support the potential application of biologic targets in asthma pharmacotherapy for CRS management. In both asthma and CRS, IL-4 and IL-13 are important cytokines that drive Th2 cell differentiation, activate type 2 inflammatory responses via IgE synthesis and eosinophil, mast cell, and basophil recruitment, and also modulate airway remodeling through mucus hypersecretion and matrix deposition.(48, 49) In the asthma literature, clinical studies have previously assessed the IL-4/IL-13 signaling pathway and its effects on varying outcome parameters, such as measures of pulmonary function, levels of downstream inflammatory mediators, and symptom-based scores. Past examples of these biologic therapies for asthma have included pascolizumab, a monoclonal antibody targeting free IL-4; anrukinzumab and lebrikizumab, monoclonal antibodies targeting free soluble IL-13; and altrakincept, a soluble recombinant human IL-4 receptor (IL-4R), which specifically binds and competitively inhibits free IL-4.(50–53) Unfortunately, these clinical studies displayed equivocal results with varying therapeutic benefits for asthmatic patients.
More recent research on biologic therapies for asthma has focused on the simultaneous targeting of both IL-4 and IL-13, as opposed to targeting of either cytokine individually, in order to cause a more comprehensive inhibition of the type 2 inflammatory pathway. Given that the alpha subunit of IL-4R (IL-4Rα) is common to both the IL-4 and IL-13 receptors and that monoclonal antibodies that target IL-4Rα thereby block the actions of both cytokines, several clinical trials have shown a potential role for targeting IL-4Rα to improve clinical endpoints in patients with asthma and atopic dermatitis.(54–56) In particular, dupilumab, a humanized monoclonal antibody directed against IL-4Rα, has recently demonstrated overall therapeutic efficacy in a randomized, double-blinded, placebo-controlled trial for patients with CRSwNP refractory to intranasal corticosteroids.(57) In the clinical trial by Bachert et al, the addition of subcutaneous dupilumab to mometasone furoate nasal sprays for a 16-week period was found to reduce nasal polyp burden, decrease Lund-Mackay scores, and improve both olfactory and disease-specific quality-of-life questionnaire scores, when compared to use of mometasone sprays alone. These encouraging results suggest that biologic therapy specifically targeting IL-4Rα may become a viable treatment option for a variety of inflammatory conditions, including well-defined CRS endotypes.
Targeting the epithelial cell-derived cytokine pathway
Increasing research on the regulation of the type 2 inflammatory response in CRSwNP has additionally focused on the role of the mucosal epithelium, which not only provides a mechanical barrier to the external environment, but also actively stimulates the host innate and acquired immune responses through cytokine production. These epithelial cell-derived cytokines, including thymic stromal lymphopoietin (TSLP), IL-33, and IL-25, have shown the capacity to activate type 2 innate lymphoid cells (ILC2s), a subset of innate immune cells which release significant amounts of type 2 cytokines, including IL-5 and IL-13, in the absence of specific immune activation.(58–61) TSLP, IL-33, and IL-25 also influence acquired immune responses by fostering Th2 lymphocyte differentiation with an amplified type 2 cytokine response.(62, 63) Overall, epithelial cell-derived cytokines promote important upstream mechanisms that drive the type 2 inflammation observed in CRSwNP. Targeting these key biomolecules involved in these immunologic pathways may ultimately offer more effective pharmacologic methods to alter the inflammatory responses in CRSwNP.
For one, TSLP is a cytokine derived from epithelial cells of various organ systems that is linked to such inflammatory disorders as atopic dermatitis, eosinophilic esophagitis, and asthma. TSLP upregulates the expression of OX40 ligand on dendritic cells, which by interacting with the OX40 receptor on CD4 T cells, mediates the differentiation of naïve T-cells into Th2 cells. TSLP also augments the function and activity of ILC2s, which highly express the TSLP surface receptor.(58, 64) Furthermore, TSLP synergizes the inflammatory effects of IL-1 in activating the production of Th2 cytokines by mast cells. In a double-blind, placebo-controlled study, AMG 157, a human monoclonal antibody that binds human TSLP and prevents its receptor interaction, showed efficacy in decreasing allergen-induced bronchoconstriction and eosinophil levels in the sputum and blood of asthmatic patients.(65) Increased levels of TSLP and OX40 ligand activity in CRSwNP also raise the possibility of use of anti-TSLP, anti-TSLP receptor, and anti-OX40L therapy for CRSwNP.(58, 64, 66) At present, clinical trials examining the efficacy of anti-TSLP treatments in CRSwNP are lacking.
Like TSLP, IL-33 is closely linked to the type 2 inflammatory cascade of various autoimmune and inflammatory disorders through the modulation of innate and adaptive immune cells.(67) As a member of the IL-1 family of cytokines, IL-33 is released by epithelial cells to signal pathogen- or stress-related injury at the surface epithelium of the respiratory tract, skin, and gastrointestinal tract.(68, 69) Following its release, IL-33 directly recruits, induces, and enhances the survival of different immune cells, including Th2 cells, mast cells, and basophils, all of which highly express the IL-33 surface receptor, composed of ST2 and the IL-1 receptor accessory protein. Importantly, IL-33 also activates ILC2s and enhances the production of IL-13, a key mediator that drives type 2 inflammation.(59) Given its crucial role in initiating type 2 inflammatory responses, the IL-33 pathway provides a potential pharmacologic target for the management of such conditions as asthma, atopic dermatitis, and CRSwNP. In the murine model of allergic asthma, both anti-IL-33 and anti-ST2 monoclonal antibodies have been found to reduce eosinophilic airway inflammation and Th2 cytokine production.(70, 71) The effects of blocking the IL-33 pathway are further undergoing investigation in a phase I clinical trial utilizing AMG 282, a monoclonal antibody that inhibits binding of IL-33 to the ST2 receptor, for possible therapeutic use in atopic asthma and CRSwNP.
IL-25, which is a member of the IL-17 cytokine family, also contributes to the pathophysiology of various inflammatory diseases, including asthma and atopic dermatitis. IL-25 plays an active role in triggering a type 2 inflammatory response through the upregulation of the IL-25 surface receptor on Th2 cells, subsequent recruitment of eosinophils, and production of corresponding cytokines, including IL-4, IL-5, IL-13, and eotaxin.(72–74) Shin et al highlighted the important role of IL-25 in CRSwNP by showing that nasal polyp tissue from human CRSwNP subjects is associated with elevated expression of IL-25 in the mucosa and also an upregulation of the IL-25 receptor on effector immune cells.(75) Furthermore, in this same study, an IL-25 neutralizing antibody was tested in the murine model, resulting in a lower number of nasal polypoid lesions; a reduced thickness in the polyp mucosa; and a suppressed expression of IL-25 and associated cytokines. These study findings suggest a potential use of anti-IL-25 therapy as a novel treatment strategy for CRSwNP, although this directed approach requires further investigation into its efficacy and safety in human patients with CRSwNP.
Other directions in biologic therapy for CRS
For CRS pharmacotherapy, platelets have increasingly emerged as promising biologic targets for patients with aspirin-exacerbated respiratory disease (AERD), a chronic inflammatory condition characterized by the triad of CRSwNP, asthma, and sensitivity to aspirin and other drugs that inhibit the cyclooxygenase-1 enzyme. AERD etiology conventionally implicates defects in the metabolism of arachidonic acids with distinctive patterns of inflammatory markers at the histological and biochemical levels, including an intense accumulation of eosinophils and mast cells in the sinonasal and respiratory mucosa and increased cysteinyl leukotriene, a class of lipid inflammatory mediators that are produced by the 5-lipoxygenase and leukotriene C4 synthase enzymatic pathway.(1) Increasing evidence suggests that activated platelets also strongly influence the inflammatory state of AERD by amplifying the generation of cysteinyl leukotriene, forming aggregates with circulating levels of inflammatory leukocytes, and enhancing leukocyte recruitment to local tissue sites.(76–78) Given the potential role of activated platelets in the development of AERD, several clinical trials are ongoing to assess the effects of platelet-targeted therapies on various clinical endpoints of AERD. Such investigational therapies presently include prasugrel and ifetroban, which selectively inhibit the P2Y12 receptors and T prostanoid receptors, respectively, and thereby block the downstream platelet-associated mechanisms of inflammation in AERD.(76)
Future research interest may furthermore continue to expand, targeting effector cells involved with type 2 inflammatory responses, such as basophils, mast cells, and eosinophils. To this point, the sialic acid immunoglobulin-like lectin (Siglec) group of cell-surface proteins might present such a target. Among them, Siglec-8 is uniquely expressed by human eosinophils, mast cells, and basophils. Engaging this structure with antibodies has the therapeutic potential to neutralize all three cell types and thus address a wide array of type 2 inflammatory disorders.(79, 80) In particular, targeting of Siglec-8 has been found to result in apoptosis in human eosinophils and inhibition of mediator release from human mast cells without affecting their survival.(79) At present, anti-Siglec-8 treatments are entering phase II of clinical trials for use in CRSwNP patients. Continued research related to therapies for CRS subpopulations will likely provide valuable direction in the understanding of pathophysiologic pathways underlying the inflammation in CRS.
Conclusion
In CRS, the varying immunologic and histologic profiles reflect the pathophysiologic mechanisms by which inflammation is initiated and propagated. Improved understandings of these pathophysiologic pathways have expanded the recognition of CRS subsets, thereby enhancing the diagnostic criteria for CRS endotypes and raising the possibility of using pharmacotherapy to target the pathways present in individual patients.
Recent developments and clinical studies on various biologic therapies, including those targeting the IgE, IL-5, IL-4/IL-13, and epithelial cell-derived cytokine pathways, further open the door to more innovative strategies for the management of CRS in general and CRSwNP in particular. Biologic therapy for CRS thus provides a shift in the paradigm of medical treatments, which traditionally has relied on nonspecific therapies but has progressively focused more on target-specific treatments that may be efficacious in patients with certain CRS phenotypic and endotypic characteristics.
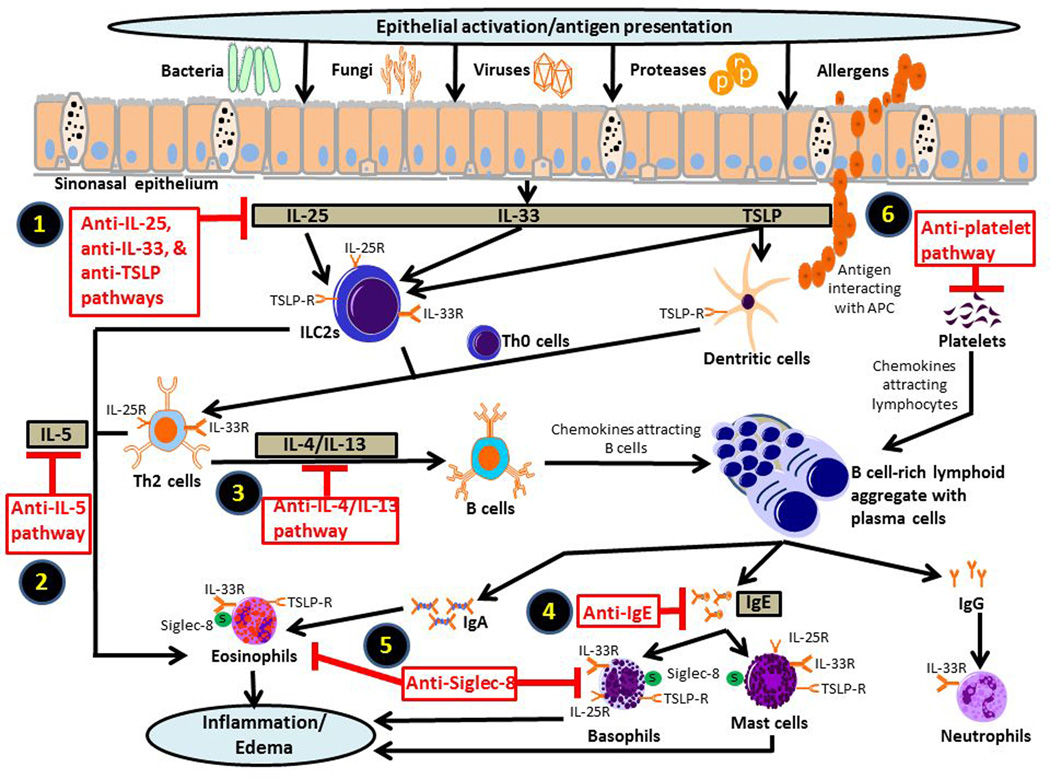
Figure 1.
Current and future targets for biologic therapy in chronic rhinosinusitis (CRS). Chronic inflammation associated with CRS is linked to various pathophysiologic mechanisms in the innate and adaptive immune systems. While the nature of the inflammatory response likely varies across different CRS phenotypes, the typical case of CRS with nasal polyposis in the United States and Europe characteristically follows a type 2 inflammatory profile. Potential therapeutic targets that influence a type 2 inflammation include (1) epithelial cell-derived cytokines, including IL-25, IL-33, and TSLP; (2) IL-5; (3) IL-4 and IL-13; and (4) IgE. Due to the importance of these upstream mediators in instigating tissue inflammation along the sinonasal tract, they have served as promising targets for inhibition in the management CRS. Furthermore, the cytokine receptors for the IL-33, TSLP, IL-5, IL-4, and IL-13 are important signaling components of the inflammatory pathways and have thus provided additional therapeutic targets for research. (5) Siglec-8 proteins, which are found on eosinophils, mast cells, and basophils, may also be an effective target for diminishing effector cell activation. (6) Specifically in patients with aspirin-exacerbated respiratory disorder, platelets may also play a significant role in the pathophysiology of disease through the recruitment of effector cells to the local tissue, thereby warranting special attention for biologic targeting.
Acknowledgments
None
Research funding: AL is supported by the Center for Clinical and Translational Sciences, which is funded by National Institutes of Health Clinical and Translational Award UL1 TR000371 and KL2 TR000370 from the National Center for Advancing Translational Science. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Science or the National Institutes of Health.
Footnotes
Conflict of interest: None
References
- 1.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;(23):3. preceding table of contents, 1–298. Epub 2012/07/07. [PubMed] [Google Scholar]
- 2.Schlosser RJ, Soler ZM. Evidence-based treatment of chronic rhinosinusitis with nasal polyps. American journal of rhinology & allergy. 2013;27(6):461–466. doi: 10.2500/ajra.2013.27.3982. Epub 2013/11/28. [DOI] [PubMed] [Google Scholar]
- 3.Lal D, Scianna JM, Stankiewicz JA. Efficacy of targeted medical therapy in chronic rhinosinusitis, and predictors of failure. American journal of rhinology & allergy. 2009;23(4):396–400. doi: 10.2500/ajra.2009.23.3334. Epub 2009/08/13. [DOI] [PubMed] [Google Scholar]
- 4.Dubin MG, Liu C, Lin SY, Senior BA. American Rhinologic Society member survey on "maximal medical therapy" for chronic rhinosinusitis. American journal of rhinology. 2007;21(4):483–488. doi: 10.2500/ajr.2007.21.3047. Epub 2007/09/22. [DOI] [PubMed] [Google Scholar]
- 5.Sylvester DC, Carr S, Nix P. Maximal medical therapy for chronic rhinosinusitis: a survey of otolaryngology consultants in the United Kingdom. International forum of allergy & rhinology. 2013;3(2):129–132. doi: 10.1002/alr.21084. Epub 2012/10/06. [DOI] [PubMed] [Google Scholar]
- 6.Baguley C, Brownlow A, Yeung K, Pratt E, Sacks R, Harvey R. The fate of chronic rhinosinusitis sufferers after maximal medical therapy. International forum of allergy & rhinology. 2014;4(7):525–532. doi: 10.1002/alr.21315. Epub 2014/03/13. [DOI] [PubMed] [Google Scholar]
- 7.Smith KA, Smith TL, Mace JC, Rudmik L. Endoscopic sinus surgery compared to continued medical therapy for patients with refractory chronic rhinosinusitis. International forum of allergy & rhinology. 2014;4(10):823–827. doi: 10.1002/alr.21366. Epub 2014/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith TL, Kern R, Palmer JN, Schlosser R, Chandra RK, Chiu AG, et al. Medical therapy vs surgery for chronic rhinosinusitis: a prospective, multi-institutional study with 1-year follow-up. International forum of allergy & rhinology. 2013;3(1):4–9. doi: 10.1002/alr.21065. Epub 2012/06/28. [DOI] [PubMed] [Google Scholar]
- 9.Smith SS, Evans CT, Tan BK, Chandra RK, Smith SB, Kern RC. National burden of antibiotic use for adult rhinosinusitis. The Journal of allergy and clinical immunology. 2013;132(5):1230–1232. Epub 2013/08/31. [Google Scholar]
- 10.Fajt ML, Wenzel SE. Biologic therapy in asthma: entering the new age of personalized medicine. J Asthma. 2014:1–8. doi: 10.3109/02770903.2014.910221. Epub 2014/04/10. [DOI] [PubMed] [Google Scholar]
- 11.Bochner BS, Gleich GJ. What targeting eosinophils has taught us about their role in diseases. The Journal of allergy and clinical immunology. 2010;126(1):16–25. doi: 10.1016/j.jaci.2010.02.026. quiz 6–7. Epub 2010/05/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachert C, Zhang N. Chronic rhinosinusitis and asthma: novel understanding of the role of IgE 'above atopy'. Journal of internal medicine. 2012;272(2):133–143. doi: 10.1111/j.1365-2796.2012.02559.x. Epub 2012/05/30. [DOI] [PubMed] [Google Scholar]
- 13.Tajiri T, Matsumoto H, Hiraumi H, Ikeda H, Morita K, Izuhara K, et al. Efficacy of omalizumab in eosinophilic chronic rhinosinusitis patients with asthma. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2013;110(5):387–388. doi: 10.1016/j.anai.2013.01.024. Epub 2013/04/30. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Lane AP. Chronic rhinosinusitis as a multifactorial inflammatory disorder. Curr Infect Dis Rep. 2011;13(2):159–168. doi: 10.1007/s11908-011-0166-z. Epub 2011/03/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan BK, Schleimer RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Current opinion in otolaryngology & head and neck surgery. 2010;18(1):21–26. doi: 10.1097/MOO.0b013e3283350053. Epub 2009/12/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam K, Schleimer R, Kern RC. The Etiology and Pathogenesis of Chronic Rhinosinusitis: a Review of Current Hypotheses. Current allergy and asthma reports. 2015;15(7):41. doi: 10.1007/s11882-015-0540-2. Epub 2015/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. American journal of rhinology. 2008;22(6):549–559. doi: 10.2500/ajr.2008.22.3228. Epub 2008/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. The Journal of allergy and clinical immunology. 2013;131(6):1479–1490. doi: 10.1016/j.jaci.2013.02.036. Epub 2013/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachert C, Zhang N, van Zele T, Gevaert P. Chronic rhinosinusitis: from one disease to different phenotypes. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2012;23(Suppl 22):2–4. doi: 10.1111/j.1399-3038.2012.01318.x. Epub 2012/08/01. [DOI] [PubMed] [Google Scholar]
- 20.Lam K, Kern R. The use of immunologic and histopathologic characteristics to classify chronic rhinosinusitis subtypes. Otorinolaringologia. 2014;64(2):39–43. [Google Scholar]
- 21.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–725. doi: 10.1038/nm.2678. Epub 2012/05/09. [DOI] [PubMed] [Google Scholar]
- 22.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. American journal of respiratory and critical care medicine. 2009;180(5):388–395. doi: 10.1164/rccm.200903-0392OC. Epub 2009/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarvis D, Newson R, Lotvall J, Hastan D, Tomassen P, Keil T, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012;67(1):91–98. doi: 10.1111/j.1398-9995.2011.02709.x. Epub 2011/11/05. [DOI] [PubMed] [Google Scholar]
- 24.Pearlman AN, Chandra RK, Chang D, Conley DB, Tripathi-Peters A, Grammer LC, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. American journal of rhinology & allergy. 2009;23(2):145–148. doi: 10.2500/ajra.2009.23.3284. Epub 2009/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam K, Hirsch AG, Tan BK. The association of premorbid diseases with chronic rhinosinusitis with and without polyps. Current opinion in otolaryngology & head and neck surgery. 2014;22(3):231–241. doi: 10.1097/MOO.0000000000000052. Epub 2014/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. The Journal of allergy and clinical immunology. 2010;126(5):962–968. 8 e1–8 e6. doi: 10.1016/j.jaci.2010.07.007. Epub 2010/09/03. [DOI] [PubMed] [Google Scholar]
- 27.Hsu J, Avila PC, Kern RC, Hayes MG, Schleimer RP, Pinto JM. Genetics of chronic rhinosinusitis: state of the field and directions forward. The Journal of allergy and clinical immunology. 2013;131(4):977–993. 93 e1–93 e5. doi: 10.1016/j.jaci.2013.01.028. Epub 2013/04/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: developing guidance for clinical trials. The Journal of allergy and clinical immunology. 2006;118(5 Suppl):S17–S61. doi: 10.1016/j.jaci.2006.09.005. Epub 2006/11/07. [DOI] [PubMed] [Google Scholar]
- 29.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61(11):1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. Epub 2006/09/28. [DOI] [PubMed] [Google Scholar]
- 30.Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. The Journal of allergy and clinical immunology. 2009;124(3):478–484. 84 e1–84 e2. doi: 10.1016/j.jaci.2009.05.017. Epub 2009/06/23. [DOI] [PubMed] [Google Scholar]
- 31.Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. The Journal of allergy and clinical immunology. 2008;121(6):1435–1441. 41 e1–41 e3. doi: 10.1016/j.jaci.2008.02.018. Epub 2008/04/22. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda K, Shiozawa A, Ono N, Kusunoki T, Hirotsu M, Homma H, et al. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. The Laryngoscope. 2013;123(11):E1–E9. doi: 10.1002/lary.24154. Epub 2013/05/15. [DOI] [PubMed] [Google Scholar]
- 33.Kim YM, Munoz A, Hwang PH, Nadeau KC. Migration of regulatory T cells toward airway epithelial cells is impaired in chronic rhinosinusitis with nasal polyposis. Clin Immunol. 2010;137(1):111–121. doi: 10.1016/j.clim.2010.05.013. Epub 2010/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. The Journal of allergy and clinical immunology. 2008;122(5):961–968. doi: 10.1016/j.jaci.2008.07.008. Epub 2008/09/23. [DOI] [PubMed] [Google Scholar]
- 35.Caruso M, Crisafulli E, Lizzio R, Polosa R. Biologic therapy for atopic asthma and beyond. Current opinion in allergy and clinical immunology. 2013;13(6):677–685. doi: 10.1097/ACI.0000000000000012. Epub 2013/10/25. [DOI] [PubMed] [Google Scholar]
- 36.Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131(1):110–116. e1. doi: 10.1016/j.jaci.2012.07.047. Epub 2012/10/02. [DOI] [PubMed] [Google Scholar]
- 37.Pinto JM, Mehta N, DiTineo M, Wang J, Baroody FM, Naclerio RM. A randomized, double-blind, placebo-controlled trial of anti-IgE for chronic rhinosinusitis. Rhinology. 2010;48(3):318–324. doi: 10.4193/Rhino09.144. Epub 2010/11/03. [DOI] [PubMed] [Google Scholar]
- 38.Chipps BE, Figliomeni M, Spector S. Omalizumab: an update on efficacy and safety in moderate-to-severe allergic asthma. Allergy and asthma proceedings : the official journal of regional and state allergy societies. 2012;33(5):377–385. doi: 10.2500/aap.2012.33.3599. Epub 2012/10/03. [DOI] [PubMed] [Google Scholar]
- 39.Long A, Rahmaoui A, Rothman KJ, Guinan E, Eisner M, Bradley MS, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. The Journal of allergy and clinical immunology. 2014 doi: 10.1016/j.jaci.2014.02.007. Epub 2014/04/01. [DOI] [PubMed] [Google Scholar]
- 40.Hamilos DL, Leung DY, Wood R, Meyers A, Stephens JK, Barkans J, et al. Chronic hyperplastic sinusitis: association of tissue eosinophilia with mRNA expression of granulocyte-macrophage colony-stimulating factor and interleukin-3. The Journal of allergy and clinical immunology. 1993;92(1 Pt 1):39–48. doi: 10.1016/0091-6749(93)90035-e. Epub 1993/07/01. [DOI] [PubMed] [Google Scholar]
- 41.Hamilos DL, Leung DY, Wood R, Cunningham L, Bean DK, Yasruel Z, et al. Evidence for distinct cytokine expression in allergic versus nonallergic chronic sinusitis. The Journal of allergy and clinical immunology. 1995;96(4):537–544. doi: 10.1016/s0091-6749(95)70298-9. Epub 1995/10/01. [DOI] [PubMed] [Google Scholar]
- 42.Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele T, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118(5):1133–1141. doi: 10.1016/j.jaci.2006.05.031. Epub 2006/11/08. [DOI] [PubMed] [Google Scholar]
- 43.Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele T, Acke F, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. The Journal of allergy and clinical immunology. 2011;128(5):989–995. e1–e8. doi: 10.1016/j.jaci.2011.07.056. Epub 2011/10/01. [DOI] [PubMed] [Google Scholar]
- 44.Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. The Journal of allergy and clinical immunology. 2013;132(5):1086–1096. e5. doi: 10.1016/j.jaci.2013.05.020. Epub 2013/07/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Busse WW, Katial R, Gossage D, Sari S, Wang B, Kolbeck R, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. The Journal of allergy and clinical immunology. 2010;125(6):1237–1244. e2. doi: 10.1016/j.jaci.2010.04.005. Epub 2010/06/02. [DOI] [PubMed] [Google Scholar]
- 46.Ghazi A, Trikha A, Calhoun WJ. Benralizumab--a humanized mAb to IL-5Ralpha with enhanced antibody-dependent cell-mediated cytotoxicity--a novel approach for the treatment of asthma. Expert opinion on biological therapy. 2012;12(1):113–118. doi: 10.1517/14712598.2012.642359. Epub 2011/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahdavinia M, Carter RG, Ocampo CJ, Stevens W, Kato A, Tan BK, et al. Basophils are elevated in nasal polyps of patients with chronic rhinosinusitis without aspirin sensitivity. The Journal of allergy and clinical immunology. 2014;133(6):1759–1763. doi: 10.1016/j.jaci.2013.12.1092. Epub 2014/03/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamilos DL, Leung DY, Wood R, Bean DK, Song YL, Schotman E, et al. Eosinophil infiltration in nonallergic chronic hyperplastic sinusitis with nasal polyposis (CHS/NP) is associated with endothelial VCAM-1 upregulation and expression of TNF-alpha. American journal of respiratory cell and molecular biology. 1996;15(4):443–450. doi: 10.1165/ajrcmb.15.4.8879177. Epub 1996/10/01. [DOI] [PubMed] [Google Scholar]
- 49.Nonaka M, Nonaka R, Woolley K, Adelroth E, Miura K, Okhawara Y, et al. Distinct immunohistochemical localization of IL-4 in human inflamed airway tissues. IL-4 is localized to eosinophils in vivo and is released by peripheral blood eosinophils. J Immunol. 1995;155(6):3234–3244. Epub 1995/09/15. [PubMed] [Google Scholar]
- 50.Hart TK, Blackburn MN, Brigham-Burke M, Dede K, Al-Mahdi N, Zia-Amirhosseini P, et al. Preclinical efficacy and safety of pascolizumab (SB 240683): a humanized anti-interleukin-4 antibody with therapeutic potential in asthma. Clinical and experimental immunology. 2002;130(1):93–100. doi: 10.1046/j.1365-2249.2002.01973.x. Epub 2002/09/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noonan M, Korenblat P, Mosesova S, Scheerens H, Arron JR, Zheng Y, et al. Dose-ranging study of lebrikizumab in asthmatic patients not receiving inhaled steroids. The Journal of allergy and clinical immunology. 2013;132(3):567–574. e12. doi: 10.1016/j.jaci.2013.03.051. Epub 2013/06/04. [DOI] [PubMed] [Google Scholar]
- 52.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. The New England journal of medicine. 2011;365(12):1088–1098. doi: 10.1056/NEJMoa1106469. Epub 2011/08/05. [DOI] [PubMed] [Google Scholar]
- 53.Oh CK, Geba GP, Molfino N. Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma. European respiratory review : an official journal of the European Respiratory Society. 2010;19(115):46–54. doi: 10.1183/09059180.00007609. Epub 2010/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. American journal of respiratory and critical care medicine. 2010;181(8):788–796. doi: 10.1164/rccm.200909-1448OC. Epub 2010/01/09. [DOI] [PubMed] [Google Scholar]
- 55.Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007;370(9596):1422–1431. doi: 10.1016/S0140-6736(07)61600-6. Epub 2007/10/24. [DOI] [PubMed] [Google Scholar]
- 56.Beck LA, Thaci D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. The New England journal of medicine. 2014;371(2):130–139. doi: 10.1056/NEJMoa1314768. Epub 2014/07/10. [DOI] [PubMed] [Google Scholar]
- 57.Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of Subcutaneous Dupilumab on Nasal Polyp Burden in Patients With Chronic Sinusitis and Nasal Polyposis: A Randomized Clinical Trial. Jama. 2016;315(5):469–479. doi: 10.1001/jama.2015.19330. Epub 2016/02/03. [DOI] [PubMed] [Google Scholar]
- 58.Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. The Journal of allergy and clinical immunology. 2013;132(3):593–600. e12. doi: 10.1016/j.jaci.2013.04.005. Epub 2013/05/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. American journal of respiratory and critical care medicine. 2013;188(4):432–439. doi: 10.1164/rccm.201212-2227OC. Epub 2013/06/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517(7534):293–301. doi: 10.1038/nature14189. Epub 2015/01/17. [DOI] [PubMed] [Google Scholar]
- 61.Kim BS, Wojno ED, Artis D. Innate lymphoid cells and allergic inflammation. Current opinion in immunology. 2013;25(6):738–744. doi: 10.1016/j.coi.2013.07.013. Epub 2013/09/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. The Journal of allergy and clinical immunology. 2009;123(2):472–478. doi: 10.1016/j.jaci.2008.10.022. Epub 2008/12/10. [DOI] [PubMed] [Google Scholar]
- 63.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nature immunology. 2011;12(11):1055–1062. doi: 10.1038/ni.2104. Epub 2011/09/13. [DOI] [PubMed] [Google Scholar]
- 64.Liu T, Li TL, Zhao F, Xie C, Liu AM, Chen X, et al. Role of thymic stromal lymphopoietin in the pathogenesis of nasal polyposis. The American journal of the medical sciences. 2011;341(1):40–47. doi: 10.1097/MAJ.0b013e3181f20489. Epub 2010/10/07. [DOI] [PubMed] [Google Scholar]
- 65.Gauvreau GM, O'Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. The New England journal of medicine. 2014;370(22):2102–2110. doi: 10.1056/NEJMoa1402895. Epub 2014/05/23. [DOI] [PubMed] [Google Scholar]
- 66.Boita M, Garzaro M, Raimondo L, Riva G, Mazibrada J, Pecorari G, et al. Eosinophilic inflammation of chronic rhinosinusitis with nasal polyps is related to OX40 ligand expression. Innate immunity. 2015;21(2):167–174. doi: 10.1177/1753425914523460. Epub 2014/03/04. [DOI] [PubMed] [Google Scholar]
- 67.Reh DD, Wang Y, Ramanathan M, Jr, Lane AP. Treatment-recalcitrant chronic rhinosinusitis with polyps is associated with altered epithelial cell expression of interleukin-33. American journal of rhinology & allergy. 2010;24(2):105–109. doi: 10.2500/ajra.2010.24.3446. Epub 2010/03/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saluja R, Khan M, Church MK, Maurer M. The role of IL-33 and mast cells in allergy and inflammation. Clinical and translational allergy. 2015;5:33. doi: 10.1186/s13601-015-0076-5. Epub 2015/10/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Theoharides TC, Petra AI, Taracanova A, Panagiotidou S, Conti P. Targeting IL-33 in autoimmunity and inflammation. The Journal of pharmacology and experimental therapeutics. 2015;354(1):24–31. doi: 10.1124/jpet.114.222505. Epub 2015/04/25. [DOI] [PubMed] [Google Scholar]
- 70.Liu X, Li M, Wu Y, Zhou Y, Zeng L, Huang T. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochemical and biophysical research communications. 2009;386(1):181–185. doi: 10.1016/j.bbrc.2009.06.008. Epub 2009/06/11. [DOI] [PubMed] [Google Scholar]
- 71.Kearley J, Buckland KF, Mathie SA, Lloyd CM. Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway. American journal of respiratory and critical care medicine. 2009;179(9):772–781. doi: 10.1164/rccm.200805-666OC. Epub 2009/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iinuma T, Okamoto Y, Yamamoto H, Inamine-Sasaki A, Ohki Y, Sakurai T, et al. Interleukin-25 and mucosal T cells in noneosinophilic and eosinophilic chronic rhinosinusitis. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2015;114(4):289–298. doi: 10.1016/j.anai.2015.01.013. Epub 2015/02/24. [DOI] [PubMed] [Google Scholar]
- 73.Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. The Journal of allergy and clinical immunology. 2006;118(3):606–614. doi: 10.1016/j.jaci.2006.04.051. Epub 2006/09/05. [DOI] [PubMed] [Google Scholar]
- 74.Tamachi T, Maezawa Y, Ikeda K, Iwamoto I, Nakajima H. Interleukin 25 in allergic airway inflammation. International archives of allergy and immunology. 2006;140(Suppl 1):59–62. doi: 10.1159/000092713. Epub 2006/06/15. [DOI] [PubMed] [Google Scholar]
- 75.Shin HW, Kim DK, Park MH, Eun KM, Lee M, So D, et al. IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. The Journal of allergy and clinical immunology. 2015;135(6):1476–1485. e7. doi: 10.1016/j.jaci.2015.01.003. Epub 2015/03/03. [DOI] [PubMed] [Google Scholar]
- 76.Laidlaw TM, Boyce JA. Platelets in patients with aspirin-exacerbated respiratory disease. The Journal of allergy and clinical immunology. 2015;135(6):1407–1414. doi: 10.1016/j.jaci.2015.02.005. quiz 15. Epub 2015/06/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012;119(16):3790–3798. doi: 10.1182/blood-2011-10-384826. Epub 2012/01/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu T, Laidlaw TM, Katz HR, Boyce JA. Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(42):16987–16992. doi: 10.1073/pnas.1313185110. Epub 2013/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacology & therapeutics. 2012;135(3):327–336. doi: 10.1016/j.pharmthera.2012.06.005. Epub 2012/07/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ilmarinen P, Kankaanranta H. Eosinophil apoptosis as a therapeutic target in allergic asthma. Basic & clinical pharmacology & toxicology. 2014;114(1):109–117. doi: 10.1111/bcpt.12163. Epub 2013/10/24. [DOI] [PubMed] [Google Scholar]