Abstract
Background
The UNAIDS “90-90-90” global treatment target aims to achieve 73% virologic suppression among HIV-infected persons worldwide by 2020.
Objective
Using a microsimulation model of HIV detection, disease and treatment, we estimate the clinical and economic value of reaching this ambitious goal in South Africa.
Design
We model: the Current Pace strategy, simulating existing scale-up efforts and gradual increases in overall virologic suppression from 24% to 36% in 5 years; and the UNAIDS Target strategy, simulating 73% suppression in 5 years.
Data Sources
Published estimates and South African survey data inform HIV transmission rates (0.16–9.03/100PY), HIV-specific age-stratified fertility rates (1.0–9.1/100PY), and costs (ART: $11–31/month, routine care: $20–157/month).
Target population
South African HIV-infected population, including incident infections over the next ten years
Perspective
Modified societal perspective, excluding time and productivity costs
Time Horizon
Five and ten years
Interventions
Aggressive HIV case-detection, efficient linkage to care, rapid treatment scale-up and adherence/retention interventions toward the UNAIDS Target strategy
Outcome Measures
HIV transmissions, deaths, years of life saved (YLS), maternal orphans, costs (2014USD), and cost-effectiveness
Base Case Analysis
Compared to Current Pace over (5- and) 10-years, the UNAIDS Target strategy would avert (873,000) 2,051,000 HIV transmissions, (1,174,000) 2,478,000 deaths, and (726,000) 1,689,000 maternal orphans, while saving (3,002,000) 13,340,000 life-years. The additional budget required for the UNAIDS Target strategy would be ($7.965) $15.979 billion, yielding an incremental cost-effectiveness ratio (ICER) of ($2,720/YLS) $1,260/YLS, (<50%) <20% of South Africa per capita GDP.
Results of Sensitivity Analysis
Outcomes generally varied <20% from base case outcomes when we varied key input parameters within plausible ranges.
Limitations
Several pathways may lead to 73% overall suppression which were examined in sensitivity analysis.
Conclusions
Reaching the “90-90-90” HIV suppression target would be costly but both extraordinarily effective and cost-effective in South Africa. Global health policy makers should mobilize the political and economic support to make the 90-90-90 aspiration a reality.
Keywords: HIV, treatment cascade, orphans, survival, HIV treatment scale-up
INTRODUCTION
In September 2014, the Joint Nations Program on HIV/AIDS (UNAIDS) launched “90-90-90: An ambitious treatment target to help end the AIDS epidemic” by 2020. The 90-90-90 goal aims to: diagnose 90% of HIV-infected persons worldwide; link 90% of these to antiretroviral therapy (ART); and achieve 90% virologic suppression among ART recipients. Such coverage would produce virologic suppression in 73% (=0.90*0.90*0.90) of patients worldwide, a marked increase from current estimates of 29% (1). 90-90-90 addresses the so-called “HIV treatment cascade,” documenting attrition in the continuum of care leading from HIV diagnosis, through linkage to care, to suppression on ART and retention. Current South African estimates suggest that 67% of HIV-infected patients are undiagnosed and/or unlinked, 33% receive ART, and only 24% are virologically suppressed (2).
While much has been said and written about the moral and public health imperative to implement 90-90-90 (1, 3), comparatively little is known about how reaching its treatment targets will translate into broader, downstream health and social outcomes of interest: survival, new transmissions, numbers of children becoming orphans, and costs. Given the magnitude of the investment that this initiative will require, policy makers and international partners (e.g., the President’s Emergency Plan for AIDS Relief, the Global Fund, and the World Bank) deserve to know what return they can anticipate on their investment.
METHODS
Analytic Overview
We use the Cost-effectiveness of Preventing AIDS Complications–International (CEPAC-I) model and South African data to compare the Current Pace strategy to the UNAIDS Target strategy. We project the progress of cohorts through the continuum of care for ten years, taking into account new infections, diagnoses, ART initiations, and ART-associated virologic outcomes. Over both 5- and 10-year horizons, we project the following outcomes: cumulative HIV transmissions, deaths, years of life saved, numbers of children becoming orphans, lifetime costs (in 2014USD), and incremental cost-effectiveness. Outcomes are reported both undiscounted (for clinical and budget impact purposes) and discounted at 3% annually (for cost-effectiveness evaluation).
Cohort Definitions
The current population of HIV-infected adults (≥15 years) in South Africa (the “prevalent cohort”) is assigned at model outset to stages in the care continuum: 67% undiagnosed, unlinked to care, or otherwise not on ART; 9% unsuppressed on ART; and 23% and 1% of the total HIV-infected population suppressed on first- and second-line ART (2, 4). Each year, we introduce an incident cohort and thereafter combine outcomes of the 2016 prevalent cohort and all later incident cohorts.
The CEPAC-I Model
CEPAC-I is a microsimulation of HIV disease progression and treatment in resource-limited settings (5). CEPAC-I aggregates the experience of large numbers of individual patients to project the population-level impact of alternative treatment scale-up policies (6). Individual patient characteristics (e.g., age, gender, CD4 count, HIV RNA) are generated by random draws from distributions, some of which (e.g., CD4) are dependent on the simulated patient’s stage in the HIV continuum of care at model outset (i.e. not in care, on ART suppressed, on ART not suppressed, Table 1, top). CEPAC-I simulates the success and failure of disease detection, linkage to care, ART initiation, virologic suppression, and retention in care. It also simulates risks of opportunistic infections (OI), which increase with falling CD4 counts. Undetected or otherwise unlinked patients experience immunologic/CD4 decline. HIV is detected through testing or the development of an OI. Only patients achieving virologic suppression on ART experience CD4 increases; unsuppressed ART patients achieve no CD4 recovery and continue to face reduced CD4-specific OI risk while accruing ART costs (5).
Table 1.
Selected model input values for an analysis of 90-90-90 in South Africa
| Cohort | Mean Age (SD), years | Mean CD4 (SD), × 109 cells/L* | Weighted Distribution, % | Reference |
|---|---|---|---|---|
| Incident | 30 (8) | 0.667 (0.134) | N/A | (7,8) |
| Prevalent undiagnosed | 36 (16) | 0.434 (0.255) | 45.3 | (2,22) |
| Prevalent, on first-line ART | 39 (16) | 0.454 (0.246) | 31.8 | (2,22) |
| Prevalent, on second-line ART | 41 (16) | 0.454 (0.246) | 1.7 | (2,4,22) |
| Prevalent diagnosed, not in care | 41 (16) | 0.257 (0.080) | 21.2 | (2,9,22) |
| Cohort Characteristics | Value | Reference |
|---|---|---|
| Gender distribution, % male | 42.3 | (22) |
| HIV RNA distribution after acute infection, % | (17) | |
| >100,000 copies/ml | 42 | |
| 30,001–100,000 copies/ml | 28 | |
| 10,001–30,000 copies/ml | 18 | |
| 3,001–10,000 copies/ml | 8 | |
| ≤3,000 copies/ml | 4 | |
| Mean ART efficacy, % virologic suppression at 48 weeks | 72 | (2,10) |
| Loss to follow-up, probability in care at 5 (1) years, % | (12–14) | |
| Current Pace strategy | 80 (96) | |
| UNAIDS Target strategy | 88 (98) | |
| Return to care | ||
| Return to care probability after one year monthly % | 1.0 | Assumption |
| Return to care probability upon WHO stage 3/4 OI, % | 50 | Assumption |
| Transmission rates (per 100PY), by disease stage and viral load | (15,16) | |
| Incident infection (6 months post infection) | 7.25 × 9.03† | |
| Late stage disease (CD4 <2.00 × 109 cells/L) | 9.03 | |
| >100,000 copies/ml | 9.03 | |
| 10,001–100,000 copies/ml | 8.12 | |
| 3,001–10,000 copies/ml | 4.17 | |
| 501–3,000 copies/ml | 2.06 | |
| 21–500 copies/ml | 0.16 | |
| ≤20 copies/ml | 0.16 | |
|
| ||
|
Orphans
| ||
| HIV-specific age-stratified fertility rates (per 100PY)‡ | (20,22,23) | |
| 15–19 years | 5.8 | |
| 20–29 years | 9.1 | |
| 30–39 years | 6.6 | |
| 40–49 years | 1.0 | |
|
| ||
|
Costs, 2014 USD
| ||
| ART Costs | (25) | |
| First-line ART, monthly (annually) | 11 (137) | |
| Second-line ART, monthly (annually) | 31 (375) | |
| Monitoring costs | (30) | |
| HIV viral load, cost per test | 36 | |
| CD4 count, cost per test | 7 | |
| Routine care cost, monthly (ranges by CD4 count) | (26,30) | |
| CD4 > 0.500 × 109 cells/L | 20 | |
| CD4 0.350 – 0.500 × 109 cells/L | 27 | |
| CD4 0.200 – 0.350 × 109 cells/L | 32 | |
| CD4 0.050 – 0.200 × 109 cells/L | 70 | |
| CD4 < 0.050 × 109 cells/L | 157 | |
| OI treatment costs (ranges by OI) | (27) | |
| WHO stage 3–4 AIDS defining diseases | ||
| Visceral | 810 | |
| Muco-cutaneous | 520 | |
| Other | 430 | |
| Tuberculosis | 770 | |
| Severe Bacterial infections | 770 | |
| Mild fungal disease | 360 | |
| Mild other disease | 240 | |
| UNAIDS Target Strategy only | (31) | |
| HIV test cost per negative test | 7 | |
| HIV test cost per positive test | 20 | |
| Adherence/retention intervention cost, annual per patient on ART§ | 155 | |
Abbreviations: SD, standard deviation; ART, antiretroviral therapy; PY, person years; OI, opportunistic infection; WHO, World Health organization; USD, United States Dollars
When reported as medians and interquartile ranges, CD4 cell counts were converted to means and standard deviations for reporting purposes.
During the period of acute infection, the transmission rate is 7.25 times the transmission rate of the highest viral load stratum (>100,000 copies/mL).
Using age-specific HIV prevalence as well as age-specific fertility rate risk ratios between HIV-infected and uninfected, we derived HIV- and age-specific fertility rates. These rates/100PY translate into a total fertility rate (TFR) for HIV-infected: 2.0, TFR for HIV-uninfected: 2.7 (20, 22, 23)
Adherence/retention intervention affects ART efficacy (% suppressed) and attenuates loss to follow-up as described in the Methods.
For those initiating ART, the 48-week virologic suppression rate is 72% (2, 10). HIV RNA is monitored according to South African guidelines (11). Patients in whom ART failure is detected may be switched to a second-line regimen, if one is available. Patients in care are at an adherence-dependent risk of loss-to-follow-up at monthly rates ranging from 0.2–1.1%, resulting in an 80% probability of remaining in care at 5 years (12–14). Additional model detail – including model figures, flowcharts, state space definitions, user guides, data fields, protocols for data assembly, and stopping rules – can be found at: http://www.massgeneral.org/mpec/cepac/.
Transmissions
To estimate HIV transmission, we use the model’s unique capacity to report monthly, patient-level information on HIV RNA. We make the assumption that HIV viral load drives the rate at which infected individuals transmit HIV to others. We link published estimates of viral-load-specific transmission (15, 16) to monthly CEPAC-I output on each patient’s viral load (Table 1). This method -- and the published data employed -- does not consider age, gender or risk-group but instead uses aggregated transmission events stratified by viral load. In the model, patient viral load depends on the baseline HIV RNA value (17) and whether the patient is acutely infected (18); virologically suppressed; either becoming suppressed or rebounding; or at an advanced disease stage (CD4 ≤0.200 × 109 cells/L). During the 6-month period of acute infection, we amplify transmissions 7.25-fold (16). The cumulative viral load estimated by the model across all patients and all 12 months is employed to estimate the yearly number of incident infections over a 10-year horizon.
Clinical Outcomes
For each of the yearly cohorts, the CEPAC-I Model provides the monthly distribution of all living patients – including those newly-infected – across the various stages of the cascade. The output also provides, for each year: the numbers of deaths, the number of persons alive at each stage in the continuum of HIV care (i.e. not in care, on ART not suppressed, on ART suppressed), and the cumulative years of life accrued to both prevalent and incident cohorts.
Maternal Orphans
We consider the children of HIV-infected women in 2016 (current children) and children projected to be born to HIV-infected women over the next ten years (new children). We define maternal orphans as children <18 years old whose mother has died from HIV/AIDS (19). We also report results for particularly vulnerable orphans <5 years of age. Numbers of orphans and their ages are estimated using country- and age-specific fertility rates, derived from the United Nations and Statistics South Africa, corrected for women’s HIV prevalence by age (20–22). We adjust our estimate to take into account: reductions in fertility due to HIV infection (23); the risk of child mortality, both prior to and after becoming orphaned (24); and the possibility that an orphan will attain the age of 18 years (and thus no longer meet the definition of orphanhood), at some point in the 10-year time horizon. We tally both cumulative orphans (i.e., all children orphaned between 2016–2026) and current orphans (i.e., living orphans <18 years old at a given time).
Costs
First- and second-line ART costs are $137 and $375/year (25). HIV-related care costs include OI treatment, laboratory monitoring, and CD4-stratified routine care costs (Table 1) (26, 27). When necessary, year-specific Gross Domestic Product (GDP) deflators are used to first update all South African costs to 2014 Rand and subsequently converted to 2014 USD (28, 29).
Strategies
The Current Pace strategy reflects current HIV detection, treatment, and retention activities in South Africa, resulting in gradual increases in overall virologic suppression from 24% to 36% and 44% over the next 5 and 10 years (Table 1).
For the UNAIDS Target Strategy, we adjust parameters in the CEPAC-I model to achieve the objectives of the 90-90-90 initiative in South Africa. Model output demonstrates that both testing, on average, every two years and linking 50% of identified cases, are required to achieve 90% diagnosis and 90% on ART in 5 years. Average CD4 at ART initiation among those in the previously undetected prevalent and incident cohorts increases to 0.305 and 0.364 × 109 cells/L (from 0.124 and 0.167 × 109 cells/L), respectively. To achieve overall virologic suppression of 73%, we assume the routine implementation of a highly effective, monthly adherence/retention intervention. Such a program might require intensive, door-to-door services costing as much as $13/person on ART/month, a figure reported by a study of a comparably exhaustive, community-based intervention (31). Although there are no currently available reports of an intervention of this scope and efficacy, we assume here that this intervention could increase both 48-week ART suppressive efficacy from 72% (under Current Pace) to 87% and the 5-year probability of retention in care from 80% to 88%.
The UNAIDS Target strategy adds substantial costs for case detection. The costs of extensive outreach are assumed to be $20 per positive test and $7 per negative test (31). The number of persons tested each year is estimated by dividing the number of persons linked to treatment by the proportion of the population >15yo with undiagnosed HIV. As undiagnosed prevalence decreases from 8% to ~2% over ten years, an increased number of negative tests are required to find each unlinked HIV-infected person.
Sensitivity Analyses
To examine the robustness of our findings in the face of changes in the underlying data, we conduct numerous one-way and multi-way sensitivity analyses, varying input parameters across a broad range of plausible alternative values. Parameters of interest include: the duration of acute infection (base value = 6 months; plausible range = 3–12 months); the efficiency of testing and linkage (base linkage rate = 50%; plausible range = 25%–90%); annual ART costs (base values = $137 and $375 for 1st- and 2nd-line, respectively; plausible ranges = 50–100% of base values); the cost of adherence/retention interventions (base value = $155; plausible range = 50–200% of base value). We also consider alternative pathways leading to the UNAIDS virologic suppression target.
RESULTS
Cascade Results for the Two Strategies
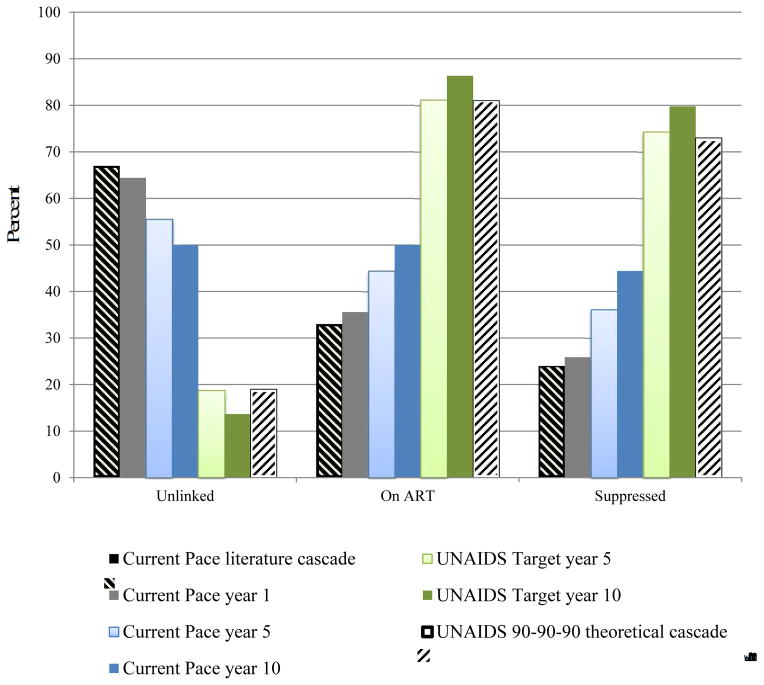
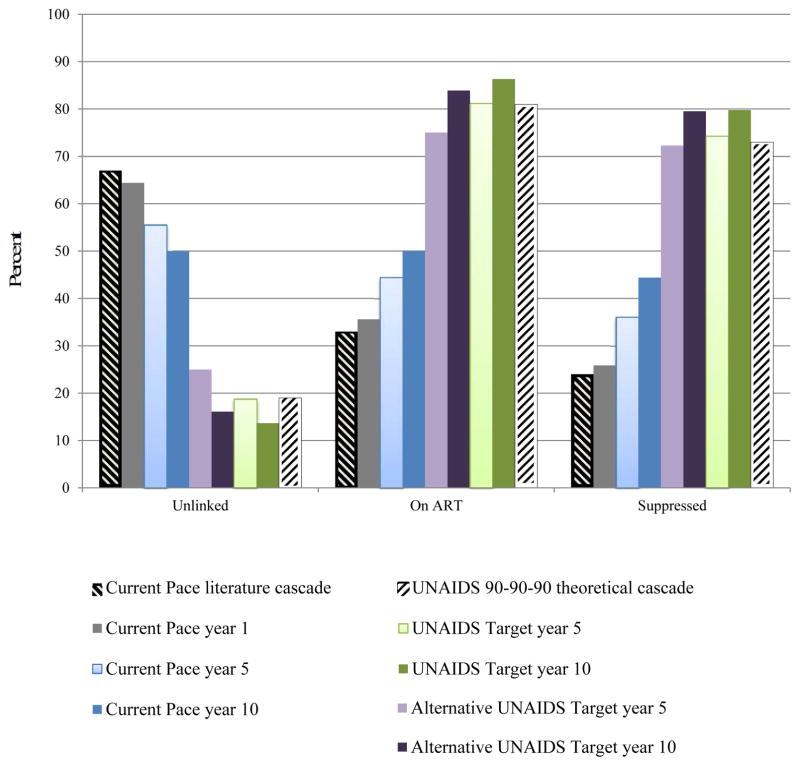
Our model predictions of the fraction of the population in each cascade stage at the end of year 1 under the Current Pace strategy are calibrated to published estimates and are within an absolute 3% at each stage (Figure 1, gray bars versus hatched black bars) (2). The Current Pace strategy results in gradually improved cascade outcomes; by year 10, 44% of living HIV-infected persons are suppressed (dark blue bars). The UNAIDS Target strategy achieves 81% diagnosis and 74% virologic suppression (light green bars) by year 5; by year 10, the suppression rate among living HIV-infected persons is 80% (dark green bars).
Figure 1. HIV treatment cascade by cascade strategy.
This figure demonstrates the results of the HIV treatment cascade over time, examining the percent of patients alive (vertical axis) who are unlinked (undiagnosed or previously linked and now lost-to-follow-up), on ART, or virologically suppressed (horizontal axis). Current Pace strategy: The hatched black bar on the left demonstrates literature-derived data on the current South Africa cascade of care. The grey bar represents model-based results after 1 year of the current cascade and demonstrates a near match to the black bar, except for some anticipated modest improvement over time, with slight decreases among those unlinked and concomitant increases in those suppressed. Bars in blue provide Current Pace results over 5-year (light blue) and 10-year horizons (dark blue) and further demonstrate modest improvements in the cascade. At 10-years, the Current Pace shows 44% of those alive are virologically suppressed. UNAIDS Target strategy: The hatched white bar on the far right demonstrates the aspirational UNAIDS Target strategy with 73% virologically suppressed. Model output demonstrates UNAIDS Target strategy results that might be achieved in 5 years (light green). The screening, linkage, adherence and retention parameters of the model were adjusted to force the light green bars to reach 90-90-90 target values; we were able to achieve 80% of patients alive with virologic suppression in 10 years. Importantly, the denominators (number alive) in the two strategies over time differ due to an increased number of transmissions and deaths in the Current Pace strategy.
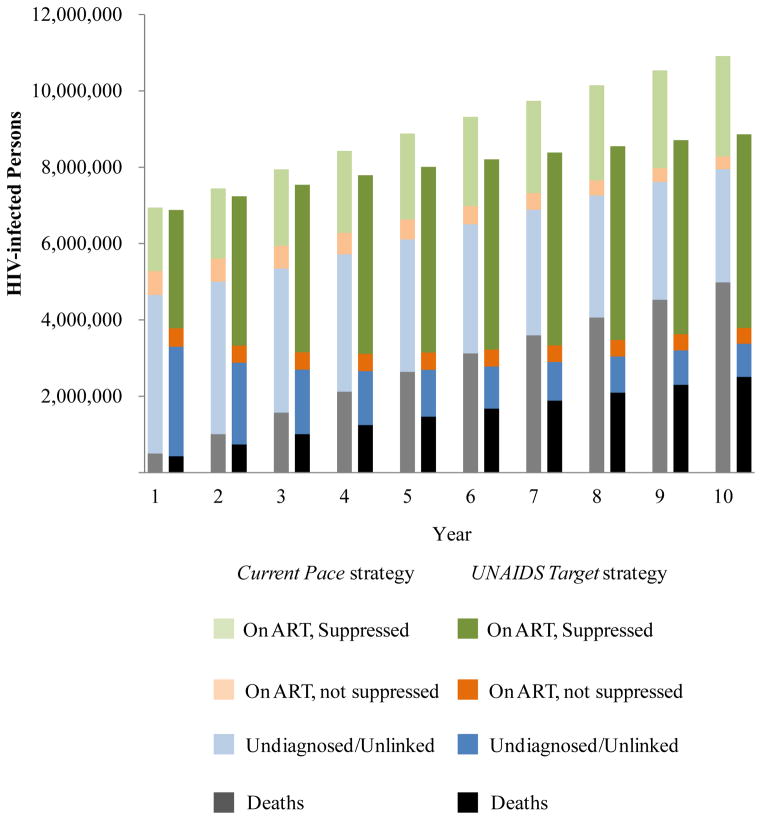
Taking into account incident cases and deaths over the next decade, we project under the Current Pace strategy, that 2.632 million South Africans will be suppressed on ART, and 2.963 million will remain undiagnosed or unlinked to care (Figure 2, light green and light blue). Under the UNAIDS Target strategy, we project more than 2 million fewer persons undiagnosed or not in care (870,000) and almost 2.5 million more on suppressive ART (5.070 million, Figure 2, dark blue and dark green).
Figure 2. Comparison of survival outcomes over time by cascade strategy.
This figure provides a side-by-side comparison of the cumulative outcomes over time between the Current Pace strategy (light bars) and the UNAIDS Target strategy (dark bars). The cumulative number of HIV-infected persons, including those who have died, is on the vertical axis. Black/gray shading indicate the number who have died; blue shading indicates the number who are undiagnosed and/or unlinked; orange shading indicates the number who are unsuppressed on ART; and green shading indicates the number who are suppressed on ART. The difference in the height of the bars in each year indicates the cumulative number of additional transmissions in the Current Pace strategy compared to the UNAIDS Target strategy.
HIV Transmissions
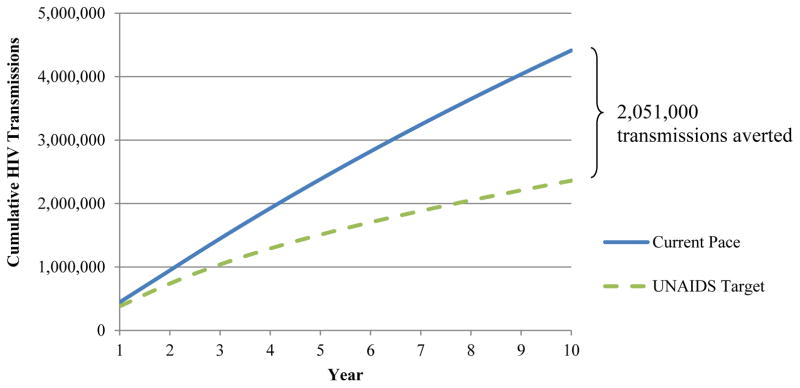
Under the Current Pace strategy, we project 450,000 HIV transmissions in year 1 (32), yielding an annual incidence of 1.80%. We validated this model-driven incidence by comparing it to the annual incidence among 15–49 year-olds, which the 2012 South Africa HIV National survey reports as 1.72% (22). Over ten years, we project 4.413 million new infections (Table 2; Appendix Figure 1, blue line). Under the UNAIDS Target strategy, 2.362 million new infections are projected over ten years (Table 2), suggesting 2.051 million transmissions averted (Appendix Figure 1, green line).
Table 2.
Clinical and economic outcomes of the Current Pace strategy and the UNAIDS Target strategy at five and ten years.*
| Total transmissions | Cumulative 5 year | Cumulative 10 year | ||
|---|---|---|---|---|
| Current Pace | 2,384,000 | 4,413,000 | ||
| UNAIDS Target | 1,511,000 | 2,362,000 | ||
|
| ||||
| Transmissions averted | 873,000 | 2,051,000 | ||
|
| ||||
| Cumulative deaths | Cumulative mortality 5 year | Cumulative mortality 10 year | ||
|
| ||||
| Current Pace | 2,639,000 | 4,985,000 | ||
| UNAIDS Target | 1,466,000 | 2,507,000 | ||
|
| ||||
| Deaths averted | 1,174,000† | 2,478,000 | ||
|
| ||||
| Years of life | Life years over 5 years | Life years over 10 years | ||
|
| ||||
| Current Pace | 31,673,000 | 61,209,000 | ||
| UNAIDS Target | 34,675,000 | 74,549,000 | ||
|
| ||||
| Years of life saved | 3,002,000 | 13,340,000 | ||
|
| ||||
| Cumulative orphans‡ | 0–5yo, 5 year | 0–5yo, 10 year | 0–18yo, 5 year | 0–18yo, 10 year |
|
| ||||
| Current Pace | 211,000 | 460,000 | 1,538,000 | 2,979,000 |
| UNAIDS Target | 105,000 | 184,000 | 812,000 | 1,290,000 |
|
| ||||
| Orphans averted | 106,000 | 276,000 | 726,000 | 1,689,000 |
|
| ||||
| Total costs ($ million)§ | 5 year | 10 year | ||
|
| ||||
| Current Pace | $18,674 | $38,353 | ||
| UNAIDS Target | $26,639 | $54,332 | ||
|
| ||||
| UNAIDS Target additional cost | $7,965 | $15,979 | ||
|
| ||||
| ICER ($/YLS)§ | 5 year | 10 year | ||
|
| ||||
| UNAIDS Target | $2,720/YLS | $1,260/YLS | ||
Abbreviations: ICER, incremental cost-effectiveness ratio; YLS, years of life saved; yo, years old
For each set of results, the third row highlights the difference between UNAIDS Target and the Current Pace strategies
Subtraction is done prior to rounding, which accounts for any discrepancy.
We define maternal orphans as children <18 years (216 months) whose mother has died from HIV/AIDS. We also report results for particularly vulnerable orphans <5 years (60 months) of age.
Clinical outcomes and total costs are reported undiscounted; ICERs are calculated as Δ$/ΔYLS of UNAIDS Target strategy compared to Current Pace strategy. Discounted values at 3% per year are used for incremental cost-effectiveness ratios.
Deaths and Life-Years Saved
Over the next ten years and inclusive of each year’s newly transmitted cases, the Current Pace and the UNAIDS Target strategies will result in 4.985 million and 2.507 million cumulative deaths among HIV-infected persons. Over 5- and 10-year horizons, the UNAIDS Target strategy will result in 3.002 million and 13.340 million additional years of life saved (YLS), compared to the Current Pace strategy (Table 2).
Maternal Orphans
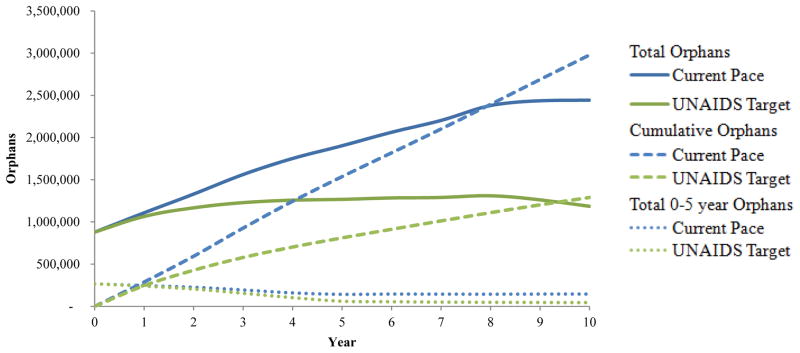
In 2013, there were an estimated 900,000 living maternal orphans due to HIV/AIDS in South Africa (19, 33). Under the Current Pace strategy, 2.979 million more children will be orphaned due to HIV infection within 10 years; in 2026, accounting for orphan mortality and “aging out,” we project 2.443 million living maternal orphans. In contrast, reaching the UNAIDS Target strategy, we project a cumulative 1.290 million children orphaned with 1.185 million living orphans in 10 years, a cumulative difference of 1.689 million orphans (Table 2, Figure 3).
Figure 3. Outcomes related to maternal orphans by cascade strategy.
The vertical axis represents the number of maternal orphans (defined as children <18 years old whose mother has died of HIV/AIDS) over time (horizontal axis). Results from the Current Pace strategy are indicated by blue lines and results of the UNAIDS Target strategy are indicated by green lines. Cumulative orphans are denoted by the dashed lines, which demonstrate the cumulative number of children ever orphaned by HIV/AIDS during the time horizon of the analysis (see Methods). Solid lines indicate the projected number of current orphans in a given year, accounting for both death among orphans after orphanhood and for aging out of being considered an orphan. The kinks in the solid lines at year ~8 demonstrate the aging out of a large majority of prevalent orphans aged 9–14 years old at model initiation. The dotted lines indicate the number of living orphans <5 years old.
Costs and Cost-effectiveness
Model output demonstrates an average annual cost of $930 for patients in care and on ART, consistent with annual patient costs previously reported in South Africa (34). Total 5- and 10-year costs for the Current Pace strategy are $18.674 billion and $38.353 billion, of which $1.986 billion (11%) and $4.415 billion (12%) are ART costs (Table 2). Total 5- and 10-year costs for the UNAIDS Target strategy are $26.639 billion and $54.332 billion, of which $3.409 billion (13%) and $8.032 billion (15%) are ART costs, $1.182 billion (4%) and $2.045 billion (4%) are testing costs, and $3.604 billion (14%) and $7.858 billion (14%) are adherence/retention intervention costs. The incremental cost-effectiveness ratio (ICER) of the UNAIDS Target compared to the Current Pace strategy (Δcosts/ΔYLS) is $2,720/YLS and $1,260/YLS over 5- and 10-years. These ICERs represent 42% and 19% of the South African per capita GDP ($6,500) (28), suggesting that the UNAIDS Target strategy is very cost-effective by international standards in this South African setting (35).
Sensitivity Analyses
Changing the duration of acute HIV infection from 6 months to 12 (3) months resulted in larger (smaller) incremental benefits from the UNAIDS Target strategy in terms of cumulative deaths, years of life saved, and orphans averted. Most variations in these sensitivity analyses remained <20% from the base case (Appendix Table 1).
With lower testing and linkage rates but increased ART adherence in the UNAIDS Target strategy, we achieved 72% virologic suppression at year 5 via an alternative pathway (Appendix Figure 2, light purple bars). This alternative UNAIDS Target strategy nearly reached the 73% suppression endpoint but failed to meet the interim targets of 90% diagnosed and 90% on ART, resulting in less favorable UNAIDS Target outcomes due to the decreased proportion of persons receiving ART and HIV care (Appendix Table 1).
With a halving of testing/linkage efficiency (only 25% of HIV-infected patients link to care each time they test positive) and a doubling of per-person adherence/retention costs, the UNAIDS Target strategy remained both very expensive ($31.445 billion, $64.370 billion), and also very cost-effective ($4,330/YLS, $2,040/YLS) at 5 and 10 years. Conversely, if testing/linkage efficiency reached 90% and adherence/retention intervention and ART costs were both halved, the 5- and 10-year costs of the UNAIDS Target strategy would be $21.487 billion and $42.726 billion, and the strategy would be substantially more cost-effective ($1,490/YLS, $600/YLS, Appendix Table 2).
CONCLUSIONS
UNAIDS is right to label the 90-90-90 initiative “a momentous opportunity” to “end the AIDS epidemic.” (1) We considered a wide range of clinical, epidemiological, and social performance standards by which policymakers and donors might measure return on investment. By every one of these measures, achieving the UNAIDS Target strategy could have a transformative impact: averting millions of new HIV infections; saving millions of lives and tens of millions of years of life; preventing millions of children from becoming orphans; and thereby curtailing the global pandemic. And while it will not be easy and it certainly will not be inexpensive, we also find that it will be highly cost-effective and well worth trying.
It will not be easy. Our analysis suggests that the 90-90-90 performance targets can only be attained if every step in the continuum leading from HIV detection to durable viral suppression can be made to function sustainably at “best practice” levels. For example, we find that 90% rates of diagnosis and linkage will require aggressive HIV screening, likely as often as once every two years, across the South African population. Further, achieving 73% overall virologic suppression will require individual 48-week ART suppression rates of ~87% accompanied by 5-year retention rates of nearly 90%. These performance levels are as good as those reported in the most rigorous of US-based and international clinical trials, which generally bias enrollment to the most adherent (36). While we emphasize that these are ambitious performance targets, they are plausible: emerging evidence suggests that best practices can be implemented and sustained, both in resource-rich and resource-constrained settings. For example, very high virologic suppression rates at the population level have already been reported from Australia (62%), United Kingdom (58%), and the higher-burdened Botswana (70%) (37, 38).
It will not be inexpensive. We estimate a cost in South Africa of $54 billion over the next 10 years, an increase of $16 billion over current HIV detection and treatment efforts. To place this in perspective, the 2014–2015 South Africa National Strategic Plan proposed $2.8 billion needed in 2014–2015 for combined efforts in HIV, tuberculosis and sexually transmitted infection (STI) prevention and treatment (39). This would require a 14% annual budget increase over ten years ($54 billion total) for the required investment to be met. However, this analysis also highlights that such investments would yield extraordinary returns. Scale-up would be as cost-effective as ART itself (5, 26, 40, 41), and may be even more cost-effective in settings with higher HIV prevalence, especially among the young. This compelling cost-effectiveness case holds even though our analysis excludes both the social and economic value of keeping mothers alive and the productivity costs saved by curbing the HIV epidemic over time. In short, we find that while reaching the UNAIDS Target strategy exceeds current budgetary allocations, it would be a very cost-effective proposition.
Like any model-based exploration of a yet-to-be-implemented intervention, our analysis is limited by uncertainties in the input data and by some inevitable simplifications in our portrayal of the system. Key uncertainties and limitations include: omission of alternative plausible pathways that might lead to 73% virologic suppression; use of a single parameter to characterize phenomena that may vary by gender (e.g. differences in rates of testing or transmission); exclusion of orphans’ outcomes in the calculation of deaths and years of life saved; exclusion of any increased non-HIV medical clinical outcomes and costs (related to, for example, cardiovascular disease) resulting from reduced transmission and prolonged survival; and exclusion of other social and economic effects (e.g., labor force participation, economic productivity, financial protection and impoverishment) as outcomes of interest. Additionally, we highlight that this analysis is specific to both the South African setting and time in the epidemic. While we have made every effort to report these uncertainties transparently and to bias the analysis whenever possible against the cost-effectiveness conclusions, it is the striking robustness of our findings (+/−20%) in the face of the many uncertainties that is notable.
We find that if the 90-90-90 targets for HIV diagnosis, on ART, and virologic suppression can each be achieved in South Africa, enormous population and clinical benefits will follow. Broad variation in our key input parameters across their plausible ranges produced relatively small swings in overall outcomes and did little to undermine the overall conclusion that 90-90-90 can work, that it offers decision makers and donors a superb return on investment, and that there is nothing overstated about the UNAIDS suggestion that it could “lay the foundation for a healthier, more just and equitable world for future generations.” (1)
Acknowledgments
Financial Support: This research was funded by the National Institutes of Health (R01 AI058736, R37 AI093269, R01 HD079214, K01 HL123349, R01 MH105203, R01 DA015612) and by the Steve and Deborah Gorlin MGH Research Scholars Award (Executive Committee on Research to RPW).
Role of Funding Source: This research was funded by the National Institutes of Health (R01 AI058736, R37 AI093269, R01 HD079214, K01 HL123349, R01 MH105203, R01 DA015612) and by the Steve and Deborah Gorlin MGH Research Scholars Award (Executive Committee on Research to RPW). The funding sources had no role in the design, analysis, or interpretation of the study or in the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Walensky had access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Appendix
Appendix Table 1.
Sensitivity of clinical outcomes to variation in key input parameters in a modeling analysis of 90-90-90 in South Africa.
| Strategy | Total Transmissions | Cumulative Deaths | Years of life | 0–5, 5year |
Cumulative Orphans | Total,
10 year |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 year | 10 year | 5 year | 10 year | 5 year | 10 year | 0–5, 10 year |
Total, 5 year |
|||
| Basecase | ||||||||||
|
| ||||||||||
| Current Pace | 2,384,000 | 4,413,000 | 2,639,000 | 4,985,000 | 31,673,000 | 61,209,000 | 211,000 | 460,000 | 1,538,000 | 2,979,000 |
| UNAIDS Target | 1,511,000 | 2,362,000 | 1,466,000 | 2,507,000 | 34,675,000 | 74,549,000 | 105,000 | 184,000 | 812,000 | 1,290,000 |
| Difference | 873,000 | 2,051,000 | 1,174,000 | 2,478,000 | 3,002,000 | 13,340,000 | 106,000 | 276,000 | 726,000 | 1,689,000 |
|
| ||||||||||
| Duration of acute infection, 3 months | ||||||||||
|
| ||||||||||
| Current Pace | 2,010,000 | 3,474,000 | 2,618,000 | 4,799,000 | 30,796,000 | 57,518,000 | 207,000 | 433,000 | 1,522,000 | 2,831,000 |
| UNAIDS Target | 1,254,000 | 1,880,000 | 1,453,000 | 2,455,000 | 33,800,000 | 70,394,000 | 103,000 | 176,000 | 802,000 | 1,249,000 |
| Difference | 756,000 | 1,594,000 | 1,165,000 | 2,344,000 | 3,004,000 | 12,876,000 | 104,000 | 257,000 | 720,000 | 1,582,000 |
|
| ||||||||||
| Duration of acute infection, 12 months | ||||||||||
|
| ||||||||||
| Current Pace | 3,547,000 | 8,187,000 | 2,700,000 | 5,598,000 | 34,235,000 | 74,362,000 | 219,000 | 547,000 | 1,585,000 | 3,469,000 |
| UNAIDS Target | 2,353,000 | 4,391,000 | 1,501,000 | 2,696,000 | 37,317,000 | 89,402,000 | 110,000 | 210,000 | 839,000 | 1,440,000 |
| Difference | 1,194,000 | 3,796,000 | 1,199,000 | 2,902,000 | 3,082,000 | 15,040,000 | 109,000 | 337,000 | 746,000 | 2,029,000 |
|
| ||||||||||
| Alternative 90-90-90 Cascade, 75% on ART, 72% virologically suppressed | ||||||||||
|
| ||||||||||
| Alternative Target | 1,559,000 | 2,438,000 | 1,685,000 | 2,705,000 | 33,998,000 | 72,780,000 | 125,000 | 208,000 | 953,000 | 1,453,000 |
| Difference | 825,000 | 1,975,000 | 954,000 | 2,280,000 | 2,325,000 | 11,571,000 | 86,000 | 252,000 | 585,000 | 1,526,000 |
Red numbers indicate changes for UNAIDS Target strategy that are smaller than in the base case (worse differences in outcomes); Blue numbers indicate changes for the UNAIDS Target strategy that are greater than in the base case (better differences in outcomes). Bolded numbers indicate instances where the values differ from the base case by >20%.
Appendix Table 2.
Sensitivity of economic outcomes to variation in key input parameters in a modeling analysis of 90-90-90 in South Africa.*
| Basecase | 5 yr | 10 yr |
|---|---|---|
| Current Pace total cost | $18,674 | $38,353 |
| UNAIDS Target total cost | $26,639 | $54,332 |
|
| ||
| UNAIDS Target additional cost | $7,965 | $15,979 |
|
| ||
| ICER ($/YLS) | $2,720 | $1,260 |
|
| ||
| Testing/linkage efficiency (25%), Adherence/retention intervention cost (200%) | 5 yr | 10 yr |
|
| ||
| Current Pace total cost | $18,674 | $38,353 |
| UNAIDS Target total cost | $31,445 | $64,370 |
|
| ||
| UNAIDS Target additional cost | $12,771 | $26,016 |
|
| ||
| ICER ($/YLS) | $4,330 | $2,040 |
|
| ||
| Testing/linkage efficiency (90%), Adherence/retention intervention cost (50%) | 5 yr | 10 yr |
|
| ||
| Current Pace total cost | $18,674 | $38,353 |
| UNAIDS Target total cost | $24,343 | $49,533 |
|
| ||
| UNAIDS Target additional cost | $5,668 | $11,180 |
|
| ||
| ICER ($/YLS) | $1,940 | $890 |
|
| ||
| ART costs (50%) | 5 yr | 10 yr |
|
| ||
| Current Pace total cost | $17,002 | $34,579 |
| UNAIDS Target total cost | $23,784 | $47,525 |
|
| ||
| UNAIDS Target additional cost | $6,782 | $12,947 |
|
| ||
| ICER ($/YLS) | $2,270 | $980 |
|
| ||
| ART costs (50%), testing/linkage efficiency (90%), Adherence/retention intervention cost (50%) | 5 yr | 10 yr |
|
| ||
| Current Pace total cost | $17,002 | $34,579 |
| UNAIDS Target total cost | $21,487 | $42,726 |
|
| ||
| UNAIDS Target additional cost | $4,485 | $8,147 |
|
| ||
| ICER ($/YLS) | $1,490 | $600 |
Abbreviations: ICER, incremental cost-effectiveness ratio; YLS, years of life saved
All costs are reported in 2014 USD. Total costs are reported undiscounted, in millions; ICERs are calculated as Δ$/ΔYLS of UNAIDS Target strategy compared to Current Pace strategy. Discounted values at 3% per year are used for incremental cost-effectiveness ratios.
Appendix Figure 1. Cumulative HIV transmissions.
The cumulative number of HIV transmissions (vertical axis) over the 10-year modeled horizon (horizontal axis). Results for the Current Pace strategy are shown by the blue solid curve and for the UNAIDS Target strategy are shown by the green dashed curve. By the end of 2025, the UNAIDS Target strategy results in 2.051 million fewer transmission events.
Appendix Figure 2. HIV treatment cascade by cascade strategy; sensitivity analysis of an alternative way of reaching virologic suppression goals for the UNAIDS Target strategy.
This demonstrates results of the HIV treatment cascade over time, examining the proportion of patients alive (vertical axis) who are unlinked, on ART, or virologically suppressed (horizontal axis). Current Pace strategy: The hatched black bar on the left demonstrates literature-based data to inform the current South Africa cascade of care. The grey bar represents model-based results after 1 year of the current cascade and demonstrates a near match to the black bar, except for some anticipated modest improvement over time, with slight decreases among those unlinked with concomitant increases in those suppressed. Bars in blue provide current cascade results over 5-year (light blue) and 10-year (dark blue) and further demonstrate modest improvements in the cascade. At 10 years, the current cascade shows 44% of those alive are virologically suppressed. UNAIDS Target strategy and Alternative UNAIDS Target strategy. This figure utilizes alternative input parameters (lower testing and linkage rates and higher rates of virologic suppression), compared to the UNAIDS Target strategy base case to achieve viral suppression goals in the Alternative UNAIDS Target strategy, without 81% of persons on ART. The hatched white bar on the far right demonstrates the aspirational 90-90-90 cascade with 73% virologically suppressed. Model output demonstrates 90-90-90 basecase results that might be achieved in 5 and 10 years (in light and dark green) and cascade states at 5 and 10 years in the Alternative UNAIDS Target strategy (light and dark purple). While the UNAIDS Target strategy and the Alternative UNAIDS Target strategy have similar viral suppression rates (~73%), they differ in the proportion of persons on ART (81% in the UNAIDS Target strategy and 75% in the Alternative UNAIDS Target strategy). The denominators (number alive) in these three strategies over time differ as a result of differences in the number of transmissions and deaths.
References
- 1.90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2014. [Accessed March 30, 2016]. at http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf. [Google Scholar]
- 2.Kranzer K, Lawn SD, Johnson LF, Bekker L-G, Wood R. Community viral load and CD4 count distribution among people living with HIV in a South African township: implications for treatment as prevention. J Acquir Immune Defic Syndr. 2013;63(4):498–505. doi: 10.1097/QAI.0b013e318293ae48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piot P, Abdool Karim SS, Hecht R, Legido-Quigley H, Buse K, Stover J, et al. Defeating AIDS—advancing global health. Lancet. 2015;386(9989):171–218. doi: 10.1016/S0140-6736(15)60658-4. [DOI] [PubMed] [Google Scholar]
- 4.Long L, Fox M, Sanne I, Rosen S. The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. AIDS. 2010;24(6):915–9. doi: 10.1097/QAD.0b013e3283360976. [DOI] [PubMed] [Google Scholar]
- 5.Walensky RP, Ross EL, Kumarasamy N, Wood R, Noubary F, Paltiel AD, et al. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. N Engl J Med. 2013;369(18):1715–25. doi: 10.1056/NEJMsa1214720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walensky RP, Paltiel AD, Losina E, Morris BL, Scott CA, Rhode ER, et al. Test and treat DC: forecasting the impact of a comprehensive HIV strategy in Washington DC. Clin Infect Dis. 2010;51(4):392–400. doi: 10.1086/655130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mossong J, Grapsa E, Tanser F, Bärnighausen T, Newell M-L. Modelling HIV incidence and survival from age-specific seroprevalence after antiretroviral treatment scale-up in rural South Africa. AIDS. 2013;27(15):2471. doi: 10.1097/01.aids.0000432475.14992.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis. 2015;60(7):1120–7. doi: 10.1093/cid/ciu1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barth RE, van der Loeff MFS, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2010;10(3):155–66. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 11.South African National AIDS Council. The South African Antiretroviral Treatment Guidelines. Pretoria, South Africa: Department of Health for the Republic of South Africa; 2010. [Google Scholar]
- 12.Hyle EP, Jani IV, Lehe J, Su AE, Wood R, Quevedo J, et al. The clinical and economic impact of point-of-care CD4 testing in Mozambique and other resource-limited settings: a cost-effectiveness analysis. PLoS Med. 2014;11(9):e1001725. doi: 10.1371/journal.pmed.1001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15(s1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinkhof M, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4(6):e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23(11):1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 16.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 17.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19(18):2109–16. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 18.Novitsky V, Essex M. Using HIV viral load to guide treatment-for-prevention interventions. Curr Opin HIV AIDS. 2012;7(2):117–24. doi: 10.1097/COH.0b013e32834fe8ff. [DOI] [PubMed] [Google Scholar]
- 19.Hall K, Meintjes H. Statistics on children in South Africa: demography - orphanhood. Cape Town, South Africa: Children Count; 2015. [Accessed March 30, 2016]. at http://www.childrencount.ci.org.za. [Google Scholar]
- 20.World Fertility Data 2012. Geneva, Switzerland: United Nations, Department of Economic and Social Affairs, Population Division; 2013. [Accessed March 30, 2016]. at http://www.un.org/esa/population/publications/WFD2012/MainFrame.html. [Google Scholar]
- 21.Census 2011: fertility in South Africa. Pretoria, South Africa: Statistics South Africa; 2015. [Accessed March 30, 2016]. at http://www.statssa.gov.za/publications/Report-03-01-63/Report-03-01-632011.pdf. [Google Scholar]
- 22.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, Labadarios D, Onoya D, et al. South African national HIV prevalence, incidence and behaviour survey, 2012. Cape Town, South Africa: HSRV Press; 2014. [Accessed March 30, 2016]. at http://www.hsrc.ac.za/uploads/pageContent/4565/SABSSM%20IV%20LEO%20final.pdf. [DOI] [PubMed] [Google Scholar]
- 23.Chen W-J, Walker N. Fertility of HIV-infected women: insights from Demographic and Health Surveys. Sex Transm Infect. 2010;86(Suppl 2):ii22–ii7. doi: 10.1136/sti.2010.043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortality and causes of death in South Africa, 2014: findings from death notification. Pretoria, South Africa: Statistics South Africa; 2015. [Accessed March 30, 2016]. at http://www.statssa.gov.za/publications/P03093/P030932014.pdf. [Google Scholar]
- 25.ARV ceiling price list: 2014. Boston, MA: The Clinton Health Access Initiative (CHAI); 2015. [Accessed March 30, 2016]. at http://www.clintonhealthaccess.org/chai-arv-ceiling-price-list-2014/ [Google Scholar]
- 26.Cleary S, Boulle A, McIntyre D, Coetzee D. Cost-effectiveness of antiretroviral treatment for HIV-positive adults in a South African township. Durban, South Africa: Health Systems Trust; 2004. [Google Scholar]
- 27.Anglaret X, Chêne G, Attia A, Toure S, Lafont S, Combe P, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d’Ivoire: a randomised trial. Lancet. 1999;353(9163):1463–8. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 28.World Development Indicators. Washington, D.C: World Bank; [Accessed March 30, 2016]. at http://data.worldbank.org/) [Google Scholar]
- 29.Historical Currency Converter. [Accessed 29 April 2016];Oanda Solutions for Business. at https://www.oanda.com/solutions-for-business/historical-rates-beta/hcc.html.
- 30.aids2031 Costs and Financing Working Group. The long run costs and financing of HIV/AIDS in South Africa. Washington DC: Results for Development Institute; 2010. [Accessed March 30, 2016]. at http://www.resultsfordevelopment.org/sites/resultsfordevelopment.org/files/aids2031%20South%20Africa%20Report_Published%202010.pdf. [Google Scholar]
- 31.Smith JA, Sharma M, Levin C, Baeten JM, van Rooyen H, Celum C, et al. Cost-effectiveness of community-based strategies to strengthen the continuum of HIV care in rural South Africa: a health economic modelling analysis. Lancet HIV. 2015;2(4):e159–e68. doi: 10.1016/S2352-3018(15)00016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.AIDSinfo. Geneva, Switzerland: UNAIDS; [Accessed March 30, 2016]. at http://aidsinfo.unaids.org/ [Google Scholar]
- 33.UNAIDS. [Accessed April 28, 2016];HIV and AIDS estimates South Africa, 2014. at http://www.unaids.org/en/regionscountries/countries/southafrica.
- 34.Meyer-Rath G, Miners A, Santos AC, Variava E, Venter WD. Cost and resource use of patients on antiretroviral therapy in the urban and semiurban public sectors of South Africa. J Acquir Immune Defic Syndr. 2012;61(3):e25–32. doi: 10.1097/QAI.0b013e31826cc575. [DOI] [PubMed] [Google Scholar]
- 35.Ochalek J, Lomas J, Claxton K. Cost per DALY averted thresholds for low- and middle- income countries: evidence from cross country data. York, Great Britain: Centre for Health Economics, University of York; 2015. [Accessed March 30, 2016]. at https://www.york.ac.uk/che/news/2015/che-research-paper-122/ [Google Scholar]
- 36.Sax PE, Wohl D, Yin MT, Post F, DeJesus E, Saag M, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–15. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- 37.Gaolathe T, Wirth KE, Holme MP, Makhema J, Moyo S, Chakalisa U, et al. Botswana’s progress toward achieving the 2020 UNAIDS 90-90-90 antiretroviral therapy and virological suppression goals: a population-based survey. Lancet HIV. 2016;2(5):e221–e230. doi: 10.1016/S2352-3018(16)00037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raymond A, Hill A, Pozniak A. Large disparities in HIV treatment cascades between eight European and high-income countries–analysis of break points. [Accessed March 30, 2016];J Int AIDS Soc. 2014 doi: 10.7448/IAS.17.4.19507. at http://www.jiasociety.org/jias/index.php/jias/article/view/19507. [DOI] [PMC free article] [PubMed]
- 39.Cohen S, Guthrie T. Financing the South African national strategic plan for HIV, STIs, and TB 2012–2016: an analysis of fundings, funding gaps, and financial considerations. Pretoria, South Africa: SANAC Costing Technical Team & Strategic Development Consultants; 2014. [Google Scholar]
- 40.Braithwaite RS, Tsevat J. Is antiretroviral therapy cost-effective in South Africa? PLoS Med. 2006;3(1):e60. doi: 10.1371/journal.pmed.0030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walensky RP, Wolf LL, Wood R, Fofana MO, Freedberg KA, Martinson NA, et al. When to start antiretroviral therapy in resource-limited settings. Ann Intern Med. 2009;151(3):157–66. doi: 10.7326/0003-4819-151-3-200908040-00138. [DOI] [PMC free article] [PubMed] [Google Scholar]