Abstract
The cough reflex is evoked by noxious stimuli in the airways. Although this reflex is essential for health, it can be triggered chronically in inflammatory and infectious airway disease. Neuronal transient receptor potential (TRP) channels such as ankyrin 1 (TRPA1) and vanilloid 1 (TRPV1) are polymodal receptors expressed on airway nociceptive afferent nerves. Reactive oxygen species (ROS) and other reactive compounds are associated with inflammation, from either NADPH oxidase or mitochondria. These reactive compounds cause activation and hyperexcitability of nociceptive afferents innervating the airways, and evidence suggests key contributions of TRPA1 and TRPV1.
Keywords: Sensory nerve, afferent, airway, lung, cough, irritant, reactive oxygen species, ROS, electrophiles, TRPA1, TRPV1, mitochondria, H2O2
Graphical abstract
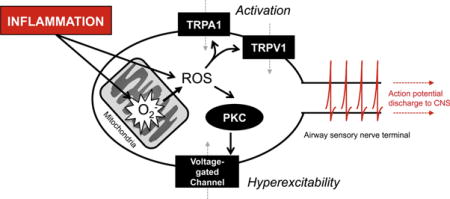
Introduction
The cough reflex serves to clear the airways of obstruction, thus protecting the airways during breathing. The cough reflex is triggered by stimulation of afferent (sensory) nerve terminals within the airways, resulting in the central co-ordination of motor fibers innervating the glottis, chest wall and diaphragm. Appropriate activation of the cough reflex is protective, but inappropriate and persistent activation of cough is a serious health burden. Cough is the most common reason for seeking medical attention, with it accounting for ~40% of visits to primary health care physicians [1]. Present treatments for cough are largely ineffective [2–5]. Cough is clinical associated with infectious and inflammatory diseases [2, 4, 6, 7], which likely are the source of neuroactive agents that cause excessive/inappropriate activation of airway afferent nerves. Recent evidence suggests that oxidative stress can evoke profound effects on airway afferent nerves, particularly through the modulation of neuronal transient receptor potential (TRP) channels. Although mammals share similarities in their respiratory physiology and airway afferent pharmacology, the cough reflex is only reliably present in guinea pigs and larger mammals [8–10]. Rats and mice do not possess a recognizable cough reflex, although these mammals have proved useful in elucidating aspects of airway afferent neurophysiology.
Sensory afferent nerves involved in cough
The airways (larynx, trachea, bronchi, bronchioles and alveoli) are densely innervated by sensory/afferent fibers, whose cell bodies reside in the vagal ganglia (nodose and jugular) [11–13]. These nerves synapse with brainstem neurons in the medulla, and modulate breathing rhythms, autonomic function and defensive airway reflexes [14–17]. Vagal airway afferents are heterogeneous with respect to embryological source, anatomical distribution, structure, myelination, protein expression, connectivity and function. Most vagal neurons reside in the vagal nodose ganglia and are embryologically derived from the placodes; the remaining neurons reside in the vagal jugular ganglia and are derived from the neural crest [18, 19]. Nodose and jugular neurons are under differential neurotrophic control [20], which impacts their gene expression and function [21–23].
The vast majority of vagal airway afferents are unmyelinated C fibers that innervate the epithelial layer [10, 13, 24, 25]. These nerves are sensitive to a wide-range of noxious stimuli (e.g. heat, cold, non-isotonicity, low pH (<6), irritants and inflammatory mediators) and are often termed as ‘nociceptors’ [22, 26–30]. Airway nociceptive C fibers are synonymous with C fibers projected from the dorsal root ganglia (DRG) that innervate the skin and viscera and initiate painful sensations when activated by noxious stimuli. Nevertheless, activation of airway C fibers does not lead to pain; rather it can evoke dyspnea, cough, apnea, tachypnea, bronchospasm and bradycardia [24]. Airway C fibers are projected from both nodose and jugular ganglia [21–23]. Airway C fibers overwhelmingly express the capsaicin-sensitive TRP vanilloid 1 (TRPV1) channel [30–32], the cinnamaldehyde-sensitive TRP ankyrin 1 (TRPA1) channel (Fig. 1) [33, 34] and metabotropic receptors for bradykinin [22, 35] and trypsin [36]. Few airway vagal C fibers express the menthol-sensitive TRP melastatin 8 (TRPM8) channel [33], although its expression is more common in the larynx and nasal airways [37, 38]. Nodose C fibers also express P2X2/3 channels, whereas jugular C fibers express peptide neurotransmitters such as substance P [21–23].
Figure 1.
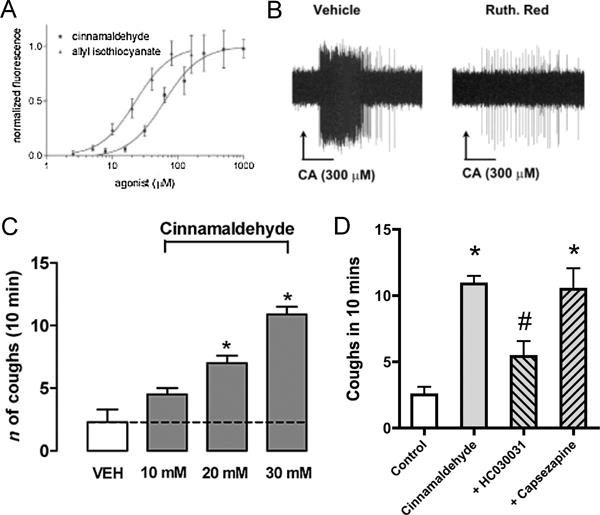
Cinnamaldehyde, the TRPA1 agonist, activates airway C fibers and causes cough. A, Dose-response curve of cinnamaldehyde and allyl isothiocyanate on mouse TRPA1-expressing CHO cells using FLIPR. Each datapoint represents an average of four to eight independent readings. Taken from [76]. B, Representative action potential discharge in mouse bronchopulmonary C fiber (recorded ex vivo) in response to cinnamaldehyde (CA) during perfusion with vehicle and after 15 min pretreatment with the TRP channel blocker ruthenium red (30 μM). Taken from [33]. C and D, Mean ± SEM coughs evoked by aerosolized cinnamaldehyde-induced cough in guinea pigs. C, Dose-response relationship. D, Effect of aerosolized TRPA1 inhibitor HC-030031 (HC, 0.3 mM) or TRPV1 inhibitor capsazepine (CPZ, 10 μM) on cough induced by cinnamaldehyde (10 mM) in guinea pig. *P < 0.05, significant difference versus vehicle (VEH, 5% ethanol and 3.5% Tween-80 in isotonic saline). # P < 0.05, significant difference to cinnamaldehyde without inhibitor. Taken from [103].
Stimulation of jugular C fibers causes cough in conscious animals, but not under ketamine anesthesia [39–44]. The most common tussive stimuli studied are capsaicin, citric acid and bradykinin. Capsaicin and extracellular acidification are direct stimulators of TRPV1 [32, 45] and activation of bradykinin B2 receptors has been shown to gate TRPV1 channels via second messenger systems [35, 46, 47], thus inhibition of TRPV1 reduces cough by these agents. In humans, inhalation of C fiber stimulants evokes cough and ‘urge-to-cough’ sensations [48, 49]. However, selective stimulation of nodose C fibers fails to evoke cough [44]. The mechanisms underlying this disparity are unknown, although recent evidence suggests that jugular and nodose C fibers modulate distinct brainstem networks [50, 51].
The airways are also innervated by myelinated afferents that are typically sensitive to mechanical forces but insensitive to noxious stimuli. In the lungs, heavily myelinated afferents (Aβ fibers) detect mechanical changes induced by breathing and feedback to brainstem circuits that control breathing [52, 53]. In the larynx, trachea and main bronchi, there is a subtype of partially myelinated nodose afferents (Aδ fibers) that innervate the smooth muscle layer and that are exquisitely sensitive to punctate mechanical force, low pH (<6.5) and hypotonicity [54, 55]. Activation of these nodose Aδ fibers evokes cough [39, 56], which is not abolished by ketamine anesthesia. Nodose Aδ fibers do not express TRPV1 or TRPA1 and are not sensitive to temperature, irritants and inflammatory mediators [54, 55, 57]. The receptor responsible for acid sensitivity in nodose Aδ fibers is unknown but is possibly a member of the Acid Sensing Ion Channel (ASIC) family.
Thus cough can be evoked by activation of two distinct vagal afferent groups innervating the airways: jugular C fibers and nodose Aδ fibers. Based upon their activation profile it is likely that Aδ fiber cough is evoked by aspiration and C fiber cough is evoked by endogenous inflammation/infection and by inhalation of irritants. Nevertheless, functional evidence suggests that these distinct pathways converge within the brainstem. Activation of airway C fibers augments Aδ fiber-evoked cough [58, 59]. Furthermore there is evidence that activation of TRPM8-expressing afferents innervating the larger airways can reduce the cough reflex [37, 38].
Sensitivity of airway afferent nerves to ROS
There are no published reports of the effect of H2O2 (or other ROS) on the cough reflex in vivo. Nevertheless, inhalation of H2O2 caused canonical reflex changes in respiratory rate in mice [60] and rats [61] consistent with reports that H2O2 activates airway nociceptive C fibers [62–64]. Furthermore, H2O2 also augmented changes in respiratory rate evoked by other noxious stimuli [65], consistent with data demonstrating that H2O2 causes hyperexcitability in airway nociceptive C fibers [64, 65]. Overall, the available data suggests that ROS have the potential to profoundly increase the activity of airway C fibers associated with cough.
ROS have the capacity to evoke these acute effects via the direct and indirect modulation of a wide range of cellular components, including ligand-gated ion channels, voltage-gated ion channels, organelle ion channels, transporters, enzymes, signaling molecules and lipids [66–73]. As such, H2O2-induced activation and hyperexcitability are likely complex interactions of multiple pathways, most of which have not been studied in airway nociceptive neurons. Some of these ROS-mediated effects may oppose others. For example oxidation of KCNQ channels, which are widely expressed in vagal sensory neurons [74], causes increased ‘M-currents’, which would be expected to decrease neuronal excitability [70]. Here, I shall focus on those mechanisms that have been directly studied in airway nociceptive nerves.
Transient Receptor Potential Ankyrin 1 (TRPA1)
In the airways, TRPA1 is a non-selective cation channel that is expressed on the majority of nociceptive C fibers [33, 34]. Activation of TRPA1 leads to neuronal depolarization and action potential discharge in the afferent fiber. TRPA1 is a polymodal channel that is directly stimulated by a wide range of stimuli: including noxious cold, cysteine-modifying reactive electrophiles, intracellular Ca2+, 2-APB, thymol, menthol and d9-tetrahydrocannabinol [75–80].
The two major sources of cysteine-modifying reactive electrophiles for airway afferent TRPA1 channels are pollutants and oxidative stress [81]. TRPA1, expressed in either heterologous systems or in nociceptive neurons, is activated by pollutants such as acrolein, ozone, diisocyanates, isothiocyanates, formaldehyde and crotonaldehyde [77, 82–86]. Similarly, TRPA1 is activated by ROS such as superoxide and H2O2 (Fig. 2), by downstream products of lipid peroxidation such as 4-hydroxynonenal, 4-oxononenal, 9-nitrooleate and by dehydrated prostanoids [34, 60, 87–93]. In fact, all cysteine-modifying agents that can access the cytosol can activate TRPA1, suggesting intracellular targets on the channel [79]. Point mutations of select Cys residues in TRPA1 have demonstrated reduced electrophile-induced activation, while retaining functional responses to non-electrophilic activators. However, there is some disparity with C414 and C421 being important in mouse TRPA1 responses [79] and C621, C641 and C665 being important for human TRPA1 responses [78, 91]. Chemically, unsaturated α,β carbonyl compounds such as acrolein, 4-hydroxynonenal, 15d-Δ12 prostaglandin J2 and cinnamaldehyde, cause irreversible adduction of Cys. ROS-mediated TRPA1 activation is reversible [90] and is reported to coincide with the formation of disulfide bonds within TRPA1 [94].
Figure 2.
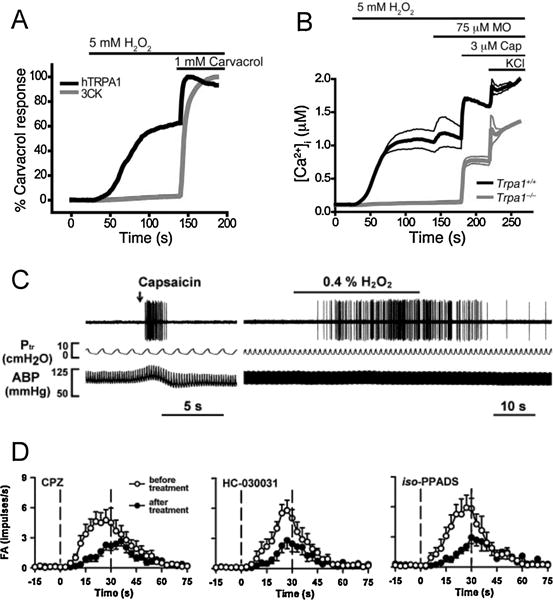
H2O2 activates nociceptive nerves via TRPA1. A, Requirement of covalent agonist acceptor sites for TRPA1 activation by H2O2. [Ca2+]ichanges were compared between HEK293t cells expressing human TRPA1 wildtype channels (black lines) and cells expressing TRPA1 channels with mutated interaction sites (C619, C639, C663, and K708; denoted 3CK; grey lines). Values denote percent maximal response to carvacrol, nonreactive TRPA1 agonist (n = 60 cells/trace). B, Activation of Ca2+influx by H2O2 into DRG neurons plotted against time. Average [Ca2+]i concentration of neurons activated by application of H2O2 followed by mustard oil (a.k.a. allyl isothiocyanate), capsaicin, and 65 mM KCl. Thick and thin lines denote mean and ± SEM, respectively. N = 189 Trpa1+/+ neurons (black line) and 146 Trpa1−/− neurons (grey lines). Taken from [60]. C, Representative action potential discharge in rat bronchopulmonary C fiber (recorded in vivo) to intravenous capsaicin (1 μg/kg, 0.1 ml, left), and aerosolized H2O2 (0.4%, right). Also shown: tracheal pressure (Ptr), and arterial blood pressure (ABP). D, Effect of TRPV1 inhibitor capsazepine (CPZ, 3 mg/kg), TRPA1 inhibitor HC-030031 (3 mg/kg) and P2X inhibitor iso-PPADS (15mg/kg) on action potential discharge by bronchopulmonary C fibers to 0.4% H2O2. Taken from [62].
TRPA1 activation has also been demonstrated following the activation of other neuronal receptors. In particular, stimulation of Gq coupled receptors such as Bradykinin B2, protease-activated receptor (PAR) 2, the bile receptor TGR5 and two members of the Mas-related G protein-coupled receptor family, MrgprA3 and MrgprC11, causes activation of TRPA1 in nociceptive neurons and heterologous systems [76, 82, 95–99]. In most cases, TRPA1 is activated downstream of phospholipase C activation; and diacylglycerol, cytosolic Ca2+, PKC and the hydrolysis of PIP2 have all been implicated. Recent studies have identified TRPA1 as a downstream target of toll-like receptor signaling [100–102]. TLR7 was shown to interact directly with TRPA1 in excised patch recordings [100], whereas LPS was shown to directly stimulate TRPA1 independently of TLR4 [101].
Airway C fibers are activated by cinnamaldehyde, allyl isothiocyanate (AITC), ozone, 4-oxononenal and 9-nitrooleate [33, 34, 57, 85, 93], and these responses are abolished by genetic knockout or pharmacological inhibition of TRPA1. Inhalation of TRPA1 agonists causes cough in conscious guinea pigs and humans [57, 103, 104]. Furthermore bradykinin evokes cough in conscious guinea pigs [39, 105], which is inhibited by TRPA1 inhibitors [97]. The role of TRPA1 in H2O2-mediated nociceptor activation is concentration dependent: at concentrations lower than 10mM, H2O2 responses were co-localized to TRPA1-expressing neurons [60, 64, 89, 90] and were abolished by TRPA1 knockout [60, 90]; at higher concentrations (up to 120mM), inhibition of TRPA1 has only a partial inhibitory effect [62], suggesting the involvement of other pathways. Thus TRPA1 is a likely mechanism involved in cough associated with inflammation, oxidative stress and air pollution.
Other neuronal proteins
Although TRPA1 is the main mechanism through which ROS and downstream products of lipid peroxidation activate airway C fibers, both TRPV1 and P2X receptors have also been implicated. In heterologous systems, TRPV1 is activated by some electrophilic cysteine-modifiers including allicin and 4-oxononenal [34, 106, 107], albeit at concentrations higher than those capable of TRPA1 activation. Knockout of TRPV1 partially reduced nocifensive behaviors evoked by allicin [107]. However, no evidence was found for a role of TRPV1 in the 4-oxononenal-mediated activation of airway C fibers [34]. Oxidation of select TRPV1 cysteines can cause channel activation [108, 109], and action potential discharge in airway C fibers evoked by high concentrations of H2O2 were reduced by capsazepine [62, 63], a TRPV1 inhibitor. Thus TRPV1 is a potential mediator of ROS-stimulation of cough-associated C fibers.
Inhibition of P2X channels and the scavenging of ATP have been shown to reduce action potential discharge in airway C fibers evoked by high concentrations of H2O2 [62, 63]. Although H2O2 may directly modulate P2X channel function [110], it is likely that such high concentrations of H2O2 cause cellular damage leading to the release of cytosolic ATP [62]. Regardless of the mechanism involved, it is unlikely that P2X channels contribute significantly to oxidative stress-mediated cough. Only C fibers from the nodose ganglion express the functional heteromeric channel P2X2/3 [21, 23], and stimulation of nodose C fibers fails to evoke cough [44]. Whereas the cough-associated jugular C fibers only express the rapidly inactivating P2X3 homomeric channel [21, 23], whose stimulation by ATP analogs is unable to evoke significant nerve discharge [22].
In addition to causing C fiber activation, H2O2 also causes hyperexcitability in airway nociceptive C fibers [64, 65]. Scavenging of ROS prevented this response. The role of TRPV1 and TRPA1 in oxidant-induced hyperexcitability of airway nociceptors is unclear: in one study inhibition of either TRPV1 or TRPA1 partially diminished the H2O2-induced augmentation of α,β methylene ATP (P2X2/3 selective agonist)-induced apneic reflexes [65]; however we found that oxidant-induced hyperexcitability of airway nociceptors did not correlate with TRPA1 expression nor was it reduced by either inhibition or knockout of TRPV1 [64]. Instead, we found that oxidant-induced airway C fiber hyperexcitability was prevented by inhibition of protein kinase C using BIM I (but not by the inactive analog BIM V) [64]. Activation of PKC causes its translocation to the plasma membrane, and consistent with this, H2O2 caused acute translocation of PKC to the plasma membrane in dissociated vagal neurons. The mechanism underlying PKC-mediated oxidant-induced nociceptor hyperexcitability is presently unclear, although direct stimulation of PKC using phorbel-12-myristate-13-acetate has been shown repeatedly to induce temporary nociceptor hyperexcitability [64, 111–114], likely via its phosphorylation of voltage-gated Na+ channels, which increases their voltage-sensitivity and currents. Nociceptive neurons express multiple PKC isoforms [111], many of which are known to be activated by ROS [72, 115, 116].
Sources of neuronal ROS
ROS are produced during inflammation, particularly downstream of NADPH oxidase activation in infiltrating immune cells [117, 118]. ROS may evoke responses through direct interactions with neuronal proteins or indirectly via electrophiles such as acrolein, 4-hydroxynonenal and 4-oxononenal downstream of lipid peroxidation. However, there is evidence that ROS can be produced within nociceptive neurons. NADPH oxidase subunits have been demonstrated in nociceptive neurons [119–121], with evidence suggesting that their activation contributes to neuronal excitability.
The mitochondrial electron transport chain (ETC) is another potent source of ROS [122], particularly during the early stages of apoptotic cascades. However it is becoming increasingly clear that mitochondrial ROS play critical roles in physiological signaling downstream of multiple pathways [123], including hypoxia [124, 125], TNFα [126], neurotrophins via p75NTR [127], Toll-like Receptors [128, 129] and TGFβ [130]. Importantly, afferent terminals innervating the peripheral tissue, including the airways, are densely packed with mitochondria [131–133]. Thus both mitochondria and afferent receptors are co-localized. Antimycin, an inhibitor of the ETC complex III, causes significant mitochondrial ROS production [122]. Consistent with the hypothesis that mitochondrial ROS could modulate airway nociceptor activity, antimycin caused both activation and hyperexcitability of airway C fibers [64, 134]. Inhibition of complex III will also depolarize the mitochondrial membrane potential, thus disrupting ATP production. Selective inhibition of mitochondrial ATP production using oligomycin (ATP synthase inhibitor) failed to alter nociceptor activity, suggesting that mitochondrial ATP levels were not acutely critical to the antimycin-induced responses. Antimycin-induced nociceptor activation was substantially reduced by knockout or selective inhibition of TRPA1, although TRPV1 was also implicated to a lesser extent [134]. In addition, antimycin activated heterologously expressed TRPA1 and this was inhibited by a combination of tempol and MnTMPyP, indicating the role of ROS. Antimycin-induced hyperexcitability was only observed in nociceptive afferent fibers innervating the airways [64]. Despite its correlation with TRPV1 expression, Antimycin-induced hyperexcitability was not reduced by knockout or selective inhibition of TRPV1. Instead, mitochondrial ROS caused airway C fiber hyperexcitability via the activation (and translocation) of PKC [64].
Interactions of TRPA1 and TRPV1
TRPA1 and TRPV1 are co-expressed in many nociceptive neurons [33, 34, 77, 82] including jugular C fibers innervating the airways [57]. Numerous studies have investigated the potential interaction between the two channels. Co-stimulation of TRPA1 and TRPV1 produced synergistic increases in C fiber action potential firing and synergistic increases in apneic responses [135]. Such synergy was recapitulated in whole cell patch clamp studies of dissociated vagal nociceptors and was absent in Ca2+-free conditions [136]. This synergy was specific to TRPA1/TRPV1 interactions, and was not observed with simultaneous treatments with activators of P2X2/3 and 5HT3 channels, suggesting specific interactions (physical or functional) between TRPA1 and TRPV1. Pretreatment with TRPA1 agonists also increases subsequent TRPV1 responses in nociceptive neurons and heterologous systems via Ca2+ and PKA signaling [137]. There is also evidence in DRG nociceptors of a complex physical interaction between TRPA1 and TRPV1 subunits under the regulation of Tmem100 [138], although whether this happens in airway C fibers is unknown.
Potential role of ROS in chronic plasticity
Previous studies have highlighted the possibility that airway sensory nerve protein expression is plastic and can be chronically modulated by pathways associated with inflammation and infection [139–144]. These changes in ion channel and neuropeptide expression are hypothesized to induce long lasting changes in the function of airway afferents associated with cough [10, 145]. A direct role of ROS in chronic plasticity has not been studied. Nevertheless, studies in other neuronal subtypes suggest that ROS have the potential to initiate long-term changes in transcription/translation. For example, ROS are stimulators of nuclear factor erythroid 2-related factor 2 (Nrf2) [146], hypoxia-inducible factor 1α (HIF1α) [125, 147], myocyte enhancer factor 2 (MEF2) [148], and neurotrophin receptors [127, 149]. Alternatively, oxidative stress can activate NFAT and ERK1/2 indirectly via modulation of mitochondrial Ca2+ handling [150, 151]. How ROS impacts signaling of these transcription factors in cough afferents is yet to be studied.
Open questions and challenges
TRPA1 and TRPV1 are expressed on airway C fibers and their activation leads to cough. Furthermore, there is sufficient evidence to suggest that inflammation in the airways is associated with oxidative stress, and that ROS and associated products can activate TRPA1, TRPV1 and other neuronal targets. However there are substantial gaps in our knowledge of the role of TRP channels and oxidative stress in chronic cough.
Firstly, most in vivo animal studies have thus far tested pharmacological treatments on cough evoked by acute treatment with jugular C fiber or nodose Aδ fiber stimulants. For example both TRPA1 and TRPV1 have been implicated in bradykinin-evoked cough in conscious guinea pigs. However, it is likely that these models do not adequately resemble clinical presentation of cough, where coughs are spontaneously evoked during or following airway inflammation (allergic or viral or a combination). As such we have little evidence to suggest that TRP channels actually contribute to aberrant cough signaling. A role for nociceptive afferents and TRPA1 itself has been shown in the airway hyperreactivity associated with ovalbumin-induced allergic airway disease [152–154], although the link between aberrant afferent signaling and aberrant bronchial resistance, presumably dependent on parasympathetic reflexes, has not been clarified.
Secondly, the effect of chronic inflammation in clinical populations on airway afferent function is not well understood. Inflammation is associated with increased neurotrophin signaling [155, 156], which can alter expression of neuropeptides and receptor channels [20, 139], thus potentially altering afferent functionality. The effect of neurotrophin signaling on afferent mitochondrial function and oxidative stress is unknown. The mechanisms underlying subacute/chronic changes in afferent excitability in human airway disease [157, 158] have not been identified.
Thirdly, there is little definitive proof that afferent terminals undergo oxidative stress during inflammatory/infectious disease states. Partly this due to the technical challenges associated with measuring ROS in micrometer-wide structures within the complex three-dimensional structure of the airways. Furthermore, the therapeutic use of antioxidants have shown little clinical effectiveness in the treatment of chronic cough [159, 160]. It is possible that glutathione mimetics such as n-acetylcysteine could fail to block TRPA1 activation by electrophiles due to kinetic considerations. Indeed, micromolar electrophiles have been consistently shown to activate TRPA1 despite the presence of millimolar glutathione within the cytosol.
These are some of the critical gaps of our knowledge. It is perhaps fair to say that oxidative stress and TRP signaling can evoke cough, but whether they are critical to the clinical presentation of cough is yet to be determined.
Figure 3.
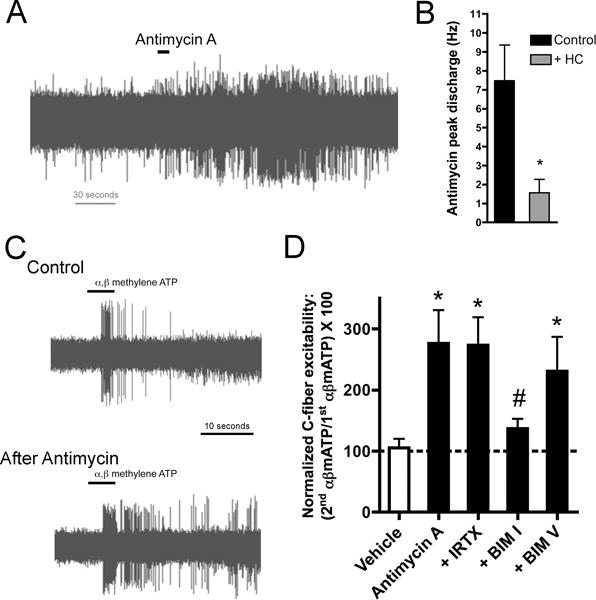
Antimycin A, mitochondrial complex III inhibitor, causes activation and hyperexcitability in bronchopulmonary C fibers. A, representative trace showing action potential discharge to antimycin A (20 μM) in an individual TRPA1-expressing mouse bronchopulmonary C fiber. B, Mean ± SEM peak discharge of TRPA1-expressing bronchopulmonary C fibers to antimycin A with and without pretreatment with TRPA1 inhibitor HC-030031 (30 μM). * Significant difference (p<0.05). Adapted from [134]. C, Representative traces of action potential discharge evoked by 10-second challenge with α,β-methylene ATP (P2X2/3 agonist, 30 μM) in a mouse bronchopulmonary C fiber before (control) and 10 minutes after treatment with antimycin A (20 μM). D, mean ± SEM action potential discharge response to 2nd application of α,β mATP (30 μM) normalized to response to 1st application of α,β mATP prior to either vehicle (white bar) or antimycin A (20 μM, black bars) in mouse nociceptive bronchopulmonary C fibers. The role of TRPV1 was determined using TRPV1 antagonist iodoresiniferatoxin (IRTX, 1μM). The role of PKC was determined using PKC inhibitor BIM I (1 μM) and the inactive analog BIM V (1 μM). * Significant difference from vehicle (p<0.05). # Significant difference from antimycin A (p<0.05). Adapted from [64].
Highlights.
Cough is triggered by the activation of specific airway afferent subtypes.
ROS cause activation of airway C fibers via TRPA1.
ROS increase the excitability of airway C fibers through multiple mechanisms
Acknowledgments
This work was supported by the NHLBI (R01HL119802).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schappert SM, Burt CW. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 2001–02. Vital Health Stat. 2006;13:1–66. [PubMed] [Google Scholar]
- 2.Barnes PJ. The problem of cough and development of novel antitussives. Pulm Pharmacol Ther. 2007;20:416–422. doi: 10.1016/j.pupt.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Smith SM, Schroeder K, Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev. 2008:CD001831. doi: 10.1002/14651858.CD001831.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Chung KF. Chronic ‘cough hypersensitivity syndrome’: a more precise label for chronic cough. Pulm Pharmacol Ther. 2011;24:267–271. doi: 10.1016/j.pupt.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Irwin RS, French CT, Lewis SZ, Diekemper RL, Gold PM. Overview of the management of cough: CHEST Guideline and Expert Panel Report. Chest. 2014;146:885–889. doi: 10.1378/chest.14-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morice AH. Epidemiology of cough. In: Chung F, Widdicombe J, Boushey HA, editors. Cough: Causes, Mechanisms and Therapy. Blackwell; 2003. pp. 11–16. [Google Scholar]
- 7.Tatar M, Plevkova J, Brozmanova M, Pecova R, Kollarik M. Mechanisms of the cough associated with rhinosinusitis. Pulm Pharmacol Ther. 2009;22:121–126. doi: 10.1016/j.pupt.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Canning BJ. Encoding of the cough reflex. Pulm Pharmacol Ther. 2007;20:396–401. doi: 10.1016/j.pupt.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canning BJ. The cough reflex in animals: relevance to human cough research. Lung. 2008;186(Suppl 1):S23–28. doi: 10.1007/s00408-007-9054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor-Clark TE. Peripheral neural circuitry in cough. Curr Opin Pharmacol. 2015;22:9–17. doi: 10.1016/j.coph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paintal AS. Vagal sensory receptors and their reflex effects. Physiol Rev. 1973;53:159–227. doi: 10.1152/physrev.1973.53.1.159. [DOI] [PubMed] [Google Scholar]
- 12.Coleridge HM, Coleridge JC. Pulmonary reflexes: neural mechanisms of pulmonary defense. Annu Rev Physiol. 1994;56:69–91. doi: 10.1146/annurev.ph.56.030194.000441. [DOI] [PubMed] [Google Scholar]
- 13.Carr MJ, Undem BJ. Bronchopulmonary afferent nerves. Respirology. 2003;8:291–301. doi: 10.1046/j.1440-1843.2003.00473.x. [DOI] [PubMed] [Google Scholar]
- 14.Canning BJ. Reflex regulation of airway smooth muscle tone. J Appl Physiol. 2006;101:971–985. doi: 10.1152/japplphysiol.00313.2006. [DOI] [PubMed] [Google Scholar]
- 15.Canning BJ. Functional implications of the multiple afferent pathways regulating cough. Pulm Pharmacol Ther. 2011;24:295–299. doi: 10.1016/j.pupt.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Bolser DC, Gestreau C, Morris KF, Davenport PW, Pitts TE. Central neural circuits for coordination of swallowing, breathing, and coughing: predictions from computational modeling and simulation. Otolaryngol Clin North Am. 2013;46:957–964. doi: 10.1016/j.otc.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Driessen AK, Farrell MJ, Mazzone SB, McGovern AE. Multiple neural circuits mediating airway sensations: Recent advances in the neurobiology of the urge-to-cough. Respir Physiol Neurobiol. 2015 doi: 10.1016/j.resp.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Lindsay RM. Role of neurotrophins and trk receptors in the development and maintenance of sensory neurons: an overview. Philos Trans R Soc Lond B Biol Sci. 1996;351:365–373. doi: 10.1098/rstb.1996.0030. [DOI] [PubMed] [Google Scholar]
- 19.Baker CV, Bronner-Fraser M. Establishing neuronal identity in vertebrate neurogenic placodes. Development. 2000;127:3045–3056. doi: 10.1242/dev.127.14.3045. [DOI] [PubMed] [Google Scholar]
- 20.Lieu T, Kollarik M, Myers AC, Undem BJ. Neurotrophin and GDNF family ligand receptor expression in vagal sensory nerve subtypes innervating the adult guinea pig respiratory tract. Am J Physiol Lung Cell Mol Physiol. 2011;300:L790–798. doi: 10.1152/ajplung.00449.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nassenstein C, Taylor-Clark TE, Myers AC, Ru F, Nandigama R, Bettner W, Undem BJ. Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J Physiol. 2010;588:4769–4783. doi: 10.1113/jphysiol.2010.195339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–917. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwong K, Kollarik M, Nassenstein C, Ru F, Undem BJ. P2X2 Receptors Differentiate Placodal vs Neural Crest C-fiber Phenotypes Innervating Guinea Pig Lungs and Esophagus. Am J Physiol Lung Cell Mol Physiol. 2008;295:L858–865. doi: 10.1152/ajplung.90360.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- 25.Lee LY. Respiratory sensations evoked by activation of bronchopulmonary C-fibers. Respir Physiol Neurobiol. 2009;167:26–35. doi: 10.1016/j.resp.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleridge HM, Coleridge JC, Luck JC. Pulmonary afferent fibres of small diameter stimulated by capsaicin and by hyperinflation of the lungs. J Physiol. 1965;179:248–262. doi: 10.1113/jphysiol.1965.sp007660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coleridge HM, Coleridge JC, Ginzel KH, Baker DG, Banzett RB, Morrison MA. Stimulation of ‘irritant’ receptors and afferent C-fibres in the lungs by prostaglandins. Nature. 1976;264:451–453. doi: 10.1038/264451a0. [DOI] [PubMed] [Google Scholar]
- 28.Lee LY, Kou YR, Frazier DT, Beck ER, Pisarri TE, Coleridge HM, Coleridge JC. Stimulation of vagal pulmonary C-fibers by a single breath of cigarette smoke in dogs. J Appl Physiol. 1989;66:2032–2038. doi: 10.1152/jappl.1989.66.5.2032. [DOI] [PubMed] [Google Scholar]
- 29.Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol. 2001;127:113–124. doi: 10.1016/s0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- 30.Kollarik M, Dinh QT, Fischer A, Undem BJ. Capsaicin-sensitive and -insensitive vagal bronchopulmonary C-fibres in the mouse. J Physiol. 2003;551:869–879. doi: 10.1113/jphysiol.2003.042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Undem BJ, Kollarik M. Characterization of the vanilloid receptor 1 antagonist iodo-resiniferatoxin on the afferent and efferent function of vagal sensory C-fibers. J Pharmacol Exp Ther. 2002;303:716–722. doi: 10.1124/jpet.102.039727. [DOI] [PubMed] [Google Scholar]
- 32.Kollarik M, Undem BJ. Activation of bronchopulmonary vagal afferent nerves with bradykinin, acid and vanilloid receptor agonists in wild-type and TRPV1−/− mice. J Physiol. 2004;555:115–123. doi: 10.1113/jphysiol.2003.054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol. 2008;586:1595–1604. doi: 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, Carr MJ, Undem BJ. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol. 2008;586:3447–3459. doi: 10.1113/jphysiol.2008.153585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carr MJ, Kollarik M, Meeker SN, Undem BJ. A role for TRPV1 in bradykinin-induced excitation of vagal airway afferent nerve terminals. J Pharmacol Exp Ther. 2003;304:1275–1279. doi: 10.1124/jpet.102.043422. [DOI] [PubMed] [Google Scholar]
- 36.Kwong K, Nassenstein C, de Garavilla L, Meeker S, Undem BJ. Thrombin and trypsin directly activate vagal C-fibres in mouse lung via protease-activated receptor-1. J Physiol. 2010;588:1171–1177. doi: 10.1113/jphysiol.2009.181669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buday T, Brozmanova M, Biringerova Z, Gavliakova S, Poliacek I, Calkovsky V, Shetthalli MV, Plevkova J. Modulation of cough response by sensory inputs from the nose - role of trigeminal TRPA1 versus TRPM8 channels. Cough. 2012;8:11. doi: 10.1186/1745-9974-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plevkova J, Kollarik M, Poliacek I, Brozmanova M, Surdenikova L, Tatar M, Mori N, Canning BJ. The role of trigeminal nasal TRPM8-expressing afferent neurons in the antitussive effects of menthol. J Appl Physiol (1985) 2013;115:268–274. doi: 10.1152/japplphysiol.01144.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004;557:543–558. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laude EA, Higgins KS, Morice AH. A comparative study of the effects of citric acid, capsaicin and resiniferatoxin on the cough challenge in guinea-pig and man. Pulm Pharmacol. 1993;6:171–175. doi: 10.1006/pulp.1993.1023. [DOI] [PubMed] [Google Scholar]
- 41.Lalloo UG, Fox AJ, Belvisi MG, Chung KF, Barnes PJ. Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J Appl Physiol (1985) 1995;79:1082–1087. doi: 10.1152/jappl.1995.79.4.1082. [DOI] [PubMed] [Google Scholar]
- 42.Trevisani M, Milan A, Gatti R, Zanasi A, Harrison S, Fontana G, Morice AH, Geppetti P. Antitussive activity of iodo-resiniferatoxin in guinea pigs. Thorax. 2004;59:769–772. doi: 10.1136/thx.2003.012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka M, Maruyama K. Mechanisms of capsaicin- and citric-acid-induced cough reflexes in guinea pigs. J Pharmacol Sci. 2005;99:77–82. doi: 10.1254/jphs.fpj05014x. [DOI] [PubMed] [Google Scholar]
- 44.Muroi Y, Ru F, Chou YL, Carr MJ, Undem BJ, Canning BJ. Selective inhibition of vagal afferent nerve pathways regulating cough using Nav 1.7 shRNA silencing in guinea pig nodose ganglia. Am J Physiol Regul Integr Comp Physiol. 2013;304:R1017–1023. doi: 10.1152/ajpregu.00028.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 46.Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2002;99:10150–10155. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 48.Forsberg K, Karlsson JA, Theodorsson E, Lundberg JM, Persson CG. Cough and bronchoconstriction mediated by capsaicin-sensitive sensory neurons in the guinea-pig. Pulm Pharmacol. 1988;1:33–39. doi: 10.1016/0952-0600(88)90008-7. [DOI] [PubMed] [Google Scholar]
- 49.Kanezaki M, Ebihara S, Gui P, Ebihara T, Kohzuki M. Effect of cigarette smoking on cough reflex induced by TRPV1 and TRPA1 stimulations. Respir Med. 2012;106:406–412. doi: 10.1016/j.rmed.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 50.McGovern AE, Driessen AK, Simmons DG, Powell J, Davis-Poynter N, Farrell MJ, Mazzone SB. Distinct brainstem and forebrain circuits receiving tracheal sensory neuron inputs revealed using a novel conditional anterograde transsynaptic viral tracing system. J Neurosci. 2015;35:7041–7055. doi: 10.1523/JNEUROSCI.5128-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Driessen AK, Farrell MJ, Mazzone SB, McGovern AE. The Role of the Paratrigeminal Nucleus in Vagal Afferent Evoked Respiratory Reflexes: A Neuroanatomical and Functional Study in Guinea Pigs. Front Physiol. 2015;6:378. doi: 10.3389/fphys.2015.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schelegle ES, Green JF. An overview of the anatomy and physiology of slowly adapting pulmonary stretch receptors. Respir Physiol. 2001;125:17–31. doi: 10.1016/s0034-5687(00)00202-4. [DOI] [PubMed] [Google Scholar]
- 53.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ricco MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol. 1996;496(Pt 2):521–530. doi: 10.1113/jphysiol.1996.sp021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol. 2002;543:591–600. doi: 10.1113/jphysiol.2002.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazzone SB, Reynolds SM, Mori N, Kollarik M, Farmer DG, Myers AC, Canning BJ. Selective expression of a sodium pump isozyme by cough receptors and evidence for its essential role in regulating cough. J Neurosci. 2009;29:13662–13671. doi: 10.1523/JNEUROSCI.4354-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brozmanova M, Mazurova L, Ru F, Tatar M, Kollarik M. Comparison of TRPA1-versus TRPV1-mediated cough in guinea pigs. Eur J Pharmacol. 2012;689:211–218. doi: 10.1016/j.ejphar.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol. 2005;569:559–573. doi: 10.1113/jphysiol.2005.093153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Canning BJ, Mori N. An essential component to brainstem cough gating identified in anesthetized guinea pigs. Faseb J. 2010;24:3916–3926. doi: 10.1096/fj.09-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruan T, Ho CY, Kou YR. Afferent vagal pathways mediating respiratory reflexes evoked by ROS in the lungs of anesthetized rats. J Appl Physiol. 2003;94:1987–1998. doi: 10.1152/japplphysiol.01047.2002. [DOI] [PubMed] [Google Scholar]
- 62.Lin YJ, Hsu HH, Ruan T, Kou YR. Mediator mechanisms involved in TRPV1, TRPA1 and P2X receptor-mediated sensory transduction of pulmonary ROS by vagal lung C-fibers in rats. Respir Physiol Neurobiol. 2013;189:1–9. doi: 10.1016/j.resp.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 63.Ruan T, Lin YS, Lin KS, Kou YR. Sensory transduction of pulmonary reactive oxygen species by capsaicin-sensitive vagal lung afferent fibres in rats. J Physiol. 2005;565:563–578. doi: 10.1113/jphysiol.2005.086181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hadley SH, Bahia PK, Taylor-Clark TE. Sensory Nerve Terminal Mitochondrial Dysfunction Induces Hyperexcitability in Airway Nociceptors via Protein Kinase C. Mol Pharmacol. 2014;85:839–848. doi: 10.1124/mol.113.091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruan T, Lin YJ, Hsu TH, Lu SH, Jow GM, Kou YR. Sensitization by pulmonary reactive oxygen species of rat vagal lung C-fibers: the roles of the TRPV1, TRPA1, and P2X receptors. PLoS One. 2014;9:e91763. doi: 10.1371/journal.pone.0091763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kozai D, Ogawa N, Mori Y. Redox regulation of transient receptor potential channels. Antioxid Redox Signal. 2014;21:971–986. doi: 10.1089/ars.2013.5616. [DOI] [PubMed] [Google Scholar]
- 67.Hidalgo C. Cross talk between Ca2+ and redox signalling cascades in muscle and neurons through the combined activation of ryanodine receptors/Ca2+ release channels. Philos Trans R Soc Lond B Biol Sci. 2005;360:2237–2246. doi: 10.1098/rstb.2005.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sahoo N, Hoshi T, Heinemann SH. Oxidative modulation of voltage-gated potassium channels. Antioxid Redox Signal. 2014;21:933–952. doi: 10.1089/ars.2013.5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bogeski I, Kilch T, Niemeyer BA. ROS and SOCE: recent advances and controversies in the regulation of STIM and Orai. J Physiol. 2012;590:4193–4200. doi: 10.1113/jphysiol.2012.230565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gamper N, Ooi L. Redox and nitric oxide-mediated regulation of sensory neuron ion channel function. Antioxid Redox Signal. 2015;22:486–504. doi: 10.1089/ars.2014.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sag CM, Wagner S, Maier LS. Role of oxidants on calcium and sodium movement in healthy and diseased cardiac myocytes. Free Radic Biol Med. 2013;63:338–349. doi: 10.1016/j.freeradbiomed.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 72.Cosentino-Gomes D, Rocco-Machado N, Meyer-Fernandes JR. Cell Signaling through Protein Kinase C Oxidation and Activation. Int J Mol Sci. 2012;13:10697–10721. doi: 10.3390/ijms130910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rahman I, Yang SR, Biswas SK. Current concepts of redox signaling in the lungs. Antioxid Redox Signal. 2006;8:681–689. doi: 10.1089/ars.2006.8.681. [DOI] [PubMed] [Google Scholar]
- 74.Wladyka CL, Kunze DL. KCNQ/M-currents contribute to the resting membrane potential in rat visceral sensory neurons. J Physiol. 2006;575:175–189. doi: 10.1113/jphysiol.2006.113308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 76.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 77.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 78.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 80.Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taylor-Clark TE, Undem BJ. Sensing pulmonary oxidative stress by lung vagal afferents. Respir Physiol Neurobiol. 2011;178:406–413. doi: 10.1016/j.resp.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 83.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor-Clark TE, Undem BJ. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol. 2010;588:423–433. doi: 10.1113/jphysiol.2009.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Taylor-Clark TE, Kiros F, Carr MJ, McAlexander MA. Transient receptor potential ankyrin 1 mediates toluene diisocyanate-evoked respiratory irritation. Am J Respir Cell Mol Biol. 2009;40:756–762. doi: 10.1165/rcmb.2008-0292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A. An ion channel essential for sensing chemical damage. J Neurosci. 2007;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sawada Y, Hosokawa H, Matsumura K, Kobayashi S. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur J Neurosci. 2008;27:1131–1142. doi: 10.1111/j.1460-9568.2008.06093.x. [DOI] [PubMed] [Google Scholar]
- 90.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takahashi N, Mizuno Y, Kozai D, Yamamoto S, Kiyonaka S, Shibata T, Uchida K, Mori Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels (Austin) 2008;2:287–298. doi: 10.4161/chan.2.4.6745. [DOI] [PubMed] [Google Scholar]
- 92.Taylor-Clark TE, Undem BJ, Macglashan DW, Jr, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-Induced Activation of Nociceptive Neurons via Direct Interaction with Transient Receptor Potential A1 (TRPA1) Mol Pharmacol. 2008;73:274–281. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- 93.Taylor-Clark TE, Ghatta S, Bettner W, Undem BJ. Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol. 2009;75:820–829. doi: 10.1124/mol.108.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang L, Cvetkov TL, Chance MR, Moiseenkova-Bell VY. Identification of in vivo disulfide conformation of TRPA1 ion channel. J Biol Chem. 2012;287:6169–6176. doi: 10.1074/jbc.M111.329748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007 doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grace M, Birrell MA, Dubuis E, Maher SA, Belvisi MG. Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax. 2012;67:891–900. doi: 10.1136/thoraxjnl-2011-201443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Than JY, Li L, Hasan R, Zhang X. Excitation and modulation of TRPA1, TRPV1, and TRPM8 channel-expressing sensory neurons by the pruritogen chloroquine. J Biol Chem. 2013;288:12818–12827. doi: 10.1074/jbc.M113.450072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lieu T, Jayaweera G, Zhao P, Poole DP, Jensen D, Grace M, McIntyre P, Bron R, Wilson YM, Krappitz M, Haerteis S, Korbmacher C, Steinhoff MS, Nassini R, Materazzi S, Geppetti P, Corvera CU, Bunnett NW. The bile acid receptor TGR5 activates the TRPA1 channel to induce itch in mice. Gastroenterology. 2014;147:1417–1428. doi: 10.1053/j.gastro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park CK, Xu ZZ, Berta T, Han Q, Chen G, Liu XJ, Ji RR. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron. 2014;82:47–54. doi: 10.1016/j.neuron.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernandez-Pena C, Talavera A, Kichko T, Navia B, Sanchez A, Senaris R, Reeh P, Perez-Garcia MT, Lopez-Lopez JR, Voets T, Belmonte C, Talavera K, Viana F. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun. 2014;5:3125. doi: 10.1038/ncomms4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mukhopadhyay I, Kulkarni A, Aranake S, Karnik P, Shetty M, Thorat S, Ghosh I, Wale D, Bhosale V, Khairatkar-Joshi N. Transient receptor potential ankyrin 1 receptor activation in vitro and in vivo by pro-tussive agents: GRC 17536 as a promising anti-tussive therapeutic. PLoS One. 2014;9:e97005. doi: 10.1371/journal.pone.0097005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andre E, Gatti R, Trevisani M, Preti D, Baraldi PG, Patacchini R, Geppetti P. Transient receptor potential ankyrin receptor 1 is a novel target for pro-tussive agents. Br J Pharmacol. 2009;158:1621–1628. doi: 10.1111/j.1476-5381.2009.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Birrell MA, Belvisi MG, Grace M, Sadofsky L, Faruqi S, Hele DJ, Maher SA, Freund-Michel V, Morice AH. TRPA1 Agonists Evoke Coughing in Guinea-pig and Human Volunteers. Am J Respir Crit Care Med. 2009;180:1042–1047. doi: 10.1164/rccm.200905-0665OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smith JA, Hilton EC, Saulsberry L, Canning BJ. Antitussive effects of memantine in guinea pigs. Chest. 2012;141:996–1002. doi: 10.1378/chest.11-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 107.Salazar H, Llorente I, Jara-Oseguera A, Garcia-Villegas R, Munari M, Gordon SE, Islas LD, Rosenbaum T. A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat Neurosci. 2008;11:255–261. doi: 10.1038/nn2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ogawa N, Kurokawa T, Fujiwara K, Polat OK, Badr H, Takahashi N, Mori Y. Functional and Structural Divergence in Human TRPV1 Channel Subunits by Oxidative Cysteine Modification. J Biol Chem. 2015 doi: 10.1074/jbc.M115.700278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- 110.Coddou C, Codocedo JF, Li S, Lillo JG, Acuna-Castillo C, Bull P, Stojilkovic SS, Huidobro-Toro JP. Reactive oxygen species potentiate the P2X2 receptor activity through intracellular Cys430. J Neurosci. 2009;29:12284–12291. doi: 10.1523/JNEUROSCI.2096-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- 112.Matsumoto S, Yoshida S, Ikeda M, Tanimoto T, Saiki C, Takeda M, Shima Y, Ohta H. Effect of 8-bromo-cAMP on the tetrodotoxin-resistant sodium (Nav 1.8) current in small-diameter nodose ganglion neurons. Neuropharmacology. 2007;52:904–924. doi: 10.1016/j.neuropharm.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 113.Baker MD. Protein kinase C mediates up-regulation of tetrodotoxin-resistant, persistent Na+ current in rat and mouse sensory neurones. J Physiol. 2005;567:851–867. doi: 10.1113/jphysiol.2005.089771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu DF, Chandra D, McMahon T, Wang D, Dadgar J, Kharazia VN, Liang YJ, Waxman SG, Dib-Hajj SD, Messing RO. PKCepsilon phosphorylation of the sodium channel NaV1.8 increases channel function and produces mechanical hyperalgesia in mice. J Clin Invest. 2012;122:1306–1315. doi: 10.1172/JCI61934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gopalakrishna R, Anderson WB. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci U S A. 1989;86:6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Knapp LT, Klann E. Superoxide-induced stimulation of protein kinase C via thiol modification and modulation of zinc content. J Biol Chem. 2000;275:24136–24145. doi: 10.1074/jbc.M002043200. [DOI] [PubMed] [Google Scholar]
- 117.Karihtala P, Soini Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. Apmis. 2007;115:81–103. doi: 10.1111/j.1600-0463.2007.apm_514.x. [DOI] [PubMed] [Google Scholar]
- 118.Blair IA. Endogenous glutathione adducts. Curr Drug Metab. 2006;7:853–872. doi: 10.2174/138920006779010601. [DOI] [PubMed] [Google Scholar]
- 119.Kallenborn-Gerhardt W, Schroder K, Del Turco D, Lu R, Kynast K, Kosowski J, Niederberger E, Shah AM, Brandes RP, Geisslinger G, Schmidtko A. NADPH oxidase-4 maintains neuropathic pain after peripheral nerve injury. J Neurosci. 2012;32:10136–10145. doi: 10.1523/JNEUROSCI.6227-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kentish SJ, O’Donnell TA, Wittert GA, Page AJ. Diet-dependent modulation of gastro-oesphageal vagal afferent mechanosensitivity by endogenous nitric oxide. J Physiol. 2014;592:3287–3301. doi: 10.1113/jphysiol.2014.272674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ibi M, Matsuno K, Shiba D, Katsuyama M, Iwata K, Kakehi T, Nakagawa T, Sango K, Shirai Y, Yokoyama T, Kaneko S, Saito N, Yabe-Nishimura C. Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. J Neurosci. 2008;28:9486–9494. doi: 10.1523/JNEUROSCI.1857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stowe DF, Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 125.Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, Chandel NS. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Corda S, Laplace C, Vicaut E, Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol. 2001;24:762–768. doi: 10.1165/ajrcmb.24.6.4228. [DOI] [PubMed] [Google Scholar]
- 127.Pehar M, Vargas MR, Robinson KM, Cassina P, Diaz-Amarilla PJ, Hagen TM, Radi R, Barbeito L, Beckman JS. Mitochondrial superoxide production and nuclear factor erythroid 2-related factor 2 activation in p75 neurotrophin receptor-induced motor neuron apoptosis. J Neurosci. 2007;27:7777–7785. doi: 10.1523/JNEUROSCI.0823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Djafarzadeh S, Vuda M, Takala J, Ochs M, Jakob SM. Toll-like receptor-3-induced mitochondrial dysfunction in cultured human hepatocytes. Mitochondrion. 2011;11:83–88. doi: 10.1016/j.mito.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 130.Michaeloudes C, Sukkar MB, Khorasani NM, Bhavsar PK, Chung KF. TGF-{beta} regulates Nox4, MnSOD and catalase expression and IL-6 release in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2010 doi: 10.1152/ajplung.00134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hung KS, Hertweck MS, Hardy JD, Loosli CG. Innervation of pulmonary alveoli of the mouse lung: an electron microscopic study. Am J Anat. 1972;135:477–495. doi: 10.1002/aja.1001350404. [DOI] [PubMed] [Google Scholar]
- 132.Hung KS, Hertweck MS, Hardy JD, Loosli CG. Ultrastructure of nerves and associated cells in bronchiolar epithelium of the mouse lung. J Ultrastruct Res. 1973;43:426–437. doi: 10.1016/s0022-5320(73)90019-1. [DOI] [PubMed] [Google Scholar]
- 133.von During M, Andres KH. Structure and functional anatomy of visceroreceptors in the mammalian respiratory system. Prog Brain Res. 1988;74:139–154. doi: 10.1016/s0079-6123(08)63008-3. [DOI] [PubMed] [Google Scholar]
- 134.Nesuashvili L, Hadley SH, Bahia PK, Taylor-Clark TE. Sensory nerve terminal mitochondrial dysfunction activates airway sensory nerves via transient receptor potential (TRP) channels. Mol Pharmacol. 2013;83:1007–1019. doi: 10.1124/mol.112.084319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin YJ, Lin RL, Ruan T, Khosravi M, Lee LY. A synergistic effect of simultaneous TRPA1 and TRPV1 activations on vagal pulmonary C-fiber afferents. J Appl Physiol (1985) 2015;118:273–281. doi: 10.1152/japplphysiol.00805.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hsu CC, Lee LY. Role of calcium ions in the positive interaction between TRPA1 and TRPV1 channels in bronchopulmonary sensory neurons. J Appl Physiol (1985) 2015;118:1533–1543. doi: 10.1152/japplphysiol.00043.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Spahn V, Stein C, Zollner C. Modulation of transient receptor vanilloid 1 activity by transient receptor potential ankyrin 1. Mol Pharmacol. 2014;85:335–344. doi: 10.1124/mol.113.088997. [DOI] [PubMed] [Google Scholar]
- 138.Weng HJ, Patel KN, Jeske NA, Bierbower SM, Zou W, Tiwari V, Zheng Q, Tang Z, Mo GC, Wang Y, Geng Y, Zhang J, Guan Y, Akopian AN, Dong X. Tmem100 Is a Regulator of TRPA1-TRPV1 Complex and Contributes to Persistent Pain. Neuron. 2015;85:833–846. doi: 10.1016/j.neuron.2014.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fischer A, McGregor GP, Saria A, Philippin B, Kummer W. Induction of tachykinin gene and peptide expression in guinea pig nodose primary afferent neurons by allergic airway inflammation. J Clin Invest. 1996;98:2284–2291. doi: 10.1172/JCI119039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hunter DD, Myers AC, Undem BJ. Nerve growth factor-induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med. 2000;161:1985–1990. doi: 10.1164/ajrccm.161.6.9908051. [DOI] [PubMed] [Google Scholar]
- 141.Carr MJ, Hunter DD, Jacoby DB, Undem BJ. Expression of tachykinins in nonnociceptive vagal afferent neurons during respiratory viral infection in guinea pigs. Am J Respir Crit Care Med. 2002;165:1071–1075. doi: 10.1164/ajrccm.165.8.2108065. [DOI] [PubMed] [Google Scholar]
- 142.de Vries A, Engels F, Henricks PA, Leusink-Muis T, McGregor GP, Braun A, Groneberg DA, Dessing MC, Nijkamp FP, Fischer A. Airway hyper-responsiveness in allergic asthma in guinea-pigs is mediated by nerve growth factor via the induction of substance P: a potential role for trkA. Clin Exp Allergy. 2006;36:1192–1200. doi: 10.1111/j.1365-2222.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 143.Zhang G, Lin RL, Wiggers M, Snow DM, Lee LY. Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. J Physiol. 2008;586:5771–5786. doi: 10.1113/jphysiol.2008.161042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lieu TM, Myers AC, Meeker S, Undem BJ. TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am J Physiol Lung Cell Mol Physiol. 2012;302:L941–948. doi: 10.1152/ajplung.00366.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Undem BJ, Taylor-Clark T. Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol. 2014;133:1521–1534. doi: 10.1016/j.jaci.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 148.Brusco J, Haas K. Interactions between mitochondria and the transcription factor myocyte enhancer factor 2 (MEF2) regulate neuronal structural and functional plasticity and metaplasticity. J Physiol. 2015;593:3471–3481. doi: 10.1113/jphysiol.2014.282459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huang YZ, Mcnamara JO. Neuroprotective effects of reactive oxygen species mediated by BDNF-independent activation of TrkB. J Neurosci. 2012;32:15521–15532. doi: 10.1523/JNEUROSCI.0755-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim MS, Usachev YM. Mitochondrial Ca2+ cycling facilitates activation of the transcription factor NFAT in sensory neurons. J Neurosci. 2009;29:12101–12114. doi: 10.1523/JNEUROSCI.3384-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li Z, Ji G, Neugebauer V. Mitochondrial reactive oxygen species are activated by mGluR5 through IP3 and activate ERK and PKA to increase excitability of amygdala neurons and pain behavior. J Neurosci. 2011;31:1114–1127. doi: 10.1523/JNEUROSCI.5387-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Trankner D, Hahne N, Sugino K, Hoon MA, Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci U S A. 2014;111:11515–11520. doi: 10.1073/pnas.1411032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D’Amours M, Witek JS, Fanger CM, Chong JA, Hayward NJ, Homer RJ, Cohn L, Huang X, Moran MM, Jordt SE. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A. 2009;106:9099–9104. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.McAlexander MA, Gavett SH, Kollarik M, Undem BJ. Vagotomy reverses established allergen-induced airway hyperreactivity to methacholine in the mouse. Respir Physiol Neurobiol. 2015;212–214:20–24. doi: 10.1016/j.resp.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bonini S, Lambiase A, Levi-Schaffer F, Aloe L. Nerve growth factor: an important molecule in allergic inflammation and tissue remodelling. Int Arch Allergy Immunol. 1999;118:159–162. doi: 10.1159/000024055. [DOI] [PubMed] [Google Scholar]
- 156.Tortorolo L, Langer A, Polidori G, Vento G, Stampachiacchere B, Aloe L, Piedimonte G. Neurotrophin overexpression in lower airways of infants with respiratory syncytial virus infection. Am J Respir Crit Care Med. 2005;172:233–237. doi: 10.1164/rccm.200412-1693OC. [DOI] [PubMed] [Google Scholar]
- 157.Doherty MJ, Mister R, Pearson MG, Calverley PM. Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax. 2000;55:643–649. doi: 10.1136/thorax.55.8.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jesenak M, Babusikova E, Petrikova M, Turcan T, Rennerova Z, Michnova Z, Havlicekova Z, Villa MP, Banovcin P. Cough reflex sensitivity in various phenotypes of childhood asthma. J Physiol Pharmacol. 2009;60(Suppl 5):61–65. [PubMed] [Google Scholar]
- 159.Mallet P, Mourdi N, Dubus JC, Bavoux F, Boyer-Gervoise MJ, Jean-Pastor MJ, Chalumeau M. Respiratory paradoxical adverse drug reactions associated with acetylcysteine and carbocysteine systemic use in paediatric patients: a national survey. PLoS One. 2011;6:e22792. doi: 10.1371/journal.pone.0022792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chalumeau M, Duijvestijn YC. Acetylcysteine and carbocysteine for acute upper and lower respiratory tract infections in paediatric patients without chronic broncho-pulmonary disease. Cochrane Database Syst Rev. 2013;5:CD003124. doi: 10.1002/14651858.CD003124.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]


