Abstract
Chondrocyte dedifferentiation presents a major barrier in engineering functional cartilage constructs. To mitigate the effects of dedifferentiation, this study employed a post-expansion aggregate culture step to enhance the chondrogenic phenotype of passaged articular chondrocytes (ACs) before their integration into self-assembled neocartilage constructs. The objective was two-fold: 1) To explore how passage number (P2, P3, P4, P5, P6, and P7), with or without aggregate culture, affect construct properties; and 2) to determine the highest passage number that can form neocartilage with functional properties. Juvenile leporine ACs were passaged to P2–P7, with or without aggregate culture, and self-assembled into 5 mm discs in non-adhesive agarose molds without using any exogenous scaffolds. Construct biochemical and biomechanical properties were assessed. With aggregate culture, neocartilage constructs had significantly higher collagen content, higher tensile properties, and flatter morphologies. These beneficial effects were most obvious at higher passage numbers. Specifically, collagen content, Young’s modulus, and instantaneous compressive modulus in the P7, aggregate group were 53%, 116%, and 178% higher than those in the P7, non-aggregate group. Most interestingly, these extensively passaged P7 ACs (expansion factor of 85,000), which are typically highly dedifferentiated, were able to form constructs with properties similar to or higher than those formed by lower passage number cells. This study not only demonstrated that post-expansion aggregate culture can significantly improve the properties of self-assembled neocartilage, but also that chondrocytes of exceedingly high passage numbers, expanded using the methods in this study, can be used in cartilage engineering applications.
Keywords: chondrocyte expansion, cell passaging, aggregate culture, cartilage tissue engineering, chondrocyte redifferentiation
Graphical Abstract
1. Introduction
Tissue engineered cartilage has the potential to alleviate several shortcomings of current articular cartilage repair therapies. In a study examining 25,000 knee arthroscopies, 60% of knees had presence of articular lesions [1]. From a surgical perspective, an estimated 250,000 articular cartilage repair procedures of the knee are performed annually in the U.S. [2]. Current cartilage repair therapies, however, do not consistently produce hyaline repair tissue, fill the entire defect, or integrate repair tissue with adjacent native tissue. Microfracture has been shown to form biomechanically inferior repair tissue, leading to its deterioration after 1.5–5 years [3–6]. Autologous chondrocyte implantation (ACI) and its matrix-assisted variants can result in inconsistent repair tissue; only 15–30% of treated defects were shown to develop hyaline-like repair tissue, while the rest developed a fibrocartilaginous fill [7–9]. These inconsistencies may arise from the placement of cells, which is surgeon-dependent, and maturation of the repair tissue, which is patient-dependent. Through a tissue engineering approach, functional neocartilage constructs can be consistently fabricated in vitro and used to replace damaged cartilage, potentially overcoming the deficiencies of current therapies.
One method of engineering cartilage is using the self-assembling process to generate scaffold-free neocartilage [10]. Advantages of a scaffold-free approach include unobstructed matrix formation by scaffold-associated chemistry, complete biocompatibility, and potentially good integration due to the construct’s high cellularity. Previously, scaffold-free neocartilage has been generated by the self-assembling process, using passaged articular chondrocytes (ACs) as a cell source. These constructs contained mostly type II collagen (little to no type I collagen) and had biomechanical properties close to juvenile native cartilage [11–13]. To form these constructs, ACs were first expanded under chondrogenically-tuned conditions, which involved the use of serum-free, FGF-2-supplemented medium and prolonged culture past cell confluence [11]. Cells were then cultured in aggregate suspensions to enhance redifferentiation of the dedifferentiated chondrocytes [12, 14]. This aggregate culture step, central to the present study, will be discussed later. Finally, cells were dissociated and self-assembled in non-adherent agarose wells, where they secrete an abundance of cartilage-specific matrix and form a neocartilage construct [15]. In a previous study, optimization of the cell seeding density, at 2 million cells per 5 mm diameter disc, allowed the formation of homogeneous neotissues with hyaline-like matrix composition and tensile properties on par with native tissue values [13]. These scaffold-free neocartilage constructs can potentially be used to repair articular cartilage defects.
Because scaffold-free neocartilage constructs typically require high cell numbers for construct formation (e.g., 10 million cells/cm2), expansion of chondrocytes to high passage numbers will be advantageous to overcome cell-source limitations or to create large constructs. However, a caveat of chondrocyte expansion is the rapid loss of the chondrogenic phenotype after the first passage (P1) [16]. Chondrocyte dedifferentiation is marked by a progression from rounded to fibroblastic cell morphologies, an increase in cell size, and a decrease in secretion of cartilage-specific matrix [17, 18]. Chondrogenic genes (e.g., SZP, COMP, aggrecan, collagen II, and SOX 9) are downregulated, while fibroblastic or mesenchymal genes (e.g., collagen I, collagen X, tenascin, and versican) are upregulated [16, 19–23]. Although dedifferentiation is rapid, gene expression changes after each subsequent passage have been measured up to P6, indicating that progressive cellular changes still occur long after P1 [24]. Fortunately, chondrocyte redifferentiation can be induced by prolonged 3D culture (e.g., pellet culture, alginate encapsulation, suspension culture, culture within a scaffold, etc.). Chondrocytes expanded too extensively (approximately > P4), however, have been shown to lose their ability to partially or completely redifferentiate [19, 25–28], rendering them unusable for cartilage engineering applications. Thus, chondrocyte dedifferentiation still poses a major barrier toward expanding chondrocytes to high passage numbers.
In a previous study, a post-expansion aggregate culture step was shown to enhance the ability of P4 ACs in forming self-assembled neocartilage with higher matrix content and biomechanical properties than neocartilage derived from cells that had not undergone the aggregate culture step [12]. Furthermore, these P4 AC-derived neocartilage constructs possessed equal or higher properties than constructs formed by lower passaged ACs, specifically, P0 and P3 ACs. These results suggest that ACs of even higher passage numbers (> P4), with aggregate culture, could potentially be used to form functional neocartilage. Employment of this aggregate culture step can potentially overcome the current notion that high-passage chondrocytes (> P4) are not suitable for use in cartilage engineering.
Allogeneic juvenile ACs were used in this study because this cell source has exhibited promising translational potential. Juvenile ACs have been shown to have significantly higher type II collagen gene expression [29, 30] and drastically higher glycosaminoglycan (GAG) production than adult chondrocytes [29, 31]. Juvenile ACs, after monolayer expansion, have also been shown to retain superior capabilities in secreting cartilage specific-matrix compared to expanded adult chondrocytes or stem cells [29]. Furthermore, an allogeneic source has been shown to be non-immunogenic [32] and require only one surgical procedure, as opposed to two for autologous approach. Currently, allogeneic juvenile ACs are used in the FDA-approved product DeNovo® NT and the Phase III clinical product RevaFlex™ for cartilage repair. Therefore, allogeneic juvenile ACs are a cell source with high translational potential.
In this study, the effects of passage number (P2, P3, P4, P5, P6, and P7) – as modulated by a post-expansion, aggregate culture step – on the properties of self-assembled neocartilage were investigated in a full-factorial design. In addition to explore comprehensively the effects of passage number and aggregate culture on neocartilage properties, another objective was to determine the highest passage number that can be used to form constructs that still maintained functional biomechanical properties. At the end of 4 weeks of culture, constructs were evaluated for their gross morphology, matrix content, and biomechanical properties (compressive and tensile). Constructs formed with cells of higher passage numbers were hypothesized to exhibit diminished properties, as the cells become more dedifferentiated and less capable of synthesizing cartilage-specific matrix. The aggregate culture step was hypothesized to stimulate chondrocyte redifferentiation and rescue construct properties at the high passage numbers. The ability to create functional neocartilage with extensively passaged chondrocytes may be a boon towards reducing donor site morbidity, increasing feasibility to treat large articular lesions, and removing barriers for new treatments that might typically require high chondrocyte numbers.
2. Materials and methods
2.1. Chondrogenic medium formulation
The chondrogenic medium used throughout the study was composed of DMEM (25 mM glucose/GlutaMAX™; Life Technologies, Carlsbad, CA), 1% PSF (penicillin-streptomycin-fungizone; Lonza, Basel, Switzerland), 1% ITS (insulin-transferrin-selenium; BD Biosciences, San Jose, CA), 1% NEAA (nonessential amino acids; Life Technologies), 100 μg/mL sodium pyruvate (Thermo Fischer Scientific, Waltham, MA), 50 μg/mL ascorbate-2-phosphate (Sigma, St. Louis, MO), 40 μg/mL L-proline (Sigma), and 100 nM dexamethasone (Sigma).
2.2. Isolation of juvenile rabbit articular chondrocytes
ACs were isolated from full-thickness cartilage of the femoral condyle, trochlear groove, and tibial plateau of 6- to 8-week-old, juvenile New Zealand White rabbits (Jones Rabbit Farm, Santa Rosa, CA). Cells were pooled from 10 animals of mixed genders. Minced cartilage tissue was washed with PBS and digested with 500 units/mL collagenase type 2 (Worthington Biochemical, Lakewood, NJ) in chondrogenic medium + 3% FBS (Atlanta Biologicals, Lawrenceville, GA) for 18 h at 37 °C/10% CO2. It was recommended by the manufacturer that 10% CO2 be used based on the bicarbonate concentration in the medium. Cells were then strained through a 70 μm filter, washed three times with medium, counted, and cryopreserved in chondrogenic medium + 20% FBS + 10% DMSO (Sigma). Cell viability observed by trypan blue was > 95%. Each knee yielded ~3×106 ACs.
2.3. Chondrocyte passaging
Primary ACs were passaged in monolayers to P2, P3, P4, P5, P6, and P7 in chondrogenic medium + 5 ng/mL bFGF (Peprotech, Rocky Hill, NJ) in a chondrogenically tuned process, as previously described [11]. Briefly, P0 ACs were thawed and seeded into T225 flasks at ~25,000 cells/cm2. For the first 24 h, 10% FBS was supplemented to promote cell adhesion. The medium was changed every 3–4 days. All cultures took place at 37 °C/10% CO2. When cells reached 95% confluence, the monolayers were cultured for an additional 4 days. Cell sheets were then lifted with 20 min incubation in 0.25% trypsin/EDTA (Invitrogen) and the sheets digested with 500 units/mL collagenase in chondrogenic medium + 3% FBS for 30 min. Cells were filtered through a 70 μm cell strainer, washed three times, counted with a hemocytometer and Coulter counter (Beckman-Dickinson Z-100), and either passaged again or subjected to aggregate culture, as described next. All passaging metrics are presented in Table 1. Cell diameter was determined through analysis with a Coulter counter. The following formulas were used for calculating the cell expansion metrics: cell doubling number = log(expansion factor)/log(2) (i.e., the number of times a cell population has doubled); expansion factor = initial number of cells/final number of cells.
Table 1.
Monolayer cell expansion metrics before and after each passaging event for the rabbit articular chondrocytes used in this study.
| Passage | Cells Seeded per Flask (million) | Cell Seeding Density (cells/cm2) | # of 225cm2 Flasks | PASSAGING EVENT | Days in Culture | Cell Yield per Flask | Final Cell Density (cells/cm2) | Expansion Factor/Passage | Cumulative Expansion Factor | Cumulative Cell Doubling Number |
|---|---|---|---|---|---|---|---|---|---|---|
| P0→P1 | 5.50 | 24,444 | 5 | ≫≫ | 9 | 40.6 | 180,444 | 7.38 | 7.38 | 2.88 |
| P1→P2 | 5.66 | 25,185 | 6 | ≫≫ | 9 | 36.7 | 162,963 | 6.47 | 47.8 | 5.58 |
| P2→P3 | 5.63 | 25,000 | 6 | ≫≫ | 8 | 37.5 | 166,667 | 6.67 | 318 | 8.31 |
| P3→P4 | 5.66 | 25,185 | 6 | ≫≫ | 7 | 19.0 | 84,370 | 3.35 | 1067 | 10.1 |
| P4→P5 | 5.63 | 25,000 | 6 | ≫≫ | 8 | 18.5 | 82,222 | 3.29 | 3508 | 11.8 |
| P5→P6 | 5.63 | 25,000 | 6 | ≫≫ | 9 | 27.5 | 122,222 | 4.89 | 17152 | 14.1 |
| P6→P7 | 5.63 | 25,000 | 6 | ≫≫ | 8 | 28.0 | 124,444 | 4.98 | 85380 | 16.4 |
2.4. Aggregate culture
Passaged ACs were redifferentiated in aggregate cultures, as previously described [12]. Briefly, 10 cm petri dishes were coated with a thin layer of 2% agarose to prevent cell adhesion. In each dish, 15 million cells in 15 mL chondrogenic medium + 10 ng/mL TGF-β1 (Peprotech) were added. Dishes were put on an orbital shaker at 55 rpm for 24 h and then cultured statically for 6 days. Medium was changed every 2 days. At day 7, cell aggregates were digested with 0.25% trypsin/EDTA for 20 min, the trypsin removed, and then digested in 500 units/mL collagenase in chondrogenic medium + 3% FBS for 60–90 min. Cells were filtered through a 70 μm cell strainer, washed three times, counted, and used for the self-assembly of neocartilage constructs. Aggregates were dissociated to single cells because direct use of un-dissociated aggregates were not amenable to forming homogeneous self-assembled neocartilage tissues (data not shown).
2.5. Self-assembly of neocartilage constructs
Disk-shaped, 5 mm diameter neocartilage constructs were grown via the self-assembling process [33]. Custom machined, stainless steel pegs were used as a negative cast to create 5 mm diameter wells made of 2% agarose in 24-well plates. Several changes of chondrogenic medium were added to the wells over 3 days to saturate the agarose with medium. Empty wells were each seeded with 100 μL medium containing 2 million cells. After 4 h incubation, a loose construct was formed and 500 μL medium was carefully added to each well (t=0 days). Every 24 h, 500 μL medium was changed until day 14. At day 14, constructs were gently unconfined [34] from the wells and placed into 6-well plates (5 constructs per 10 mL medium per well) with medium changes every 48 h. After 4 weeks, constructs were removed from culture, their wet weights taken, and photographed. Each construct was then sectioned into appropriately sized pieces for histology, biochemical testing, and biomechanical testing.
2.6. Histology and immunohistochemistry
Tissue samples (~1×1×1 mm) were cryoembedded in HistoPrep (Thermo Fisher Scientific) and sectioned at 16 μm thickness. After fixation in formalin, slides were stained for GAGs with Safranin O, Fast Green, and Weigert’s hematoxylin. Picrosirius Red was used to stain for all collagens. Collagen I and II immunohistochemistry were conducted, as described previously [35]. Color was developed using the Vectastain ABC reagents and DAB (Vectastain).
2.7. Biochemical analysis
Neocartilage samples, ~3×1×1 mm were weighed to obtain wet weights (WW), lyophilized for 3 days, and weighed again to obtain dry weights (DW). Water content (%) was calculated as (WW-DW)/WW × 100%. Each sample was digested in 900 μL 1.1 mg/mL pepsin (Sigma)/0.05 M acetic acid/0.44 M NaCl for 7 days at 4 °C on a rocker. Undigested tissues were then homogenized with an ultrasonicator (Misonix XL-2000; Qsonica, Newtown, CT). Samples were then neutralized with 100 μL 10× TBS and treated with elastase (0.09 mg/mL; Sigma) for 2 days at 4 °C on a rocker.
Total collagen content was determined by measuring hydroxyproline content using a modified chloramine-T colorimetric assay [36]. For each sample, 100 μL of the digested sample solution was diluted into 100 μL 1× TBS, hydrolyzed with 200 μL 4N NaOH at 120 °C for 15 min in an autoclave, and neutralized with 200 μL 4N HCl. Each sample was then added with 1.25 mL of 0.062 M chloramine T (Sigma) in an acetate-citrate buffer (0.45 M NaOH, 0.45 M sodium acetate, 0.14 M citric acid, and 0.11 M acetic acid), incubated for 20 min at room temperature, added with 1.25 mL of 1.2 M Ehrlich’s reagent (Sigma) in 30% perchloric acid/70% isopropanol, and incubated for 20 min incubation at 65 °C for color development. Samples were plated in duplicates and absorbance measured at 550 nm with a microplate reader. Equal amounts of digest solution had been added to the standards (bovine collagen from the Sircol Collagen Assay; Biocolor, Carrickfergus, U.K.) and samples to ensure consistency.
Total sulfated GAG content was measured using the Blyscan GAG Assay kit (Biocolor) following manufacturer’s instructions. Briefly, 10 μL of digested sample was incubated with 500 μL Dye Reagent for 30 min with intermittent vortexing, the precipitate centrifuged, and the pellet dissolved in 500μL Dissociated Reagent for absorbance measurement at 650 nm.
2.8. Biomechanical analysis
For tensile testing, dog bone-shaped samples were created with a scalpel and a 3 mm biopsy punch. The samples were photographed, glued to paper tabs [37], and subjected to uniaxial tension using an Instron model 5565 (Instron, Canton, MA). A strain rate of 1% the gauge length/s was used. The gauge length of 1.27 mm was set as the distance between the paper tabs. Cross-sectional areas were calculated in ImageJ from front- and side-view photographs of the dog bone. The Young’s modulus was obtained from the linear region of the stress-strain curve. The ultimate tensile strength (UTS) was defined as the maximum stress reached.
For compressive testing, a 3 mm diameter tissue sample was taken from the middle of the construct with a biopsy punch. The sample was placed in a PBS bath at room temperature and subjected to an unconfined, incremental stress-relaxation test, as described previously [38]. Briefly, sample heights were determined by lowering the platen until a load change of 0.02 N was detected. Samples were compressed to 10% strain at a rate of 1% the sample height/s, held for 200 s, compressed to 20% strain, and held for 450 s. The instantaneous modulus and relaxation modulus of the 20% strain curve were determined by curve fitting parameters into the standard linear solid (SLS), finite deformation (FD) model [38] using MatLab software.
2.9. Statistics
All analyses represent data collected from one experiment. To determine significant differences 1) between the no aggregate and aggregate groups and 2) among the passage number groups, all results were analyzed with a two-factor ANOVA and Tukey’s post hoc test (p<0.05) using JMP 10 software (SAS Institute). Errors bars represent standard deviations around the means with a sample size of n=5–7 per group. Significant differences between the no aggregate and aggregate groups are denoted by a “Y” and “Z” with the “Y” representing the higher-value group; significant differences exist in the passage number groups not sharing the same lower-case Greek letters. To determine significant differences within each of the no aggregate and aggregate groups, a one-factor ANOVA was performed with Tukey’s post hoc test (p<0.05). Significant differences exist among means not sharing the same lower-case and uppercase Roman letters, respectively. Groups without letters indicate that no significant differences were measured among any of the groups.
3. Results
3.1. Chondrocyte Expansion Metrics
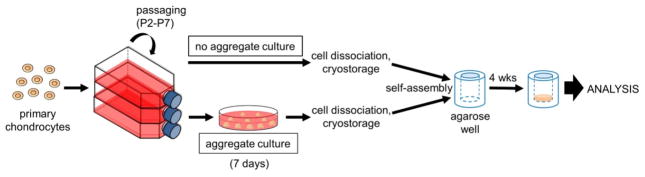
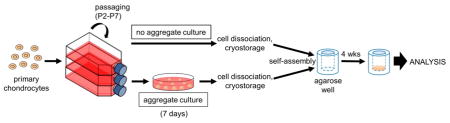
Articular chondrocytes pooled from 10 rabbit knees were passaged in monolayer up to P7. Cell yields and culture durations for each passage are summarized in Table 1. Generally, chondrocytes were cultured for 7–9 days between passages (3–5 days were typically needed to reach 90% confluence); chondrocytes were then passaged 4 days later, as per the chondrogenically tuned protocol [11]. Cell yields after each passage, as represented by the final cell density in the culture flasks, were observed to decrease after P3 as the cells became larger (see Table 2) and/or became more spread in 2D culture as they developed a more fibroblastic phenotype. At P7, chondrocytes had undergone significant expansion, reaching an expansion factor of 85,000 and a cell doubling number of 16.4. Other than the chondrocytes becoming progressively fibroblastic, another morphological change as passage number increased was an increase in cell size, as shown in Table 2. After the aggregate culture step, which is known to promote chondrocyte redifferentiation [14], chondrocytes decreased in diameter. The entire experimental flow from chondrocyte expansion, to aggregate culture, and finally to the self-assembly of neocartilage constructs is shown in Fig. 1.
Table 2.
Changes in chondrocyte diameter (population medium) over passage number and after aggregate culture.
| Passage | Cell diameter before aggregate (μm) | Cell diameter after aggregate (μm) |
|---|---|---|
| P0 | 7.3 | NA |
| P1 | 10.2 | NA |
| P2 | 14.5 | 13.1 |
| P3 | 15.2 | 13.2 |
| P4 | 14.8 | 12.9 |
| P5 | 15.8 | 13.3 |
| P6 | 15.1 | 13.2 |
| P7 | 14.9 | 14.1 |
Figure 1.
The experimental setup in this study first involved expanding primary rabbit articular chondrocytes to P2, P3, P4, P5, P6, and P7. Passaged cells were then either subjected to aggregate culture or cryopreserved. Cells were then thawed and self-assembled without exogenous scaffolds in agarose wells to form neocartilage constructs.
3.2. Neocartilage Gross Morphology
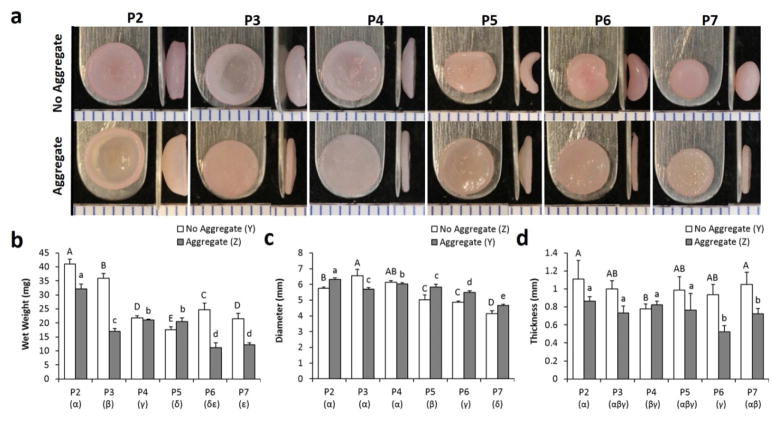
Both passage number and the aggregate culture step were significant factors in dictating neocartilage morphology and growth (Fig. 2). Over increasing passage numbers, constructs tended to have lower wet weights and smaller diameters (range of 11.2–40.0 mg and 4.1–6.6 mm, respectively). The aggregate culture step led to constructs having a lower wet weight and thickness but a larger diameter. Construct thickness (range of 0.5–1.1 mm) was largely unaffected by passage number, although it is noted that P6 and P7 constructs within the aggregate group were markedly thin constructs. Changes of construct morphology were evident as a function of passage number. Without aggregate culture, the constructs progressed from bowl-shaped (P2, P3, and P4), to saddle-shaped (P5 and P6) and then to a spherical shaped morphology (P7). Strikingly, the aggregate culture step induced formation of constructs that were mostly flat. The bowl-shaped morphology of the P2, aggregate culture group appears to be due to overgrowth of the construct in a confined well.
Figure 2.
Gross morphology and growth metrics of self-assembled neocartilage constructs. (A) Frontal and side images of neocartilage constructs. 1 mm tick marks. Wet weight (B), diameter (C), and thickness (D) measurements of the construct.
3.3. Histology and Immunohistochemistry
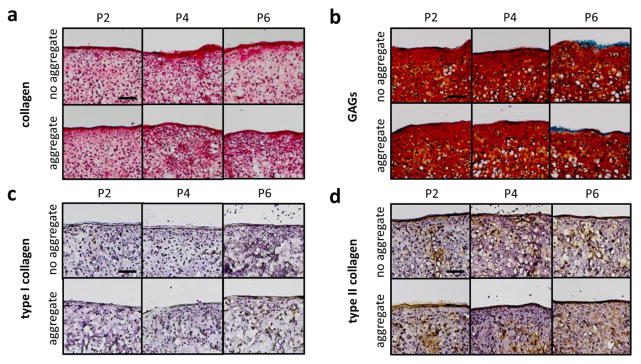
Histology revealed a homogeneous distribution of GAGs (Saf-O/Fast Green stain) and total collagen (Picrosirius Red stain) throughout the neocartilage constructs (Fig. 3). Differences in staining intensity and staining distribution were not observed among any groups. However, as presented in the next section, quantitative biochemistry yielded differences in matrix content among several groups. Collagen I and II immunohistochemistry revealed presence of type II collagen in all constructs; traces of type I collagen were evident, but the stains were faint and not homogeneously distributed in the construct. Histological characteristics qualitatively support the formation of hyaline-like cartilage in all constructs.
Figure 3.
Histological analysis of constructs indicates that constructs have a matrix rich in collagen (A), as stained by Picrosirius Red, and GAGs (B), as stained by Saf-O/Fast Green. Immunohistochemistry stains show little to no type I collagen (C) and presence of type II collagen (D) in all constructs. Scale bar = 100 μm.
3.4. Neocartilage Biochemical Properties
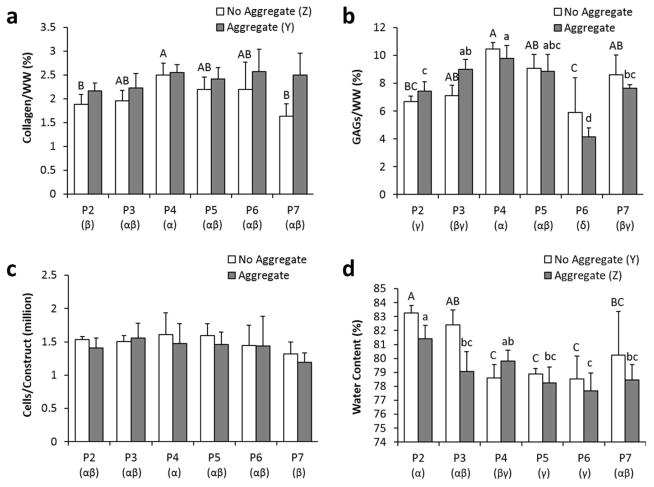
Employment of the aggregate culture step significantly increased the collagen/WW content of the neocartilage constructs (Fig. 4). Interestingly, when examining passage number as single factor (Greek letters below the bars) or when only examining the no aggregate group (capitalized letters above the bars), P4 constructs had significantly higher collagen/WW(2.5±0.2%) than that of P2 constructs (2.0±0.2%). Within the aggregate group, collagen production was unchanged over passages.
Figure 4.
Biochemical properties of the self-assembled neocartilage constructs at the end of 4 weeks of culture, as characterized by collagen/WW (A), GAGs/WW (B), cells/construct (C), and water content (D). Groups not sharing the same letters are statistically different (p < 0.05).
Aggregate culture did not influence GAG content, as measured by GAG/WW, of neocartilage constructs. Passage number significantly affected GAG content, but no clear trends were observed. As seen in trends for collagen/WW, P4 constructs again exhibited higher GAG/WW (10.2±0.8%) than constructs formed by less-passaged chondrocytes (6.7±0.4 to 9.0±0.7%). Of note, the P6 constructs contained the lowest GAG content (5.1±0.2%).
Total matrix content was obtained by multiplying the matrix/WW values (Figure 3) with the corresponding WW (Figure 2). Total collagen (mg) values for groups P2, P3, P4, P5, P6, and P7 were 0.77±0.09, 0.71±0.08, 0.54±0.06, 0.38±0.05, 0.54±0.02, and 0.35±0.06 mg for the no aggregate group and 0.70±0.06, 0.38±0.06, 0.54±0.04, 0.49±0.06, 0.29±0.07, and 0.30±0.06 for the aggregate group, respectively. Total GAG (mg) values for these groups were 2.7±0.2, 2.6±0.3, 2.3±0.9, 1.6±0.2, 1.5±0.7, and 1.9±0.4 mg for the no aggregate group and 2.4±0.3, 1.5±0.1, 2.1±0.2, 1.7±0.4, 0.5±0.1, and 0.9±0.6 for the aggregate group, respectively. The GAG/WW:Collagen/WW ratios of the neocartilage were also calculated and were found to be 3.5±0.2, 3.7±0.5, 4.2±0.3, 4.1±0.4, 3.0±0.8, and 5.0±0.8 for the no aggregate group and 3.4±0.4, 4.0±0.7, 3.9±0.6, 3.8±0.4, 1.7±0.3, and 3.2±0.6 for the aggregate group, respectively. The GAG/WW:collagen/WW ratio of native articular cartilage was found to range from 0.8 to 1.2 [39].
The cellularity among all constructs remained largely similar, except for a significantly lower cellularity in P7 constructs when compared to P4 constructs. Water content of the neocartilage constructs, an indicator of tissue density, showed significant differences among the groups: aggregate culture decreased water content, while higher passages trended with lower water content. When considering these biochemical factors as a whole, P4 and P5 chondrocytes seemed to form denser constructs with higher matrix content.
3.4. Neocartilage Biomechanical Properties
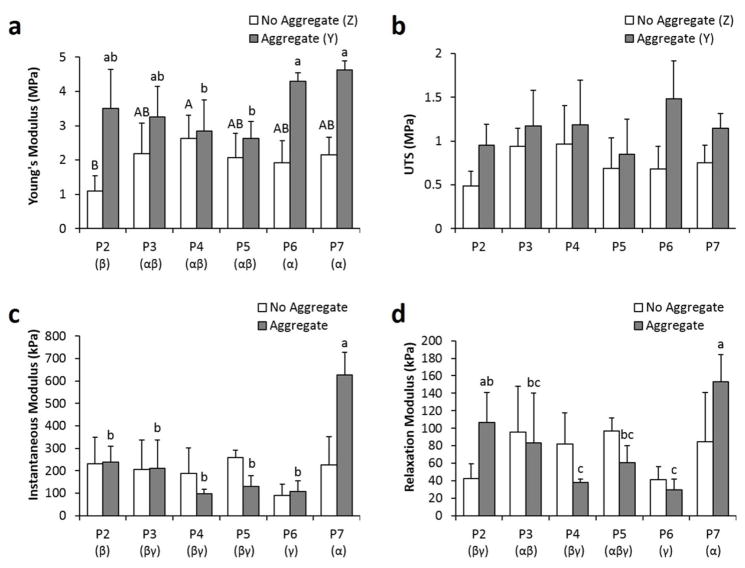
The aggregate culture step, when examined as a single factor in a two-way ANOVA test, significantly increased the Young’s modulus and UTS of the constructs (Fig. 5). When passage number was examined as a single factor, both P6 and P7 constructs had significantly higher Young’s modulus than P2 constructs. The higher tensile properties at higher passages were especially evident in the aggregate group (2.6±0.5 to 3.5±1.1 MPa for P2–P5 constructs compared to 4.3±0.3 to 4.6±0.2 MPa for P6–P7 constructs).
Figure 5.
Tensile and compressive properties of the neocartilage constructs at the end of 4 weeks of culture. Young’s modulus (A) and UTS (B) were used to assess tissue tensile properties, while instantaneous modulus (C) and relaxation modulus (D) were used to assess tissue compressive properties. Groups not sharing the same letters are statistically different (p < 0.05).
In terms of compressive properties, the aggregate culture step, as a single factor, did not have any effect. In addition, no clear trends were observed with passage number. However, when considering P7 constructs, aggregate culture noticeably increased the instantaneous and relaxation modulus by 178% and 81%, respectively. On the other hand, P6 constructs possessed lower compressive properties, despite having high tensile properties.
When considering both tensile and compressive properties, P7 aggregate constructs were the most mechanically robust. However, because these constructs were significantly smaller in wet weight and diameter than other constructs, they may not be ideal for clinical application. Although P6 constructs possessed high tensile properties, their compressive properties were the lowest. Within the aggregate group, which is the group of more interest, P2 and P3 constructs trended with higher overall biomechanical properties than P4 and P5 constructs, although the differences were not statistically significant.
4. Discussion
This study explored the effects of passage number (P2, P3, P4, P5, P6, and P7) and a post-expansion aggregate culture step on the properties of self-assembled neocartilage constructs. The aggregate culture step was shown to be a significant factor in enhancing many functional properties (e.g., collagen/WW, Young’s modulus, UTS, tissue density as measured by water content, and tissue morphology) of tissue engineered cartilage. The beneficial effects of aggregate culture were especially pronounced at the higher passage numbers. For example, P7 aggregate constructs had 53% higher collagen/WW, 116% higher Young’s modulus, 52% higher UTS, 178% higher instantaneous modulus, and 81% higher relaxation modulus than P7 no aggregate constructs. Results from this study also demonstrated that extensively passaged chondrocytes (up to P7; expansion factor of 85,000; population doubling of 16), which are typically highly dedifferentiated, can be used to form neocartilage constructs with properties similar to or higher than those formed by lower-passaged chondrocytes. This study demonstrated that, with the culturing protocols presented in this paper, chondrocytes can be expanded to high cell numbers while maintaining their chondrogenic potential. Obtaining high cell yields can potentially reduce donor site morbidity or help treat large cartilage lesions. Having the ability to acquire high chondrocyte numbers may be especially significant in removing barriers for scaffold-free approaches in repairing cartilage lesions, as such strategies employ typically high chondrocyte numbers.
Construct morphology was prominently affected by both passage number and aggregate culture. In both the no aggregate and aggregate groups, cells of increasing passage number formed constructs with smaller wet weights and diameters. Reasons for this may include 1) incomplete integration of all cells or cell death at higher passages, as the P7 group had lower cellularity than the other passage numbers; and 2) less total matrix secretion at higher passages. One of the most striking effects of aggregate culture was that constructs developed a flatter morphology at each respective passage number. This flatter morphology may be due to a general enhancement of the chondrogenic phenotype, as primary chondrocytes are also known to self-assemble into flat discs [40]. Overall, P3 aggregate, P4 aggregate, and possibly P5 aggregate constructs were the flattest, in addition to having the largest diameters, and could be considered as having the best morphologies.
Aggregate culture significantly affected both neocartilage biochemical and biomechanical properties. When examined as a single-factor, aggregate culture increased the constructs’ collagen/WW and decreased their water content, the latter indicating formation of a denser tissue matrix. The ability for aggregate culture to increase collagen/WW seemed more prominent at higher passages, such as at P6 and P7, where 17% and 53% increases over the no aggregate constructs of the same passage number, respectively, were observed. These enhanced effects at higher passages can also be interpreted as an ability of aggregate culture to rescue constructs from the adverse effects of cell dedifferentiation; in the no aggregate group, collagen/WW significantly decreased from P4 to P7, while, in the aggregate group, P7 constructs maintained similar collagen content to P4 constructs. The ability for aggregate culture to increase neocartilage collagen/WW was reflected in a concomitant increase in tensile properties (i.e., Young’s modulus and UTS). These effects were also most prominent at the higher passages; P6 aggregate and P7 aggregate groups had a Young’s modulus 123% and 116% higher and a UTS 119% and 52% higher than P6 no aggregate and P7 no aggregate groups, respectively. Although aggregate culture as a single factor did not affect compressive properties, aggregate culture within the P7 group increased the instantaneous and relaxation modulus by 178% and 81%, respectively. Large error bars in the compression data may have masked any other potential trends; difficulty in obtaining flat constructs and variability in construct curvature may have contributed to such errors. From the overall biochemical and biomechanical results, the P7 aggregate group possessed the best properties. These overall results also prominently demonstrate that the aggregate culture step was able to increase the matrix content and functional biomechanical properties of tissue engineered neocartilage constructs.
The authors note that GAG and collagen contents have been sometimes shown not to correlate with compressive and tensile properties, respectively. Many factors that compromise the matrix can affect tensile or compressive properties. For example, collagen content and cross-linking can affect both tensile and compressive measurements [41, 42]. Collagen content, not only aggrecan content, can contribute significantly to compressive properties [41]. Furthermore, some evidence suggests that hyaluronan content can also contribute to compressive properties [43]. Thus, in general, the amount of matrix may not correlate with the observed biomechanical properties.
The ability for aggregate culture to increase the collagen/WW content of construct and tensile properties is presumably due to the enhanced chondrogenic potential of the cells after having undergone the initial 3D culture stage. Culture of chondrocytes in 3D – whether in alginate gels, pellets, scaffold-free cultures, suspension aggregate culture, and in scaffolds – is known to induce a degree of chondrocyte redifferentiation [12, 16, 25, 27, 44]. Chondrocytes in aggregate suspension culture, as employed in the present study, were also shown to induce chondrocyte redifferentiation, as indicated by enhanced expression of cartilage-specific genes (e.g., SOX9 and Col2A1) and cartilage-specific matrix (e.g., type II collagen and GAGs) [14, 45]. The mechanism for redifferentiation in 3D culture may be related to the actin cytoskeleton network within the chondrocyte. Preservation of a rounded cell morphology, as opposed to a fibroblastic morphology, was found to be conducive towards a chondrogenic phenotype [46, 47]. Furthermore, inhibition of actin polymerization with various agents was also shown to enhance redifferentiation of dedifferentiated chondrocytes [48]. Therefore, chondrocyte redifferentiation in aggregate culture may fundamentally be due to the cell’s inability to maintain, during 3D culture, the strong actin networks that it had developed in monolayer culture. Employment of the aggregate culture step after post-expansion is, thus, fundamental toward recapturing the chondrogenic phenotype of passaged cells.
Passage number was also shown to significantly affect construct biomechanical and biochemical properties, although no clear monotonic increases or decreases were observed in most measured parameters. One interesting trend seemed to be that P4 constructs generally had higher collagen/WW and GAG/WW content than P2 constructs, indicating that use of P4 cells may actually be more beneficial than passaging chondrocytes twice. In a previous study, P4 constructs with aggregate culture were shown to have higher matrix content and biomechanical properties than those formed by P0 constructs, further supporting this notion [12]. Furthermore, in the present study, P2 constructs were not matrix-dense tissues, as indicated by their high water content. The low GAG/WW content of P6 constructs is difficult to explain; the thinness of the construct may be a contributing factor, as GAGs can diffuse out of the construct during sample processing. It is noted that P7 constructs had significantly higher tensile and compressive properties than P2 constructs. The low compressive properties of P6 constructs was expected due to their low GAG/WW content. After P7 constructs, the next best passage number in terms of biomechanical properties was P5. Several studies have shown that excessive passaging of chondrocytes (> P4) may render them unable to partially or completely redifferentiate [19, 25–28]. Currently, many clinical chondrocyte-based therapies limit their expansion to P3 [49]. However, in the present study, both P5 and P7 constructs aided by the beneficial effect of aggregate culture evidently have properties on par with lower-passaged constructs. Overall, the biochemical and biomechanical results support not only that higher-passaged chondrocytes were potentially more superior in forming neocartilage with higher biomechanical properties, but that exceedingly high passage numbers (P5 to P7) can potentially be used in cartilage tissue engineering applications.
As per the objective on this study, a maximum passage number that allowed the formation of functional neocartilage was identified. P7 aggregate constructs had a matrix content on par with constructs formed by the other passages and had superior biomechanical properties, thus making this group the most desirable for clinical application. In addition, P7 aggregate constructs were flat and homogeneous tissues. However, one caveat of the P7 aggregate group was that the constructs’ wet weight and diameter were less than that of the other groups; thus, its total matrix content was lower than the other groups. However, because the size of the construct may be less of a concern than its actual functional properties, P7 aggregate constructs can potentially be used for translational applications. When considering gross morphological properties, in addition to functional properties, P5 aggregate constructs represent a group with morphologies similar to the lower-passage groups and functional properties second to those of the P7 group.
Remarkably, the neocartilage constructs presented in this study have tensile modulus approaching that of native, juvenile bovine articular cartilage (4–5 MPa) tested under similar conditions [50, 51]. Collagen/WW (%) and GAG/WW (%) contents of native tissues measured that study were 5–20% and 10–18%, respectively, compared to 2.5% and 10.2% found in the present study. When compared to native, adult human cartilage, the constructs in the present study have about 1/3 the collagen content and tensile modulus within the range of native tissue values (5–25 MPa) [52, 53]. Unfortunately, the articular cartilage of juvenile rabbit specimens was too thin to be biomechanically tested using the stated protocols. Because the neocartilage constructs in this study were only cultured for 4 weeks, they were still in an immature state. As studies have shown that self-assembled neocartilage can increase in biomechanical properties over longer in vitro [15] and in vivo [54] culture, the possibility of these constructs gaining full biomechanical functionality appears promising.
Passaging young ACs to such high passage numbers can potentially open new avenues for the improvement of current therapies or development of new ones. Young allogeneic ACs are an emerging promising cell source for cartilage repair, as they possess a higher chondrogenic potential than adult ACs [29, 31]. The use of young allogenic ACs has been demonstrated in the FDA-approved product DeNovo® NT and the product RevaFlex™, which is currently in clinical trials. Use of these cells can potentially be even more widespread if they are used to replace the autologous cell source used in current chondrocyte-based repair strategies or those in clinical trials [49]. Expansion of young ACs to high numbers could potentially improve current therapies. Current products use a seeding density of 1–1.5 million cells/mL. This can be increased to potentially improve neocartilage formation and healing. The ability to acquire large cell numbers is especially beneficial for generating scaffold-free neocartilage constructs (e.g., RevaFlex™), which typically require high cell numbers for their formation. For example, the self-assembled neocartilage constructs presented in this study use 10 million cells per cm2. Finally, if adult ACs can also be expanded to high numbers using the protocols described in this study, these advantages can also be applied to autologous therapies, along with potential reduction of donor site morbidity. Future studies are being carried out in expanding adult ACs to similar levels. Therefore, the ability to use chondrocytes of high passages in cartilage engineering applications would be highly beneficial in improving current chondrocyte-based therapies and removing barriers for the development of scaffold-free approaches.
5. Conclusion
The present study explored the effects of aggregate culture and passage number (P2–P7) on the properties of self-assembled neocartilage constructs. Results show that P4 chondrocytes were able to form constructs with higher matrix content and functional biomechanical properties than those formed by P2 chondrocytes, indicating that the use of higher passage numbers may not always be detrimental to neocartilage formation. Employment of a post-expansion, aggregate culture step to the chondrocytes before their self-assembly into constructs was shown to improve the morphology, matrix content, and biochemical properties of the constructs. Excitingly, this study demonstrates that, with the employment of aggregate culture, P7 chondrocytes were able to form constructs with higher or similar functional properties than those formed by lower passage numbers. These results indicate that extensively expanded chondrocytes (expansion factor up to 85,000) could be used for cartilage engineering applications. Use of a higher number of cells per defect area could potentially improve current therapies and remove barriers for the development of large scaffold-free, engineered cartilage constructs.
Acknowledgments
This work was supported by the National Institutes of Health [grant number R01AR067821] for funding this work.
Footnotes
Disclosures
The author declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14(3):177–82. doi: 10.1016/j.knee.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.McCormick F, Harris JD, Abrams GD, Frank R, Gupta A, Hussey K, Wilson H, Bach B, Jr, Cole B. Trends in the surgical treatment of articular cartilage lesions in the United States: an analysis of a large private-payer database over a period of 8 years. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2014;30(2):222–6. doi: 10.1016/j.arthro.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, Sudkamp N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14(11):1119–25. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee surgery sports traumatology, arthroscopy : official journal of the ESSKA. 2014;22(9):1986–96. doi: 10.1007/s00167-013-2676-8. [DOI] [PubMed] [Google Scholar]
- 5.Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2013;29(9):1579–88. doi: 10.1016/j.arthro.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 6.Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. The American journal of sports medicine. 2009;37(10):2053–63. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640–5. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 8.Shekkeris A, Perera J, Bentley G, Flanagan A, Miles J, Carrington R, Skinner J, Briggs T. HISTOLOGICAL RESULTS OF 406 BIOPSIES FOLLOWING ACI/MACI PROCEDURES FOR OSTEOCHONDRAL DEFECTS IN THE KNEE. Journal of Bone & Joint Surgery. 2012;94-B(SUPP XXXVI):12. [Google Scholar]
- 9.Ringe J, Burmester GR, Sittinger M. Regenerative medicine in rheumatic disease-progress in tissue engineering. Nat Rev Rheumatol. 2012;8(8):493–498. doi: 10.1038/nrrheum.2012.98. [DOI] [PubMed] [Google Scholar]
- 10.Athanasiou KA, Eswaramoorthy R, Hadidi P, Hu JC. Self-organization and the self-assembling process in tissue engineering. Annual review of biomedical engineering. 2013;15:115–36. doi: 10.1146/annurev-bioeng-071812-152423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huey DJ, Hu JC, Athanasiou KA. Chondrogenically tuned expansion enhances the cartilaginous matrix-forming capabilities of primary, adult, leporine chondrocytes. Cell transplantation. 2013;22(2):331–40. doi: 10.3727/096368912X657648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huey DJ, Athanasiou KA. Alteration of the fibrocartilaginous nature of scaffoldless constructs formed from leporine meniscus cells and chondrocytes through manipulation of culture and processing conditions. Cells, tissues, organs. 2013;197(5):360–71. doi: 10.1159/000346252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang BJ, Huey DJ, Hu JC, Athanasiou KA. Engineering biomechanically functional neocartilage derived from expanded articular chondrocytes through the manipulation of cell seeding density and dexamethasone concentration. Journal of tissue engineering and regenerative medicine. 2016 doi: 10.1002/term.2132. In press. [DOI] [PubMed] [Google Scholar]
- 14.Murphy MK, Huey DJ, Hu JC, Athanasiou KA. TGF-beta1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem cells. 2015;33(3):762–73. doi: 10.1002/stem.1890. [DOI] [PubMed] [Google Scholar]
- 15.Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA. Matrix development in self-assembly of articular cartilage. PloS one. 2008;3(7):e2795. doi: 10.1371/journal.pone.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2005;23(2):425–32. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Schulze-Tanzil G. Activation and dedifferentiation of chondrocytes: implications in cartilage injury and repair. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 2009;191(4):325–38. doi: 10.1016/j.aanat.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Demoor M, Ollitrault D, Gomez-Leduc T, Bouyoucef M, Hervieu M, Fabre H, Lafont J, Denoix JM, Audigie F, Mallein-Gerin F, Legendre F, Galera P. Cartilage tissue engineering: molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochimica et biophysica acta. 2014;1840(8):2414–40. doi: 10.1016/j.bbagen.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 19.Dell’Accio F, De Bari C, Luyten FP. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis and rheumatism. 2001;44(7):1608–19. doi: 10.1002/1529-0131(200107)44:7<1608::AID-ART284>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 20.Barlic A, Drobnic M, Malicev E, Kregar-Velikonja N. Quantitative analysis of gene expression in human articular chondrocytes assigned for autologous implantation. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2008;26(6):847–53. doi: 10.1002/jor.20559. [DOI] [PubMed] [Google Scholar]
- 21.Kaps C, Frauenschuh S, Endres M, Ringe J, Haisch A, Lauber J, Buer J, Krenn V, Haupl T, Burmester GR, Sittinger M. Gene expression profiling of human articular cartilage grafts generated by tissue engineering. Biomaterials. 2006;27(19):3617–30. doi: 10.1016/j.biomaterials.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Karlsen TA, Shahdadfar A, Brinchmann JE. Human primary articular chondrocytes, chondroblasts-like cells, and dedifferentiated chondrocytes: differences in gene, microRNA, and protein expression and phenotype. Tissue engineering. Part C, Methods. 2011;17(2):219–27. doi: 10.1089/ten.TEC.2010.0200. [DOI] [PubMed] [Google Scholar]
- 23.Lin Z, Fitzgerald JB, Xu J, Willers C, Wood D, Grodzinsky AJ, Zheng MH. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2008;26(9):1230–7. doi: 10.1002/jor.20523. [DOI] [PubMed] [Google Scholar]
- 24.Ma B, Leijten JC, Wu L, Kip M, van Blitterswijk CA, Post JN, Karperien M. Gene expression profiling of dedifferentiated human articular chondrocytes in monolayer culture. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21(4):599–603. doi: 10.1016/j.joca.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30(1):215–24. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 26.Giovannini S, Diaz-Romero J, Aigner T, Mainil-Varlet P, Nesic D. Population doublings and percentage of S100-positive cells as predictors of in vitro chondrogenicity of expanded human articular chondrocytes. Journal of cellular physiology. 2010;222(2):411–20. doi: 10.1002/jcp.21965. [DOI] [PubMed] [Google Scholar]
- 27.Schulze-Tanzil G, de Souza P, Villegas Castrejon H, John T, Merker HJ, Scheid A, Shakibaei M. Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell and tissue research. 2002;308(3):371–9. doi: 10.1007/s00441-002-0562-7. [DOI] [PubMed] [Google Scholar]
- 28.Stokes DG, Liu G, Dharmavaram R, Hawkins D, Piera-Velazquez S, Jimenez SA. Regulation of type-II collagen gene expression during human chondrocyte de-differentiation and recovery of chondrocyte-specific phenotype in culture involves Sry-type high-mobility-group box (SOX) transcription factors. The Biochemical journal. 2001;360(Pt 2):461–70. doi: 10.1042/0264-6021:3600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adkisson HDt, Martin JA, Amendola RL, Milliman C, Mauch KA, Katwal AB, Seyedin M, Amendola A, Streeter PR, Buckwalter JA. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. The American journal of sports medicine. 2010;38(7):1324–33. doi: 10.1177/0363546510361950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smeriglio P, Lai JH, Dhulipala L, Behn AW, Goodman SB, Smith RL, Maloney WJ, Yang F, Bhutani N. Comparative potential of juvenile and adult human articular chondrocytes for cartilage tissue formation in three-dimensional biomimetic hydrogels. Tissue engineering. Part A. 2015;21(1–2):147–55. doi: 10.1089/ten.TEA.2014.0070. [DOI] [PubMed] [Google Scholar]
- 31.Barbero A, Grogan S, Schafer D, Heberer M, Mainil-Varlet P, Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2004;12(6):476–84. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Adkisson HD, Milliman C, Zhang X, Mauch K, Maziarz RT, Streeter PR. Immune evasion by neocartilage-derived chondrocytes: Implications for biologic repair of joint articular cartilage. Stem cell research. 2010;4(1):57–68. doi: 10.1016/j.scr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue engineering. 2006;12(4):969–79. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 34.Elder BD, Athanasiou KA. Effects of confinement on the mechanical properties of self-assembled articular cartilage constructs in the direction orthogonal to the confinement surface. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2008;26(2):238–46. doi: 10.1002/jor.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy MK, Huey DJ, Reimer AJ, Hu JC, Athanasiou KA. Enhancing post-expansion chondrogenic potential of costochondral cells in self-assembled neocartilage. PloS one. 2013;8(2):e56983. doi: 10.1371/journal.pone.0056983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Archives of biochemistry and biophysics. 1961;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 37.Natoli RM, Skaalure S, Bijlani S, Chen KX, Hu J, Athanasiou KA. Intracellular Na(+) and Ca(2+) modulation increases the tensile properties of developing engineered articular cartilage. Arthritis and rheumatism. 2010;62(4):1097–107. doi: 10.1002/art.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen KD, Athanasiou KA. Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression. J Biomech. 2006;39(2):312–322. doi: 10.1016/j.jbiomech.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Paschos NK, Makris EA, Hu JC, Athanasiou KA. Topographic variations in biomechanical and biochemical properties in the ankle joint: an in vitro bovine study evaluating native and engineered cartilage. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2014;30(10):1317–26. doi: 10.1016/j.arthro.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 40.Revell CM, Reynolds CE, Athanasiou KA. Effects of initial cell seeding in self assembly of articular cartilage. Annals of biomedical engineering. 2008;36(9):1441–8. doi: 10.1007/s10439-008-9524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williamson AK, Chen AC, Sah RL. Compressive properties and function-composition relationships of developing bovine articular cartilage. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2001;19(6):1113–21. doi: 10.1016/S0736-0266(01)00052-3. [DOI] [PubMed] [Google Scholar]
- 42.Makris EA, Responte DJ, Paschos NK, Hu JC, Athanasiou KA. Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(45):E4832–41. doi: 10.1073/pnas.1414271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Responte DJ, Natoli RM, Athanasiou KA. Identification of potential biophysical and molecular signalling mechanisms underlying hyaluronic acid enhancement of cartilage formation. Journal of the Royal Society, Interface / the Royal Society. 2012;9(77):3564–73. doi: 10.1098/rsif.2012.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandl EW, van der Veen SW, Verhaar JA, van Osch GJ. Multiplication of human chondrocytes with low seeding densities accelerates cell yield without losing redifferentiation capacity. Tissue engineering. 2004;10(1–2):109–18. doi: 10.1089/107632704322791754. [DOI] [PubMed] [Google Scholar]
- 45.Lee TJ, Bhang SH, La WG, Yang HS, Seong JY, Lee H, Im GI, Lee SH, Kim BS. Spinner-flask culture induces redifferentiation of de-differentiated chondrocytes. Biotechnology letters. 2011;33(4):829–36. doi: 10.1007/s10529-010-0488-1. [DOI] [PubMed] [Google Scholar]
- 46.Zhou M, Yuan X, Yin H, Gough JE. Restoration of chondrocytic phenotype on a two-dimensional micropatterned surface. Biointerphases. 2015;10(1):011003. doi: 10.1116/1.4913565. [DOI] [PubMed] [Google Scholar]
- 47.Daniels K, Solursh M. Modulation of chondrogenesis by the cytoskeleton and extracellular matrix. Journal of cell science. 1991;100(Pt 2):249–54. doi: 10.1242/jcs.100.2.249. [DOI] [PubMed] [Google Scholar]
- 48.Loty S, Forest N, Boulekbache H, Sautier JM. Cytochalasin D induces changes in cell shape and promotes in vitro chondrogenesis: a morphological study. Biology of the cell / under the auspices of the European Cell Biology Organization. 1995;83(2–3):149–61. doi: 10.1016/0248-4900(96)81303-7. [DOI] [PubMed] [Google Scholar]
- 49.Huang BJ, Hu JC, Athanasiou KA. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1–22. doi: 10.1016/j.biomaterials.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eleswarapu SV, Responte DJ, Athanasiou KA. Tensile properties, collagen content and crosslinks in connective tissues of the immature knee joint. PloS one. 2011;6(10):e26178. doi: 10.1371/journal.pone.0026178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paschos NK, Makris EA, Hu JC, Athanasiou KA. Topographic Variations in Biomechanical and Biochemical Properties in the Ankle Joint: An In Vitro Bovine Study Evaluating Native and Engineered Cartilage. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2014 doi: 10.1016/j.arthro.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 52.Temple MM, Bae WC, Chen MQ, Lotz M, Amiel D, Coutts RD, Sah RL. Age- and site-associated biomechanical weakening of human articular cartilage of the femoral condyle. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007;15(9):1042–52. doi: 10.1016/j.joca.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 53.Athanasiou KA, Darling EM, Hu JC. Articular Cartilage Tissue Engineering. Synthesis Lectures on Tissue Engineering. 2009;1(1):1–182. [Google Scholar]
- 54.Responte DJ, Arzi B, Natoli RM, Hu JC, Athanasiou KA. Mechanisms underlying the synergistic enhancement of self-assembled neocartilage treated with chondroitinase-ABC and TGF-beta1. Biomaterials. 2012;33(11):3187–94. doi: 10.1016/j.biomaterials.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]