Abstract
We report the first structure of heptaprenyl diphosphate synthase from Staphylococcus aureus (SaHepPPS), together with an investigation of its mechanism of action, and inhibition. The protein is involved in the formation of menaquinone, a key electron transporter in many bacteria, including pathogens. SaHepPPS consists of a “catalytic” subunit (SaHepPPS-2) having two “DDXXD” motifs and a “regulatory” subunit (SaHepPPS-1) that lacks these motifs. High concentrations of the substrates, isopentenyl diphosphate and farnesyl diphosphate, inhibit the enzyme, which is also potently inhibited by bisphosphonates. The most active inhibitors (Ki ~ 200 nM) were N-alkyl analogs of zoledronate containing ~C6 alkyl side-chains. They were modestly active against S. aureus cell growth, and growth inhibition was partially “rescued” by addition of menaquinone-7. Since SaHepPPS is essential for S. aureus cell growth, its structure is of interest in the context of the development of menaquinone biosynthesis inhibitors as potential antibiotic leads.
Introduction
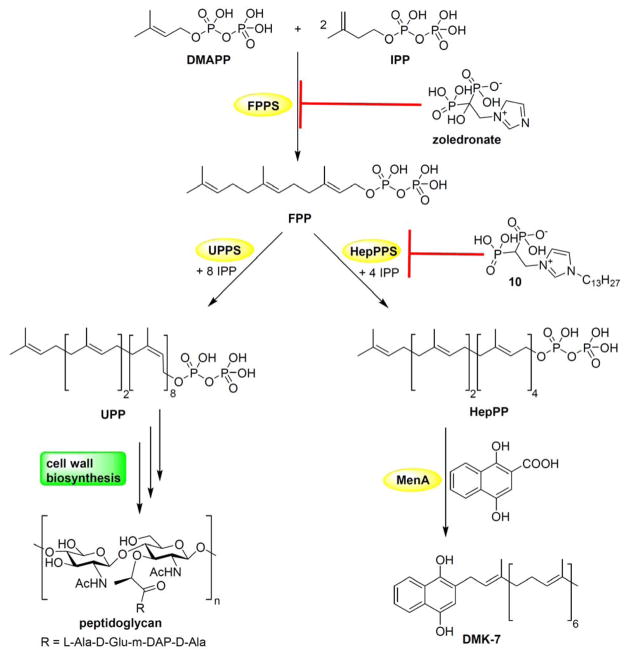
With the increasing rise in resistance to antibiotics, there is a need for new drug targets, and inhibitors. Isoprenoid biosynthesis is of interest in this context because isoprenoids are involved in the production of a diverse range of compounds including sterols, membrane lipids, carotenoids, quinones, hemes, as well as in bacterial cell wall biosynthesis.[1] Moreover, many important commercial drugs such as statins, azoles, and bisphosphonates, act in these pathways. Isoprenoid biosynthesis in general begins with the condensation of dimethylallyl diphosphate (DMAPP) with two molecules of isopentenyl diphosphate (IPP) to produce farnesyl diphosphate (FPP) in a reaction catalyzed by farnesyl diphosphate synthase (FPPS, Figure 1).[2] FPP is then converted to e.g. the C55-isoprenoid undecaprenyl diphosphate (involved in cell wall biosynthesis); to virulence factors such as staphyloxanthin or tuberculosinyl adenosine, or to quinones such as menaquinone and ubiquinone, used in electron transport. Many of the enzymes involved in these reactions are present only in bacteria and are essential for survival or virulence, so they are drug targets.[3] In Staphylococcus aureus, the major quinone is menaquinone-7 (MK-7), which contains a C35 side-chain (made from 7 isoprenoid C5 subunits). Because S. aureus does not contain ubiquinone (CoQ), menaquinone is the only quinone electron transporter.[4] MK-7 is produced via the condensation of 1,4-dihydroxy-2-naphthoic acid (Figure 1) with heptaprenyl diphosphate in a reaction catalyzed by MenA, and the heptaprenyl diphosphate substrate is produced by the condensation of FPP with four molecules of IPP in a reaction catalyzed by heptaprenyl diphosphate synthase (HepPPS, Figure 1). HepPPS from S. aureus is a heterodimeric trans-prenyl transferase that consists of a regulatory protein (190 residues) and a catalytic protein (319 residues), called here SaHepPPS-1 and SaHepPPS-2, respectively. In this work, we report the X-ray structure of the heterodimeric protein (SaHepPPS), its activity, its inhibition by a series of compounds, together with the inhibition of S. aureus cell growth, growth rescue by menaquinone, as well as synergy assays with a range of known antibiotics.
Figure 1.
Schematic illustration of the biosynthesis of several isoprenoids in Staphylococcus aureus. Condensation of one DMAPP and two IPP to produce FPP is carried out by FPPS. Condensation of 8 IPP with FPP yields a C55-isoprenoid, undecaprenyl diphosphate, which is an essential intermediate in cell wall biosynthesis. Sequential condensation of FPP with 4 IPP yields heptaprenyl diphosphate, which then alkylates dihydroxynaphthoic acid in a reaction catalyzed by MenA to generate demethylmenaquinol (DMK-7), an intermediate in menaquinone biosynthesis.
Results and Discussion
Sequence Similarity Network for SaHepPPS
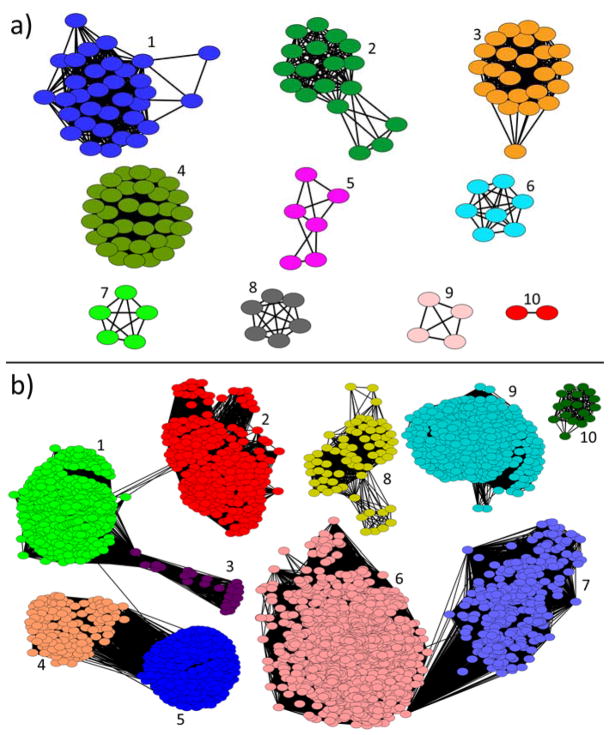
Sequence similarity networks for HepPPS-1 and HepPPS-2 were generated by using enzyme similarity tools in the Enzyme Function Initiative (EFI-EST).[5] The sequences of close relatives were obtained by using BLAST searches[6] and were then used to generate an output network in Cytoscape 3.1.0. Results for the putative regulatory domain (HepPPS-1) and the putative catalytic domain (HepPPS-2) are shown in Figures 2a, b, respectively. There are 3,414 relevant nodes in the HepPPS-2 network (Figure 2b), but only 212 in the HepPPS-1 network (Figure 2a).
Figure 2.
Sequence similarity networks for SaHepPPS-1 and SaHepPPS-2. a) Network based on regulatory domain (SaHepPPS-1) sequence constructed with E=10−50. Major clusters are: 1, HepPPS from Staphylococcus spp.; 2, HepPPS from Geobacillus spp.; 3, HepPPS from Paenibacillus spp.; 4, HepPPS from Bacillus spp.; 5, ParB-like protein from Burkholderia and Ralstonia spp.; 6, HepPPS from Anoxybacillus spp.; 7, HepPPS from Planomicrobium spp.; 8, tyrosine recombinase from Rhodopirellula spp.; 9, peptidase from Prevotella spp.; 10, HexPPS from Micrococcus spp. b) Network based on the catalytic domain (SaHepPPS-2) sequence constructed with an E=10−70. Major clusters are: 1, HepPPS and polyprenyl pyrophosphate synthase (PPPPS) from Bacillus, Staphylococcus and sulfate reducing bacteria; 2, HepPPS and PPPPS from Lactococcus, Enterococcus, Streptococcus, and Lactobacillus spp.; 3, PPPPS from Selanomonas, Thermosinus and Paelosinus spp.; 4, eukaryotic DPPS from Drosophila and Polysphondylium spp.; 5, solanesyl pyrophosphate synthase (SPPS) from eukaryotes and bacteria, plant species and Cyanobacteria; 6, octaprenyl pyrophosphate synthase (OPPS) from soil bacteria and Pseudomonas spp.; 7, PPPPS from flavobacteria, Prevotella and Bacteriodes spp.; 8, FPPS and GGPPS from methanogenic archaea, 9, GGPPS and PPPPS from Streptomyces, Micrococcus and Mycobacterium spp.; 10, GGPPS from sulfate reducing Desulfitobacteria spp.
As can be seen in Figure 2b, there are many sequences (from a broad range of organisms) with close sequence similarity to SaHepPPS and the vast majority are annotated as polyprenyl diphosphate synthases with ≥ C20 side-chains, many being found in Bacillus species. Shorter chain synthases, such as farnesyl diphosphate synthase (FPPS), are largely absent. No human genes were identified as close relatives in the HepPPS-2 network. However, a BLAST search for SaHepPPS-2 against the Homo sapiens genome did reveal one homolog, decaprenyl diphosphate synthase (HsDPPS-1) having a 22% sequence identity as calculated using MultiSeq.[7] In contrast, the BLAST analysis revealed no significant sequence similarities between SaHepPPS-1 and any human enzymes. This is of potential interest since as we show below, both SaHepPPS-1 and SaHepPPS-2 are essential for SaHepPPS activity, suggesting the possibility that selective inhibition might be possible by targeting the regulatory domain and/or its interaction with the catalytic domain.
Structure of S. aureus Heptaprenyl Diphosphate Synthase
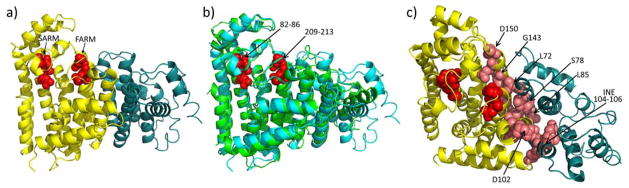
We next determined the structure of the heterodimeric protein, SaHepPPS, as described in the Experimental Section. Full data acquisition and refinement details are given in Table S1. The SaHepPPS (SaHepPPS-1 plus SaHepPPS-2) structure is shown in Figure 3a and contains two Asp-rich motifs: the first Asp-rich motif (FARM), DDVID, and the second Asp-rich motif (SARM) DDVLD, involved in catalysis, shown by the red spheres. As expected for a head-to-tail trans-prenyl transferase, the catalytic domain is highly helical. Using the SSM server,[8] we find that the closest known structure is that of hexaprenyl diphosphate synthase from Micrococcus luteus (PDB:3AQB)[9] where there is a 1.27 Å Cα RMSD for the catalytic domain (over 304 residues) and a 2.33 Å Cα RMSD for the regulatory domain (over 128 residues).[10] Structural superpositions of the two proteins are shown in Figure 3b. The catalytic domains are clearly more similar than are the regulatory domains, but also of interest, there is a cluster of highly conserved residues (pink spheres, Figure 3c) that lie at the interface between the two proteins. Partial sequence alignments of a series of regulatory (Figure 4a) and catalytic (Figure 4b) proteins annotated as heptaprenyl or hexaprenyl diphosphate synthases from S. aureus, S. haemolyticus, S. epidermidis, Streptococcus pneumoniae, Bacillus subtilis, and Micrococcus luteus (Figure 4) reveal that 9 out of 13 residues that are conserved in these regulatory proteins are located at the interface of HepPPS-1 and HepPPS-2. As expected, the majority of the conserved residues in the catalytic subunits are in close proximity to the substrate binding sites (FARM and SARM) but the others are at the interface, Figures 3c, 4b. So, even though there is low overall sequence homology between the different regulatory proteins, most of the residues that are conserved are located at the interface and are close to conserved (non-active site) residues in the catalytic proteins, implying that they play an important role in catalysis.
Figure 3.
X-ray structures of heptaprenyl and hexaprenyl diphosphate synthases. a) Structure of the dimeric protein SaHepPPS (PDB:5H9D) where HepPPS-1 is shown in teal. and HepPPS-2 is shown in yellow. The FARM and SARM Asp-rich motifs in the active site are shown as red spheres and are in the HepPPS-2 catalytic protein. b) Superimposition of SaHepPPS (cyan) and MlHexPPS (green; PDB:3AQB) structures. c) View of the highly conserved residues (in pink) that are located at or near the interface between SaHepPPS-1 (teal) and SaHepPPS-2 (yellow); FARM/SARM motifs are shown in red. The conserved residues at or near the interface were deduced from the ClustalW alignments shown in Figure 4.
Figure 4.
ClustalW alignments of regulatory and catalytic proteins in heptaprenyl and hexaprenyl diphosphate synthase heterodimers. a) S. aureus, S. epidermidis, S. haemolyticus, S. pneumoniae, B. subtilis, and M. luteus HepPPS-1 and HexPPS-1 sequences. Conserved residues in the interface of the two subunits are shown in orange. This is the regulatory protein. b) S. aureus, S. epidermidis, S. haemolyticus, S. pneumoniae, B. subtilis, and M. luteus HepPPS-2 and HexPPS-2 sequences. Conserved residues at the interface of the two subunits are shown in orange. This is the protein that contains the Asp-rich domains involved in catalysis.
We then sought to see how the SaHepPPS monomer structures compared with the structures of a broad range of other prenyl synthases by using QH[11] scores, obtained by using the MultiSeq program.[7] The QH score is a structural homology score that attempts to account for errors in structural alignments when the numbers of residues in target and query proteins differ. Homologous proteins have QH>0.4. When QH has a low value (0.1–0.3), structures are considered to have low homology. As can be seen in Figure S1A, the regulatory protein SaHepPPS-1 clusters with the M. luteus protein, MlHexPPS-1, but the cluster is isolated and the QH = 0.31 value indicates low homology. On the other hand, the catalytic domain of SaHepPPS-2 is very highly homologous (QH = 0.80) to that found in M. luteus HexPPS-2 (Figure S1B), and is also clustered with other long-chain (C40, C50) prenyl transferases. These observations reinforce the idea that SaHepPPS-1 has an unusual structure.
Enzyme Kinetics and Substrate Inhibition
We next investigated SaHepPPS catalytic activity, and its inhibition. In SaHepPPS, we find that the two components are not active individually, but form a heterodimeric complex that is catalytically active in the presence of IPP, FPP and Mg2+. Proteins that were separately expressed using the pET-28a vector had the same activity as proteins co-expressed using the pRSFDuet-1 vector. As detailed in the Experimental Section, we used a coupled assay in which diphosphate released in isoprenoid biosynthesis is converted by pyrophosphatase (baker’s yeast) to monophosphate, which in the presence of purine nucleotide phosphorylase (PNP) converts 7-methyl-6-thioguanosine to 7-methyl-6-thioguanine (E = 11,000 M−1•cm−1 at 360 nm), and the rate of increase in absorbance at 360 nm is taken to reflect the rate of HepPP synthesis.[12]
The observation that the two individual HepPPS components were inactive individually, but were active when mixed, is basically as observed with MlHexPPS[9] and other heterodimeric prenyl synthases.[13] However, the activity of the SaHepPPS heterodimeric complex at high substrate concentrations deviated very considerably from the hyperbolic dependence of velocity on substrate concentration seen with most enzymes, decreasing with increases in either IPP or FPP substrate concentration. One possible explanation for this behavior is formation of a ternary substrate-enzyme-substrate (SES) complex in which a second molecule of substrate binds to the ES complex, forming an inactive, ternary SES complex.[14] The rates of reaction observed with the active SaHepPPS heterodimer as a function of IPP and FPP substrate concentrations are shown in Figure 5. If this mechanism of substrate inhibition (i.e. negative feedback) is correct, then the effect of second substrate binding (and SES complex formation) on activity will be given by[14] the following equation, where Kd represents the dissociation constant for the inhibitory SES ternary complex:
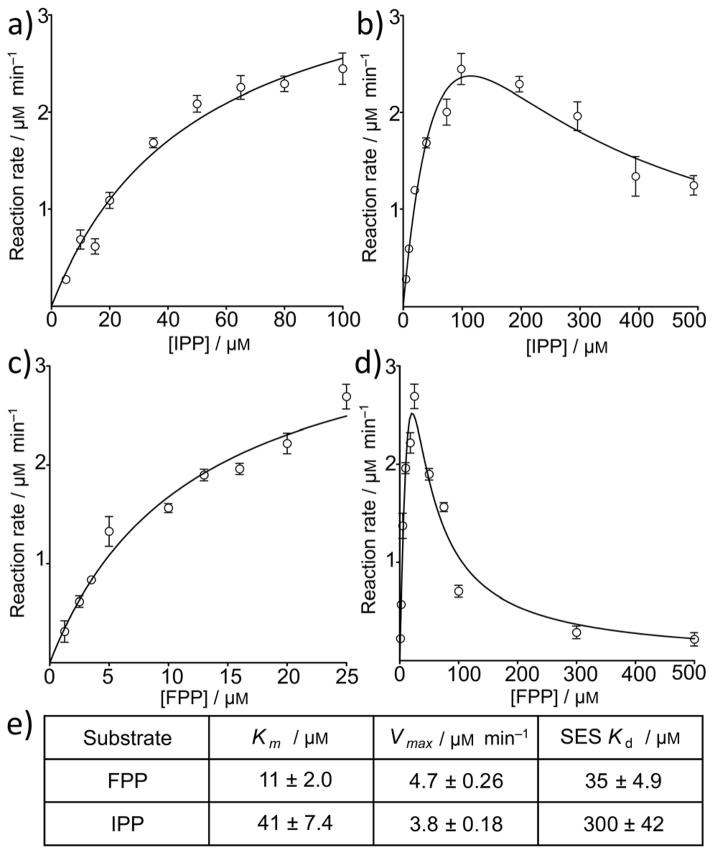
Figure 5.
Rates of reaction of SaHepPPS as a function of substrate (IPP, FPP) concentration. a) Low [IPP]. b) Low [FPP]. c) High [IPP]. d) High [FPP]. e) Kinetic parameters for SaHepPPS. Data points are shown as mean±SD, for duplicate experiments.
As shown in Figure 5, we estimated the Km and Kd values by splitting the velocity function domain at the maximum, because at low substrate concentrations HepPPS follows Michaelis-Menten kinetics (Figures 5a, b). The results suggest strong substrate inhibition, in particular with FPP. As can be seen in Figures 5c, d, the experimental results for both IPP as well as FPP inhibition (at high concentrations) can be fit by using the above equation using a Kd of 300 ± 40 μM for IPP, and 35 ± 5 μM for FPP (Figure 5c–e). We interpret these results to indicate that—at high concentrations—both FPP as well as IPP may bind to the regulatory protein, SaHepPPS-1, and indeed, Suzuki et al. have found, using atomic force microscopy, that FPP can bind to the regulatory domain in a Bacillus subtilis HepPPS.[15] It is thus possible that at high levels, FPP disrupts the HepPPS-1/HepPPS-2 protein-protein interaction required for catalysis. There might also, in principle, be feedback inhibition by HepPP (or other, not fully-elongated) products. However, this latter possibility seems unlikely since the longer (C20) chain species geranylgeranyl diphosphate (GGPP) did not exhibit the substrate-inhibition effect at high concentrations, having a lower Vmax and a much larger Km (Vmax = 2.2 μM/min and Km = 652 μM) than did FPP (Figure S2). As controls, we also investigated whether high FPP and IPP might inhibit the coupling enzymes (phosphatase and purine nucleoside phosphorylase), but as can be seen in Figure S3, this is not the case. It thus appears that FPP may play a role in regulating menaquinone biosynthesis, in S. aureus.
SaHepPPS Inhibition by Other Compounds
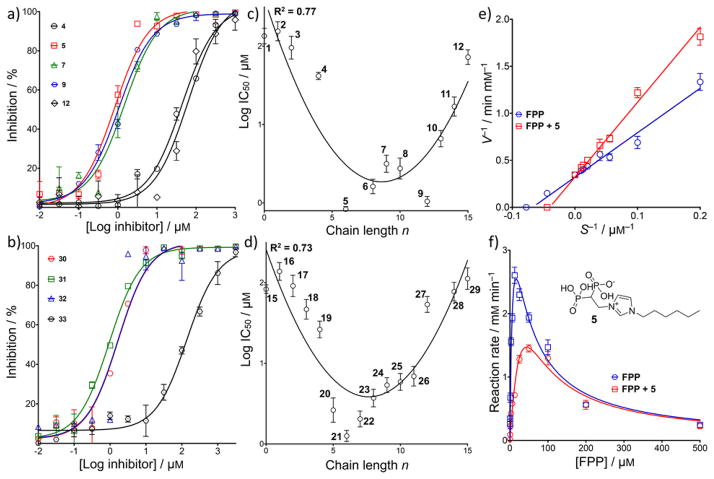
We next investigated a series of compounds that in earlier work were found to inhibit a diverse range of prenyl synthases since one of the objectives of our work is to find new drug leads having new targets. The structures of the 55 compounds (1–55) tested are given in Table S2, with their dose-response curves shown in Figure S4 and their syntheses and characterization were previously reported.[3a, 16] Of the several families of compounds tested—benzoic acids, sulfonic acids, diketo acids, bisphosphonates, and dicationic species—only the lipophilic bisphosphonates were active. Representative dose-response curves for imidazolium and pyridinium bisphosphonates are shown in Figures 6 a, b, and Table S2 shows more extensive data for zoledronate (15; Figure 1) and 28 analogs (containing 1-H and 1-OH groups, and varying N-alkyl substituents). The effects of chain length on activity for both 1-H and 1-OH analogs are illustrated in Figures 6 c, d.
Figure 6.
Dose-response curves and chain-length dependence of log(IC50) for HepPPS by bisphosphonates. a) Dose-response curves for SaHepPPS inhibition by 1-H analogs of zoledronate. b) Dose-response curves for SaHepPPS inhibition by pyridinium bisphosphonates. c) Effects of chain length on SaHepPPS inhibition (log IC50) for 1-H analogs of zoledronate. d) Effect of chain length on SaHepPPS inhibition (log IC50) for zoledronate analogs. e) Lineweaver-Burk plot for SaHepPPS in the presence of 1 μM 5. F) Enzyme kinetics of SaHepPPS in the presence of 1 μM 5. Data points are shown as mean±SD, for duplicate experiments.
What is clear from the results shown in Figure 6 (and Table S2) is that the most potent inhibitors have ~C6–C8 side-chains, which results in an overall length of ~22 Å, about that of the FPP substrate (~24 Å). Surprisingly, zoledronate itself (and its short chain N-alkyl chain-containing analogs) had very little activity with IC50 values of ~100 μM.
Zoledronate (15) has potent activity against human FPPS[17] as well as a (bifunctional) Plasmodium geranylgeranyl diphosphate synthase (P. vivax GGPPS; PvGGPPS)[18] and both of these proteins have similar folds. However, zoledronate has much less activity against human GGPPS due, it has been suggested, to the lack of the third Asp in the second aspartate rich motif (DDYAN) that coordinates to Mg2+ in the catalytic site.[19] So, the pattern of inhibition activity we observe is far more similar to that found in human GGPPS than in human FPPS or PvGGPPS. We also find that the lipophilic bisphosphonates are competitive inhibitors of HepPPS, as shown in Figure 6e for 5, an N-alkyl analog of zoledronate with a C6 side-chain addition (and no 1-OH group). As shown in Figure 6f, the reaction rate is significantly decreased in the presence of 1 μM 5 and does not increase at high FPP concentrations, due to the FPP substrate-induced inhibition discussed above.
Effects of Bisphosphonates on S. aureus Cell Growth
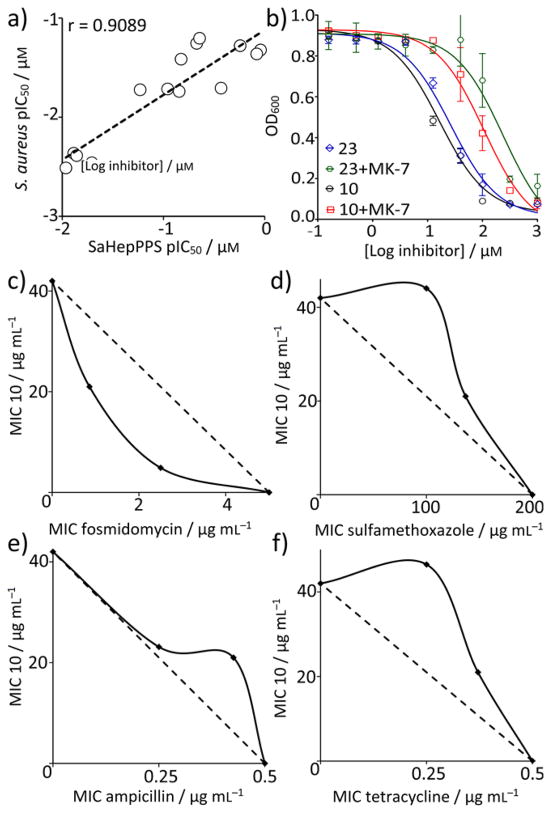
We next tested zoledronate and 28 analogs against S. aureus cell growth. 14 out of 28 inhibitors showed modest activity with a similar chain-length dependence to that seen with SaHepPPS enzyme inhibition, the most active inhibitors (IC50 ~ 16–23 μM; ~ 7–10 μg/mL) being the C8–C13 N-alkyl analogs (Table S3). Dose-response curves for the 14 bisphosphonates inhibiting S. aureus cell growth are shown in Figure S5 and their structures are shown in Table S3. As can be seen in Figure 7a, there is a correlation between the pIC50 (= −log10IC50 [μM]) values for SaHepPPS and S. aureus cell growth inhibition (a Pearson r-value correlation-coefficient r = 0.91). This correlation suggests that HepPPS might be a target, but there could be other targets, for example enzymes such as farnesyl diphosphate synthase (which is known to be inhibited by bisphosphonates), undecaprenyl diphosphate synthase (involved in cell wall biosynthesis and inhibited by some bisphosphonates) as well as MenA, another enzyme involved in quinone biosynthesis.
Figure 7.
Identification of targets of bisphosphonates inhibiting S. aureus cell growth. a) Correlation between S. aureus cell growth inhibition and SaHepPPS inhibition based on pIC50 (= −log10IC50 [μM]) results. b) Addition of 200 μM menaquinone-7 (MK-7) to the growth medium increases the IC50 of 10 and 23 for cell growth inhibition by a factor of ~8. Data points are shown as mean±SD, for duplicate experiments. c) 10+fosmidomycin in B. subtilis showing synergy (FICI=0.27) of 10 with a cell wall biosynthesis inhibitor (that targets DXR, 1-deoxy-D-xylulose-5-phosphate reductoisomerase, in the non-mevalonate pathway). d) 10+sulfamethoxazole in B. subtilis showing an indifferent effect (FICI=1.73) of 10 with a nucleic acid biosynthesis inhibitor (that targets dihydropteroate synthase). e) 10+ampicillin in S. aureus showing an indifferent effect (FICI=1.40) of 10 and a cell wall biosynthesis inhibitor (that targets transpeptidase). f) 10+tetracycline in S. aureus showing an indifferent effect (FICI=1.85) of 10 with a protein synthesis inhibitor (that targets ribosome function).
To test whether FPPS or UPPS inhibition might be contributing to S. aureus cell growth inhibition, we investigated synergistic interactions with a series of known drugs that target, or do not target, bacterial cell wall biosynthesis since synergistic activity would be expected with FPPS or UPPS inhibitors.[20] We carried out 12 fractional inhibitory concentration index (FICI) determinations using one of the most potent bisphosphonates 10 (zoledronate containing an N-C13 alkyl substituent and a 1-H group; IC50 = 7μg/mL; Figure 7b) together with either a known antibacterial isoprenoid/cell wall biosynthesis inhibitor (fosmidomycin, carbenicillin, vancomycin, ampicillin, bacitracin, cefotaxime, fosfomycin) or an antibacterial compound that does not target these pathways (kanamycin, tetracycline, chloramphenicol, spectinomycin, sulfamethoxazole, trimethoprim). We obtained FICI values for each combination using the FICI formula:[21]
where FICA and FICB are fractional inhibitory concentrations of drugs A and B, MIC(A) and MIC(B) are the MIC values of drugs A and B acting alone, and MIC(AB) and MIC(BA) are the MIC values of the most effective combination of drug A or B in the presence of drug B and A. FICI values of <0.5 are taken here to represent synergy, >0.5 and <1.0 represent additivity, >1 and <2 represent an indifferent effect, and ≥2 represents antagonism.[22] In addition, we evaluated isobolograms.[23] FICI values are shown in Table S4 and isobolograms are in Figures S6 and S7. We actually obtained data on two organisms: B. subtilis, chosen because it utilizes the non-mevalonate (MEP) pathway and is inhibited by fosmidomycin (which targets 1-deoxy-D-xylulose-5-phosphate reductoisomerase, DXR) as well as of course S. aureus, which uses the mevalonate pathway. The results shown in Table S4 show that in both organisms, there is—in almost all cases—an indifferent interaction with drugs in combination with 10 (FICIavg = 1.43 ± 0.37, n = 24). The only exception is with fosmidomycin where there is a synergistic interaction, in B. subtilis (FICI = 0.29 ± 0.094) (Figure 7c–f). The observation of a synergistic interaction between fosmidomycin and 10 but a lack of any synergistic interaction with the other cell wall biosynthesis inhibitors suggests that FPPS and UPPS are not major targets for 10 since these cell wall biosynthesis inhibitors would be expected to act in synergy with an FPPS or UPPS inhibitor. However, HepPPS and MenA remain as possible candidates because fosmidomycin targets 1-deoxy-D-xylulose 5-phosphate reductoisomerase, reducing FPP levels and thence, HepPP biosynthesis.
We next tested whether S. aureus cell growth inhibition was “rescued” by addition of menaquinone-7 (MK-7). As can be seen in Figure 7b, we investigated the effects of 200 μM MK-7 on S. aureus growth inhibition by the two most potent S. aureus cell growth inhibitors (10 and 23) finding that there was, on average, an ~8x increase in the IC50 for cell growth inhibition, consistent with an HepPPS target. However, this is not indicative of HepPPS being the sole target since we also find that 23 inhibits MenA (from E. coli) with an IC50 of ≈10 μM (Figure S8), and MK-7 should show a similar rescue effect if SaMenA were being targeted. It thus appears that, based on the totality of the evidence: SaHepPPS inhibition, MenA inhibition, fosmidomycin synergy and MK-7 rescue experiments, that both HepPPS as well as MenA are being targted by the lipophilic bisphosphonates, in cells.
Conclusions
The results we have described above are of interest for several reasons. We obtained the first structure of Staphylococcus aureus heptaprenyl diphosphate synthase, HepPPS, an anti-infective drug target. The protein crystallizes as a heterodimer containing a catalytic (HepPPS-2) as well as a regulatory (HepPPS-1) chain. In addition to the highly conserved (DDXXD) residues in the catalytic protein we found 22 highly conserved residues that exist at the interface between the two subunits. HepPPS was only catalytically active when both subunits were present and we found substrate inhibition with FPP with a Kd ~ 35 μM. We also discovered several μM inhibitors, N-alkyl zoledronate analogs, whose activity was dependent on alkyl side-chain length with Ki as low as ~200 nM. These compounds had modest activity against S. aureus cell growth (best IC50 = 7 μg/mL), which was partially rescued by menaquinone-7. These inhibitors acted synergistically with fosmidomycin but not with other, known cell-wall-targeting (or other) antibiotics, indicating that FPPS and UPPS are not being targeted. This is consistent with a HepPPS target although since we also find activity against a MenA (from E. coli), multi-site targeting is likely. Since SaHepPPS has high structural homology to only M. luteus hexaprenyl diphosphate synthase, not the more common trans-prenyl transferases such as human FPPS and GGPPS, our results open up the possibility of future in silico screening targeting both the catalytic and regulatory domains, to find selective SaHepPPS inhibitors as new drug leads.
Experimental Section
Clones and Plasmids
Staphylococcus aureus genomic DNA (ATCC, #700699) was used to amplify the gene sequences of the two components of HepPPS. PCR amplification of HepPPS-1 and HepPPS-2 was accomplished using gene-specific (IDT, Coralville, IA) primers designed from sequences identified in the S. aureus genome. HepPPS-1 forward 5′-GCCGCTAGCATGGAAACAACTGTTAGCAAATTGGAAAGACAAATAGAAGAAAGA and HepPPS-1 reverse 5′-GCCCTCGAGTAATTACCTCTACTTTTTAAATAACTTTTTTGGATATCGTGTAAGTAATGCTTTACTTCACT were used to amplify the HepPPS-1 sequence. HepPPS-2 forward 5′-GCCGCTAGCATGGCAAAGTTAAACATGAACAATGAAATTA AGAAAGTGGAACAAC and HepPPS-2 reverse 5′-GCCCTCGAGCTATGTGTTTCTTGACCCCATTTTTTTCGTCAAACTTAAAAGTAGT were used to amplify the HepPPS-2 sequence. Both HepPPS-1 and HepPPS-2 amplified sequences were incorporated into a pET28-a vector (Novagen, #69864-3). For co-expression of both components, HepPPS-1 forward 5′-GCCGGATCCCTGGTGCCGCGCGGCAGCATGGAAACAACTGTTAGCAAATTGGAAAGACAAATAGAAGAAAGA, HepPPS-1 reverse 5′-GCCGCGGCCGCTAATTACCTCTACTTTTTAAATAACTTTTTTGGATATCGTGTAAGTA ATGCTTTACTTCACT, HepPPS-2 forward 5′-GCCAGATCTATGGCAAAGTTAAACATGAACAATGAAATTAAGAAAGTGGAACAAC, and HepPPS-2 reverse 5′-GGCCTCGAGCTGGGCCGCGCGGCAGCTGTGTTTCTTGACCCCATTTTTTTCGTCAAACTTAAAAGTAGT primers were used. HepPPS-1 and HepPPS-2 genes were incorporated into multi-cloning sites 1 and 2 of a pRSFDuet-1 vector (Novagen, #71341-3), respectively, with thrombin cleavage site-containing linker sequences (DPLVPRGS) inserted between the N-terminal His6-tag and HepPPS-1, and between the C-terminal S-tag and HepPPS-2. The cloned products were transformed into E. coli BL21-CodonPlus (DE3)-RIPL cells, plated on LB containing 50 μg/mL kanamycin and 34 μg/mL chloramphenicol, and cultured overnight at 37°C to select kanamycin/chloramphenicol resistant clones, which were then confirmed by DNA sequencing (ACGT Inc., Wheeling, IL).
Recombinant protein expression and purification
A single colony was used to inoculate 100 mL of LB broth containing 50 μg/mL kanamycin and 34 μg/mL chloramphenicol at 37°C, overnight. The overnight culture was transferred to six 1 L flasks of LB broth containing the same antibiotic concentrations. Flasks were shaken at 200 RPM at 37°C until the OD600 reached ~0.5. Recombinant protein expression was induced with 1 mM IPTG and cells were incubated at room temperature, overnight. Cells were then harvested by centrifugation at 10,800 ×g for 10 minutes. Protein was obtained by lysing the cells by three freeze-thaw cycles, followed by sonication for 6 minutes. Cell lysates were centrifuged at 57,200×g for 30 minutes. Recombinant proteins were purified from the supernatant by Ni-NTA affinity chromatography using 10 mM HEPES, pH 7.5 buffer containing 500 mM NaCl, 35 mM imidazole, then eluted with increasing concentrations of imidazole in a gradient (35 mM to 500 mM) in the same buffer. The purified protein fractions were run on a 4–20% SDS polyacrylamide gel to ascertain size and purity. SaHepPPS-1 had an apparent MW of 22 kDa and SaHepPPS-2 had an apparent MW 36 kDa. For co-expression of the two components using the pRSFDuet-1 vector, basically the same protocol was used except that the kanamycin concentration was reduced to 30 μg/mL, and protein expression was induced with 0.3 mM IPTG. For purification of co-expressed proteins, 20 mM HEPES, pH 7.0 buffer containing 150 mM NaCl, 2 mM CHAPS, 10 mM β-mercaptoethanol, 35 mM imidazole, 6% glycerol was used, and protein was eluted with increasing concentrations of imidazole in a gradient (35 mM to 500 mM) in the same buffer. SaHepPPS-1 was cloned with a His6-tag and SaHepPPS-2 with an S-tag, however, Ni-NTA affinity chromatography alone was sufficient to purify both enzymes in the heterodimeric structure because of strong intermolecular binding affinity. For the crystallization experiments, the HepPPS-1 gene was inserted into an ampicillin resistant pET46 EK/LIC (Novagen) by using an EK/LIC cloning kit, creating a pET46-HepPPS-1 construct which expressed HepPPS-1 with an N-terminal His6-tag. The HepPPS-2 gene was inserted into a kanamycin resistant pET28a (Novagen) and the pET28a-HepPPS-2 construct expressed HepPPS-2 without any tag. Both plasmids were transformed into E. coli BL21(DE3) cells and target proteins were expressed by inducing with IPTG. Cells overexpressing HepPPS-1 and HepPPS-2 were mixed and harvested by centrifugation at 5,000×g for 20 minutes. The mixed cells were re-suspended in lysis buffer containing 50 mM Tris-HCl, pH 7.5, 300 mM NaCl, 10 mM imidazole and 10% glycerol. Cells were disrupted by a French press and the debris removed by centrifugation at 17,000×g for 1 hour. The supernatant was then loaded onto a Ni-NTA column. HepPPS-1 and HepPPS-2 formed a complex and the target proteins eluted at ~150 mM imidazole. The purified protein fractions were run on a 4–12% SDS-PAGE to ascertain size and purity. The complex showed two bands: at 22 kDa for SaHepPPS-1 and at 36 kDa for SaHepPPS-2. Proteins were concentrated to 1 mL and applied to a size exclusion column (Sephacryl S-200 26/60, 320 mL, GE Healthcare) equilibrated with buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl) at a flow rate of 1 mL/min. Unknown proteins, HepPPS complex, and HepPPS-1 showed three peaks on the elution profile. The second peak contained the HepPPS complex. The purified proteins were then concentrated to 5 mg/mL in 25 mM Tris-HCl, pH 7.5, 150 mM NaCl buffer.
Crystallization, data collection, structure determination and refinement
SaHepPPS crystals were obtained using the No. 40 solution of the PEG/Ion 2 screen kit (Hampton Research) (0.03 M citric acid, 0.07 M bis-tris propane / pH 7.6, 20% w/v polyethylene glycol 3350) using the sitting-drop vapor method. In general, 1 μL 5 mg/mL SaHepPPS was mixed with 1 μL of reservoir solution in 48-well Cryschem plates, then equilibrated against 100 μL of the reservoir at 25°C. Within 3 days, the crystals reached dimensions suitable for X-ray diffraction. X-ray diffraction datasets were collected at beam lines BL13B1, BL13C1, and BL15A1 of the National Synchrotron Radiation Research Center (NSRRC, Hsinchu, Taiwan) and were processed by using the HKL2000 program.[24] Prior to structure refinement, 5 % randomly selected reflections were set aside for calculating Rfree as a monitor of model quality.[25] The SaHepPPS structure was solved by using molecular replacement (MR) using the crystal structure of hexaprenyl diphosphate synthase from Micrococcus luteus B-P 26, which shares 44% identity with SaHepPPS-2 (PDB:3AQB), as template. Model building was carried out by using Coot.[26] Refinement employed PHENIX[27] or REFMAC.[28] The final MR model gave R and Rfree values of 0.24 and 0.29 respectively at 2.68 Å resolution after refinement against the native SaHepPPS dataset using the CNS program.[29] All graphics for the protein structures were prepared by using the PyMOL program (http://pymol.sourceforge.net/.)
Enzyme kinetics and Inhibition
All putative inhibitors were prepared in 10 mM stock solutions in either DMSO or sodium carbonate buffer, and were then serially diluted in a gradient from 1 mM to 1 nM. Inhibitors were incubated with 25 μg of co-expressed enzymes at room temperature for 10 minutes in assay buffer (10 mM HEPES, 150 mM NaCl, 1 mM MgCl2, pH 7.5) before adding “reaction mixture” containing 20 μM FPP, 80 μM IPP, 3 U/mL purine nucleoside phosphorylase, 1 U/mL phosphatase, and ~ 600 μM MESG, again in the assay buffer. Reactions were monitored for 15 minutes. The rate of increase in absorbance at 360 nm was taken as the rate of HepPP synthesis. The 50% inhibitory concentration (IC50) values were calculated by using Prism 5 (GraphPad Software, Inc., La Jolla, CA).
MenA Inhibition Assay
The MenA inhibition assay was carried out as described previously. [30]
S. aureus and B. subtilis Growth Inhibition Assay
IC50 values for S. aureus cell growth inhibition were determined by using a microdilution method. An overnight starter culture of S. aureus (Newman strain) in tryptic soy broth was diluted 1000-fold in fresh tryptic soy media to create a “working solution”. 200 μL of working solution was transferred into each well of a 96-well culture plate (Corning 3370). Inhibitors were then added at 1 mM and sequentially diluted 3× to 46 nM, keeping volume and culture broth composition constant. Plates were incubated for 12 hours at 37°C, shaking at 200 RPM, then absorbance at 600 nm was measured to assess bacterial cell growth. IC50 values were determined using nonlinear regression whereas minimum inhibitory concentration (MIC) values in the synergy assays were calculated by using a Gompertz function in Prism 5 (GraphPad Software, Inc., La Jolla, CA). For the B. subtilis cell growth inhibition assay, an overnight starter culture (in LB broth) of B. subtilis (subsp. subtilis (Ehrenberg) Cohn ATCC 6051) was diluted 1000-fold (in fresh LB media) to create a “working solution”. Working solutions were then transferred into flat-bottom 96-well plates and inhibitors were added at 1 mM and sequentially diluted 3× to 46 nM. Plates were incubated at 37°C, shaking at 200 RPM overnight. The OD600 was then measured to determine bacterial growth inhibition.
Synergy/Antagonism Assays
In order to investigate possible synergistic interactions between compound 10 and a range of antibiotics, we carried out two-drug combination assays. Bacteria were incubated with a 3× gradient of antibiotic typically ranging from 40 μg/mL to 18 ng/mL (200 μg/mL to 90 ng/mL for bacitracin, fosfomycin, and sulfamethoxazole) in the presence of half-MIC concentrations of 10, in addition to a 3× gradient of 10 ranging from 40 μg/mL to 18 ng/mL in the presence of half-MIC concentrations of each antibiotic. New MIC values were calculated by using a Gompertz function in Prism 5 (GraphPad Software, Inc, La Jolla, CA).
Supplementary Material
Acknowledgments
This work was supported by the United States Public Health Service (NIH grants CA158191 and GM065307), a Harriet A. Harlin Professorship, the University of Illinois Foundation/Oldfield Research Fund, and the National Natural Science Foundation of China (grants 31200053, 31300615, 31400678 and 31470240) J. D. was supported by the NIGMS-NIH Molecular Biophysics Training Grant (GM008276). The authors thank Guodong Rao for performing the MenA inhibition assays.
References
- 1.Oldfield E. Acc Chem Res. 2010;43:1216–1226. doi: 10.1021/ar100026v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oldfield E, Lin FY. Angew Chem Int Ed Engl. 2012;51:1124–1137. doi: 10.1002/anie.201103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Zhu W, Zhang Y, Sinko W, Hensler ME, Olson J, Molohon KJ, Lindert S, Cao R, Li K, Wang K. Proc Natl Acad Sci USA. 2013;110:123–128. doi: 10.1073/pnas.1219899110. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shang N, Li Q, Ko TP, Chan HS, Li J, Zheng Y, Huang CH, Ren F, Chen CC, Zhu Z. PLoS Pathogens. 2014;10:e1004114. doi: 10.1371/journal.ppat.1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hamamoto H, Urai M, Ishii K, Yasukawa J, Paudel A, Murai M, Kaji T, Juranaga T, Hamase K, Katsu T. Nat Chem Biol. 2015;11:127–133. doi: 10.1038/nchembio.1710. [DOI] [PubMed] [Google Scholar]
- 4.Bentley R, Meganathan R. Microbiol Rev. 1982;46:241–280. doi: 10.1128/mr.46.3.241-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerlt JA, Bouvier JT, Davidson DB, Imker HJ, Sadkhin B, Slater DR, Whalen KL. Biochim Biophys Acta. 2015;1854:1019–1037. doi: 10.1016/j.bbapap.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. Nucleic Acids Res. 2008;36:W5–9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts E, Eargle J, Wright D, Luthey-Schulten Z. BMC Bioinformatics. 2006;7:382. doi: 10.1186/1471-2105-7-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krissinel E, Henrick K. Acta Crystallogr D. 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki D, Fujihashi M, Okuyama N, Kobayashi Y, Noike M, Koyama T, Miki K. J Biol Chem. 2011;286:3729–3740. doi: 10.1074/jbc.M110.147991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shindyalov IN, Bourne PE. Nucleic Acids Res. 2011;29:228–229. doi: 10.1093/nar/29.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Donoghue P, Luthey-Schulten Z. Microbio Mol Biol Rev. 2003;67:550–573. doi: 10.1128/MMBR.67.4.550-573.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webb MR. Proc Natl Acad Sci USA. 1992;89:4884–4887. doi: 10.1073/pnas.89.11.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang YW, Li XY, Toyama T. Biochemistry. 2000;39:12717–12722. doi: 10.1021/bi001311p. [DOI] [PubMed] [Google Scholar]
- 14.Copeland RA. Enzymes. Wiley-VCH; Weinheim: 2000. pp. 137–141. [Google Scholar]
- 15.Suzuki T, Zhang YW, Toyama T, Sasaki DY, Kurihara K. J Am Chem Soc. 2006;128:15209–15214. doi: 10.1021/ja061822k. [DOI] [PubMed] [Google Scholar]
- 16.a) Zhang YH, Zhu W, Liu YL, Wang H, Wang K, Li K, No JH, Ayong L, Gulati A, Pang R. ACS Med Chem Lett. 2013;4:423–427. doi: 10.1021/ml4000436. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang Y, Cao R, Yin F, Hudock MP, Guo RT, Krysiak K, Mukherjee S, Gao YG, Robinson H, Song Y. J Am Chem Soc. 2009;131:5153–5162. doi: 10.1021/ja808285e. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chen CKM, Hudock MP, Zhang YH, Guo RT, Cao R, No JH, Liang PH, Ko TP, Chang TH, Chang SC. J Med Chem. 2008;51:5594–5607. doi: 10.1021/jm800325y. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Zhang Y, Cao R, Yin F, Lin F, Wang H, Krysiak K, No JH, Mukkamala D, Houlihan K, Li J, Morita C, Oldfield E. Angew Chem Int Ed. 2009;49:1136–1138. doi: 10.1002/anie.200905933. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Lin FY, Zhang Y, Hensler M, Liu YL, Chow OA, Zhu W, Wang K, Pang R, Thienphrapa W, Nizet V, Oldfield E. ChemMedChem. 2012;7:561–564. doi: 10.1002/cmdc.201100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell RG. Ann N Y Acad Sci. 2006;1068:367–401. doi: 10.1196/annals.1346.041. [DOI] [PubMed] [Google Scholar]
- 18.No JH, de Macedo Dessin F, Zhang Y, Liu YL, Zhu W, Feng X, Yoo JA, Lee E, Wang K, Hui R. Proc Natl Acad Sci USA. 2012;109:4058–4063. doi: 10.1073/pnas.1118215109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arts D, Wernimont AK, Dunford JE, Schapira M, Dong A, Zhao Y, Lew J, Russell RG, Ebetino FH, Oppermann U, Hui R. J Biol Chem. 2010;286:3315–3322. doi: 10.1074/jbc.M109.027235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farha MA, Czarny TL, Myers CL, Worrall LJ, French S, Conrady DG, Wang Y, Oldfield E, Strynadka NC, Brown ED. Proc Natl Acad Sci USA. 2015;112:11048–11053. doi: 10.1073/pnas.1511751112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.a) Eliopolous GM, RC . In: Moellering in Antibiotics in Laboratory Medicine. Lorian V, editor. Williams & Wilkins Publishing Co; 1998. pp. 330–396. [Google Scholar]; b) Singh PK, Tack BF, McCray PB, Welsh MJ. Am J Physiol Lung Cell Mol Physiol. 2000;279:L799–805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- 22.European Committee for Antimicrobial Susceptibility Testing (EUCAST) Clin Microbiol Infect. 2000;6:503–508. doi: 10.1111/j.1469-0691.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- 23.Berenbaum MC. Pharmacol Rev. 1989;41:93–141. [PubMed] [Google Scholar]
- 24.Otwinowski Z, Minor W. Macromole Crystallogr A. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 25.Brünger AT. Acta Crystallogr D. 1993;49:24–36. doi: 10.1107/S0907444992007352. [DOI] [PubMed] [Google Scholar]
- 26.Emsley P, Cowtan K. Acta Crystallogr D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 27.Borbulevych OY, Plumley JA, Martin RI, Merz KM, Jr, Westerhoff LM. Acta Crystallogr D. 2014;70:1233–1247. doi: 10.1107/S1399004714002260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murshudov GN, Skubak P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F, Vagin A. Acta Crystallogr D. 2011;67:355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 30.Li K, Schurig-Briccio L, Feng X, Upadhyay A, Pujari V, Lechartier B, Fontes FL, Yang H, Rao G, Zhu W. J Med Chem. 2014;57:3126–3139. doi: 10.1021/jm500131s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. Nature Protocols. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.