Abstract
Hypospadias results from a failure of urethral closure in the male phallus and affects 1 in 200–300 boys. It is thought to be due to a combination of genetic and environmental factors. The development of the penis progresses in 2 stages: an initial hormone-independent phase and a secondary hormone-dependent phase. Here, we review the molecular pathways that contribute to each of these stages, drawing on studies from both human and mouse models. Hypospadias can occur when normal development of the phallus is disrupted, and we provide evidence that mutations in genes underlying this developmental process are causative. Finally, we discuss the environmental factors that may contribute to hypospadias and their potential immediate and transgen erational epigenetic impacts.
Keywords: Disorders of sexual development, Hypospadias, Penis, Phallus, Urethra
Hypospadias is a congenital anomaly in which the urethral opening is not correctly positioned at the tip of the penis. It is the second most common congenital disorder in boys after cryptorchidism and the most frequent malformation of the penis [Ságodi et al., 2014], affecting one in 200–300 boys [Blaschko et al., 2012]. The severity of hypospadias can vary from minor to severe depending on where the urethral opening is located (fig. 1) and often requires surgery [Snodgrass et al., 2011]. Given the frequency and on-going implications, hypospadias is an important health issue and can be a substantial burden on health-care resources. Indeed, because of a high risk of complications, such as recurrent stenosis or fistulae, the most severe cases often require several surgeries. In addition, a significant percentage of patients suffer from cosmetic or functional difficulties that can affect urinary and sexual function [Aulagne et al., 2010]. In the last consensus on definition of disorders of sexual development (DSDs), hypospadias was included as a form of 46,XY DSD [Hughes et al., 2006]. Numerous DSDs have a genetic cause [Eggers and Sinclair, 2012], and a number of genes have been broadly implicated in hypospadias; it is thought that a combination of environmental influences and genetic susceptibility cause this anomaly [Kalfa et al., 2011a; Marrocco et al., 2015]. Only 30% of hypospadias cases have a clear genetic cause [Ságodi et al., 2014]. In this review, we will discuss the current knowledge of the genetic contribution to hypospadias in humans and consider candidate genes implicated through mouse studies. Finally, we discuss the role of the environment and epigenetics in hypospadias risk.
Fig. 1.
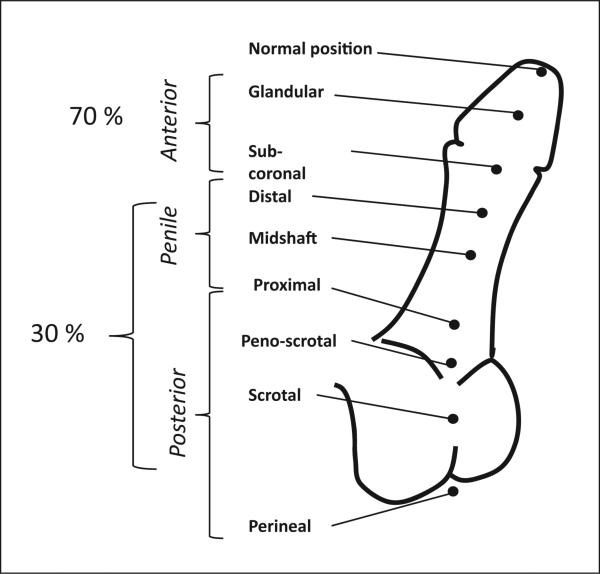
Clinical classification of the severity of hypospadias. Hypospadias severity is classified according to the position of the urethral meatus. Anterior hypospadias is also referred to as distal or minor, and posterior as proximal or severe. The respective prevalence for each form is noted.
Hypospadias-Associated Malformations and Syndromes
In the majority of cases, hypospadias presents as an isolated condition, but it can occur in association with other abnormalities, especially in the urogenital tract. The most common associated abnormalities are: uni- or bilateral cryptorchidism, a condition where one or both of the testes have not descended properly into the scrotum, and micropenis, where the dorsal length of the neonate penis is less than 2.5 cm. The occurrence of these co-morbidities often indicates an androgen deficiency, which can be tested by hormone assays [Snodgrass et al., 2011]. In addition, hypospadias has been described in over 200 other syndromes [Kalfa et al., 2011a]. Among these, are WAGR [Wilms’ tumour (WT), aniridia, genitourinary abnormalities and mental retardation], Denys-Drash syndrome (genitourinary malformations and sus ceptibility to WT) and Smith-Lemli-Opitz syndrome (malformations of the heart, lungs, kidneys, gastrointestinal tract, and genitalia). In these syndromic cases, there are often well-defined genetic causes [reviewed in Shih and Graham, 2014] such as mutations in WT1 in Denys-Drash syndrome or in DHCR7 for Smith-Lemli-Opitz. In this review, we will concentrate on the molecular genetics underlying non-syndromic hypospadias.
Isolated Hypospadias and Known Genetic Causes
Numerous lines of evidence suggest an important genetic component in hypospadias with the presence of an affected family member being the biggest risk factor so far identified [Thorup et al., 2014]. Overall, the heritability of hypospadias is suggested to be between 57 and 77% [Stoll et al., 1990; Schnack et al., 2008], with equal transmission through both maternal and paternal lines [Schnack et al., 2008]. In non-syndromic (isolated) forms of hypospadias, most cases are idiopathic [Carmichael et al., 2012]; however, familial aggregation can be found in ~ 10% of cases [Kalfa et al., 2011a]. For example, the brother of a male with hypospadias has a 9–17% risk of also having the condition [Schnack et al., 2008]. Heritability appears to vary with severity, with several studies showing that anterior or middle hypospadias occurs more frequently in familial clusters than proximal hypospadias [Fredell et al., 2002; Brouwers et al., 2010]. However, it is hard to formally distinguish this from a lack of fecundity or appropriate sexual function in the most severe cases. Recent advances in massively parallel sequencing technologies and linkage studies using large cohorts are uncovering novel hypospadias-associated genes and potentially causative variants [Achermann et al., 2015; Kon et al., 2015]. In addition, the use of animal models has shed light on the molecular genetics underlying many aspects of normal and abnormal penile development.
Normal and Abnormal Penile Development in Humans
Hypospadias is caused by abnormal or incomplete urethral closure during the early weeks of embryonic development. In humans, the development of external genitalia occurs in 2 phases: an early hormone-independent phase (5–8 weeks of gestation) and a later hormone-dependent stage (weeks 8–12) [Blaschko et al., 2012] (fig. 2).
Fig. 2.
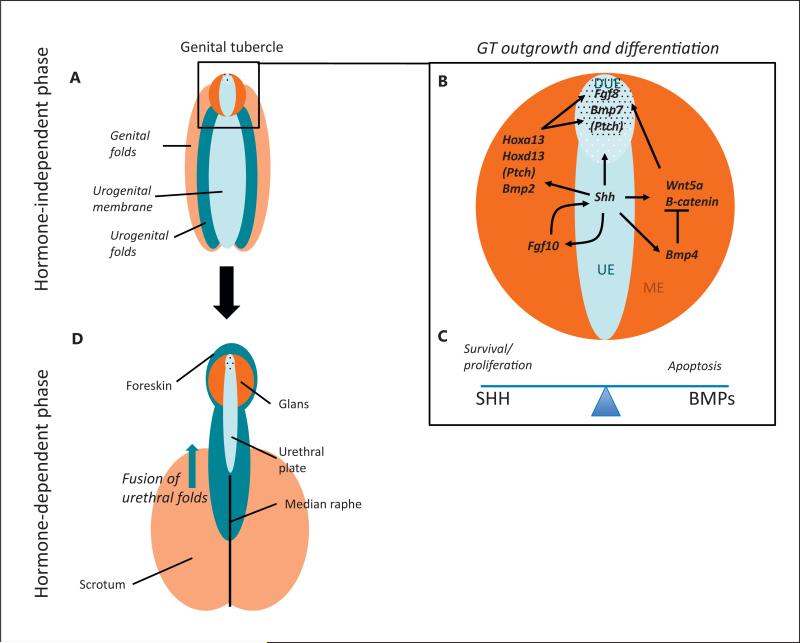
The molecular pathways underlying the development of the male external genitalia. A–C The hormone-independent phase where the urogenital sinus is indifferent. This phase occurs following the division of the cloacal membrane into the urogenital sinus and anal membrane. A At this stage the tissue is made up of the urethral folds (dark blue), the urethral epithelium (light blue), the labioscrotal swellings (light orange), and the mesenchyme of the GT (dark orange). B The GT (enlarged in box) consists of a urethral plate epithelium (UE), including the distal urethral epithelium (DUE), surrounded by a bilateral mesenchyme (ME). Numerous signalling molecules contribute to GT outgrowth and differentiation during this first phase. A central cue is SHH, which is expressed in the UE, and activates its pathway via its receptor Patched (PTCH). In the bilateral mesenchyme the HH pathway activates the expression of Hoxa13 and Hoxd13, Wnt5a and its pathway (including β-catenin). SHH signalling also activates Fgf8 and Bmp7 in the DUE. SHH has a positive feedback loop with FGF10 in the mesenchyme. SHH signalling is required for both GT outgrowth and cell survival. SHH targets Fgf8 and Wnt5a are also necessary for outgrowth. Opposing this, BMP4 (and other BMPs) repress outgrowth, perhaps via their repression of Wnt5a and Fgf8. C BMP4 also represses proliferation while activating cell apoptosis, a balance that is counteracted by SHH signaling. D The later hormone-dependent stage of development after masculinisation has occurred. During masculinisation, testosterone secreted by the testis causes the GT to elongate and urogenital membrane to transform into urethral groove. Following this, all components of the penis start to close ventrally, giving rise to the median raphe. A balance between testosterone and oestrogen signalling regulates these processes. The colour of each tissue represents its origin in the previous stages.
During the hormone-independent phase, the external genitalia are indistinguishable between males and females as sexual differentiation of the gonads is not yet complete. During the 5th week of gestation, mesodermal cells spread along the cloacal membrane to form a pair of swellings – the cloacal folds. These fuse along the midline, anteriorly to the cloacal membrane, to form the genital tubercle (GT) [Schoenwolf et al., 2009]. These cloacal folds then divide into urogenital folds flanking the urogenital sinus and anal folds posteriorly. Labioscrotal folds then develop on each side of the urogenital folds. The GT consists of lateral plate mesoderm, surface face ectoderm and endodermal urethral epithelium that derives from the urogenital sinus (fig. 2). The distal most part of this urethral plate epithelium (DUE) acts as a signalling centre to stimulate GT outgrowth and differentiation. From week 8 onwards, when the gonads have differentiated into testes in XY males, the hormone-dependent phase of sexual differentiation begins. The presence of testosterone produced by the testis causes the GT to elongate and the urethral groove appears, laterally defined by the urethral folds. The distal portion of the urethral groove terminates in a solid epithelial plate, called the urethral plate, which extends into the glans penis. Fusion of the urethral folds is the key step in the formation of the penile urethra. Eventually, in males, the GT gives rise to the glans and the labioscrotal folds fuse to form the scrotum (fig. 2). Finally, the penile skin, including the foreskin, develops from ectodermal tissue externally covering the entire penile length, and the corporeal bodies arise from mesenchymal condensations. Closure of these structures on the ventral midline gives rise to a ridge of tissue referred to as the median raphe.
Perturbation of the genes or signalling pathways involved in phallus development and urethral closure can result in hypospadias, which typically refers to an anomaly of closure of the ventral aspect of the penis. This results in an abnormal location of the urethral opening on the ventral aspect of the penis (fig. 1). This anomaly can also be associated with (1) a variable degree of ventral curvature of the penis, known as chordee, and (2) an abnormal foreskin, usually incomplete, referred to as hooded foreskin. Although the criteria used to evaluate the severity of hypospadias are not well defined, it is generally accepted that the position of the urethral meatus alone is not sufficient and that the level of division of corpus spongiosum, an inner component of the penis, should also be taken into account. For this reason, a definitive classification can only be completed during surgery [Snodgrass et al., 2011]. Indeed, during surgery, a portion of closed but hypoplastic urethra between the proximal division of the corpus spongiosum and the position of the urethral meatus may be uncovered and require refashioning [Snodgrass et al., 2011]. Nevertheless, hypospadias is usually classified solely on the position of the urethral opening into distal (anterior), midshaft (middle) or proximal (posterior), with some subcategories further defining the position of the malformation (fig. 1). Anterior forms of hypospadias represent 70% of cases, midshaft and posterior cases account for the remaining 30% [Carmichael et al., 2012].
Genes Implicated in Early Patterning of the GT and Hypospadias
Development of the external genitalia during mammalian evolution was crucial to enable internal fertilisation and colonisation of the land. All therian mammals have external genitalia, and development of the GT is often compared to that of the limbs [Yamada et al., 2006; Cohn, 2011]. Indeed, the morphogenesis of both structures appears to involve similar genetic pathways including canonical Wingless-type MMTV integration site family (WNT) signalling, Hedgehog (HH) signalling, bone morphogenetic protein (BMP) signalling and Hox genes.
During embryonic development, the DUE is thought to be the major signalling centre, and regulates the growth of the GT via several signalling pathways [Haraguchi et al., 2000; Perriton et al., 2002]. Animal models are useful in understanding the potential role of these pathways. However, interpretation of mouse hypospadias pheno-types is confounded by differences in morphology between the mouse and human penis [Cunha et al., 2015]. As opposed to the human penis, which is fully developed at birth, rat and mouse penis development occurs both pre- and postnatally [Cunha et al., 2015]. The undifferentiated GT of embryonic or newborn mice is immature and subtle malformations in the external genitalia are often not manifest until several weeks after birth [Cunha et al., 2015]. Recently, however, a detailed annotation of the adult mouse penis has provided a better understanding of its development and relevance to humans [Phillips et al., 2015]. This important resource will enable an accurate comparison of murine and human hypospadias pheno-types in future studies [Phillips et al., 2015].
In humans, the majority of hypospadias association studies have either been mutation analyses in key genes or association/linkage studies in large affected cohorts. While these do not prove pathogenicity, they are valuable. Some expression analysis has also been performed, but not always in the affected tissue (i.e. in foreskin tissue removed from hypospadias patients and not the urethral epithelium). Below, we draw evidence from both animal models and human studies for the involvement of several different signalling pathways and genes in normal GT development and hypospadias.
The Hedgehog Signalling Pathway
The Hh signalling pathway plays numerous important roles during embryogenesis [Tang and Cheng, 2014]. In mammals, 3 HH genes can activate this pathway, including sonic hedgehog (SHH) , desert hedgehog (DHH) and Indian hedgehog (IHH). In mice, SHH plays a role in both the initial development of embryonic external genitalia and also in genital masculinisation [Haraguchi et al., 2001; Perriton et al., 2002]. In the hormone-independent phase, SHH from the urethral plate epithelium is required for outgrowth, patterning, and cell survival in the developing GT [Perriton et al., 2002] (fig. 2). Mice with a targeted deletion of Shh show initiation of the genital swellings, but outgrowth is not maintained, meaning that external genitalia are absent [Haraguchi et al., 2001]. It is thought that SHH signalling modulates the balance between apoptosis and proliferation [Haraguchi et al., 2001] (fig. 2). The SHH signal is mediated through its receptor Patched which is upregulated by SHH in the DUE, and the pathway activator Smoothened which controls the activity of the GLI transcription factors (GLI1–3). GLI1 and GLI2 are thought to be the main transcriptional activators for the pathway. In Gli2, knockout mice outgrowth of the GT is initiated, but urethral formation was lacking [Miyagawa et al., 2011]. The critical role of SHH signalling during this hormone-independent phase is relayed either directly or indirectly by the expression of its targets Fgf8 and Bmp7 in the DUE or Fgf10, Bmp2, Bmp4, Hoxa13, and Hoxd13 in the mesenchyme (fig. 2).
In the human foetal penis, SHH, PTCH1, SMO, and GLI1 are all expressed around the time of urethral closure [Shehata et al., 2011], indicating that the role of this pathway is conserved. Variants in both SHH itself, and also in downstream components of its signalling pathway, have been associated with hypospadias in humans. In a large Californian cohort of patients with hypospadias, several SNPs in SHH, GLI1, GLI2, and GLI3 were associated with an increased risk [Carmichael et al., 2013a] (table 1). Interestingly, some SNPs showed an association only among Caucasian patients and some SNPs in GLI1 were associated with a decreased risk [Carmichael et al., 2013a] (table 1).
Table 1.
Polymorphisms associated with hypospadias in humans
| Gene | Variants | Effect | Reference | |
|---|---|---|---|---|
| Hedgehog pathway | SHH | rs9333613 (A:G) | ↑ (M) | Carmichael et al., 2013a |
| GLI1 | rs10783827 (T:G), rs4760259 (C:T), rs2228226 (C:G) rs3825077 (G:A), rs3782126 (G:A), rs2292657 (C:T) |
↑ (M) ↓ |
||
| GLI2 | rs4848125 (A:G), rs4848126 (G:A) rs4143116 (G:A) |
2 ↑ (M, Cauc) 2 ↑ (M) |
||
| GLI3 | rs6974655 (G:T) rs9886211 (A:G) rs3801223 (C:T) |
↑ (A) ↑ (P) ↑ (M) |
||
| FGF pathway | FGF8 | rs3218238 (G:A) | ↑ | Beleza-Meireles et al., 2007a |
| FGF10 | rs16901816 (T:G), rs2973644 (T:C), rs2973646 (C:A) rs6892212 (A:C) |
2– 4 ↑ (M, Cauc) 3 ↑ (M, Cauc) |
Carmichael et al., 2013a | |
| FGFR2 | c.382 + 52→G, c.550 + 27C>T, c.727 + 180T>G M186T (hetero) |
↑ ↑ (M) |
Beleza-Meireles et al., 2007a | |
| BMP | BMP7 | rs6070007 (C:A) rs6127978 (A:G), rs6127985 (G:A) rs6127980 (G:A) |
↑ (M) ↑ ↑ (P) |
Carmichael et al., 2013a |
| WT1 | WT1 | rs12293750 (C:A) rs1799937 (T:C), rs5030277 (T:A), rs16754 (A:G), rs5030234 (C:A), rs385449 (G:A) |
4 ↑ (A) ↑ (P) |
Carmichael et al., 2013a |
| Additional genes in GT patterning | DGKK | rs1934179 (A), rs7063116 (A) | 2 ↑ (A, M) | van der Zanden et al., 2011 |
| rs1934179, rs7063116, rs4143304, rs9969978, rs1934190, rs1934188 | ↑ (A, M) | Ma et al., 2014 | ||
| rs5961179, rs7882950, rs12556919, rs17003341, rs1934190, rs4143304, rs1934188, rs17328236, rs9969978, rs1934179, rs5961183, rs7876567, rs7063116 | ↑ (A, M) | Carmichael et al., 2013b | ||
| rs1934183, rs6614511 | ↓ | |||
| Androgen production and signalling | HSD3B1 | rs6203(C:T) | 2 – 3 ↑ (M, Hisp) | Carmichael et al., 2014 |
| HSD17B3 | rs12552648 (C:T) rs8190566 (A:G) rs8190557 (C:T) rs2026001 (C:A) |
↑ (M) 2 ↑ (P) ↑ (P) ↓ (A) |
Carmichael et al., 2014 | |
| rs2066479 | 3 – 4 ↑ (P) | Sata et al., 2010 | ||
| SRD5A2 | rs1042578 (G:A), rs9332975 (A:G), rs2281546 (T:G), rs28383032 (C:T) rs6543634 (T:G), rs2268794, rs7562326 (T:C) rs519704 (G:A) rs725631 (C:A), rs765138 (C:A) |
1, 5 ↑ (M) ↑ (M) 3 – 4 ↑ (P) ↓ (P) |
Carmichael et al., 2014 | |
| V89L | ↓ | Thai et al., 2005 | ||
| V89L (G:C) | 3 ↑ (P) | Sata et al., 2010 | ||
| AR | GGN repeats >22 | ↑ (M) |
Radpour et al., 2007
Aschim et al., 2004 |
|
| GGN repeats >24 | ↑ (M) | |||
| Oestrogen production and signalling | ESR1 | rs 926779 (G:A), rs 3020364 (A:G), rs 6932902 (G:A), rs 3020371 (C:T), rs 3020375 (C:A) | haplotype AGATA strongly associated | Watanabe et al., 2007 |
| rs11155820 (A:G), rs3798573 (A:G), rs9479190 (A:G), rs1709183 (A:G), rs3020371 (C:T), rs2347923 (A:C), rs750686 (G:A), rs3798758 (G:T), rs3778099 (T:C), rs3020407 (A:G), rs726281, (A:G), rs7743290 (T:G), rs9397463 (C:T), rs3020364 (A:G), rs3020375 (A:C), rs6932902 (G:A), rs926777 (C:A), rs9479188 (G:A), rs11155819 (T:C), rs9340931 (T:C), rs2459107 (G:A), rs2347867 (G:A), rs6901451 (G:A), rs13203975 (G:A), rs13219476 (T:G), rs3798568 (G:A), rs3822990 (C:T), rs3778090 (C:T), rs12664544 (G:C), rs985695 (C:T), rs9340995 (G:C), s7755185 (A:G), rs2982683 (C:T) | ↑ (Cauc and Hisp) | Choudhry et al., 2014 | ||
| rs2234693 (T:C) | 2 ↑ (M) | Ban et al., 2008 | ||
| rs9340799 (A:G) | ↓ (M) | |||
| ESR2 | rs944050 (A:G) | ↓ (M) | Ban et al., 2008 | |
| rs944050 (A:G), rs3832949 (266_267insC) | ↑ | Beleza-Meireles et al., 2006 | ||
| ↑ CA repeat | ↑ | |||
| rs2987983 (T:C), rs10483774 (A:G), (CA)n | ↑ | Beleza-Meireles et al., 2007c | ||
Genes are grouped with their respective pathways. In many cases the reference SNP number and the associated allele or nucleotide change is shown. The effect on hypospadias risk (increased or decreased), fold, and associated severity are shown. The number indicated the fold change. ↑ indicates an increased and ↓ indicates a decreased risk of hypospadias. A = Associated with anterior hypospadias; Cauc = the association was found only in Caucasians; Hisp = the association was found only in Hispanic population; M = associated with penile hypospadias; P = associated with proximal hypospadias. The reference for each study is shown.
The WNT Signalling Pathway
Like SHH, signalling by the WNT pathway is required for numerous processes during embryo development, and the 2 pathways are often intricately linked. WNT signalling has a role in both early hormone-independent GT development and later during the hormone-dependent signalling phase (see below). In mice, Wnt5a is highly expressed in the genital primordia from 10.5 dpc, and its expression is graded along the outgrowing axis of the GT, with the highest levels distally. In the GT, Wnt5a is a transcriptional target of SHH signalling [Perriton et al., 2002] (fig. 2). In Wnt5a knockout mice, the GT appears to be specified; however, outgrowth is affected, meaning that the GT is stunted or absent [Yamaguchi et al., 1999].
Many WNT ligands bind to their receptor Frizzled to activate canonical WNT signalling controlling the stabilisation of the transcription factor β-catenin. Frizzled1 is expressed in the urethral epithelium [Li et al., 2006]. Mice with β-catenin (Ctnnb1) loss of function have an abnormal prepuce with no fusion at the ventral midline. WNT/β-catenin signalling appears to be required in the endodermal urethra to activate and maintain the expression of Fgf8, directing GT outgrowth (fig. 2). β-Catenin is also required in the mesenchyme to promote cell proliferation and in the ectoderm to maintain tissue integrity. Knockout of β-catenin in any of these tissues causes severe hypospadias in adult mice [Lin et al., 2008].
In humans, mutations in WNT5A have been found in patients with Robinow syndrome, who often have hypospadias [Person et al., 2010]. However, there is no report of mutations in patients with isolated hypospadias. This may be due to lethality associated with the disruption of such a vital signalling pathway, and searches may be better focused on regulatory regions or pathway effectors.
The Fibroblast Growth Factor Signalling Pathway
Signalling by the fibroblast growth factors (FGFs) is suggested to regulate epithelial to mesenchymal interactions during GT growth. In mice, Fgf8 is expressed in the DUE during early development under the control of WNT and SHH signalling [Haraguchi et al., 2000; Perriton et al., 2002] (fig. 2). This may be through its indirect upregulation by Homeobox A13 (Hoxa13) [Morgan et al., 2003]. FGF8 is required for GT proximal-distal outgrowth, as abolishing FGF8 or its receptors leads to GT agenesis in mice [Morgan et al., 2003]. It is thought that while WNTs and FGF8 signalling work in a linear fashion to direct proximo-distal outgrowth, SHH signalling works in parallel to control growth [Lin et al., 2008] (fig. 2). Fgf10 is expressed in the mesenchyme of the GT, also under the control of SHH signalling [Perriton et al., 2002]. In Fgf10-deficient male mice, the fusion of the urethral folds fails, leading to an open urethral groove [Haraguchi et al., 2000; Yucel et al., 2004]. In addition, mutations in the FGF receptor Fgfr2IIIb, that binds FGF10 in mice, cause severe hypospadias [Petiot et al., 2005]. Variants in FGF8 and FGFR2 have been associated with hypospadias [Beleza-Meireles et al., 2007a] (table 1). However, no mutations in the FGF10 gene have yet been associated with hypospadias in humans.
The BMP Signalling Pathway
Various BMP signalling molecules are expressed in the DUE and surrounding tissues, where they regulate different epithelial-mesenchymal interactions during GT formation [Suzuki et al., 2003] (fig. 2). Bmp7 is expressed in the urethral epithelium in mice [Morgan et al., 2003], where it is upregulated by HOXA13. A second ligand, Bmp2, is expressed in the urethral epithelium and the proximal mesenchyme and Bmp4 is expressed in both the mesenchyme and the urethral epithelium [Suzuki et al., 2003] (fig. 2). All of these BMP molecules appear to have a role in GT development. Exogenous BMP4 downregulates the expression of several other genes implicated in GT formation, such as Fgf8 and Wnt5a. Null mice for Bmp7 have an abnormal urethral plate and urethral opening [Wu et al., 2009]. BMP receptor 1a (Bmpr1a) mutant mice have decreased apoptosis and increased Fgf8 expression in the DUE – causing GT hyperplasia [Suzuki et al., 2003]. Thus, BMP signalling may act to negatively regulate GT outgrowth and control apoptosis (fig. 2).
In humans, screening in hypospadias patients revealed mutations in BMP7 [Chen et al., 2006; Beleza-Meireles et al., 2007a] (table 2) and SNPs in BMP7 associated with a 2-fold increased risk of hypospadias, regardless of the severity [Carmichael et al., 2013a] (table 1). In addition, exome sequencing revealed 3 missense mutations in BMP4 (table 2) in middle or proximal hypospadias patients [Chen et al., 2006]. These mutations cause changes to highly conserved amino acids. In contrast, no link between SNPs in BMP4 and hypospadias was found in a large cohort [Carmichael et al., 2013a].
Table 2.
Variants identified in hypospadias in humans
| Gene | Variants | Phenotype | Reference | |
|---|---|---|---|---|
| BMP pathway | BMP4 | c.619C>G (het), c.668G>A (het) c.751C>T (het) |
penoscrotal penile |
Chen et al., 2006 |
| BMP7 | c.907C>T (het) c.597G>A (het), c.1567A>G (het) c.1465T>A (het) |
glandular penile penoscrotal |
||
| WT1 | WT1 | N130N (het) A131T (het) S159S (het) |
penoscrotal or penile penile glandular |
Wang et al., 2004 |
| Homeobox | HOXA4 | c.385G>T (het) c.869C>G (het) |
penoscrotal penile |
Chen et al., 2006 |
| HOXB6 | c.367T>C (het) c.124C>A (het) |
penile scrotal |
||
| Gonad development and signalling | MAP3K1 | c.2416G>A (het) | penoscrotal | Das et al., 2013 |
| CHD7 | not specified | penoscrotal | Baxter et al., 2015 | |
| NR5A1 (SF1) | c.319C>T (het), c.103-3C>A (het), c.31G>T (het) | penoscrotal | Köhler et al., 2009 | |
| p.Arg313Cys | glandular | Allali et al., 2011 | ||
| c.184C>T, c.361delGAGACAGG, c.460G>A p.Arg62Cys (het), p.Glu121AlafsX25 (het), p.Ala154Thr (het) |
penile penile |
Tantawy et al., 2014 | ||
| W279X (het), g3314-3317delTCTC | perineal | Warman et al., 2011 | ||
| p.Y25C (het) | penoscrotal | Wu et al., 2013 | ||
| MAMLD1 | E124X (hemi), Q197X (hemi), R653X (hemi) | penoscrotal | Fukami et al., 2006 | |
| V432A (hemi), L121X (hemi) CAG 10 > 13 |
proximal coronal |
Kalfa et al., 2008a | ||
| S143X, P384L | proximal | Kalfa et al.,2012 | ||
| p.P286S, p.V432A, p.N589S, p.531ins3Q, p.Q529K | not specified | Chen Y et al., 2010 | ||
| p.K609fsX1070 | not specified | Igarashi et al., 2015 | ||
| Steroidogenesis | HSD3B2 | p.S213T (het), p.S284R (het) | proximal | Codner et al., 2004 |
| p.A10T (hom) | proximal | Kon et al., 2015 | ||
| CYP11A1 | p.R360W, p.R405X (comp het) | penoscrotal | Parajes et al., 2012 | |
| L222P (hom) | midshaft | Rubtsov et al., 2009 | ||
| AKR1C3 | p.Ala215Thr (het) | penile | Söderhäll et al., 2014 | |
| Androgen production and signalling | SRD5A2 | R246Q (hom), Q6X (hom), G203S + L224H (het), 656delT (het) | scrotal | Wang et al., 2004 |
| R227Q (hom), G203S (hom) | penile or scrotal | |||
| R227Q (het) | glandular | |||
| L113V (het), H231R (het) | proximal | Silver and Russell, 1999 | ||
| G196S (het) | midshaft | Thai et al., 2005 | ||
| AR | 1991C>T (hemi) | glandular | Wang et al., 2004 | |
| 2577C>A (hemi) | penile | |||
| 2519G>A (hemi), 2525T>C (hemi), 2564G>A (hemi) | perineal, micropenis, bifid scrotum | |||
| Q798E (hemi) | scrotal | Thai et al., 2005 | ||
| V870A (hemi) | penoscrotal | Hiort et al., 1994 | ||
| G566V (hemi) | perineal | Alléra et al., 1995 | ||
| P546S (hemi) | midshaft | Sutherland et al., 1996 | ||
| S597T (hemi) | severe | Nordenskjöld et al., 1999 | ||
| Oestrogen pathway | ATF3 | A90G (het), c.817C>T (het) | moderate | Beleza-Meireles et al., 2008 |
| C53070T, C53632A Ins53943A |
various | Kalfa et al., 2008b | ||
| L23M (het) | anterior | |||
| Others | BNC2 | p.P306A (het), p.P579L (het), p.Q152R (het), p.E240G (het) p.R283G (het) |
not detailed | Bhoj et al., 2011 |
Gene variants and mutations proposed to be pathogenic in hypospadias. Genes are grouped into signalling pathways. The cDNA or amino acid changes and the inheritance are indicated based on the original publication. The phenotype of the patient(s) carrying these variants is indicated. The references are shown for each study. hemi = Hemizygous; het = heterozygous; hom = homozygous.
WT1 and Its Associated Pathway
WT1 is a transcriptional regulator with various functions including signalling in both the embryonic kidneys and gonads. Mutations in this zinc finger transcription factor are associated with Denys-Drash or Frasier syndromes, which result in a broad range of malformations including hypospadias. While WT1 plays an important role in indifferent gonad development, and later in testicular differentiation, it may also play a more localised role in the external genitalia. Studies in human foetal samples showed that WT1 and an androgen receptor (AR) are co-expressed in the mesenchyme surrounding the urogenital sinus at 7 weeks. In vitro, WT1 can modulate AR expression [Köhler et al., 2007]. Indeed, mutations in WT1 have been described in boys with isolated hypospadias [Wang et al., 2004]. In a large hypospadias cohort, SNPs in WT1 were associated with a 2-fold increased risk of severe hypospadias [Carmichael et al., 2013a]. In addition, SNPs in WTAP , a nuclear protein contributing to WT1 function [Little et al., 2000], are suggested to be associated with hypospadias [Carmichael et al., 2013a].
Homeobox Genes
Homeobox (or Hox) genes convey positional information in developing embryos and are involved in numerous congenital malformations [Acampora et al., 1989; McGinnis and Krumlauf, 1992]. In mice, Hoxa13 and Hoxd13 are expressed in the mesenchyme of the GT. As mentioned above, Hoxa13 regulates the expression of various morphogens such as Fgf8 and Bmp7 (fig. 2). These decrease in the urethral plate epithelium in Hoxa13 knockout mice, causing hypospadias [Morgan et al., 2003]. Hoxa13/Hoxd13 double-knockout mice fail to develop a GT [Warot et al., 1997].
In humans, HOX genes have been implicated in both syndromic and isolated hypospadias. Beside its involvement in hand-foot-genital syndrome [Halal, 1988], where hypospadias is a frequent feature, HOXA13 is a candidate gene for isolated hypospadias. HOXA13 expression is increased in the prepuce and urethral plate of hypospadias patients, relative to the position of the urethral meatus [Wang C et al., 2013]. In addition, 2 missense mutations in HOXA4 have been found in cases of isolated hypospadias [Chen et al., 2006] (table 2), and recent genome-wide analyses showed increased HOXA4 expression in a hypospadias risk allele [Geller et al., 2014]. Although mutations in HOXB6 have been found in Chinese boys with hypospadias [Chen et al., 2006] (table 2), a recent study failed to establish a linkage between HOXB6 and hypospadias [Carmichael et al., 2013a].
Eph-Ephrin Genes
EfnB2 is a bidirectional signalling molecule that mediates cell adhesion and patterning events occurring at the midline, including scrotal bulge fusion, urethral closure and palate fusion (which happen by an analogous process to urethral closure) [Baskin et al., 2001]. Deletion of EfnB2 in mice is embryonic lethal at E11.0 due to severe patterning defects [Wang et al., 1998]. However, mutations that specifically disrupt EfnB2 reverse signalling in mice result in a severe penoscrotal hypospadias phenotype [Dravis et al., 2004]. Similarly, mutations in the EfnB2 ligands, EphB2 and EphB3 result in severe hypospadias [Yucel et al., 2007]. EFNB2 has also been identified at the key causative gene responsible for hypospadias and genitourinary malformations in 13q-deletion syndrome in humans, confirming its role as key mediator of urethral closure [Garcia et al., 2006].
Additional Genes
Diacylglycerol kinase K (DGKK) is an enzyme that phosphorylates diacylglycerol, converting it to phosphatidic acid. In mice, Dgkk is expressed in the differentiating squamous epithelial cells of the urethral plate/groove and the epidermis during development [Shen et al., 2015]. While little is known about the function of this gene during this time, in humans there is a strong association between DGKK and hypospadias risk [van der Zanden et al., 2011; Carmichael et al., 2013b]. Individual genotyping of 2 SNPs in DGKK showed strong association in various European groups [van der Zanden et al., 2011] (table 2), and expression of DGKK in preputial tissues is significantly lower in carriers of the risk allele [van der Zanden et al., 2011]. However, the risk may depend on ethnicity as these variants have not been found in a Chinese cohort [Ma et al., 2014].
The transcriptional regulator CHD7 binds to enhancer elements in the nucleus and can positively regulate the biogenesis of ribosomal RNA [Zentner et al., 2010]. Beside its implication in CHARGE syndrome, where signs of hypogonadism can be seen, exome sequencing of a male patient with penoscrotal hypospadias identified a variant in a regulatory region for this gene (table 2). This variant, however, remains of uncertain significance [Baxter et al., 2015].
Genes Implicated in Testis Development, Masculinisation and Hypospadias
Proper formation of the penis through masculinisation relies on the secretion of male hormones, such as testosterone, from the testis. Mutations that affect the ability of the gonads to produce these hormones, or the cell's sensitivity to these hormones can result in anomalies of male development including hypospadias.
Testis Signalling Pathways
Sex-determining region Y (SRY) is the Y-linked transcription factor that sits at the top of the testis developmental cascade and is sufficient to trigger male differentiation [Berta et al., 1990; Sinclair et al., 1990; Koopman, 1999; Tanaka and Nishinakamura, 2014]. While mutations in SRY have not been shown to directly cause hypospadias [Wang et al., 2004], sex chromosome anomalies have [Moreno-Garcia and Miranda, 2002]. In addition, genes that regulate SRY expression have been implicated. MAP3K1, also called MEKK1, is a mitogen-activated protein kinase [Schlesinger et al., 2002]. This gene and its pathway regulate numerous processes including upregulation of SRY in the undifferentiated gonad [Warr et al., 2014]. While mutations in this gene lead to complete sex reversal in 46,XY individuals [Baxter et al., 2015], one missense pathogenic variant in MAP3K1 has been identified in 4 boys with hypospadias [Das et al., 2013] (table 2).
SRY activates the transcription of another SRY box-related gene, SOX9, in the developing gonad. SOX9 is required for testis differentiation, as its loss results in 46,XY gonadal dysgenesis. Following this, several other important male pathway regulators are activated. These include FGF9, which works in both a feedback loop with SOX9 and also acts to repress the ovarian development pathway. Mutations in these essential male genes tend to cause gonadal dysgenesis and sex reversal rather than milder phenotypes such as isolated hypospadias. Consistently, in a Chinese cohort of patients with hypospadias, no mutation was found in SOX9 [Wang et al., 2004]. SOX9 is responsible for the proper differentiation and function of the Sertoli cells, the first cells to differentiate in the testis. Sertoli cells secrete the anti-Müllerian hormone (AMH), which triggers Müllerian duct regression. Sertoli cells are also required for the specification of the hormone producing Leydig cells. This requires several genes including DHH. DHH knockout mice have a failure of foetal Leydig cell differentiation, and in humans, mutations in DHH can cause 46,XY gonadal dysgenesis with hypospadias [Clark et al., 2000]. Much of the steroidogenic pathway takes place in the Leydig cells, which secrete testosterone – a key step in the masculinisation of the external genitalia. Below, we highlight the genes involved in the hormone-dependent stage of penile development and how their disruption results in hypospadias.
Nuclear Receptor Subfamily 5 Group A Member 1
The gene nuclear receptor subfamily 5 group A member 1 (NR5A1), which encodes steroidogenic factor 1, plays a major role in the development of both the kidneys and urogenital system [Eggers et al., 2015]. Steroidogenic factor 1 acts in the gonads to positively regulate the expression of several key testis genes including SOX9 and AMH [Allali et al., 2011] as well as many of the genes involved in steroidogenesis [Scott et al., 2009]. Mutations in NR5A1 have been reported in association with a wide spectrum of 46,XY DSD phenotypes: from complete testicular dysgenesis to undescended testes and isolated hypospadias [Köhler et al., 2009; Allali et al., 2011]. Heterozygous mutations in NR5A1 have been described in several families and individuals with hypospadias [Warman et al., 2011; Wu et al., 2013], and loss-of-function mutations in NR5A1 tend to be associated with more severe forms of hypospadias [Köhler et al., 2009] (table 2). However, the relative importance of different NR5A1 polymorphisms in hypospadias may depend on ethnicity. Variants in NR5A1 were frequently associated with hypospadias in Chinese patients, but not in Swedish Caucasians [Adamovic et al., 2012]. Two novel NR5A1 mutations with aberrant biological activity were also found in Egyptian patients [Tantawy et al., 2014] (table 2).
Mastermind-Like Domain-Containing 1
Mastermind-like domain-containing 1 (MAMLD1 or CXORF6) is an X-linked transcriptional co-activator. It is expressed in mice foetal Sertoli and Leydig cells around the critical period for sexual development (E12.5–E14.5) [Miyado et al., 2012], where it is probably regulated by Sf1 [Fukami et al., 2008]. MAMLD1 has been implicated in various forms of human DSDs, including hypospadias. Mutations in MAMLD1 were initially described in severe forms of hypospadias but have subsequently been described in milder forms [Fukami et al., 2006; Kalfa et al., 2008a, 2012], including intronic variants [Chen Y et al., 2010] and splice errors [Igarashi et al., 2015]. In silico modelling has predicted pathogenicity in at least 2 of these variants [Kalfa et al., 2011b]. Importantly, the role of MAMLD1 in hypospadias may be restricted to some ethnic groups as an association was not found in a Chinese population study [Zhuang et al., 2012]. MAMLD1 is thought to regulate the expression of steroidogenic pathway genes, such as Nr0b1 and Cyp17a1 in Leydig cells to permit production of a sufficient amount of testosterone for male sex development [Ogata et al., 2009; Nakamura et al., 2011]. Mamld1 null male mice have normal male development and fertility, but show a mild decrease in the expression of the Leydig-associated genes Star, Cyp11a1, Cyp17a1, Hsd3b1, and Insl3. It is interesting to note that MAMLD1 is also implicated in hypospadias in both a horse [De Lorenzi et al., 2010] and a dog [Switonski et al., 2012].
Androgens and Hypospadias
Androgen Production
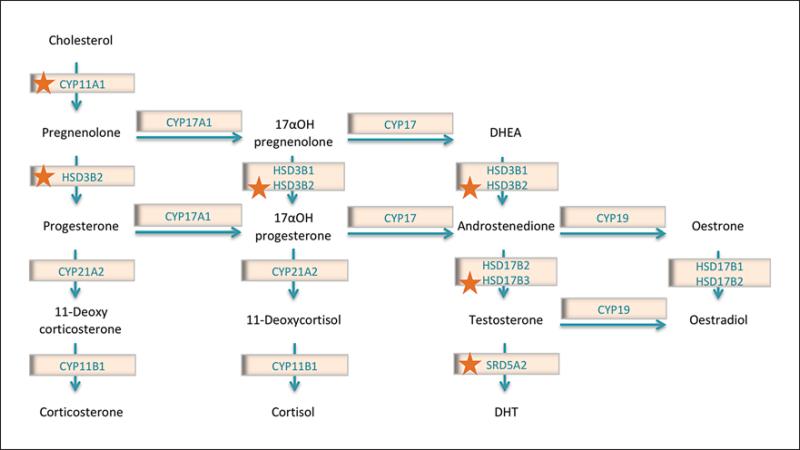
Mutations that specifically affect androgen synthesis in the testis can lead to hypospadias. In males, the enzymatic steps that contribute to steroidogenesis mainly take place in the Leydig cells of the differentiated testis. Firstly, cholesterol is converted into pregnenolone by the cyto-chrome P450 side-chain cleavage enzyme (CYP11A1) (fig. 3). Mutations in CYP11A1 are thought to cause severe hypospadias with late-onset adrenal insufficiency [Rubtsov et al., 2009; Parajes et al., 2012] (table 2). Following this, 3β-hydroxysteroid dehydrogenase 2 (HSD3B2) catalyses the oxidation and isomerisation of Δ5-3β-hydroxysteroid precursors into Δ4-ketosteroids (i.e. pregnenolone into progesterone) (fig. 3), thus leading to the genesis of all classes of steroid hormones. HSD3B2 is expressed almost exclusively in the adrenal glands and gonads [Lachance et al., 1991]. A deficiency in this enzyme (usually caused by homozygous or compound heterozygous mutations) can lead to acute adrenal crisis, salt wasting and male pseudohermaphroditism [Mendonça et al., 1994; Russell et al., 1994]. Although uncommon, heterozygous [Codner et al., 2004] and a putative pathogenic homozygous mutation [Kon et al., 2015] have been found in patients with hypospadias (table 2). Androstenedione is converted to testosterone in the Leydig cells by several enzymes. The AKR1C3 gene encodes one of the 4 human aldo-keto reductases, which function in vitro as 3α-hydroxysteroid dehydrogenases. They can convert potent sex hormones into their inactive metabolites or act in the reverse direction to produce potent hormones by oxidising inactive forms (fig. 3). Tissue distribution studies in human showed a strong expression of AKR1C3 in genitourinary tissues [Azzarello et al., 2008]. One mutation has been described in the AKR1C3 gene in hypospadias (table 2). In silico studies predicted this mutation to damage protein function or structure [Söderhäll et al., 2015]. HSD17B3 encodes testosterone 17β-dehydro genase 3, another enzyme required for conversion of androstenedione into testosterone. A reduced activity of this enzyme can lead to male pseudohermaphroditism and gynecomastia. While the contribution of HSD17B3 to these cases of DSDs is well characterised, its association with hypospadias is not yet well understood. In a study of 633 hypospadias cases, significant association of 4 SNPs and 2 haplotype blocks in HSD17B3 was found [Carmichael et al., 2014] (table 1). In a Japanese study of 89 hypospadias patients and 291 controls, the researchers found that in subjects carrying homozygous HSD17B3 +913A alleles (289S) the risk of hypospadias was significantly higher, including the risk of severe hypospadias [Sata et al., 2010] (table 1). They found that the mRNA expression levels of this form of HSD17B3 were lower, suggesting this polymorphism may be a risk modifier for hypospadias [Sata et al., 2010]. Following HSD17B3 bio-synthesis of testosterone, the enzyme steroid 5α reductase type 2 (SRD5A2) converts testosterone into dihydrotestosterone (fig. 3). Dihydrotestosterone binds to the AR with higher affinity than testosterone and is therefore a more potent masculinisation signal for the external genitalia [Manson and Carr, 2003]. In human foetal GT samples, the expression of SRD5A2 is localised to the stroma surrounding the urethra, especially the urethral seam area in the ventral portion of the remodelling urethra [Kim et al., 2002]. Like HSD17B3, mutations in SRD5A2 can lead to a variety of phenotypes [Akcay et al., 2014], and the role of SRD5A2 in 46,XY DSD has been studied for almost 40 years. Indeed, various mutations in this gene have been linked with an increased risk of hypospadias [Silver and Russell, 1999; Wang et al., 2004; Samtani et al., 2011; Wang R et al., 2013; Carmichael et al., 2014] and one hypospadias-associated variant, the SRD5A2 V89L polymorphism, is less active in vitro [Thai et al., 2005]. Differences in the activity of SRD5A2 variants may explain why some are associated with hypospadias [Silver and Russell, 1999], while others are reported to reduce the risk of both mild and severe hypospadias [Thai et al., 2005] (tables 1, 2).
Fig. 3.
The steroidogenic pathway. The synthesis of androgens occurs in the Leydig cells of the testis via the steroidogenic pathway. This pathway converts cholesterol into sex hormones, especially testosterone and dihydrotestosterone (DHT). These are necessary for the hormone-dependent stage of penis development, and disruptions in their production can cause hypospadias. Enzymes marked with a star have been implicated in human hypospadias.
Androgen Receptor and Associated Genes
Following secretion from the testis, testosterone and dihydrotestosterone both bind the AR to activate a transcriptional response within the developing external sex organs. AR is expressed throughout the developing human penis including the urethra [Kim et al., 2002]. In human foetal genitalia between 12 and 14 weeks of gestation, AR is expressed in the epithelial cells of the urethral plate in the glans, the tubular urethra of the penile shaft, and stromal tissue surrounding the urethral epithelium. Later, between 16 and 20 weeks’ gestation, the density of AR expression is greatest in urethral epithelial cells, where it is stronger along the ventral aspect than the dorsal aspect [Kim et al., 2002]. Administration of an AR antagonist to pregnant mice leads to hypospadias in the male offspring [Kojima et al., 2002; Uda et al., 2004]. Hemizygous mutations (as AR is X-linked) have been reported in hypospadias patients in several studies [van der Zanden et al., 2012], predominantly in proximal hypospadias and often associated with other features of under-masculinisation, such as micropenis or bifid scrotum [Hiort et al., 1994; Alléra et al., 1995; Sutherland et al., 1996; Nordenskjöld et al., 1999; Wang et al., 2004; Thai et al., 2005] (tables 1, 2). The contribution of several polymorphisms in AR are yet to be confirmed, with some authors reporting that an expanded CAG repeat could be relevant in isolated hypospadias [Lim et al., 2001; van der Zanden et al., 2012], whereas others found an association between the length of GGN repeats and hypospadias, especially in proximal forms [Aschim et al., 2004; Radpour et al., 2007]. These reported polymorphisms may change the ability of AR to bind testosterone rather than the levels of AR, as mouse studies have reported no direct association between changes in AR mRNA expression and the presence or absence of hypospadias [Agras et al., 2006].
Additional genes that work in conjunction with AR or downstream of it are also implicated in hypospadias. The FK506-binding protein-52 (FKBP52), encoded by the FKBP4 gene, is a co-chaperone of AR and regulates its activity [Cheung-Flynn et al., 2005; Yong et al., 2007]. Targeted ablation in mice results in ectopic urethral seam fusion [Chen H et al., 2010] and a proximal urethral opening [Yong et al., 2007]. In humans, FKBP52 is expressed in the foreskin of prepubertal boys, and while this gene has been suggested as a candidate for hypospadias, no difference in the expression of this gene was observed between boys with hypospadias and controls [Beleza-Meireles et al., 2007b]. In addition, mutation screening in 91 boys with hypospadias did not identify any mutations, and association studies in a larger cohort failed to show a link between polymorphisms in FKBP52 and hypospadias [Beleza-Meireles et al., 2007b].
FGF/WNT and HH Signalling
Unlike FGF8, which plays an early role in GT formation, FGF10 and its receptor (FGFR2 IIIb) appear to be involved in the hormone-dependent phase [Petiot et al., 2005]. FGF10-deficient mutant male mice have a failure in ventral fusion of the urethral plate, consistent with hypospadias [Yucel et al., 2004]. AR antagonism leads to a loss of FGF10 expression in the urethra and an associated hypospadias phenotype in mice [Petiot et al., 2005], suggesting that this gene is a target of AR signalling. In a large association study, 5 SNPs for FGF10 were associated with a 3- to 4-fold increased risk of hypospadias, regardless of severity. Four of these SNPs were restricted to Caucasians [Carmichael et al., 2013a]. FGFR2 has also been associated with hypospadias, although results are contradictory [Beleza-Meireles et al., 2007a; Carmichael et al., 2013a]. Fgfr2IIIb is thought to be a downstream target of AR, and antagonism of AR may cause hypospadias through a loss of its expression [Petiot et al., 2005]. However, this mouse study only analysed the phenotype during the embryonic and neonatal period, without verification of its persistence into adulthood.
HH signalling is a key factor not only for initial development, but also for masculinisation of the external genitalia in males. During development, SHH expressed in the urethral plate epithelium regulates GT mesenchymal differentiation through GLI2 [Miyagawa et al., 2011] and Gli2 mutant mice have defective urethral formation and are feminised [Haraguchi et al., 2001; Yamada et al., 2003]. It is thought that HH signalling facilitates the masculinisation processes by regulating androgen responsiveness by its regulation of AR [Miyagawa et al., 2011]. In marsupials, SHH also plays an important role during these processes, and recent studies suggest that SHH plays a role in the early hormone-independent patterning, followed by a hormone-dependent role [Chew et al., 2014]. In addition to FGF and HH, mouse studies have also shown that the WNT/β-catenin pathway interacts with the androgen pathway to regulate masculinisation of the GT in the hormone-dependent phase [Miyagawa et al., 2009].
Oestrogens and Hypospadias
Oestrogen Production
Oestrogens (oestrone, oestradiol) are synthesised in the gonads from androstenedione or testosterone by the P450 aromatase (CYP19A1) enzyme. Aromatase insufficiency can result in virilisation of female patients. A novel homozygous mutation has recently been described to cause aromatase deficiency in a 46,XX newborn (with enlarged GT and fusion of labioscrotal folds) and hypospadias and cryptorchidism in the 46,XY sibling [Bouchoucha et al., 2014]. Like reduced testosterone levels, elevated oestrogen levels may also be a risk factor for hypospadias [Staib et al., 1994]. The source of oestrogen can be external, and the risk of hypospadias increases in male babies of women exposed to diethylstilbestrol, a potent synthetic oestrogen, while in utero [Klip et al., 2002].
Indeed, it appears that oestrogens play a critical role in both penile and clitoral development, and maintaining the balance between androgen and oestrogen levels is essential for normal penile development. Studies in hyena, in which females have extreme masculinisation of the external genitalia, have shown that oestrogens play a critical role in the positioning of the urethral orifice, determining elasticity of the urethral meatus, and facilitating epithelial-epithelial fusion events during distal urethra/urogenital sinus and prepuce formation [Cunha et al., 2015].
Oestrogen Receptors and Associated Genes
In humans, there are 2 oestrogen receptors, ESR1 (ER-α) and ESR2 (ER-β), which interact to form homo- or heterodimer ER complexes. ER bound by oestrogen translocates to the nucleus to activate oestrogen-responsive genes. ESR1 and ESR2 are expressed in most cells of the male urethra [Dietrich et al., 2004], and studies in human foetal penile smooth muscle cells have shown that active ER is expressed [Crescioli et al., 2003]. Interestingly, while testosterone can masculinise the female foetus, oestrogen cannot generally feminise. Various polymorphisms in ER genes have been reported in hypospadias. Watanabe et al. [2007] showed a strong association of one haplotype (covering 5 SNPS) of ESR1 with hypospadias (table 1). Interestingly, while some variants of ESR1 have been reported to decrease the risk of hypospadias, others have been described to increase it [Ban et al., 2008; Tang et al., 2011]. Various SNPs in ESR1 are associated with an increased risk of hypospadias relative to severity [Choudhry et al., 2014]. While SNPs in ESR2 are not associated with an increased risk of hypospadias in this same cohort [Choudhry et al., 2014], a different study found that prolonged CA repeats or heterozygous mutations in ESR2 increased the risk of hypospadias [Beleza-Meireles et al., 2006, 2007c]. In a Norwegian population, no association was found between hypospadias and 2 polymorphisms of ESR2 [Aschim et al., 2005]. The expression of both ER genes is reduced in foreskins from hypospadias patients versus controls [Qiao et al., 2011], consistent with aberrant oestrogenic effects playing a role in the aetiology of hypospadias.
Factors that affect the level or activity of ER may also play a role. In boys with congenital genitourinary tract masculinisation disorders, including hypospadias, 1.35% of cases showed a de novo duplication of a region of the X chromosome containing the VAMP7 gene [Tannour-Louet et al., 2014]. Transgenic mice with an increased dose of VAMP7 phenocopied the defective urogenital traits such as cryptorchidism and hypospadias as well as reduced penile length and subfertility. VAMP7 is a member of the SNARE family of proteins and is involved in vesicle trafficking from early endosomes to lysosomes [Advani et al., 1999]. VAMP7 may control the processing/turnover of select proteins, including ESR1, and VAMP7 positively regulates ESR1 levels meaning its duplication results in an increase in the transcription of oestrogen-responsive genes including Atf3, Ctgf and Cyr61 . These genes have all been shown to be upregulated in the foreskin of individuals with hypospadias [Wang et al., 2007]. It will be interesting to see if VAMP7 or other vesicle-trafficking proteins are associated with isolated cases of hypospadias in the future.
Oestrogen-Responsive Genes
Like androgens, oestrogen via its receptors can up-regulate target genes in the developing external genitalia. The expression of various oestrogen-responsive genes, including CYR61, GADD45β, and ZEB1, is increased in the foreskin of patients with hypospadias [Wang et al., 2007]. Oestrogen activates the expression of ZEB1, a transcription factor [Qiao et al., 2011], and patients with severe hypospadias have increased expression of ZEB1 in the basal layer of the foreskin epidermis [Wang et al., 2007]. Zeb1 mutant mice exhibit various developmental defects, although no defect of the sex organs has yet been described, nor have variants in this gene been associated with hypospadias. Like ZEB1 , the transcription factor ATF is an oestrogen-responsive gene that is strongly upregulated in the penile skin of patients with hypospadias [Liu et al., 2005; Wang et al., 2007; Zhou et al., 2009; Gurbuz et al., 2010; Karabulut et al., 2013]. Furthermore, it is overexpressed in the urethral plate surrounding the ectopic orifice of the urethra in human foetuses with hypospadias [Kalfa et al., 2008b]. Genomic variants of ATF3 accounted for 10% of patients with hypospadias in one cohort [Kalfa et al., 2008b]. However, other reports showed that ethnicity may have an influence, as upregulation of ATF3 was not found in tissues from Japanese patients [Takahashi et al., 2013]. Finally, 2 variants in ATF3 have been found in 2 patients with moderate forms of hypospadias [Beleza-Meireles et al., 2008] (table 2).
Connective tissue growth factor (CTGF) is a mitogen secreted by vascular endothelial cells and plays a role in chondrocyte proliferation and differentiation as well as cell adhesion. CTGF enhances FGF-induced DNA synthesis. The expression of CTGF is significantly upregulated in the tissues of patients with hypospadias when they are exposed to oestradiol, compared to a control group [Zhou et al., 2009], an effect relative to the severity of hypospadias [Wang et al., 2007]. Indeed, it has been proposed that CTGF may be a candidate gene for hypospadias via the oestrogen regulation pathway [Zhou et al., 2009]. While null mice die at birth, mice with CTGF over-expression have small testes and reduced fertility [Nakanishi et al., 2001]. Polymorphisms in this gene are yet to be linked to hypospadias in humans.
Candidate Hypospadias Genes
Several other candidate hypospadias genes have been described. Midline 1 (MID1), encodes an E3 ubiquitin ligase involved in Opitz G/BBB syndrome, which can include hypospadias in its phenotype [Lu et al., 2013]. Mild changes in the MID1 protein may cause hypospadias alone, and one study found a SNP associated with hypospadias [Zhang et al., 2011]. Although of unknown function, basonuclein 2 (BNC2) is evolutionary conserved and expressed in human periurethral tissue. Mutations have been found to be associated with hypospadias [Bhoj et al., 2011] (table 2). Finally, a human gene expression array in hypospadias and control penile tissues found 24 genes to be upregulated. Apart from ATF3 and CYR61, already reported and discussed earlier, genes were involved in apoptosis (FOS), apoptosis and signalling (NR4A1), metabolism (PTGS2), protein binding (RTN4), receptor activity (CD69), signalling (DUSP1, SOCS3, NR4A2, EGR1, RGS1, HBEGF, CD9), transcription (FOSB, JUN, JUNB, IER2, ZFP36, KLF2, BTG2, HNRNPUL1) , trans lation (EIF4A1), and transporter activities (SLC25A25) [Karabulut et al., 2013]. The sample size, however, was small, and the significance will need to be confirmed in a larger cohort.
Environmental Factors and Hypospadias
Exogenous Endocrine-Disrupting Chemicals and Oestrogen-Like Compounds
The process of urethral closure is known to be exquisitely responsive to the hormonal environment. Oestrogenic and anti-androgenic compounds are well established to induce hypospadias in humans and mice. The increasing incidence of hypospadias, particularly in developed countries has led to the hypothesis that our elevated exposure to oestrogenic and anti-androgenic environmental factors may contribute to its aetiology [Lund et al., 2009; Kalfa et al., 2011a]. This may include exposure to molecules that interfere with synthesis, transport or metabolism of endogenous hormones, such as xenoestrogens or endocrine disrupting compounds (EDCs) [Mar-rocco et al., 2015]. Indeed, a recent prospective study has shown that exposure to EDCs during foetal life significantly increases the risk of developing hypospadias [Kalfa et al., 2015]. One type of EDCs are phthalates, and an association between phthalate levels in patients and hypospadias has been demonstrated [Choi et al., 2012], perhaps due to their ability to repress testosterone biosyn-thesis [Giordano et al., 2010]. Phthalates can act in a variety of different ways; dichlorodiphenyltrichloroethane (DDT) and its metabolites block AR and linuron reduces expression of the luteinising hormone (LH) receptor, affecting, in turn, testosterone production. Dioxin is suspected to lower LH secretion, repressing StAR and CYP17.
Many other chemicals have been identified as toxic for the urogenital tract, such as phytoestrogens, mycoestrogens, epichlorohydrin, atrazine, and furans [Nassar et al., 2010], and many of them have pro-oestrogenic or anti-androgenic effects [Luccio-Camelo and Prins, 2011]. In a recent study, the expression of WT1, LHR, 17βHSD3, and SRD5A2 were downregulated after exposure to bisphenol A [Liu et al., 2015].
Contraceptives and Assisted Reproductive Technologies
Although they are high in oestrogens, oral contraceptives have not been associated with an increased risk of hypospadias [Nørgaard et al., 2009]. Conversely, an increased risk of hypospadias may be associated with both intracytoplasmic sperm injection and in vitro fertilisation – the former higher than the latter – compared to natural conception [Bonduelle et al., 2005]. However, the data is controversial, and paternal subfertility, as well as epigenetics, such as methylation of the AR gene have been suggested as confounding factors [Fauser et al., 2014]. Questions also exist regarding the risk of oestrogen treatment used in these medical interventions [Carmichael et al., 2005; Sørensen et al., 2005].
Diet, Lifestyle and Maternal Environment
Vegetarian diet with iron supplementation in pregnant women has been associated with a higher risk of hypospadias, perhaps due to greater exposure to phytoestrogens [North and Golding, 2000] or to the increased ingestion of pesticides and herbicides [Giordano et al., 2008]. Iron supplementation itself has also been suggested as a risk factor [Brouwers et al., 2007]. Similarly, an increased risk of genital malformations has been found in greenhouse workers or farmers [Kalfa et al., 2011a] and hairdressers [Ormond et al., 2009]. These findings are however contradictory [Brouwers et al., 2007]. Regarding parental exposure to pollutants, offspring of mothers working in the leather industry [García and Fletcher, 1998] or military personnel [Araneta et al., 2003] have an increased risk of hypospadias. As for the paternal side, some jobs carry increased risks such as vehicle operators, police officers or fire fighters [Schnitzer et al., 1995] and men exposed to dust from grinding metals [Brouwers et al., 2010]. For people living close to hazardous waste-disposal sites, the risk was first described as minor [Elliott et al., 2001], but this has also been refuted [Morris et al., 2003].
A strong association between low birth weight and hypospadias exists, especially in the proximal forms [Giordano et al., 2010]. This has been attributed to in utero growth retardation [Hussain et al., 2002]. Moreover, placental dysfunction may lower chorionic gonadotropin production and influence external genitalia differentiation [Fujimoto et al., 2008]. Maternal smoking is typically associated with low birth weight [Varvarigou et al., 2009], and one study has suggested an alteration of the Sertoli cell-specific gene DHH by maternal smoking [Fowler et al., 2008]. Some studies, however, associate maternal smoking with a negative risk of hypospadias [Håkonsen et al., 2014], perhaps through the anti-oestrogenic effect of tobacco smoke [Michnovicz et al., 1988]. Maternal hypertension during pregnancy, preeclampsia and preterm delivery is also associated with hypospadias [van der Zanden et al., 2012]. An increased risk of hypospadias has been found in overweight and obese mothers [Marengo et al., 2013], which is attributed to an increased level of free circulating oestrogens or larger quantities of chemicals stored in the fatty tissue. Again, however, some reports are contradictory [Adams et al., 2011] and low maternal weight has also been associated with an increased risk of hypospadias [Rankin et al., 2010].
Consumption of several drugs, such as valproate [Rodríguez-Pinilla et al., 2008], loperamide [Källén et al., 2008] or paroxetine [Reis and Källén, 2010] may confer an increased risk of hypospadias in offspring.
One must note, however, that in most cases it is difficult to establish direct association between hypospadias risk and these factors as confounding elements such as the effect of genetic or ethnic background on susceptibility may interfere.
Environmental-Genetic Interactions and Epigenetics
Gene polymorphisms seem to play a role in the impact of EDCs on the pathophysiology of hypospadias. As many pollutants are metabolised by detoxifying enzymes, allelic variations in these enzymes may change the impact of chemicals and their metabolites on the developing urogenital tissue [van der Zanden et al., 2012]. Cytochrome P4501A1 (or CYP1A1) metabolises various toxins, and an association between CYP1A1 variants and mothers of boys with hypospadias has been shown [Kurahashi et al., 2005]. Similarly, glutathione S-transferases (GSTM1 and GSTT1) are involved in toxin metabolism, and an association between hypospadias and deletion of these 2 genes in mothers has been described [Kurahashi et al., 2005; Shekharyadav et al., 2011].
Environmental factors can change transcriptional activity via epigenetic modifications to the genome. A genome-wide DNA methylation study of foreskin samples from 12 patients and 8 controls identified 14 CpG islands associated with hypospadias. Differentially methylated CpG islands were identified in association with the SCARB1 and MYBPH genes, suggesting these as potential candidates for hypospadias [Choudhry et al., 2012]. DNA methylation of the AR was also found to be higher in fore-skin samples from hypospadias patients than in controls [Vottero et al., 2011]. Diethylstilbestrol (DES), thought to be an anti-abortive medication and used until 1971, is associated with an increased risk of hypospadias in males exposed in utero, and this effect extends into the second generation [Klip et al., 2002; Brouwers et al., 2007]. However, for the effects of DES to be considered truly transgenerational, an increased risk of hypospadias must persist into the F3 generation as the F2 are derived from germ cells directly exposed to DES in utero themselves. While this data is not yet available, it does raise the concern that certain environmental exposures may not only induce hypospadias in the exposed individual, but also create long-lasting epigenetic changes that continue to increase the hypospadias risk for generations to come. It is well known that DES interferes with histone methylation [Baccarelli and Bollati, 2009], thus suggesting an epigenetic mechanism for the action of DES [Bredfeldt et al., 2010]. Oestrogen receptor activation is also well known to alter the epigenetic landscape [Wong and Walker, 2013], suggesting that oestrogenic compounds known to cause hypospadias are equally likely to be making long-lasting changes to the transcriptional environment.
Future Directions
While the studies highlighted above have suggested a role for both genetics and environment in hypospadias, further research is needed to better understand this complex disorder. Identifying candidate genes and regulatory regions is confounded by the complex interaction between environmental exposures and gene polymorphisms in the manifestation and severity of hypospadias in humans. The advances in massively parallel sequencing and their reduced costs will be extremely important for the discovery of novel pathogenic variants or genes associated with hypospadias. In addition, large-scale epigenetic screens in the affected tissues will shed light on the interplay between environment and genetics. Given that numerous morphogenic pathways are involved in external genitalia development, screening for mutations in these genes is unlikely to identify mutations that cause hypospadias alone because of their numerous developmental roles. Instead, focus on the regulatory regions that control the external genitalia-specific action of these genes, as well as identification of genital-specific effector proteins, will be important. Animal models are essential for our understanding of the development of the external genitalia. Advances in genome editing, such as CRISPR technologies, will allow rapid screening for novel genes and mutations that cause hypospadias. Together, these technologies will allow a more comprehensive understanding of the environmental, epigenetic and genetic factors that contribute to hypospadias and provide avenues for prevention or intervention.
References
- Acampora D, D'Esposito M, Faiella A, Pannese M, Migliaccio E, et al. The human HOX gene family. Nucleic Acids Res. 1989;17:10385–10402. doi: 10.1093/nar/17.24.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achermann JC, Domenice S, Bachega TASS, Nishi MY, Mendonca BB. Disorders of sex development: effect of molecular diagnostics. Nat Rev Endocrinol. 2015;11:478–488. doi: 10.1038/nrendo.2015.69. [DOI] [PubMed] [Google Scholar]
- Adamovic T, Chen Y, Thai HTT, Zhang X, Markljung E, et al. The p.G146A and p.P125P polymorphisms in the steroidogenic factor-1 (SF-1) gene do not affect the risk for hypospadias in Caucasians. Sex Dev. 2012;6:292–297. doi: 10.1159/000343782. [DOI] [PubMed] [Google Scholar]
- Adams SV, Hastert TA, Huang Y, Starr JR. No association between maternal pre-pregnancy obesity and risk of hypospadias or cryptorchidism in male newborns. Birth Defects Res A Clin Mol Teratol. 2011;91:241–248. doi: 10.1002/bdra.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani RJ, Yang B, Prekeris R, Lee KC, Klumperman J, Scheller RH. VAMP-7 mediates vesicular transport from endosomes to lysosomes. J Cell Biol. 1999;146:765–776. doi: 10.1083/jcb.146.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agras K, Willingham E, Liu B, Baskin LS. Ontogeny of androgen receptor and disruption of its mRNA expression by exogenous estrogens during morphogenesis of the genital tubercle. J Urol. 2006;176:1883–1888. doi: 10.1016/S0022-5347(06)00613-6. [DOI] [PubMed] [Google Scholar]
- Akcay T, Fernandez-Cancio M, Turan S, Güran T, Audi L, Bereket A. AR and SRD5A2 gene mutations in a series of 51 Turkish 46,XY DSD children with a clinical diagnosis of androgen insensitivity. Andrology. 2014;2:572–578. doi: 10.1111/j.2047-2927.2014.00215.x. [DOI] [PubMed] [Google Scholar]
- Allali S, Muller JB, Brauner R, Lourenço D, Boudjenah R, et al. Mutation analysis of NR5A1 encoding steroidogenic factor 1 in 77 patients with 46,XY disorders of sex development (DSD) including hypospadias. PLoS One. 2011;6:e24117. doi: 10.1371/journal.pone.0024117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alléra A, Herbst MA, Griffin JE, Wilson JD, Schweikert HU, McPhaul MJ. Mutations of the androgen receptor coding sequence are infrequent in patients with isolated hypospadias. J Clin Endocrinol Metab. 1995;80:2697–2699. doi: 10.1210/jcem.80.9.7673412. [DOI] [PubMed] [Google Scholar]
- Araneta MRG, Schlangen KM, Edmonds LD, Destiche DA, Merz RD, et al. Prevalence of birth defects among infants of Gulf War veterans in Arkansas, Arizona, California, Georgia, Hawaii, and Iowa, 1989–1993. Birth Defects Res A Clin Mol Teratol. 2003;67:246–260. doi: 10.1002/bdra.10033. [DOI] [PubMed] [Google Scholar]
- Aschim EL, Nordenskjöld A, Giwercman A, Lundin KB, Ruhayel Y, et al. Linkage between cryptorchidism, hypospadias, and GGN repeat length in the androgen receptor gene. J Clin Endocrinol Metab. 2004;89:5105–5109. doi: 10.1210/jc.2004-0293. [DOI] [PubMed] [Google Scholar]
- Aschim EL, Giwercman A, Ståhl O, Eberhard J, Cwikiel M, et al. The RsaI polymorphism in the estrogen receptor-beta gene is associated with male infertility. J Clin Endocrinol Metab. 2005;90:5343–5348. doi: 10.1210/jc.2005-0263. [DOI] [PubMed] [Google Scholar]
- Aulagne MB, Harper L, de Napoli-Cocci S, Bondonny JM, Dobremez E. Long-term outcome of severe hypospadias. J Pediatr Urol. 2010;6:469–472. doi: 10.1016/j.jpurol.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Azzarello J, Fung KM, Lin HK. Tissue distribution of human AKR1C3 and rat homolog in the adult genitourinary system. J Histochem Cytochem. 2008;56:853–861. doi: 10.1369/jhc.2008.951384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr Opin Pediatr. 2009;21:243–251. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban S, Sata F, Kurahashi N, Kasai S, Moriya K, et al. Genetic polymorphisms of ESR1 and ESR2 that may influence estrogen activity and the risk of hypospadias. Hum Reprod. 2008;23:1466–1471. doi: 10.1093/humrep/den098. [DOI] [PubMed] [Google Scholar]
- Baskin LS, Erol A, Jegatheesan P, Li Y, Liu W, Cunha GR. Urethral seam formation and hypospadias. Cell Tissue Res. 2001;305:379–387. doi: 10.1007/s004410000345. [DOI] [PubMed] [Google Scholar]
- Baxter RM, Arboleda VA, Lee H, Barseghyan H, Adam MP, et al. Exome sequencing for the diagnosis of 46,XY disorders of sex development. J Clin Endocrinol Metab. 2015;100:E333–E344. doi: 10.1210/jc.2014-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beleza-Meireles A, Omrani D, Kockum I, Frisén L, Lagerstedt K, Nordenskjöld A. Polymorphisms of estrogen receptor beta gene are associated with hypospadias. J Endocrinol Invest. 2006;29:5–10. doi: 10.1007/BF03349170. [DOI] [PubMed] [Google Scholar]
- Beleza-Meireles A, Lundberg F, Lagerstedt K, Zhou X, Omrani D, et al. FGFR2, FGF8 , FGF10 and BMP7 as candidate genes for hypospadias. Eur J Hum Genet. 2007a;15:405–410. doi: 10.1038/sj.ejhg.5201777. [DOI] [PubMed] [Google Scholar]
- Beleza-Meireles A, Barbaro M, Wedell A, Töhönen V, Nordenskjöld A. Studies of a co-chaperone of the androgen receptor, FKBP52, as candidate for hypospadias. Reprod Biol Endocrinol. 2007b;5:8. doi: 10.1186/1477-7827-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beleza-Meireles A, Kockum I, Lundberg F, Söderhäll C, Nordenskjöld A. Risk factors for hypospadias in the estrogen receptor 2 gene. J Clin Endocrinol Metab. 2007c;92:3712–3718. doi: 10.1210/jc.2007-0543. [DOI] [PubMed] [Google Scholar]
- Beleza-Meireles A, Töhönen V, Söderhäll C, Schwentner C, Radmayr C, et al. Activating transcription factor 3: a hormone responsive gene in the etiology of hypospadias. Eur J Endocrinol. 2008;158:729–739. doi: 10.1530/EJE-07-0793. [DOI] [PubMed] [Google Scholar]
- Berta P, Hawkins JB, Sinclair AH, Taylor A, Griffiths BL, et al. Genetic evidence equating SRY and the testis-determining factor. Nature. 1990;348:448–450. doi: 10.1038/348448A0. [DOI] [PubMed] [Google Scholar]
- Bhoj EJ, Ramos P, Baker LA, Garg V, Cost N, et al. Human balanced translocation and mouse gene inactivation implicate Basonuclin 2 in distal urethral development. Eur J Hum Genet. 2011;19:540–546. doi: 10.1038/ejhg.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschko SD, Cunha GR, Baskin LS. Molecular mechanisms of external genitalia development. Differentiation. 2012;84:261–268. doi: 10.1016/j.diff.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonduelle M, Wennerholm UB, Loft A, Tarlatzis BC, Peters C, et al. A multi-centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conception. Hum Reprod. 2005;20:413–419. doi: 10.1093/humrep/deh592. [DOI] [PubMed] [Google Scholar]
- Bouchoucha N, Samara-Boustani D, Pandey AV, Bony-Trifunovic H, Hofer G, et al. Characterization of a novel CYP19A1 (aromatase) R192H mutation causing virilization of a 46,XX newborn, undervirilization of the 46,XY brother, but no virilization of the mother during pregnancies. Mol Cell Endocrinol. 2014;390:8–17. doi: 10.1016/j.mce.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Bredfeldt TG, Greathouse KL, Safe SH, Hung MC, Bedford MT, Walker CL. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol. 2010;24:993–1006. doi: 10.1210/me.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers MM, Feitz WFJ, Roelofs LAJ, Kiemeney LALM, de Gier RPE, Roeleveld N. Risk factors for hypospadias. Eur J Pediatr. 2007;166:671–678. doi: 10.1007/s00431-006-0304-z. [DOI] [PubMed] [Google Scholar]
- Brouwers MM, van der Zanden LFM, de Gier RPE, Barten EJ, Zielhuis GA, et al. Hypospadias: risk factor patterns and different pheno-types. BJU Int. 2010;105:254–262. doi: 10.1111/j.1464-410X.2009.08772.x. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Laurent C, Croughan MS, Olney RS, Lammer EJ. Maternal progestin intake and risk of hypospadias. Arch Pediatr Adolesc Med. 2005;159:957–962. doi: 10.1001/archpedi.159.10.957. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Lammer EJ. Environmental and genetic contributors to hypospadias: a review of the epidemiologic evidence. Birth Defects Res A Clin Mol Teratol. 2012;94:499–510. doi: 10.1002/bdra.23021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Ma C, Choudhry S, Lammer EJ, Witte JS, Shaw GM. Hypospadias and genes related to genital tubercle and early urethral development. J Urol. 2013a;190:1884–1892. doi: 10.1016/j.juro.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Mohammed N, Ma C, Iovannisci D, Choudhry S, et al. Diacylglycerol kinase K variants impact hypospadias in a California study population. J Urol. 2013b;189:305–311. doi: 10.1016/j.juro.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Witte JS, Ma C, Lammer EJ, Shaw GM. Hypospadias and variants in genes related to sex hormone biosynthesis and metabolism. Andrology. 2014;2:130–137. doi: 10.1111/j.2047-2927.2013.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Yong W, Hinds TD, Jr, Yang Z, Zhou Y, et al. Fkbp52 regulates androgen receptor transactivation activity and male urethra morphogenesis. J Biol Chem. 2010;285:27776–27784. doi: 10.1074/jbc.M110.156091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Li Q, Xu J, Ding K, Wang Y, et al. Mutation screening of BMP4, BMP7, HOXA4 and HOXB6 genes in Chinese patients with hypospadias. Eur J Hum Genet. 2006;15:23–28. doi: 10.1038/sj.ejhg.5201722. [DOI] [PubMed] [Google Scholar]
- Chen Y, Thai HTT, Lundin J, Lagerstedt-Robinson K, Zhao S, et al. Mutational study of the MAMLD1 -gene in hypospadias. Eur J Med Genet. 2010;53:122–126. doi: 10.1016/j.ejmg.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Cheung-Flynn J, Prapapanich V, Cox MB, Riggs DL, Suarez-Quian C, Smith DF. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol. 2005;19:1654–1666. doi: 10.1210/me.2005-0071. [DOI] [PubMed] [Google Scholar]
- Chew KY, Pask AJ, Hickford D, Shaw G, Renfree MB. A dual role for SHH during phallus development in a marsupial. Sex Dev. 2014;8:166–177. doi: 10.1159/000357927. [DOI] [PubMed] [Google Scholar]
- Choi H, Kim J, Im Y, Lee S, Kim Y. The association between some endocrine disruptors and hypospadias in biological samples. J Environ Sci Health Part A Tox Hazard Subst Environ Eng. 2012;47:2173–2179. doi: 10.1080/10934529.2012.680387. [DOI] [PubMed] [Google Scholar]
- Choudhry S, Deshpande A, Qiao L, Beckman K, Sen S, Baskin LS. Genome-wide DNA methylation profiling of CpG islands in hypospadias. J Urol. 2012;188:1450–1455. doi: 10.1016/j.juro.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry S, Baskin LS, Lammer EJ, Witte JS, Dasgupta S, et al. Genetic polymorphisms in ESR1 and ESR2 genes and risk of hypospadias in a multiethnic study population. J Urol. 2014;193:1625–1631. doi: 10.1016/j.juro.2014.11.087. [DOI] [PubMed] [Google Scholar]
- Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod. 2000;63:1825–1838. doi: 10.1095/biolreprod63.6.1825. [DOI] [PubMed] [Google Scholar]
- Codner E, Okuma C, Iñiguez G, Boric MA, Avila A, et al. Molecular study of the 3 beta-hydroxysteroid dehydrogenase gene type II in patients with hypospadias. J Clin Endocrinol Metab. 2004;89:957–964. doi: 10.1210/jc.2002-020873. [DOI] [PubMed] [Google Scholar]
- Cohn MJ. Development of the external genitalia: conserved and divergent mechanisms of appendage patterning. Dev Dyn. 2011;240:1108–1115. doi: 10.1002/dvdy.22631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescioli C, Maggi M, Vannelli GB, Ferruzzi P, Granchi S, et al. Expression of functional estrogen receptors in human fetal male external genitalia. J Clin Endocrinol Metab. 2003;88:1815–1824. doi: 10.1210/jc.2002-021085. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Sinclair A, Risbridger G, Hutson J, Baskin LS. Current understanding of hypospadias: relevance of animal models. Nat Rev Urol. 2015;12:271–280. doi: 10.1038/nrurol.2015.57. [DOI] [PubMed] [Google Scholar]
- Das DK, Rahate SG, Mehta BP, Gawde HM, Tamhankar PM. Mutation analysis of mitogen activated protein kinase 1 gene in Indian cases of 46,XY disorder of sex development. Indian J Hum Genet. 2013;19:437–442. doi: 10.4103/0971-6866.124372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzi L, Genualdo V, Iannuzzi A, Di Meo GP, Perucatti A, et al. Cytogenetic and genetic studies in a hypospadic horse (Equus caballus, 2n = 64). Sex Dev. 2010;4:352–357. doi: 10.1159/000319527. [DOI] [PubMed] [Google Scholar]
- Dietrich W, Haitel A, Huber JC, Reiter WJ. Expression of estrogen receptors in human corpus cavernosum and male urethra. J Histochem Cytochem. 2004;52:355–360. doi: 10.1177/002215540405200306. [DOI] [PubMed] [Google Scholar]
- Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, et al. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Eggers S, Sinclair A. Mammalian sex determination –insights from humans and mice. Chromosome Res. 2012;20:215–238. doi: 10.1007/s10577-012-9274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers S, Smith KR, Bahlo M, Looijenga LH, Drop SL, et al. Whole exome sequencing combined with linkage analysis identifies a novel 3 bp deletion in NR5A1. Eur J Hum Genet. 2015;23:486–493. doi: 10.1038/ejhg.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott P, Briggs D, Morris S, de Hoogh C, Hurt C, et al. Risk of adverse birth outcomes in populations living near landfill sites. BMJ. 2001;323:363–368. doi: 10.1136/bmj.323.7309.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser BCJM, Devroey P, Diedrich K, Balaban B, Bonduelle M, et al. Health outcomes of children born after IVF/ICSI: a review of current expert opinion and literature. Reprod Biomed Online. 2014;28:162–182. doi: 10.1016/j.rbmo.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Fowler PA, Cassie S, Rhind SM, Brewer MJ, Collinson JM, et al. Maternal smoking during pregnancy specifically reduces human fetal desert hedgehog gene expression during testis development. J Clin Endocrinol Metab. 2008;93:619–626. doi: 10.1210/jc.2007-1860. [DOI] [PubMed] [Google Scholar]
- Fredell L, Iselius L, Collins A, Hansson E, Holmner S, et al. Complex segregation analysis of hypospadias. Hum Genet. 2002;111:231–234. doi: 10.1007/s00439-002-0799-y. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Suwa T, Kabe K, Adachi T, Nakabayashi M, Amamiya T. Placental insufficiency in early gestation is associated with hypospadias. J Pediatr Surg. 2008;43:358–361. doi: 10.1016/j.jpedsurg.2007.10.046. [DOI] [PubMed] [Google Scholar]
- Fukami M, Wada Y, Miyabayashi K, Nishino I, Hasegawa T, et al. CXorf6 is a causative gene for hypospadias. Nat Genet. 2006;38:1369–1371. doi: 10.1038/ng1900. [DOI] [PubMed] [Google Scholar]
- Fukami M, Wada Y, Okada M, Kato F, Katsumata N, et al. Mastermind-like domain-containing 1 (MAMLD1 or CXorf6) transactivates the Hes3 promoter, augments testosterone production, and contains the SF1 target sequence. J Biol Chem. 2008;283:5525–5532. doi: 10.1074/jbc.M703289200. [DOI] [PubMed] [Google Scholar]
- García AM, Fletcher T. Maternal occupation in the leather industry and selected congenital malformations. Occup Environ Med. 1998;55:284–286. doi: 10.1136/oem.55.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia NM, Allgood J, Santos LJ, Lonergan D, Batanian JR, et al. Deletion mapping of critical region for hypospadias, penoscrotal transposition and imperforate anus on human chromosome 13. J Pediatr Urol. 2006;2:233–242. doi: 10.1016/j.jpurol.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller F, Feenstra B, Carstensen L, Pers TH, van Rooij IALM, et al. Genome-wide association analyses identify variants in developmental genes associated with hypospadias. Nat Genet. 2014;46:957–963. doi: 10.1038/ng.3063. [DOI] [PubMed] [Google Scholar]
- Giordano F, Carbone P, Nori F, Mantovani A, Taruscio D, Figà-Talamanca I. Maternal diet and the risk of hypospadias and cryptorchidism in the offspring. Paediatr Perinat Epidemiol. 2008;22:249–260. doi: 10.1111/j.1365-3016.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- Giordano F, Abballe A, De Felip E, di Domenico A, Ferro F, et al. Maternal exposures to endocrine disrupting chemicals and hypospadias in offspring. Birth Defects Res A Clin Mol Teratol. 2010;88:241–250. doi: 10.1002/bdra.20657. [DOI] [PubMed] [Google Scholar]
- Gurbuz C, Demir S, Zemheri E, Canat L, Kilic M, Caskurlu T. Is activating transcription factor 3 up-regulated in patients with hypospadias? Korean J Urol. 2010;51:561–564. doi: 10.4111/kju.2010.51.8.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkonsen LB, Ernst A, Ramlau-Hansen CH. Maternal cigarette smoking during pregnancy and reproductive health in children: a review of epidemiological studies. Asian J Androl. 2014;16:39–49. doi: 10.4103/1008-682X.122351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halal F. The hand-foot-genital (hand-foot-uterus) syndrome: family report and update. Am J Med Genet. 1988;30:793–803. doi: 10.1002/ajmg.1320300312. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Suzuki K, Murakami R, Sakai M, Kamikawa M, et al. Molecular analysis of external genitalia formation: the role of fibro-blast growth factor (Fgf) genes during genital tubercle formation. Development. 2000;127:2471–2479. doi: 10.1242/dev.127.11.2471. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, et al. Unique functions of Sonic hedgehog signaling during external genitalia. Development. 2001;128:4241–4250. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- Hiort O, Klauber G, Cendron M, Sinnecker GH, Keim L, et al. Molecular characterization of the androgen receptor gene in boys with hypospadias. Eur J Pediatr. 1994;153:317–321. doi: 10.1007/BF01956409. [DOI] [PubMed] [Google Scholar]
- Hughes IA, Houk C, Ahmed SF, Lee PA. Lawson Wilkins Pediatric Endocrine Society/European Society for Paediatric Endocrinology Consensus Group: Consensus statement on management of intersex disorders. J Pediatr Urol. 2006;2:148–162. doi: 10.1016/j.jpurol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Hussain N, Chaghtai A, Herndon CDA, Herson VC, Rosenkrantz TS, McKenna PH. Hypospadias and early gestation growth restriction in infants. Pediatrics. 2002;109:473–478. doi: 10.1542/peds.109.3.473. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Wada Y, Kojima Y, Miyado M, Nakamura M, et al. Novel splice site mutation in MAMLD1 in a patient with hypospadias. Sex Dev. 2015;9:130–135. doi: 10.1159/000380842. [DOI] [PubMed] [Google Scholar]
- Kalfa N, Liu B, Klein O, Audran F, Wang MH, et al. Mutations of CXorf6 are associated with a range of severities of hypospadias. Eur J Endocrinol. 2008a;159:453–458. doi: 10.1530/EJE-08-0085. [DOI] [PubMed] [Google Scholar]
- Kalfa N, Liu B, Klein O, Wang MH, Cao M, Baskin LS. Genomic variants of ATF3 in patients with hypospadias. J Urol. 2008b;180:2183–2188. doi: 10.1016/j.juro.2008.07.066. [DOI] [PubMed] [Google Scholar]
- Kalfa N, Philibert P, Baskin LS, Sultan C. Hypospadias: interactions between environment and genetics. Mol Cell Endocrinol. 2011a;335:89–95. doi: 10.1016/j.mce.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Kalfa N, Cassorla F, Audran F, Oulad Abdennabi I, Philibert P, et al. Polymorphisms of MAMLD1 gene in hypospadias. J Pediatr Urol. 2011b;7:585–591. doi: 10.1016/j.jpurol.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Kalfa N, Fukami M, Philibert P, Audran F, Pienkowski C, et al. Screening of MAMLD1 mutations in 70 children with 46,XY DSD: identification and functional analysis of two new mutations. PLoS One. 2012;7:e32505. doi: 10.1371/journal.pone.0032505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalfa N, Paris F, Philibert P, Orsini M, Broussous S, et al. Is hypospadias associated with prenatal exposure to endocrine disruptors? A French collaborative controlled study of a cohort of 300 consecutive children without genetic defect. Eur Urol. 2015;68:1023–1030. doi: 10.1016/j.eururo.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Källén B, Nilsson E, Otterblad Olausson P. Maternal use of loperamide in early pregnancy and delivery outcome. Acta Paediatr Oslo Nor 1992. 2008;97:541–545. doi: 10.1111/j.1651-2227.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- Karabulut R, Turkyilmaz Z, Sonmez K, Kumas G, Ergun S, et al. Twenty-four genes are upregulated in patients with hypospadias. Balkan J Med Genet. 2013;16:39–44. doi: 10.2478/bjmg-2013-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Liu W, Cunha GR, Russell DW, Huang H, et al. Expression of the androgen receptor and 5 alpha-reductase type 2 in the developing human fetal penis and urethra. Cell Tissue Res. 2002;307:145–153. doi: 10.1007/s004410100464. [DOI] [PubMed] [Google Scholar]
- Klip H, Verloop J, van Gool JD, Koster META, Burger CW, et al. Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet. 2002;359:1102–1107. doi: 10.1016/S0140-6736(02)08152-7. [DOI] [PubMed] [Google Scholar]
- Köhler B, Delezoide AL, Boizet-Bonhoure B, McPhaul MJ, Sultan C, Lumbroso S. Coexpression of Wilms' tumor suppressor 1 (WT1) and androgen receptor (AR) in the genital tract of human male embryos and regulation of AR promoter activity by WT1. J Mol Endocrinol. 2007;38:547–554. doi: 10.1677/JME-06-0020. [DOI] [PubMed] [Google Scholar]
- Köhler B, Lin L, Mazen I, Cetindag C, Bieber-mann H, et al. The spectrum of phenotypes associated with mutations in steroidogenic factor 1 ( SF-1 , NR5A1 , Ad4BP) includes severe penoscrotal hypospadias in 46,XY males without adrenal insufficiency. Eur J Endocrinol. 2009;161:237–242. doi: 10.1530/EJE-09-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Hayashi Y, Mizuno K, Mogami M, Sasaki S, Kohri K. Spermatogenesis, fertility and sexual behavior in a hypospadiac mouse model. J Urol. 2002;167:1532–1537. [PubMed] [Google Scholar]
- Kon M, Suzuki E, Dung VC, Hasegawa Y, Mitsui T, et al. Molecular basis of non-syndromic hypospadias: systematic mutation screening and genome-wide copy-number analysis of 62 patients. Hum Reprod. 2015;30:499–506. doi: 10.1093/humrep/deu364. [DOI] [PubMed] [Google Scholar]
- Koopman P. Sry and Sox9 : mammalian testis-determining genes. Cell Mol Life Sci. 1999;55:839–856. doi: 10.1007/PL00013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi N, Sata F, Kasai S, Shibata T, Moriya K, et al. Maternal genetic polymorphisms in CYP1A1 , GSTM1 and GSTT1 and the risk of hypospadias. Mol Hum Reprod. 2005;11:93–98. doi: 10.1093/molehr/gah134. [DOI] [PubMed] [Google Scholar]
- Lachance Y, Luu-The V, Verreault H, Dumont M, Rhéaume E, et al. Structure of the human type II 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase (3 beta-HSD) gene: adrenal and gonadal specificity. DNA Cell Biol. 1991;10:701–711. doi: 10.1089/dna.1991.10.701. [DOI] [PubMed] [Google Scholar]
- Li J, Willingham E, Baskin LS. Gene expression profiles in mouse urethral development. BJU Int. 2006;98:880–885. doi: 10.1111/j.1464-410X.2006.06435.x. [DOI] [PubMed] [Google Scholar]
- Lim HN, Nixon RM, Chen H, Hughes IA, Hawkins JR. Evidence that longer androgen receptor polyglutamine repeats are a causal factor for genital abnormalities. J Clin Endocrinol Metab. 2001;86:3207–3210. doi: 10.1210/jcem.86.7.7674. [DOI] [PubMed] [Google Scholar]
- Lin C, Yin Y, Long F, Ma L. Tissue-specific requirements of beta-catenin in external genitalia development. Development. 2008;135:2815–2825. doi: 10.1242/dev.020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little NA, Hastie ND, Davies RC. Identification of WTAP, a novel Wilms' tumour 1-associating protein. Hum Mol Genet. 2000;9:2231–2239. doi: 10.1093/oxfordjournals.hmg.a018914. [DOI] [PubMed] [Google Scholar]
- Liu B, Wang Z, Lin G, Agras K, Ebbers M, et al. Activating transcription factor 3 is up-regulated in patients with hypospadias. Pediatr Res. 2005;58:1280–1283. doi: 10.1203/01.pdr.0000187796.28007.2d. [DOI] [PubMed] [Google Scholar]
- Liu D, Shen L, Tao Y, Kuang Y, Cai L, et al. Alterations in gene expression during sexual differentiation in androgen receptor knockout mice induced by environmental endocrine disruptors. Int J Mol Med. 2015;35:399–404. doi: 10.3892/ijmm.2014.2015. [DOI] [PubMed] [Google Scholar]
- Lu T, Chen R, Cox TC, Moldrich RX, Kurniawan N, et al. X-linked microtubule-associated protein, Mid1, regulates axon development. Proc Natl Acad Sci USA. 2013;110:19131–19136. doi: 10.1073/pnas.1303687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccio-Camelo DC, Prins GS. Disruption of androgen receptor signaling in males by environmental chemicals. J Steroid Biochem Mol Biol. 2011;127:74–82. doi: 10.1016/j.jsbmb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund L, Engebjerg MC, Pedersen L, Ehrenstein V, Nørgaard M, Sørensen HT. Prevalence of hypospadias in Danish boys: a longitudinal study, 1977–2005. Eur Urol. 2009;55:1022–1026. doi: 10.1016/j.eururo.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Ma Q, Tang Y, Lin H, Xu M, Xu G, et al. Diacylglycerol kinase K (DGKK) variants and hypospadias in Han Chinese: association and meta-analysis. BJU Int. 2014;116:634–640. doi: 10.1111/bju.12965. [DOI] [PubMed] [Google Scholar]
- Manson JM, Carr MC. Molecular epidemiology of hypospadias: review of genetic and environmental risk factors. Birth Defects Res A Clin Mol Teratol. 2003;67:825–836. doi: 10.1002/bdra.10084. [DOI] [PubMed] [Google Scholar]
- Marengo L, Farag NH, Canfield M. Body mass index and birth defects: Texas, 2005–2008. Matern Child Health J. 2013;17:1898–1907. doi: 10.1007/s10995-012-1214-5. [DOI] [PubMed] [Google Scholar]
- Marrocco G, Grammatico P, Vallasciani S, Gulia C, Zangari A, et al. Environmental, parental and gestational factors that influence the occurrence of hypospadias in male patients. J Pediatr Urol. 2015;11:12–19. doi: 10.1016/j.jpurol.2014.10.003. [DOI] [PubMed] [Google Scholar]
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Mendonça BB, Russell AJ, Vasconcelos-Leite M, Arnhold IJ, Bloise W, et al. Mutation in 3 beta-hydroxysteroid dehydrogenase type II associated with pseudohermaphroditism in males and premature pubarche or cryptic expression in females. J Mol Endocrinol. 1994;12:119–122. doi: 10.1677/jme.0.0120119. [DOI] [PubMed] [Google Scholar]
- Michnovicz JJ, Naganuma H, Hershcopf RJ, Bradlow HL, Fishman J. Increased urinary catechol estrogen excretion in female smokers. Steroids. 1988;52:69–83. doi: 10.1016/0039-128x(88)90218-8. [DOI] [PubMed] [Google Scholar]
- Miyado M, Nakamura M, Miyado K, Morohashi KI, Sano S, et al. Mamld1 deficiency significantly reduces mRNA expression levels of multiple genes expressed in mouse fetal Leydig cells but permits normal genital and reproductive development. Endocrinology. 2012;153:6033–6040. doi: 10.1210/en.2012-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa S, Satoh Y, Haraguchi R, Suzuki K, Iguchi T, et al. Genetic interactions of the androgen and Wnt/β-catenin pathways for the masculinization of external genitalia. Mol Endocrinol. 2009;23:871–880. doi: 10.1210/me.2008-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa S, Matsumaru D, Murashima A, Omori A, Satoh Y, et al. The role of sonic hedgehog-Gli2 pathway in the masculinization of external genitalia. Endocrinology. 2011;152:2894–2903. doi: 10.1210/en.2011-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Garcia M, Miranda EB. Chromosomal anomalies in cryptorchidism and hypospadias. J Urol. 2002;168:2170–2172. doi: 10.1016/S0022-5347(05)64346-7. [DOI] [PubMed] [Google Scholar]
- Morgan EA, Nguyen SB, Scott V, Stadler HS. Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development. 2003;130:3095–3109. doi: 10.1242/dev.00530. [DOI] [PubMed] [Google Scholar]
- Morris SE, Thomson AO, Jarup L, de Hoogh C, Briggs DJ, Elliott P. No excess risk of adverse birth outcomes in populations living near special waste landfill sites in Scotland. Scott Med J. 2003;48:105–107. doi: 10.1177/003693300304800403. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Fukami M, Sugawa F, Miyado M, Nonomura K, Ogata T. Mamld1 knockdown reduces testosterone production and Cyp17a1 expression in mouse Leydig tumor cells. PloS One. 2011;6:e19123. doi: 10.1371/journal.pone.0019123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T, Yamaai T, Asano M, Nawachi K, Suzuki M, et al. Overexpression of connective tissue growth factor/hypertrophic chondrocyte-specific gene product 24 decreases bone density in adult mice and induces dwarfism. Biochem Biophys Res Commun. 2001;281:678–681. doi: 10.1006/bbrc.2001.4379. [DOI] [PubMed] [Google Scholar]
- Nassar N, Abeywardana P, Barker A, Bower C. Parental occupational exposure to potential endocrine disrupting chemicals and risk of hypospadias in infants. Occup Environ Med. 2010;67:585–589. doi: 10.1136/oem.2009.048272. [DOI] [PubMed] [Google Scholar]
- Nordenskjöld A, Friedman E, Tapper-Persson M, Söderhäll C, Leviav A, et al. Screening for mutations in candidate genes for hypospadias. Urol Res. 1999;27:49–55. doi: 10.1007/s002400050088. [DOI] [PubMed] [Google Scholar]
- Nørgaard M, Wogelius P, Pedersen L, Rothman KJ, Sørensen HT. Maternal use of oral contraceptives during early pregnancy and risk of hypospadias in male offspring. Urology. 2009;74:583–587. doi: 10.1016/j.urology.2009.04.034. [DOI] [PubMed] [Google Scholar]
- North K, Golding J. A maternal vegetarian diet in pregnancy is associated with hypospadias. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. BJU Int. 2000;85:107–113. doi: 10.1046/j.1464-410x.2000.00436.x. [DOI] [PubMed] [Google Scholar]
- Ogata T, Laporte J, Fukami M. MAMLD1 (CXorf6) : a new gene involved in hypospadias. Horm Res. 2009;71:245–252. doi: 10.1159/000208797. [DOI] [PubMed] [Google Scholar]
- Ormond G, Nieuwenhuijsen MJ, Nelson P, Toledano MB, Iszatt N, et al. Endocrine disruptors in the workplace, hair spray, folate supplementation, and risk of hypospadias: case-control study. Environ Health Perspect. 2009;117:303–307. doi: 10.1289/ehp.11933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parajes S, Chan AOK, But WM, Rose IT, Taylor AE, et al. Delayed diagnosis of adrenal insufficiency in a patient with severe penoscrotal hypospadias due to two novel P450 side-change cleavage enzyme (CYP11A1) mutations (p.R360W; p.R405X). Eur J Endocrinol. 2012;167:881–885. doi: 10.1530/EJE-12-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol. 2002;247:26–46. doi: 10.1006/dbio.2002.0668. [DOI] [PubMed] [Google Scholar]
- Person AD, Beiraghi S, Sieben CM, Hermanson S, Neumann AN, et al. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev Dyn. 2010;239:327–337. doi: 10.1002/dvdy.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petiot A, Perriton CL, Dickson C, Cohn MJ. Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development. 2005;132:2441–2450. doi: 10.1242/dev.01778. [DOI] [PubMed] [Google Scholar]
- Phillips TR, Wright DK, Gradie PE, Johnston LA, Pask AJ. A comprehensive atlas of the adult mouse penis. Sex Dev. 2015;9:162–172. doi: 10.1159/000431010. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Tasian GE, Zhang H, Cunha GR, Baskin L. ZEB1 is estrogen responsive in vitro in human foreskin cells and is over expressed in pe-nile skin in patients with severe hypospadias. J Urol. 2011;185:1888–1893. doi: 10.1016/j.juro.2010.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radpour R, Rezaee M, Tavasoly A, Solati S, Saleki A. Association of long polyglycine tracts (GGN repeats) in exon 1 of the androgen receptor gene with cryptorchidism and penile hypospadias in Iranian patients. J Androl. 2007;28:164–169. doi: 10.2164/jandrol.106.000927. [DOI] [PubMed] [Google Scholar]
- Rankin J, Tennant PWG, Stothard KJ, Bythell M, Summerbell CD, Bell R. Maternal body mass index and congenital anomaly risk: a cohort study. Int J Obes (Lond) 2010;34:1371–1380. doi: 10.1038/ijo.2010.66. [DOI] [PubMed] [Google Scholar]
- Reis M, Källén B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychol Med. 2010;40:1723–1733. doi: 10.1017/S0033291709992194. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Pinilla E, Mejías C, Prieto-Merino D, Fernández P, Martínez-Frías ML. ECEMC Working Group: Risk of hypospadias in newborn infants exposed to valproic acid during the first trimester of pregnancy: a case-control study in Spain. Drug Saf. 2008;31:537–543. doi: 10.2165/00002018-200831060-00008. [DOI] [PubMed] [Google Scholar]
- Rubtsov P, Karmanov M, Sverdlova P, Spirin P, Tiulpakov A. A novel homozygous mutation in CYP11A1 gene is associated with late-onset adrenal insufficiency and hypospadias in a 46,XY patient. J Clin Endocrinol Metab. 2009;94:936–939. doi: 10.1210/jc.2008-1118. [DOI] [PubMed] [Google Scholar]
- Russell AJ, Wallace AM, Forest MG, Donaldson MD, Edwards CR, Sutcliffe RG. Mutation in the human gene for 3 beta-hydroxysteroid dehydrogenase type II leading to male pseudo-hermaphroditism without salt loss. J Mol Endocrinol. 1994;12:225–237. doi: 10.1677/jme.0.0120225. [DOI] [PubMed] [Google Scholar]
- Ságodi L, Kiss A, Kiss-Tóth E, Barkai L. Prevalence and possible causes of hypospadias (in Hungarian). Orv Hetil. 2014;155:978–985. doi: 10.1556/OH.2014.29858. [DOI] [PubMed] [Google Scholar]
- Samtani R, Bajpai M, Vashisht K, Ghosh PK, Saraswathy KN. Hypospadias risk and polymorphism in SRD5A2 and CYP17 genes: case-control study among Indian children. J Urol. 2011;185:2334–2339. doi: 10.1016/j.juro.2011.02.043. [DOI] [PubMed] [Google Scholar]
- Sata F, Kurahashi N, Ban S, Moriya K, Tanaka KD, et al. Genetic polymorphisms of 17β-hydroxysteroid dehydrogenase 3 and the risk of hypospadias. J Sex Med. 2010;7:2729–2738. doi: 10.1111/j.1743-6109.2009.01641.x. [DOI] [PubMed] [Google Scholar]
- Schlesinger TK, Bonvin C, Jarpe MB, Fanger GR, Cardinaux JR, et al. Apoptosis stimulated by the 91-kDa caspase cleavage MEKK1 fragment requires translocation to soluble cellular compartments. J Biol Chem. 2002;277:10283–10291. doi: 10.1074/jbc.M106885200. [DOI] [PubMed] [Google Scholar]
- Schnack TH, Zdravkovic S, Myrup C, Westergaard T, Christensen K, et al. Familial aggregation of hypospadias: a cohort study. Am J Epidemiol. 2008;167:251–256. doi: 10.1093/aje/kwm317. [DOI] [PubMed] [Google Scholar]
- Schnitzer PG, Olshan AF, Erickson JD. Paternal occupation and risk of birth defects in off-spring. Epidemiology. 1995;6:577–583. doi: 10.1097/00001648-199511000-00003. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Bleyl SB, Brauer PR, Francis-West PH, editors. Larsen's Human Embryology. ed 4 Churchill Livingstone, Elsevier; New York: 2009. [Google Scholar]
- Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30:883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- Shehata BM, Elmore JM, Bootwala Y, Steelman CK, Bare JB, et al. Immunohistochemical characterization of sonic hedgehog and its downstream signaling molecules during human penile development. Fetal Pediatr Pathol. 2011;30:244–251. doi: 10.3109/15513815.2011.555809. [DOI] [PubMed] [Google Scholar]
- Shekharyadav C, Bajpai M, Kumar V, Ahmed RS, Gupta P, Banerjee BD. Polymorphism in CYP1A1 , GSTMI , GSTT1 genes and organo-chlorine pesticides in the etiology of hypospadias. Hum Exp Toxicol. 2011;30:1464–1474. doi: 10.1177/0960327110392402. [DOI] [PubMed] [Google Scholar]
- Shen J, Liu B, Sinclair A, Cunha G, Baskin LS, Choudhry S. Expression analysis of DGKK during external genitalia formation. J Urol. 2015:S0022–5347. doi: 10.1016/j.juro.2015.06.098. [DOI] [PubMed] [Google Scholar]
- Shih EM, Graham JM., Jr Review of genetic and environmental factors leading to hypospadias. Eur J Med Genet. 2014;57:453–463. doi: 10.1016/j.ejmg.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Silver RI, Russell DW. 5alpha-reductase type 2 mutations are present in some boys with isolated hypospadias. J Urol. 1999;162:1142–1145. doi: 10.1016/S0022-5347(01)68102-3. [DOI] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Snodgrass W, Macedo A, Hoebeke P, Mouriquand PDE. Hypospadias dilemmas: a round table. J Pediatr Urol. 2011;7:145–157. doi: 10.1016/j.jpurol.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Söderhäll C, Körberg IB, Thai HT, Cao J, Chen Y, et al. Fine mapping analysis confirms and strengthens linkage of four chromosomal regions in familial hypospadias. Eur J Hum Genet. 2015;23:516–522. doi: 10.1038/ejhg.2014.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen HT, Pedersen L, Skriver MV, Nørgaard M, Nørgård B, Hatch EE. Use of clomifene during early pregnancy and risk of hypospadias: population based case-control study. BMJ. 2005;330:126–127. doi: 10.1136/bmj.38326.606979.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staib P, Kau N, Romalo G, Schweikert HU. Oestrogen formation in genital and non-genital skin fibroblasts cultured from patients with hypospadias. Clin Endocrinol (Oxf) 1994;41:237–243. doi: 10.1111/j.1365-2265.1994.tb02536.x. [DOI] [PubMed] [Google Scholar]
- Stoll C, Alembik Y, Roth MP, Dott B. Genetic and environmental factors in hypospadias. J Med Genet. 1990;27:559–563. doi: 10.1136/jmg.27.9.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RW, Wiener JS, Hicks JP, Marcelli M, Gonzales ET, et al. Androgen receptor gene mutations are rarely associated with isolated penile hypospadias. J Urol. 1996;156:828–831. doi: 10.1097/00005392-199608001-00077. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Bachiller D, Chen YP, Kamikawa M, Ogi H, et al. Regulation of outgrowth and apoptosis for the terminal appendage: external genitalia development by concerted actions of BMP signaling [corrected]. Development. 2003;130:6209–6220. doi: 10.1242/dev.00846. [DOI] [PubMed] [Google Scholar]
- Switonski M, Payan-Carreira R, Bartz M, Nowacka-Woszuk J, Szczerbal I, et al. Hypospadias in a male (78,XY; SRY-positive) dog and sex reversal female (78,XX; SRY-negative) dogs: clinical, histological and genetic studies. Sex Dev. 2012;6:128–134. doi: 10.1159/000330921. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Shimotakahara A, Miyahara K, Lane GJ, Yamataka A. Activating transcription factor 3 is not up-regulated in hypospadias patients in Japan. Afr J Paediatr Surg. 2013;10:371–373. doi: 10.4103/0189-6725.125451. [DOI] [PubMed] [Google Scholar]
- Tanaka SS, Nishinakamura R. Regulation of male sex determination: genital ridge formation and Sry activation in mice. Cell Mol Life Sci. 2014;71:4781–4802. doi: 10.1007/s00018-014-1703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang KF, Zheng JZ, Xing JP. Molecular analysis of SNP12 in estrogen receptor α gene in hypospadiac or cryptorchid patients from Northwestern China. Urol Int. 2011;87:359–362. doi: 10.1159/000330902. [DOI] [PubMed] [Google Scholar]
- Tang Y, Cheng S. Progress on Hedgehog signaling transduction (in Chinese). Sheng Li Xue Bao. 2014;66:415–422. [PubMed] [Google Scholar]
- Tannour-Louet M, Han S, Louet JF, Zhang B, Romero K, et al. Increased gene copy number of the vesicle SNARE VAMP7 disrupts human male urogenital development through altered estrogen action. Nat Med. 2014;20:715–724. doi: 10.1038/nm.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantawy S, Mazen I, Soliman H, Anwar G, Atef A, et al. Analysis of the gene coding for steroidogenic factor 1 (SF1, NR5A1 ) in a cohort of 50 Egyptian patients with 46,XY disorders of sex development. Eur J Endocrinol. 2014;170:759–767. doi: 10.1530/EJE-13-0965. [DOI] [PubMed] [Google Scholar]
- Thai HTT, Kalbasi M, Lagerstedt K, Frisén L, Kockum I, Nordenskjöld A. The valine allele of the V89L polymorphism in the 5-alpha-reductase gene confers a reduced risk for hypospadias. J Clin Endocrinol Metab. 2005;90:6695–6698. doi: 10.1210/jc.2005-0446. [DOI] [PubMed] [Google Scholar]
- Thorup J, Nordenskjöld A, Hutson JM. Genetic and environmental origins of hypospadias. Curr Opin Endocrinol Diabetes Obes. 2014;21:227–232. doi: 10.1097/MED.0000000000000063. [DOI] [PubMed] [Google Scholar]
- Uda A, Kojima Y, Hayashi Y, Mizuno K, Asai N, Kohri K. Morphological features of external genitalia in hypospadiac rat model: 3-dimensional analysis. J Urol. 2004;171:1362–1366. doi: 10.1097/01.ju.0000100140.42618.54. [DOI] [PubMed] [Google Scholar]
- van der Zanden LFM, van Rooij IAL, Feitz WFJ, Knight J, Donders ART, et al. Common variants in DGKK are strongly associated with risk of hypospadias. Nat Genet. 2011;43:48–50. doi: 10.1038/ng.721. [DOI] [PubMed] [Google Scholar]
- van der Zanden LFM, van Rooij IAL, Feitz WFJ, Franke B, Knoers NV, Roeleveld N. Aetiology of hypospadias: a systematic review of genes and environment. Hum Reprod Update. 2012;18:260–283. doi: 10.1093/humupd/dms002. [DOI] [PubMed] [Google Scholar]
- Varvarigou AA, Asimakopoulou A, Beratis NG. Impact of maternal smoking on birth size: effect of parity and sex dimorphism. Neonatology. 2009;95:61–67. doi: 10.1159/000151756. [DOI] [PubMed] [Google Scholar]
- Vottero A, Minari R, Viani I, Tassi F, Bonatti F, et al. Evidence for epigenetic abnormalities of the androgen receptor gene in foreskin from children with hypospadias. J Clin Endocrinol Metab. 2011;96:E1953–E1962. doi: 10.1210/jc.2011-0511. [DOI] [PubMed] [Google Scholar]
- Wang CH, Zeng Q, Jiang XZ, Wan B, He LY. Distribution of HoxA13 in the prepuce and urethral plate in different types of hypospadias and its implication (in Chinese). Zhonghua Nan Ke Xue. 2013;19:806–810. [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wang R, Dong Z, Wang W, Xiao Y, Ni J, Wang D. Mutation analysis of the SRD5A2 , AR and SF-1 genes in 52 Chinese boys with hypospadias. J Pediatr Endocrinol Metab. 2013;26:887–893. doi: 10.1515/jpem-2012-0316. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li Q, Xu J, Liu Q, Wang W, et al. Mutation analysis of five candidate genes in Chinese patients with hypospadias. Eur J Hum Genet. 2004;12:706–712. doi: 10.1038/sj.ejhg.5201232. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu BC, Lin GT, Lin CS, Lue TF, et al. Up-regulation of estrogen responsive genes in hypospadias: microarray analysis. J Urol. 2007;177:1939–1946. doi: 10.1016/j.juro.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Warman DM, Costanzo M, Marino R, Berensztein E, Galeano J, et al. Three new SF-1 (NR5A1) gene mutations in two unrelated families with multiple affected members: within-family variability in 46,XY subjects and low ovarian reserve in fertile 46,XX subjects. Horm Res Paediatr. 2011;75:70–77. doi: 10.1159/000320029. [DOI] [PubMed] [Google Scholar]
- Warot X, Fromental-Ramain C, Fraulob V, Chambon P, Dollé P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997;124:4781–4791. doi: 10.1242/dev.124.23.4781. [DOI] [PubMed] [Google Scholar]
- Warr N, Siggers P, Carré GA, Bogani D, Brixey R, et al. Transgenic expression of Map3k4 rescues T-associated sex reversal (Tas) in mice. Hum Mol Genet. 2014;23:3035–3044. doi: 10.1093/hmg/ddu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Yoshida R, Ueoka K, Aoki K, Sasagawa I, et al. Haplotype analysis of the estrogen receptor 1 gene in male genital and reproductive abnormalities. Hum Reprod. 2007;22:1279–1284. doi: 10.1093/humrep/del513. [DOI] [PubMed] [Google Scholar]
- Wong RLY, Walker CL. Molecular pathways: environmental estrogens activate nongenomic signaling to developmentally reprogram the epigenome. Clin Cancer Res. 2013;19:3732–3737. doi: 10.1158/1078-0432.CCR-13-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, McGown IN, Lin L, Achermann JC, Harris M, et al. A novel NR5A1 variant in an infant with elevated testosterone from an Australasian cohort of 46,XY patients with disorders of sex development. Clin Endocrinol (Oxf) 2013;78:545–550. doi: 10.1111/cen.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Ferrara C, Shapiro E, Grishina I. Bmp7 expression and null phenotype in the urogenital system suggest a role in re-organization of the urethral epithelium. Gene Expr Patterns. 2009;9:224–230. doi: 10.1016/j.gep.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada G, Satoh Y, Baskin LS, Cunha GR. Cellular and molecular mechanisms of development of the external genitalia. Differentiation. 2003;71:445–460. doi: 10.1046/j.1432-0436.2003.7108001.x. [DOI] [PubMed] [Google Scholar]
- Yamada G, Suzuki K, Haraguchi R, Miyagawa S, Satoh Y, et al. Molecular genetic cascades for external genitalia formation: an emerging organogenesis program. Dev Dyn. 2006;235:1738–1752. doi: 10.1002/dvdy.20807. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yong W, Yang Z, Periyasamy S, Chen H, Yucel S, et al. Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J Biol Chem. 2007;282:5026–5036. doi: 10.1074/jbc.M609360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel S, Liu W, Cordero D, Donjacour A, Cunha G, Baskin LS. Anatomical studies of the fibro-blast growth factor-10 mutant, Sonic Hedge Hog mutant and androgen receptor mutant mouse genital tubercle. Adv Exp Med Biol. 2004;545:123–148. doi: 10.1007/978-1-4419-8995-6_8. [DOI] [PubMed] [Google Scholar]
- Yucel S, Dravis C, Garcia N, Henkemeyer M, Baker LA. Hypospadias and anorectal malformations mediated by Eph/ephrin signaling. J Pediatr Urol. 2007;3:354–363. doi: 10.1016/j.jpurol.2007.01.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentner GE, Hurd EA, Schnetz MP, Handoko L, Wang C, et al. CHD7 functions in the nucleolus as a positive regulator of ribosomal RNA biogenesis. Hum Mol Genet. 2010;19:3491–3501. doi: 10.1093/hmg/ddq265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen Y, Zhao S, Markljung E, Nordenskjöld A. Hypospadias associated with hyper-telorism, the mildest phenotype of Opitz syndrome. J Hum Genet. 2011;56:348–351. doi: 10.1038/jhg.2011.17. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang H, Peng YB, Chen Q, Da J, et al. Expressions of ATF3 and CTGF and their regulation by estradiol in the prepuce of hypospadias patients (in Chinese). Zhonghua Nan Ke Xue. 2009;15:1075–1080. [PubMed] [Google Scholar]
- Zhuang LK, Fu QH, Wang J, Sun J. Single-nucleotide polymorphisms of MAMLD1 and hypospadias in Chinese (in Chinese). Zhonghua Nan Ke Xue. 2012;18:727–730. [PubMed] [Google Scholar]