Abstract
Objective
To investigate the effect of laryngopharyngeal neuromuscular electrical stimulation (NMES) on dysphonia in patients with dysphagia caused by stroke or traumatic brain injury (TBI).
Methods
Eighteen patients participated in this study. The subjects were divided into NMES (n=12) and conventional swallowing training only (CST, n=6) groups. The NMES group received NMES combined with CST for 2 weeks, followed by CST without NMES for the next 2 weeks. The CST group received only CST for 4 weeks. All of the patients were evaluated before and at 2 and 4 weeks into the study. The outcome measurements included perceptual, acoustic and aerodynamic analyses. The correlation between dysphonia and swallowing function was also investigated.
Results
There were significant differences in the GRBAS (grade, roughness, breathiness, asthenia and strain scale) total score and sound pressure level (SPL) between the two groups over time. The NMES relative to the CST group showed significant improvements in total GRBAS score and SPL at 2 weeks, though no inter-group differences were evident at 4 weeks. The improvement of the total GRBAS scores at 2 weeks was positively correlated with the improved pharyngeal phase scores on the functional dysphagia scale at 2 weeks.
Conclusion
The results demonstrate that laryngopharyngeal NMES in post-stroke or TBI patients with dysphonia can have promising effects on phonation. Therefore, laryngopharyngeal NMES may be considered as an additional treatment option for dysphonia accompanied by dysphagia after stroke or TBI.
Keywords: Dysphonia, Deglutition disorders, Electrical stimulation therapy, Stroke, Brain injuries
INTRODUCTION
Dysphonia is a voice disorder of the larynx usually manifesting as hoarseness. Its etiology can be neurologic, organic, or functional [1]. Those with dysphonia can be affected socially and vocationally as they develop communication issues with friends and colleagues. Indeed, they might experience social isolation, depression and impaired quality of life [2]. Another speech issue is dysarthria, in which the muscles used for speech have been weakened or are not properly controllable. Cohen et al. [1] maintained that cerebral hemisphere strokes often result in dysarthria that affects all of the cranial nerves innervating the speech and voice musculature, resulting, in turn, in slow speech, imprecise articulation, and a strained quality of voice. In cases of acquired brain injury, speech is typically one of the important rehabilitation concerns in terms of a patient's quality of life. As such, various types of therapy are used clinically for dysphonia and dysarthria. For these rehabilitation programs, however, there are as yet no distinct guidelines [3,4,5].
Swallowing and phonation are different functions that share a common structure in the form of the aerodigestive tract. Consequently, if either function is impaired, it is highly probable that the other also is affected. Traditionally, therapeutic methods have been applied to each function separately; however, given the recent discovery of cross-system interaction between those two functions, it has been considered that a therapeutic benefit for one might also offer improvement for the other [6,7,8]. In this regard, laryngopharyngeal neuromuscular electrical stimulation (NMES), a recent dysphagia therapy that is effective in improving swallowing function (significant improvements in laryngeal elevation, epiglottic closure, and the pharyngeal transit time of the pharyngeal phase have been reported [9]) has been shown to effect positive changes in phonation in addition to those of swallowing function [6,10]. Another, acoustic evaluation reported improved voice intensity and stabilized jitter and shimmer after laryngopharyngeal NMES [11]. The precise mechanism of action here, however, remains unclear. A possible explanation is that contraction of the superficial cricothyroid muscles is facilitated, thereby increasing vocal fold tension, improving glottal closure and contributing in turn to improved voice performance [6]. Another study, meanwhile, failed to demonstrate any intrinsic laryngeal muscle activation or improved vocal fold closure [12].
Thus far there have been only a few clinical trials that have objectively—perceptually, acoustically, aerodynamically—evaluated the effect of laryngopharyngeal NMES therapy on phonation. The aim of the present study was to evaluate, in perceptual, acoustic and aerodynamic aspects, the effect of laryngopharyngeal NMES therapy on the phonation of patients diagnosed with dysphagia after stroke and traumatic brain injury (TBI), specifically by comparison with those who had undergone conventional swallowing training (CST) only. The correlation between dysphonia and swallowing function was also evaluated.
MATERIALS AND METHODS
Subjects
Eighteen patients admitted to our rehabilitation department for stroke or TBI between November 2011 and May 2015 participated in this study. After the onset of stroke, all the subjects manifested symptoms of dysphonia, including hoarseness and/or a breathy or rough voice. The subjects were medically stable and able to participate in the therapy programs. For patients with dysphagia diagnosed by videofluoroscopic swallowing study (VFSS), a speech therapist performed a voice analysis. The excluded patients were those with an organic voice disorder involving polyps, tumors or paralysis of the vocal cords, those who had dysphagia before disease onset, and those who could not participate in therapy due to cognitive dysfunction or a pre-existing psychiatric condition, those who were unable to undergo electrotherapy due to cardiac pacemakers, and those who could not phonate due to tracheostomy. The subjects were divided into the following two groups: an experimental group, which received both CST and laryngopharyngeal NMES therapy (n=12), and a control group that received only CST (n=6). This study was approved by the Institutional Review Board of Dankook University in Korea.
Treatment protocol
Both the NMES and CST groups underwent 4 weeks of CST, 5 times per week and once per day for 60 minutes each. The CST consisted of tongue retraction exercise, jaw and lip range-of-motion exercise, bolus manipulation exercise, Shaker exercise, thermotactile stimulation and Mendelsohn maneuver. If necessary, patients were educated in compensatory techniques.
The NMES group received laryngopharyngeal NMES stimulation using the VitalStim Therapy System (Chattanooga Group, Austin, TX, USA) 5 times per week and once per day for 60 minutes during the initial 2 weeks while undergoing CST. The NMES stimulation was applied through two independent channels, pads having been placed on both sides of the thyroid notch and at two points on the hyoid bone, respectively. The optimal biphasic wave and pulse duration were determined to be 80 Hz and 300 µs, respectively, as described in Freed et al. [13]. To prevent laryngeal muscle spasm, stimulation was applied for 50 seconds, before and after which there were 1 second rise and fall times, respectively, followed by a pause of 8 seconds. The intensity of stimulation ranged between 3 mA and a maximum value selected according to the subject's capacity.
Voice analysis
The patients' voice data were obtained in Dankook University Hospital's Vocal Language Treatment room using the Kay-PENTAX Real-Time electroglottograph (EGG) Analysis program (Model 5138, Version 3.1.6; PENTAX Medical Company, Tokyo, Japan). The patient was seated in an easy posture with EGG (Model 6103, Kay-PENTAX) electrodes attached to both sides of the thyroid cartilage, and were asked to continue phonating the vowel a at maximum length for as long as possible while maintaining a 5-cm distance between the mouth and the microphone. The voice-sampling rate was 44,100 Hz with 16-bit quantization. For perceptual voice quality evaluation, the universally employed GRBAS scale with its five parameters (Appendix 1) was used [14]. Each parameter was rated on a 4-point scale ranging from 0 (normal) to 3 (severe). For acoustic evaluation, the fundamental frequency ('Fo'), variation of frequency ('jitter'), variation of vocal amplitude ('shimmer'), noise-to-harmonic ratio (NHR) and soft phonation index (SPI), which are representative parameters of vocal cord vibration stability, were assessed using the Multi-Dimensional Voice Program (MDVP) of a computerized speech laboratory (Model 4500, Kay-PENTAX) [15]. For the purposes of an aerodynamic evaluation, the maximal phonation time (MPT), mean flow rate (MFR), sound pressure level (SPL), subglottic pressure (Psub), phonation efficiency (PE) and phonation resistance (PR) were assessed by Aerophone II (Kay-PENTAX). Voice analysis was performed on all patients before as well as 2 and 4 weeks after the start of the study.
Swallowing function
All of the patients were assessed by VFSS before as well as 2 and 4 weeks after the start of the study. The VFSS was performed according to a modified Logemann protocol [16]. The procedure involved having the subject seated with fluoroscopic video images in lateral projection. Massive aspiration was screened by swallowing a 2-mL volume of thin liquid before beginning the main test procedure with five sequential swallows of 5 mL of thick and thin liquids. The images were analyzed frame-by-frame by an experienced physiatrist.
For the assessment of overall swallowing function, the functional dysphagia scale (FDS) was used [17]. The FDS consists of 11 items with weighted values representing 4 oral and 7 pharyngeal functions that can be observed by VFSS (Appendix 2). For evaluation of the severity of penetration and aspiration, the penetration-aspiration scale (PAS) was applied, with scores ranging from 1 to 8 [18]. Higher FDS and PAS scores indicate poor swallowing function. Additionally, the patients were assessed on the American Speech-Language-Hearing Association National Outcome Measurement System (ASHA NOMS) swallowing scale, a 7-point rating system in which lower levels indicate more severe dysphagia. It was clinically evaluated by integrating all information regarding the diet level and the supervision level required [19].
Statistical analysis
Statistical analysis was performed using SPSS ver. 12.0K for Windows (SPSS Inc., Chicago IL, USA). To compare the baseline characteristics between the groups, the Mann-Whitney U and Fisher's exact tests were applied for continuous and categorical variables, respectively. A repeated-measures ANOVA with post-hoc pairwise comparisons (Bonferroni corrected) were used to compare voice analysis factor and swallowing function changes between the groups over time (group-by-time interaction). Statistical significance was set at p<0.05.
RESULTS
Characteristics of subjects
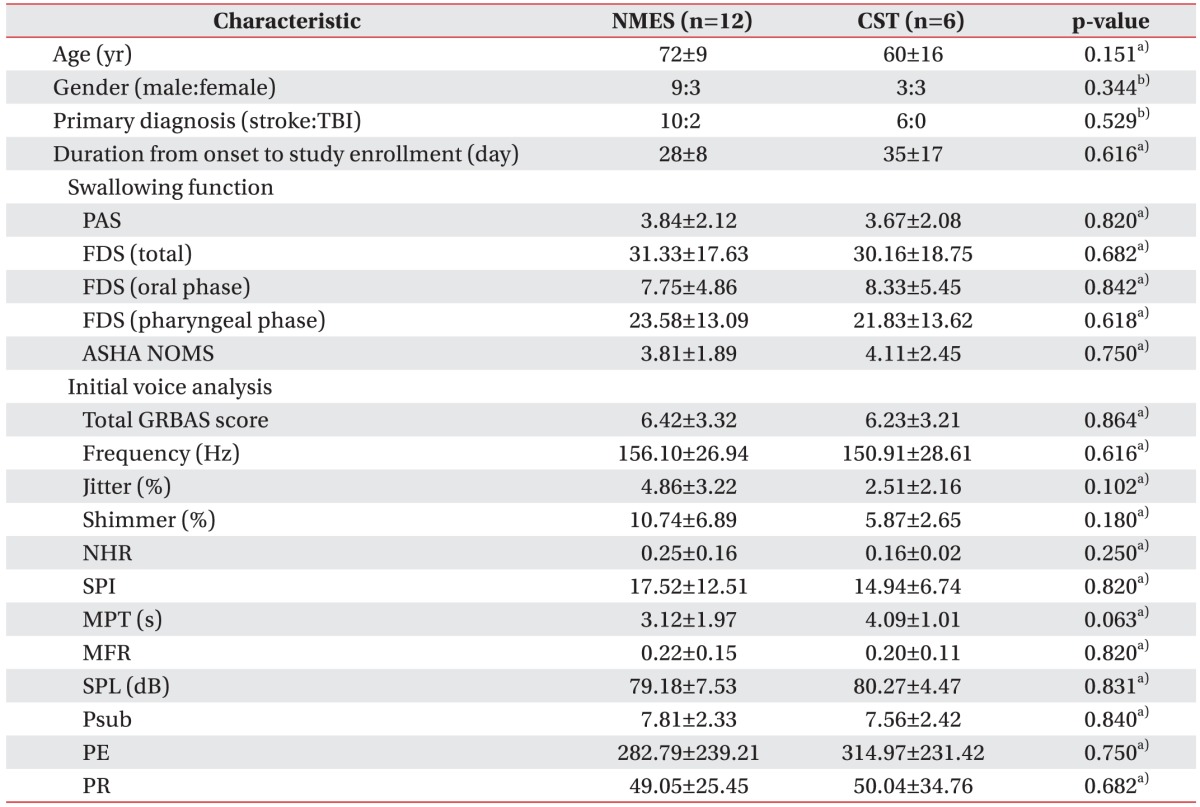
This study was conducted with a total of 18 subjects, 12 males and 6 females. There were no statistically significant differences between the groups with respect to age, gender, primary diagnosis, duration from onset to study enrollment or the initial swallowing function and voice analysis parameters (Table 1).
Table 1. Baseline characteristics of patients.
Values are presented as mean±standard deviation or numbers.
NEST, neuromuscular electrical stimulation; CST, conventional swallowing training; TBI, traumatic brain injury; PAS, penetration-aspiration scale; FDS, functional dysphagia scale; ASHA NOMS, American Speech-Language-Hearing Association National Outcome Measurement System swallowing scale; GRBAS, grade, roughness, breathiness, asthenia, and strain; NHR, noise-to-harmonic ratio; SPI, soft phonation index; MPT, maximal phonation time; MFR, mean flow rate; SPL, sound pressure level; Psub, subglottic pressure; PE, phonation efficiency; PR, phonation resistance
a)Mann-Whitney U test, b)Fisher's exact test.
Voice analysis
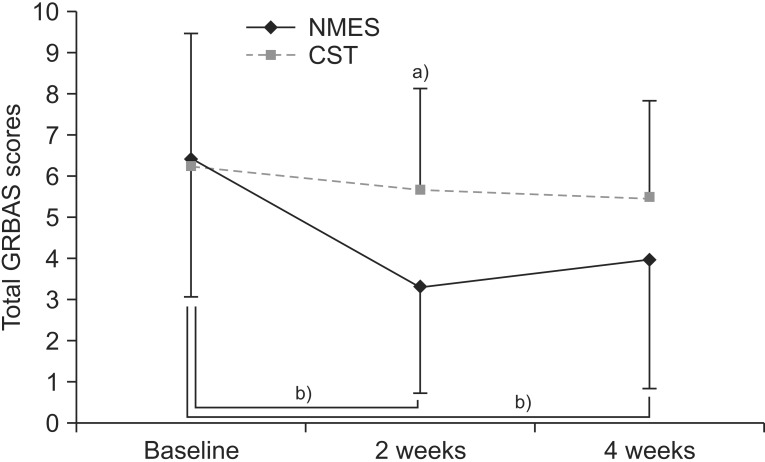
Fig. 1 shows the total GRBAS scores in the NMES and CST groups. Statistically significant intra-group differences were also observed over time (main effect of time, p<0.01). Additionally, there was a group-by-time interaction, which indicated a score difference between the groups over time (p<0.05). A post-hoc analysis by Mann-Whitney U test with Bonferroni correction was performed at each time point. The NMES group revealed a significant score improvement relative to the CST group at 2 weeks (p=0.01), although by 4 weeks, no difference was evident. Meanwhile, in the within-group analysis by Wilcoxon signed-rank test, the NMES group showed a statistically significant improvement from baseline to 2 weeks (p<0.01) and from baseline to 4 weeks (p<0.01). Contrastingly, in the CST group, there were no significant improvements over time.
Fig. 1. Schematization of perceptual evaluation changes: total GRBAS score at baseline and at 2 and 4 weeks after initiation of the study in each group. GRBAS, grade, roughness, breathiness, asthenia, and strain. a)p<0.017 by Mann-Whitney U test with Bonferroni correction. b)p<0.025 by Wilcoxon signed-rank test with Bonferroni correction.
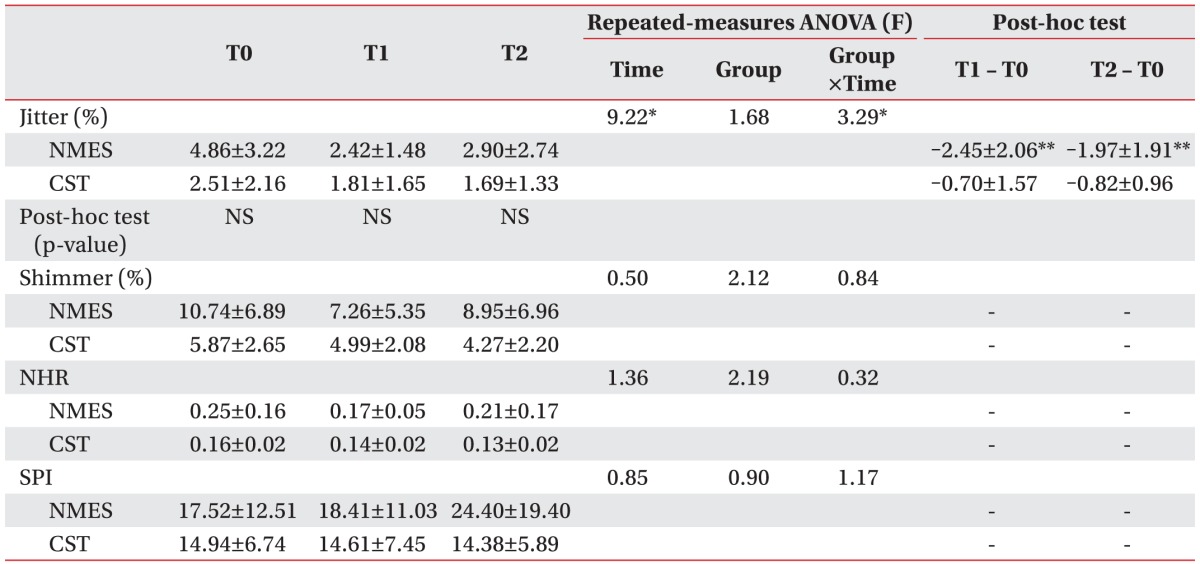
Table 2 shows the changes in the acoustic evaluation results for the NMES and CST groups. Within both groups, statistically significant differences in jitter over time (main effect of time, p<0.01) were observed. Also, a group-by-time interaction indicating a score difference between the groups over time was found (p<0.05). However, post-hoc analysis at each time point revealed no significant differences for any of the time intervals, which signaled a low therapeutic effect. In the within-group analysis, the NMES group showed statistically significant jitter improvement from baseline to 2 weeks (p<0.01) and from baseline to 4 weeks (p<0.01). In the CST group, however, there was no significant improvement in jitter over time. Regarding the other acoustic parameters, there were no significant group-by-time interaction, effect of time or group differences.
Table 2. Improved acoustic evaluation results.
Values are presented as mean±standard deviation.
NEST, neuromuscular electrical stimulation; CST, conventional swallowing training; NHR, noise-to-harmonic ratio; SPI, soft phonation index; T0, baseline; T1, 2 weeks after initiation of study; T2, 4 weeks after initiation of study; NS, non-specific.
*p<0.05 by repeated-measures ANOVA.
**p<0.025 by Wilcoxon signed-rank test with Bonferroni correction.
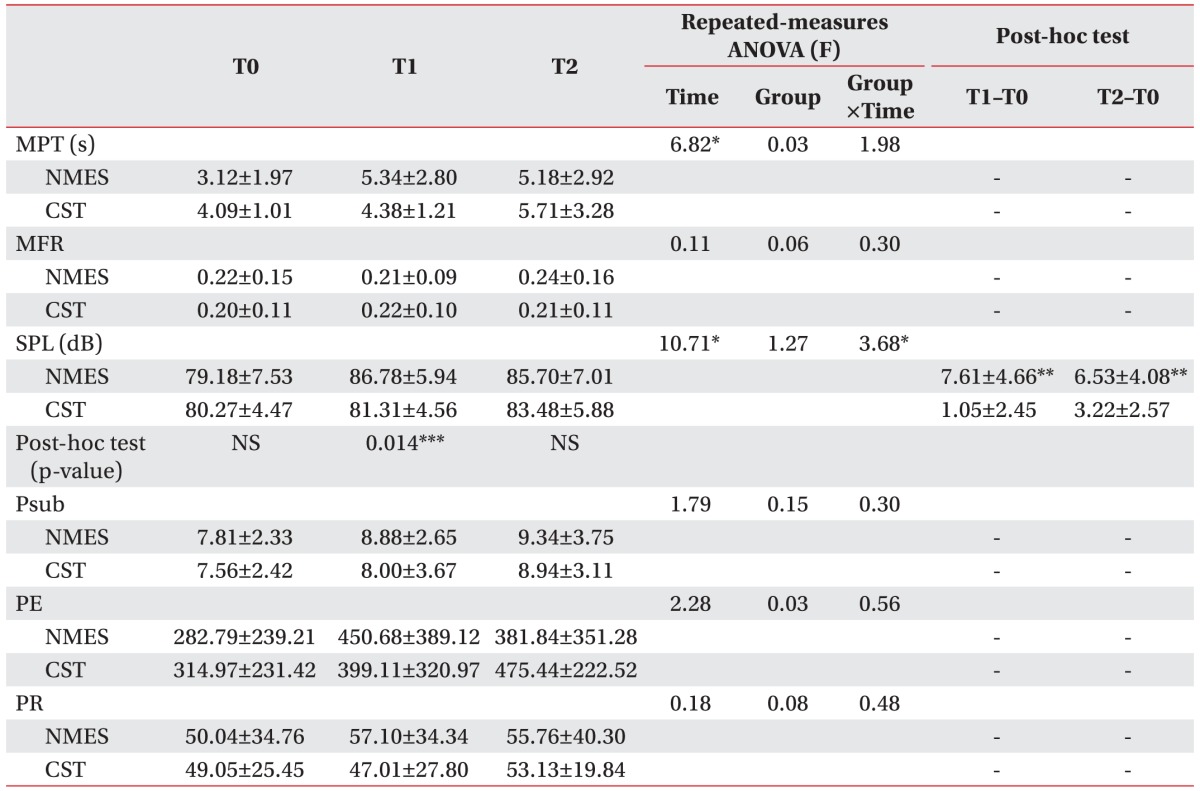
Table 3 shows the results of the intra- and inter-group aerodynamic evaluations. Within both groups, statistically significant differences were observed in SPL over time (main effect of time, p<0.01). There was also a group-by-time interaction indicating a score difference between the groups over time (p<0.05). For the NMES group, post hoc analysis at each time point revealed a significant improvement in SPL at 2 weeks (p=0.01), though by 4 weeks, no score difference was evident between the groups. In the within-group analysis, the NMES group showed a statistically significant improvement in SPL from baseline to 2 weeks (p<0.01) and from baseline to 4 weeks (p<0.01). In the CST group by contrast, there were no significant improvements in SPL over time, although an improving trend was evident.
Table 3. Improved aerodynamic evaluation results.
Values are presented as mean±standard deviation.
NEST, neuromuscular electrical stimulation; CST, conventional swallowing training; MPT, maximal phonation time; MFR, mean flow rate; SPL, sound pressure level; Psub, subglottic pressure; PE, phonation efficiency; PR, phonation resistance; T0, baseline; T1, 2 weeks after initiation of study; T2, 4 weeks after initiation of study; NS, non-specific.
*p<0.05 by repeated-measures ANOVA.
**p<0.025 by Wilcoxon signed-rank test with Bonferroni correction.
***p<0.017 by Mann-Whitney U test with Bonferroni correction.
The MPT similarly improved over time in both groups (main effect of time, p<0.01). As for the MFR, Psub, PE and PR parameters, there were no significant group-by-time interaction, effect of time or group differences.
Swallowing function
Table 4 lists the swallowing function results for the two groups. All of the measurements in both groups showed similar improvements over time.
Table 4. Improved swallowing function results.

Values are presented as mean±standard deviation.
NEST, neuromuscular electrical stimulation; CST, conventional swallowing training; PAS, penetration-aspiration scale; FDS, functional dysphagia scale; ASHA NOMS, American Speech-Language-Hearing Association National Outcome Measurement System swallowing scale; T0, baseline; T1, 2 weeks after initiation of study; T2, 4 weeks after initiation of study.
*p<0.05 by repeated-measures ANOVA.
Correlation between dysphonia and swallowing function
The relevant data are presented in Table 5. The improvement in the total GRBAS scores at 2 weeks was positively correlated with those for the pharyngeal phase on the FDS at 2 weeks (p<0.05). No other correlations between improved total GRBAS scores and any swallowing function parameter at 2 or 4 weeks were found. This result suggests that the dysphonia improvement was moderately related to that for the pharyngeal phase during swallowing.
Table 5. Correlation between improvement in the total GRBAS score and swallowing function at 2 weeks.

Values are Spearman's rank correlation coefficients.
PAS, penetration-aspiration scale; FDS, functional dysphagia scale; ASHA NOMS, American Speech-Language-Hearing Association National Outcome Measurement System swallowing scale.
*p<0.05.
DISCUSSION
Speech is generated by respiration, phonation, resonation, articulation and neurologic integration factors. Among these, phonation is the sound energy generated by the vocal fold vibration that is produced by the laryngeal muscle. In a stroke patient, however, the laryngeal muscle is frequently paralyzed, resulting in impaired vocal fold movement and, thus, vocalization abnormalities [11,20]. In this study, the MDVP was used for acoustic evaluation of the dysphonia patients. The MDVP is a widely used clinical tool, as enables rapid analysis, digitization and standardization of the characteristics of individual voices [21,22]. In so doing, the MDVP evaluates a total of 33 parameters, which are divided into eight groups including Fo, jitter, shimmer, NHR, SPI, and others [15]. All of these are representative acoustic parameters related to vocal fold vibration; as such, they are especially useful for evaluating voice disorders according to criteria that determine the stability of vocal fold vibration.
In an acoustic evaluation, Wang et al. [23], employing the MDVP program to analyze the voices of 10 patients with stroke-related dysarthria and comparing the results with 10 neurologically healthy controls, reported that fundamental frequency, jitter, shimmer and NHR were significantly changed. Actually, vocal fold instability is closely related to laryngeal muscle weakness, and many treatments can be applied for strengthening of such muscles, among which laryngopharyngeal NMES has been vigorously researched in the clinical setting [6,7,8,10,11]. In the present study, the NMES group showed a significant group-by-time interaction with regard to jitter, which represents the frequency perturbation of vocal fold vibration. This finding is similar to those reported by Byeon [11]. Such a result, in fact, is supported by the following mechanism. As laryngopharyngeal NMES recovers the functions of the muscles that control vocal fold vibration, the strengthening of muscles attached to the hyoid bone, such as the mylohyoid and thyrohyoid muscles, induces an increase in laryngeal elevation and consequently improves excessive quaver upon speaking [24,25].
For analysis of the physiologic pattern of an individual voice, not only acoustic but also aerodynamic evaluation is required. The most commonly employed tool for aerodynamic evaluation, which provides laryngeal valving activity information, is the Aerophone II [26]. This instrument evaluates, indirectly and non-invasively, Psub, MFR, MPT, SPL, PR and PE as well as lung volumes and capacities, and is frequently utilized to analyze phonatory-respiratory function and airflow patterns of the upper airway and voice [26,27,28]. In the present study, aerodynamic evaluation using Aerophone II showed significant SPL improvement for the NMES group at 2 weeks. SPL is a key parameter that is controlled by vocal fold thickness, glottal configuration, vocal ligament and thyroarytenoid muscle tension as well as pulmonary driving pressure. The SPL improvement in this study was the effect of the laryngopharyngeal NMES; this fact suggests very strongly that by such treatment, vocal ligament and thyroarytenoid muscle tension are strengthened to improve glottal resistance [29].
The improvement of the total GRBAS scores at 2 weeks was positively correlated with those for the pharyngeal phase on the FDS at 2 weeks, but not at 4 weeks. The most plausible explanation is that the NMES is superior to CST in respect of suprahyoid muscle strengthening. Thus, the more improved is dysphonia, the more improved is swallowing function, especially in the pharyngeal phase including laryngeal elevation. However, the fact that the improvements in total GRBAS score and SPL were not maintained in the NMES group over time might be related either to the short duration of the treatment or to a weak treatment effect.
This study has several limitations. First, because laryngopharyngeal NMES treatment is expensive, and given also that some patients experience physical discomfort when receiving it, patients with mild dysphagia on VFSS were denied the treatment, which made randomization of the study impossible. Second, as there was no available comparator group that had not received either NMES or CST, no assay on the improvement of phonation by natural recovery could be performed. As all of the subjects were acute or subacute patients who urgently required treatment for dysphagia, including a completely non-treatment group in the study would not have been ethical. Third, during the study period, no voice training or compensational treatment to control for factors affecting voice change other than NMES and CST was conducted, but rather, both groups were only coached in swallowing; as such, not all of the factors affecting or potentially affecting voice change were controlled. Fourth, for a more complete picture of the improvements observed, re-innervation of the nerves and recovery of muscle function should have been checked by laryngeal electromyography or computer tomography at the cervical level.
In conclusion, this study demonstrated the effect of laryngopharyngeal NMES therapy on phonation in a group of post-stroke and TBI patients who complained of dysphonia and underwent VFSS evaluation of their dysphagia. The results indicate that laryngopharyngeal NMES therapy had a positive effect on phonation in these patients. This effect can be accomplished by NMES to improve vocal fold vibration/tension or restore the function of the muscles for laryngeal elevation or both. The results of this study suggest laryngopharyngeal NMES to be an additional treatment option for dysphonia accompanied by dysphagia after stroke and TBI. To clearly identify the anatomical cause-and-effect relationship of laryngopharyngeal NMES with phonation, a large well-designed randomized trial coupled with laryngeal electromyography or computed tomography at the cervical level might be required.
Appendix 1
The grade, roughness, breathiness, asthenia, and strain scale

Appendix 2
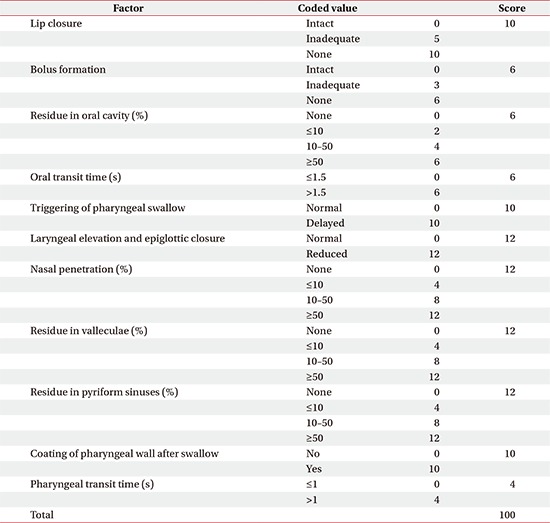
The functional dysphagia scale
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Cohen SM, Elackattu A, Noordzij JP, Walsh MJ, Langmore SE. Palliative treatment of dysphonia and dysarthria. Otolaryngol Clin North Am. 2009;42:107–121. doi: 10.1016/j.otc.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Cohen SM, Dupont WD, Courey MS. Quality-of-life impact of non-neoplastic voice disorders: a meta-analysis. Ann Otol Rhinol Laryngol. 2006;115:128–134. doi: 10.1177/000348940611500209. [DOI] [PubMed] [Google Scholar]
- 3.Lee JW, Jang SJ, Kim DA, Park SW, Jung WK, Yoo JH, et al. Development and application of cognitive perceptual assessment for driving of people with brain injury: comparison with cognitive behavioral driver's inventory. J Korean Acad Rehabil Med. 2004;28:523–531. [Google Scholar]
- 4.Ertzgaard P, Ward AB, Wissel J, Borg J. Practical considerations for goal attainment scaling during rehabilitation following acquired brain injury. J Rehabil Med. 2011;43:8–14. doi: 10.2340/16501977-0664. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka T, Kudo A, Sugihara S, Izumi T, Maeda Y, Kato N, et al. A study of upper extremity training for patients with stroke using a virtual environment system. J Phys Ther Sci. 2013;25:575–580. doi: 10.1589/jpts.25.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagorio LA, Carnaby-Mann GD, Crary MA. Cross-system effects of dysphagia treatment on dysphonia: a case report. Cases J. 2008;1:67. doi: 10.1186/1757-1626-1-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McFarland DH, Tremblay P. Clinical implications of cross-system interactions. Semin Speech Lang. 2006;27:300–309. doi: 10.1055/s-2006-955119. [DOI] [PubMed] [Google Scholar]
- 8.El Sharkawi A, Ramig L, Logemann JA, Pauloski BR, Rademaker AW, Smith CH, et al. Swallowing and voice effects of Lee Silverman Voice Treatment (LSVT): a pilot study. J Neurol Neurosurg Psychiatry. 2002;72:31–36. doi: 10.1136/jnnp.72.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon YS, Lim JT, Yun SB, Ohm BY, Kang JY, Lim HY, et al. The effect of functional electrical stimulation on swallowing function in stroke patients with dysphagia. J Korean Acad Rehabil Med. 2006;30:417–423. [Google Scholar]
- 10.Fowler LP, Gorham-Rowan M, Hapner ER. An exploratory study of voice change associated with healthy speakers after transcutaneous electrical stimulation to laryngeal muscles. J Voice. 2011;25:54–61. doi: 10.1016/j.jvoice.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Byeon H. Voice change associated with swallowing disorder caused by a stroke after neuromuscular electrical stimulation. J Korea Acad Ind Coop Soc. 2012;13:1665–1671. [Google Scholar]
- 12.Humbert IA, Poletto CJ, Saxon KG, Kearney PR, Ludlow CL. The effect of surface electrical stimulation on vocal fold position. Laryngoscope. 2008;118:14–19. doi: 10.1097/MLG.0b013e318155a47d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freed ML, Freed L, Chatburn RL, Christian M. Electrical stimulation for swallowing disorders caused by stroke. Respir Care. 2001;46:466–474. [PubMed] [Google Scholar]
- 14.Hirano M. Clinical examination of voice. New York: Springer; 1981. [Google Scholar]
- 15.Ko DH. A study of extracting acoustic parameters for individual speakers. Speech Sci. 2003;10:129–143. [Google Scholar]
- 16.Logemann JA. Evaluation and treatment of swallowing disorders. 2nd ed. Austin: Pro-Ed; 1998. [Google Scholar]
- 17.Han TR, Paik NJ, Park JW. Quantifying swallowing function after stroke: a functional dysphagia scale based on videofluoroscopic studies. Arch Phys Med Rehabil. 2001;82:677–682. doi: 10.1053/apmr.2001.21939. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 19.Wesling M, Brady S, Jensen M, Nickell M, Statkus D, Escobar N. Dysphagia outcomes in patients with brain tumors undergoing inpatient rehabilitation. Dysphagia. 2003;18:203–210. doi: 10.1007/s00455-002-0098-8. [DOI] [PubMed] [Google Scholar]
- 20.Lee KW, Kim SB, Lee JH, Kim YD, Han DW, Kim TH, et al. Relationship between the incidence of vocal cord palsy and aspiration risk in acute ischemic stroke patients. J Korean Acad Rehabil Med. 2010;34:15–19. [Google Scholar]
- 21.Kent RD, Vorperian HK, Kent JF, Duffy JR. Voice dysfunction in dysarthria: application of the Multi-Dimensional Voice Program. J Commun Disord. 2003;36:281–306. doi: 10.1016/s0021-9924(03)00016-9. [DOI] [PubMed] [Google Scholar]
- 22.Yu P, Ouaknine M, Revis J, Giovanni A. Objective voice analysis for dysphonic patients: a multiparametric protocol including acoustic and aerodynamic measurements. J Voice. 2001;15:529–542. doi: 10.1016/S0892-1997(01)00053-4. [DOI] [PubMed] [Google Scholar]
- 23.Wang YT, Chung YM, Chen HH, Chia LY, Lu HJ. Voice acoustic analysis of Taiwanese adults with dysarthria following stroke. Can Acoust. 2010;38:142–143. [Google Scholar]
- 24.Stepp CE. Characterization and improvement of the clinical assessment of vocal hyperfunction [dissertation] Cambridge: Massachusetts Institute of Technology; 2009. [Google Scholar]
- 25.Burnett TA, Mann EA, Cornell SA, Ludlow CL. Laryngeal elevation achieved by neuromuscular stimulation at rest. J Appl Physiol (1985) 2003;94:128–134. doi: 10.1152/japplphysiol.00406.2002. [DOI] [PubMed] [Google Scholar]
- 26.Zraick RI, Smith-Olinde L, Shotts LL. Adult normative data for the KayPENTAX Phonatory Aerodynamic System Model 6600. J Voice. 2012;26:164–176. doi: 10.1016/j.jvoice.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Mehta RP, Goldman SN, Orloff LA. Long-term therapy for spasmodic dysphonia: acoustic and aerodynamic outcomes. Arch Otolaryngol Head Neck Surg. 2001;127:393–399. doi: 10.1001/archotol.127.4.393. [DOI] [PubMed] [Google Scholar]
- 28.Lu FL, Presley S, Lammers B. Efficacy of intensive phonatory-respiratory treatment (LSVT) for presbyphonia: two case reports. J Voice. 2013;27:786.e11–786.e23. doi: 10.1016/j.jvoice.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 29.McHenry MA, Reich AR. Effective airway resistance and vocal sound pressure level in cheerleaders with a history of dysphonic episodes. Folia Phoniatr (Basel) 1985;37:223–231. doi: 10.1159/000265802. [DOI] [PubMed] [Google Scholar]