Abstract
Understanding conservation and livelihood threats in park landscapes is important to informing conservation policy. To identify threats, we examined perceived risks of residents living near three national parks in Uganda. We used cross-sectional household data to document, rank, and measure severity of perceived risks. Three risk categories, grouped into protected area, climate, and health, were cited by 80 % of respondents and received the highest severity scores. Elevation, proximity to the park, local forest loss, recent population change, and measures of poverty were the most important variables in predicting whether or not an individual identified these risks as the most or second most severe risk. Health issues were cited throughout the landscape, while problems attributed to climate (mainly insufficient rainfall) were reported to be most severe farther from the park. Increased population density was associated with increased perceived risk of health challenges, but decreased perceived risks attributed to the park and climate. Participatory risk mapping provides the opportunity to make standardized comparisons across sites, to help identify commonalities and differences, as a first step to examining the degree to which conservation management might address some of these local challenges and where mitigation techniques might be transferable between different sites or conflict scenarios.
Electronic supplementary material
The online version of this article (doi:10.1007/s13280-016-0775-8) contains supplementary material, which is available to authorized users.
Keywords: Risk perception, Protected areas, Population growth, Albertine Rift, Climate variability
Introduction
Protected areas (PAs) are often effective in reducing land cover change within their borders (Andam et al. 2008; Campbell et al. 2008) but do not always address social problems (e.g., resource scarcity, population growth) outside their boundaries. The PA model for biodiversity protection and conservation has a defining impact on wild areas remaining in the world, and a significant impact on the way in which local people interact with protected lands and landscapes that surround them. Thus, PAs cannot be viewed in isolation; the establishment of PAs invariably affects the livelihoods of people living in surrounding communities or within their boundaries (Ferraro and Hanauer 2014). Most biodiverse regions within the tropics are or soon will be agricultural lands managed by high-density populations surrounding a few remaining islands of natural area (DeFries et al. 2007; Wittemyer et al. 2008). A major concern is that PAs will not be sustainable due to population pressure and land-use intensification outside their boundaries (Cincotta et al. 2000). Increased population density leads to increased land conversion and land-use intensification surrounding parks, altering ecological function and biodiversity within parks (Hansen and DeFries 2007). This juxtaposition of biodiversity preservation and agriculture in biodiversity hotspots greatly challenges conservation mechanisms and adds risk to local livelihoods near PAs.
There is debate about the virtues of PAs, and local consequences of PAs can be contrary and variable (Terborgh and van Schaik 2002; Adams et al. 2004; Hutton et al. 2005; Brockington and Wilkie 2015). Undeniably, there are winners and losers; regulations associated with PAs constrain people’s livelihood activities and can increase conflict between humans and wildlife (West et al. 2006), despite local people being highly dependent on natural resources. Tensions can arise from eviction from PAs through the loss of rights and privileges, corruption, disruption in land tenure, little or no compensation, and unwelcome attention (Hoffman et al. 2011, Goldman 2011). PAs can also attract migrants and stimulate land-use change (Wittemyer et al. 2008), serve as economic engines and prompt development projects directed at poverty alleviation (Ferraro et al. 2011), and provide important ecosystem services. Domesticated landscapes outside PAs are important because they represent reservoirs of land, resources, and economic opportunity for people (Hayes 2006), and are often viewed as buffers by PA managers (Schonewald-Cox and Bayless 1986; Gaston et al. 2008). Conservation success, often predicated on local support for the PA, is influenced by local experiences and opinions of PA management (Bennett and Dearden 2014), and the reality that potential or perceived benefits may not outweigh the negative relationships, histories, problems, risks, and conflicts involved in living near a PA (Davis 2011). Given the pervasiveness of the PA model in conservation initiatives worldwide, understanding the concerns of local people living near PAs is important for managing conservation landscapes and creating greater respect for and inclusivity of neighboring communities, and devising, prioritizing, and targeting risk mitigation strategies.
The complex interactions between PAs and smallholder farmers are exemplified in national park landscapes of the Albertine Rift in Sub-Saharan Africa. The Albertine Rift, which encompasses 313 000 km2 and extends from northern Uganda to northern Zambia, is one of the most important conservation regions in Africa. It is a biodiversity hotspot that is home to the greatest number of endemic African vertebrate species (Plumptre et al. 2007) but severely threatened due to intensive smallholder agriculture and subsequent high rates of habitat loss and conversion (Fisher and Christopher 2007). The majority of the greater than 40 million people living in the Rift are farmers, who rely completely on adequate rainfall and soil fertility, and their intensity of land use is rapidly increasing as a result of some of the highest human population growth rates in the world (PRB 2012).
The transition from park to domesticated landscape in the Albertine Rift is abrupt since local populations are often excluded from parks, and communities cultivate land right up to park boundaries, providing easy but illegal access to park resources that may help mitigate livelihood vulnerabilities, particularly for poorer households (MacKenzie and Hartter 2013). However, living next to a park also exposes households to risks that affect economic stability, food security, and their lives. For example, many people complain of crop loss and livestock predation by park-protected animals. These human–wildlife encounters are blamed for loss of food and seed sources, zoonotic disease transmission (Salyer et al. 2012), and poor childhood education resulting from children guarding crops instead of attending school (MacKenzie et al. 2015). Climate variability exacerbates these risks because the timing and amount of precipitation from seasonal rains directly affect yields and food security. While it has been argued that proximity to the park has no effect on productive assets (Naughton-Treves et al. 2011), people living near parks face important challenges and may seek retribution for lost crops and livestock by poaching park resources.
Given the potential for conflict around parks in East Africa and given the history of establishment using fortress conservation (Neumann 1998; Brockington 2002; Hartter and Goldman 2011), an understanding of the entangled relationships of parks and neighbors is important. Knowing how local people perceive their problems in PA landscapes is crucial to pinpointing and mitigating potential conflicts. In this paper, we hypothesize that perceptions of risk (SI text 1) depend on the distance to the park boundary, and a combination of social, demographic, and environmental factors. Using cross-sectional household data, we examine the perceived risks by local residents living near three national parks in Uganda, and the risk mitigation measures they employ, using participatory risk mapping (Smith et al. 2000). Studying three parks allows us to control for physical differences in park landscape (savannah versus forest) and provides a wider perspective of the national park system in the Ugandan Albertine Rift. By calculating measures of risk severity and incidence, and mapping these respondent-identified risks, coupled with satellite-derived measures of land cover, population density, and other parameters, we examine the key predictors of perceived risk in households near the parks in order to identify critical locations where conflict between local residents and park management may be more likely to occur.
Study sites
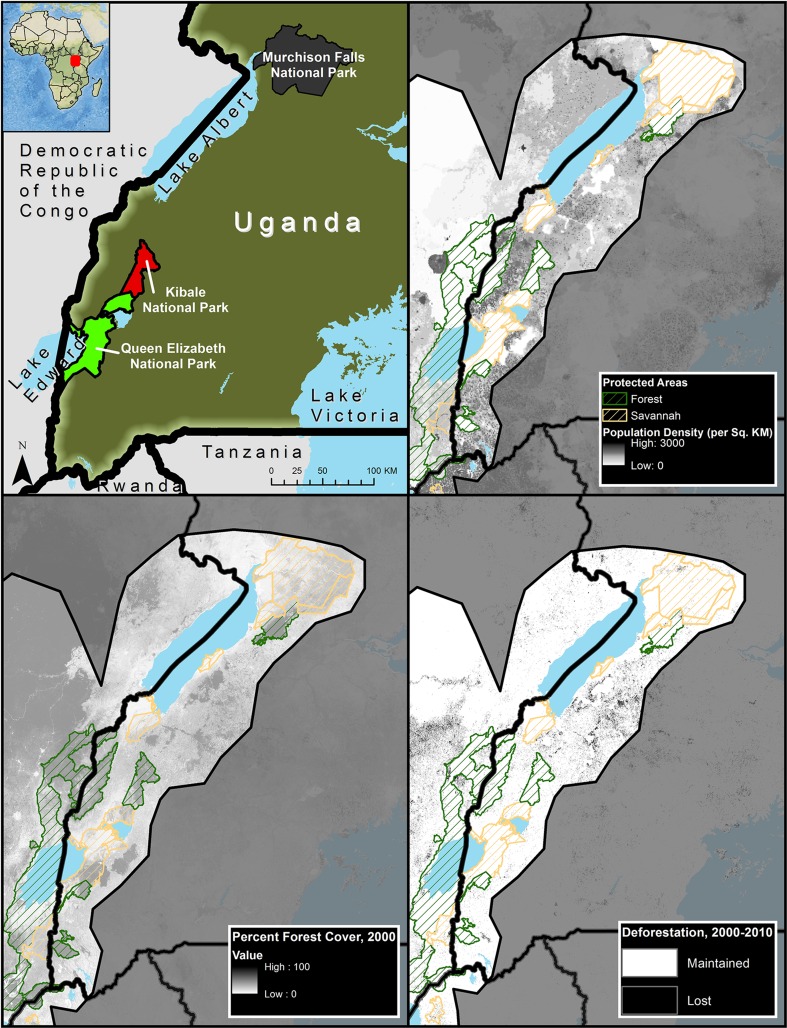
We conducted our research near three national parks in the Albertine Rift of Uganda: Kibale National Park (KNP), Murchison Falls National Park (MFNP), and Queen Elizabeth National Park (QENP) (Fig. 1). None of the park boundaries are fenced, leading to direct interaction between the park and local communities. Agriculture is the dominant livelihood near all of these parks, but the geographies of the three parks vary. KNP is a relatively small forest park, while QENP and MFNP are predominately savannah. Human population density is significantly higher and agriculture more intensive near QENP and KNP compared to MFNP. The north–south movement of the Intertropical Convergence Zone (ITCZ) and the associated rainbelt controls strongly the intra-annual variability of rainfall at the three parks (Nicholson and Grist 2003). Therefore, the parks typically have two rainy seasons: QENP receives much less rainfall than the other two parks, especially during the long rains (i.e., September–November), and MFNP receives much more rainfall during boreal summer (Diem et al. 2014a).
Fig. 1.
Upper left National parks where surveys were conducted; upper right park boundaries overlaid on population density showing the high human population surrounding Ugandan parks; lower left forest cover in 2000 illustrating the island character of Ugandan parks; lower right deforestation between 2000 and 2010; forest parks outlined in green and savannah parks outlined in orange. Gray area is the area outside of the Albertine Rift, and the shading was used to draw readers attention to the Albertine rift area
Kibale National Park
(1100–1600 m a.s.l., 795 km2) lies just north of the equator and is a remnant of a transitional forest between mid-altitude tropical forest (north) and savannah (south), surrounded by a large subsistence agricultural population. KNP, gazetted as a national park in 1993, is situated in one of the most densely human-populated areas in Uganda (Hartter et al. 2015), estimated at over 260 individuals-km−2 in 2006 and ranging as high as 600 individuals-km−2 in some locales by 2009 (MacKenzie and Ahabyona 2012).
Murchison falls National Park
(500–1290 m a.s.l., 3840 km2) is at the northern terminus of the Albertine Rift, with savannah in the north and forest and woodlands to the south. The national park was first gazetted as a game reserve in 1926, then as a National Park in 1952, making it the oldest, largest, and most visited park in Uganda (UWA 2012). Human population density surrounding MFNP has risen from an estimated average of 18 individuals-km−2 in 1959 to 111 individuals-km−2 in 2014 (Uganda and East Africa High Commission 1961; UBoS 2014).
Queen Elizabeth National Park
(940–1350 m, a.s.l., 2080 km2) was established in 1952 and is Uganda’s second most visited and second largest national park. It lies along the equator and is connected to the southern edge of KNP. Situated in the Albertine Rift Valley floor, QENP habitats include savannahs, wetlands, and lowland and gallery forests. The area around the park is densely settled by more than 400 000 people (UBoS 2005) who are farmers, and a small minority who keep cattle. Since 1959, the average human population density surrounding QENP has risen from 46 individuals-km2 to 107 individuals-km2 in 2014 (Uganda and East Africa High Commission 1961; UBoS 2014).
Materials and Methods
Household surveys
Between 2011 and 2013, a total of 795 household surveys were conducted in communities within 5 km of park boundaries (310 KNP, 308 QENP, 177 MFNP). We used a 5-km boundary, as it was previously shown to encompass the localized effects of park presence (Hartter and Goldman 2011). We used the geographic sampling strategy of designating “superpixels,” as in previous work (e.g., Hartter and Goldman 2011). Since we were examining the park-neighbor interaction, ranger stations and entrance gates served as anchor points for 5 km buffers within which we conducted our research. Within each of those 5-km areas, ten superpixels were randomly generated (9 ha circle areas, with radii of 170 m). If superpixels overlapped, fell into urban areas (since land holdings and livelihoods are widely diversified, and direct connection to the national park is limited), water, or in restricted spaces (e.g., United Nations airfield, prison lands), a new random superpixel centroid location was generated. Five households were selected from among all landholders (those who rent, borrow, or own) in each superpixel. Respondents could be either the household head or their spouse. The survey instrument had two parts: (1) a series of fixed-choice questions to characterize demographics and land use, and (2) a series of open-ended questions for respondents to self-identify and rank risks. Interview location (the house or family compound) was recorded using a handheld global positioning system receiver. All interviews were conducted in local tribal languages, Kiswahili, or English by a Ugandan field assistant. Our prior experience in Uganda found interviewer subjectivity could influence participant responses (MacKenzie 2016). As a result, we ensured no westerners attended interviews as they are seen as potential benefactors, informed consents identified that interviewers are not as a government employees. We also conducted subjectivity interviews with field assistants to understand potential sources of bias. Before conducting any interviews, the appropriate permissions were obtained from the local government, village leaders, and survey respondents. Table 1 provides a summary of respondent characteristics.
Table 1.
Summary of respondent characteristics from each of the three park areas
| Respondent characteristics | Kibale National Park | Queen Elizabeth National Park | Murchison Falls National Park |
|---|---|---|---|
| Sample size | 308 | 309 | 177 |
| Household elevation range | 936–1550 m | 939–1556 m | 650–1079 m |
| Age | |||
| Mean | 41 | 41.3 | 39.7 |
| Range | 18–98 | 19–83 | 20–82 |
| Household distance from park (KM) | |||
| Mean | 1.2 | 2.7 | 1.9 |
| Range | 0–4.2 | 0–5.0 | 0–4.1 |
| Sex | |||
| Male | 129 | 145 | 88 |
| Female | 179 | 164 | 89 |
| Education | |||
| Some primary school (PS) | 93 | 114 | 72 |
| Completed PS | 181 | 124 | 70 |
| Some secondary school (SS) | 27 | 68 | 32 |
| Completed SS | 7 | 3 | 3 |
| Ethnicity | |||
| Acholi | 0 | 0 | 68 |
| Alur | 0 | 0 | 51 |
| Bafumbira | 7 | 16 | 0 |
| Bagungu | 0 | 0 | 39 |
| Bakiga | 139 | 16 | 0 |
| Bakonjo | 10 | 196 | 0 |
| Basongora | 12 | 54 | 0 |
| Batoro | 120 | 13 | 0 |
| Other | 20 | 14 | 19 |
| Identified protected area as a top risk | 223 | 212 | 148 |
| Identified health as a top risk | 130 | 92 | 83 |
| Identified climate as a top risk | 157 | 141 | 30 |
Risk severity
We used the participatory risk procedure described by Smith et al. (2000) and modified by Baird et al. (2009), to create incidence and severity scores for each risk, valued from 0 to 1. Respondents were free to name whatever risks they felt were important to them and their household, and there was no limit on the number of risks that participants could name. After respondents named and ranked all risks, they were asked how they prevent or mitigate the effects of each named risk. Risks were first coded as a threat to life, livelihoods, or lifestyle (SI Text S2). Within each of these categories, the identified risks were thematically coded. We then calculated the overall risk severity for each named risk for each respondent. The result of this formula is a number showing increasing severity from 0 to 1 and allows for comparison between the severity scores among all risks. The severity index versus incidence scores was then plotted for each of the three national parks (SI Text 2).
Spatially explicit datasets
We used spatial information and datasets for population, forest cover, and deforestation as variables in our statistical analysis. Population, poverty, birth, and pregnancy data were extracted from WorldPop (www.worldpop.org.uk), using average density (people per hectare) from 2005 and 2010, proportion of population living under $1.25 per day from the year 2011, as well as live births and pregnancies per grid square from 2000 and 2012. All WorldPop data had an average spatial resolution at the equator of 100 meters. For forest cover and deforestation, we used Landsat derived Global Forest Change data, available only for the year 2000 (www.earthenginepartners.appspot.com/science-2013-global-forest), with forest cover or trees defined as vegetation taller than 5 m and a percent of each 30 m pixel is reported. As determined by Hansen et al. (2013), deforestation was the loss or change of a pixel from a forested to a non-forested state and is reported as a binary change. Both of these spatial layers are approximately 30 m resolution at the equator SI Text 2).
Modeling
We used Conditional Random Forests to examine predictors of a binary response of the three main risks being named as the most severe or second most severe of the risks a household faces. Probability weights were applied (Lee and Forthofer 2006) to correct for design bias related to local population near each park. The ‘party’ package (Hothorn et al. 2006; Strobl et al. 2008, 2007) in R (R Core Team 2015) was used to judge variable importance over 5000 unbiased conditional inference trees. As Random Forests that are built from individual classification trees are biased toward selecting variables with more categories over variables with only a few, we used unbiased conditional inference trees, sampled without replacement as recommended by Strobl et al. (2007). This allowed for variables, such as Park (3 unique values), to have the potential to be just as important as variables with many unique values, such as elevation. We illustrated the average impact of each variable on the response over the 5000 conditional inference trees for model interpretation using partial dependence plots in the ‘edarf’ R package (Jones and Linder 2015). The parameters used in model creation are described in Table S2. Multicollinearity was checked and corrected using the ‘usdm’ package (Naimi 2015) in R using the variance inflation factor (VIF) to remove the terms with the highest multicollinearity through an iterative process until all VIF values were less than 10 (Hair et al. 1995). This resulted in a subset of 12 of the original 20 potential regressors used in the Random Forest model generation. We then used out-of-the-bag (OOB) error to choose the best model for each risk. A benefit of OOB error and Random Forests is that OOB error does not require a test set, as the OOB estimate is derived from the 36.8 % of samples not included in the building of each inference tree, and has been shown to be just as accurate as a cross validation set (Breiman 1996).
Results
What do people worry about most?
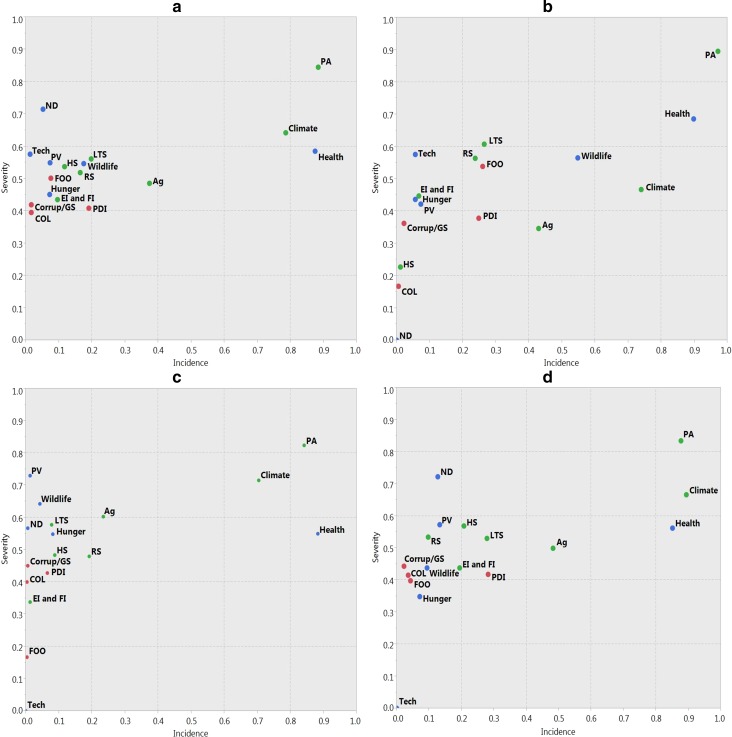
Ninety-two unique risks were named and subsequently binned into categories of risk typology (life, livelihoods, and lifestyle) and then thematic sub-categories (protected area, health, and climate) (SI Text S1, Table S1). Some risks were named once, while others were named many times. These differences may be attributable to translation, or due to distinct risks perceived by different respondents. In general, risks to lifestyle (e.g., poor infrastructure and cost of living) received the lowest composite risk scores, were mentioned relatively infrequently, and when mentioned, tended to affect households less than other categories. Risks to life (e.g., food security, wildlife attack) were ranked as more severe risks, though not necessarily the most often mentioned. Risks to livelihoods (e.g., land tenure security and crop yields) were generally named more often and had higher severity scores than risks to lifestyle and risks to life (Fig. 2).
Fig. 2.
Risk map for all parks (a), MFNP (b), KNP (c), and QENP (d). Risks to life colored in blue, risks to livelihoods colored in green, and risks to lifestyle colored in red. All abbreviations for risks are provided in supplementary Table S1
Despite the somewhat different social and environmental contexts of the three national parks, the same three main categories of risks stood out in all three parks with high incidence and high severity: protected area, climate, and human health. Combining data from all three parks, protected area had a risk severity value of 0.845, climate had value of 0.641 and health had a value of 0.585. Agricultural and pastoral risk were also widely cited and had a relatively high severity score (37 % of all respondents across all parks, risk severity value of 0.485). Uniquely, at MFNP, wildlife attacks were more widely cited than at the other two parks, but there was also more fear of outsiders and worry about land tenure and resources. People have recently returned from internally displaced people’s camps following the expulsion of the Lord’s Resistance Army in northern Uganda only to face land tenure conflict with outsiders, drawn by the potential of abandoned land, and oil exploration in and around the park, grabbing untitled land.
Protected area
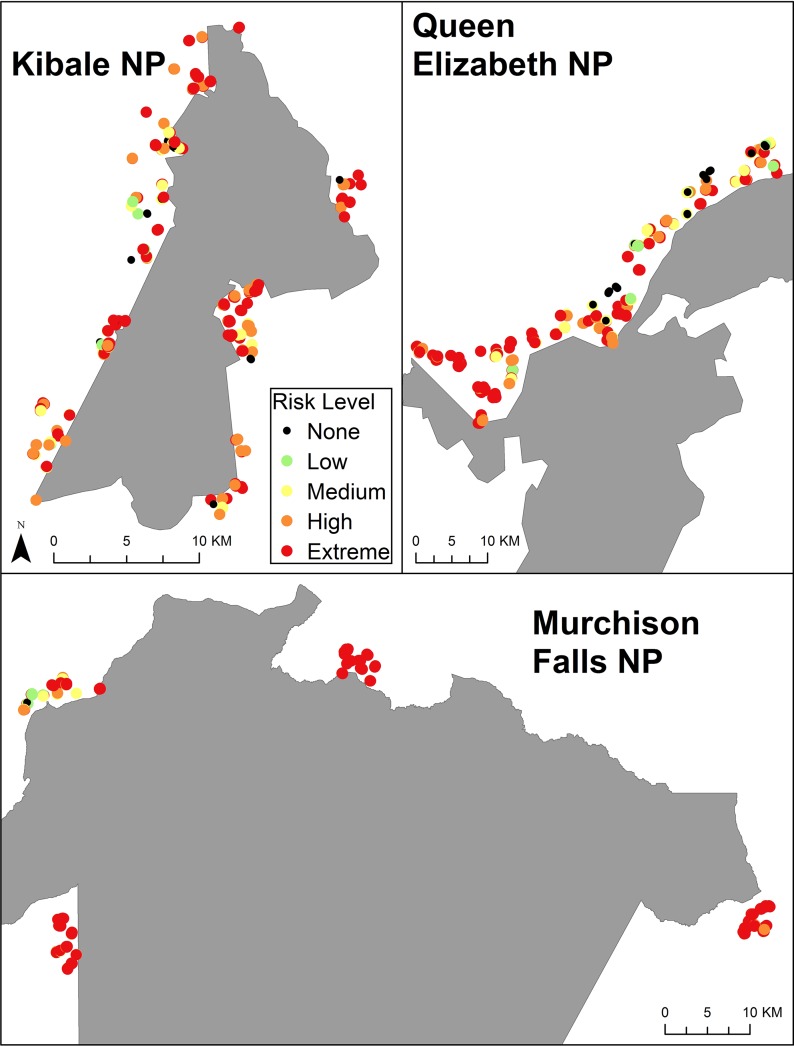
Risks attributed to the presence of the protected area had the highest severity score at all three sites and were reported by 88 % of respondents; 84 % at KNP, 97 % at MFNP, and 88 % at QENP. Crop raiding by wildlife (mentioned by 81 % of respondents) was reported most often as a park-based risk and, when named, had a high severity score. At KNP, 72 % of households said that the protected area represented a high or extreme risk, 69 % at QENP, and 84 % at MFNP. While there were minor spatial variations in risk levels at KNP, the other two parks had some noticeable east–west differences: (1) risk severity near MNFP was lowest in northwest communities, near the Nile River/Lake Albert confluence; (2) risk severity near QENP was highest around the northwest edge of the park near the Congo border (Fig. 3).
Fig. 3.
Risk map of “protected area” risk perceived by households near National Parks. No risk = that particular risk was not named by the respondent. Low risk had a severity score ranging from 0.1 to 0.3; medium risk 0.31–0.6; high risk 0.61–0.9; and extreme risk from 0.91 to 1.0
Table S2 shows variable importance for the protected area Random Forest model. In general, individual factors (e.g., residence time, sex, age, and education) tend to have lower importance than distance to park, overall population change, and natural population growth for risk perception. Elevation, proportion of people living on less than $1.25 per day, deforestation, and which park respondents live near were also important predictors (Figure S2). Respondents were more likely to designate the protected area as a primary or secondary risk, controlling for other variables, if they lived in areas with the following characteristics: closer to the park (<3.75 km), higher birthrate increases (2000–2012), higher proportion of people living on less than $1.25/day, low to medium elevation, and lower population growth. While in many areas birthrate and population growth can often increase simultaneously, there are areas where migration is stronger determinant of population growth than birthrate, or vice versa. Since there was no severe multicollinearity between these variables, we can assume that they contained unique information from one another. People near KNP and QENP were less likely to designate protected area as a #1 or #2 risk than those living near MFNP.
Climate
Overall, 79 % of respondents named climate as a risk. Residents near QENP named climate more often (89 %) than those near KNP (70 %) and MFNP (74 %), with the most frequently mentioned climate risk being drought (49 % overall, 48 % QENP, 55 % KNP, and 42 % MFNP). Climate was identified as the primary or secondary risk by 48 % at QENP, 51 % at KNP, and only 17 % at MFNP, with severity ranked as high or extreme by 51 % of respondents at KNP, 46 % at QENP, but only 17 % at MFNP, much lower than in communities near the other two parks. No apparent spatial clustering emerged around any of the three parks (Figure S5).
Table S2 shows that elevation was the most important variables in the Random Forest model to predict which residents perceive climate to be one of their top two risks. Those who live in areas of higher elevation (above 1000 m) are more likely to see climate as a high risk (Figure S3). In addition, areas that have lower rates of poverty and those who live farther from the park perceive climate as a #1 or #2 important risk; perhaps as crop raiding diminishes farther from the park, residents are less preoccupied with the risk of raiding and their concern over climatic conditions heightens. Where population change has been lower, respondents are also more likely to perceive climate as an important risk. Compared to MFNP, people living near KNP and QENP are more likely to rank climate as an important risk, but probably for different reasons; climatic conditions are drier around QENP, whereas rainfall was reported to be more variable around KNP compared to MFNP.
Health
Health was also an important risk category; named by 87 % of respondents (88 % KNP, 85 % QENP, and 90 % MFNP). Most people mentioned “fever” and malaria. While health was mentioned frequently, it was only the primary or secondary risk in 38 % of households (42 % KNP, 47 % MFNP, and 30 % QENP; Figure S6). Residents in areas where population density has increased at a higher rate are more likely to report health as a top risk. Additionally, those living in either high (above 1400 m) or low (below 750 m) elevations have a higher perceived health risk than those living in middle elevations (Figure S4). Respondents near MFNP have a higher likelihood of reporting health as a top risk compared to the other parks, perhaps because they are less concerned about climate risk.
Risk mitigation strategies
The most prevalent theme in the risk mitigation responses was the expectation that government or Uganda Wildlife Authority (UWA) should solve respondents’ problems. They looked to the government to help prepare for year-to-year changes in rainfall, stop crop raiding, provide infrastructure, create jobs, provide tree seedlings, land, and irrigation, and even to clear snakes from their gardens. For crop raiding, respondents suggested UWA employ more rangers to guard crops, build crop-raiding defenses, or provide compensation to cover losses. However, 63 % of households spent time guarding against crop-raiding animals (73 % MFNP, 76 % KNP, 56 % QENP), 13 % had dogs to scare away wild animals (27 % MFNP, 1 % KNP, 17 % QENP), and 12 % said they set fires and made noise to keep wild animals away (24 % MFNP, 0 % KNP, 17 % QENP). Eight percent of survey households (12 % MFNP, 11 % QENP) said UWA should allow farmers to kill raiding animals, admitting they already set traps to do so, although this practice was rarely mentioned around KNP (1.3 %). To combat the risk of food insecurity, resulting from crop raiding or climate change, respondents said they needed more land (19 %). All of the 52 respondents wanting more land around MFNP planned to buy or rent, but 79 (13 %) respondents around KNP and QENP suggested that it was the government’s responsibility to make more land available with five respondents near QENP suggesting park land be given or sold to them for crop cultivation. To mitigate the impacts of climate variability, respondents suggested waiting for the rains and planting at different times (11 %), planting trees (3 %), using drought tolerant seed (2 %), or using irrigation (2 %). Twenty respondents said they would resort to poaching park resources (firewood, meat, fish, and grazing their cattle). To mitigate health risks, 50 % of respondents said they would visit clinics and take drugs, although 9 % of respondents mentioned they would take herbal medications but that these herbs were hard to get outside the park. Since malaria is so prevalent in the Albertine Rift, 15 % of respondents planned to use nets to protect themselves. The health risk posed by the lack of clean drinking water led 9 % of respondents to boil water or to use purification tablets, while 9 % of respondents said that the government should be building water pumps and boreholes.
Discussion
People living near PAs bear higher crop-raiding costs than those living farther away (MacKenzie and Ahabyona 2012), but two other important risk categories emerged that may be less obvious: climate and health. Our results concur with research near Tarangire National Park in Tanzania, where human disease, drought, and conservation were identified as risks, and respondents were particularly concerned that conservation policies might limit land use or confiscate land for park expansion (Baird et al. 2009). Quinn et al. (2003) found disease and water availability to be two of the most important risks in semi-arid villages in central and northeast Tanzania, while Webber and Hill (2014) found high crop-raiding severity in communities near Budongo Forest Reserve, adjacent to MFNP.
As human population grows, more land is used for farming, fewer resources are available, the landscape fills in, and PAs become insularized. Thus, population growth and forest loss prove useful predictors for conservation risks. Human risk perception provides another predictor in these conservation landscapes, because perception influences behavior (Baird et al. 2009). If communities proximate to PAs decide to access park resources or intensify resource use on the land adjoining PAs, then conservation objectives can be threatened (Oates 1999; MacKenzie and Hartter 2013). Although there is no consensus as to whether people preferentially move within close proximity of PAs (Wittemyer et al. 2008), Hartter et al. (2015) found the population density around KNP to be 1.5 times higher than farther from the park boundary over the span of 43 years, and Zommers and MacDonald (2012) found areas close to MFNP were more populated. Human population growth near these PAs means more opportunity for interaction with the park (i.e., resource collection, human–wildlife interaction), and these landscapes are no longer frontiers. Mitigation and avoidance become difficult because nearly everyone is farming and little unclaimed land remains.
Protected area
Crop raiding presents a risk to people near PAs, with financial losses (e.g., crops and seed sources) incurred far outweighing benefits from conservation sources (MacKenzie 2012). Park-protected wildlife damage farmers’ crops and take their food, possibly even leading to local human displacement (Chiyo and Cochrane 2005), and perceived lost opportunity costs because crop guarding reduces the time available for school or income generating activities (MacKenzie and Ahabyona 2012; MacKenzie et al. 2015). Our results confirm local perceptions that raiding intensifies near more forested areas. Since many animals are protected, past crop-raiding mitigation techniques, such as killing the intruding animal, are now illegal, although some local residents still set traps, lay snares, and put out poisons to stop crop-raiding animals.
The land surrounding these parks is exceptionally fertile and well suited for agriculture. Remnant forests are often cut and productive tropical wetlands drained to create land for high-density subsistence agriculture and to collect timber for fuel. As available land and resources dwindle and human population increases, park-community relationship are strained as residents want access to resources locked away within the park. Crop raiding has become more prevalent on farms next to the park edge as both human and wildlife populations have increased (MacKenzie and Ahabyona 2012). Many animals use the forest patches as travel corridors, and without them, have to cross into the agricultural matrix. However, farmers report that the best defense for crop raiding is to have more people for two reasons: less natural habitat for the wildlife to travel in and more frontline households to absorb most of the crop raiding. Although higher human population density has been linked to more frequent crop raiding, this tends to be in areas of relatively low human population density where more people increase the frequency of human–wildlife interaction (Sitati et al. 2003). Around these three parks in Uganda, human population is already high, so increases in population density buffer more of the population as frontline households take the brunt of the damage (MacKenzie and Ahabyona 2012).
Climate
Over 80 % of farmers in Uganda practice rain-fed agriculture, a practice that is sensitive to climate variability. Farmers complained of decreasing yields and were concerned about climate changes (timing, duration, and perceived erratic nature of rainy seasons, and drought). Poorer households are more concerned because drought and timing of rainfall not only leads to reduced or lost harvest for that season but also affects the abundance of seed source for the following season, further reinforcing existing vulnerabilities to food insecurity, malnutrition, and disease (Labbé et al. 2015). Moreover, the local belief that living closer to the park results in more rainfall (Hartter et al. 2015) is upheld in this study, where people farther from the park were more worried about the climate—although this claim has yet to be empirically verified. In a previous study around KNP, MacKenzie (2012) found that 97 % of respondents (n = 596) identified more rainfall next to the park. One local chairperson explained that the yield was double relative to 5kms farther from the park1. Thus, people say they would rather live closer to the park, despite the potential for park conflict (Naughton-Treves et al. 2011) because they can do something about the wildlife, but not about lack of rain (Hartter and Goldman 2011). The lack of climate variability buffering strategies proposed by our participants highlights the vulnerability of smallholder households to climate variability and the lack of adaptive capacity to deal with these changes. Although traditional knowledge, migration, and crop diversification have provided resilience to change in the past, it is unclear if these approaches will be sufficient in light of rapidly increasing human population density and land shortage (Niang et al. 2014). This is disconcerting, since there has been a drying trend in western Uganda—and thus less soil moisture—over the past several decades (Diem et al. 2014b), and near-term projections show a decrease in soil moisture due to rising temperatures (Kirtman et al. 2013).
Interestingly, elevation was the most important predictor for perceived climate risk, with households at higher elevations (above 1000 m) more likely to highlight climate as a risk. Households near MFNP were much less concerned with climate as opposed to households around QENP and KNP because most households surveyed near QENP and KNP were above 1000 m. QENP has two distinct dry seasons, but MFNP does not have a true dry boreal summer (see Diem et al. 2014a), so people near MFNP should be less concerned about available precipitation, and less likely to report drought. Further, QENP sits in the rain shadow of the Rwenzori Mountains creating hotter and drier conditions, leading to much less available soil moisture. Based on the mitigation strategies suggested by respondents near KNP and QENP, park managers need to prepare for a potential rise in illegal resource extraction and boundary encroachment as yields decrease due to low soil moisture.
Health
Health was another important concern among residents in these park landscapes. The rich biodiversity of the Albertine Rift includes high abundance of ticks and other species of disease vectors and reservoirs (Lafferty 2009). MacKenzie and Ahabyona (2012) found that living next to KNP brings a perceived increase in human disease such as malaria. We found that perception of health as a top risk is more likely where human population has increased and at higher elevations. The literature strongly supports the idea that proliferation and emergence or re-emergence of infectious diseases can be driven by land-use and other environmental factors, including climate change (Labbé et al. 2015), with natural habitat loss and fragmentation from agricultural expansion being one of the most important factors (Patz et al. 2004). Land-use change may affect the number of vectors and parasites as well as their interactions with hosts, ultimately affecting disease severity and occurrence (Schotthoefer et al. 2011), and biodiversity declines have been linked with increases in parasitism and the spread of parasitic diseases (Hatcher et al. 2012).
Another possible explanation is that exposure to disease outbreaks, such as yellow fever, anthrax, and Ebola in this region have led to a raised level of awareness of health issues. Further, there have been education and sensitization campaigns by the Ministry of Health, non-profit organizations (e.g., Conservation Through Public Health, Kibale Health & Conservation Project), and researchers, which could raise concerns of local people. Our results may provide some evidence that there is a link between sickness and population growth, and that local people rely on access to clinics, modern drugs, mosquito nets, and clean water sources to mitigate this risk. It is unclear how health risk will interact with conservation, but improved legal access to medicinal plants inside PAs may be needed (Labbé et al. 2015).
Conclusion
Our results point to important stressors that are common to both conservation and local livelihoods in park landscapes: population growth, forest loss, and climate change. Residents near three parks in Uganda identified the protected area (e.g., crop raiding), climate (e.g., variable timing and amount of rainfall), and health (e.g., disease) as most frequently reported and highest severity risks, with protected area and health risks linked to population growth and forest loss. Climate variability will further exacerbate food insecurity and disease for local residents, and as wildlife becomes isolated inside PAs, animals moving to alternate suitable areas in response to climate change will be constrained by human population, resulting in more human–wildlife conflict bordering PAs and potentially, more zoonotic disease transmission.
Future analyses that examine the relationship between risk perception and information networks that mitigate risk will provide further insight into the connection between perception and action in conservation landscapes. In places with high population density and where effects of the park quickly diminish with distance, future research should address how PA management could incorporate consultation with local people to create opportunities for risk mitigation for both livelihoods and conservation objectives. Understanding how perception of risk in the past shapes objective risk in the future, and hence, the actions people will choose to employ to mitigate risk will require behavioral modelling that accounts for discounting, reciprocity, and inertia (Venkatachalam 2008). This analysis is complex, requiring much consultation and data gathering. However, future investments can be limited by focusing on the three primary risks identified in this research. Lastly, understanding the nature and spatial extent of perceived risks would aid in the geographic targeting of intervention strategies. This could be particularly helpful with respect to health in developing a systematic, comprehensive, and spatially explicit syndromic surveillance that would identify environmental, cultural, and socio-economic factors that contribute to perceived health risk.
Participatory risk mapping provides the opportunity to make standardized comparisons across sites, to help identify commonalities and differences, to examine the degree to which conservation management might address some local challenges, and where mitigation techniques might be transferable between different sites or conflict scenarios. The method provides a first pass at identifying the main areas of concern for people and is useful in conservation landscapes where the dialogue can be informed by a holistic perspective across sites. Understanding the nature of local residents’ perceived risks will allow conservation managers to adapt strategies when risk mitigation strategies of local residents might threaten protected wildlife. Given the commonality and interaction between livelihood and conservation stressors found in this study, adaptive conservation strategies may also help support local livelihoods, if those strategies mitigate crop raiding, and protect tree cover; but health care provisioning and delivery and access to medicinal plants must also be addressed (Chapman et al. 2014).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by the National Science Foundation (1114977) and National Geographic Committee on Research and Exploration grants. We are grateful to our Ugandan field assistants for their hard work and dedication, Irene Feretti and Brian Devine for data entry, and our study participants. The authors give a big shout out to Tim Baird for his assistance. Makerere University Biological Field Station, Uganda Wildlife Authority, Uganda National Council for Science and Technology, and many local officials provided useful assistance and granted permission for this research.
Biographies
Joel Hartter
Joel Hartter is an Associate Professor and Associate Director of Professional Education in the Environmental Studies Program at the University of Colorado. His research interests include protected areas, land science, and coupled human and natural systems.
Nicholas Dowhaniuk
is a graduate student at the University of New Hampshire. His interests lie in protected areas, land use/land cover change, and human migration.
Catrina A. MacKenzie
is an Affiliate Member of the Department of Geography at McGill University and an Adjunct Lecturer in the Department of Geography at the University of Vermont. Her research interests include the ecological economics of protected area management, conservation theory in Sub-Saharan Africa, and qualitative methods.
Sadie J. Ryan
is an Assistant Professor of Medical Geography at the University of Florida, in the Department of Geography and the Emerging Pathogens Institute. Her research interests include quantitative landscape ecology, conservation, and disease ecology, with emphasis on tropical environments and the social–ecological context.
Jeremy E. Diem
is an Associate Professor at Georgia State University. His research focuses primarily on climatology, and his main research areas are precipitation variability in the southeastern United States; urban effects on precipitation; climate literacy; air pollution; the North American monsoon; and rainfall in eastern equatorial Africa.
Michael W. Palace
is an Associate Professor in the Department of Earth Sciences and the Institute for the Study of Earth, Oceans and Space at the University of New Hampshire. He is an environmental scientist focusing on vegetation dynamics, landscape ecology, remote sensing, and geospatial science. His research ranges from field studies of forest structure to the use of satellite imagery in an effort to predict disease, understand forest dynamics and biogeochemistry, and find and interpret past human settlement patterns.
Colin A. Chapman
is a Professor in the Department of Anthropology and McGill School of Environment, where he holds a Canada Research Chair Tier 1 position in Primate Ecology and Conservation, is a Killam Research Fellow, and is a fellow of the Royal Society of Canada. He has conducted research in Kibale National Park in Uganda for over 25 years and is interested in the roles of disease, nutrition, and stress in determining primate abundance and how to best to forge a harmony between the needs of local people and conservation.
Footnotes
Research results were communicated by C.A. MacKenzie to village chairpersons around KNP in 2012. This comment was made during one of those feedback meetings, although many chairpersons concurred that the climate for agriculture was better near the park.
Contributor Information
Joel Hartter, Phone: 303-492-9164, Email: joel.hartter@colorado.edu.
Nicholas Dowhaniuk, Email: ndowhaniuk@gmail.com.
Catrina A. MacKenzie, Email: catrina.mackenzie@mail.mcgill.ca
Sadie J. Ryan, Email: sjryan@ufl.edu
Jeremy E. Diem, Email: jdiem@gsu.edu
Michael W. Palace, Email: michael.palace@unh.edu
Colin A. Chapman, Email: colin.chapman@mcgill.ca
References
- Adams WM, Aveling R, Brockington D, Dickson B, Elliot J, Hutton J, Roe D, Vira B, Wolmer W. Biodiversity conservation and the eradication of poverty. Science. 2004;306:1146–1149. doi: 10.1126/science.1097920. [DOI] [PubMed] [Google Scholar]
- Andam K, Ferraro P, Pfaff A, Sanchez- Azofeifa A, Robalino J. Measuring the effectiveness of protected area networks in reducing deforestation. Proceedings of the National Academy of Sciences. 2008;105:16089–16094. doi: 10.1073/pnas.0800437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird TD, Leslie PW, McCabe JT. The effect of wildlife conservation on local perceptions of risk and behavioral response. Human Ecology. 2009;37:463–474. doi: 10.1007/s10745-009-9264-z. [DOI] [Google Scholar]
- Bennett NJ, Dearden P. Why local people do not support conservation: Community perceptions of marine protected area livelihood impacts, governance and management in Thailand. Marine Policy. 2014;44:107–116. doi: 10.1016/j.marpol.2013.08.017. [DOI] [Google Scholar]
- Breiman, L. 1996. Out-of-bag estimation. Technical report, Department of Statistics, University of California, Berkeley.
- Brockington D. Fortress conservation: the preservation of the Mkomazi Game Reserve, Tanzania. Bloomington: Indiana University Press; 2002. [Google Scholar]
- Brockington D, Wilkie D. Protected areas and poverty. Philosophical Transactions of the Royal Society B. 2015;370:2014071. doi: 10.1098/rstb.2014.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A, Clark S, Coad L, Miles L, Bolt K, Roe D. Protecting the future: Carbon, forests, protected areas and local livelihoods. Cambridge: UNEP World Conservation Monitoring Centre; 2008. [Google Scholar]
- Chapman CA, van Bavel B, Bleeker J, Boodman C, Ghai RR, Gogarten JF, Goldberg T, Hartter J, Mechak LE, Omeja PA, Poonawala S, Zastavniouk C. Providing health care to promote people-park relations. Oryx. 2014 doi: 10.1017/S0030605313001592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiyo PI, Cochrane EP. Population structure and behavior of crop-raiding elephants in Kibale National Park, Uganda. African Journal of Ecology. 2005;43:233–241. doi: 10.1111/j.1365-2028.2005.00577.x. [DOI] [Google Scholar]
- Cincotta RP, Wisnewski J, Engelman R. Human population in the biodiversity hotspots. Nature, 2000;404(6781):990–992. doi: 10.1038/35010105. [DOI] [PubMed] [Google Scholar]
- Davis A. ‘Ha! What is the benefit of living next to the park?’ Factors limiting in-migration next to Tarangire National Park. Tanzania. Conservation and Society. 2011;9:25–34. doi: 10.4103/0972-4923.79184. [DOI] [Google Scholar]
- DFID. 1999. Sustainable Livelihoods Guidance Sheets. Retrieved November 15, 2010, from National Strategies for Sustainable Development: http://www.nssd.net/references/SustLiveli/DFIDapproach.htm#Guidance.
- DeFries R, Hansen A, Turner BL, Reid R, Liu J. Land use change around protected areas: management to balance human needs and ecological function. Ecological Applications. 2007;17:1031–1038. doi: 10.1890/05-1111. [DOI] [PubMed] [Google Scholar]
- Diem J, Hartter J, Ryan SJ, Palace M. Validation of satellite-based rainfall products for western Uganda. Journal of Hydrometeorology. 2014;15:2030–2038. doi: 10.1175/JHM-D-13-0193.1. [DOI] [Google Scholar]
- Diem J, Ryan SJ, Hartter J, Palace MW. A recent drying trend in Central Equatorial Africa. Climatic Change. 2014;126:263–272. doi: 10.1007/s10584-014-1217-x. [DOI] [Google Scholar]
- Ferraro PJ, Hanauer MM. Quantifying causal mechanisms to determine how protected areas affect poverty through changes in ecosystem services and infrastructure. PNAS. 2014;111:4332–4337. doi: 10.1073/pnas.1307712111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro PJ, Hanauer MM, Sims KRE. Conditions associate with protected area success in conservation and poverty reduction. PNAS. 2011;108:13913–13918. doi: 10.1073/pnas.1011529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Christopher T. Poverty and biodiversity: measuring the overlap of human poverty and the biodiversity hotspots. Ecological Economics. 2007;62:93–101. doi: 10.1016/j.ecolecon.2006.05.020. [DOI] [Google Scholar]
- Gaston KJ, Jackson SF, Cantú-Salazar L, Cruz-Piñón G. The ecological performance of protected areas. Annual Review of Ecology Evolution and Systematics. 2008;39:93–113. doi: 10.1146/annurev.ecolsys.39.110707.173529. [DOI] [Google Scholar]
- Goldman MJ. Strangers in their own land: Maasai and wildlife conservation in northern Tanzania. Conservation and Society. 2011;9:65–79. doi: 10.4103/0972-4923.79194. [DOI] [Google Scholar]
- Hair JF, Anderson RE, Tatham RL, Black WC. Multivariate data analysis: with readings. Englewood Cliffs: Prentice-Hall; 1995. [Google Scholar]
- Hansen AJ, DeFries R. Ecological mechanisms linking protected areas to surrounding lands. Ecological Applications. 2007;17:974–988. doi: 10.1890/05-1098. [DOI] [PubMed] [Google Scholar]
- Hansen MC, Potapov PV, Moore R, Hancher M, Turubanova SA, Tyukavina A, Thau D, Stehman SV, Goetz SJ, Loveland TR, Kommareddy A, Egorov A, Chini L, Justice CO, Tonshend JRG. High-resolution global maps of 21st-century forest cover change. Science. 2013;342:850–853. doi: 10.1126/science.1244693. [DOI] [PubMed] [Google Scholar]
- Hartter J, Ryan SJ, MacKenzie CA, Goldman A, Dowhaniuk N, Palace MW, Diem JE, Chapman CA. Now there is no land: A story of ethnic migration in a protected area landscape in western Uganda. Population and Environment. 2015;36:452–479. doi: 10.1007/s11111-014-0227-y. [DOI] [Google Scholar]
- Hartter J, Goldman A. Local responses to a forest park in western Uganda: Alternative narratives on fortress conservation. Oryx. 2011;45:60–68. doi: 10.1017/S0030605310000141. [DOI] [Google Scholar]
- Hatcher MJ, Dick JTA, Dunn AM. Disease emergence and invasions. Functional Ecology. 2012;26:1275–1287. doi: 10.1111/j.1365-2435.2012.02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TM. Parks, people, and forest protection: an institutional assessment of the effectiveness of protected areas. World Development. 2006;34:2064–2075. doi: 10.1016/j.worlddev.2006.03.002. [DOI] [Google Scholar]
- Hoffman DM, Fay D, Joppa L. Introduction: human migration to protected area edges in Africa and Latin America: Questioning large-scale statistical analysis. Conservation & Society. 2011;9:1–7. doi: 10.4103/0972-4923.79177. [DOI] [Google Scholar]
- Hothorn T, Buehlmann P, Dudoit S, Molinaro A, Van Der Laan M. Survival ensembles. Biostatistics. 2006;7:355–373. doi: 10.1093/biostatistics/kxj011. [DOI] [PubMed] [Google Scholar]
- Hutton J, Adams WM, Murombedzi JC. Back to the barriers? Changing narratives in biodiversity conservation. Forum for Development Studies. 2005;2:341–370. doi: 10.1080/08039410.2005.9666319. [DOI] [Google Scholar]
- Jones, Z.M., Linder, F. 2015. edarf: Exploratory data analysis using random forests. R package version 0.1.
- Kirtman B, Power SB, Adedoyin JA, Boer GJ, Bojariu R, Camilloni I, Doblas-Reyes FJ, Fiore AM, Kimoto M, Meehl GA, Prather M, Sarr A, Schär C, Sutton R, van Oldenborgh GJ, Vecchi G, Wang HJ. Near-term climate change: projections and predictability. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors. Climate change 2013: The physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- Labbé J, Ford JD, Berrang-Ford L, Donnelly B, Lwasa S, Namanya DB, Twesigomwe S, Research Team IHACC, Harper SL. Vulnerability to the health effects of climate variability in rural southwestern Uganda. Mitigation and Adaptation Strategies for Global Change. 2015 [Google Scholar]
- Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- Lee Eun Sul, Forthofer Ronald N. Analyzing complex survey data. 2. Thousand Oaks: Sage; 2006. [Google Scholar]
- MacKenzie CA, Hartter J. Demand and proximity: Drivers of illegal forest resource extraction. Oryx. 2013;47:288–297. doi: 10.1017/S0030605312000026. [DOI] [Google Scholar]
- MacKenzie CA, Ahabyona P. Elephants in the garden: Financial and social costs of crop raiding. Ecological Economics. 2012;75:72–82. doi: 10.1016/j.ecolecon.2011.12.018. [DOI] [Google Scholar]
- MacKenzie CA, Sengupta RR, Kaoser R. Chasing baboons or attending class: Protected areas and childhood education in Uganda. Environmental Conservation. 2015;42:373–383. doi: 10.1017/S0376892915000120. [DOI] [Google Scholar]
- MacKenzie CA. Filtered meaning: Appreciating linguistic skill, social position and subjectivity of interpreters in cross-language research. Qualitative Methods. 2016;16:167–182. [Google Scholar]
- MacKenzie CA. Accruing benefit or loss from a protected area: Location matters. Ecological Economics. 2012;76:119–129. doi: 10.1016/j.ecolecon.2012.02.013. [DOI] [Google Scholar]
- Maslow AH. A theory of human motivation. Psychological Review. 1943;50:370–396. doi: 10.1037/h0054346. [DOI] [Google Scholar]
- Naimi, B. 2015. Usdm: Uncertainty analysis for species distribution models. R package version 1.1-15. https://CRAN.R-project.org/package=usdm.
- Naughton-Treves L, Alix-Garcia J, Chapman CA. Lessons about parks and poverty from a decade of forest loss and economic growth around Kibale National Park, Uganda. PNAS. 2011;108:13919–13924. doi: 10.1073/pnas.1013332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann RP. Imposing wilderness: struggles over livelihood and nature preservation in Africa. Berkeley: University of California Press; 1998. [Google Scholar]
- Niang, I., Ruppel, O.C., Abdrabo, M.A., Essel, A., Lennard, C., Padgham, J., Urquhart, P. 2014. Africa. Climate Change 2014: Impacts, Adaptation and Vulnerability. Part B: Regional Aspects. In Contribution of working group II to the fifth assessment report of the Intergovernmental Panel on Climate Change, eds Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., Girma, B., Kissel, E.S., Levy, A.N., MacCracken, S., Mastrandrea, P.R., White, L.L. 1199-1265, Cambridge: Cambridge University Press.
- Nicholson SE, Grist JP. The seasonal evolution of the atmospheric circulation over West Africa and Equatorial Africa. Journal of Climate. 2003;16:1013–1030. doi: 10.1175/1520-0442(2003)016<1013:TSEOTA>2.0.CO;2. [DOI] [Google Scholar]
- Oates JF. Myth and reality in the rain forest. Berkeley: University of California Press; 1999. [Google Scholar]
- Patz JA, Daszak P, Tabor GM, Aguirre AA, Pearl A, Epstein J, Wolfe ND, Kilpatrick AM, Foufopoulos J, Molyneux D, Bradley DJ. Unhealthy landscapes: Policy recommendations on land use change and infectious disease emergence. Environmental Health Perspectives. 2004;112:1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumptre AJ, Davenport TRB, Behangana M, Kityo R, Eilu G, Ssegawa P, Ewango C, Meirte D, Kahindo C, Herremans M, Peterhans JK, Pilgrim JD, Wilson M, Languy M, Moyer D. The biodiversity of the Albertine Rift. Biological Conservation. 2007;134:178–194. doi: 10.1016/j.biocon.2006.08.021. [DOI] [Google Scholar]
- Population Reference Bureau (PRB). 2012. 2012 World Population Data Sheet. Washington DC. http://www.prb.org/pdf09/09wpds_eng.pdf].
- Quinn CH, Huby M, Kiswala H, Lovett JC. Local perceptions to livelihood in semi-arid Tanzania. Journal of Environmental Management. 2003;68:111–119. doi: 10.1016/S0301-4797(03)00013-6. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
- Salyer SJ, Gillespie TR, Rwego IB, Chapman CA, Goldberg TL. Epidemiology and molecular relationships of Cryptosporidium spp. in people, primates, and livestock from western Uganda. PLOS Neglected Tropical Diseases. 2012;6:e1597. doi: 10.1371/journal.pntd.0001597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonewald-Cox CM, Bayless JW. The boundary model: a geographic analysis of design and conservation of nature reserves. Biological Conservation. 1986;38:305–322. doi: 10.1016/0006-3207(86)90057-1. [DOI] [Google Scholar]
- Schotthoefer AM, Rohr JR, Cole RA, Koehler AV, Johnson CM, Johnson LB, Beasley VR. Effects of wetland versus landscape variables on parasite communities of Rana pipiens: Links to anthropogenic factors. Ecological Applications. 2011;21:1257–1271. doi: 10.1890/10-0374.1. [DOI] [PubMed] [Google Scholar]
- Sitati NW, Walpole MJ, Smith RJ, Leader-Williams N. Predicting spatial aspects of human-elephant conflict. Journal of Applied Ecology. 2003;40:667–677. doi: 10.1046/j.1365-2664.2003.00828.x. [DOI] [Google Scholar]
- Smith K, Barrett CB, Box PW. Participatory risk mapping for targeting research and assistance: With an example from East African pastoralists. World Development. 2000;28:1945–1959. doi: 10.1016/S0305-750X(00)00053-X. [DOI] [Google Scholar]
- Strobl C, Boulesteix AL, Zeileis A, Hothorn T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinformatics. 2007;8:1. doi: 10.1186/1471-2105-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobl C, Boulesteix AL, Kneib T, Augustin T, Zeileis A. Conditional variable importance for random forests. BMC Bioinformatics. 2008;9:1. doi: 10.1186/1471-2105-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terborgh J, van Schaik C. Why the World Needs Parks. In: Terborgh T, van Schaik C, Davenport L, Rao M, editors. Making parks work: Strategies for preserving tropical nature. Washington D.C.: Island Press; 2002. pp. 3–14. [Google Scholar]
- Uganda Bureau of Statistics (UBoS) The 2002 Uganda population and housing census, main report. Kampala: Uganda Bureau of Statistics; 2005. [Google Scholar]
- Uganda Bureau of Statistics (UBoS) National population and housing census 2014: Provisional results. Kampala: Uganda Bureau of Statistics; 2014. [Google Scholar]
- Uganda and East Africa High Commission . Uganda census, 1959, African population. Nairobi: Statistics Branch, Ministry of Economic Affairs; 1961. [Google Scholar]
- Uganda Wildlife Authority (UWA) 2012. UWA official website: Murchison Falls National Park: http://www.ugandawildlife.org/explore-our-parks/parks-by-name-a-z/murchisonfalls-national-park.
- Venkatachalam L. Behavioural economics for environmental policy. Ecological Economics. 2008;67:640–645. doi: 10.1016/j.ecolecon.2008.01.018. [DOI] [Google Scholar]
- Webber AD, Hill CM. Using participatory risk mapping (PRM) to identify and understand people’s perceptions of crop loss to animals in Uganda. Plos One. 2014;9:e102912. doi: 10.1371/journal.pone.0102912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West P, Igoe J, Brockington D. Parks and people: the social impact of protected areas. Annual Review of Anthropology. 2006;35:251–277. doi: 10.1146/annurev.anthro.35.081705.123308. [DOI] [Google Scholar]
- Wittemyer G, Elsen P, Bean WT, Coleman A, Burton O, Brashares J. Accelerated human population growth at protected area edges. Science. 2008;321:123–126. doi: 10.1126/science.1158900. [DOI] [PubMed] [Google Scholar]
- Zommers Z, MacDonald DW. Protected areas as frontiers for human migration. Conservation Biology. 2012;26:547–556. doi: 10.1111/j.1523-1739.2012.01846.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.